Abstract
This review of the basics of cancer metabolism focuses on exploiting the metabolic differences between normal and cancer cells. The first part of the review covers the different metabolic pathways utilized in normal cells to generate cellular energy, or ATP, and the glycolytic intermediates required to build the cellular machinery. The second part of the review discusses aerobic glycolysis, or the Warburg effect, and the metabolic reprogramming involving glycolysis, tricarboxylic acid cycle, and glutaminolysis in the context of developing targeted inhibitors in cancer cells. Finally, the selective targeting of cancer mitochondrial metabolism using positively charged lipophilic compounds as potential therapeutics and their ability to mitigate the toxic side effects of conventional chemotherapeutics in normal cells are discussed. I hope this graphical review will be useful in helping undergraduate, graduate, and medical students understand how investigating the basics of cancer cell metabolism could provide new insight in developing potentially new anticancer treatment strategies.
Abbreviations: ATP, adenosine triphosphate; BrP, bromopyruvate; CO2, carbon dioxide; CoA, acetyl-coenzyme A; 2-DG, 2-deoxyglucose; ETC, electron transport chain; FAO, fatty acid oxidation; FAD, flavin adenine dinucleotide; FDG, fluorinated deoxyglucose; FFA, free fatty acid; GAPDH, glyceraldehyde-3-phosphate; GIAN, Global Initiative of Academic Networks; G-6P, glucose-6-phosphate; GLUT, glucose transporters; H2O, water; HK, hexokinase; HIF-1, hypoxia-inducible factor 1; IDH-2, isocitrate dehydrogenase-2; KRAS, V-Ki-ras2 Kirsten rat sarcoma; LDH, lactate dehydrogenase; LN, lonidamine; MAGL, monoacylglycerol lipase; MCT, monocarboxylate transporter; MDR, multidrug resistance; NAD+, nicotinamide adenine dinucleotide; NADPH, nicotinamide adenine dinucleotide phosphate; OXPHOS, oxidative phosphorylation; PHGDH, 3-phosphoglycerate dehydrogenase; PDH, pyruvate dehydrogenase; PDK, pyruvate dehydrogenase kinase; PET, positron emission tomography; PK, pyruvate kinase; TCA, tricarboxylic acid; TPP+, triphenylphosphonium; VDAC, voltage-dependent anion channel
Highlights
-
•
Exploiting biochemical and metabolic differences between normal and cancer cells.
-
•
Mitigating reverse Warburg effect in the tumor stroma or microenvironment to hinder tumor growth.
-
•
Dual targeting of glycolysis and mitochondrial metabolism to inhibit tumor cell proliferation.
1. Introduction
A few months ago, I was invited to teach a course on “targeting cancer metabolism” to students at Anna University in Chennai, Tamil Nadu, India (Fig. 1). These lectures were part of Anna University's recently launched Global Initiative of Academic Networks (GIAN) program, a project of the Ministry of Human Resource Development, Government of India. One of the goals of GIAN is to provide a platform to initiate future teaching and research collaborations between Anna University and the Medical College of Wisconsin in Milwaukee, USA. Teaching this course was especially gratifying to me, because it allowed me to “give back” to the city where I grew up and was educated from first grade through receipt of my Bachelor's degree. My visit was hosted by Dr. Anuradha Dhanasekaran, director and head of the Center for Biotechnology at Anna University. I gave six lectures covering the basics of cancer metabolism, redox signaling, metabolic imaging, and the side effects of standard-of-care antitumor drugs. In this review, I will focus on the basics of cancer metabolism.
Fig. 1.
Map showing the location of Chennai in India. Chennai (formerly Madras), located on the coast of the Bay of Bengal, is the capital of the state of Tamil Nadu. Also dubbed as the “Detroit of India” due to a thriving automotive industry, the population of Chennai including the suburbs is nearly 10 million.
2. Targeting cancer cell metabolism: new anticancer treatment strategies
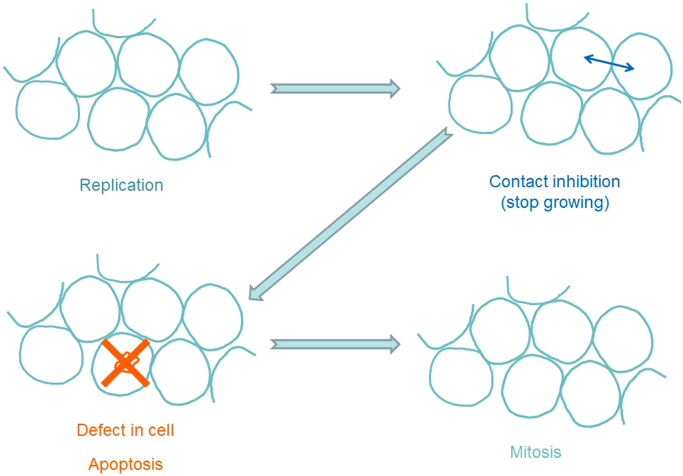
Cancer is not a single disease. It is caused by the uncontrolled growth of abnormal or mutated cells in the body, and cancer cells are characterized by their ability to rapidly grow and divide, and to undergo uncontrolled proliferation [1], [2]. Whereas normal cells follow a set of organized metabolic programs, cancer cells do not obey this “law and order.” Most normal cells in our body replicate in an orderly fashion through a process called mitosis (Fig. 2). When these cells get crowded during growth, they stop growing through contact inhibition. If there is a defect or mutation in a cell, it is removed by the cellular machinery through programmed cell death (called “apoptosis”), and the mutated dead cell is replaced by a new cell (Fig. 2). The number of mutations that occur in normal cells every day is not very high – about a thousand or so. Considering the enormous number of cells (100 trillion or so) that exist in the human body and the huge number of cells that are formed every day, the number of mutations that occur daily in normal cells is not very high [3].
Fig. 2.
Mitosis of normal cells – a process by which normal cells replicate in an orderly fashion.
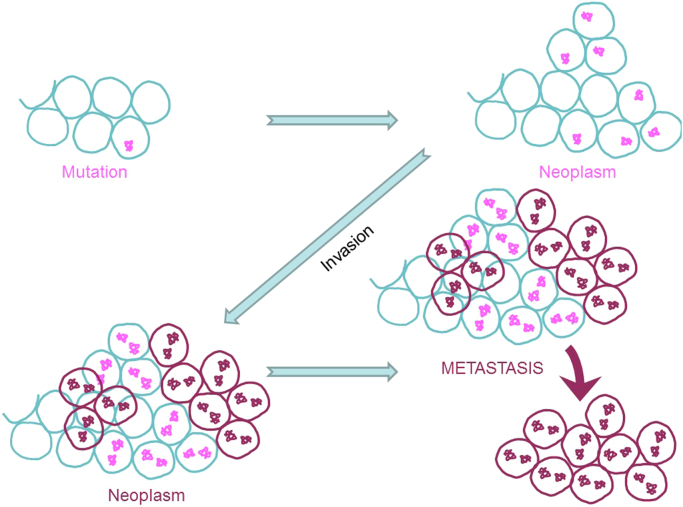
However, in some situations, mutations within the cells' DNA make them resistant to apoptosis and/or cause them to undergo faster replication. Under these conditions, new cells that are formed by replication still contain the same mutation, ultimately resulting in a lump of abnormal cells (neoplasms) (Fig. 3). Neoplastic cells are considered to be benign as long as they remain as a lump and do not grow out of control. However, under conditions where cells contain broken genetic information, the cells tend to “run amuck” and invade neighboring cells. These invasive, mutated cells are cancerous, and the collection of such cells forms a tumor. Cancer cells are uninhibited by contact, are fast replicating, exhibit genetic abnormality, escape cellular checkpoints, develop resistance to cell death, and consume a vast amount of cellular nutrients [2].
Fig. 3.
Formation of neoplasms, invasion, and metastasis of cancer cells.
In another scenario, which occurs in most cancers, the invasive cancer cells break off, get into blood circulation, and migrate to distant organs where they take up residence. This process (i.e., the ability of cancer cells to spread away from the primary site) is called tumor metastasis (Fig. 3). Metastatic cells may have a completely different set of mutations and, therefore, may respond to chemotherapy much differently from the primary tumors. Thus, cancer is indeed a multiple disease, which makes completely treating and curing it more challenging.
To meet the increased energy demands, cancer cells strive to acquire additional nutrients, by hook or crook, using multiple mechanisms [3], [4]. With every division, a cancer cell must assemble essential cellular components such as DNA, phospholipids, and other organelle. The metabolic characteristics of cancer cells, and the pathways through which they acquire and replenish their metabolic needs, are different from those of normal cells because of the metabolic reprogramming [5]. Emerging research suggests that selectively starving cancer cells of their insatiable metabolic needs, using drugs that are relatively nontoxic (to normal cells), can selectively inhibit cancer cell proliferation, thus delaying or suppressing tumor growth [6], [7]. Thus, a better understanding of cancer cell metabolism may pave the way for designing targeted anticancer drugs that are more effective and less toxic (to normal cells). Recent developments in cancer drug discovery focus on inhibitors of metabolic pathways exploited by cancer cells [8], [9], [10]. As opposed to cytotoxic therapies (chemotherapy and radiation) that kill cancer cells and result in tumor shrinkage quickly after therapy, targeted therapies do not immediately kill cancer cells [11]. Rather, by altering or slowing the cancer metabolism, the new targeted therapies interrupt cancer cell growth, inhibiting cancer cells' glucose uptake with time; changes or regression of the tumor occur many days or months later. Ultimately, cancer cells treated with targeted therapies die.
