Abstract
Relaxin‐3 is a member of a superfamily of structurally‐related peptides that includes relaxin and insulin‐like peptide hormones. Soon after the discovery of the relaxin‐3 gene, relaxin‐3 was identified as an abundant neuropeptide in brain with a distinctive topographical distribution within a small number of GABAergic neuron populations that is well conserved across species. Relaxin‐3 is thought to exert its biological actions through a single class‐A GPCR – relaxin‐family peptide receptor 3 (RXFP3). Class‐A comprises GPCRs for relaxin‐3 and insulin‐like peptide‐5 and other peptides such as orexin and the monoamine transmitters. The RXFP3 receptor is selectively activated by relaxin‐3, whereas insulin‐like peptide‐5 is the cognate ligand for the related RXFP4 receptor. Anatomical and pharmacological evidence obtained over the last decade supports a function of relaxin‐3/RXFP3 systems in modulating responses to stress, anxiety‐related and motivated behaviours, circadian rhythms, and learning and memory. Electrophysiological studies have identified the ability of RXFP3 agonists to directly hyperpolarise thalamic neurons in vitro, but there are no reports of direct cell signalling effects in vivo. This article provides an overview of earlier studies and highlights more recent research that implicates relaxin‐3/RXFP3 neural network signalling in the integration of arousal, motivation, emotion and related cognition, and that has begun to identify the associated neural substrates and mechanisms. Future research directions to better elucidate the connectivity and function of different relaxin‐3 neuron populations and their RXFP3‐positive target neurons in major experimental species and humans are also identified.
Linked Articles
This article is part of a themed section on Recent Progress in the Understanding of Relaxin Family Peptides and their Receptors. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.10/issuetoc
Abbreviations
- AgRP
agouti‐related peptide
- BNST
bed nucleus of the stria terminalis
- CRF
corticotrophin‐releasing factor
- HCN
hyperpolarisation‐activated cyclic nucleotide‐gated channels
- IGL
intergeniculate leaflet
- MCH
melanin‐concentrating hormone
- NPY
neuropeptide‐Y
- POMC
pro‐opiomelanocortin (ACTH)
- PVN
paraventricular nucleus of hypothalamus
Tables of Links
| TARGETS | |
|---|---|
| GPCRs a | Enzymes b |
| 5‐HT1A receptor | ChAT |
| CRF1 receptor | ERK1 |
| CRF2 receptor | ERK2 |
| D2 receptor | GAD |
| OX2 receptor | PKA |
| RXFP1 receptor | Tryptophan hydroxylase |
| RXFP2 receptor | |
| RXFP3 receptor | |
| RXFP4 receptor |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a, bAlexander et al., 2015a, 2015b).
Introduction
Discovery of relaxin‐3 and RXFP3 receptors
Relaxin‐3 was discovered in 2001 by searching for homologues of the relaxin gene in the Celera Discovery System and Celera Genomics databases (Bathgate et al., 2002) and due to its predominant expression in brain, was subsequently classified as a neuropeptide. Relaxin‐3, like other relaxin and insulin‐like peptide family members, is a 5 kDa peptide that consists of two chains with three disulphide bonds (Bathgate et al., 2002; Liu et al., 2003b; Hossain et al., 2013). All family members contain the characteristic sequence ‘RXXXRXX(I/V)’ within their B‐chain, which is essential for binding the different cognate receptors (Bullesbach and Schwabe, 2000; Bathgate et al., 2002; 2006b).
The cognate receptor for relaxin‐3 is relaxin‐family peptide 3 receptor (RXFP3) (Bathgate et al., 2006a). Also known as GPCR135 (Liu et al., 2003b), it was first discovered in 2000 and named somatostatin‐ and angiotensin‐like peptide receptor, due to its high amino acid similarity with somatostatin receptor‐5 and the angiotensin AT1 receptor (Matsumoto et al., 2000). RXFP3 is a Gi/o‐protein‐coupled receptor, and its activation produces inhibition of intracellular cAMP accumulation and activation of ERK1/2 (Liu et al., 2003b; van der Westhuizen et al., 2007). Although in cell‐based studies, relaxin‐3 can bind and activate three related GPCRs – RXFP3, RXFP1 (originally named LGR7; Sudo et al., 2003) and RXFP4 (originally named GPCR142; Liu et al., 2003a), there is considerable evidence that RXFP3 is the native receptor for relaxin‐3. Firstly, RXFP3 displays the highest affinity for relaxin‐3 (Bathgate et al., 2006b), and the genes encoding the peptide and protein have phylogenetically co‐evolved (Hsu et al., 2005; Wilkinson et al., 2005). Furthermore, there is a strong overlap between the distribution of relaxin‐3‐positive nerve fibres and RXFP3 mRNA/binding sites throughout the rat (Sutton et al., 2004; Ma et al., 2007), mouse (Smith et al., 2010) and macaque brain (Ma et al., 2009b, c). Moreover, RXFP4 is primarily expressed within the gastrointestinal tract and is largely absent from brain (Sutton et al., 2006) and is, in fact, a pseudogene in rat (Chen et al., 2005). Also, although RXFP1 is expressed widely throughout the rodent brain (Ma et al., 2006), its distribution pattern does not correspond with that of the relaxin‐3 innervation, and relaxin is expressed by distinct forebrain neuron populations (Ma et al., 2006). Finally, relaxin‐3 is the only relaxin‐peptide family member that can activate RXFP3 (Liu et al., 2003b), whereas relaxin is the preferred ligand for RXFP1 and also binds to RXFP2 (Sudo et al., 2003). The related insulin‐like peptide 5 is uniquely expressed in enteroendocrine L‐cells of the colon, and it is the cognate ligand for RXFP4 (Liu et al., 2005b; Sutton et al., 2006; Grosse et al., 2014).
Distribution of relaxin‐3 and RXFP3 in the brain – a road map to function
Relaxin‐3/RXFP3 systems conserved across various mammalian species
The brain is the main site of relaxin‐3 mRNA synthesis, with high levels of expression observed in various species including zebrafish (Donizetti et al., 2009), mouse (Bathgate et al., 2002; Smith et al., 2010), rat (Burazin et al., 2002; Tanaka et al., 2005), macaque (Ma et al., 2009b) and human (Liu et al., 2003b). The presence and anatomical distribution of relaxin‐3‐producing neurons has been best studied in rat and mouse brain, with the largest population observed in the brainstem nucleus incertus (Figure 1; Bathgate et al., 2002; Burazin et al., 2002; Tanaka et al., 2005; Ma et al., 2007; Smith et al., 2010; Ryan et al., 2011). Relaxin‐3 neurons, which use GABA as their primary transmitter, are also present in smaller populations in the pontine raphé nucleus (~350 neurons) medial and ventrolateral periaqueductal grey (~550 neurons), and in an area dorsal to the substantia nigra (~350 neurons), relative to the ~2000 relaxin‐3‐positive neurons in the rat nucleus incertus (Tanaka et al., 2005; Ma et al., 2007; Smith et al., 2010).
Figure 1.
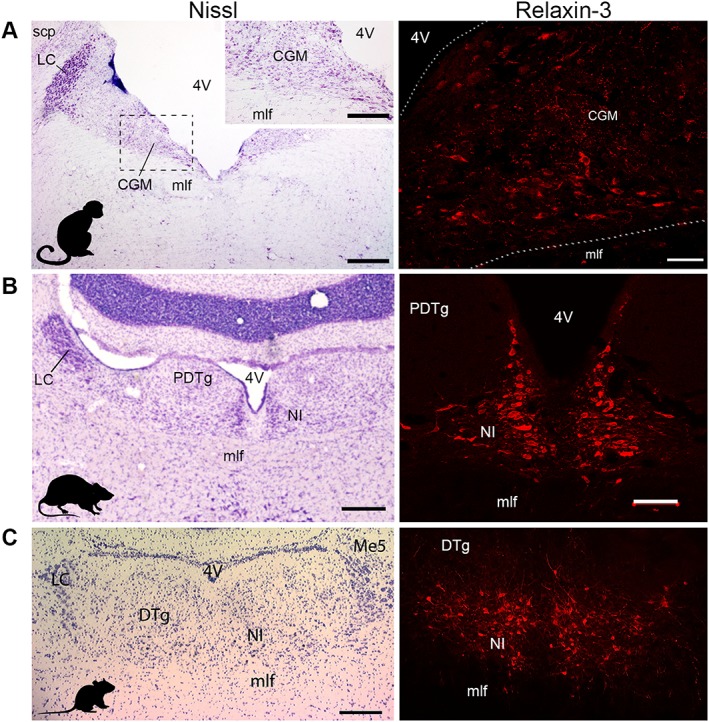
The nucleus incertus and its relaxin‐3 neurons are similarly located in the midline periventricular central grey of non‐human primate (macaque), rat and mouse brain and are conserved across species (adapted from Ma et al., 2007; 2009b; Smith et al., 2010). Nucleus incertus (NI) is the primary source of neurons expressing relaxin‐3 mRNA and abundant relaxin‐3 immunoreactivity, which are located in the midline periventricular central grey at the base of the fourth ventricle (4V) of (A) macaque, (B) rat and (C) mouse. Abbreviations: CGM, mid central grey; DTg, dorsal tegmental nucleus; LC, locus coeruleus; Me5, mesencephalic trigeminal nucleus; mlf, medial longitudinal fasciculus; PDTg, posterodorsal tegmental nucleus; scp, superior cerebellar peduncle. Scale bars, (A) Nissl, 0.6 mm, inset, 80 μm, relaxin‐3, 0.2 mm; (B) Nissl, 0.3 mm, relaxin‐3, 0.1 mm; (C) 0.2 mm.
Major inputs to the nucleus incertus, which lies in the midline periventricular central grey, arise from the prefrontal cortex, lateral habenula, interpeduncular nucleus, median raphe and lateral hypothalamus (see Ma and Gundlach, 2015 for review), but only limited data are available regarding the specific inputs to the relaxin‐3 and non‐relaxin‐3 neurons in the area. Additionally, the proximity of the nucleus incertus to the fourth ventricle in rodents, primates and humans (Ma and Gundlach, 2015) makes it a potential target for neurohumoral signals, as described for similarly located structures like the dorsal raphe nucleus (Torterolo et al., 2008). Nonetheless, the neural inputs identified point to a likely role for nucleus incertus/relaxin‐3/RXFP3 networks in the integration of multiple physiological functions, including energy and endocrine homeostasis, circadian rhythmicity, reward and emotional processing (Figure 2). Moreover, nucleus incertus neurons broadly innervate cortical and subcortical structures, such as the prefrontal and cingulate cortex, septum, hippocampus, thalamus and hypothalamus, and innervate the brainstem (Goto et al., 2001; Olucha‐Bordonau et al., 2003; Ma and Gundlach, 2015), suggesting that nucleus incertus relaxin‐3 neurons integrate behavioural and physiological responses to internal and external stimuli.
