Linked Articles
This article is part of a themed section on Recent Progress in the Understanding of Relaxin Family Peptides and their Receptors. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.10/issuetoc
Tables of Links
| TARGETS | |
|---|---|
| GPCRs a | Enzymes b |
| RXFP1 receptor | ERK1/2 |
| RXFP2 receptor | MMP2 |
| RXFP3 receptor | |
| RXFP4 receptor |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a,b).
The 7th International Conference in the series on ‘Relaxin and related peptides’ covered all aspects of the basic biology of relaxin (human H2‐relaxin), its potential clinical applications and the current clinical trials in acute heart failure. Additionally, the evolution, chemistry, pharmacology, physiology and potential clinical applications of the other members of the relaxin peptide family including, relaxin‐3, insulin‐like peptide 3 (INSL3) and INSL5 were also covered. Relaxin pharmacology has reached an important stage with the recombinant form of the peptide now in extended Phase III clinical trials for the treatment of acute heart failure (see articles by Unemori 2017 and Sarwar et al., 2017) having been identified as one of few approaches with the potential to improve cardiovascular and all‐cause mortality in this condition.
The pleiotropic actions of relaxin have stimulated a wide variety of studies in physiology and pharmacology including, in this issue, actions on the musculo‐skeletal sytem, cancer, fibrosis, reproduction and the cardiovascular system. The chemical structure of the relaxins, all closely related to insulin, is complex and has presented many challenges (Figure 1), but many relaxin peptides have now been synthesized. The challenges and successes achieved in the synthesis of relaxin family peptides are nicely summarized in the article by Akhter Hossain and colleagues (Patil et al., 2017) who identify, from a series of studies in which many different relaxin analogues were synthesized, key residues for activation of the RXFP1 receptors by relaxin. There has been limited success in synthesizing peptide analogues with antagonist activity with BR13/17K being about the best available so far. Much more success has been achieved in studies determining the minimum active relaxin structure with A(4‐24)(B7‐24) H2 relaxin having similar efficacy although lower potency than relaxin. More recently a soluble and easily synthesized single chain peptide, B7‐33, that has a B‐chain with an N‐terminal truncation and C‐terminal elongation has been produced that has very interesting pharmacology. While a relatively weak agonist with low efficacy for cAMP production compared to relaxin, B7‐33 is a potent full agonist for ERK1/2 phosphorylation that promotes matrix metalloproteinase 2 (MMP2) expression with similar potency to relaxin. This suggests that this peptide may have powerful anti‐fibrotic activity but lack the side effects associated with increased cAMP. The anti‐fibrotic actions of relaxin have long been a focus of studies by Chrishan Samuel and colleagues (Samuel et al., 2017). They emphasize the importance of fibrosis as an endpoint in many diseases and estimate that the condition contributes to 45–50% of deaths in Western societies. There are no really effective anti‐fibrotic agents used clinically, but relaxin has a number of properties that suggest it has promise. In particular, relaxin inhibits disease‐related fibrosis, but not normal or unstimulated, fibroblast proliferation, differentiation and matrix production. Relaxin also increases matrix degradation by up‐regulating release and activation of matrix‐degrading MMPs and down‐regulating tissue inhibitor of metalloproteinase (TIMP) activity. The peptide also has