Current chemotherapies often are associated with acute and delayed toxic side effects [12], [13]. Most conventional chemotherapeutic drugs are potently cytotoxic to cancer cells and normal cells, although newer targeted therapies exhibit a higher therapeutic index (increased chemotherapeutic efficacy and decreased side effects). A key objective in cancer metabolic chemotherapy is to use a combinatorial chemotherapy such that tumor cell cytotoxicity is enhanced by combining metabolic inhibitors with “standard-of-care” chemotherapeutic agents and mitigating cytotoxicity to normal cells.
3. Nutrients, metabolism, and cellular energy
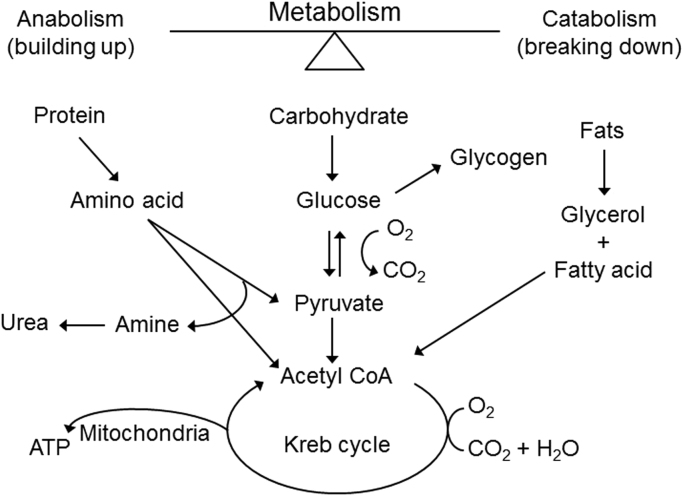
Cells in our body obtain energy through a series of chemical reactions or metabolism. Metabolism can be viewed as a balance between anabolism (building up) and catabolism (breaking down). Food (carbohydrates, proteins, and fat) is converted to energy (or adenosine triphosphate [ATP]) through metabolism (Fig. 4) [3]. In normal cells, ATP (the cellular energy currency) is generated through catabolism of carbohydrates, proteins, and fats, as shown. Carbohydrates are broken down to glucose that is converted to pyruvate and acetyl-coenzyme A (CoA). Acetyl-CoA is metabolized to a series of intermediates in the tricarboxylic acid (TCA) pathway, or “Krebs cycle,” releasing ATP and high-energy carriers (nicotinamide adenine dinucleotide phosphate [NADPH]) that result in additional ATPs. Energy is released in the form of ATPs during aerobic glucose oxidation to carbon dioxide and water. Protein is broken down to amino acids that help build pyruvate and acetyl-CoA molecules. Fats are catabolized to a small-chain three-carbon molecule, glycerol, and fatty acids that help build pyruvate and acetyl-CoA molecules. Both anabolism and catabolism pathways are regulated by several hormones (glucagon, glucocorticoids, insulin, and growth hormone) [3].
Fig. 4.
Cellular energy formed from metabolism of carbohydrates, proteins, and fats in normal cells.
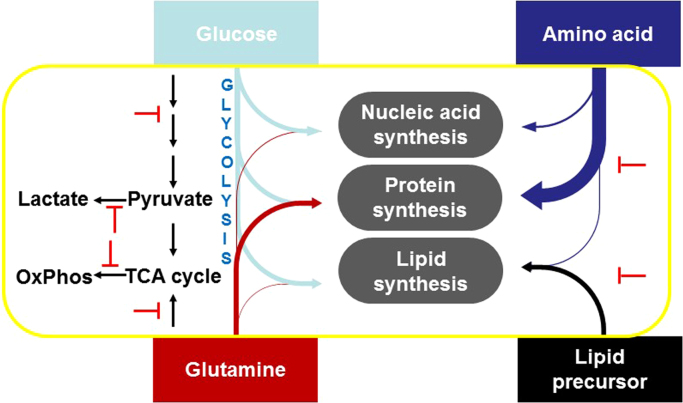
From a therapeutic perspective, the need to understand cancer cell metabolism is critically important. Therapeutic strategies for cancer treatment involve targeting several metabolic pathways, including glycolysis, the Krebs cycle, oxidative phosphorylation (OXPHOS), glutamine metabolism, fatty acid oxidation, nucleic acid synthesis, lipid synthesis, and amino acid metabolism (Fig. 5).
Fig. 5.
Targeting the various energetic pathways in cancer metabolism. (Modified; reprinted from Developmental Cell, 36/5, Hosios AM, Hecht VC, Danai LV, Johnson MO, Rathmell JC, Steinhauser ML, Manalis SR, Vander Heiden MG, Amino Acids Rather than Glucose Account for the Majority of Cell Mass in Proliferating Mammalian Cells, 540-9, Copyright (2016) [22], with permission from Elsevier.)
4. The Warburg effect: the origin of metabolic differences between normal and cancer cells
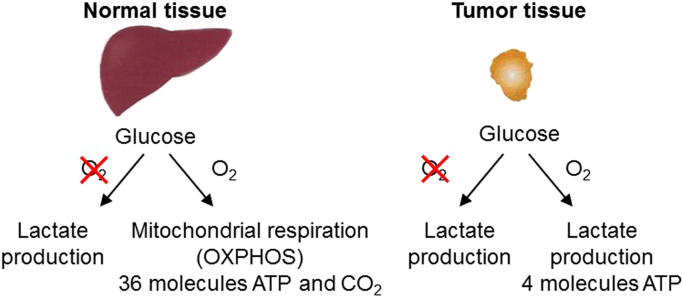
What type of metabolic reprogramming occurs in cancer cells? In order to sustain the uncontrolled growth rate, cancer cells have adopted a different yet unconventional mechanism to derive energy from outside. Normal cells do not metabolize glucose to lactate when oxygen is available. Only when the oxygen is absent or limiting do normal cells resort to anaerobic glycolysis or metabolism of glucose to lactic acid. In contrast, cancer cells metabolize glucose to lactate even in the presence of oxygen (aerobic glycolysis). The idea that cancer cells exhibit an altered metabolism was originally conceived by Otto Warburg nearly 100 years ago (reviewed by Koppenol et al.) [14].
The propensity of cancer cells under aerobic (well-oxygenated) conditions to metabolize glucose to lactate (aerobic glycolysis) is known as the Warburg effect (Fig. 6). Warburg made the seminal observation that tumor slices consume glucose and secrete lactate at a higher rate than normal tissues, even in the presence of air (aerobic conditions) [14]. Normal cells rely on mitochondrial OXPHOS to produce ATP. Under aerobic conditions, normal cells and tissues metabolize glucose to carbon dioxide (CO2) and water (H2O) through OXPHOS and convert glucose to lactate (glycolysis) under hypoxic (poorly oxygenated) conditions. However, tumor tissues metabolize glucose to lactate even under aerobic conditions. Most cancer cells depend on glycolysis, even in the presence of plenty of oxygen, to generate a considerably lesser amount of ATP with a decreased use of the OXPHOS mechanism [15]. This metabolic change is one of the first recognized biochemical hallmarks of cancer cells. Usually, cancer cells are highly glycolytic (glucose addiction) and have a “sweet tooth” in that they avidly take up more glucose than do normal cells from outside. The increased uptake of glucose is facilitated by the overexpression of several isoforms of membrane glucose transporters (GLUTs). Although the Warburg effect was discovered decades ago, use of the glycolytic pathway as a potential target for anticancer drug development was not recognized by the scientific community. However, the Warburg effect was used in cancer diagnoses and in evaluating the drug response in cancer treatment. Experimental confirmation for the higher uptake of glucose in tumors and metastasis came from positron emission tomography (PET) imaging, one of the most commonly used imaging techniques for diagnosing tumor growth and response to therapy [16]. The most frequently used PET tracer in oncology is fluorinated deoxyglucose (18-FDG). FDG, an analog of glucose, is not metabolized in the glycolytic pathway; rather, it is transported by glucose transporters (GLUT 1, GLUT 3, etc., that exhibit differing affinity for glucose) located in the plasma membrane. FDG-PET uptake and GLUT expression are positively correlated. The increased uptake of FDG indirectly measures the glucose metabolism of cancer and other proliferating cells; therefore, it is used as an imaging biomarker for glucose metabolism and the metabolic capability of cancer [17]. It also correlates with a poor cancer prognosis. Thus, Warburg's century old original hypothesis – that cancer cells have high glycolytic rates compared to normal resting tissue – has certainly stood the test of time.
Fig. 6.
Metabolic differences in normal and cancer cells and tissues.