Figure 2.
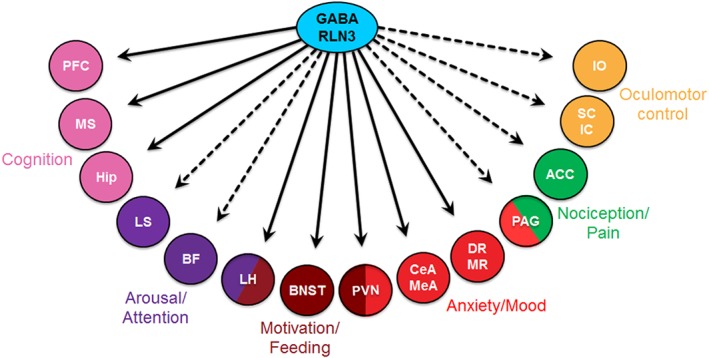
Schematic illustration of some of the major downstream neural targets of relaxin‐3 neurons and the likely ‘tested’ ( ) or putative untested (
) or putative untested (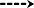 ) functional roles of relaxin‐3/RXFP3 signalling in the coordinated regulation of modalities including cognition, arousal, motivation, anxiety, mood, pain and oculomotor control. Abbreviations: ACC, anterior cingulate cortex; BF, basal forebrain; CeA, central amygdala; DR, dorsal raphe; Hip, hippocampus; IC, inferior colliculus; IO, inferior olive; LH, lateral hypothalamus; LS, lateral septum; MeA, medial amygdala; MR, median raphe; MS, medial septum; PAG, periaqueductal grey; PFC, prefrontal cortex; PVN, paraventricular hypothalamic nucleus; SC, superior colliculus.
) functional roles of relaxin‐3/RXFP3 signalling in the coordinated regulation of modalities including cognition, arousal, motivation, anxiety, mood, pain and oculomotor control. Abbreviations: ACC, anterior cingulate cortex; BF, basal forebrain; CeA, central amygdala; DR, dorsal raphe; Hip, hippocampus; IC, inferior colliculus; IO, inferior olive; LH, lateral hypothalamus; LS, lateral septum; MeA, medial amygdala; MR, median raphe; MS, medial septum; PAG, periaqueductal grey; PFC, prefrontal cortex; PVN, paraventricular hypothalamic nucleus; SC, superior colliculus.
In the rat, the distribution of relaxin‐3‐containing fibres throughout the brain largely parallels that of nucleus incertus efferent projections assessed by anterograde neural tract‐tracing (Goto et al., 2001; Olucha‐Bordonau et al., 2003), suggesting that a substantial component of axonally‐transported relaxin‐3 originates from nucleus incertus. However, there is evidence for distinct relaxin‐3 pathways arising from the smaller populations outside nucleus incertus. For example, the thalamic intergeniculate leaflet (IGL) receives dense relaxin‐3 projections (Tanaka et al., 2005; Ma et al., 2007; Smith et al., 2010) that arise from neurons in the periaqueductal grey, not the nucleus incertus. Indeed, RXFP3 agonist peptides depolarise neuropeptide‐Y (NPY) neurons in the IGL (Figure 3; Blasiak et al., 2013), which are known to modulate suprachiasmatic nucleus function and associated circadian rhythms. Therefore, further studies are required to establish the detailed projection patterns of the different relaxin‐3 neuron populations, a task that may eventually be facilitated by genetic and/or viral based methods (see e.g. Schwarz et al., 2015).
Figure 3.
Activation of RXFP3 receptors excites or inhibits intergeniculate leaflet neurons, depending on their neurochemical nature (adapted from Blasiak et al., 2013). (A) A zero current‐clamp recording illustrating the depolarising effect of bath‐applied RXFP3 agonist, R3/I5 (100 nM, horizontal bar). Upwards deflections represent truncated action potentials present on top of calcium spikes evoked by membrane potential recovery from hyperpolarisation induced by current injection (downward deflections), and a confocal projection image of the neuron depolarised by R3/I5 stained for biocytin injected into the neuron (red) and neuropeptide Y (NPY) immunoreactivity (yellow) revealing the NPY nature of the neuron recorded. Scale bar, 10 μm. (B) A zero current‐clamp recording illustrating the hyperpolarising effect of bath‐applied R3/I5 (100 nM, horizontal bar) on the membrane potential and firing properties of another intergeniculate leaflet neuron, and a confocal projection image of the neuron hyperpolarised by R3/I5 stained for biocytin (red) and NPY immunoreactivity (yellow) revealing the NPY‐negative nature of the neuron recorded. Scale bar, 10 μm.
The distribution of relaxin‐3‐containing nerve fibres is similar in rat, mouse and macaque brain (Ma et al., 2009b), and ‘matches’ the distribution of RXFP3, as reflected by the distribution of RXFP3 mRNA, and binding sites for a relaxin‐3 agonist analogue, [125I]‐R3/I5 (Sutton et al., 2004; Ma et al., 2007; Smith et al., 2010). The relaxin‐3/RXFP3 system can be generally viewed as being closely associated with functional circuits involving the septum and hippocampus (septohippocampal system) and hippocampal‐modulating regions, the hypothalamus, limbic areas and the thalamus/cortex. For further details, see Ma and Gundlach (2007) and Smith et al. (2011).
Notably, however, the presence of a strong relaxin‐3 innervation to the infralimbic, prelimbic and anterior cingulate and posterior retrosplenial areas of the cortex in rat and mouse was not observed in the macaque brain (Ma et al., 2009b). Otherwise, the distribution of the relaxin‐3 innervation largely parallels that of nucleus incertus projections, which have been demonstrated to be positioned to modulate various higher‐cognitive brain circuits, related to behavioural planning and state, motivation, emotion, and learning and memory (Goto et al., 2001; Olucha‐Bordonau et al., 2003). With respect to learning and memory, the dense relaxin‐3 innervation of the septum (Olucha‐Bordonau et al., 2012) and hippocampus further suggests the relaxin‐3/RXFP3 system modulates cognition via the septohippocampal system and associated effects on hippocampal function (Ma et al., 2009a). To date, however, anatomical and functional studies in human are limited, although in a preliminary study, relaxin‐3‐like immunoreactivity was reported to be present in neurons in the dorsal raphe and pontine reticular nuclei, and regions of the dorsal and ventral tegmental nucleus, with immunoreactive fibres in the ventrolateral tegmental area, basis pontis, pontine nucleus and pontocerebellar tracts (Silvertown et al., 2010), and confirmatory studies are now required.
In other studies, human neocortex lysates from Alzheimer's disease patients were reported to contain a moderately higher level of RXFP3 protein detected by immunoblotting, which correlated with longitudinal scores of depression (Lee et al., 2016), and the RXFP3 antiserum was shown to recognize an appropriate sized protein, although tissues from Rxfp3 gene knockout mice were not tested. Also, in a cohort of patients treated with antipsychotics, two RXFP3 polymorphisms and a relaxin‐3 gene polymorphism displayed significant associations with hypercholesterolaemia, suggesting a role for relaxin‐3/RXFP3 signalling in metabolic disturbances linked to antipsychotic treatment (Munro et al., 2012). In a cohort of female patients, a moderate increase in serum relaxin‐3 levels was correlated with component traits of metabolic syndrome (Ghattas et al., 2013), although in this study, the specificity of the assay for relaxin‐3 detection was not fully demonstrated, so further confirmation of such links is required. A further issue, given the growing preclinical evidence for a role of relaxin‐3/RXFP3 signalling in modulating central processes underlying cognition and behaviour, is a need for more comprehensive studies of the system in human brain and its potential involvement in, or therapeutic impact on, dementia, neurodegeneration and neuropsychiatric disorders (see Kumar et al., 2017).
Physiology of relaxin‐3 and RXFP3 in the brain
Responsiveness to stress
Substantial anatomical and functional data (e.g. Potter et al., 1994; Bittencourt and Sawchenko, 2000; Banerjee et al., 2010) suggest the nucleus incertus and its relaxin‐3 neuron population are highly ‘stress‐reactive’ (see Ryan et al., 2011 for review). Notably, while dorsal raphe serotonergic neurons express corticotrophin‐releasing factor (CRF) receptors 1 and 2 (CRF1/2) (Kirby et al., 2008), the nucleus incertus expresses higher levels of CRF1 than CRF2 receptors (Bittencourt and Sawchenko, 2000; Van Pett et al., 2000; Justice et al., 2008). Neurogenic stress in rats resulting from forced‐swim, increased relaxin‐3 heteronuclear RNA and mRNA levels in the nucleus incertus, via a CRF1 receptor‐dependent action (Banerjee et al., 2010). A major nucleus incertus neuron population expressing CRF1 receptors (including relaxin‐3‐containing neurons) exhibited a long‐lasting and non‐desensitizing depolarisation response to CRF (Ma et al., 2013). These responses differ from those within the neighbouring dorsal raphe nucleus, where serotonergic and non‐serotonergic neurons display differential, dose‐dependent responses to CRF that are rapidly desensitised (Kirby et al., 2008). Similarly, relaxin‐3 neurons exhibited increased firing frequency following i.c.v. infusion of CRF (1–3 μg), whereas decreased firing was only observed in relaxin‐3 negative neurons (Figure 4; Ma et al., 2013). These findings suggest that distinct neural populations in the nucleus incertus respond differentially to the stress hormone, but relaxin‐3 neurons are robustly stimulated by CRF. The stress reactivity of other relaxin‐3 neuron populations has yet to be investigated.
Figure 4.
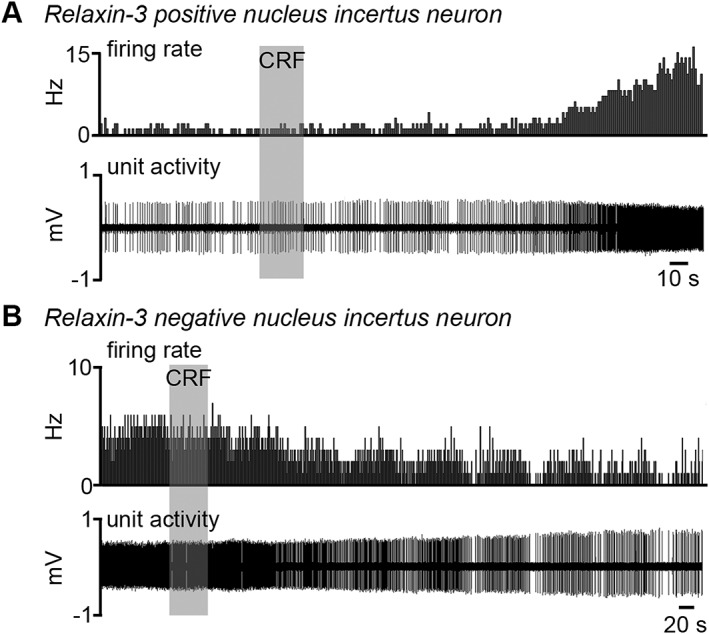
Variations in the response of different types of nucleus incertus neurons to CRF in vivo (adapted from Ma et al., 2013). Extracellular recording and juxtacellular‐filling of nucleus incertus neurons in rat revealed that (A) relaxin‐3 neurons increased firing in response to i.c.v. administration of CRF, whereas (B) some non‐relaxin‐3 neurons exhibited decreased firing in response to CRF, suggesting specific but complex responses to the stress hormone.
