Abstract
Children with neurofibromatosis type 1 (NF1) cancer predisposition syndrome are prone to the development of low-grade brain tumors (gliomas) within the optic pathway (optic gliomas). One of the key obstacles to developing successful therapeutic strategies for these tumors is the striking lack of information about the mechanical properties that characterize these tumors relative to non-neoplastic optic nerve tissue. To study the physical changes that may occur when an optic nerve glioma is present, we employed atomic force microscopy to measure the stiffness of healthy versus tumor-bearing optic nerve tissue. We found that the average elastic moduli of non-neoplastic and tumor-bearing optic nerves were ∼3 and ∼6 kPa, respectively. Based on previous studies implicating changes in extracellular matrix remodeling in other, related optic nerve pathological states, we found decreased expression of one major metalloproteinase protein (MMP-2) and unchanged expression of lysyl oxidase and a second metalloproteinase, MMP-9, in murine optic gliomas relative to normal non-neoplastic optic nerve. Collectively, these observations suggest a productive interplay between physical properties of mouse optic nerve gliomas and the extracellular matrix.
Main Text
Brain tumors represent the most common solid tumor in children, with low-grade gliomas (LGGs) accounting for the majority of these neoplasms (1, 2, 3). Among these LGGs, many arise within the optic pathway (optic pathway gliomas (OPGs)). Although most of these OPGs arise in the absence of a predisposing genetic syndrome, the presence of an OPG should raise suspicion for neurofibromatosis type 1 (NF1), a nervous system cancer predisposition syndrome that causes tumors in the optic pathway (4, 5, 6). Of the population of children affected by NF1 syndrome, 15–20% of affected children will develop an OPG.
With the identification of the Nf1 gene, it became possible to develop small-animal models of NF1-associated OPG (7). The generation of these strains represented a critical first step toward the identification of efficacious treatments for these tumors, as NF1-OPGs are rarely resected and renewable human biological specimens are not available. In this regard, numerous molecular targets have been discovered using these Nf1 genetically engineered mouse (GEM) models, several of which have been evaluated as potential chemotherapies in children with NF1-OPG (8, 9, 10).
However, NF1-OPGs, similar to their sporadic pilocytic astrocytoma counterparts, are complex ecosystems composed of both cellular and acellular components. Analysis of human LGGs, including rare NF1 pilocytic astrocytomas, has demonstrated that 30–50% of the cells in these tumors are immune-system-like cells (macrophages and microglia) embedded within a rich extracellular matrix (11, 12). Although intense study of the role of macrophages and microglia has revealed additional targets for stroma-directed therapies, the contribution of the extracellular matrix (ECM) remains unexplored.
In many types of cancer, including breast, brain, and colorectal cancers (13, 14, 15), cell growth and ECM remodeling that occur during tumor development alter the mechanical properties of the tumor. As such, breast and colorectal tumors have been found to be stiffer than their surrounding healthy tissue (13, 15). Lymph nodes also have been found to increase in stiffness when invaded by metastatic lung carcinoma cells (14). This rise in stiffness is also associated with enhanced metastatic potential of cancer cells (16, 17). Although such relationships, i.e., between tumor development and stiffness, have been established for a variety of cancer types, the mechanical properties of optic nerve glioma, as well as their underlying acellular causes, are unknown.
The normal optic nerve is a complex tissue composed of numerous distinct cellular and extracellular components. Similarly, optic nerve gliomas harbor a unique collection of neoplastic (astrocytes and stem cells) and non-neoplastic (endothelial cells and microglia) cell types embedded within a rich ECM. In this manner, the normal and neoplastic optic nerve tissues represent distinct ecological systems. As such, future therapies might leverage the physical properties unique to the optic glioma ecosystem as a means of targeting the specialized determinants of the tumor. This information is critical to the design of future in vitro platforms to perform drug-screening studies for these optic nerve tumors.