However, the reasons for increased glycolysis in cancer cells (the Warburg effect) are not entirely clear. Warburg hypothesized that defects in mitochondrial function could be the reason why cancer cells depend on glycolysis for the survival [14], [18]. However, subsequent studies have clearly implicated a robust role for mitochondrial metabolism in cancer cells [19], [20], [21]. Recent research suggests that a major advantage of the Warburg effect lies not in the generation of glycolytic ATP but in generating many glycolytic intermediates that are precursors to anabolic processes such as pentose phosphate pathway generating NADPH, ribose-6-phosphate, amino acid, lipids, and other cellular sources of energy (de novo syntheses of carbohydrates, proteins, and fats) [8], [9], [22]. Because both glycolysis and respiration are energy-producing processes, inhibiting one or both pathways using selectively targeted drugs potentially would serve as an anticancer mechanism. It has been suggested that a Warburg-like mechanism also is operative in other rapidly proliferating cells and tissues (e.g., T-lymphocytes, embryonic tissues) [23], [24].
5. The glycolytic pathway
From the above discussion, it is apparent that cancer cells need to maintain a high level of glycolytic activity for survival and growth. Thus, inhibiting glycolytic metabolites (and ATP generation) could have a devastating effect on cancer cells. The glycolytic pathway that occurs in the cytosol presents a unique array of potential therapeutic targets for anticancer drug development. Detailed understanding of the steps involved in the glycolytic pathway may help develop new metabolic anticancer strategies [25].
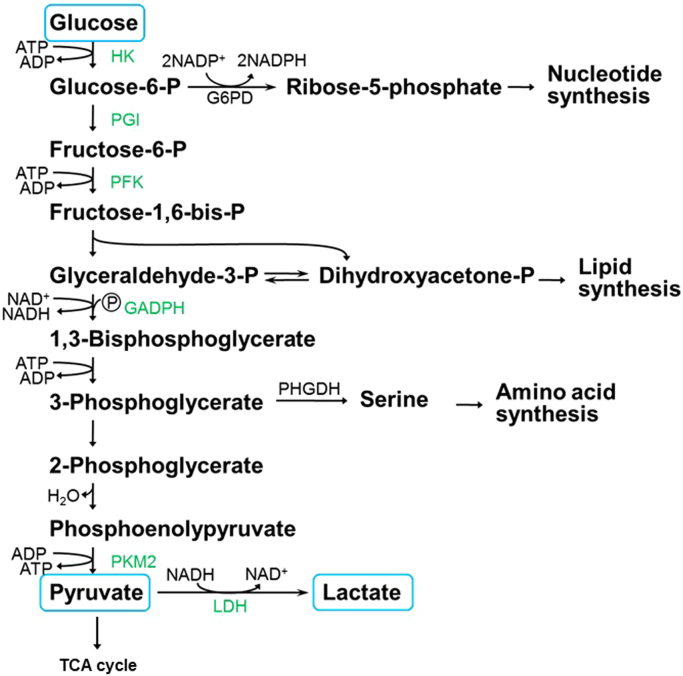
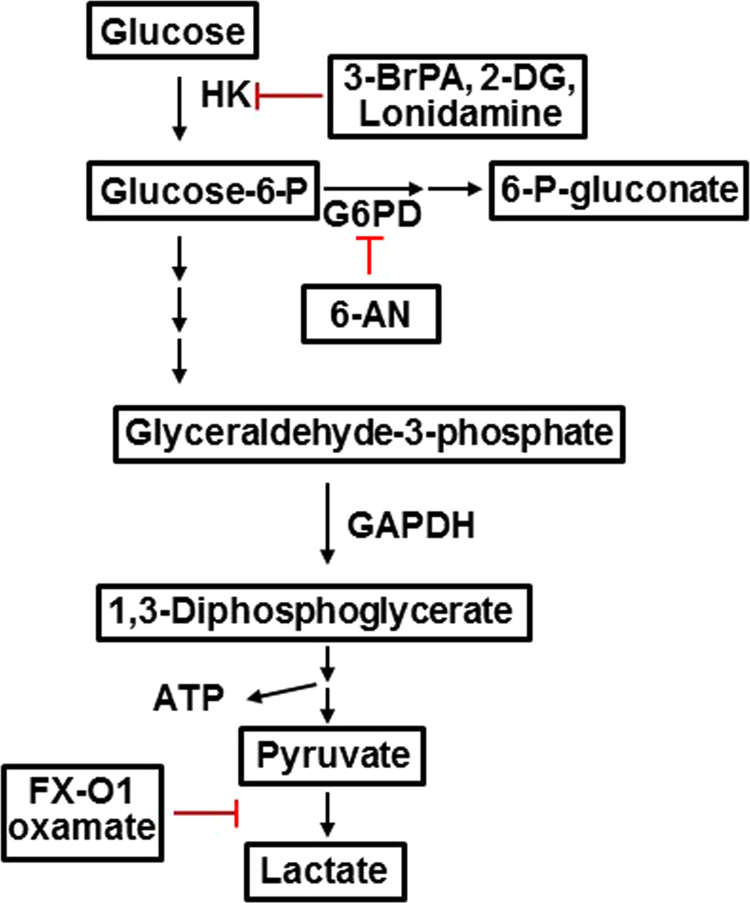
The glycolytic pathway involves the catabolism of glucose to pyruvate in a series of oxidation reactions (10 to be exact) catalyzed by ATP and nicotinamide adenine dinucleotide (NAD+) (Fig. 7) [3]. The first step is the conversion of the nonionic glucose to the anion, glucose-6-phosphate (G-6P), that is trapped in the cells. This reaction is catalyzed by an isoform of hexokinases (HK). G-6P is converted to fructose-6-phosphate by phosphoglucose isomerase. Using ATP, fructose-6-phosphate is converted to fructose-1,6-bisphosphate. The enzyme aldolase catalyzes the conversion of fructose-1,6-bisphosphate to glyceraldehyde-3-phosphate (GAPDH) and dihydroxyacetone phosphate. The GAPDH enzyme coupled with NAD+ converts GAPDH to 1,3-bisphosphoglycerate. Until this point, this glycolytic pathway represents the “investment” phase, using up two ATPs as a source of energy [26].
Fig. 7.
The glycolytic pathway showing catabolic and anabolic intermediates and products derived from glucose.
Other nucleotides, fatty acid, and amino acid are synthesized from the glycolytic pathway (Fig. 7). For example, G-6P can be diverted via the pentose phosphate pathway catalyzed by the enzyme G-6P dehydrogenase generating the precursor molecule, ribose-5-phosphate, for nucleotide and DNA biosynthesis and NADPH. Glyceraldehyde-3-phosphate or dihydroxyacetone phosphate is a precursor for biosynthesis of cell membrane components, phospholipids, and triglycerols. Amino acids (e.g., serine), cysteine, and glycine derived from serine are generated from the 3-phosphoglycerate dehydrogenase (PHGDH)-catalyzed conversion of 3-phosphoglycerate into serine. As discussed previously, cancer cells switch to aerobic glycolysis; however, because of the poor efficiency of ATP generation, which is nearly 18-fold lower in glycolysis relative to OXPHOS, the reason for this type of reprogramming initially was unclear. The functional role for this switch in cancer cells became clearer in light of the new perspective that enhanced glucose metabolism or glycolysis facilitates the biosynthesis of the nucleotides and amino acids needed for cell assembly and growth (Fig. 7) [26].
In the “payoff” phase of the glycolytic pathway, two ATPs (total) are formed during phosphoglycerate kinase-catalyzed oxidation of 1,3-bisphosphoglycerate to 3-phosphoglycerate. Enolase is responsible for converting 2-phosphoglycerate to phosphoenolpyruvate. These glycolytic reactions do not require molecular oxygen. Pyruvate kinase (PK) catalyzes the formation of pyruvate from phosphoenolpyruvate. Pyruvate is the critical metabolite of the glycolytic pathway and is responsible for the formation of lactic acid catalyzed by lactate dehydrogenase (LDH) in cancer cells. Alternatively, pyruvate can enter into the Krebs cycle and stimulate mitochondrial metabolism, as discussed below.
