Abstract
Background and Purpose
3,4‐Methylenedioxypyrovalerone (MDPV) is a synthetic cathinone with powerful psychostimulant effects. It selectively inhibits the dopamine transporter (DAT) and is 10–50‐fold more potent as a DAT blocker than cocaine, suggesting a high abuse liability. The main objective of the present study was to assess the consequences of an early (adolescence) MDPV exposure on the psychostimulant, rewarding and reinforcing effects induced by cocaine in adult mice.
Experimental Approach
Twenty‐one days after MDPV pretreatment (1.5 mg·kg−1, s.c., twice daily for 7 days), adult mice were tested with cocaine, using locomotor activity, conditioned place preference and self‐administration (SA) paradigms. In parallel, dopamine D2 receptor density and the expression of c‐Fos and ΔFosB in the striatum were determined.
Key Results
MDPV treatment enhanced the psychostimulant and conditioning effects of cocaine. Acquisition of cocaine SA was unchanged in mice pretreated with MDPV, whereas the breaking point achieved under a progressive ratio programme and reinstatement after extinction were higher in this group of mice. MDPV decreased D2 receptor density but increased ΔFosB expression three‐fold. As expected, acute cocaine increased c‐Fos expression, but MDPV pretreatment negatively influenced its expression. ΔFosB accumulation declined during MDPV withdrawal, although it remained elevated in adult mice when tested for cocaine effects.
Conclusion and Implications
MDPV exposure during adolescence induced long‐lasting adaptive changes related to enhanced responsiveness to cocaine in the adult mice that seems to lead to a higher vulnerability to cocaine abuse. This particular behaviour correlated with increased expression of ΔFosB.
Abbreviations
- CPP
conditioned place preference
- DAT
dopamine transporter
- HLA
horizontal locomotor activity
- MDPV
3,4‐methylenedioxypyrovalerone
- NPS
new psychoactive substances
- SA
self‐administration
Tables of Links
| TARGETS |
|---|
| GPCRs |
| Dopamine D2 receptor |
| LIGANDS |
|---|
| Cocaine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015).
Introduction
In recent years, the illicit drug‐market has changed remarkably and several new psychoactive substances (NPS), such as the synthetic cathinones, have been identified. The popularity of synthetic cathinones has increased due to their ease of access, price and initial legal status (Bijlsma et al., 2015; Katselou et al., 2015). 3,4‐Methylenedioxypyrovalerone (MDPV) is considered as one of the most abused synthetic cathinone and the main ingredient of ‘bath salts’ (Zuba and Byrska, 2013; Johnson and Johnson, 2014) and able of triggering powerful psychostimulant effects (Baumann et al., 2013).
Cocaine is also a powerful psychostimulant, and its repeated use could lead to a substance use disorder and is often associated with other severe psychiatric and medical complications (Pozzi et al., 2008; Walsh et al., 2009). Despite the invasion of the illegal market by the NPS, the illicit use of cocaine is still a persistent health problem worldwide (UNODC, n.d.). Similarly, MDPV shows cocaine‐like properties and selectively inhibits dopamine (DAT) and noradrenaline transporters, being 10‐ to 50‐fold more potent than cocaine, as a DAT blocker (Simmler et al., 2013; Baumann et al., 2013). Furthermore, it shows rewarding and reinforcing effects (King et al., 2014), pointing to a similar abuse liability to that of cocaine.
Although MDPV use could be considered as a transient trend in drug abuse, the long‐lasting consequences of its repeated consumption are still unknown. Considering this, it is relevant to determine whether the use of MDPV will lead to an increased sensitivity and subsequent vulnerability to cocaine abuse. Adolescents and young adults use MDPV as a cheaper and easily obtained alternative to classical psychostimulants. Conversely, cocaine is a more widely and currently used psychostimulant and is generally consumed in adulthood. Consequently, the main objective of the present study was to assess the consequences of early and repeated MDPV exposure on the responses of adult mice to the psychostimulant cocaine.
A repeated (7 days) moderate dose (1.5 mg·kg−1, twice, daily) of MDPV eliciting hyperlocomotion was chosen for this study. After this MDPV schedule, adult mice were tested to cocaine responses. Hence, hyperlocomotion to an acute dose of cocaine was assayed as an indicative of its psychostimulant effect. In a second experiment, we investigated whether MDPV schedule could enhance the rewarding effects of cocaine, using the conditioned place preference (CPP). Next, we evaluated the reinforcing cocaine effects on the self‐administration (SA) paradigm. Earlier studies have shown that dopamine D2 receptors played a role in the development and expression of behavioural sensitization (Thompson et al., 2010). Moreover, the expression of c‐Fos and deltaFosB (ΔFosB) in some brain areas is induced by acute or chronic exposure to virtually all drugs of abuse and regulates their psychomotor and rewarding effects. Therefore, we have assessed the D2 receptor density and the expression of c‐Fos and ΔFosB in dorsal and ventral striatum.
Hence, we have performed behavioural procedures (hyperlocomotion, CPP and SA) and biochemical analyses that allowed us to characterize the liability for cocaine abuse shown by adult mice who were pretreated, as adolescents, with MDPV.
Methods
Animals
All animal care and experimental protocols were approved by the Animal Ethics Committee of the University of Barcelona and PRBB, respectively, under the supervision of the Autonomic Government of Catalonia, following the guidelines of the European Community Council (2010/63/EU). All efforts were made to minimize animal suffering and to reduce the number of animals used. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). Animals were housed four per cage (polycarbonate with wood‐derived bedding) at 22 ± 1°C under a 12 h light/dark cycle with free access to food and drinking water.
Our model was based on the following considerations. Adolescence is a period of particular vulnerability to drug addiction (Cass et al., 2013), being a period of life in which different psychiatric disorders emerge (Paus et al., 2008). For this reason, we used for our study adolescent (PND 41–44) male Swiss CD‐1 mice (Charles River, Spain), which are equivalent to the beginning of peri‐adolescence (Spear and Brake, 1983), and assayed cocaine effects after 21 days of withdrawal, when animals had reached adulthood. The CD‐1 mouse strain was selected for its optimal sensitivity to the reinforcing and psychostimulating effects of cocaine (McKerchar et al., 2005).
Drug administration protocols and experimental design
In administration regime A, MDPV (1.5 mg·kg−1) or saline (5 mg·kg−1) was injected s.c. to mice twice daily (4 h apart) for three consecutive days and, thereafter, they were exposed to the cocaine‐sensitization protocol (Figure 1A). In administration regime B, animals were also treated with MDPV (1.5 mg·kg−1) or saline (5 mL·kg−1) twice daily for seven consecutive days and were then housed in their home cages until reaching adulthood (PND 69–72), when they were tested for cocaine‐induced horizontal locomotor activity (HLA), CPP and SA experiments as described below (Figure 1B). This dose is equivalent to a dose in humans of about 6 mg twice a day (Reagan‐Shaw et al., 2008), in which threshold dosages are around 1–5 mg and strong effects are shown with 10–25 mg (2014). Re‐dosing is a typical pattern of consumption followed by consumers of such substances to avoid an unpleasant comedown (Ross et al., 2012).
Figure 1.