Alternatively, the activity of hypothalamic CRF neurons has been reported to be influenced by central administration of relaxin‐3, although the nature of these actions is currently unclear. I.c.v. infusion of relaxin‐3 has been shown to increase c‐fos (a marker of neuronal activation) and CRF mRNA expression in CRF neurons in the rat paraventricular nucleus of the hypothalamus (PVN) (Watanabe et al., 2010), and to elevate plasma adrenocorticotropic hormone levels (Watanabe et al., 2010; McGowan et al., 2014). Thus, there appears to exist a reciprocal interaction between relaxin‐3 and CRF systems, but further studies are required to determine the nature of any direct or indirect effects of relaxin‐3 inputs on the activity of CRF neurons and related physiological/behavioural measures of hypothalamic CRF neural activity. Studies are also required to catalogue the location and identity of the CRF neurons that innervate nucleus incertus relaxin‐3 neurons as there are many candidate extrahypothalamic CRF neuron populations that may do so (Lenglos et al., 2013; Ma et al., 2013; Walker et al., 2016). More generally, there is a need to identify and characterise other neurochemical/neural inputs to relaxin‐3 neurons that are altered by acute or chronic stressors, such as the hypothalamic orexinergic neurons (Blasiak et al., 2015; Kastman et al., 2016).
Pharmacological effects of RXFP3 activation
Neurophysiological effects
Relaxin‐3 activation of its cognate receptor, RXFP3, leads to the inhibition of intracellular cAMP accumulation and activation of the ERK1/2 enzyme in cell‐based assays (Liu et al., 2003b; van der Westhuizen et al., 2007). Regulation of the cAMP pathway is a common intracellular signalling cascade target for neuropeptides (e.g. CRF, vasoactive intestinal peptide and calcitonin gene‐related peptide) (Haug and Storm, 2000) and other transmitters, including catecholamines (Pedarzani and Storm, 1995). cAMP activates the PKA enzyme, which phosphorylates target proteins, including ion channels that can mediate suppression of membrane ion currents (e.g. the slow calcium‐activated potassium current) (Haug and Storm, 2000; Hu et al., 2011). Moreover, cAMP can exert direct effects on ion channels independent of PKA, such as hyperpolarization‐activated cyclic nucleotide‐gated (HCN) channels, whereby activation increases non‐selective Ih cation currents that lead to membrane depolarisation (Pedarzani and Storm, 1995; Sun et al., 2003). Currently, there are few published reports of the direct impact of RXFP3 activation on the physiological or neurochemical activity of target neurons, but these studies are underway, and there are several candidate target areas/neurons for investigation.
For example, the medial septum component of the septohippocampal system is a major innervation target of relaxin‐3 neurons and contains a high density of RXFP3 mRNA expressing neurons (Sutton et al., 2004; Ma et al., 2007). Electrical stimulation of the nucleus incertus in anaesthetized rats evoked hippocampal θ oscillations and lesions of the nucleus incertus abolished θ rhythm evoked by brainstem stimulation (Nunez et al., 2006). Moreover, selective activation of RXFP3 receptors in the medial septum promoted hippocampal θ rhythm, as well as spatial memory and exploratory activity (Ma et al., 2009a). In this regard, HCN h‐currents exist in septal fast‐spiking GABAergic and, to a lesser extent, fast‐firing glutamatergic neurons (Sotty et al., 2003); rhythmic firing at θ frequency is characteristic of all HCN‐expressing neurons (Varga et al., 2008). Therefore, the role of relaxin‐3 in the regulation of septohippocampal activity may rely on RXFP3‐dependent modulation of cAMP in GABAergic septal neurons, which play a critical role in synchronizing the hippocampal neuron network at θ frequency (Toth et al., 1997). Importantly, inhibition of cAMP accumulation reduces neuronal excitability and produces membrane hyperpolarisation (Molosh et al., 2013) and RXFP3 activation inhibits a population of IGL neurons in vitro (Figure 3B; Blasiak et al., 2013).
In addition to GABAergic neurons, hippocampal θ rhythm is also regulated by cholinergic pacemaker neurons of the medial septum (Yoder and Pang, 2005). A recent study reported that i.c.v. administration of the selective RXFP3 agonist, RXFP3‐A2, increased ERK phosphorylation in septal cholinergic neurons (20 and 60 min post‐injection) and impaired spatial working memory in a spontaneous alternation test assessed 5 min post‐treatment (Albert‐Gasco et al., 2016). ERK1/2 activation is capable of increasing neuronal excitability through inhibition of transient potassium (A‐type) currents (Fu et al., 2008), but the recent study did not assess the direct or indirect nature of the excitatory/inhibitory effect of RXFP3 activation on different septal neurons, as the site of peptide administration was outside the septum (Albert‐Gasco et al., 2016). Moreover, these recent behavioural findings contrast with those from earlier studies, which reported an increase in the power of hippocampal θ activity following infusion of the RXFP3 agonist, R3/I5, directly into the medial septum, and an impairment in spatial memory performance in the spontaneous alternation task with intra‐septal infusion of an RXFP3 antagonist, R3(BΔ23–27)R/I5 (Ma et al., 2009a). Thus, additional studies are required to investigate the precise nature of relaxin‐3/RXFP3 signalling within the medial septum, which may differ depending on the neural circuits and the neuronal cell types involved when using different ‘pharmacological’ approaches. Notably, however, a key goal is to determine the physiological/behavioural effects of ‘global’ RXFP3 modulation initiated via a peripheral route of administration, as this is vital in a therapeutic context.
Feeding and other motivated behaviours
The first reported pharmacological effect of relaxin‐3 on behaviour in rats was a potent orexigenic action (McGowan et al., 2005; see also Calvez et al., 2017). In satiated rats, relaxin‐3 injected into the lateral cerebral ventricle (180 pmol) or the PVN (18 pmol) during the early light phase, produced a marked increase in food intake. This orexinergic response did not appear to involve classical peptidergic feeding pathways, as no change in NPY, pro‐opiomelanocortin (POMC) or agouti‐related peptide (AgRP) mRNA levels was produced by the peptide. Later studies indicated that chronic intra‐PVN relaxin‐3 injections (180 pmol, twice a day for 7 days) also promoted food intake, an effect associated with an increase in plasma leptin levels and decreased thyroid‐stimulating hormone levels (McGowan et al., 2006). Similar effects were produced by chronic (14‐day) relaxin‐3 infusion into the cerebral ventricles via osmotic minipumps (Hida et al., 2006), which in addition to the increase in food intake and body weight, caused severe hyperleptinaemia and hyperinsulinaemia – symptoms that accompany obesity in humans (Leon‐Cabrera et al., 2013). A caveat of these early studies was the possible activation of RXFP3 and RXFP1 receptors by exogenously administered relaxin‐3, as both are expressed in the hypothalamus and PVN (Sutton et al., 2004; Ma et al., 2006; Bathgate et al., 2006b; Ganella et al., 2013b).
Studies using the first selective RXFP3 agonist, R3/I5 (Liu et al., 2005a; Sutton et al., 2009) and the ‘next generation’ minimised agonist, RXFP3‐A2 (Shabanpoor et al., 2012) confirmed the involvement of RXFP3 receptors in promoting feeding in rats. Furthermore, the likely involvement of oxytocin and vasopressin signalling in the orexigenic action of relaxin‐3 was revealed as viral‐mediated, chronic secretion of R3/I5 in the PVN region (Ganella et al., 2013a), which produced a robust reduction in whole hypothalamic oxytocin and vasopressin mRNA levels (50% and 25% decrease relative to control, respectively). Importantly, chronic activation of RXFP3 receptors in this study led to a modest, but significant, increase in body weight and in daily food intake, and so similar studies using an RXFP3 antagonist to determine its ability to attenuate feeding in rats would be of interest. In this regard, RXFP3 antagonist peptides are capable of blocking acute agonist‐induced feeding (Kuei et al., 2007; Haugaard‐Kedstrom et al., 2011) and stress‐induced increase in sucrose intake in binge‐like eating prone, but not binge‐like eating resistant, female rats (Calvez et al., 2016, 2017). Therefore, while these studies suggest a lack of a strong direct influence of RXFP3 activation on hypothalamic NPY, AgRP and POMC neurons, the mechanisms and hypothalamic neural circuits underlying relaxin‐3‐induced feeding including effects via oxytocin and/or vasopressin, and other feeding‐related peptides, such as orexins, require further investigation. These studies should also examine other experimental species such as mice and non‐human primates and investigate the impact of stress and different diet compositions on outcomes.
Other motivation and stress‐sensitive behaviours are also influenced by relaxin‐3/RXFP3 signalling, including alcohol seeking and self‐administration, and stress‐induced relapse to alcohol seeking following abstinence in alcohol‐preferring (iP) rats (Ryan et al., 2013b). Infusion of the RXFP3‐selective antagonist, R3(B1–22)R, into the lateral cerebral ventricle or directly into the bed nucleus of the stria terminalis (BNST) of iP rats significantly attenuated lever pressing for alcohol, and cue‐ and stress‐induced reinstatement of lever pressing (Ryan et al., 2013b). Importantly, these rats display increased stress/CRF responsiveness, and decreased brain CRF levels (Ehlers et al., 1992); and relaxin‐3 mRNA levels in the nucleus incertus are positively correlated with their alcohol and sucrose intake (Ryan et al., 2014). Together, these findings suggest relaxin‐3/RXFP3 signalling in key hypothalamic and limbic circuits is capable of integrating stress‐related external and internal information, by regulating the networks responsible for orexigenic and goal‐directed (motivated) behaviours.
