Abstract
Programmed death‐ligand 1 (PD‐L1) expression either indicates immune inhibitory status or concurrent immune response. Although the relationship between PD‐L1 and clinical outcomes has been studied widely in recent years, its role in prognosis of esophageal squamous cell carcinoma (ESCC) remains unclear. Here, we assessed the significance of PD‐L1 in ESCC and its association with epidermal growth factor receptor (EGFR) and radiation response. We found that PD‐L1 was present both on the surface of tumor cells and tumor‐infiltrating immune cells. Patients with tumor‐infiltrating immune cell PD‐L1 expression had better survival. PD‐L1 expression on immune cells was an independent prognostic factor for patients with ESCC. PD‐L1 expression either on tumor‐infiltrating immune cells or tumor cells was negatively associated with EGFR expression. EGFR/PD‐L1 pairs could separate the survival between EGFR low/PD‐L1 positive and EGFR high/PD‐L1 negative groups. In ESCC cell lines with EGFR high expression, PD‐L1 expression was induced significantly when EGFR signaling was activated by radiation and was dramatically inhibited by an EGFR tyrosine kinase inhibitor. In conclusion, tumor‐infiltrating immune cell PD‐L1 expression is an independent prognostic factor for ESCC, and the association between EGFR and PD‐L1 is vital to determining survival. It is important to consider radiotherapy‐induced imbalance of pro‐tumor and anti‐tumor immune response. A combination of radiotherapy and PD‐L1‐targeted therapy could be a promising therapeutic strategy for ESCC patients.
Keywords: Epidermal growth factor receptor, esophageal squamous cell carcinoma, programmed death‐ligand 1, radiation, tumor‐infiltrating immune cell
Esophageal cancer is the 6th leading cause of cancer‐related mortality and 8th most common cancer in the world, with an estimated 456,000 new cases per year worldwide.1, 2 In China, esophageal cancer is the 4th leading cause of cancer‐related mortality, and esophageal squamous cell carcinoma (ESCC) is the predominant histological type, accounting for more than 90% of the cases of esophageal cancer. Esophagectomy remains the standard treatment for resectable esophageal cancer.3 Although neoadjuvant chemoradiotherapy may improve survival rates, only a few studies have found long‐term survival rates exceeding 40%.4 More than 50% of patients experience local‐regional recurrence or distant metastasis.5, 6 Thus, new prognostic and therapeutic strategies are urgently needed.
Programmed death‐ligand 1 (PD‐L1; also called B7‐H1 or CD274), which is expressed on many cancer cells and tumor‐infiltrating immune cells (ICs), plays an important role in tumor immune escape by binding its receptors programmed death‐1 (PD‐1) and B7.1 (CD80), both of which are negative regulators of T lymphocyte activation. Several studies have shown that PD‐L1 expression on tumor cells (TCs) is associated with worse prognosis in patients with renal cell carcinoma and cancers of the lung, breast, pancreas, liver, and ovary.7 However, recent studies have reported inconsistent results. In non‐small cell lung cancer,8, 9 breast cancer,10 metastatic melanoma,11 and colorectal cancer,12 PD‐L1 expression has been associated with improved survival rates, indicating that induction of the PD‐1/PD‐L1 pathway may present an occurred or ongoing adaptive antitumor response. To date, the study of the role of PD‐L1 and its related immune status in prognosis of human esophageal cancer is not sufficient.
Multiple studies in recent years have focused on the relationship between PD‐1/PD‐L1 and epidermal growth factor receptor (EGFR) activation, but the results have been controversial.13, 14, 15, 16, 17 In studies of non‐small cell lung cancer, it has been reported that PD‐L1‐positive status was significantly associated with the presence of EGFR mutations.13, 14 Activation of an EGFR mutant gene induced PD‐L1 expression that contributed to immune escape.15 However, another study reported no significant relationship between PD‐L1 and EGFR/Kirsten rat sarcoma viral oncogene homolog (KRAS) expression in lung adenocarcinoma.16 A recent study of head and neck cancers indicated that overexpressed EGFR mediated immune evasion by upregulating PD‐L1 expression.17 Although EGFR is the most studied gene in esophageal cancer research, its association with PD‐L1 remains to be determined.
We previously reported that patients with high EGFR expression benefited from postoperative radiotherapy;18 however, the survival rate in these patients was still lower than in those with low EGFR expression who received surgery only. Though radiation exposure could induce or strengthen adaptive antitumor response, PD‐L1 also increased immediately in response to interferon‐γ (IFN‐γ) which was produced by activated lymphocytes in irradiated tissue, leading to the development of adaptive resistance19 and might reduce therapeutic effect. Nonetheless, the direct effects of radiotherapy on PD‐L1 expression in EGFR‐overexpressing ESCC tumor cells are not yet known.
This study aimed to determine the role of immunity in the prognosis of ESCC patients. We assessed the prognostic potential of PD‐L1 expression in patients with ESCC. We also investigated the association between EGFR and PD‐L1 in tumor tissues and the effect of radiation exposure on PD‐L1 expression in ESCC cell lines with high EGFR expression. Our study provides new insight into identifying a potential predictor for prognosis of ESCC and combination of radiotherapy with PD‐1/PD‐L1 targeted immunotherapy.
Materials and Methods
Tissue samples
The resected tumor samples had been collected for our previous study conducted between September 1986 and December 1997.18, 20 We retrieved 344 formalin‐fixed and paraffin‐embedded archived tissue samples, with 197 samples from patients who received surgery alone and 147 from patients who received postoperative radiotherapy. The Institutional Review Board of Cancer Hospital, Chinese Academy of Medical Science, approved the study protocol. All the experiments were conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all subjects.