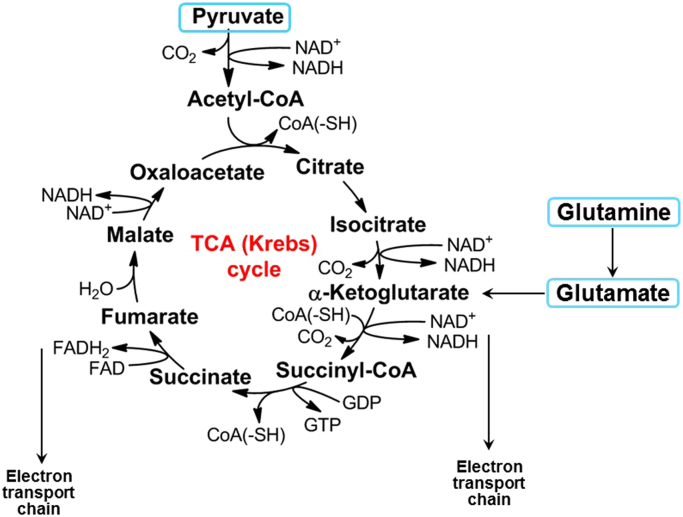
6. The tricarboxylic acid cycle
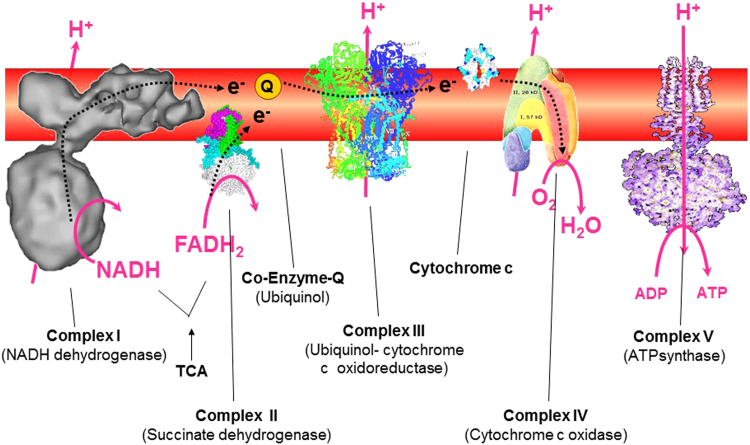
Pyruvate generated from the glycolytic pathway is oxidized by the enzyme pyruvate dehydrogenase (PDH) to the acetyl-CoA, which combines with an oxaloacetate molecule to form citric acid or citrate [3]. This is the start of the TCA cycle (Fig. 8). The TCA cycle intermediates are precursors to fatty acids, nucleotides, hemes, and porphyrins, which are essential building blocks for macromolecules (DNA, proteins, and lipids). Important regulators of the TCA cycle that are formed from citric acid are alpha-ketoglutarate formed from isocitrate, succinate from succinyl-CoA, fumarate and malonate, and ultimately oxaloacetate that combines with acetyl-CoA to keep the TCA cycle going. The oxidation of acetyl-CoA to CO2 during aerobic respiration is accompanied by the reduction of NAD+ and flavin adenine dinucleotide (FAD) during the TCA cycle. The intermediate products, NADH and FADH2, serve as electron transport chain (ETC) coenzymes for OXPHOS. Electrons are subsequently transferred from NADH and FADH2 to oxygen via the mitochondrial complexes located within the inner mitochondrial membrane, ultimately catalyzing the formation of ATP (Fig. 9).
Fig. 8.
The Krebs cycle or the TCA cycle fueled by pyruvate derived from glucose and glutamine.
Fig. 9.
Mitochondrial electron transport chain complexes involved in oxidative phosphorylation. (Modified from a slide obtained from Paul Brooks.)
7. Glutaminolysis
Cancer cells also take up and metabolize glutamine. To sustain the functioning of the TCA uninterruptedly, metabolites can be fed into this cycle. For example, glutaminolysis involves the glutaminase-catalyzed conversion of glutamine to glutamate, which subsequently forms alpha-ketoglutarate that enters the TCA cycle (Fig. 9). Glutamine is also a substrate for fatty acid synthesis in hypoxic cells, presumably via hypoxia-inducible factor 1 (HIF-1) activation. Glutamine is the most abundant circulating nonessential amino acid, and glutamate generated from glutamine is also a precursor of other nonessential amino acids (e.g., aspartate, alanine, arginine, and proline). Glutamine is the source of nitrogen for the biosynthesis of glycosylated molecules and nucleotide, and it serves as a substrate for fatty acid synthesis in hypoxic cells or cells with HIF-1-alpha activation [27].
Blood glutamine levels are enhanced in certain cancer patients [28]. In normal mitochondria, the TCA cycle continues clockwise and forms additional oxaloacetate, which drives the cycle. However, with dysfunctional mitochondria (arising from protein mutations in the Krebs cycle, where pyruvate oxidation to the acetyl-CoA is prevented), the alpha-ketoglutarate formed from glutamine is converted into citrate through increased reductive carboxylation in an isocitrate dehydrogenase-2 (IDH-2) dependent reaction [29], [30]. Reductive carboxylation of glutamine to citrate is also an alternative pathway for lipid synthesis. Glutamine is responsible for synthesis of glutathione, an important component of cellular antioxidant machinery.
V-Ki-ras2 Kirsten rat sarcoma (KRAS), the viral oncogene, stimulates cancer cell growth through enhanced formation of alpha-ketoglutarate via the TCA cycle. Increased activity of the enzyme, glutamate pyruvate transaminase, was observed in cancer. Thus, glutamine/glutamate metabolism provides an attractive therapeutic target. Inhibitors of glutaminase activity (e.g., BPTES [bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide]) are being tested for anticancer activity [31].
8. Oxidative phosphorylation/electron transport chain
OXPHOS refers to the generation of ATP from ADP and phosphate by ATP synthase (or complex V) using the proton gradient established across the inner mitochondrial membrane. The ETC, or respiratory chain, consists of complex I or NADH dehydrogenase, complex II, complex III, and complex IV present in the inner mitochondrial membrane. NADH and FADH2, the reducing equivalents generated by the TCA cycle, are oxidized to NAD+ and FAD, respectively. This generates energy that is used to pump protons or hydrogen ions from the mitochondrial matrix into the inner membrane phase by complex I, III, and IV and, ultimately, the terminal electron acceptor (oxygen) to generate two molecules of water (Fig. 9). No proton pumping occurs at complex II, coenzyme Q, or cytochrome c. The proton gradients generate a large electrical component (membrane potential), facilitating ATP generation by complex V. Most of the ATP is generated during this process—oxidation of NADH and FADH2 (proton gradients established by mitochondrial complexes across the inner mitochondrial membrane) and reduction of oxygen to water.
9. Serine biosynthesis pathway
Synthesis of serine involves both glycolysis and glutaminolysis intermediates via coupling of 3-phosphoglycerate-derived 3-phosphohydroxypyruvate with glutamate. Both glycolysis and glutaminolysis pathways are activated in several cancers. Thus, the serine biosynthesis pathway is essential in breast cancer and associated with poor five-year survival in breast cancer patients [32]. Recently, attention is focused on the enzyme PHGDH. Flux analysis showed that nearly 9% of glucose is shuttled into the PHGDH pathway in PHGDH-dependent cells, compared with 1% of glucose in PHGDH-insensitive cells. The serine biosynthesis pathway is an important regulator of glycolysis/glutaminolysis pathways in cancer.
10. Role of respiration in aspartate biosynthesis from glutamine
Mitochondrial respiration serves as an ATP-generating catabolic powerhouse in nonproliferating cells. However, recent reports suggest that respiration has an anabolic role in that it stimulates aspartate biosynthesis in proliferating cancer cells [33], [34]. Aspartate is an amino acid that is one of the fundamental building blocks of cellular proteins. Aspartate is required in ample supply for nucleotide (DNA and RNA) and protein biosynthesis in proliferating cells. Circulating blood cannot provide the aspartate needed to build the cellular machinery [35].
11. Reverse Warburg effect
The glycolytic product, lactic acid, secreted by cancer cells or fibroblasts is also used by neighboring cancer cells to make citric acid and sustain cancer progression. The “reverse Warburg effect” is a new term for “parasitic” cancer metabolism (Fig. 10) [36], [37], [38]. It was proposed that cancer cells act as metabolic parasites in that they obtain nutrients from host cells by inducing catabolic processes. One such process is aerobic glycolysis in host cells. This phenomenon is similar to what happens in parasitic diseases such as malaria and Chagas disease, where the intracellular parasite extracts its fuel supply from host cells following induction of oxidative stress.
Fig. 10.
The reverse Warburg effect in cancer metabolism. (Modified from Martinez-Outschoorn UE et al., Cell Cycle, 10:4208-16, 2011 [36].)
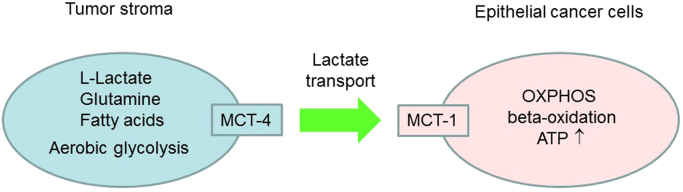
The tumor-host interactions, or metabolic coupling, is a well-accepted phenomenon in cancer progression [39]. First proposed in 1889, the “seed and soil” hypothesis states that cancer cells act as seeds; they grow best in a host organ microenvironment that provides “fertile soil” for their growth [39]. However, when tumors take residence, the neighborhood goes amuck. The tumor stroma or the tumor microenvironment, composed of fibroblasts, adipocytes, endothelial cells, and macrophages, becomes the source of fuel for tumor growth. Tumors “steal” energy-rich metabolites from the microenvironment. Tumor cells constantly interact with their microenvironment [40]. In addition to glycolysis, cancer cells will use fatty acids from adipocyte tissues for energy. Other stromal-derived metabolites that promote oxidative mitochondrial metabolism and ATP production in epithelial cancer cells are glutamine and ketones. Monocarboxylate transporters (MCTs) shuttle L-lactate between cancer-associated stromal cells and cancer cells [41]. MCT4, present in fibroblasts, is responsible for exporting L-lactate out of stromal cells, and MCT1, localized in epithelial cancer cells, is responsible for L-lactate uptake.