Although most relaxin‐3‐related pharmacological research to date has been conducted in rats, studies in mice have contributed to our knowledge of relaxin‐3 biology. In agreement with a role in motivated feeding, which is well‐established in rats, i.c.v. infusion of the RXFP3 antagonist, R3(B1–22)R in mice reduced the consumption of palatable food and of regular chow during the early dark phase and following mild food deprivation (Smith et al., 2014a). Furthermore, i.c.v. infusion of this same RXFP3 antagonist reduced the consumption of NaCl (salt) in sodium‐depleted mice (Smith et al., 2015), and Rxfp3 gene knockout mice displayed reduced motivation to consume sucrose compared to wildtype controls (Walker et al., 2015b). Despite a clear ability of relaxin‐3/RXFP3 signalling to modulate feeding in both rats and mice, it is interesting that central infusion of RXFP3 agonists (or native relaxin‐3 peptide) potently increases food consumption in rats (e.g. Shabanpoor et al., 2012), but not mice (Smith et al., 2013b; 2014a). The reason for this species discrepancy is not obvious, as, for example, both species display strong and roughly equivalent regional patterns of RXFP3 expression within hypothalamic feeding centres (Ma et al., 2007; Smith et al., 2010). However, the neurochemical identity of RXFP3‐positive neurons within each of these regions, and their efferent and afferent connectivity, remains to be determined in each species. For example, differences exist between rat and mouse hypothalamic melanin‐concentrating hormone (MCH) neurons as reflected by their gene expression and projection patterns, birthdates and a divergence in their developmental differentiation, which may underlie the observed species‐specific effects of MCH signalling in the control of feeding behaviour and the sleep/wake cycle (Croizier et al., 2010).
Another consummatory behaviour relaxin‐3 signalling is able to modulate in both rats and mice is alcohol consumption. In line with rat studies, in which i.c.v. infusion of the RXFP3 antagonist R3(B1–22)R reduced alcohol seeking (Ryan et al., 2013b), Rxfp3 gene knockout mice on a C57BL/6J background displayed reduced alcohol preference relative to wildtype controls following chronic stress (Walker et al., 2015a). This study also demonstrated that basal alcohol preferences were equivalent between genotypes; while a recent study reported that male Rln3 gene knockout mice on a C57BL/6N background displayed increased baseline alcohol intake compared with wildtype controls (Shirahase et al., 2016). These differences may be attributable to genetic differences in the C57BL/6 mice used, as it has been established that substrains of these mice display marked behavioural differences (Kiselycznyk and Holmes, 2011). Again, further studies are required to explore these possibilities and clarify the true nature and biological importance of the alcohol consumption differences observed.
Circadian rhythm and arousal
An ability of relaxin‐3/RXFP3 signalling to promote a range of consummatory behaviours is in line with its likely primary role in driving arousal and motivated behaviour more broadly (Smith et al., 2011; Ma and Gundlach, 2015). For example, male and female relaxin‐3 (Rln3) (Smith et al., 2012) and Rxfp3 gene knockout mice (Hosken et al., 2015) display reduced circadian dark phase running wheel activity compared to wildtype controls (Figure 5). Furthermore, acute i.c.v. injection of the RXFP3 antagonist, R3(B1–22)R, reduced food anticipatory activity displayed by pre‐conditioned mice (Smith et al., 2014a), and viral vector‐mediated chronic secretion of an RXFP3 agonist within the mouse cerebral ventricular system reduced locomotor habituation to a novel environment (Smith et al., 2013a). Central arousal systems are also strongly involved in mediating the response to stress (Smith et al., 2014b), and similar to rats (Ryan et al., 2013a), i.c.v. injection of an RXFP3 agonist reduced (elevated) anxiety‐like behaviour in mice (Zhang et al., 2015). Although subtle signs of altered anxiety‐like behaviour have been detected in Rln3 (Watanabe et al., 2011) and Rxfp3 knockout mice (Hosken et al., 2015), life‐long relaxin‐3 or RXFP3 deletion did not alter depressive‐like behaviours relative to wildtype controls during methamphetamine withdrawal (Haidar et al., 2016). Although the mechanisms underlying the ability of relaxin‐3/RXFP3 signalling to promote arousal and modulate stress responses in mice are not known, based on the similar distribution of ligand and receptor in both species (Ma et al., 2007; Smith et al., 2010), mechanisms identified in rats (such as modulation of the septohippocampal system, amygdala and PVN; see above) are likely to be involved.
Figure 5.
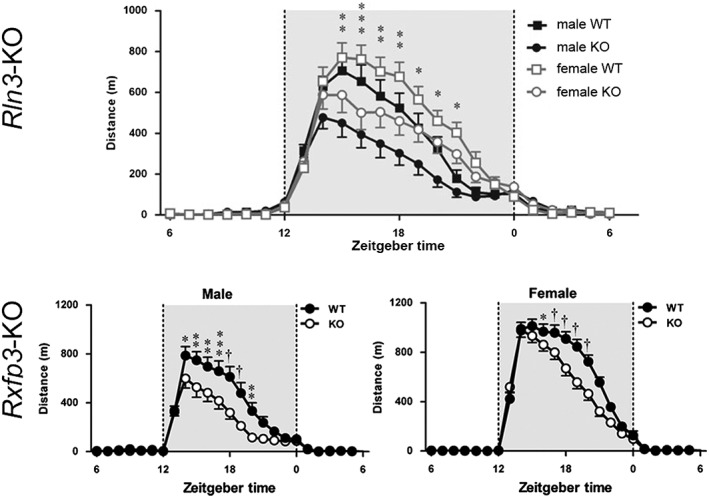
‘Whole‐of‐life’ deletion (knockout) of the relaxin‐3 or the Rxfp3 gene results in circadian hypoactivity in adult mice (adapted from Smith et al., 2012; Hosken et al., 2015). The distance travelled on home‐cage voluntary running wheels by male and female Rln3 (Smith et al., 2012) and Rxfp3 (Hosken et al., 2015) gene knockout mice is markedly less than their wildtype littermates, possibly reflecting a reduced level of sustained attention or motivation. Distance is shown as m·h–1 during an average 24 h day. Grey shading indicates the dark phase.
Furthermore, in the context of arousal, recent studies have demonstrated that nucleus incertus relaxin‐3 neurons receive an excitatory orexinergic innervation from the lateral hypothalamus and perifornical area, and that orexin‐A produces depolarisation and action potential firing of neurons in vitro via the OX2 receptor (Blasiak et al., 2015). Conversely, nucleus incertus relaxin‐3 neurons also express inhibitory D2 dopamine receptors, which, when pharmacologically activated, result in decreased locomotor activity in rats (Kumar et al., 2015, 2017).
Among the brain sites that might underlie the relaxin‐3/RXFP3 signalling modulation of arousal patterns, the IGL, which is a primary regulator of circadian rhythm, is a candidate. The largely GABAergic and NPY‐expressing IGL neurons have strong projections to the suprachiasmatic nucleus, which is considered to be the main circadian ‘pacemaker’ in the circadian timing system (Morin and Blanchard, 2005; Moore, 2013). The IGL displays dense RXFP3 mRNA levels and relaxin‐3‐immunoreactive nerve fibres (Tanaka et al., 2005; Ma et al., 2007), but is not a target of nucleus incertus projections (Goto et al., 2001; Olucha‐Bordonau et al., 2003). Thus, retrograde neural tract‐tracing studies identified that a large population of relaxin‐3 neurons in the periaqueductal grey innervate the IGL (Blasiak et al., 2013). Furthermore, in vitro electrophysiological studies of these neurons revealed that RXFP3 activation led to excitation or inhibition of neurons (Figure 3), depending on their neurochemical nature; suggesting that the actions of relaxin‐3/RXFP3 signalling can be bidirectional/opposing within different neural circuits (Blasiak et al., 2013).
Other findings that support a putative involvement of relaxin‐3/RXFP3 in arousal arise from studies of the nucleus incertus, which has been described as a ‘key GABAergic projection hub for the regulation of cortical arousal’ (Brown and McKenna, 2015). Consistent with this hypothesis, our laboratory has recently demonstrated that chemogenetic activation of the nucleus incertus network in rats led to long‐lasting wakefulness, and enhanced EEG measures of cortical arousal/desynchronisation that was independent of movement; and enhanced vigilance in response to impending threat (Ma et al., 2016). Similarly, unilateral electrical stimulation of the nucleus incertus induced forward locomotion and rotation, accompanied by an increase in movement velocity (Farooq et al., 2016). In both studies, it was suggested that the promotion of arousal and movement may be via the septohippocampal system, as glutamatergic neuron activation in the medial septum controls the initiation and velocity and locomotion, and associated entrainment of hippocampal θ oscillations (Fuhrmann et al., 2015; Robinson et al., 2016). Furthermore, the septohippocampal system also underlies anxiety‐related hippocampal θ rhythm (Wells et al., 2013). Thus, further studies examining the impact of nucleus incertus (and relaxin‐3) neurons in modulating stress‐associated arousal and related behaviours will be of immense interest.
Learning, memory and hippocampal θ rhythm
Neural substrates underlying learning and memory chiefly reside in the hippocampus and associated brain regions that regulate its activity, particularly an activity known as hippocampal θ rhythm, which are distinct oscillations at θ frequency (4–12 Hz) that reflect mnemonic processing (Vertes, 2005). The θ rhythm is detectable in the EEG recording of brain activity in many mammals, and the temporal aspects and behavioural correlations of these brain rhythms detected are highly conserved (Buzsaki et al., 2013). In addition to memory, hippocampal θ rhythm has also been associated with arousal states, exploratory behaviour and spatial navigation, rapid eye movement sleep and anxiety‐related behaviours (Vertes, 1984; 2005; McNaughton and Gray, 2000; Stujenske et al., 2014).
The ‘septohippocampal system’ is an important regulator of hippocampal θ rhythm, whereby GABAergic and cholinergic neurons located in the medial septum function as ‘pacemakers’ for the genesis and pacing of hippocampal θ rhythm (Vertes and Kocsis, 1997; Simon et al., 2006; Hangya et al., 2009). Both septum and hippocampus receive a dense relaxin‐3 innervation, and relaxin‐3‐positive nerve fibres make close contacts (putative synapses) with various types of pacemaker cells, including ChAT, and inhibitory GAD67‐positive neurons, and those containing the calcium‐binding proteins parvalbumin, calbindin and calretinin (Olucha‐Bordonau et al., 2012). In addition, medial septum calretinin‐positive neurons project to the nucleus incertus (Sanchez‐Perez et al., 2015), forming a closed‐loop neural circuit, although the function of this bidirectional feedback is still not known. The effects of relaxin‐3 on cognitive performance and EEG markers of septohippocampal activity have been investigated in rats, whereby the RXFP3‐selective agonist, R3/I5, or antagonist, R3(BΔ23–27)R/I5, were locally infused into the medial septum. Infusion of the RXFP3 agonist significantly enhanced, whereas the antagonist attenuated hippocampal θ power in freely‐moving rats, and impaired spatial working memory performance in a spontaneous alternation task (Ma et al., 2009a).
In electrophysiological studies in anaesthetised rats, hippocampal θ oscillations were induced by electrical stimulation of the nucleus incertus (Nunez et al., 2006). In contrast, brainstem‐induced hippocampal θ rhythm was blocked by electrolytic lesion of, or muscimol injection into, the nucleus incertus (Nunez et al., 2006), suggesting it may act as a key relay node between the brainstem and forebrain θ‐pacing regions (Brown and McKenna, 2015). Notably in this regard, nucleus incertus relaxin‐3 neurons exhibit spontaneous firing activity that is coherent with the early ascending phase of θ oscillations (while other neurons do not), further supporting the proposed functional link (Ma et al., 2013).