anti‐inflammatory, antioxidant, antihypertrophic, anti‐apoptotic, angiogenic, wound healing and vasodilator properties that contribute to the minimization of fibrosis. The limitations to develop relaxin as a therapeutic are also discussed. Most notably, relaxin is less effective in reversing well‐established fibrosis than preventing its development, has a short half life (10 min) and can be administered only parenterally. Newer drugs such as ML290 (Agoulnik et al., 2017) or B7–33 (Patil et al., 2017) may offer solutions to these problems, but their efficacy has yet to be demonstrated. Richard Ivell and colleagues (Ivell et al., 2017) examine the roles of relaxin family peptides in male reproduction. Relaxin was long regarded as a female sex hormone but its pleiotropic effects in the CNS, in fibrosis and in the cardiovascular system together with the discovery of the RXFP1 receptor signalosome with sensitivity to sub‐picomolar concentrations of relaxin suggest that it has much wider roles. In the male reproductive system, they show that relaxin is expressed in the prostate and appears in semen. A role for relaxin in prostate cancer is discussed. In prostate cancer cell lines, relaxin increases proliferation, migration and invasiveness whereas in immunocompromised mice, human cancer xenograft growth is suppressed by relaxin siRNA or a peptide antagonist. The authors inject a note of caution by describing the widely differing roles played by relaxin across species. The emergence of relaxin as a potent therapeutic agent for the treatment of heart disease has further encouraged studies on its actions in the cardiovascular system. Laura Parry and her colleagues (Leo et al., 2017) bring together the classical studies showing that relaxin plays an important role in the cardiovascular changes that occur with pregnancy. Relaxin reduced peripheral resistance associated with vasodilatation, increased arterial compliance, leading to increased GFR and renal plasma flow. More recent work, however, shows that relaxin is also present in non‐pregnant females and male rats suggesting additional roles. It is now becoming clear that relaxin shows great heterogeneity with respect to its actions on blood vessels with distinct signalling mechanisms across different cell types, different localization patterns of RXFP1 receptors and a variety of responses in particular vascular beds. Over the longer term evidence is presented showing that relaxin also has remodelling effects on the vasculature. Mohsin Sarwar and colleagues (Sarwar et al., 2017) continue this theme and integrate their studies using human vascular cells with animal models of disease and with the early clinical observations with relaxin. They emphasize the variety of signalling pathways activated by relaxin (Figure 1), the differences between endothelial cells, smooth muscle cells and fibroblasts and the variation observed between similar cell types taken from different regions. The cellular models also reflect one of the major concerns with relaxin therapy – bell‐shaped concentration–response relationships – and provide an explanation if not a solution to this problem. In animal models of cardiovascular disease, ischaemia/reperfusion, myocardial infarction, hypertensive heart disease and cardiomyopathy, relaxin has cardioprotective effects. In humans, infusion of the peptide is safe and is associated with favourable haemodynamic changes with the most recent phase III RELAX‐AHF trial being extended to detect mortality differences. The review concludes by highlighting the current knowledge gaps associated with the clinical use of relaxin.
Figure 1.
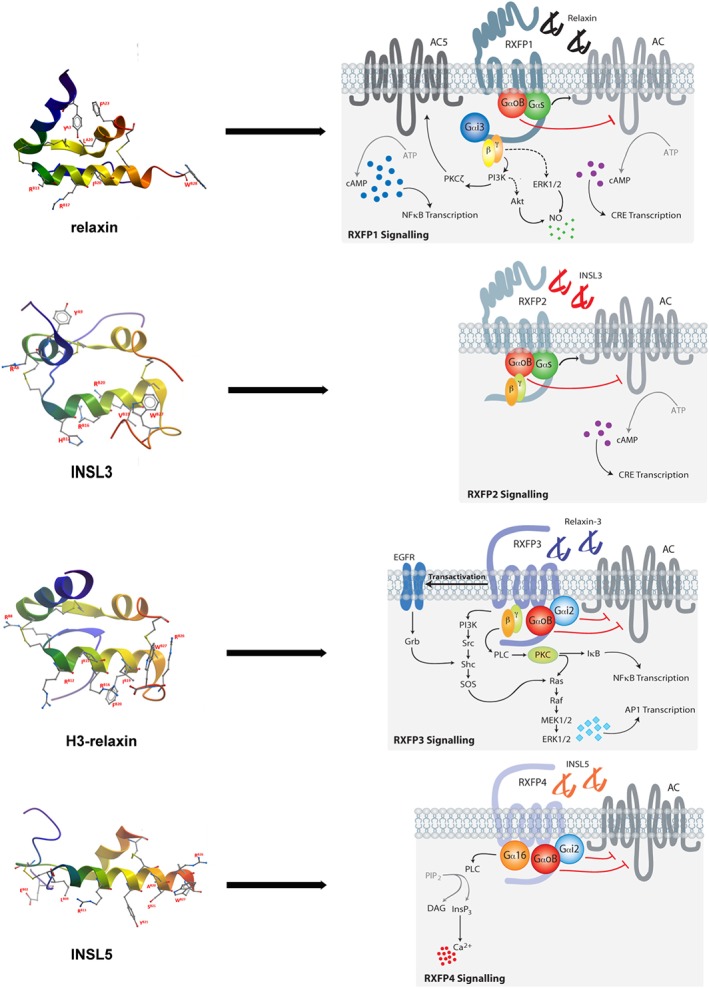
Relaxin family peptides with their cognate receptors and major signalling pathways.