12. Tumor hypoxia and the Warburg effect: lactate shuttle
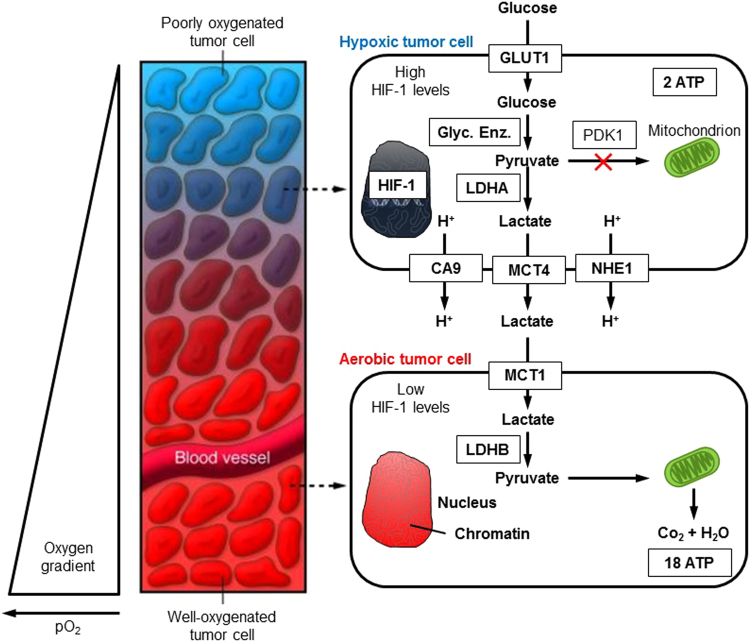
Tumors are heterogeneous, containing aerobic and hypoxic regions. The existence of an oxygen gradient in tumor tissues is a well-accepted fact. As shown in Fig. 11, tumor cells surrounding the blood vessel are well oxygenated, whereas the tumor cells located further away from the blood vessel are poorly oxygenated. Recently, the existence of a “metabolic symbiosis” between hypoxic and aerobic regions of cancer cells was demonstrated [42], [43]. Lactate generated from glycolysis in hypoxic cells is secreted; it is taken up by aerobic cancer cells through MCT1 and converted to pyruvate by LDH-B. The substrate pyruvate then undergoes OXPHOS in aerobic tumor cells generating ATP. By this mechanism, more glucose is left for hypoxic cancer cell metabolism and survival. However, when MCT1 is inhibited, aerobic cancer cells can no longer take up lactate, compete with hypoxic cancer cells for glucose, and deprive hypoxic cancer cells of adequate glucose use. Thus, the MCT1 inhibitor could potentially act as a targeted chemotherapeutic agent. Drugs targeting glycolysis and/or mitochondrial metabolism in cancer cells or catabolism in neighboring stromal cells would be effective in inhibiting tumor progression and metastasis.
Fig. 11.
Lactate shuttle between tumor cells and normal cells. (Modified; republished with permission of American Society for Clinical Investigation, from Tumor Metabolism: Cancer Cells Give and Take Lactate, Gregg L. Semenza, 118, 12, 2008 [42]; permission conveyed through Copyright Clearance Center, Inc.)
13. Fatty acid oxidation/role of fat on tumor metabolism
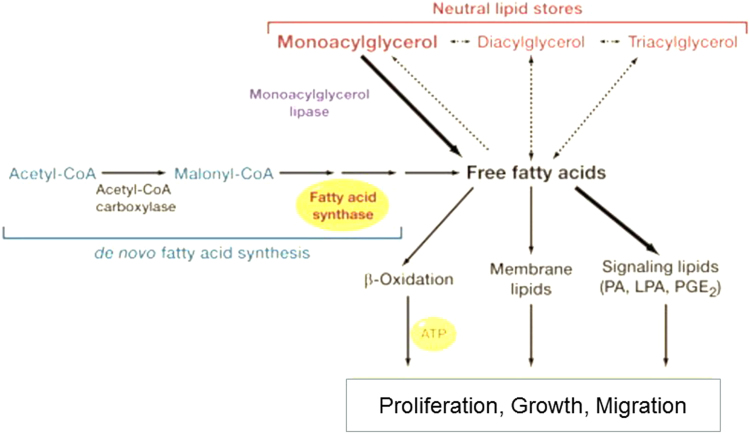
If glucose consumption does not increase proportionately to keep up with the energy demands of rapidly growing cancer cells, the cells resort to fatty acid oxidation (FAO). One of the most abundantly used bioenergetic pathways in prostate cancer cells is enhanced uptake of palmitate and FAO. Inhibition of FAO using the drug etornoxir, which inhibits the carnitine palmitoyltransferase enzyme, blocked tumor growth in a breast cancer xenograft mouse model. Normal cells typically acquire fatty acids through dietary sources. In contrast, tumor cells exhibit a marked increase in de novo fatty acid synthesis. More importantly, some tumor cells (ovarian tumor tissues) have increased monoacylglycerol lipase (MAGL) activity [44]. MAGL was elevated in patients with advanced ovarian cancer. This enzyme hydrolyzes monoglycerols and releases glycerol and free fatty acids (FFAs) (Fig. 12). Aggressive cancers use this mechanism to acquire FFAs. MAGL have been shown to promote tumorigenesis by increasing the FFA levels. As shown in Fig. 12, MAGL induced specific FFA-derived signaling lipids such as phosphatidic acid, lysophosphatidic acid, and prostaglandin E2. These lipid molecules activate tumor signaling mechanisms. De novo lipogenesis involves conversion of acetyl-CoA to palmitate catalyzed by fatty acid synthase. The newly synthesized FFAs presumably are converted into neutral lipid stores and released by MAGL. In addition to glycolysis, which serves as a predominant mechanism, the oxidation of fatty acid provides an alternate route for meeting the bioenergetics needs of tumor cells. Studies suggest that MAGL is a promising targeted therapeutic cancer treatment [45].
Fig. 12.
Release of fatty acid and factors controlling cancer onset. (Modified; reprinted from Cell, 140/1, Jessica L. Yecies, Brendan D. Manning, Chewing the Fat on Tumor Cell Metabolism, 28–30, Copyright (2010) [44], with permission from Elsevier.)
14. Inhibiting glycolysis as a potential antitumor strategy
Glucose is one of the most abundant sources of energy in cancer cells. During the breakdown of one molecule of glucose in the presence of oxygen, six molecules of carbon dioxide and water are released in addition to energy in the form of ATPs. Nearly 34–38 ATP molecules are produced during glycolysis (four ATPs), the Krebs cycle (two ATPs), and mitochondrial respiration (34 ATPs). Thus, targeting glycolytic and mitochondrial metabolism is a strategic approach to slow or inhibit the growth of cancer cells [6], [46], [47].
15. Inhibiting glucose uptake
The transport of glucose into cells is facilitated by a family of membrane-bound proteins called GLUTs. There are multiple forms of GLUT proteins (GLUT 1, GLUT 3, etc.,) that are upregulated in several cancers and, for the most part, increased expression of GLUTs is associated with poor prognosis and survival of cancer patients. As glucose uptake into cancer cells is the rate-limiting step in glycolysis, targeting GLUTs by blocking their glucose transport channel with small molecules should be a viable mechanism of nutrient deprivation in tumors. Currently, there exist several GLUT inhibitors, including cytochalasin B and selected tyrosine kinase inhibitors. Some of these compounds are relatively less toxic to normal cells, showing promise in tumor treatment [25].
16. Targeting hexokinase
16.1. 2-Deoxyglucose
One of the first antiglycolytic antitumor strategies is the use of 2-deoxyglucose (2-DG) (Fig. 13) [48]. HK-2 plays a pivotal role in the Warburg mechanism [49]. HK-2 has low expression levels in normal cells but is highly expressed in many cancer cells and insulin-sensitive cells, such as muscle and adipose cells, consistent with the increased needs for glucose. As shown in Fig. 13, the first step in glucose metabolism is HK-catalyzed, ATP-dependent phosphorylation of glucose to G-6P. G-6P is a metabolite critical for the generation of ATP as well as other building blocks for the nucleotides, amino acids, and fatty acids needed for enzymatic reactions and for macromolecules in highly proliferating cancer cells. HK has four isoforms. The three isoforms HK1-3 have a 250-fold higher affinity for glucose than HK-4. HK-2 is bound to the voltage-dependent anion channel (VDAC) located in the outer mitochondrial membrane. The tumor cells have a higher expression level of VDAC.
Fig. 13.
Inhibitors of the glycolytic pathway. (Modified; reprinted by permission from Macmillan Publishers Ltd: Oncogene (Pelicano H et al. Ongogene 25:4633-46, 2006), copyright (2006) [48].)