Patient follow‐up
Patients had been instructed to return for follow‐up, including a clinical examination, barium swallow test, chest radiography, abdominal ultrasonography, and thoracic computed tomography, at 3‐ to 6‐month intervals for 1 year, and then every 6–12 months thereafter. All patients were followed through the end of December 2010. The patient demographic and other clinical data from a prospectively maintained database. Details have been described in a previous study.18
Immunochemistry
The paraffin‐embedded tumor samples were sectioned to 5 μm slices. The tissue section slides were deparaffinized and rehydrated. For PD‐L1 immunochemistry staining, antigen retrieval was achieved in boiled ethylenediaminetetraacetic acid buffer (pH 8.0) for 10 min. The tissue section slices were incubated with peroxidase blocking reagent (3% H2O2 solution) for 10 min and then washed in phosphate‐buffered saline (PBS) solution three times to block the endogenous peroxidase. The slices were incubated with the primary anti‐PD‐L1 antibody (clone SP142; Spring Bioscience, Pleasanton, CA, USA) in a humidified chamber overnight at 4°C. After being washed with PBS/0.05% Tween three times, the slices were incubated with MaxVision enhanced regent (catalog no. KIT‐5020, Fuzhou Maixin Biotech. Co., Ltd., Fuzhou, China) for 20 min at room temperature and washed again in PBS/0.05% Tween. The slices were then incubated with MaxVision HRP‐polymer anti‐mouse/rabbit secondary antibody (catalog no. KIT‐5020, Fuzhou Maixin Biotech) chamber for 30 min. We then stained the slices with 3,3ʹ‐diaminobenzidine (catalog no. ZLI‐9018; Zhongshan, Beijing, China). PD‐L1 expression was evaluated on both TCs and tumor‐infiltrating ICs. The scoring was performed by two experienced pathologists who were blinded to the clinical outcome data independently.
PD‐L1 immunostaining was semiquantitatively evaluated as previously described both on TCs and tumor‐infiltrating ICs.21 The proportion of PD‐L1 positive cells was estimated as the percentage of total TCs or ICs: 0, 0%–1%; 1, 1%–5%; 2, 5%–10%; 3, >10%. PD‐L1 expression was scored in at least five microscopic fields. PD‐L1 positivity was defined as ≥5% of ICs or TCs with staining for PD‐L1 (Fig. 1). The mean percentage of PD‐L1 positive cells in each sample of the five fields was calculated.
Figure 1.
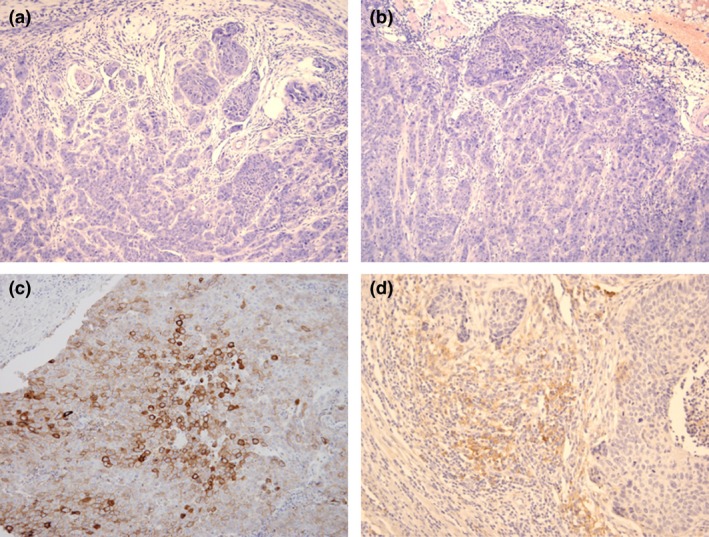
Immunohistochemical staining for programmed death‐ligand 1 expression in esophageal squamous cell carcinoma. (a) Negative staining. (b) Positive staining on tumor cells. (c) Positive staining on tumor‐infiltrating immune cells. Magnification: 200 ×
The result of EGFR expression in tumor cells was from our previous study.18 Briefly, for EGFR score, the intensity was classified as follows: 0, negative staining; 1, weak staining; 2, moderate staining; 3, strong staining. The rate of positive cells was recorded: 1, 0%–25%; 2, 26%–50%; 3, 51%–75%; 4, greater than 75%. A final score was achieved by multiplication of the intensity (0, 1, 2, and 3) and the rate of positive cells (1, 2, 3, and 4). For data analysis, scores of less than 8 were defined as low expression and scores of 8 or more, as high expression.
Cell culture
Esophageal squamous cell lines with wild‐type EGFR expression kyse30, TE1, and CaEs‐17 were purchased from ATCC. All cells were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 2 mmol/L L‐glutamine, 100 units/mL penicillin (Hyclone, GE Healthcare Life Sciences, Logan, UT, USA), 100 μg/mL streptomycin (Hyclone), and 10% fetal calf serum (FCS) (Gibco, ThermoFisher Scientific, Grand Island, NY, USA). Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2 and used in the experiments when in the logarithmic growth phase.
Radiation exposure
Complete culture media (10% FCS in RPMI 1640 media) was replaced by RPMI 1640 media w/o EGFR tyrosine kinase inhibitor AG1478 (10 μM) before exposure of cells to radiation for 30 min. Then cells were exposed to 6 MV X‐rays at 0, 2, 4 or 8 Gy. 24 h later, the cells were collected and tested using flow cytometry or Western blot.