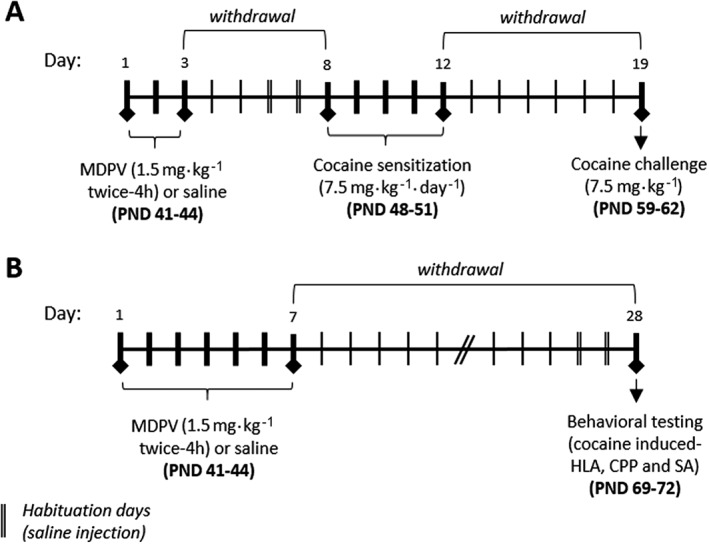
Drug exposure protocol and experimental design. (A) In administration regime A, animals were treated with MDPV or saline twice daily for 3 days and, 5 days after, they were exposed to a cocaine sensitization protocol. (B) In administration regime B, animals were treated with MDPV or saline twice daily for 7 days and, 21 days later, cocaine‐induced HLA, CPP and SA experiments were performed.
Sensitization to the locomotor responses induced by a repeated cocaine administration
Locomotor activity was evaluated by placing the mice individually in the actimeter boxes (24 × 24 × 24 cm) (LE881 IR, Panlab, Barcelona, Spain) provided with 14 axes (Y and X) in a low‐luminosity room. Animals were treated with saline (n = 10) or MDPV (n = 11), according to the administration regime A. The sensitization procedure consisted of three phases over 14 days: habituation, treatment and challenge. In the habituation phase (Days 6–7), mice were placed on the actimeter boxes for 30 min immediately after an i.p. saline injection. Treatment phase consisted of five sessions (Days 8–12). In each session, mice received daily an i.p. injection of cocaine (7.5 mg·kg−1) immediately before being placed in the apparatus for 15 min. Finally, following a 7 day drug‐free period after the last cocaine injection, mice were tested (Day 19 – challenge phase) with a cocaine injection (7.5 mg·kg−1, i.p.) in the same actimeter boxes, and the locomotor activity was registered for 15 min.
Cocaine‐induced HLA
The HLA response induced by a single cocaine injection was video‐monitored (Smart 3.0, Panlab, s.l.u., Barcelona, Spain) for 15 min in a black Plexiglass open field arena (25 × 25 × 40 cm) under low‐light conditions. Two days before testing, the animals were handled for 10 min and placed in the black arena for habituation. Two groups of animals (n = 12 per group) were pretreated in their home cage according to administration regime B, and after 21 days of withdrawal, when they reached adulthood (PND 69–72), both groups were challenged with saline (5 mL·kg−1, i.p.) (Day 27) and cocaine (7.5 mg·kg−1 i.p.) (Day 28), and locomotor activity was registered.
Cocaine‐induced CPP
The cocaine potential to induce approaching behaviours toward drug‐related stimuli was determined using an unbiased place conditioning paradigm, as described by Soria et al. (2006). The apparatus consisted of two main conditioning compartments (30 × 29 × 35 cm) connected by a smaller, central compartment (Cibertec S.A., Madrid, Spain). The conditioning compartments were disposed with differences in visual and tactile cues. All the compartments were equipped with infrared emitter/detector pairs along the length of the box.
Saline‐ and MDPV‐pretreated animals according to administration regime B (n = 16 per group) were subjected to the CPP procedure after a 21 day long drug‐free period. During the preconditioning phase (day 28), initial unconditioned preference for the stimulus alternatives was determined. In this test, mice were placed in the central compartment and had free access to both compartments of the apparatus for 18 min. During the conditioning phase (days 29–34), mice received an i.p. injection of cocaine 10 mg·kg−1 immediately before being placed into one of the two conditioning compartments for 20 min on days 29, 31 and 33. On the alternate days (30, 32 and 35), mice were placed in the other compartment for 20 min after being given a saline injection. Treatments were counterbalanced as much as possible between compartments. Control animals received saline every day. The preference test was conducted exactly as the preconditioning phase. A CPP score was calculated for each subject as the difference between times spent in the drug‐paired and the saline‐paired compartments during the pre‐conditioning and the preference tests.
Cocaine operant SA
Acquisition of cocaine SA
The SA experiments were carried out in eight mouse operant chambers (Model ENV‐307A‐CT, Med Associates, Inc. Cibertec S.A., Madrid, Spain), as previously described by Soria et al. (2006). Saline‐ and MDPV‐pretreated mice according to administration regime B (n = 16 per group) were trained for 2 h·day−1 to nosepokes in order to receive 1 mg·kg−1 cocaine infusions on 10 consecutive days under a fixed ratio 1 (FR1).
Surgical implantation of the catheter into the jugular vein was performed following anaesthesia with a mixture of ketamine (100 mg·mL−1) and xylazine (20 mg·kg−1). The anaesthetic solution was injected in a volume of 0.15 mL·10 g−1, i.p. (Soria et al., 2006; Tourino et al., 2012). Mice were housed individually and allowed to recover for at least 3 days. During recovery, mice were treated daily with an analgesic (meloxicam 0.5 mg·kg−1, injected in a volume of 0.1 mL·10 g−1, i.p.) and an antibiotic solution (enrofloxacin 7.5 mg·kg−1, injected in a volume of 0.03 mL·10 g−1, i.p.). The home cages were placed upon thermal blankets to avoid post‐anaesthesia hypothermia.
SA procedures started 21 days after the last day of administration regime B (Day 28). Active and inactive nosepokes were assigned randomly. Cocaine was delivered in a 20 μL injection for 2 s via a syringe mounted on a microinfusion pump (PHM‐100A, Med‐Associates, Georgia, VT, USA) connected to single‐channel liquid swivel (375/25, Instech Lab, Plymouth Meeting, PA, USA) and the mouse's intravenous catheter. All FR1 sessions started with a cocaine priming infusion. When mice responded on the active hole, the stimulus lights (one located inside the nosepoke and the other above it) lit up for 4 s and a cocaine infusion was delivered automatically. Each infusion was followed by a 30 s time‐out period in which a nosepoke on the active hole had no consequences. Mice were considered to have acquired stable SA behaviour when the following criteria were met in two consecutive FR1 sessions: (a) 80% stability in reinforcements (the number of reinforces in each day deviated by <20% from the mean number of reinforces in two consecutive days); (b) ≥ 65% of responses were received at the active hole; and (c) a minimum of five responses in the active hole. After 10 days of training (Day 38), mice that achieved the acquisition criteria (n = 9 per group) were moved to a progressive ratio (PR) session. In the PR session (2 h), the response requirement to earn an injection escalated throughout the following series: 1–2–3–5–12–18–27–40–60–90–135–200–300–450–675–1000.
Extinction and reinstatement
All the animals that reached the acquisition criteria were subjected to an extinction phase. The extinction procedure was adapted from Soria et al. (2008). Nosepokes in the active hole produced neither cocaine infusion nor stimulus light presentation. Extinction sessions (2 h) were conducted once a day, 5 days·week−1 until reaching the extinction criteria. These criteria were achieved when mice made a mean number of responses in two consecutive extinction sessions of less than 40% of the responses performed during the last day of the cocaine‐training phase. Twenty‐four hours after achieving the extinction criteria, mice underwent a cocaine‐primed reinstatement session, as previously described (Soria et al., 2008). In order to recover the extinguished cocaine‐seeking behaviour, saline‐ and MDPV‐pretreated mice (n = 9 per group) were confined to the operant chambers for 2 h immediately after receiving an i.p. injection of cocaine 10 mg·kg−1. Nosepokes had no consequences in any of the holes.
Tissue sample preparations
Mice pretreated according to the administration regime B were killed by cervical dislocation 24 h after the treatment (Day 8) or after saline/cocaine challenge (Day 28) for the analysis of ΔFosB expression and D2 receptor density or 2 h after saline/cocaine challenge for the determination of c‐Fos expression. The inclusion of a saline challenge group allowed us to study also the long‐term effects of MDPV treatment. Ventral (including NAcc), dorsal or the whole striatum, when appropriate, were quickly dissected out and stored at −80°C until use.