The compound 2-DG is a competitive inhibitor of glucose metabolism. 2-DG is taken up into cells through the same mechanism as glucose and phosphorylated by HK to 2-DG-P. 2-DG-P cannot be further metabolized by the phosphohexose isomerase to the fructose-6-phosphate analog. As a result, glycolysis is inhibited at the first step, and the net result is lowered ATP levels in cancer cells treated with 2-DG and inhibited proliferation. One of the side effects of 2-DG is toxicity to normal cells, skeletal muscles, and neuronal cells, which also utilize glucose for energy. 2-DG only partially blocks glucose utilization; consequently, glycolysis is only partially inhibited. 2-DG as a mono-therapeutic agent in cancer treatment has not been successful but shows promise in multimodal cancer therapy [50], [51].
16.2. 3-Bromopyruvate
3-Bromopyruvate (3-BrP) is an alkylating agent and a small-molecule analog of pyruvate that inhibits the HK enzyme and glucose from entering the glycolytic pathway [52]. Alkylation targets of 3-BrP are many, including mt-HNK, SERCA-1, MCT1, GAPDG, and others. Recently, administration of 3-BrP was reported to cause increased mortality in cancer patients [53]. Clearly, additional research on the mechanisms of side effects and toxicity in normal tissues is needed prior to using this drug alone or in combination with other conventional chemotherapeutics.
16.3. Lonidamine
Lonidamine (LN), originally used as an antispermatogenic agent, now is recognized as a potential antitumor agent alone and in combination with other conventional antitumor therapies [54]. The mechanism of action is not completely known, but reports suggest that LN inhibits glycolysis and/or mitochondrial respiration, as well as mitochondrial HK-2 activity, leading to a decrease in extracellular lactic acid. However, recent reports suggest that LN inhibits the monocarboxylate transporter enzyme responsible for L-lactate efflux, effectively inhibiting L-lactate efflux. LN also inhibits mitochondrial respiration, resulting in decreased mitochondrial uptake of pyruvate via the mitochondrial pyruvate carrier and inhibition of complex II and complex I [55].
17. Targeting pyruvate oxidation
Pyruvate is viewed as a “hub” metabolite (being at the interface of glycolysis and mitochondrial metabolism) in cell metabolism and especially plays a central role in regulating metabolic reprogramming in cancer cells [56]. PK catalyzes the transformation of phosphoenolpyruvate to pyruvate during the final step of aerobic glycolysis. The synthesis of pyruvate during glycolysis in cancer cells is catalyzed by a splice variant of PK (PKM2) leading to decreased formation. Several forms of PK (PKM1, PKM2, etc.) are overexpressed in tumorigenesis [57]. PDH represents a complex of enzymes that converts cytosolic pyruvate into mitochondrial acetyl-CoA, the first substrate for the Krebs cycle. PDH is negatively regulated by pyruvate dehydrogenase kinase (PDK) with which it forms a complex, and this mechanism shifts glucose from oxidative metabolism to glycolytic metabolism [58]. Dichloroacetate (DCA), a drug that has shown promising chemotherapeutic effect in preclinical tumor xenograft studies and in humans with glioblastoma (an aggressive form of brain tumor), presumably inhibits PDK, thereby shifting the glucose metabolism from a glycolytic to oxidative pathway [59]. Thus, DCA activates PDH by inhibiting PDK, causing increased mitochondrial metabolism [60]. Several clinical trials for testing DCA as an anticancer agent are ongoing.
Under hypoxic conditions, HIF-1-alpha facilitates the transition from oxidative to glycolytic reprogramming by activating the transcription of the following genes regulating glycolytic metabolism: GLUTs and PDK1 that inactivate PDH responsible for converting pyruvate to acetyl CoA, and lactate dehydrogenase A (LDHA) that oxidizes pyruvate to lactate. The MCT4 that exports pyruvate out of the cell is also upregulated in cancer cells.
18. Targeting lactate dehydrogenase A
LDHA is a major glycolytic enzyme responsible for converting pyruvate to lactate coupled with NAD+ recycling. LDHA is encoded by a target gene of c-Myc and HIF-1-alpha and is linked to the activation of oncogenes (Myc, Ras, and Akt). LDHA is overexpressed in several tumor cells [61], [62]. LDH is a tetrameric enzyme that consists of two major subunits, A and/or B, resulting in five isozymes that can catalyze the forward and backward conversion of pyruvate to lactate. Studies indicate that inhibition of LDHA markedly delays tumor formation. FXII, a catechol-containing, small-molecular-weight compound that inhibits LDHA, inhibited tumor growth in xenografts. The mechanism of action of LDHA inhibition is likely very similar to that of DCA in that pyruvate is “forced” to enter the mitochondria and undergo decarboxylation to acetyl-CoA, resulting in enhanced mitochondrial activity.
19. Role of mitochondria in cancer metabolism
The reason why cancer cells choose to pursue aerobic glycolytic metabolism for energy remains enigmatic. Warburg hypothesized that cancer cell mitochondria are dysfunctional, thus forcing the cells to rely exclusively on glycolysis for energy. Early on, Chance and coworkers clearly showed that cancer cells have functional mitochondria because they perform mitochondrial respiration, and that respiratory deficiency is not the reason why cancer cells exhibit high glycolytic activity [63]. An alternative explanation is that the rate of ATP generation through glycolysis is higher than that acquired through mitochondrial respiration. Since cancer cells have multiple pathways through which high levels of glucose are consumed, the glycolytic pathway may be sufficient to keep up with the increased energetic needs (ATP, nucleotide, and protein biosynthesis).
The functional role of mitochondria in tumor cells became clearer when cancer cells treated with mitochondria-targeted compounds showed repressed respiration, decreased cell proliferation, and ATP formation. Mitochondrial hyperpolarization is a common feature of many tumor cell lines [64].
20. Targeting mitochondrial metabolism: selective accumulation of delocalized lipophilic cations
Delocalized phosphonium cations were shown to exhibit antitumor activity in many tumor cell lines [65], [66], [67]. Mitochondria-targeted delocalized lipophilic cations selectively accumulate into tumor cells due to enhanced lipophilicity, hydrophobic alkyl chain length, and the potential of tumor cells to have more negative mitochondrial membranes [68]. On the other hand, accumulation of mitochondria-targeted drugs in normal cell mitochondria is significantly less; therefore, they do not significantly affect normal cell proliferation. Thus, the relatively higher accumulation of mitochondria-targeted drugs in cancer cells, combined with their decreased proliferation and enhanced cytotoxicity, hypersensitizes the tumor cell to antiglycolytic agents, resulting in synergistically enhanced toxicity in tumor cells. Mitochondria-targeted metformin (mito-metformin) is synthesized by conjugating metformin to a triphenylphosphonium (TPP+) group via an alkyl chain of varying lengths [69]. Metformin is one of the most prescribed antidiabetic drugs in the world and is being repurposed as an antitumor drug. As James Black, the Noble Laureate, famously said, “the best way to develop a new drug is to start with an old drug” [70]. Targeting cancer mitochondria using TPP+-based modification of an old drug is an extension of that idea.
Tumor cells have increased levels of a multidrug resistant, plasma-membrane-bound p-glycoprotein that acts as an efflux pump for positively charged compounds or drugs [71], [72]. This is a clinically significant, p-glycoprotein-mediated multidrug resistance (MDR). Not all cationic compounds are MDR-recognizable and, with highly lipophilic analogs, the positive charge becomes less important for recognition and the subsequent effluxing mechanism by MDR [73]. Studies also suggest that cancer cell phenotypes that do not express MDR and have greater mitochondrial membrane potential are more likely to be sensitive to delocalized lipophilic cations and mitochondria-targeted cationic agents [74], [75].
21. Concluding remarks and future perspectives
Despite the fact that glycolysis in cancer cells is substantially elevated, antiglycolytic targeting using 2-DG as a mono-therapeutic agent in clinical trials has not been successful. This reflects the metabolic plasticity of cancer cells and their tendency to escape glucose dependency to other metabolic pathways through metabolic reprogramming. Clearly, new and novel combinatorial therapeutic strategies are required to inhibit tumor growth or proliferation. 2-DG and other glycolytic inhibitors are more potent in combined therapy with “standard-of-care” antitumor drugs (e.g., doxorubicin, gemcitabine) or radiation therapy.