Flow cytometry
The cells were collected and washed twice in cold flow cytometry staining buffer (pH7.4 PBS containing 0.2% (w/v) bovine serum albumin). The cells were then resuspended in cold staining buffer to a final concentration of 107 cells/mL. We aliquoted 100 μL of the cell suspension into each tube and added 5 μL of directly conjugated primary antibodies (PE‐EGFR, catalog no. 55997; APC‐PD‐L1, catalog no. 563741, BD Biosciences, San Jose, CA, USA). The cells were incubated for 30 min on ice in the dark, then washed twice with staining buffer to remove the unbound antibody. The samples were analyzed by flow cytometry immediately.
To test the whole expression of EGFR on both cell surface and in cytoplasma, we performed the fixation and permeabilization with a BD Cytofix/Cytofirm kit (catalog no. 554714; BD Biosciences) according to the manufacturer's instructions after the surface staining. Then, we stained the intracellular EGFR protein.
Western blot
Cells were lysed and protein was extracted using mammalian protein extraction agent (ThermoFisher Scientific Pierce, Waltham, MA, USA) plus Halt protease inhibitor cocktail (ThermoFisher Scientific Pierce). Protein concentrations were determined using a bicinchoninic acid assay (ThermoFisher Scientific Pierce). Aliquots of protein lysates were separated on sodium dodecyl sulfate‐polyacrylamide gels (SDS‐PAGE) and transferred onto a nitrocellulose membrane, which was blocked with 5% blotting grade milk (BioRad, Hercules, CA, USA) in PBST (0.1% Tween 20 in PBS). The membrane was then hybridized with primary antibodies to human EGFR and phospho‐EGFR (Tyr1068). We also tested the level of STAT3 and phospho‐STAT3 (Tyr705) as STAT3 has been identified to regulate PD‐L1 transcriptionally. Then, with the corresponding secondary antibodies conjugated with horseradish peroxidase, and detected using a chemiluminescence assay (EMD Millipore, Temecula, CA, USA), membranes were exposed to X‐ray film (Kodak China Investment, Shanghai, China) to visualize the bands. β‐actin was used as loading control. All primary and secondary antibodies used in the Western blotting were purchased from Cell Signaling Technology (Danvers, MA, USA).
Statistical methodology
Statistical analysis was performed using SPSS version 23.0 software (SPSS, Chicago, IL, USA). The survival rate was examined using the Kaplan–Meier method, and differences were assessed using the log‐rank test. Associations were analyzed using Pearson's χ2 test. The multivariate analysis was performed using a Cox regression model. The survival curves were plotted using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA). Survival time was calculated from the date of surgery to date of death or last follow‐up. The disease‐free survival (DFS) rate was calculated from the date of surgery to the date of first local or distant recurrence or last date of follow‐up. To compare the two PD‐L1‐positive groups by radiation exposure, we used one‐way ANOVA followed by Least‐significant Difference test, with P < 0.05 considered significant.
Results
Patient characteristics and immunohistochemistry
A total of 344 patients with ESCC were included in the study. Patient demographic and clinicopathologic characteristics are summarized in Table 1. PD‐L1 staining was observed on TCs and on tumor‐infiltrating ICs (Fig. 1). PD‐L1‐positive tumor‐infiltrating ICs were more common than PD‐L1‐positive TCs (ICs, 85 samples [24.7%]; TCs, 50 samples [14.5%]). Low EGFR expression was observed in 86 samples (25.5%) and high EGFR expression in 258 samples (75.0%).18 PD‐L1 expression on both TCs and tumor‐infiltrating ICs was negatively correlated with EGFR expression (TCs, P = 0.02; ICs, P = 0.01) (Table 1). There was a positive association between TC and IC PD‐L1 expression (Table S1).
Table 1.
Demographic and clinical characteristics and programmed death‐ligand 1 expression in 344 patients with esophageal cancer