Tissue samples for Western blot analysis were processed as described (Pubill et al., 2013), with minor modifications. Briefly, for nuclear c‐Fos Western blot analysis, dorsal striatum tissue samples were homogenized at 4°C in 400 μL of buffer (5 mM Tris–HCl, 320 mM sucrose) with the protease inhibitor cocktail. The homogenates were centrifuged at 1000 × g for 15 min at 4°C, and the pellets were resuspended in buffer (Tris–HCl 50 mM) with the protease inhibitor cocktail.
For ΔFosB Western blot analysis, ventral striatum tissue samples were thawed and homogenized at 4°C in 20 volumes of lysis buffer (20 mM Tris–HCl, pH = 8, 1% NP40, 137 mM NaCl, 10% glycerol, 2 mM EDTA) with the protease inhibitor cocktail. The homogenates were shaken and rolled for 120 min at 4°C and centrifuged at 15 000 × g for 30 min at 4°C. Aliquots of resulting supernatants (total lysate) were stored at −80°C until use.
For [3H]raclopride binding assays, crude membrane preparation from the whole striatum was prepared as described by Martínez‐Clemente et al., (2012). Briefly, tissue samples were thawed and homogenized at 4°C in 20 volumes of buffer (5 mM Tris–HCl, 320 mM sucrose) with the protease inhibitor cocktail. The homogenates were centrifuged at 15000 × g for 30 min at 4°C. The pellets were resuspended in buffer and incubated at 37°C for 5 min to remove endogenous neurotransmitters. The protein samples were recentrifuged, and the final pellets were resuspended in the appropriate buffer and stored at −80°C until use. Protein content was determined using the Bio‐Rad Protein Reagent (BioRad, Inc., Madrid, Spain).
Western blotting and immunodetection
ΔFosB and c‐Fos Western blot analyses were performed as described by Buenrostro‐Jáuregui et al. (2016) with minor modifications. Briefly, for each sample, 10 or 20 μg of protein was mixed with loading buffer [0.5 M Tris–HCl, pH = 6.8, 10% glycerol, 2% (w/v) SDS, 5% (v/v) 2‐β‐mercaptoethanol, 0.05% bromophenol blue], boiled for 5 min and loaded onto a 10% acrylamide gel. Proteins were then transferred to PVDF sheets (Immobilion‐P, Millipore). PVDF membranes were blocked for 1 h at room temperature with 5% defatted milk in Tris‐buffer plus 0.05% Tween‐20 and incubated overnight at 4°C with mouse primary antibody anti‐FosB (1:250) or rabbit primary antibody anti‐c‐Fos (1:200). After washing, membranes were incubated for 1 h at room temperature with a peroxidase‐conjugated (1:2500) antimouse or antirabbit (1:2000) IgG antibody. Immunoreactive protein was visualized using a chemoluminescence‐based detection kit (Immobilion Western, Millipore) and a BioRad ChemiDoc XRS gel documentation system (BioRad, Inc., Madrid, Spain). Scanned blots were analysed using BioRad Image Software, and dot densities were expressed as a proportion of those taken from control. As a control for load, β‐tubulin (1:2500) or GAPDH (1:5000) antibody was used.
D2 receptor density
The density of D2 receptors in striatal membranes was measured by [3H]raclopride binding assays as described (Martínez‐Clemente et al., 2014). Assays were performed in tubes containing 2 nM [3H]raclopride and 50 μg of membranes. Incubation was carried out at 25°C for 1 h in a Tris–HCl buffer. Sulpiride (300 μM) was used to determine non‐specific binding. The incubation was ended by rapid filtration under vacuum through Whatman GF/B glass fibre filters. The radioactivity in the filters was measured by liquid scintillation spectrometry.
Data acquisition and statistical analysis
The data and statistical analysis in this study comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Data were expressed as mean ± SEM. Data from biochemical analyses were normalized with 100% defined as the mean of the technical replicates in the control group, and the SEM was normalized appropriately. Animals were randomly assigned to an experimental group. During the behavioural manipulations, researchers were not aware of the pretreatment that each animal had received.
Differences between groups were compared using two‐way ANOVA or Student's t‐test for independent samples where appropriate. The α error probability was set at 0.05. Significant differences (P < 0.05) were analysed using the Tukey's post hoc test for multiple comparison measures (InVivoStat software package) only if F achieved the necessary level of statistical significance (P < 0.05) and no significant variance inhomogeneity was observed. The exact group size for the individual experiments is shown in the corresponding figure legends. To analyse the acquisition of cocaine SA during the 10 day training, extinction of the operant behaviour and reinstatement, a three‐way ANOVA was calculated, with nosepoke (active or inactive), treatment with MDPV or saline, and day (or session) as factors of variation. Subsequent Tukey's post hoc tests were calculated when required. Breaking point achieved at the end of the PR sessions was analysed using the non‐parametric Mann–Whitney U‐test.
Materials
Pure racemic MDPV·HCl was synthesized and characterized in our laboratory as described (Novellas et al., 2015). Cocaine was provided by the Spanish National Institute of Toxicology. MDPV and cocaine solutions for injection were prepared in 0.9% NaCl (saline, pH = 7.4) immediately before administration. Mouse monoclonal ΔFosB antibody and the protease inhibitor cocktail were purchased from Abcam (Cambridge, UK) and rabbit polyclonal c‐Fos antibody from Santa Cruz Biotechnology (Santa Cruz, CA, USA). [3H]raclopride was obtained from Perkin Elmer Life Sci. (Boston, MA, USA). Ketamine was from Rhône Merieux (Lyon, France). Xylazine, sulpiride and all buffer reagents (analytical grade) were obtained from Sigma‐Aldrich (St. Louis, MO, USA).
Results
Sensitization to the hyperlocomotor responses induced by repeated cocaine administration
To evaluate the behavioural sensitization to cocaine 5 days after MDPV pretreatment (regime A), we assessed the hyperlocomotion induced by repeated cocaine administration (Figure 2). During the treatment phase (Figure 2 inset), cocaine induced an acute hyperlocomotion that increased with repeated daily exposure. Two‐way ANOVA revealed effect of the day (F4,76 = 8.791) and the pretreatment factor (F1,19 = 6.025,) without interaction between factors.
Figure 2.
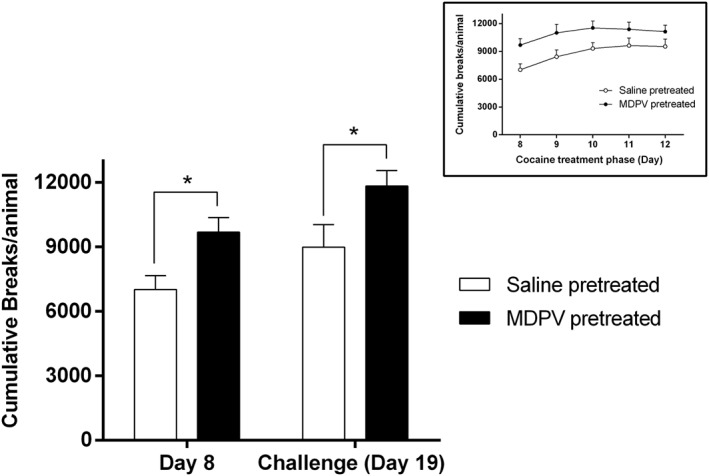
Effect of MDPV treatment (regime A) on cocaine‐induced sensitization. Bars represent mean (±SEM) of cumulative breaks per animal after cocaine (7.5 mg·kg−1, i.p.) injection on the first day of the sensitization protocol (Day 8) and the challenge day (Day 19) (saline‐pretreated group, n = 10 and MDPV‐pretreated group, n = 11). *P < 0.05, compared with the saline‐pretreated group. Inset: Cumulative breaks per animal during the treatment phase of the cocaine‐induced sensitization protocol in saline‐ and MDPV‐pretreated mice.