Recent research implicates that mitochondrial metabolism is vital for tumor growth [76]. Targeting mitochondrial metabolism is an emerging area of research in cancer biology. Cancer cell mitochondrial membrane potential is much more negative than normal cell mitochondria. Consequently, cationic drugs can be more selectively targeted to cancer cells. Several TPP+-based compounds such as Mito-CP (mitochondria-targeted carboxy-proxyl), Mito-Tempol, Mito-Vit-E (mitochondria-targeted vitamin E), Mito-Q (mitoquinone), and Mito-metformin are nearly 1000-times more potent than their “untargeted” counterparts (carboxyproxyl, vitamin E, coenzyme Q, or metformin) in inhibiting tumor cell proliferation. These compounds are relatively nontoxic and can greatly exacerbate the antitumor efficacy of conventional chemotherapeutics [77]. Unlike rotenone, a well-known mitochondrial toxin that is toxic both to normal and cancer cells, mitochondria-targeted TPP+-based cationic and antioxidant drugs selectively target tumor mitochondria because of increased negative mitochondrial membrane potential. Rotenone uptake and accumulation in mitochondria is not dependent on mitochondrial membrane potential. In addition, mitochondria-targeted antioxidant drugs mitigate the toxic side effects of antitumor drugs in normal cells (e.g., cardiomyocytes). For example, Mito-Q and other mitochondria-targeted antioxidants mitigate doxorubicin-induced cardiotoxicity while exacerbating its antitumor effects in breast cancer cells and in preclinical breast cancer xenografts [77]. Mitochondria-targeted compounds can potentially increase the therapeutic window of chemotherapeutics. Emerging research suggests that selective targeting of anticancer drugs (cis-platin and doxorubicin) helps overcome the drug resistance [78], [79]. This is an exciting time for cancer cell metabolism research that is becoming increasingly relevant in the clinical setting [80], [81]. Specific aspects of mitochondria-targeted drugs in chemotherapy, alone and in combination with antiglycolytics and inhibitors of the Krebs cycle, will be discussed in a future article.
Acknowledgements
I am greatly indebted to my collaborators and coworkers, too numerous to list here, for giving me this opportunity to become involved in cancer biology research. I am also grateful for the support from the National Cancer Institute at the National Institutes of Health and the Cancer Center of the Medical College of Wisconsin. I am thankful to Lydia Washechek for editing and preparing the manuscript and to Jane Thelaner for preparing the figures.
References
- 1.Galluzzi L., Morselli E., Kepp O. Mitochondrial gateways to cancer. Mol. Asp. Med. 2010;31(1):1–20. doi: 10.1016/j.mam.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Deberardinis R.J., Sayed N., Ditsworth D., Thompson C.B. Brick by brick: metabolism and tumor cell growth. Curr. Opin. Genet. Dev. 2008;18(1):54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michalopoulou E., Bulusu V., Kamphorst J.J. Metabolic scavenging by cancer cells: when the going gets tough, the tough keep eating. Br. J. Cancer. 2016;115(6):635–640. doi: 10.1038/bjc.2016.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward P.S., Thompson C.B. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21(3):297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheong J.H., Park E.S., Liang J. Dual inhibition of tumor energy pathway by 2-deoxyglucose and metformin is effective against a broad spectrum of preclinical cancer models. Mol. Cancer Ther. 2011;10(12):2350–2362. doi: 10.1158/1535-7163.MCT-11-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shafaee A., Dastyar D.Z., Islamian J.P., Hatamian M. Inhibition of tumor energy pathways for targeted esophagus cancer therapy. Metabolism. 2015;64(10):1193–1198. doi: 10.1016/j.metabol.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Hamanaka R.B., Chandel N.S. Targeting glucose metabolism for cancer therapy. J. Exp. Med. 2012;209(2):211–215. doi: 10.1084/jem.20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lunt S.Y., Vander Heiden M.G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Enriquez S., Gallardo-Perez J.C., Hernandez-Resendiz I. Canonical and new generation anticancer drugs also target energy metabolism. Arch. Toxicol. 2014;88(7):1327–1350. doi: 10.1007/s00204-014-1246-2. [DOI] [PubMed] [Google Scholar]
- 11.Serkova N.J., Eckhardt S.G. Metabolic imaging to assess treatment response to cytotoxic and cytostatic agents. Front. Oncol. 2016;6:152. doi: 10.3389/fonc.2016.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Icli F., Karaoguz H., Dincol D. Severe vascular toxicity associated with cisplatin-based chemotherapy. Cancer. 1993;72(2):587–593. doi: 10.1002/1097-0142(19930715)72:2<587::aid-cncr2820720242>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Lenneman C.G., Sawyer D.B. Cardio-oncology: an update on cardiotoxicity of cancer-related treatment. Circ. Res. 2016;118(6):1008–1020. doi: 10.1161/CIRCRESAHA.115.303633. [DOI] [PubMed] [Google Scholar]
- 14.Koppenol W.H., Bounds P.L., Dang C.V. Otto Warburg's contributions to current concepts of cancer metabolism. Nat. Rev. Cancer. 2011;11(5):325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 15.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skoura E., Datseris I.E., Platis I., Oikonomopoulos G., Syrigos K.N. Role of positron emission tomography in the early prediction of response to chemotherapy in patients with non–small-cell lung cancer. Clin. Lung Cancer. 2012;13(3):181–187. doi: 10.1016/j.cllc.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Gatenby R.A., Gillies R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 18.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 19.Ramsay E.E., Hogg P.J., Dilda P.J. Mitochondrial metabolism inhibitors for cancer therapy. Pharm. Res. 2011;28(11):2731–2744. doi: 10.1007/s11095-011-0584-5. [DOI] [PubMed] [Google Scholar]
- 20.Weinberg F., Hamanaka R., Wheaton W.W. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA. 2010;107(19):8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scatena R. Mitochondria and cancer: a growing role in apoptosis, cancer cell metabolism and dedifferentiation. Adv. Exp. Med. Biol. 2012;942:287–308. doi: 10.1007/978-94-007-2869-1_13. [DOI] [PubMed] [Google Scholar]
- 22.Hosios A.M., Hecht V.C., Danai L.V. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev. Cell. 2016;36(5):540–549. doi: 10.1016/j.devcel.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bortner C.D., Scoltock A.B., Cain D.W., Cidlowski J.A. T-cell development of resistance to apoptosis is driven by a metabolic shift in carbon source and altered activation of death pathways. Cell Death Differ. 2016;23(5):889–902. doi: 10.1038/cdd.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Ramos A.A., Poindessous V., Marchetti-Laurent C., Pallet N., Loriot M.A. The effect of immunosuppressive molecules on T-cell metabolic reprogramming. Biochimie. 2016;127:23–36. doi: 10.1016/j.biochi.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Bailey K.M., Wojtkowiak J.W., Hashim A.I., Gillies R.J. Targeting the metabolic microenvironment of tumors. Adv. Pharmacol. 2012;65:63–107. doi: 10.1016/B978-0-12-397927-8.00004-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandel N.S. Cold Spring Harbor, Cold Springs Harbor Laboratory Press; New York: 2015. Navigating Metabolism. [Google Scholar]
- 27.Metallo C.M., Gameiro P.A., Bell E.L. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011;481(7381):380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masiar P.J., Medekova E. The role of serine and glutamine in the metabolism of malignant bone tumors and their significance in the diagnosis and prognosis of bone tumors. Neoplasma. 1988;35(2):197–206. [PubMed] [Google Scholar]
- 29.Mullen A.R., Hu Z., Shi X. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep. 2014;7(5):1679–1690. doi: 10.1016/j.celrep.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullen A.R., Wheaton W.W., Jin E.S. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2011;481(7381):385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang Y., Stine Z.E., Xia J. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J. Clin. Investig. 2015;125(6):2293–2306. doi: 10.1172/JCI75836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Possemato R., Marks K.M., Shaul Y.D. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476(7360):346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birsoy K., Wang T., Chen W.W., Freinkman E., Abu-Remaileh M., Sabatini D.M. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell. 2015;162(3):540–551. doi: 10.1016/j.cell.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan L.B., Gui D.Y., Hosios A.M., Bush L.N., Freinkman E., Vander Heiden M.G. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell. 2015;162(3):552–563. doi: 10.1016/j.cell.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie G., Zhou B., Zhao A. Lowered circulating aspartate is a metabolic feature of human breast cancer. Oncotarget. 2015;6(32):33369–33381. doi: 10.18632/oncotarget.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez-Outschoorn U.E., Pestell R.G., Howell A. Energy transfer in "parasitic" cancer metabolism: mitochondria are the powerhouse and Achilles' heel of tumor cells. Cell Cycle. 2011;10(24):4208–4216. doi: 10.4161/cc.10.24.18487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pavlides S., Whitaker-Menezes D., Castello-Cros R. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8(23):3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 38.Sotgia F., Whitaker-Menezes D., Martinez-Outschoorn U.E. Mitochondrial metabolism in cancer metastasis: visualizing tumor cell mitochondria and the "reverse Warburg effect" in positive lymph node tissue. Cell Cycle. 2012;11(7):1445–1454. doi: 10.4161/cc.19841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribatti D., Mangialardi G., Vacca A. Stephen Paget and the 'seed and soil' theory of metastatic dissemination. Clin. Exp. Med. 2006;6(4):145–149. doi: 10.1007/s10238-006-0117-4. [DOI] [PubMed] [Google Scholar]