| Characteristic | No. of patients n = 344 | PD‐L1−TCs n = 294 | PD‐L1 + TCs n = 50 | P a | PD‐L1−ICs n = 259 | PD‐L1 + ICs n = 85 | P a |
|---|---|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |||
| Sex | |||||||
| Male | 268 (77.9) | 233 (79.3) | 35 (70.0) | 0.104 | 202 (78.0) | 66 (77.6) | 0.947 |
| Female | 76 (22.1) | 61 (20.7) | 15 (30.0) | 57 (22.0) | 19 (22.4) | ||
| Age (years) | |||||||
| <60 | 226 (65.7) | 195 (66.3) | 31 (62.0) | 0.329 | 169 (65.3) | 57 (67.1) | 0.761 |
| ≥60 | 118 (34.3) | 99 (33.7) | 19 (38.0) | 90 (34.7) | 28 (32.9) | ||
| Tumor location | |||||||
| Upper esophagus | 44 (12.8) | 36 (12.2) | 8 (16.0) | 0.224 | 33 (12.7) | 11 (12.9) | 0.328 |
| Middle esophagus | 228 (66.3) | 192 (65.3) | 36 (72.0) | 167 (64.5) | 61 (71.8) | ||
| Lower esophagus | 72 (20.9) | 66 (22.4) | 6 (12.0) | 59 (22.8) | 13 (15.3) | ||
| Tumor differentiation | |||||||
| High | 109 (31.7) | 96 (32.7) | 13 (26.0) | 0.643 | 84 (32.4) | 25 (29.4) | 0.710 |
| Moderate | 198 (57.6) | 167 (56.8) | 31 (62.0) | 149 (57.5) | 49 (57.6) | ||
| Low | 37 (10.7) | 31 (10.5) | 6 (12.0) | 26 (10.0) | 11 (12.9) | ||
| T stageb | |||||||
| T1 | 1 (0.3) | 1 (0.3) | 0 (0) | 0.045 | 0 (0) | 1(1.2) | 0.100 |
| T2 | 38 (11.0) | 27 (9.2) | 11 (22.0) | 25 (9.7) | 13(15.3) | ||
| T3 | 241 (70.1) | 208 (70.7) | 33 (66.0) | 188 (72.6) | 53 (62.4) | ||
| T4 | 64 (18.6) | 58 (19.7) | 6 (12.0) | 46 (17.8) | 18 (21.2) | ||
| N stageb | |||||||
| N0 | 167 (48.5) | 143 (48.6) | 24 (48.0) | 0.933 | 120 (46.3) | 47 (55.3) | 0.151 |
| N1 | 177 (51.5) | 151 (51.4) | 26 (52.0) | 139 (53.7) | 38 (44.7) | ||
| Pathologic stageb | |||||||
| Stage IIA | 144 (41.9) | 122 (41.5) | 22 (44.0) | 0.007 | 106 (40.9) | 38 (44.7) | 0.020 |
| Stage IIB | 18 (5.2) | 11 (3.7) | 7 (14.0) | 9 (3.5) | 9 (10.6) | ||
| Stage III | 182 (52.9) | 161 (54.8) | 21 (42.0) | 144 (55.6) | 38 (44.7) | ||
| Treatment | |||||||
| Surgery alone | 197 (57.3) | 166 (56.5) | 31 (62.0) | 0.464 | 153 (59.1) | 44 (51.8) | 0.237 |
| Surgery + Radiotherapy | 147 (42.7) | 128 (43.5) | 19 (38.0) | 106 (40.9) | 41 (48.2) | ||
| EGFR status | |||||||
| Low | 86 (25.0) | 67 (22.8) | 19 (38.0) | 0.022 | 56 (21.6) | 30 (35.3) | 0.012 |
| High | 258 (75.0) | 227 (77.2) | 31 (62.0) | 203 (78.4) | 55 (64.7) | ||
EGFR, epidermal growth factor receptor; IC, immune cell; PD‐L1, programmed death‐ligand 1; TC, tumor cell.
P ≤ 0.05 was considered statistically significant.
Union for International Cancer Control, TNM Classification of Malignant Tumors.
Relationship between PD‐L1 expression and survival
Firstly, we analyzed the relationship between the overall survival (OS) rate and PD‐L1 expression on tumor‐infiltrating ICs. For the group with positive PD‐L1 expression, the 5‐year OS rate was 37.6%, with a median survival time of 31.6 months; for the group with negative PD‐L1 expression, the 5‐year OS rate was 20.5%, with a median survival time of 17.0 months. The 5‐year disease free survival (DFS) rates were 37.6% and 18.9% in the PD‐L1‐positive and PD‐L1‐negative groups, respectively. The differences of both OS and DFS rates between these two groups reached statistical significance (OS: χ2 = 8.645, P = 0.003; DFS: χ2 = 10.269, P = 0.001) (Fig. 2a,c).
Figure 2.
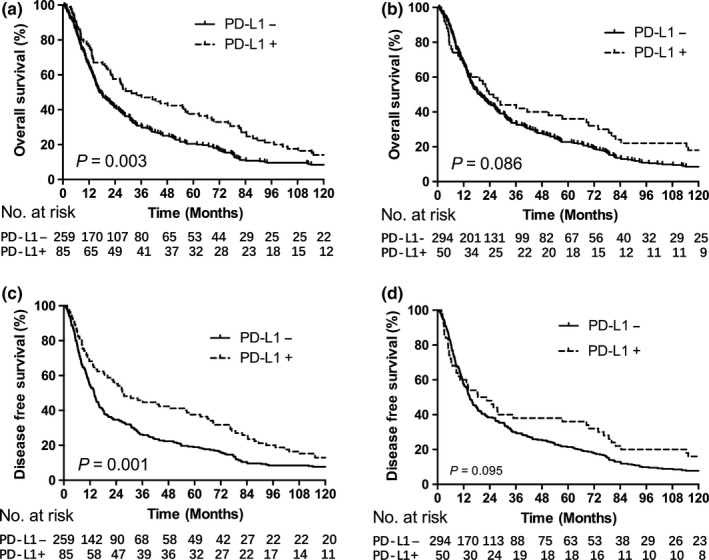
Patients with PD‐L1 positive expression showed better survival. Kaplan–Meier survival curves for PD‐L1 expression and overall survival (a and b) and disease‐free survival (c and d). PD‐L1 was expressed on tumor‐infiltrating immune cells (a and c) or tumor cells (b and d).
We also compared survival rates between the groups with positive and negative PD‐L1 expression on TCs. No significant difference was found between these two groups (OS: 36.0%, 23.6 months vs 22.8%, 19.5 months, P = 0.086; DFS: 36.0%, 18.5 months vs 21.4%, 14.7 months, P = 0.095) (Fig. 2b,d). The results suggested that PD‐L1 expressed on tumor‐infiltrating ICs, but not TCs, might indicate the prognosis of ESCC patients.