When analysing the differences between the first day of the treatment (Day 8) and the cocaine challenge (Day 19) (Figure 2), two‐way ANOVA also demonstrated a significant effect of the day (F1,19 = 9.909) and the pretreatment factor (F1,19 = 9.504) without the interaction between pretreatment and day.
Interestingly, mice pretreated with MDPV showed a significant increase in the hyperlocomotor activity induced by cocaine on both, the first day of cocaine administration (Day 8) and the challenge day (Day 19) compared with saline‐pretreated mice. Overall, these results indicate that pre‐exposure to MDPV is able to enhance the response to cocaine, without affecting the acquisition of sensitization.
Cocaine‐induced HLA
The HLA was monitored for 15 min after a single saline (5 mg·kg−1) or cocaine (7.5 mg·kg−1) i.p. injection to mice previously pretreated with saline or MDPV according to administration regime B (Figure 3). This dose of cocaine elicited a significant psychostimulant effect on saline‐ and MDPV‐pretreated animals, although a higher hyperlocomotor response was observed in the group pretreated with the psychostimulant. Two‐way ANOVA revealed significant effect of the cocaine challenge (F1,44 = 53.60) and interaction between challenge and pretreatment (F1,44 = 4.58), but no effect of the pretreatment factor was found. This means that MDPV pretreatment, by itself, is not capable of increasing locomotor activity when mice are challenged with saline, but only after challenge with cocaine.
Figure 3.
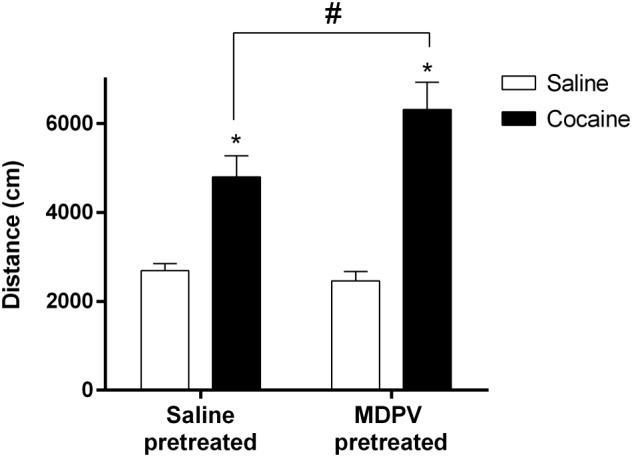
Effect of MDPV treatment (regime B) on cocaine‐induced HLA. Bars represent mean (±SEM) of the distance travelled after a single saline (5 mL·kg−1, i.p.) or cocaine (7.5 mg·kg−1, i.p) injection 21 days after treatment (n = 12 per group). *P < 0.05, compared with the corresponding saline injection. #P < 0.05, compared with the saline‐pretreated group.
Cocaine‐induced CPP
As shown in Figure 4A, saline‐ and MDPV‐pretreated mice (regime B) presented no preference for any of the compartments (Figure 4A). Only one animal from the saline‐pretreated group was withdrawn from the study due to an initial preference for one of the compartments (>65% of the total session time spent in one compartment). The repeated administration of cocaine (10 mg·kg−1, i.p.) produced a preference for the cocaine‐paired compartment (Figure 4B). Two‐way ANOVA demonstrated a significant effect of the pretreatment factor (F1,58 = 15.0), treatment effect (F1,58 = 114.4) and interaction between treatment and pretreatment (F1,58 = 7.075). Accordingly, MDPV‐pretreated mice showed an increased expression of the cocaine‐induced CPP compared with the saline‐pretreated group (Figure 4B).
Figure 4.
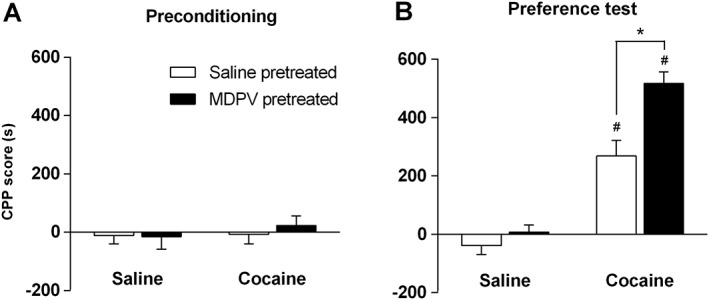
Effect of MDPV treatment (regime B) on cocaine‐induced CPP. Bars represent mean (±SEM) of CPP score (see Methods for details) during preconditioning (n = 16 per group) (Panel A) and preference test (n = 16 per group) (Panel B). *P < 0.05, compared with saline‐pretreated group; #P < 0.05, compared with its respective control group (conditioned with saline).
Self‐administration reinforced with cocaine
Acquisition of cocaine SA
The effect of the MDPV pretreatment (regime B) on the reinforcing properties of cocaine was evaluated in the SA procedure. First, both saline‐ and MDPV‐pretreated mice were trained to self‐administer cocaine (1 mg·kg−1 per infusion) during 10 days under a FR1 schedule of reinforcement. The criteria acquisition rates were equal for both groups: 56%. All the statistical analyses and data representations were performed only with mice that acquired the learning criteria. Three‐way ANOVA (pretreatment × day of training × nosepoke) of infusions on both nosepokes given along the FR1 sessions yielded no significant effects of the pretreatment factor (F1,9 = 0.56) (Figure 5A), thus indicating a lack of effect of MDPV pretreatment on the acquisition of cocaine SA behaviour. The operant procedure produced the acquisition of cocaine SA as revealed by the day of training factor (F9,9 = 2.138). Animals were able to discriminate between active and inactive nosepokes as indicated by the nosepoke factor (F1,9 = 108.8). An interaction between day of training and nosepoke factors was also found (F9,9 = 4.45). No other interactions were found. A significant effect of MDPV pretreatment was revealed in the breaking point achieved on the PR session (Mann–Whitney U = 15.5) (Figure 5B), indicating that MDPV pretreatment increased the value of cocaine as reinforcer.
Figure 5.
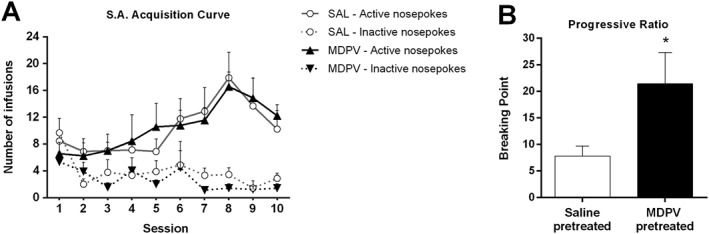
(Panel A) Effect of MDPV treatment (regime B) on the acquisition of cocaine SA behaviour for 2 h daily FR1 sessions during 10 days of training (n = 9 per group). (Panel B) Effect of MDPV treatment (regime B) on SA behaviour breaking point in a PR schedule of reinforcement (n = 9 per group). Data represent the mean (±SEM) of the last ratio achieved in the PR session during 2 h. *P < 0.05, compared with the saline‐pretreated group.
Extinction and cocaine‐primed reinstatement
Three‐way ANOVA (pretreatment × extinction day × nosepokes) showed that groups extinguished cocaine SA behaviour among the extinction sessions, as the factor extinction day had a significant effect (F11,11 = 14.78) (Figure 6A). However, the factor pretreatment had no significant influence over nosepokes along the extinction phase (F1,11 = 0.057). Animals were able to discriminate between nosepokes (F1,11 = 284.6), but as the interaction between this factor and pretreatment revealed (F1,11 = 16), both groups stopped discriminating between nosepokes in different days. Subsequent post hoc analyses indicated that MDPV‐pretreated animals discriminated between nosepokes until day 7 (Tukey), whereas SAL‐pretreated animals discriminated until day 6 (Tukey). A significant interaction between extinction day and nosepokes (F11,11 = 7645) was found. No other interactions were found.
Figure 6.