To examine the ECM and mechanical properties associated with murine Nf1 optic glioma, we employed atomic force microscopy (AFM), which allows for mechanical testing of small tissues in defined areas. In this study, we found that, similar to other LGGs, Nf1 optic glioma tissues are stiffer than healthy tissue. To establish a relationship between the ECM and mechanical properties, we also evaluated the levels of matrix metalloproteases (MMP-2 and MMP-9), which are important for ECM degradation (18), and lysyl oxidase (LOX), which is associated with ECM cross-linking (19). Consistent with the mechanical properties of our optic glioma system, we also found differing expression of MMP-2 in glioma-bearing optic nerves relative to controls.
Increased stiffness of tumor-bearing optic nerves
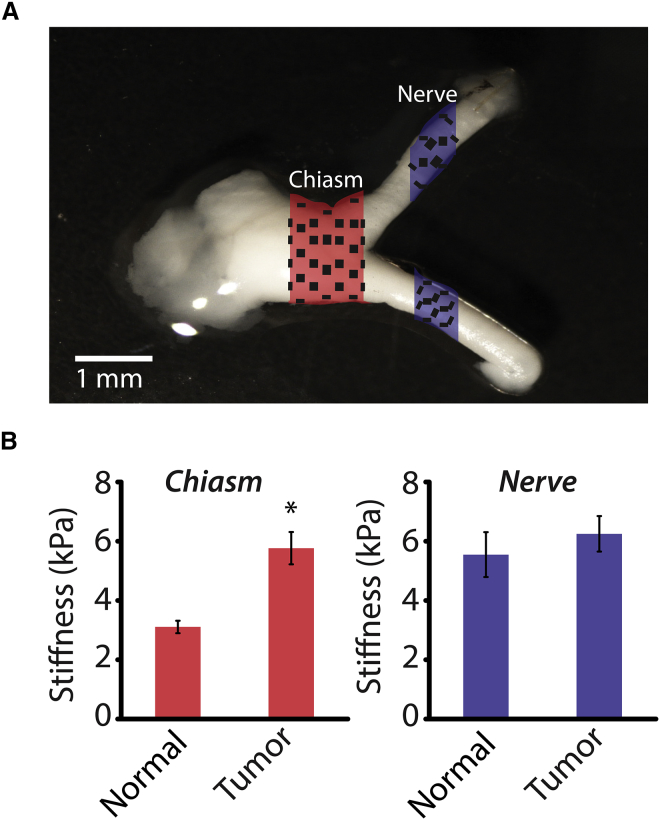
Optic nerves were harvested from 3-month-old mice at a time when optic gliomas are obvious (20), and covalently attached to glass slides precoated with poly-L-lysine. OPGs in children with NF1 typically arise in the prechiasmatic optic nerves and chiasm, similar to those arising in Nf1 mutant mice. Using AFM, the elastic modulus of the optic nerves was tested in two sections, at the chiasm and in unaffected portions of the optic nerve (≥2 mm proximal to the chiasm). Briefly, in AFM, piezoelectric actuators are used to control the x, y, and z positions of a cantilever probe, which is indented into the surface of a given tissue sample (Fig. S1). The cantilever deflection is used to calculate the indentation force of the probe and these force-deflection data are translated into the elastic modulus of the sample using a model, typically the Hertz model (21, 22). The nerves were tested at various points across the width, and the measurements were averaged (Fig. 1 A). At the chiasm, the glioma-bearing nerves were significantly stiffer than their normal (non-neoplastic) counterparts, 5.8 ± 0.5 kPa and 3.1 ± 0.6 kPa, respectively, an 85% increase (Fig. 1 B). In contrast, the stiffness of the unaffected optic nerve segments in the wild-type (6.9 ± 1.4 kPa) was similar to that in glioma-bearing nerves (6.6 ± 0.8 kPa). These findings indicate that the mechanical consequences of tumor development in the Nf1 mutant mice remain localized in the chiasm region and do not propagate into distant areas of the optic nerve tissue. These tumor-related mechanical changes in the optic nerve, along with the knowledge that NF1 tumors are characterized by ECM changes (23), could indicate that regulation of matrix-modifying proteins is a key factor in the development of these tumors.
Figure 1.
Increased stiffness of glioma-bearing optic nerves at the chiasm. (A) Diagram describing the two regions where AFM measurements were taken, the chiasm and the optic nerve (≥1 mm proximal to the chiasm). (B) Average tissue stiffness obtained using AFM. Error bars represent the mean ± SE. N > 34; ∗p < 0.05. To see this figure in color, go online.