Correlation between PD‐L1 and EGFR expression in patients with ESCC
We found significant differences in OS rates for patients with different EGFR18 and tumor‐infiltrating IC PD‐L1 expression, but not TC PD‐L1 expression. Additionally, PD‐L1 expression both on tumor‐infiltrating ICs and TCs was negatively related to EGFR expression (Table 1). We wondered whether we could improve the separation of survival rate between the groups by combining groups with high EGFR and negative PD‐L1 expression, which are both associated with lower survival rates, and groups with low EGFR and positive PD‐L1 expression, which are both associated with higher survival rates. As shown in Figure 3, the differences in survival were greater between these two groups than between groups with low versus high EGFR‐18 or negative versus positive PD‐L1 (Fig. 2). The results indicated that ESCC patients with low EGFR/positive PD‐L1 expression had much better survival rates and durations no matter whether PD‐L1 was localized on TCs or tumor‐infiltrating ICs (ICs: OS, 53.3%, 62.7 months vs 16.3%, 15.6 months, P < 0.001; DFS: 53.3%, 62.7 months vs 15.8%, 13.4 months, P < 0.001. TCs: OS, 63.2%, 80.0 months vs 18.9%, 17.7 months, P = 0.001; DFS: 63.2%, 76.4 months vs 18.5%, 14.0 months, P = 0.001) (Fig. 3). We also compared the survival among four groups which had high EGFR/negative PD‐L1, low EGFR/positive PD‐L1, high EGFR/positive PD‐L1 and low EGFR/negative PD‐L1 expression, individually. Significant difference was found among these four groups; and patients with EGFR low/PD‐L1 positive expression had the longest survival (Fig. S1).
Figure 3.
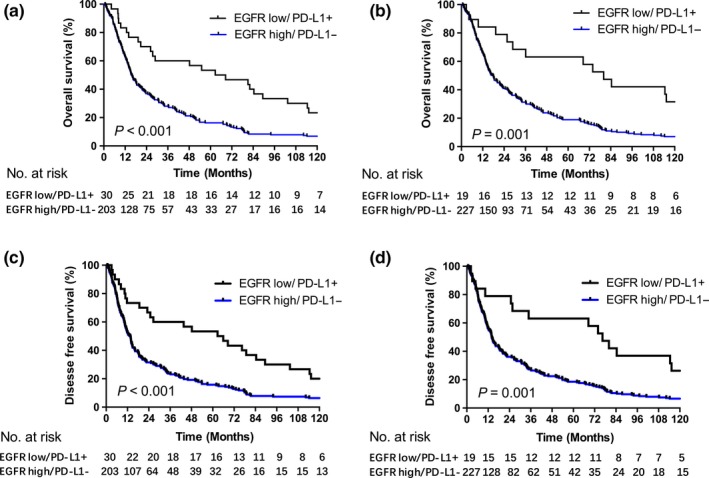
Kaplan–Meier survival curves according to PD‐L1 and EGFR expression. Overall survival and disease free survival was compared between groups with high EGFR/negative PD‐L1 and low EGFR/positive PD‐L1 expression. PD‐L1 was expressed on tumor‐infiltrating immune cells (a and c) or tumor cells (b and d).
Multivariate analysis
We performed a multivariate analysis to determine independent predictors of OS and DFS. As shown in Table 2, age, sex, T stage, N stage, histologic grade, treatment modality, PD‐L1 expression on ICs, and EGFR expression were all independent factors that predicted OS. Age, sex, T stage, N stage, histologic grade, treatment modality, and PD‐L1 expression on ICs were independent factors that predicted DFS, but EGFR expression was not an independent prognostic factor for DFS.
Table 2.
Multivariate analysis of overall survival rate and disease‐free survival rate
| Variable | Overall survival | Disease‐free survival | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | |
| Age (ref. < 60 years) | 1.393 (1.099–1.766) | 0.006 | 1.370 (1.085–1.730) | 0.008 |
| Sex (ref. male) | 0.745 (0.563–0.986) | 0.040 | 0.791 (0.599–1.045) | 0.098 |
| T stage (ref. T1) | 1.420 (1.154–1.747) | 0.001 | 1.371 (1.120–1.679) | 0.002 |
| N stage (ref. N0) | 1.907 (1.491–2.440) | 0.000 | 1.980 (1.554–2.524) | 0.000 |
| Histologic grade (ref. high) | 1.221 (1.014–1.471) | 0.035 | 1.268 (1.055–1.523) | 0.011 |
| EGFR expression (ref. low) | 1.360 (1.033–1.790) | 0.029 | 1.251 (0.948–1.650) | 0.114 |
| ICs PD‐L1 expression (ref. low) | 0.720 (0.553–0.939) | 0.015 | 0.671 (0.516–0.874) | 0.003 |
| Treatment, S or S+RT (ref. S) | 0.729 (0.577–0.921) | 0.008 | 0.684 (0.542–0.863) | 0.001 |
CI, confidence interval; EGFR, epidermal growth factor receptor; ICs, tumor‐infiltrating immune cells; S, surgery; RT, radiotherapy.
P ≤ 0.05 was considered statistically significant.
Radiation induction of PD‐L1 in ESCC cell lines with high EGFR expression
With the goal of comparing radiation‐induced PD‐L1 expression in ESCC cell lines with different level of wild‐type EGFR expression, we treated ESCC cell lines using six MV X‐rays at varying doses. We selected three ESCC cell lines of kyse30, TE1, and CaEs17, which have high, moderate, and low EGFR expression, respectively (Fig. 4a). After 24 h of radiation exposure, PD‐L1 expression on the surface of the tumor cells increased significantly in a dose‐dependent manner and corresponding with different levels of EGFR expression (Fig. 4a,b). Next, we assessed the changes of PD‐L1 expression when EGFR activation was inhibited by the EGFR tyrosine kinase inhibitor AG1478 before radiation. AG1478 inhibited radiation‐induced PD‐L1 expression dramatically, to levels even lower than those found in the un‐irradiated controls (Fig. 4c). Western blot results confirmed that radiation induced phosphorylation of EGFR in all three cell lines, and that AG1478 blocked EGFR phosphorylation. The level of phosphorylated STAT3 increased after cells were exposed to radiation and decreased after administration of AG1478 only in kyse30 and TE1 cells, not CaEs17 cells (Fig. 4d).