Emotional and anxiety‐like behaviour
Dysfunction in neural circuits controlling emotional behaviour underlies disorders such as anxiety, depression and related psychiatric illnesses. In addition to broad modulatory effects on cognition and arousal, which have interrelated importance for affective behaviour, RXFP3 receptors are also densely expressed in regions critical for emotional control, such as the amygdala, ventral hippocampus, BNST and prefrontal cortex (see Smith et al., 2014b for review). A key transmitter that is an established regulator of anxiety states and anxiety‐related behaviour is 5‐HT (serotonin), and the dorsal raphe nucleus is a major source of this monoamine (Hale et al., 2012). Early studies in rats demonstrated that most relaxin‐3 neurons of the nucleus incertus co‐express the inhibitory 5‐HT1A receptor and depletion of 5‐HT by pharmacological inhibition of tryptophan hydroxylase, resulted in increased expression of relaxin‐3, suggesting that 5‐HT normally suppresses relaxin‐3 expression (Miyamoto et al., 2008). More recent studies revealed that treatment of rats with the anxiogenic benzodiazepine, FG‐7142, resulted in enhanced anxiety‐like behaviour in the elevated plus maze that was associated with activated populations of relaxin‐3 neurons in the nucleus incertus and serotonergic neurons in the dorsal raphe (Lawther et al., 2015). Such co‐activation of serotonergic and relaxin‐3 systems suggests a functional association between these signalling systems that warrants further investigation.
Indeed, previous studies demonstrated that i.c.v. administration of relaxin‐3 (Nakazawa et al., 2013) or the RXFP3 receptor‐selective agonist, RXFP3‐A2 (Ryan et al., 2013a), resulted in anxiolytic and antidepressant‐like behavioural effects in rats, although in studies in which relaxin‐3 mRNA knockdown was achieved by viral driven expression of relaxin‐3 microRNA in nucleus incertus of rats, no overt changes in measures of anxiety‐like behaviour were observed in the light–dark box (Callander et al., 2012). However, because relaxin‐3 neurons are highly stress‐responsive, such a behavioural change may have been better observed if pre‐stressed rats were studied. Administration of typical (chlorpromazine and fluphenazine) and atypical (clozapine) antipsychotic drugs to rats activates nucleus incertus neurons, suggesting that nucleus incertus relaxin‐3 neurons are directly responsive to antipsychotic drugs of various modes of action (Rajkumar et al., 2013).
Novel technologies to investigate relaxin‐3/RXFP3 function in vivo
The recent boom in the use of viral vector technology for the dissection of complex neural circuits underlying physiology and behaviour (Schaffer et al., 2008) has revolutionised our understanding of how the brain works. Gene delivery technology, coupled with optogenetic and chemogenetic methods, now allows researchers to investigate and dissect complex neural circuit neuroanatomy and neurophysiology (Wulff and Wisden, 2005; Betley and Sternson, 2011; Deisseroth, 2015; Roth, 2016), and furthermore, gene therapy is currently being assessed for clinical applications related to CNS treatments (Ojala et al., 2015). To date, there have been limited studies using these technologies to investigate the relaxin‐3/RXFP3 system, but viral vectors have been used to determine physiological effects of relaxin‐3 mRNA knockdown in the nucleus incertus (Callander et al., 2012), and effects of chronic local secretion of a selective RXFP3 agonist peptide in hypothalamus (Ganella et al., 2013a) on feeding and body weight regulation. The effect of chemogenetic activation of the nucleus incertus on cortical and behavioural arousal (as reflected by EEG and locomotor activity changes) has also been explored (Ma et al., 2016).
Future applications of optogenetic and chemogenetics methods to study the role of relaxin‐3 and RXFP3‐regulated neurons should be greatly facilitated by the development of tools such as viral vectors driven by a cell‐specific promoter to regulate relaxin‐3 neurons and/or a relaxin‐3‐Cre or RXFP3‐Cre transgenic mouse/rat, which would allow discrete functional manipulations of relaxin‐3 neurons and their specific target neurons (Madisen et al., 2015). Furthermore, such technology could also address the importance of relaxin‐3 and GABAergic co‐transmission in brain, in studies similar to those used to evaluate histaminergic and GABAergic co‐transmission in controlling wakefulness (Yu et al., 2015).
In light of growing evidence the nucleus incertus is a heterogeneous population of relaxin‐3 positive and negative neurons that co‐express a range of inhibitory neuron markers and other neuropeptides (Ma et al., 2013), viral‐based methods could be used to map the efferent and afferent connections of relaxin‐3 neurons, which would complement and advance current mappings of the ‘whole’ nucleus incertus (Goto et al., 2001; Olucha‐Bordonau et al., 2003). The connectivity of the populations of relaxin‐3 neurons in the pontine raphé nucleus, periaqueductal grey and dorsal substantia nigra could also be characterised.
Relaxin‐3/RXFP3 related transgenic mouse strains
Although ‘whole‐body/whole‐of‐life’ Rln3 and Rxfp3 gene knockout mouse strains have been useful tools for exploring relaxin‐3/RXFP3 biology (Watanabe et al., 2011; Smith et al., 2012; Hosken et al., 2015), they potentially undergo developmental compensatory adaptations in their behaviour and brain chemistry. For example, differences in the consumption of palatable food (Smith et al., 2014a) and salt appetite (Smith et al., 2015) were detected in wildtype mice following acute injection of the RXFP3 antagonist, R3(B1–22)R, compared with vehicle, but there were no differences in these behaviours between Rxfp3 gene knockout and wildtype mice. Therefore, anticipated future studies that utilize conditional Rxfp3 gene knockout mice, which might combine the use of ‘floxed Rxfp3’ mice with viral vector‐induced expression of Cre recombinase to produce local receptor deletion, will be important, not only to avoid developmental compensation (i.e. provide temporal control) but also to allow chronic Rxfp3 gene depletion within one or more target region(s) of the brain (i.e. spatial control). Transgenic mice that express a fluorophore within RXFP3‐positive neurons would be of benefit for histological and electrophysiological studies, as a fully‐validated RXFP3 antibody is not currently available. Indeed, studies using commercially‐available RXFP3 antibodies have been conducted (Meadows and Byrnes, 2015; Albert‐Gasco et al., 2016; Lee et al., 2016), although these antibodies have not yet been tested in Rxfp3 gene knockout mice, which will be an important validation of specificity. Finally, transgenic mice that express Cre recombinase within relaxin‐3‐ or RXFP3‐positive neurons would be invaluable for facilitating viral‐vector optogenetic or designer receptors exclusively activated by designer drugs approaches to selectively activate or inhibit target neuron populations within conscious, freely‐behaving mice, as this approach has been widely adopted to study neurons of a particular neurochemical phenotype (see e.g. Krashes et al., 2014; Fuzesi et al., 2016).
Conclusions and future perspectives
In the light of the anatomical and/or functional interactions demonstrated between relaxin‐3 and multiple transmitter and neuropeptide systems (i.e.5‐HT, dopamine, CRF and orexin); evidence for a role for relaxin‐3/RXFP3 signalling in arousal, motivation and cognition, particularly in response to stress; and a range of additional putative interactions and functions, research on relaxin‐3/RXFP3 neurobiology should flourish in the future, in both basic investigations and those in relation to human neuropathology and the system's plasticity in animal models of psychiatric illness, metabolic/feeding disorders and neurodegenerative disease. For example, there is growing evidence for the impact of stress and CRF in the aetiology of neurodegenerative disorders such as Alzheimer's disease (Campbell et al., 2015; Park et al., 2015; Zhang et al., 2016), and the involvement of 5‐HT, orexin and other arousal networks in normal and abnormal cognitive processing and in the expression of comorbid symptoms of sleep dysregulation, anxiety and depression in multiple disorders (Chen et al., 2015; Kohler et al., 2016). These findings suggest there are exciting opportunities to examine the importance/involvement and/or therapeutic potential of relaxin‐3/RXFP3 signalling for the treatment of cognitive, affective and mood deficits and/or neurological disease progression in a range of clinical conditions or their validated experimental models (Smith et al., 2014b; see Kumar et al., 2017).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
Research by the authors is supported by research grants from the National Health and Medical Research Council (Australia) (1024885, 1067522, 1106330 ALG), a NARSAD Independent Investigator Award (ALG), the Polish Ministry of Science and Higher Education (N N303 569939, AB and ALG); The National Science Centre (Poland) (DEC‐2012/05/D/NZ4/02984, AB and ALG) and an EU‐funded Exchange Program (FP7‐PEOPLE‐IRSES PIRSES‐GA‐2012‐318997 NEUREN project, AB and ALG).
Ma, S. , Smith, C. M. , Blasiak, A. , and Gundlach, A. L. (2017) Distribution, physiology and pharmacology of relaxin‐3/RXFP3 systems in brain. British Journal of Pharmacology, 174: 1034–1048. doi: 10.1111/bph.13659.