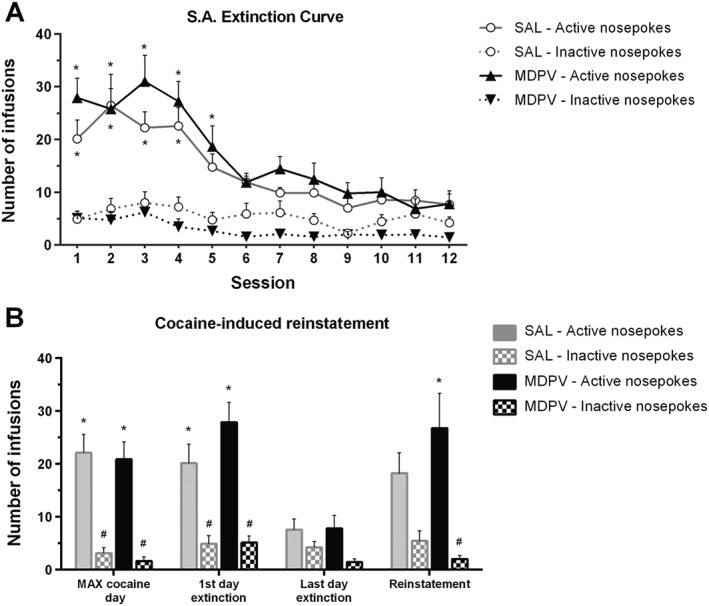
(Panel A) Extinction of cocaine SA behaviour for 2 h daily sessions during 12 days (n = 9 per group). *P < 0.05, compared with the inactive nosepokes performed by the same group in the same day of extinction phase. (Panel B) Cocaine‐primed, drug‐induced reinstatement of cocaine SA behaviour (n = 9 per group). The different phases of the experiment are showed in the X axis. *P < 0.05, compared with the active nosepokes performed by the same group in the last day of extinction. #P < 0.05, compared with the active nosepokes of the same group in the same experimental phase. Data represent the mean of nosepokes ± SEM in the active and inactive holes.
After extinction of cocaine SA, mice that acquired the extinction criteria were submitted to a cocaine‐primed (10 mg·kg−1), drug‐induced reinstatement session (Figure 6B). Three‐way ANOVA (pretreatment × session × nosepokes) showed a significant effect of day (F3,3 = 8.263) and nosepokes (F1,3 = 116.9), without pretreatment effect (F1,3 = 0.477). Significant interactions were found between days and nosepokes (F3,3 = 6.128), and pretreatment and nosepokes (F1,3 = 3.979). Post hoc analyses indicated that both groups of pretreatments did not differ in the number of active nosepokes in the reinstatement session. However, MDPV‐pretreated mice, unlike SAL‐pretreated, reinstated their previously extinguished cocaine‐SA behaviour after being drug‐primed (cocaine 10 mg·kg−1). Therefore, SAL‐pretreated mice did not reinstate previous extinguished cocaine‐SA behaviour (Tukey). Interestingly, MDPV‐pretreated mice achieved a significant difference between active nosepokes in reinstatement session versus the last day of extinction (Tukey) (Figure 6B).
c‐Fos and ΔFosB expression
Because we had seen that the pretreatment with MDPV modified the response to cocaine, we wanted to determine how the MDPV pretreatment influenced the expression of certain factors that are known to be associated with the effects of cocaine, such as c‐Fos and ΔFosB. Expression of the transcription factor ΔFosB in the brain also controls the responsiveness of an animal to the rewarding and locomotor‐activating effects of cocaine. Following regime B, we investigated the expression of ΔFosB 24 h after saline or MDPV pretreatment (Day 8) (Figure 7A) and after saline or cocaine challenge (Day 29) (Figure 7B) and also c‐Fos 2 h after saline or cocaine challenge (Day 28) (Figure 7C).
Figure 7.
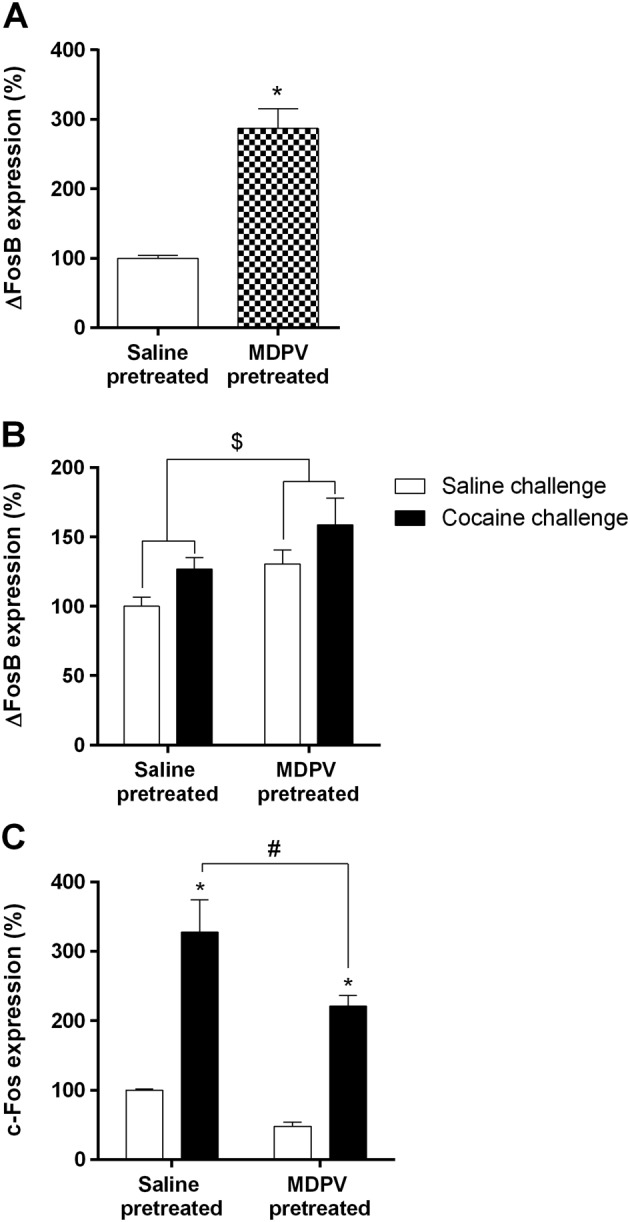
Effect of MDPV treatment (regime B) on ΔFosB expression 24 h after treatment (n = 6 per group) (Panel A) or saline and cocaine challenge (saline‐pretreated group, n = 5 per group and MDPV‐pretreated group, n = 6 per group) (Panel B). *P < 0.05, compared with the saline‐pretreated group. $P < 0.05, two‐way ANOVA pretreatment factor (F1,18 = 5.976). (Panel C) Effect of MDPV treatment (regime B) on c‐Fos expression 2 h after saline and cocaine challenge (saline‐pretreated group, n = 5 per group and MDPV‐pretreated group, n = 6 per group). *P < 0.05, compared with its corresponding saline‐challenge group. #P < 0.05, compared with the saline‐pretreated group. Results are expressed as mean ± SEM.
As shown in Figure 7A, mice pretreated with MDPV (regime B) showed a significant increase of ΔFosB expression, by 300% compared with saline‐treated mice, when measured 24 h after finishing the treatment. At day 29, 24 h after receiving the saline/cocaine challenge, the statistical analysis showed a significant effect of the pretreatment factor (F1,18 = 5.976; Figure 7B). Therefore and although the high ΔFosB expression declined during withdrawal, this factor still remained apparently elevated (132% animals pretreated with MDPV and challenged with saline). In addition, cocaine challenge also produced an increase in ΔFosB expression compared with saline injection (F1,18 = 4.699).