Figure 4.
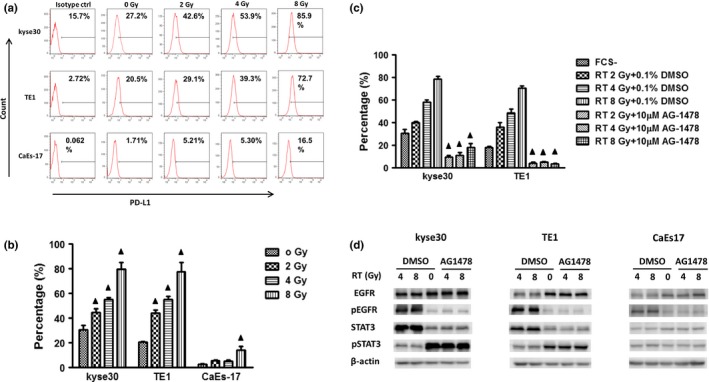
Radiation induced PD‐L1 in ESCC cells with high EGFR expression. (a,b) PD‐L1 expression increased after radiation exposure. Complete culture media (10% fetal calf serum in RPMI 1640) was replaced by RPMI 1640 before exposure of the cells to radiation for 30 min. After 24 h, the cells were collected and tested using flow cytometry. Columns indicate mean values; bars indicate standard deviation. ▲, P < 0.05 compared with data of 0 Gy. (c). AG1478 inhibited the radiation‐induced expression. AG1478 (10 μM) was added before cells were exposed to radiation for 30 min. PD‐L1 expression was tested 24 hours later by flow cytometry. ☆, P < 0.05 compared with corresponding solvent‐only control. d. Changes in protein phosphorylation by radiation. Cells were treated as above and collected 30 min after exposure to radiation for Western blot assay. All experiments were repeated three times.
Discussion
In the present study, we found that PD‐L1 expression on tumor‐infiltrating ICs is an independent prognostic factor in patients with ESCC; patients with IC PD‐L1 expression had improved survival rates and durations. PD‐L1 expression on both TCs and tumor‐infiltrating ICs was negatively correlated with tumor EGFR expression. EGFR/PD‐L1 pairs could more greatly separate the difference of the survival between groups with high EGFR/negative PD‐L1 and low EGFR/positive PD‐L1 expression when compared with that between the negative/positive PD‐L1 or high/low EGFR groups. PD‐L1 expression in ESCC cell lines with EGFR high expression increased when EGFR signaling was activated by radiation exposure.
Our present results showed that tumor‐infiltrating IC PD‐L1 expression was associated with longer OS and was an independent predictor in patients with ESCC. Several studies have shown an association between PD‐L1 protein expression and survival durations; interesting, the improved survival was also related with increased numbers of tumor‐infiltrating lymphocytes (TILs).8, 10, 11, 12 A recent study of a large and relatively homogeneous group of patients suggested that the presence of intense tumor IC infiltrates was a favorable prognostic marker for survival in patients with resected non‐small cell lung cancer22. In our study, we further pinpointed the predictive factor as PD‐L1 expression on tumor‐infiltrating ICs. The upregulation of PD‐L1 in the tumor microenvironment not only presents immune suppression but is also associated with endogenous antitumor immune activity. The patient outcomes, therefore, are influenced by both immune suppression and activation. Since the tissue samples were all collected before the patients received any anti‐tumor treatment, the PD‐L1 expression exhibited here presented naïve immune response.
We found patients with IC PD‐L1 expression, but not TC PD‐L1 expression had improved survival. Our result agreed with a recent study which showed that PD‐L1 expression on tumor cells was not correlated with PFS or OS in patients with locally advanced non‐small cell lung cancer, but CD8+ TIL density was.23 A study of multiple types of cancer using the same monoclonal antibody clone as ours (cloneSP142, Spring, USA) also indicated that patients with PD‐L1 expression on tumor‐infiltrating ICs, which stained as macrophages, dendritic cells, and T cells, had better responses to PD‐L1 blockade therapy and improved survival.21 Tumor‐infiltrating ICs in the tumor microenvironment are much closer to antitumor lymphocytes. They are probably more sensitive to PD‐L1 induction by IFN‐γ released from these cells. The presence of tumor‐infiltrating ICs expressing PD‐L1 may implicate the development of an ongoing immune response. In our study, we did not distinguish different subsets of immune cells. The effect of immune infiltrates on prognosis was mediated by both pro‐tumor and anti‐tumor immune populations, but no evidence suggested this balance could be used to predict outcomes in individual patients.22, 24 It probably exists complicated interaction among different subsets of tumor‐infiltrating ICs, which is a fascinated topic deserving to be explored in further study.
We found that PD‐L1 expression on both TCs and tumor‐infiltrating ICs was negatively related to EGFR expression. Much more sophisticated regulatory network links EGFR and PD‐L1 in vivo than in vitro. Activation of the EGFR pathway might involve suppressing the immune response in murine melanoma models, either through activating regulatory T cells or reducing the levels of the T cell chemoattractant.25 It is possible that in patients with high EGFR expression, the endogenous antitumor immune response was inhibited, which could be partially reflected by decreased PD‐L1 expression and devote to the high rates of recurrence and metastasis and shorter survival time for these patients. We found that EGFR/PD‐L1 pairs could further separate the survival between ESCC patients with high EGFR/negative PD‐L1 and low EGFR/high PD‐L1 expression. As patients with low EGFR/positive PD‐L1 tumors have their antitumor immune potential, their median OS time was much longer than that of patients with high EGFR/negative PD‐L1 expression.