- 40.Hui L., Chen Y. Tumor microenvironment: sanctuary of the devil. Cancer Lett. 2015;368(1):7–13. doi: 10.1016/j.canlet.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 41.Sanita P., Capulli M., Teti A. Tumor-stroma metabolic relationship based on lactate shuttle can sustain prostate cancer progression. BMC Cancer. 2014;14:154. doi: 10.1186/1471-2407-14-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semenza G.L. Tumor metabolism: cancer cells give and take lactate. J. Clin. Investig. 2008;118(12):3835–3837. doi: 10.1172/JCI37373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semenza G.L. Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 2011;365(6):537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 44.Yecies J.L., Manning B.D. Chewing the fat on tumor cell metabolism. Cell. 2010;140(1):28–30. doi: 10.1016/j.cell.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J., Liu Z., Lian Z. Monoacylglycerol lipase: a novel potential therapeutic target and prognostic indicator for hepatocellular carcinoma. Sci. Rep. 2016;6:35784. doi: 10.1038/srep35784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H., Hu Y.P., Savaraj N., Priebe W., Lampidis T.J. Hypersensitization of tumor cells to glycolytic inhibitors. Biochemistry. 2001;40(18):5542–5547. doi: 10.1021/bi002426w. [DOI] [PubMed] [Google Scholar]
- 47.Cheng G., Zielonka J., Dranka B.P. Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 2012;72(10):2634–2644. doi: 10.1158/0008-5472.CAN-11-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pelicano H., Martin D.S., Xu R.H., Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25(34):4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 49.Roberts D.J., Miyamoto S. Hexokinase II integrates energy metabolism and cellular protection: akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015;22(2):248–257. doi: 10.1038/cdd.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muley P., Olinger A., Tummala H. 2-Deoxyglucose induces cell cycle arrest and apoptosisin colorectal cancer cells independent of its glycolysis inhibition. Nutr. Cancer. 2015;67(3):514–522. doi: 10.1080/01635581.2015.1002626. [DOI] [PubMed] [Google Scholar]
- 51.Wang S.Y., Wei Y.H., Shieh D.B. 2-Deoxy-D-Glucose can complement doxorubicin and sorafenib to suppress the growth of papillary thyroid carcinoma cells. PLoS One. 2015;10(7):e0130959. doi: 10.1371/journal.pone.0130959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pedersen P.L. Mitochondria in relation to cancer metastasis: introduction to a mini-review series. J. Bioenergy Biomembr. 2012;44(6):615–617. doi: 10.1007/s10863-012-9470-z. [DOI] [PubMed] [Google Scholar]
- 53.Feldwisch-Drentrup H. Candidate cancer drug suspected after death of three patients at an alternative medicine clinic. Science. 2016 http://www.sciencemag.org/news/2016/08/candidate-cancer-drug-suspected-after-death-three-patients-alternative-medicine-clinic (August 12, 2016) [Google Scholar]
- 54.Di Cosimo S., Ferretti G., Papaldo P., Carlini P., Fabi A., Cognetti F. Vol. 39. 2003. Lonidamine: efficacy and safety in clinical trials for the treatment of solid tumors; pp. 157–174. (Drugs Today). [DOI] [PubMed] [Google Scholar]
- 55.Nath K., Guo L., Nancolas B. Mechanism of antineoplastic activity of lonidamine. Biochim. Biophys. Acta. 2016;1866(2):151–162. doi: 10.1016/j.bbcan.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szlosarek P.W., Lee S., Pollard P.J. Rewiring mitochondrial pyruvate metabolism: switching off the light in cancer cells? Mol. Cell. 2014;56(3):343–344. doi: 10.1016/j.molcel.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 57.Dayton T.L., Jacks T., Vander Heiden M.G. PKM2, cancer metabolism, and the road ahead. EMBO Rep. 2016;17(12):1721–1730. doi: 10.15252/embr.201643300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holness M.J., Sugden M.C. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem. Soc. Trans. 2003;31(Pt 6):1143–1151. doi: 10.1042/bst0311143. [DOI] [PubMed] [Google Scholar]
- 59.Michelakis E.D., Sutendra G., Dromparis P. Metabolic modulation of glioblastoma with dichloroacetate. Sci. Transl. Med. 2010;2(31):31ra34. doi: 10.1126/scitranslmed.3000677. [DOI] [PubMed] [Google Scholar]
- 60.Michelakis E.D., Webster L., Mackey J.R. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br. J. Cancer. 2008;99(7):989–994. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J., Wang H., Liu A., Fang C., Hao J., Wang Z. Lactate dehydrogenase A negatively regulated by miRNAs promotes aerobic glycolysis and is increased in colorectal cancer. Oncotarget. 2015;6(23):19456–19468. doi: 10.18632/oncotarget.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miao P., Sheng S., Sun X., Liu J., Huang G. Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Life. 2013;65(11):904–910. doi: 10.1002/iub.1216. [DOI] [PubMed] [Google Scholar]
- 63.Chance B. Was Warburg right? Or was it that simple? Cancer Biol. Ther. 2005;4(1):125–126. [PubMed] [Google Scholar]
- 64.Hockenbery D.M. Targeting mitochondria for cancer therapy. Environ. Mol. Mutagen. 2010;51(5):476–489. doi: 10.1002/em.20552. [DOI] [PubMed] [Google Scholar]
- 65.Fantin V.R., Berardi M.J., Scorrano L., Korsmeyer S.J., Leder P. A novel mitochondriotoxic small molecule that selectively inhibits tumor cell growth. Cancer Cell. 2002;2(1):29–42. doi: 10.1016/s1535-6108(02)00082-x. [DOI] [PubMed] [Google Scholar]
- 66.Shabaik Y.H., Millard M., Neamati N. Mechanistic evaluation of a novel small molecule targeting mitochondria in pancreatic cancer cells. PLoS One. 2013;8(1):e54346. doi: 10.1371/journal.pone.0054346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Modica-Napolitano J.S., Aprille J.R. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv. Drug Deliv. Rev. 2001;49(1–2):63–70. doi: 10.1016/s0169-409x(01)00125-9. [DOI] [PubMed] [Google Scholar]
- 68.Murphy M.P., Smith R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 69.Cheng G., Zielonka J., Ouari O. Mitochondria-targeted analogues of metformin exhibit enhanced antiproliferative and radiosensitizing effects in pancreatic cancer cells. Cancer Res. 2016;76(13):3904–3915. doi: 10.1158/0008-5472.CAN-15-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raju T.N. The nobel chronicles. 1988: James Whyte Black, (b 1924), Gertrude Elion (1918-99), and George H Hitchings (1905-98) Lancet. 2000;355(9208):1022. doi: 10.1016/s0140-6736(05)74775-9. [DOI] [PubMed] [Google Scholar]
- 71.Loetchutinat C., Saengkhae C., Marbeuf-Gueye C., Garnier-Suillerot A. New insights into the P-glycoprotein-mediated effluxes of rhodamines. Eur. J. Biochem. 2003;270(3):476–485. doi: 10.1046/j.1432-1033.2003.03403.x. [DOI] [PubMed] [Google Scholar]
- 72.Nadakavukaren K.K., Nadakavukaren J.J., Chen L.B. Increased rhodamine 123 uptake by carcinoma cells. Cancer Res. 1985;45(12 Pt 1):6093–6099. [PubMed] [Google Scholar]
- 73.Dellinger M., Pressman B.C., Calderon-Higginson C. Structural requirements of simple organic cations for recognition by multidrug-resistant cells. Cancer Res. 1992;52(22):6385–6389. [PubMed] [Google Scholar]
- 74.Jara J.A., Castro-Castillo V., Saavedra-Olavarria J. Antiproliferative and uncoupling effects of delocalized, lipophilic, cationic gallic acid derivatives on cancer cell lines. Validation in vivo in singenic mice. J. Med. Chem. 2014;57(6):2440–2454. doi: 10.1021/jm500174v. [DOI] [PubMed] [Google Scholar]
- 75.Madak J.T., Neamati N. Membrane permeable lipophilic cations as mitochondrial directing groups. Curr. Top. Med. Chem. 2015;15(8):745–766. doi: 10.2174/1568026615666150302105622. [DOI] [PubMed] [Google Scholar]
- 76.Ristow M. Oxidative metabolism in cancer growth. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9(4):339–345. doi: 10.1097/01.mco.0000232892.43921.98. [DOI] [PubMed] [Google Scholar]
- 77.Dickey J.S., Gonzalez Y., Aryal B. Mito-tempol and dexrazoxane exhibit cardioprotective and chemotherapeutic effects through specific protein oxidation and autophagy in a syngeneic breast tumor preclinical model. PLoS One. 2013;8(8):e70575. doi: 10.1371/journal.pone.0070575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buondonno I., Gazzano E., Jean S.R. Mitochondria-targeted doxorubicin: a new therapeutic strategy against doxorubicin-resistant osteosarcoma. Mol. Cancer Ther. 2016;15(11):2640–2652. doi: 10.1158/1535-7163.MCT-16-0048. [DOI] [PubMed] [Google Scholar]
- 79.Xue X., You S., Zhang Q. Mitaplatin increases sensitivity of tumor cells to cisplatin by inducing mitochondrial dysfunction. Mol. Pharm. 2012;9(3):634–644. doi: 10.1021/mp200571k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Altman B.J., Stine Z.E., Dang C.V. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer. 2016;16(10):619–634. doi: 10.1038/nrc.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chun M.G., Shaw R.J. Cancer metabolism in breadth and depth. Nat. Biotechnol. 2013;31(6):505–507. doi: 10.1038/nbt.2611. [DOI] [PubMed] [Google Scholar]