The c‐Fos expression has been used as a marker for neuronal activity. In this context, two‐way ANOVA revealed effect of the challenge factor (F1,18 = 54.21; Figure 7C). Cocaine induced a significant increase in c‐Fos expression in both groups, saline‐ and MDPV‐pretreated mice (320% and 220% respectively). Accordingly, statistical analysis also disclosed the effect of the pretreatment factor (F1,18 = 8.497). Thus, MDPV pretreatment reduces the cocaine‐induced expression of this marker. Moreover, after saline challenge, MDPV‐pretreated mice showed a decrease (52%) in c‐Fos expression compared with SAL‐pretreated mice, although such differences did not reach statistical significance. Therefore, it seemed that, at the same time when ΔFosB was increasing, c‐Fos expression was reduced.
D2 receptor density
To determine the involvement of striatal dopamine D2 receptors, we measured [3H]raclopride binding in this brain area 24 h after treatment (Figure 8A) or saline and cocaine challenge (Figure 8B). Twenty‐four hours after MDPV pretreatment, the D2 receptor density was significantly decreased (t 18 = 2.613). However, 24 h after saline or cocaine challenge, two‐way ANOVA only revealed differences of the challenge factor (F1,30 = 4.179). However, post hoc analysis did not demonstrate statistical significance.
Figure 8.
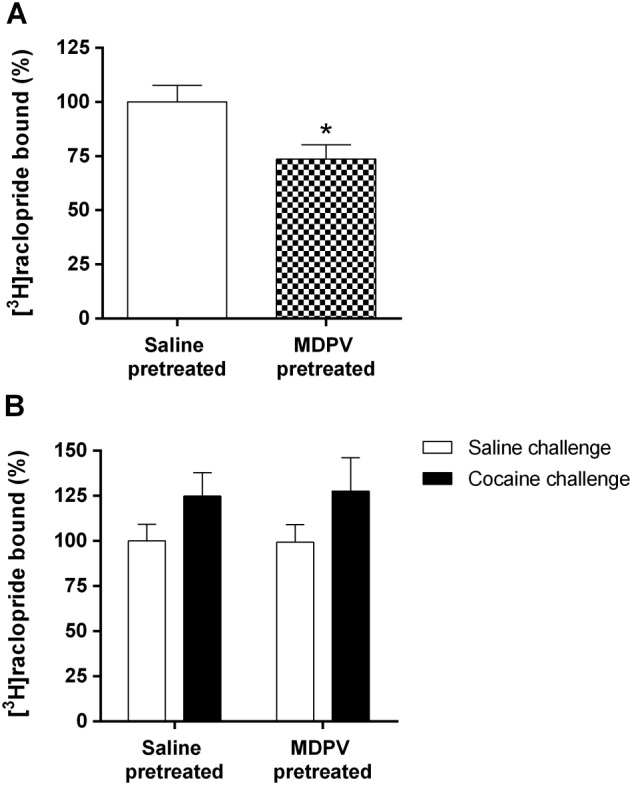
Effect of MDPV treatment (regime B) on D2 receptor density 24 h after treatment (saline‐pretreated group, n = 9 and MDPV‐pretreated group, n = 11) (Panel A), and after saline or cocaine challenge (saline‐pretreated group, n = 8 per group and MDPV‐pretreated group, n = 9 per group) (Panel B). D2 receptor density was measured as [3H]raclopride bound in the mouse striatum. Results are expressed as mean ± SEM. *P < 0.05, compared with the saline‐pretreated group.
Discussion
Cocaine abuse represents a heavy burden of disease in many countries and has become a global problem. Any factor that increases the vulnerability to cocaine abuse must be carefully evaluated. In the present study, we demonstrate that MDPV enhances the responsiveness to cocaine in all tested aspects.
In a first study (regime A), we investigated the influence of a short‐term MDPV exposure on the behavioural sensitization induced by cocaine. MDPV produced a gradual increase of cocaine hyperlocomotor effects and a high reactivity to a cocaine challenge after 7 days of cocaine withdrawal. However, mice exposed to MDPV showed a higher response to cocaine when they received this psychostimulant for the first time, and this increased hyperlocomotor effect was maintained throughout all the sensitization procedure.
In a second set of experiments, we carried out a repeated treatment (regime B) with a moderate dose of MDPV, in adolescent mice, and we investigated the influence on the acute effects of cocaine on adulthood, including motor responses, rewarding effects on the CPP, and the cocaine‐induced reinforcing effects on the SA paradigm.
We found that mice exposed to MDPV were more reactive to cocaine but not to saline injection. So this observed response was independent of the environmental context because MDPV treatment was administered in the home cages. Accordingly, an MDPV treatment with low doses (0.3 or 0.5 mg·kg−1) during 5–7 days increased responsiveness to acute cocaine (5–10 mg·kg−1) after a 10–11 days drug‐free period (Berquist et al., 2016; Buenrostro‐Jáuregui et al., 2016).
We also sought to find out if MDPV exposure resulted in increased responsiveness to cocaine in reward and reinforcing effects on mice. Our results revealed that repeated administration of MDPV in adolescent mice led to a long‐term increase of cocaine‐induced behavioural adaptations, related to its abuse potential, when tested into adulthood in the CPP. This paradigm is used to reflect the degree by which drugs of abuse establish approaching behaviours toward drug‐related stimuli (Bardo and Bevins, 2000). The observed increase in the robustness of the conditioned responses in the CPP expression seems to reflect an increase in the abuse liability of cocaine after exposure to MDPV. Thus, our findings demonstrate that MDPV exposure during adolescence can effectively potentiate cocaine abuse liability in adulthood by sensitizing the neural circuitry underlying associations between cocaine and its related stimuli. To our knowledge, this is the first study that evaluates the long‐term effects of MDPV treatment on cocaine‐induced CPP. However, similar effects of MDPV pretreatment on the development of psychostimulants‐induced reinforcing effects have been recently reported. For instance, MDPV (1.8 mg·kg−1 during 5 days) attenuated the taste avoidance induced by cocaine (18 mg·kg−1), but not those induced by LiCl, in rats (Woloshchuk et al., 2016). As mentioned above, MDPV also sensitized to cocaine locomotor response after a drug washout period (Berquist et al., 2016; Buenrostro‐Jáuregui et al., 2016). Moreover, an intermittent repeated exposure to MDPV (1 mg·kg−1 during 5 days) produced sensitization to methamphetamine challenge (0.5 mg·kg−1) in rats (Watterson et al., 2016). Nevertheless, no study has yet evaluated global MDPV effects on cocaine‐induced abuse liability and, in particular, evaluated its effects on cocaine‐induced reinforcing effects on the SA paradigm, as we present in the present study. In fact, mice repeatedly exposed to MDPV during adolescence made greater efforts to obtain a cocaine infusion in a PR schedule of reinforcement when tested in adulthood. The PR schedule of reinforcement requires behaviours specially linked to motivational functions, such as instrumental learning, execution of efforts and sustained engagement (Randall et al., 2012). The lack of difference between mice exposed to MDPV or to saline during the acquisition phase of SA could be attributed to the fact that this phase was performed under an FR1 schedule of reinforcement that seems not to be appropriate to determine the reinforcement efficiency (Richardson and Roberts, 1996). In this sense, it is a common feature of rodent cocaine SA studies to find a certain degree of inconsistency between results on FR1, PR schedules and reinstatement sessions depending on the experimental conditions (Homberg et al., 2002; Morgan et al., 2005; Zhang et al., 2005; España et al., 2011).
In addition, MDPV‐pretreated mice delayed extinction after acquisition of the operant behaviour to cocaine and reinstated after a cocaine priming injection. Reinstatement of SA behaviour reflects the reinforcing properties of drugs and its pharmacological manipulations (Shaham et al., 2003; Soria et al., 2008; Bossert et al., 2013). We have also found a drug‐seeking reinstatement behaviour in mice previously exposed to MDPV, suggesting a higher level of craving than in control mice. Thus, we suggest that MDPV exposure during adolescence will strengthen the efforts to obtain cocaine in adulthood, and this effect leads to an enhanced vulnerability to reinstate cocaine SA behaviour once extinguished.