References
- Albert‐Gasco H, Garcia‐Aviles A, Moustafa S, Sanchez‐Sarasua S, Gundlach AL, Olucha‐Bordonau FE et al. (2016). Central relaxin‐3 receptor (RXFP3) activation increases ERK phosphorylation in septal cholinergic neurons and impairs spatial working memory. Brain Struct Funct. doi:10.1007/s00429-016-1227-8. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The concise guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The concise guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Shen PJ, Ma S, Bathgate RAD, Gundlach AL (2010). Swim stress excitation of nucleus incertus and rapid induction of relaxin‐3 expression via CRF1 activation. Neuropharmacology 58: 145–155. [DOI] [PubMed] [Google Scholar]
- Bathgate RAD, Ivell R, Sanborn BM, Sherwood OD, Summers RJ (2006a). International union of pharmacology LVII: recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacol Rev 58: 7–31. [DOI] [PubMed] [Google Scholar]
- Bathgate RAD, Lin F, Hanson NF, Otvos L Jr, Guidolin A, Giannakis C et al. (2006b). Relaxin‐3: improved synthesis strategy and demonstration of its high‐affinity interaction with the relaxin receptor LGR7 both in vitro and in vivo . Biochemistry 45: 1043–1053. [DOI] [PubMed] [Google Scholar]
- Bathgate RAD, Samuel CS, Burazin TCD, Layfield S, Claasz AA, Reytomas IG et al. (2002). Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene. Novel members of the relaxin peptide family. J Biol Chem 277: 1148–1157. [DOI] [PubMed] [Google Scholar]
- Betley JN, Sternson SM (2011). Adeno‐associated viral vectors for mapping, monitoring, and manipulating neural circuits. Hum Gene Ther 22: 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt JC, Sawchenko PE (2000). Do centrally administered neuropeptides access cognate receptors? An analysis in the central corticotropin‐releasing factor system. J Neurosci 20: 1142–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasiak A, Blasiak T, Lewandowski MH, Hossain MA, Wade JD, Gundlach AL (2013). Relaxin‐3 innervation of the intergeniculate leaflet of the rat thalamus ‐ neuronal tract‐tracing and in vitro electrophysiological studies. Eur J Neurosci 37: 1284–1294. [DOI] [PubMed] [Google Scholar]
- Blasiak A, Siwiec M, Grabowiecka A, Blasiak T, Czerw A, Blasiak E et al. (2015). Excitatory orexinergic innervation of rat nucleus incertus – implications for ascending arousal, motivation and feeding control. Neuropharmacology 99: 432–447. [DOI] [PubMed] [Google Scholar]
- Brown RE, McKenna JT (2015). Turning a negative into a positive: ascending GABAergic control of cortical activation and arousal. Front Neurol 6: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullesbach EE, Schwabe C (2000). The relaxin receptor‐binding site geometry suggests a novel gripping mode of interaction. J Biol Chem 275: 35276–35280. [DOI] [PubMed] [Google Scholar]
- Burazin TCD, Bathgate RAD, Macris M, Layfield S, Gundlach AL, Tregear GW (2002). Restricted, but abundant, expression of the novel rat gene‐3 (R3) relaxin in the dorsal tegmental region of brain. J Neurochem 82: 1553–1557. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Logothetis N, Singer W (2013). Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron 80: 751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callander GE, Ma S, Ganella DE, Wimmer VC, Gundlach AL, Thomas WG et al. (2012). Silencing relaxin‐3 in nucleus incertus of adult rodents: a viral vector‐based approach to investigate neuropeptide function. PLoS One 7: e42300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvez J, de Avila C, Matte LO, Guevremont G, Gundlach AL, Timofeeva E (2016). Role of relaxin‐3/RXFP3 system in stress‐induced binge‐like eating in female rats. Neuropharmacology 102: 207–215. [DOI] [PubMed] [Google Scholar]
- Calvez J, de Ávila C, Timofeeva E (2017). Sex‐specific effects of relaxin‐3 on food intake and body weight gain. Br J Pharmacol 174: 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SN, Zhang C, Monte L, Roe AD, Rice KC, Tache Y et al. (2015). Increased tau phosphorylation and aggregation in the hippocampus of mice overexpressing corticotropin‐releasing factor. J Alzheimers Dis 43: 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kuei C, Sutton SW, Bonaventure P, Nepomuceno D, Eriste E et al. (2005). Pharmacological characterization of relaxin‐3/INSL7 receptors GPCR135 and GPCR142 from different mammalian species. J Pharmacol Exp Ther 312: 83–95. [DOI] [PubMed] [Google Scholar]
- Chen Q, de Lecea L, Hu Z, Gao D (2015). The hypocretin/orexin system: an increasingly important role in neuropsychiatry. Med Res Rev 35: 152–197. [DOI] [PubMed] [Google Scholar]
- Croizier S, Franchi‐Bernard G, Colard C, Poncet F, La Roche A, Risold PY (2010). A comparative analysis shows morphofunctional differences between the rat and mouse melanin‐concentrating hormone systems. PLoS One 5: e15471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K (2015). Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci 18: 1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donizetti A, Fiengo M, Minucci S, Aniello F (2009). Duplicated zebrafish relaxin‐3 gene shows a different expression pattern from that of the co‐orthologue gene. Dev Growth Differ 51: 715–722. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI, Wall TL, Lumeng L, Li TK, Owens MJ et al. (1992). Corticotropin releasing factor (CRF): studies in alcohol preferring and non‐preferring rats. Psychopharmacology (Berl) 106: 359–364. [DOI] [PubMed] [Google Scholar]
- Farooq U, Kumar JR, Rajkumar R, Dawe GS (2016). Electrical microstimulation of the nucleus incertus induces forward locomotion and rotation in rats. Physiol Behav 160: 50–58. [DOI] [PubMed] [Google Scholar]
- Fu Y, Han J, Ishola T, Scerbo M, Adwanikar H, Ramsey C et al. (2008). PKA and ERK, but not PKC, in the amygdala contribute to pain‐related synaptic plasticity and behavior. Mol Pain 4: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann F, Justus D, Sosulina L, Kaneko H, Beutel T, Friedrichs D et al. (2015). Locomotion, theta oscillations, and the speed‐correlated firing of hippocampal neurons are controlled by a medial septal glutamatergic circuit. Neuron 86: 1253–1264. [DOI] [PubMed] [Google Scholar]
- Fuzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, Bains JS (2016). Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat Commun 7: 11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganella DE, Callander GE, Ma S, Bye CR, Gundlach AL, Bathgate RAD (2013a). Modulation of feeding by chronic rAAV expression of a relaxin‐3 peptide agonist in rat hypothalamus. Gene Ther 20: 703–716. [DOI] [PubMed] [Google Scholar]
- Ganella DE, Ma S, Gundlach AL (2013b). Relaxin‐3/RXFP3 signaling and neuroendocrine function – a perspective on extrinsic hypothalamic control. Front Endocrinol (Lausanne) 4: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghattas MH, Mehanna ET, Mesbah NM, Abo‐Elmatty DM (2013). Relaxin‐3 is associated with metabolic syndrome and its component traits in women. Clin Biochem 46: 45–48. [DOI] [PubMed] [Google Scholar]
- Goto M, Swanson LW, Canteras NS (2001). Connections of the nucleus incertus. J Comp Neurol 438: 86–122. [DOI] [PubMed] [Google Scholar]
- Grosse J, Heffron H, Burling K, Hossain MA, Habib AM, Rogers GJ et al. (2014). Insulin‐like peptide 5 is an orexigenic gastrointestinal hormone. Proc Natl Acad Sci U S A 111: 11133–11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidar M, Lam M, Chua BE, Smith CM, Gundlach AL (2016). Sensitivity to chronic methamphetamine administration and withdrawal in mice with relaxin‐3/RXFP3 deficiency. Neurochem Res 41: 481–491. [DOI] [PubMed] [Google Scholar]
- Hale MW, Shekhar A, Lowry CA (2012). Stress‐related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cell Mol Neurobiol 32: 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangya B, Borhegyi Z, Szilagyi N, Freund TF, Varga V (2009). GABAergic neurons of the medial septum lead the hippocampal network during theta activity. J Neurosci 29: 8094–8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug T, Storm JF (2000). Protein kinase A mediates the modulation of the slow Ca(2+)‐dependent K(+) current, I(sAHP), by the neuropeptides CRF, VIP, and CGRP in hippocampal pyramidal neurons. J Neurophysiol 83: 2071–2079. [DOI] [PubMed] [Google Scholar]
- Haugaard‐Kedstrom LM, Shabanpoor F, Hossain MA, Clark RJ, Ryan PJ, Craik DJ et al. (2011). Design, synthesis, and characterization of a single‐chain peptide antagonist for the relaxin‐3 receptor RXFP3. J Am Chem Soc 133: 4965–4974. [DOI] [PubMed] [Google Scholar]
- Hida T, Takahashi E, Shikata K, Hirohashi T, Sawai T, Seiki T et al. (2006). Chronic intracerebroventricular administration of relaxin‐3 increases body weight in rats. J Recept Signal Transduct Res 26: 147–158. [DOI] [PubMed] [Google Scholar]
- Hosken IT, Sutton SW, Smith CM, Gundlach AL (2015). Relaxin‐3 receptor (Rxfp3) gene knockout mice display reduced running wheel activity: implications for role of relaxin‐3/RXFP3 signalling in sustained arousal. Behav Brain Res 278: 167–175. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Smith CM, Ryan PJ, Buchler E, Bathgate RAD, Gundlach AL et al. (2013). Chemical synthesis and orexigenic activity of rat/mouse relaxin‐3. Amino Acids 44: 1529–1536. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Semyonov J, Park JI, Chang CL (2005). Evolution of the signaling system in relaxin‐family peptides. Ann N Y Acad Sci 1041: 520–529. [DOI] [PubMed] [Google Scholar]
- Hu E, Demmou L, Cauli B, Gallopin T, Geoffroy H, Harris‐Warrick RM et al. (2011). VIP, CRF, and PACAP act at distinct receptors to elicit different cAMP/PKA dynamics in the neocortex. Cereb Cortex 21: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice NJ, Yuan ZF, Sawchenko PE, Vale W (2008). Type 1 corticotropin‐releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand‐receptor mismatch in the central corticotropin‐releasing factor system. J Comp Neurol 511: 479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastman HE, Blasiak A, Walker L, Siwiec M, Krstew EV, Gundlach AL et al. (2016). Nucleus incertus orexin‐2 receptors mediate alcohol seeking in rats. Neuropharmacology. doi:10.1016/j.neuropharm.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Freeman‐Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A et al. (2008). Corticotropin‐releasing factor increases GABA synaptic activity and induces inward current in 5‐hydroxytryptamine dorsal raphe neurons. J Neurosci 28: 12927–12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselycznyk C, Holmes A (2011). All (C57BL/6) mice are not created equal. Front Neurosci 5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Cierpinsky K, Kronenberg G, Adli M (2016). The serotonergic system in the neurobiology of depression: relevance for novel antidepressants. J Psychopharmacol 30: 13–22. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS et al. (2014). An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuei C, Sutton S, Bonaventure P, Pudiak C, Shelton J, Zhu J et al. (2007). R3(BΔ23‐27)R/I5 chimeric peptide, a selective antagonist for GPCR135 and GPCR142 over relaxin receptor LGR7: in vitro and in vivo characterization. J Biol Chem 282: 25425–25435. [DOI] [PubMed] [Google Scholar]