ECM-modifying enzyme expression is altered in glioma-bearing optic nerves
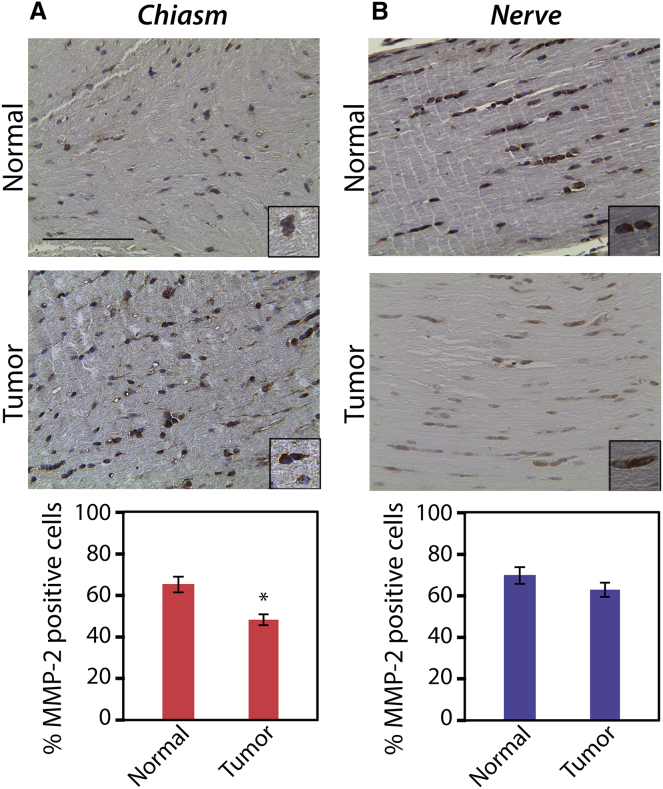
One of the hallmarks of solid tumors is the production of enzymes that modify ECM components, including MMPs (18). To examine the expression of these enzymes in the murine Nf1 optic glioma model, immunohistochemistry (IHC) was performed in 3-month-old mice with Nf1 optic glioma and in wild-type control mice using antibodies against MMP-2, MMP-9, and LOX (Figs. 2, S2, and S3, respectively). Given the localization of the glioma mainly to the chiasm and the results of the AFM experiments, the protein levels at the chiasm were compared to those in the unaffected proximal optic nerve segment. Although the intensity of the immmunostaining appears greater in glioma-bearing relative to healthy optic nerves, there was a significant decrease in the percent of MMP-2-positive cells at the chiasm in the glioma-bearing mice compared to their control counterparts (47.8 ± 1.9% and 64.1 ± 2.4%, respectively). However, in the unaffected proximal optic nerve segment of mice with Nf1 optic glioma, as compared to controls, there were no differences in MMP-2-positive cells (63.7 ± 5.0% and 68.2 ± 2.7%, respectively). There was also no difference between glioma-bearing and wild-type mice in the percent of LOX-positive cells at the chiasm (42.4 ± 7.6% and 43.3 ± 8.6%, respectively) or in the unaffected proximal optic nerve segment (50.8 ± 2.7% and 56.5 ± 5.3%, respectively). In addition, there was no difference in the percent of MMP-9-positive cells at the chiasm (48.8 ± 5.9% and 37.7 ± 3.5%) or in the unaffected proximal optic nerve segment (46.2 ± 7.5% and 39.4 ± 2.0%).
Figure 2.
Glioma-bearing optic nerves have decreased MMP-2 expression in the chiasm (A), but not in the unaffected proximal optic nerve segment (B), mirroring the effects shown with AFM. ∗p < 0.01. Error bars represent the mean ± SE. Scale bars represent 100 μm. To see this figure in color, go online.