These results lead us to hypothesize that MDPV administration induces long‐lasting adaptive changes, leading to a greater response to cocaine. ΔFosB, c‐Fos and D2 receptors are factors involved in the acute and long‐lasting effects of cocaine (Larson et al., 2010; Lee et al., 2013). For instance, studies performed using positron emission topography have consistently shown that drug abuse is accompanied by a decrease in striatal D2 receptor availability. Furthermore, studies in drug‐naïve, non‐human primates suggest that D2 receptor availability is predictive of drug‐seeking behaviour (Nader et al., 2006). In the present study, we have found that repeated MDPV exposure was associated with a decreased striatal density of D2 receptors, probably reflecting a neuroadaptive effect in response to MDPV dopaminergic stimulation, but this effect is only transient as it did not remain after 21 days of withdrawal, when D2 receptor population was observed to return to initial values. Additionally, we determined D2 receptor levels after the challenge of cocaine. As expected, an acute dose of this drug modulated D2 receptors, although without influence of MDPV pre‐exposure.
There is growing evidence for an important role of ΔFosB in animal models of drug addiction (Nestler, 2008). The Fos family of proteins is rapidly and transiently induced in the striatum after acute administration of several drugs of abuse (Graybiel et al., 1990; Hope et al., 1992; Young et al., 1991). Although most of them are highly unstable, ΔFosB is progressively accumulated after repeated drug exposure. This accumulation has been linked to cocaine‐induced reward, locomotor sensitization and SA behaviour (Colby et al., 2003; Kelz et al., 1999; McClung et al., 2004), which together suggest a role in the neural mechanisms involved in transitioning between recreational use and abuse phenomenon. In the present study, an increased expression of ΔFosB was found 24 h after the end of the MDPV exposure and levels of this factor remained raised throughout the period of abstinence. Therefore, it is reasonable to propose that the increased level of this transcription factor responded to MDPV treatment, leading to an increased responsiveness to cocaine effects. Changes in ΔFosB seem to extend the regulation of drug sensitivity toward more complex behaviours (Colby et al., 2003). Thus, according to our findings, mice overexpressing ΔFosB work harder to self‐administer cocaine in PR schedule of reinforcement in SA assays, suggesting that ΔFosB may sensitize animals to the incentive motivational properties of cocaine and thereby leading to a propensity for relapse after drug withdrawal.
Numerous putative targets for ΔFosB have been identified in brain, and some of these target genes have been related to the cellular and behavioural effects of this transcription factor (McClung et al., 2004). One of these target genes is c‐Fos. Induction of c‐Fos protein is considered an early marker of neural activation, and it is also important for behavioural responses to cocaine (Zhang et al., 2006). This factor is markedly activated by acute administration of psychostimulants, but only weakly after repeated exposure (Hope et al., 1992; Persico et al., 1993), when levels of ΔFosB are high. In this sense, Renthal et al. (2008) demonstrated that ΔFosB mediates epigenetic desensitization of the c‐Fos gene after chronic exposure to a psychostimulant. In our study, a significant increase of c‐Fos expression appeared a short time after cocaine challenge that undoubtedly reflects neuronal activation by the drug. However, this protein expression was related to MDPV pretreatment. Thus, levels of c‐Fos seemed to be inversely associated with MDPV pretreatment, when ΔFosB is significantly expressed. Results demonstrate a negative association between both factors, in accordance with the findings of Renthal et al., (2008).
In summary, MDPV increased most of the behavioural responses related to cocaine effects, including locomotor sensitization, reward and the strength of cocaine as reinforcer in a SA procedure. It is noteworthy that MDPV increased capability to reinstate cocaine SA behaviour once extinguished, presumably indicating increased craving, after MDPV exposure during adolescence. These behavioural alterations were associated with an accumulation of ΔFosB, a sustained molecular switch for cocaine addiction, providing a possible mechanism by which molecular changes induced by MDPV can persist for weeks after withdrawal and supporting the deleterious effects of MDPV on cocaine abuse liability. Therefore, these results suggest that consumption of MDPV during adolescence induces long‐lasting adaptive changes leading to a higher response to cocaine in the adulthood, predisposing to a higher vulnerability to abuse of this drug. From a clinical point of view, this feature represents a basic step to provide new knowledge about factors involved in the vulnerability to cocaine addiction.
Author contributions
O.V. and E.E. were responsible for the study concept and design. R.L.A., M.A.L. and L.D.C. carried out the experimental studies. J.C. participated in the data analysis and D.P. in immunoassays methodology. R.L.A. and M.A.L. drafted the manuscript. J.C. and D.P. participated in the interpretation of findings. O.V. and E.E. provided a critical revision of the manuscript for intellectual content. All authors critically reviewed the content and approved the final version for publication.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This study was supported by Ministerio de Economia y Competitividad (grant numbers SAF2016‐46135‐R, SAF2016‐75966‐R‐FEDER and SAF2013‐41761‐R‐FEDER), by the European Union's Horizon 2020 research and innovation programme 2014–2020 under grant agreement no 634143, Spanish Ministry of Health (RD/12/0028/0024‐FEDER and Plan Nacional sobre Drogas #2014/020). L.D.C. and M.A.L. received FPU grants from the Ministerio de Economía y Competitividad (15/02492). J.C., D.P. and E.E. belong to 2014SGR1081, and O.V. to 2014SGR34. R.L.‐A. position was funded by an institutional program of the Universitat de Barcelona in collaboration with Obra Social de la Fundació Bancària La Caixa.
López‐Arnau, R. , Luján, M. A. , Duart‐Castells, L. , Pubill, D. , Camarasa, J. , Valverde, O. , and Escubedo, E. (2017) Exposure of adolescent mice to 3,4‐methylenedioxypyrovalerone increases the psychostimulant, rewarding and reinforcing effects of cocaine in adulthood. British Journal of Pharmacology, 174: 1161–1173. doi: 10.1111/bph.13771.