- Kumar JR, Rajkumar R, Farooq U, Lee LC, Tan FC, Dawe GS (2015). Evidence of D2 receptor expression in the nucleus incertus of the rat. Physiol Behav 151: 525–534. [DOI] [PubMed] [Google Scholar]
- Kumar JR, Rajkumar R, Jayakody T, Marwari S, Hong JM, Ma S et al. (2017). Relaxin’ the brain: a case for targeting the nucleus incertus network and relaxin‐3/RXFP3 system in neuropsychiatric disorders. Br J Pharmacol 174: 1061–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther AJ, Clissold ML, Ma S, Kent S, Lowry CA, Gundlach AL et al. (2015). Anxiogenic drug administration and elevated plus‐maze exposure in rats activate populations of relaxin‐3 neurons in the nucleus incertus and serotonergic neurons in the dorsal raphe nucleus. Neuroscience 303: 270–284. [DOI] [PubMed] [Google Scholar]
- Lee JH, Koh SQ, Guadagna S, Francis PT, Esiri MM, Chen CP et al. (2016). Altered relaxin family receptors RXFP1 and RXFP3 in the neocortex of depressed Alzheimer's disease patients. Psychopharmacology (Berl) 233: 591–598. [DOI] [PubMed] [Google Scholar]
- Lenglos C, Mitra A, Guevremont G, Timofeeva E (2013). Sex differences in the effects of chronic stress and food restriction on body weight gain and brain expression of CRF and relaxin‐3 in rats. Genes Brain Behav 12: 370–387. [DOI] [PubMed] [Google Scholar]
- Leon‐Cabrera S, Solis‐Lozano L, Suarez‐Alvarez K, Gonzalez‐Chavez A, Bejar YL, Robles‐Diaz G et al. (2013). Hyperleptinemia is associated with parameters of low‐grade systemic inflammation and metabolic dysfunction in obese human beings. Front Integr Neurosci 7: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Chen J, Kuei C, Sutton SW, Nepomuceno D, Bonaventure P et al. (2005a). Relaxin‐3/insulin‐like peptide 5 chimeric peptide, a selective ligand for G protein‐coupled receptor (GPCR)135 and GPCR142 over leucine‐rich repeat‐containing G protein‐coupled receptor 7. Mol Pharmacol 67: 231–240. [DOI] [PubMed] [Google Scholar]
- Liu C, Chen J, Sutton SW, Roland B, Kuei C, Farmer N et al. (2003a). Identification of relaxin‐3/INSL7 as a ligand for GPCR142. J Biol Chem 278: 50765–50770. [DOI] [PubMed] [Google Scholar]
- Liu C, Eriste E, Sutton S, Chen J, Roland B, Kuei C et al. (2003b). Identification of relaxin‐3/INSL7 as an endogenous ligand for the orphan G‐protein coupled receptor GPCR135. J Biol Chem 278: 50754–50764. [DOI] [PubMed] [Google Scholar]
- Liu C, Kuei C, Sutton S, Chen J, Bonaventure P, Wu J et al. (2005b). INSL5 is a high affinity specific agonist for GPCR142 (GPR100). J Biol Chem 280: 292–300. [DOI] [PubMed] [Google Scholar]
- Ma S, Allocca G, Ong‐Palsson EK, Singleton CE, Hawkes D, McDougall SJ et al. (2016). Nucleus incertus promotes cortical desynchronization and behavioral arousal. Brain Struct Funct. doi:10.1007/s00429-016-1230-0. [DOI] [PubMed] [Google Scholar]
- Ma S, Blasiak A, Olucha‐Bordonau FE, Verberne AJ, Gundlach AL (2013). Heterogeneous responses of nucleus incertus neurons to corticotrophin‐releasing factor and coherent activity with hippocampal theta rhythm in the rat. J Physiol 591: 3981–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Bonaventure P, Ferraro T, Shen PJ, Burazin TCD, Bathgate RAD et al. (2007). Relaxin‐3 in GABA projection neurons of nucleus incertus suggests widespread influence on forebrain circuits via G‐protein‐coupled receptor‐135 in the rat. Neuroscience 144: 165–190. [DOI] [PubMed] [Google Scholar]
- Ma S, Gundlach AL (2015). Ascending control of arousal and motivation: role of nucleus incertus and its peptide neuromodulators in behavioural responses to stress. J Neuroendocrinol 27: 457–467. [DOI] [PubMed] [Google Scholar]
- Ma S, Gundlach AL (2007). Relaxin‐family peptide and receptor systems in brain: insights from recent anatomical and functional studies. Adv Exp Med Biol 612: 119–137. [DOI] [PubMed] [Google Scholar]
- Ma S, Olucha‐Bordonau FE, Hossain MA, Lin F, Kuei C, Liu C et al. (2009a). Modulation of hippocampal theta oscillations and spatial memory by relaxin‐3 neurons of the nucleus incertus. Learn Mem 16: 730–742. [DOI] [PubMed] [Google Scholar]
- Ma S, Sang Q, Lanciego JL, Gundlach AL (2009b). Localization of relaxin‐3 in brain of Macaca fascicularis: identification of a nucleus incertus in primate. J Comp Neurol 517: 856–872. [DOI] [PubMed] [Google Scholar]
- Ma S, Shen P‐J, Burazin TCD, Tregear GW, Gundlach AL (2006). Comparative localization of leucine‐rich repeat‐containing G‐protein‐coupled receptor‐7 (RXFP1) mRNA and [(33)P]‐relaxin binding sites in rat brain: restricted somatic co‐expression a clue to relaxin action? Neuroscience 141: 329–344. [DOI] [PubMed] [Google Scholar]
- Ma S, Shen PJ, Sang Q, Lanciego JL, Gundlach AL (2009c). Distribution of relaxin‐3 mRNA and immunoreactivity and RXFP3‐binding sites in the brain of the macaque, Macaca fascicularis . Ann N Y Acad Sci 1160: 256–258. [DOI] [PubMed] [Google Scholar]
- Madisen L, Garner AR, Shimaoka D, Chuong AS, Klapoetke NC, Li L et al. (2015). Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85: 942–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Kamohara M, Sugimoto T, Hidaka K, Takasaki J, Saito T et al. (2000). The novel G‐protein coupled receptor SALPR shares sequence similarity with somatostatin and angiotensin receptors. Gene 248: 183–189. [DOI] [PubMed] [Google Scholar]
- McGowan BM, Minnion JS, Murphy KG, Roy D, Stanley SA, Dhillo WS et al. (2014). Relaxin‐3 stimulates the neuro‐endocrine stress axis via corticotrophin‐releasing hormone. J Endocrinol 221: 337–346. [DOI] [PubMed] [Google Scholar]
- McGowan BM, Stanley SA, Smith KL, Minnion JS, Donovan J, Thompson EL et al. (2006). Effects of acute and chronic relaxin‐3 on food intake and energy expenditure in rats. Regul Pept 136: 72–77. [DOI] [PubMed] [Google Scholar]
- McGowan BM, Stanley SA, Smith KL, White NE, Connolly MM, Thompson EL et al. (2005). Central relaxin‐3 administration causes hyperphagia in male Wistar rats. Endocrinology 146: 3295–3300. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Gray JA (2000). Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. J Affect Disord 61: 161–176. [DOI] [PubMed] [Google Scholar]
- Meadows KL, Byrnes EM (2015). Sex‐ and age‐specific differences in relaxin family peptide receptor expression within the hippocampus and amygdala in rats. Neuroscience 284: 337–348. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Watanabe Y, Tanaka M (2008). Developmental expression and serotonergic regulation of relaxin 3/INSL7 in the nucleus incertus of rat brain. Regul Pept 145: 54–59. [DOI] [PubMed] [Google Scholar]
- Molosh AI, Sajdyk TJ, Truitt WA, Zhu W, Oxford GS, Shekhar A (2013). NPY Y1 receptors differentially modulate GABAA and NMDA receptors via divergent signal‐transduction pathways to reduce excitability of amygdala neurons. Neuropsychopharmacology 38: 1352–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY (2013). The suprachiasmatic nucleus and the circadian timing system. Prog Mol Biol Transl Sci 119: 1–28. [DOI] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH (2005). Descending projections of the hamster intergeniculate leaflet: relationship to the sleep/arousal and visuomotor systems. J Comp Neurol 487: 204–216. [DOI] [PubMed] [Google Scholar]
- Munro J, Skrobot O, Sanyoura M, Kay V, Susce MT, Glaser PE et al. (2012). Relaxin polymorphisms associated with metabolic disturbance in patients treated with antipsychotics. J Psychopharmacol 26: 374–379. [DOI] [PubMed] [Google Scholar]
- Nakazawa CM, Shikata K, Uesugi M, Katayama H, Aoshima K, Tahara K et al. (2013). Prediction of relaxin‐3‐induced downstream pathway resulting in anxiolytic‐like behaviors in rats based on a microarray and peptidome analysis. J Recept Signal Transduct Res 33: 224–233. [DOI] [PubMed] [Google Scholar]
- Nunez A, Cervera‐Ferri A, Olucha‐Bordonau F, Ruiz‐Torner A, Teruel V (2006). Nucleus incertus contribution to hippocampal theta rhythm generation. Eur J Neurosci 23: 2731–2738. [DOI] [PubMed] [Google Scholar]
- Ojala DS, Amara DP, Schaffer DV (2015). Adeno‐associated virus vectors and neurological gene therapy. Neuroscientist 21: 84–98. [DOI] [PubMed] [Google Scholar]
- Olucha‐Bordonau FE, Otero‐Garcia M, Sanchez‐Perez AM, Nunez A, Ma S, Gundlach AL (2012). Distribution and targets of the relaxin‐3 innervation of the septal area in the rat. J Comp Neurol 520: 1903–1939. [DOI] [PubMed] [Google Scholar]
- Olucha‐Bordonau FE, Teruel V, Barcia‐Gonzalez J, Ruiz‐Torner A, Valverde‐Navarro AA, Martinez‐Soriano F (2003). Cytoarchitecture and efferent projections of the nucleus incertus of the rat. J Comp Neurol 464: 62–97. [DOI] [PubMed] [Google Scholar]
- Park HJ, Ran Y, Jung JI, Holmes O, Price AR, Smithson L et al. (2015). The stress response neuropeptide CRF increases amyloid‐beta production by regulating gamma‐secretase activity. EMBO J 34: 1674–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF (1995). Protein kinase A‐independent modulation of ion channels in the brain by cyclic AMP. Proc Natl Acad Sci U S A 92: 11716–11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K et al. (1994). Distribution of corticotropin‐releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A 91: 8777–8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar R, See LK, Dawe GS (2013). Acute antipsychotic treatments induce distinct c‐Fos expression patterns in appetite‐related neuronal structures of the rat brain. Brain Res 1508: 34–43. [DOI] [PubMed] [Google Scholar]