Alberto Ferlin and colleagues (Ferlin et al., 2017) suggest intriguing roles for relaxin in bone and muscle pathologies, ageing and drug development. The peptide promotes bone remodelling by stimulating mineralization and bone resorption with the balance between these two processes finely tuned by the modulation of anti‐osteoclastogenesis factors released by osteoblasts. However, relaxin also facilitates muscle healing by enhancing the recruitment of satellite cells, improving vascular perfusion, inhibiting fibrosis and probably by promoting neo‐angiogenesis. They suggest that relaxin could be a new clinical candidate for conditions such as sarcopenia, muscle healing and trauma, bone disorders and fractures. Elaine Unemori (Unemori, 2017) has had a long association with relaxin, conducting some of the seminal basic research with the peptide as well as being heavily involved with many of the recent clinical trials. Here, she brings together the findings from the clinical trials and discusses the deductions that can be drawn from them. The evidence strongly supports systemic vasodilation by relaxin in humans that is well maintained with no development of tolerance. With regard to renal vasodilation the findings generally support an increase in RPF and GFR following relaxin treatment. In this regard, the effects of relaxin on renal function compare favourably with other vasodilators tested in this condition. Clinical trials performed to assess the efficacy of relaxin in scleroderma failed perhaps related to the heterogeneity of the disease process. There is also a nice summary of progress with the current RELAX‐AHF‐2 trial not only for Acute Heart failure (AHF) but also Worsening Heart Failure (WHF) and the early stage trials to treat chronic heart failure, coronary artery disease, portal hypertension and renal failure. Some of the problems with the use of relaxin as a therapeutic agent may be addressed by recent findings showing that molecules other than relaxin can interact with the RXFP1 receptor. Thomas Klonisch and colleagues (Klonisch et al., 2017) have identified a novel relationship between C1q/TNF‐related protein 8 (CTRP8) and the relaxin receptor RXFP1. Their studies reveal activation of RXFP1 receptors in human glioblastomas in the absence of relaxin leading to the identification of CTRP8 as a novel agonist of this receptor. They discuss intriguing evidence suggesting that the interaction between CTRP8 and RXFP1 receptors is involved in the invasiveness of glioblastomas and the intractable nature of these cancers. Both CTRP8 and relaxin activate similar signalling pathways including cAMP, pERK1/2 and PI3K in cells expressing RXFP1 receptors (Figure 1). Knockdown of these receptors or inhibition of their signalling pathway reduces invasiveness, suggesting new avenues (frontiers) for clinical development. Alex Agoulnik and Juan Marugan (Agoulnik et al., 2017) review their recent success in developing low MW, non‐peptide, agonists of the RXFP1 receptor (Figure 1). This receptor is an unusual GPCR with a large and complex extracellular N terminal domain to which relaxin binds with high affinity bringing it into contact with extracellular loop 2 and triggering an interaction between the LDLa module at the N terminus and extracellular loop 3, thus acting as a tethered ligand. The low MW compounds typified by ML290 are likely to interact near the tethered ligand activation site and have characteristics such as specificity, stability, bioavailability and low cost suggesting that they may be suitable for development as improved therapeutics. The review also provides a useful summary of clinical trials with relaxin to date that make a strong case for RXFP1 receptors as a therapeutic target. The clinical development of these low MW compounds has been challenged by a lack of agonist activity at the mouse receptor due to the specificity of the interaction between ML290 and the human RXFP1 allosteric activation site. This promises to be overcome by this team that has recently developed a humanized mouse model.