Two mechanisms are involved in regulation of PD‐L1 expression: native and adaptive. In adaptive resistance, PD‐L1 expression can be induced by IFN‐γ which is released from activated tumor‐specific T cells by chemotherapy and radiotherapy.26, 27, 28 In the present study, we found low levels of PD‐L1 expression in ESCC cell lines cultured in starved conditions, which was associated with EGFR expression and was sensitive to EGFR inhibitors (data not shown), indicating that in vitro condition, EGFR signaling pathways might participate in the development of intrinsic resistance of normal PD‐L1 expression in TCs. More importantly, our results showed that radiation directly and significantly deregulated PD‐L1 expression on TCs and the level of induced PD‐L1 was associated specifically with the level of EGFR overexpression. It has been reported that mutant EGFR pathway activation induced PD‐L1 expression in both bronchial epithelial cells 15 and non‐small cell lung cancer cells.29 We demonstrated here that PD‐L1 expression was upregulated following radiation induced activation of wild‐type EGFR signaling in ESCC cells and that an EGFR‐specific inhibitor could block this activation and efficiently inhibit the radiation‐induced PD‐L1 expression. This finding suggested that radiation‐induced EGFR signaling activation deregulated PD‐L1 in ESCC. The phosphorylation of STAT3 changed along with the phosphorylation of EGFR in EGFR‐high and ‐moderate cells, but did not increase in EGFR‐low cells after radiation, indicating that EGFR‐STAT3 might be involved in the regulation of PD‐L1 expression. Our study, therefore, has described an additional mechanism of radiation‐induced adaptive immune resistance via direct modulation of PD‐L1 expression in TCs. The detailed downstream signaling pathways were not explored in this study, which require further research in future.
We found that PD‐L1 expression in ICs suggested better prognosis. This PD‐L1 expression exhibited a balance between pro‐tumor and anti‐tumor capability in ESCC patients before they received any treatment (in our study, postoperative radiotherapy or not) and indicated ongoing immune response. While high EGFR expression in tumors devotes to immunological suppression. Accordingly, the negative association between EGFR and PD‐L1 expression was found in the tissue samples; and patients with high EGFR/negative PD‐L1 expression had much worse survival than that of patients with low EGFR/positive PD‐L1 expression. Interestedly, we also found induced PD‐L1 expression in radiated ESCC cells with EGFR high expression, which was directly regulated by activated EGFR signaling resulting from radiation exposure. The effect of radiotherapy on anti‐tumor immune ability has two sides. Radiation leads to cell death and promotes anti‐tumor immune response by increased release of tumor antigens, maturation of antigen processing cells and production of co‐stimulus molecules. Simultaneously, inhibitory immune factors emerge soon after radiotherapy. At this time point, the upregulated PD‐L1 expression represents negative feedback mechanism against activated anti‐tumor immune response and might be disadvantageous to patient survival. In our previous study, we found patients with high EGFR expression would benefit from postoperative radiotherapy.18 However, in the present study, we did not find significant differences of survival among patients who had either positive or negative PD‐L1 expression and received postoperative radiotherapy or not (Figs S2, S3). This result suggests that besides induced tumor cell death, negative immune mechanisms such as expression of checkpoints both on tumor and stromal cells in tumor microenvironment complicates the outcome of the treatment and consequent survival.
In conclusion, our results show that expression of PD‐L1 on tumor‐infiltrating ICs is an independent prognostic factor for patients with ESCC and the association between EGFR and PD‐L1 is vital to survival. A combination of radiotherapy and targeted therapy against PD‐L1 or its associated signaling pathway may reveal new treatment strategies for patients with ESCC.
Disclosure Statement
The authors declare that they have no competing interests.
Supporting information
Table S1. Association between tumor cell and tumor‐infiltrating immune cell PD‐L1 expression by χ2 analysis.
Fig. S1. Kaplan–Meier survival curves according to different PD‐L1 and EGFR expression.
Fig. S2. Kaplan–Meier survival curves according to tumor‐infiltrating immune cell PD‐L1 expression and postoperative radiotherapy.
Fig. S3. Kaplan–Meier survival curves according to tumor cell PD‐L1 expression and postoperative radiotherapy.
Acknowledgments
This work was supported by the Science and Technology Project of Beijing [Z121107001012004]; Capital Foundation for Medical Research and Development [2007–2012]; National Natural Science Foundation [81272512]; and Chinese Hi‐Tech R&D Program [2012AA02A503]. The authors thank Dr. Amy Ninetto for her skillful edited support.
Cancer Sci 108 (2017) 590–597
The abstract has been selected for presentation in an ORAL scientific session during the ASTRO's (American Society for Radiation Oncology) 58th Annual Meeting.
Funding Information
Science and Technology Project of Beijing, (Grant/Award Number: Z121107001012004), Capital Foundation for Medical Research and Development, (Grant/Award Number: 2007‐2012), National Natural Science Foundation, (Grant/Award Number: 81272512), Chinese Hi?Tech R&D Program, (Grant/Award Number: 2012AA02A503).
Contributor Information
Ping Wang, Email: wangping@tjmuch.com.
Zefen Xiao, Email: xiaozefen2013@163.com.