Thus, complementary to the AFM data, we found decreased MMP-2 protein levels in the chiasms of glioma-bearing optic nerves relative to their wild-type counterparts. MMPs break down fibrous ECM structures, like collagen, to create reorganization within the tumor, and they lead to changes in physical properties. Traditionally, increases in MMP expression and LOX expression are associated with high-grade tumors (24, 25). However, in the context of optic nerve glioma, we saw a decrease in MMP-2 and no change in LOX, which suggests less breakdown of collagen fibers (a greater degree of collagen cross-linking). This result is consistent with the stiffer matrix observed by AFM at the chiasm in optic nerves from Nf1 mutant mice. Although metastatic high-grade gliomas exhibit increases in MMP-2, MMP-9, and LOX, we do not expect to see the same trends in optic nerve glioma, given that they are low-grade tumors and not metastatic cancers (26). Importantly, in the unaffected proximal optic nerve segment, there was no variation in matrix stiffness between neoplastic and non-neoplastic optic nerves. IHC images from this same location also show no significant differences in MMP-2 levels, further supporting the link between decreased MMP-2 protein levels and increased stiffness at the chiasm. In addition, this decrease in MMP-2 expression likely results from loss of Nf1 gene expression in the neoplastic astrocytes, since Nf1-deficient astrocytes exhibit a 40% decrease in Mmp2 RNA levels relative to their wild-type counterparts, as determined by quantitative reverse transcriptase-polymerase chain reaction (Fig. S4; p = 0.033).
Taken together, the results leveraging both AFM and IHC show, to our knowledge, a novel relationship between the metalloproteinase protein levels present in the acellular environment and the physical properties of Nf1 optic glioma. This work warrants further studies using three-dimensional in vitro platforms to establish mechanistic connections between these acellular and physical properties and optic glioma biology.
Author Contributions
A.P., D.H.G., and S.S.-E. designed research. C.W., L.C., M.L., J.A.T., and Y.P. performed research, contributed analytic tools, and analyzed data. C.W., L.C., D.H.G., and A.P. wrote the article.
Acknowledgments
The authors thank Courtney Corman for technical assistance with the quantitative reverse transcriptase-polymerase chain reaction experiments.
This work was in part supported by grants from the National Science Foundation (CAREER Award 1454016 to A.P.), the Edward Mallinckrodt, Jr. Foundation (New Investigator Award to A.P.), the National Cancer Institute (CA195692-01 to D.H.G.), and the National Institutes of Health (T32 Interdisciplinary Training in Mechanobiology to C.W.). D.H.G. acknowledges support from The Giorgio Foundation and a National Institute of Neurological Disorders and Stroke Research Program Award (1-R35-NS097211-01).
Editor: Philip LeDuc.
Footnotes
Christopher Walter and Lindsey Crawford contributed equally to this work.
Supporting Materials and Methods and four figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30336-3.
Supporting Material
References
- 1.Dolecek T.A., Propp J.M., Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro-oncol. 2012;14(Suppl. 5):v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurney J.G., Wall D.A., Davis F.G. The contribution of nonmalignant tumors to CNS tumor incidence rates among children in the United States. Cancer Causes Control. 1999;10:101–105. doi: 10.1023/a:1008867024545. [DOI] [PubMed] [Google Scholar]
- 3.Louis D.N., Ohgaki H., Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Listernick R., Louis D.N., Gutmann D.H. Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 optic pathway glioma task force. Ann. Neurol. 1997;41:143–149. doi: 10.1002/ana.410410204. [DOI] [PubMed] [Google Scholar]
- 5.Listernick R., Ferner R.E., Gutmann D.H. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann. Neurol. 2007;61:189–198. doi: 10.1002/ana.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King A., Listernick R., Gutmann D.H. Optic pathway gliomas in neurofibromatosis type 1: the effect of presenting symptoms on outcome. Am. J. Med. Genet. A. 2003;122A:95–99. doi: 10.1002/ajmg.a.20211. [DOI] [PubMed] [Google Scholar]