The RXFP2 receptor and its cognate ligand INSL3 are closely related to RXFP1 receptors and relaxin, but they have received much less attention due probably to their specialized roles in reproduction although other functions are now being described. In their review of relaxin family peptide chemistry, Akhter Hossain and colleagues (Patil et al., 2017) point out the key residues in INSL3 required for binding. However, unlike RXFP1, it has been relatively easy to synthesize high affinity peptide antagonists of RXFP2 by shortening the N‐terminus of INSL3. Further refinement of the peptides has led to the development of a minimized analogue INSL3: B10‐24/B1‐31 (INSL3 B‐chain dimer analogue 8) an antagonist with potential as an inhibitor of both male and female fertility. Ivell et al. (2017) continue the reproductive theme bringing together evidence that shows that circulating INSL3 is derived from Leydig cells in the testis and probably modulates bone metabolism. They also emphasize the important roles of the INSL3/RXFP2 receptor system in foetal development. The roles of INSL3 and RXFP2 receptors in bone are examined in detail by Ferlin et al. (2017) who were the first to report that men with an inactivating T222P mutation of the RXFP2 gene suffered from osteoporosis suggesting that INSL3 had a role in controlling bone metabolism. This was supported by studies that showed that RXFP2−/− mice had a similar phenotype and that osteoblasts in culture expressed RXFP2 receptors and displayed a proliferative response with exposure to INSL3. More evidence is required to determine if INSL3 has anabolic effects on skeletal muscle. The authors conclude by summarizing the clinical issues surrounding INSL3 and the importance of maintaining adequate plasma levels of the hormone.
With RXFP3 receptors and the cognate ligand, relaxin‐3, attention shifts to the brain as, although relaxin‐3 is the ancestral relaxin that activates both RXFP1 and RXFP3 receptors, it is considered, on the basis of its distribution pattern, to be primarily a neuropeptide with modulatory roles in arousal, feeding, stress responses, cognition and drug addiction. Therefore, the RXFP3 receptor represents an interesting therapeutic target. Akhter Hossain and colleagues (Patil et al., 2017) again provide a detailed description of the chemistry of relaxin‐3 analogues. They show that the binding mode of relaxin‐3 to RXFP3 receptors is quite different from that of the same ligand binding to RXFP1 receptors and that greater selectivity for RXFP3/4 receptor binding is achieved by the chimeric relaxin‐3/INSL5 (R3/I5) analogue that has the A‐chain of relaxin‐3 replaced by that of INSL5. Further modifications to produce R3(BΔ23‐27)R/I5 provided a potent antagonist. Determination of the minimum active structure has identified agonist analogues that increase feeding and reduce anxiety in rats. Sherie Ma and colleagues (Ma et al., 2017) provide a detailed account of the search for the physiological role of relaxin‐3 and RXFP3 receptors. They provide striking examples of discrete localization of relaxin‐3 in neurones of monkey, rat and mouse and describe how projections from the nucleus incertus to particular areas of the brain point to likely roles of relaxin‐3/RXFP3 receptors in energy and endocrine homeostasis, circadian rhythms, reward and emotional processing. Detailed studies are being carried out to determine the relationship between relaxin‐3/RXFP3 receptors and other neurotransmitter systems (Figure 1). For instance, RXFP3 receptor agonists increase and antagonists decrease feeding; in substance abuse antagonists may reduce relapsing behaviour; circadian rhythms are known to be disturbed in relaxin‐3 or in RXFP3 receptor KO mice; and learning and memory are improved by agonists and anxiety is reduced. An increasing variety of experimental tools are being introduced to study relaxin‐3/RXFP3 receptor physiology. Juliane Calvez and colleagues (Calvez et al., 2017) review the