References
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015). The concise guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA (2000). Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 153: 31–43. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M et al. (2013). Powerful cocaine‐like actions of 3,4‐methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 38: 552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquist MD, Traxler HK, Mahler AM, Baker LE (2016). Sensitization to the locomotor stimulant effects of ‘bath salt’ constituents, 4‐methylmethcathinone (4‐MMC) and 3,4‐methylenedioxypyrovalerone (MDPV), in male Sprague‐Dawley rats. Drug Alcohol Depend 164: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlsma L, Miserez B, Ibáñez M, Vicent C, Guillamón E, Ramsey J et al. (2015). Identification and characterization of a novel cathinone derivative 1‐(2,3‐dihydro‐1H‐inden‐5‐yl)‐2‐phenyl‐2‐(pyrrolidin‐1‐yl)‐ethanone seized by customs in Jersey. Forensic Toxicol 34: 144–150. [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y (2013). The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 229: 453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro‐Jáuregui M, Ciudad‐Roberts A, Moreno J, Muñoz‐Villegas P, López‐Arnau R, Pubill D et al. (2016). Changes in CREB and deltaFosB are associated with the behavioural sensitization induced by methylenedioxypyrovalerone. J Psychopharmacol 30: 707–712. [DOI] [PubMed] [Google Scholar]
- Cass DK, Thomases DR, Caballero A, Tseng KY (2013). Developmental disruption of gamma‐aminobutyric acid function in the medial prefrontal cortex by noncontingent cocaine exposure during early adolescence. Biol Psychiatry 74: 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW (2003). Striatal cell type‐specific overexpression of DeltaFosB enhances incentive for cocaine. J Neurosci 23: 2488–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Melchior JR, Roberts DCS, Jones SR (2011). Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self‐administration. Psychopharmacology (Berl) 214: 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA (1990). Amphetamine and cocaine induce drug‐specific activation of the c‐fos gene in striosome‐matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A 87: 6912–6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, van den Akker M, Raasø HS, Wardeh G, Binnekade R, Schoffelmeer ANM et al. (2002). Enhanced motivation to self‐administer cocaine is predicted by self‐grooming behaviour and relates to dopamine release in the rat medial prefrontal cortex and amygdala. Eur J Neurosci 15: 1542–1550. [DOI] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ (1992). Regulation of immediate early gene expression and AP‐1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci U S A 89: 5764–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PS, Johnson MW (2014). Investigation of ‘bath salts’ use patterns within an online sample of users in the United States. J. Psychoactive Drugs 46: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katselou M, Papoutsis I, Nikolaou P, Spiliopoulou C, Athanaselis S (2015). α‐PVP (‘flakka’): a new synthetic cathinone invades the drug arena. Forensic Toxicol 34: 41–50. [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Whisler K, Gilden L, Beckmann AM et al. (1999). Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature 401: 272–276. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Wetzell B, Rice KC, Riley AL (2014). 3,4‐Methylenedioxypyrovalerone (MDPV)‐induced conditioned taste avoidance in the F344/N and LEW rat strains. Pharmacol Biochem Behav 126: 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Akkentli F, Edwards S, Graham DL, Simmons DL, Alibhai IN et al. (2010). Striatal regulation of ΔFosB, FosB, and cFos during cocaine self‐administration and withdrawal. J Neurochem 115: 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Oh JH, Yang JH, Youn B, Shim Y‐B, Shim I et al. (2013). Protein kinase G linked to dopamine D3 receptors in the dorsal striatum controls dopamine release, ΔFosB expression and locomotor activity after repeated cocaine administration. Neurosci Lett 541: 120–125. [DOI] [PubMed] [Google Scholar]
- Martínez‐Clemente J, Escubedo E, Pubill D, Camarasa J (2012). Interaction of mephedrone with dopamine and serotonin targets in rats. Eur Neuropsychopharmacol 22: 231–236. [DOI] [PubMed] [Google Scholar]
- Martínez‐Clemente J, López‐Arnau R, Abad S, Pubill D, Escubedo E, Camarasa J (2014). Dose and time‐dependent selective neurotoxicity induced by mephedrone in mice. PLoS One 9: e99002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ (2004). DeltaFosB: a molecular switch for long‐term adaptation in the brain. Mol Brain Res 132: 146–154. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerchar TL, Zarcone TJ, Fowler SC (2005). Differential acquisition of lever pressing in inbred and outbred mice: comparison of one‐lever and two‐lever procedures and correlation with differences in locomotor activity. J Exp Anal Behav 84: 339–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Smith MA, Roberts DCS (2005). Binge self‐administration and deprivation produces sensitization to the reinforcing effects of cocaine in rats. Psychopharmacology (Berl) 178: 309–316. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N et al. (2006). PET imaging of dopamine D2 receptors during chronic cocaine self‐administration in monkeys. Nat Neurosci 9: 1050–1056. [DOI] [PubMed] [Google Scholar]
- Nestler EJ (2008). Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci 363: 3245–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novellas J, López‐Arnau R, Carbó ML, Pubill D, Camarasa J, Escubedo E (2015). Concentrations of MDPV in rat striatum correlate with the psychostimulant effect. J Psychopharmacol 29: 1209–1128. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN (2008). Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9: 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico AM, Schindler CW, O'Hara BF, Brannock MT, Uhl GR (1993). Brain transcription factor expression: effects of acute and chronic amphetamine and injection stress. Mol Brain Res 20: 91–100. [DOI] [PubMed] [Google Scholar]
- Pozzi M, Roccatagliata D, Sterzi R (2008). Drug abuse and intracranial hemorrhage. Neurol Sci 29 (Suppl 2): S269–S270. [DOI] [PubMed] [Google Scholar]
- Pubill D, Garcia‐Ratés S, Camarasa J, Escubedo E (2013). 3,4‐Methylenedioxy‐methamphetamine induces in vivo regional up‐regulation of central nicotinic receptors in rats and potentiates the regulatory effects of nicotine on these receptors. Neurotoxicology 35: 41–49. [DOI] [PubMed] [Google Scholar]
- Randall PA, Pardo M, Nunes EJ, López Cruz L, Vemuri VK, Makriyannis A et al. (2012). Dopaminergic modulation of effort‐related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PLoS One 7: e47934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan‐Shaw S, Nihal M, Ahmad N (2008). Dose translation from animal to human studies revisited. FASEB J 22: 659–661. [DOI] [PubMed] [Google Scholar]
- Renthal W, Carle TL, Maze I, Covington HE, Truong H‐T, Alibhai I et al. (2008). Delta FosB mediates epigenetic desensitization of the c‐fos gene after chronic amphetamine exposure. J Neurosci 28: 7344–7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS (1996). Progressive ratio schedules in drug self‐administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11. [DOI] [PubMed] [Google Scholar]
- Ross EA, Reisfield GM, Watson MC, Chronister CW, Goldberger BA (2012). Psychoactive ‘bath salts’ intoxication with methylenedioxypyrovalerone. Am J Med 125: 854–858. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, Wit HD, Stewart J (2003). The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168: 3–20. [DOI] [PubMed] [Google Scholar]
- Simmler L, Buser T, Donzelli M, Schramm Y, Dieu L‐H, Huwyler J et al. (2013). Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168: 458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Barbano MF, Maldonado R, Valverde O (2008). A reliable method to study cue‐, priming‐, and stress‐induced reinstatement of cocaine self‐administration in mice. Psychopharmacology (Berl) 199: 593–603. [DOI] [PubMed] [Google Scholar]
- Soria G, Castañé A, Ledent C, Parmentier M, Maldonado R, Valverde O (2006). The lack of A2A adenosine receptors diminishes the reinforcing efficacy of cocaine. Neuropsychopharmacology 31: 978–987. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Brake SC (1983). Periadolescence: age‐dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol 16: 83–109. [DOI] [PubMed] [Google Scholar]
- Thompson D, Martini L, Whistler JL (2010). Altered ratio of D1 and D2 dopamine receptors in mouse striatum is associated with behavioral sensitization to cocaine. PLoS One 5: e11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourino C, Valjent E, Ruiz‐Medina J, Herve D, Ledent C, Valverde O (2012). The orphan receptor GPR3 modulates the early phases of cocaine reinforcement. Br J Pharmacol 167: 892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC (n.d.), World drug report 2015 Available at: http://www.unodc.org/wdr2015/ (accessed 9/15/16).
- Walsh SL, Stoops WW, Moody DE, Lin S‐N, Bigelow GE (2009). Repeated dosing with oral cocaine in humans: assessment of direct effects, withdrawal, and pharmacokinetics. Exp Clin Psychopharmacol 17: 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Taylor SB, Nemirovsky NE, Olive MF (2016). Sensitization to the motor stimulant effects of 3,4‐methylenedioxypyrovalerone (MDPV) and cross‐sensitization to methamphetamine in rats. J Drug Alcohol Res 5: pii: 235967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloshchuk CJ, Nelson KH, Rice KC, Riley AL (2016). Effects of 3,4‐methylenedioxypyrovalerone (MDPV) pre‐exposure on the aversive effects of MDPV, cocaine and lithium chloride: Implications for abuse vulnerability. Drug Alcohol Depend 167: 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ (1991). Cocaine induces striatal c‐fos‐immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci U S A 88: 1291–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Sanchez H, Kehoe P, Kosten TA (2005). Neonatal isolation enhances maintenance but not reinstatement of cocaine self‐administration in adult male rats. Psychopharmacology (Berl) 177: 391–399. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang L, Jiao H, Zhang Q, Zhang D, Lou D et al. (2006). c‐Fos facilitates the acquisition and extinction of cocaine‐induced persistent changes. J Neurosci 26: 13287–13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuba D, Byrska B (2013). Prevalence and co‐existence of active components of ‘legal highs’. Drug Test Anal 5: 420–429. [DOI] [PubMed] [Google Scholar]