References
- 1. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet 2013; 381: 400–12. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit R et al Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. [DOI] [PubMed] [Google Scholar]
- 3. Kelsen DP, Ginsberg R, Pajak TF et al Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998; 339: 1979–84. [DOI] [PubMed] [Google Scholar]
- 4. van Hagen P, Hulshof MC, van Lanschot JJ et al Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–84. [DOI] [PubMed] [Google Scholar]
- 5. Doki Y, Ishikawa O, Takachi K et al Association of the primary tumor location with the site of tumor recurrence after curative resection of thoracic esophageal carcinoma. World J Surg 2005; 29: 700–7. [DOI] [PubMed] [Google Scholar]
- 6. Smit JK, Pultrum BB, van Dullemen HM, Van Dam GM, Groen H, Plukker JT. Prognostic factors and patterns of recurrence in esophageal cancer assert arguments for extended two‐field transthoracic esophagectomy. Am J Surg 2010; 200: 446–53. [DOI] [PubMed] [Google Scholar]
- 7. McDermott DF, Atkins MB. PD‐1 as a potential target in cancer therapy. Cancer Med 2013; 2: 662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Velcheti V, Schalper KA, Carvajal DE et al Programmed death ligand‐1 expression in non‐small cell lung cancer. Lab Invest 2014; 94: 107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang CY, Lin MW, Chang YL, Wu CT, Yang PC. Programmed cell death‐ligand 1 expression is associated with a favourable immune microenvironment and better overall survival in stage I pulmonary squamous cell carcinoma. Eur J Cancer 2016; 57: 91–103. [DOI] [PubMed] [Google Scholar]
- 10. Schalper KA, Velcheti V, Carvajal D et al In situ tumor PD‐L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res 2014; 20: 2773–82. [DOI] [PubMed] [Google Scholar]
- 11. Taube JM, Anders RA, Young GD et al Colocalization of inflammatory response with B7‐h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4: 127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Droeser RA, Hirt C, Viehl CT et al Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer 2013; 49: 2233–42. [DOI] [PubMed] [Google Scholar]
- 13. Azuma K, Ota K, Kawahara A et al Association of PD‐L1 overexpression with activating EGFR mutations in surgically resected nonsmall–cell lung cancer. Ann Oncol 2014; 25: 1935–40. [DOI] [PubMed] [Google Scholar]
- 14. Tang Y, Fang W, Zhang Y et al The association between PD‐L1 and EGFR status and the prognostic value of PD‐L1 in advanced non‐small cell lung cancer patients treated with EGFR‐TKIs. Oncotarget 2015; 6: 14209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akbay EA, Koyama S, Carretero J et al Activation of the PD‐1 pathway contributes to immune escape in EGFR‐driven lung tumors. Cancer Discov 2013; 3: 1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Y, Wang L, Li Y et al Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther 2014; 7: 567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Concha‐Benavente F, Srivastava RM, Trivedi S et al Identification of the cell‐intrinsic and ‐extrinsic pathways downstream of EGFR and IFNgamma that induce PD‐L1 expression in head and neck cancer. Cancer Res 2016; 76: 1031–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang W, Zhu H, Liu X et al Epidermal growth factor receptor is a prognosis predictor in patients with esophageal squamous cell carcinoma. Ann Thorac Surg 2014; 98: 513–19. [DOI] [PubMed] [Google Scholar]
- 19. Dovedi SJ, Illidge TM. The antitumor immune response generated by fractionated radiation therapy may be limited by tumor cell adaptive resistance and can be circumvented by PD‐L1 blockade. Oncoimmunology 2015; 4: e1016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiao ZF, Yang ZY, Liang J et al Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. Ann Thorac Surg 2003; 75: 331–6. [DOI] [PubMed] [Google Scholar]
- 21. Herbst RS, Soria JC, Kowanetz M et al Predictive correlates of response to the anti‐PD‐L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brambilla E, Le Teuff G, Marguet S et al Prognostic effect of tumor lymphocytic infiltration in resectable non‐small‐cell lung cancer. J Clin Oncol 2016; 34: 1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tokito T, Azuma K, Kawahara A et al Predictive relevance of PD‐L1 expression combined with CD8 + TIL density in stage III non‐small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer 2016; 55: 7–14. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki K, Kachala SS, Kadota K et al Prognostic immune markers in non‐small cell lung cancer. Clin Cancer Res 2011; 17: 5247–56. [DOI] [PubMed] [Google Scholar]
- 25. Pivarcsi A, Muller A, Hippe A et al Tumor immune escape by the loss of homeostatic chemokine expression. Proc Natl Acad Sci USA 2007; 104: 19055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abiko K, Matsumura N, Hamanishi J et al IFN‐gamma from lymphocytes induces PD‐L1 expression and promotes progression of ovarian cancer. Br J Cancer 2015; 112: 1501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feng Y, Shen J, Gao Y et al Expression of programmed cell death ligand 1 (PD‐L1) and prevalence of tumor‐infiltrating lymphocytes (TILs) in chordoma. Oncotarget 2015; 6: 11139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bellucci R, Martin A, Bommarito D et al Interferon‐gamma‐induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD‐L1 expression. Oncoimmunology 2015; 4: e1008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen N, Fang W, Zhan J et al Upregulation of PD‐L1 by EGFR activation mediates the immune escape in EGFR‐driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol 2015; 10: 910–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association between tumor cell and tumor‐infiltrating immune cell PD‐L1 expression by χ2 analysis.
Fig. S1. Kaplan–Meier survival curves according to different PD‐L1 and EGFR expression.
Fig. S2. Kaplan–Meier survival curves according to tumor‐infiltrating immune cell PD‐L1 expression and postoperative radiotherapy.
Fig. S3. Kaplan–Meier survival curves according to tumor cell PD‐L1 expression and postoperative radiotherapy.