recent evidence suggesting that the effects of relaxin‐3 are gender specific. In relaxin‐3 knockout mice, only the females are hypoactive and in rats the effects of relaxin‐3 on food intake and body weight are greater in females than males. Repeated exposure to food restriction and stress caused increased relaxin‐3 expression and food intake in female but not in male rats and stress‐induced binge eating in female rats was blocked by an RXFP3 receptor antagonist. Centrally administered relaxin‐3 increased the expression of CRF in the paraventricular hypothalamic nucleus in male but not in female rats where relaxin‐3 increased the expression of orexin in the lateral hypothalamus. Because orexin directly activates neurons expressing relaxin‐3 in the nucleus incertus, this may intensify behavioural activation and feeding in females. There is also evidence to suggest that there is differential expression of RXFP3 receptors in the brain of male and female rats. The gender specificity of many of the effects of relaxin‐3 may suggest novel strategies for the treatment of metabolic and eating disorders.
Gavin Dawe and colleagues (Kumar et al., 2017) explore the viability of the relaxin‐3/RXFP3 receptor system as a target for the treatment of neuropsychiatric disorders. Starting from the marked changes that occur in the relaxin‐3 system with stress they go on to link these to anxiety, depression, appetite disorders and cognitive function. Central administration of RXFP3 receptor agonists has an anxiolytic effect, likely to be mediated by release of oxytocin. Relaxin‐3 may also be involved in depression since dysregulation of the 5HT system in depression is accompanied by alterations in relaxin‐3 tone. Given the well‐established role of relaxin‐3 in controlling appetite in rats, RXFP3 antagonists may have potential for the treatment of antipsychotic drug‐induced weight gain and hyperphagia associated with depression. The authors conclude by outlining the issues challenging the future development of relaxin‐3/RXFP3 as a drug target, including the lack of low MW ligands available to study the receptor – many peptide agonists and antagonists are available but currently suitable only as tool compounds. They also point to the lack of clinical studies that could help establish physiological roles and connections of the relaxin‐3/RXFP3 receptor system with neuropsychiatric disorders.
The RXFP4 receptor and its cognate ligand INSL5 has been one of the least studied of the relaxin family systems. However, as outlined in the review of the chemistry of INSL5 by Akhter Hossain and colleagues (Patil et al., 2017), the identification of its main location in the body in enteroendocrine L‐cells where it is co‐expressed with GLP‐1 has stimulated recent interest. INSL5 seems to have a role in controlling food intake and may come to be regarded as a decretin for its effects on metabolism. One obstacle to studies involving INSL5 has been the difficulty in synthesizing the human orthologue but mouse INSL5 has proved to be both simpler to synthesize and more potent. The key residues involved in interaction with RXFP4 receptors have been identified and a minimized analogue that is both easier to assemble and potent is now available. The paper by Ang et al., (2017) is one of the few published to date that examines signalling pathways activated by RXFP4 receptors (Figure 1). Not only do these receptors inhibit cAMP production but they also activate p38MAPK, ERK1/2, Akt and S6RP. The principal G proteins involved have been identified and the interaction with GRK2 and β‐arrestins suggests that RXFP4 receptors undergo desensitisation and internalisation. INSL5 also inhibited insulin secretion from MIN6 insulinoma cells and inhibited cAMP production in NCI‐H716 enteroendocrine cells. The improved understanding of RXFP4 receptor signal transduction mechanisms will underpin the development of novel anti‐obesity, anti‐diabetic and/or appetite‐modulating drugs.