- Robinson J, Manseau F, Ducharme G, Amilhon B, Vigneault E, El Mestikawy S et al. (2016). Optogenetic activation of septal glutamatergic neurons drive hippocampal theta rhythms. J Neurosci 36: 3016–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL (2016). DREADDs for neuroscientists. Neuron 89: 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PJ, Buchler E, Shabanpoor F, Hossain MA, Wade JD, Lawrence AJ et al. (2013a). Central relaxin‐3 receptor (RXFP3) activation decreases anxiety‐ and depressive‐like behaviours in the rat. Behav Brain Res 244: 142–151. [DOI] [PubMed] [Google Scholar]
- Ryan PJ, Kastman HE, Krstew EV, Rosengren KJ, Hossain MA, Churilov L et al. (2013b). Relaxin‐3/RXFP3 system regulates alcohol‐seeking. Proc Natl Acad Sci U S A 110: 20789–20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PJ, Krstew EV, Sarwar M, Gundlach AL, Lawrence AJ (2014). Relaxin‐3 mRNA levels in nucleus incertus correlate with alcohol and sucrose intake in rats. Drug Alcohol Depend 140: 8–16. [DOI] [PubMed] [Google Scholar]
- Ryan PJ, Ma S, Olucha‐Bordonau FE, Gundlach AL (2011). Nucleus incertus‐an emerging modulatory role in arousal, stress and memory. Neurosci Biobehav Rev 35: 1326–1341. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Perez AM, Arnal‐Vicente I, Santos FN, Pereira CW, ElMlili N, Sanjuan J et al. (2015). Septal projections to nucleus incertus in the rat: bidirectional pathways for modulation of hippocampal function. J Comp Neurol 523: 565–588. [DOI] [PubMed] [Google Scholar]
- Schaffer DV, Koerber JT, Lim KI (2008). Molecular engineering of viral gene delivery vehicles. Annu Rev Biomed Eng 10: 169–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE et al. (2015). Viral‐genetic tracing of the input–output organization of a central noradrenaline circuit. Nature 524: 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabanpoor F, Akhter Hossain M, Ryan PJ, Belgi A, Layfield S, Kocan M et al. (2012). Minimization of human relaxin‐3 leading to high‐affinity analogues with increased selectivity for relaxin‐family peptide 3 receptor (RXFP3) over RXFP1. J Med Chem 55: 1671–1681. [DOI] [PubMed] [Google Scholar]
- Shirahase T, Aoki M, Watanabe R, Watanabe Y, Tanaka M (2016). Increased alcohol consumption in relaxin‐3 deficient male mice. Neurosci Lett 612: 155–160. [DOI] [PubMed] [Google Scholar]
- Silvertown JD, Neschadim A, Liu HN, Shannon P, Walia JS, Kao JC et al. (2010). Relaxin‐3 and receptors in the human and rhesus brain and reproductive tissues. Regul Pept 159: 44–53. [DOI] [PubMed] [Google Scholar]
- Simon AP, Poindessous‐Jazat F, Dutar P, Epelbaum J, Bassant MH (2006). Firing properties of anatomically identified neurons in the medial septum of anesthetized and unanesthetized restrained rats. J Neurosci 26: 9038–9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CM, Blasiak A, Ganella DE, Chua BE, Layfield SL, Bathgate RAD et al. (2013a). Viral‐mediated delivery of an RXFP3 agonist into brain promotes arousal in mice. Ital J Anat Embryol 118: 42. [PubMed] [Google Scholar]
- Smith CM, Chua BE, Zhang C, Walker AW, Haidar M, Hawkes D et al. (2014a). Central injection of relaxin‐3 receptor (RXFP3) antagonist peptides reduces motivated food seeking and consumption in C57BL/6J mice. Behav Brain Res 268: 117–126. [DOI] [PubMed] [Google Scholar]
- Smith CM, Hosken IT, Downer NL, Chua BE, Hossain MA, Wade JD et al. (2013b). Pharmacological activation of RXFP3 is not orexigenic in C57BL/6J mice. Ital J Anat Embryol 118: 52–55. [PubMed] [Google Scholar]
- Smith CM, Hosken IT, Sutton SW, Lawrence AJ, Gundlach AL (2012). Relaxin‐3 null mutation mice display a circadian hypoactivity phenotype. Genes Brain Behav 11: 94–104. [DOI] [PubMed] [Google Scholar]
- Smith CM, Ryan PJ, Hosken IT, Ma S, Gundlach AL (2011). Relaxin‐3 systems in the brain‐the first 10 years. J Chem Neuroanat 42: 262–275. [DOI] [PubMed] [Google Scholar]
- Smith CM, Shen PJ, Banerjee A, Bonaventure P, Ma S, Bathgate RAD et al. (2010). Distribution of relaxin‐3 and RXFP3 within arousal, stress, affective, and cognitive circuits of mouse brain. J Comp Neurol 518: 4016–4045. [DOI] [PubMed] [Google Scholar]
- Smith CM, Walker AW, Hosken IT, Chua BE, Zhang C, Haidar M et al. (2014b). Relaxin‐3/RXFP3 networks: an emerging target for the treatment of depression and other neuropsychiatric diseases? Front Pharmacol 5: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CM, Walker LL, Chua BE, McKinley MJ, Gundlach AL, Denton DA et al. (2015). Involvement of central relaxin‐3 signalling in sodium (salt) appetite. Exp Physiol 100: 1064–1072. [DOI] [PubMed] [Google Scholar]
- Sotty F, Danik M, Manseau F, Laplante F, Quirion R, Williams S (2003). Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. J Physiol 551: 927–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stujenske JM, Likhtik E, Topiwala MA, Gordon JA (2014). Fear and safety engage competing patterns of theta‐gamma coupling in the basolateral amygdala. Neuron 83: 919–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo S, Kumagai J, Nishi S, Layfield S, Ferraro T, Bathgate RA et al. (2003). H3 relaxin is a specific ligand for LGR7 and activates the receptor by interacting with both the ectodomain and the exoloop 2. J Biol Chem 278: 7855–7862. [DOI] [PubMed] [Google Scholar]
- Sun QQ, Prince DA, Huguenard JR (2003). Vasoactive intestinal polypeptide and pituitary adenylate cyclase‐activating polypeptide activate hyperpolarization‐activated cationic current and depolarize thalamocortical neurons in vitro . J Neurosci 23: 2751–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SW, Bonaventure P, Kuei C, Nepomuceno D, Wu J, Zhu J et al. (2006). G‐protein‐coupled receptor (GPCR)‐142 does not contribute to relaxin‐3 binding in the mouse brain: Further support that relaxin‐3 is the physiological ligand for GPCR135. Neuroendocrinology 82: 139–150. [DOI] [PubMed] [Google Scholar]
- Sutton SW, Bonaventure P, Kuei C, Roland B, Chen J, Nepomuceno D et al. (2004). Distribution of G‐protein‐coupled receptor (GPCR)135 binding sites and receptor mRNA in the rat brain suggests a role for relaxin‐3 in neuroendocrine and sensory processing. Neuroendocrinology 80: 298–307. [DOI] [PubMed] [Google Scholar]
- Sutton SW, Shelton J, Smith C, Williams J, Yun S, Motley T et al. (2009). Metabolic and neuroendocrine responses to RXFP3 modulation in the central nervous system. Ann N Y Acad Sci 1160: 242–249. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Iijima N, Miyamoto Y, Fukusumi S, Itoh Y, Ozawa H et al. (2005). Neurons expressing relaxin 3/INSL 7 in the nucleus incertus respond to stress. Eur J Neurosci 21: 1659–1670. [DOI] [PubMed] [Google Scholar]
- Torterolo P, Lagos P, Sampogna S, Chase MH (2008). Melanin‐concentrating hormone (MCH) immunoreactivity in non‐neuronal cells within the raphe nuclei and subventricular region of the brainstem of the cat. Brain Res 1210: 163–178. [DOI] [PubMed] [Google Scholar]
- Toth K, Freund TF, Miles R (1997). Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. J Physiol 500 (Pt 2): 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Westhuizen ET, Werry TD, Sexton PM, Summers RJ (2007). The relaxin family peptide receptor 3 activates extracellular signal‐regulated kinase 1/2 through a protein kinase C‐dependent mechanism. Mol Pharmacol 71: 1618–1629. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C et al. (2000). Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428: 191–212. [DOI] [PubMed] [Google Scholar]
- Varga V, Hangya B, Kranitz K, Ludanyi A, Zemankovics R, Katona I et al. (2008). The presence of pacemaker HCN channels identifies theta rhythmic GABAergic neurons in the medial septum. J Physiol 586: 3893–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (1984). Brainstem control of the events of REM sleep. Prog Neurobiol 22: 241–288. [DOI] [PubMed] [Google Scholar]
- Vertes RP (2005). Hippocampal theta rhythm: a tag for short‐term memory. Hippocampus 15: 923–935. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B (1997). Brainstem‐diencephalo‐septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience 81: 893–926. [DOI] [PubMed] [Google Scholar]
- Walker AW, Smith CM, Chua BE, Krstew EV, Zhang C, Gundlach AL et al. (2015a). Relaxin‐3 receptor (RXFP3) signalling mediates stress‐related alcohol preference in mice. PLoS One 10: e0122504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AW, Smith CM, Gundlach AL, Lawrence AJ (2015b). Relaxin‐3 receptor (Rxfp3) gene deletion reduces operant sucrose‐ but not alcohol‐responding in mice. Genes Brain Behav 14: 625–634. [DOI] [PubMed] [Google Scholar]
- Walker LC, Kastman HE, Koeleman JA, Smith CM, Perry CJ, Krstew EV et al. (2016). Nucleus incertus corticotrophin‐releasing factor 1 receptor signalling regulates alcohol seeking in rats. Addict Biol. doi:10.1111/adb.12426. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Miyamoto Y, Matsuda T, Tanaka M (2010). Relaxin‐3/INSL7 regulates the stress‐response system in the rat hypothalamus. J Mol Neurosci 43: 169–174. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Tsujimura A, Takao K, Nishi K, Ito Y, Yasuhara Y et al. (2011). Relaxin‐3‐deficient mice showed slight alteration in anxiety‐related behavior. Front Behav Neurosci 5: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells CE, Amos DP, Jeewajee A, Douchamps V, Rodgers J, O'Keefe J et al. (2013). Novelty and anxiolytic drugs dissociate two components of hippocampal theta in behaving rats. J Neurosci 33: 8650–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson TN, Speed TP, Tregear GW, Bathgate RAD (2005). Coevolution of the relaxin‐like peptides and their receptors. Ann N Y Acad Sci 1041: 534–539. [DOI] [PubMed] [Google Scholar]
- Wulff P, Wisden W (2005). Dissecting neural circuitry by combining genetics and pharmacology. Trends Neurosci 28: 44–50. [DOI] [PubMed] [Google Scholar]
- Yoder RM, Pang KC (2005). Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus 15: 381–392. [DOI] [PubMed] [Google Scholar]
- Yu X, Ye Z, Houston CM, Zecharia AY, Ma Y, Zhang Z et al. (2015). Wakefulness is governed by GABA and histamine cotransmission. Neuron 87: 164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Chua BE, Yang A, Shabanpoor F, Hossain MA, Wade JD et al. (2015). Central relaxin‐3 receptor (RXFP3) activation reduces elevated, but not basal, anxiety‐like behaviour in C57BL/6J mice. Behav Brain Res 292: 125–132. [DOI] [PubMed] [Google Scholar]
- Zhang C, Kuo CC, Moghadam SH, Monte L, Campbell SN, Rice KC et al. (2016). Corticotropin‐releasing factor receptor‐1 antagonism mitigates beta amyloid pathology and cognitive and synaptic deficits in a mouse model of Alzheimer's disease. Alzheimers Dement 12: 527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]