- 7.Bajenaru M.L., Hernandez M.R., Gutmann D.H. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63:8573–8577. [PubMed] [Google Scholar]
- 8.Dasgupta B., Yi Y., Gutmann D.H. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005;65:2755–2760. doi: 10.1158/0008-5472.CAN-04-4058. [DOI] [PubMed] [Google Scholar]
- 9.Hegedus B., Banerjee D., Gutmann D.H. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008;68:1520–1528. doi: 10.1158/0008-5472.CAN-07-5916. [DOI] [PubMed] [Google Scholar]
- 10.Arun D., Gutmann D.H. Recent advances in neurofibromatosis type 1. Curr. Opin. Neurol. 2004;17:101–105. doi: 10.1097/00019052-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Rickman D.S., Bobek M.P., Hanash S.M. Distinctive molecular profiles of high-grade and low-grade gliomas based on oligonucleotide microarray analysis. Cancer Res. 2001;61:6885–6891. [PubMed] [Google Scholar]
- 12.Sharma M.K., Watson M.A., Gutmann D.H. Matrilin-2 expression distinguishes clinically relevant subsets of pilocytic astrocytoma. Neurology. 2006;66:127–130. doi: 10.1212/01.wnl.0000188667.66646.1c. [DOI] [PubMed] [Google Scholar]
- 13.McKnight A.L., Kugel J.L., Ehman R.L. MR elastography of breast cancer: preliminary results. AJR Am. J. Roentgenol. 2002;178:1411–1417. doi: 10.2214/ajr.178.6.1781411. [DOI] [PubMed] [Google Scholar]
- 14.Miyaji K., Furuse A., Omata S. The stiffness of lymph nodes containing lung carcinoma metastases: a new diagnostic parameter measured by a tactile sensor. Cancer. 1997;80:1920–1925. doi: 10.1002/(sici)1097-0142(19971115)80:10<1920::aid-cncr8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 15.Baker A.M., Bird D., Erler J.T. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene. 2013;32:1863–1868. doi: 10.1038/onc.2012.202. [DOI] [PubMed] [Google Scholar]
- 16.Swaminathan V., Mythreye K., Superfine R. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 2011;71:5075–5080. doi: 10.1158/0008-5472.CAN-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nasrollahi S., Pathak A. Topographic confinement of epithelial clusters induces epithelial-to-mesenchymal transition in compliant matrices. Sci. Rep. 2016;6:18831. doi: 10.1038/srep18831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shay G., Lynch C.C., Fingleton B. Moving targets: emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015;44-46:200–206. doi: 10.1016/j.matbio.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kise K., Kinugasa-Katayama Y., Takakura N. Tumor microenvironment for cancer stem cells. Adv. Drug Deliv. Rev. 2016;99(Pt. B):197–205. doi: 10.1016/j.addr.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Toonen J.A., Ma Y., Gutmann D.H. Defining the temporal course of murine neurofibromatosis-1 optic gliomagenesis reveals a therapeutic window to attenuate retinal dysfunction. Neuro Oncol. 2016 doi: 10.1093/neuonc/now267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lekka M., Laidler P. Applicability of AFM in cancer detection. Nat. Nanotechnol. 2009;4:72. doi: 10.1038/nnano.2009.004. author reply 72–73. [DOI] [PubMed] [Google Scholar]
- 22.Faria E.C., Ma N., Snook R.D. Measurement of elastic properties of prostate cancer cells using AFM. Analyst (Lond.) 2008;133:1498–1500. doi: 10.1039/b803355b. [DOI] [PubMed] [Google Scholar]
- 23.Daginakatte G.C., Gutmann D.H. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum. Mol. Genet. 2007;16:1098–1112. doi: 10.1093/hmg/ddm059. [DOI] [PubMed] [Google Scholar]
- 24.da Silva R., Uno M., Oba-Shinjo S.M. LOX expression and functional analysis in astrocytomas and impact of IDH1 mutation. PLoS One. 2015;10:e0119781. doi: 10.1371/journal.pone.0119781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charles N.A., Holland E.C., Kettenmann H. The brain tumor microenvironment. Glia. 2011;59:1169–1180. doi: 10.1002/glia.21136. [DOI] [PubMed] [Google Scholar]
- 26.Wang M., Wang T., Teramoto A. The expression of matrix metalloproteinase-2 and -9 in human gliomas of different pathological grades. Brain Tumor Pathol. 2003;20:65–72. doi: 10.1007/BF02483449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.