The papers in this themed issue on recent research involving relaxin family peptides and their receptors illustrate the progress that has been made during the last few years in exploring their physiological roles and clinical potential. The development of low MW compounds active at RXFP1 receptors offers further potential for the future.
Conflict of interest
The author wishes to acknowledge a conflict of interest and highlight that he is a co‐author on the papers by Sarwar et al. (2017), and Ang et al. (2017), in this issue and that he also has co‐authored papers with Akhter Hossain, Chrishan Samuel and Alexander Agoulnik.
Summers, R. J. (2017) Recent progress in the understanding of relaxin family peptides and their receptors. British Journal of Pharmacology, 174: 915–920. doi: 10.1111/bph.13778.
This themed issue of the British Journal of Pharmacology stems from the 7th International Conference on Relaxin and Related Peptides held in Kuching, Sarawak, Malaysia from 20 to 24 September 2015.
Footnotes
RELAX‐AHF‐2 did not meet its primary endpoints of reduction in cardiovascular death through Day 180 or reduced worsening heart failure through Day five when added to standard therapy in patients with AHF. https://www.novartis.com/news/media-releases/novartis-provides-update-phase-iii-study-rlx030-serelaxin-patients-acute-heart
References
- Agoulnik AI, Agoulnik IU, Hu X, Marugan J (2017). Synthetic non‐peptide low molecular weight agonists of the relaxin receptor 1. Br J Pharmacol 174: 977–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang SY, Hutchinson DS, Patil N, Evans BA, Bathgate RAD, Halls ML et al (2017). Signal transduction pathways activated by insulin‐like peptide 5 at the relaxin family peptide RXFP4 receptor. Br J Pharmacol 174: 1077–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvez J, de Ávila C, Timofeeva E (2017). Sex‐specific effects of relaxin‐3 on food intake and body weight gain. Br J Pharmacol 174: 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlin A, De Toni L, Sandri M, Foresta C (2017). Relaxin and insulin‐like peptide 3 in the musculoskeletal system: from bench to bedside. Br J Pharmacol 174: 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivell R, Agoulnik AI, Anand‐Ivell R (2017). Relaxin‐like peptides in male reproduction a human perspective. Br J Pharmacol 174: 990–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonisch T, Glogowska A, Thanasupawat T, Burg M, Krcek J, Pitz M et al (2017). Structural commonality of C1q TNF‐related proteins and their potential to activate relaxin/insulin‐like family peptide receptor 1 signalling pathways in cancer cells. Br J Pharmacol 174: 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar JR, Rajkumar R, Jayakody T, Marwari S, Hong JM, Ma S et al (2017). Relaxin' the brain: a case for targeting the nucleus incertus network and relaxin‐3/RXFP3 system in neuropsychiatric disorders. Br J Pharmacol 174: 1061–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo CH, Jelinic M, Ng HH, Marshall SA, Novak J, Tare M et al (2017). Vascular actions of relaxin: nitric oxide and beyond. Br J Pharmacol 174: 1002–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Smith CM, Blasiak A, Gundlach AL (2017). Distribution, physiology and pharmacology of relaxin‐3/RXFP3 systems in brain. Br J Pharmacol 174: 1034–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil NA, Rosengren KJ, Separovic F, Wade JD, Bathgate RAD, Hossain MA (2017). Relaxin family peptides: structure–activity relationship studies. Br J Pharmacol 174: 950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CS, Royce SG, Hewitson TD, Denton KM, Cooney TE, Bennett RG (2017). Anti‐fibrotic actions of relaxin. Br J Pharmacol 174: 962–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar M, Du X‐J, Dschietzig TB, Summers RJ (2017). The actions of relaxin on the human cardiovascular system. Br J Pharmacol 174: 933–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unemori E (2017). Serelaxin in clinical development: past, present and future. Br J Pharmacol 174: 921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]


