Abstract
Background
Vitamin D status [25(OH)D] has recently been reported to be associated with altered cellular bioenergetic profiles of peripheral blood mononuclear cells (PBMCs). No study has tracked the seasonal variation of 25(OH)D and its putative influence on whole body energy metabolism, cellular bioenergetic profiles, inflammatory markers and clinical chemistry.
Material and methods
Whole body energy metabolism and substrate utilisation were measured by indirect calorimetry. PBMCs obtained from the same subjects were isolated from whole blood, counted and freshly seeded. Bioenergetic analysis (mitochondrial stress test and glycolysis stress test) was performed using the Seahorse XFe96 flux analyser. 25(OH)D was assessed using the Architect immunoassay method.
Results
25(OH)D increased by a median (IQR) of 14.40 (20.13) nmol/L (p<0.001) from winter to summer and was accompanied by significant improvements in indices of insulin sensitivity, McAuley's index (p=0.019) and quantitative insulin sensitivity check index (p=0.028). PBMC mitochondrial parameters basal respiration, non-mitochondrial respiration, ATP production, proton leak, and maximal respiration decreased in summer compared to winter. Similarly, PBMC glycolytic parameters glycolytic activity, glucose response, and glycolytic capacity were all reduced in summer compared to winter. There was also a trend for absolute resting metabolic rate (RMR) to decrease (p=0.066). Markers of systemic inflammation MCP-1, IL-6, IL-8, IL-10, and IL-12p70 decreased significantly in summer compared to winter. Participants who entered winter with a low 25(OH)D (<50 nmol/L), had the greatest alteration in bioenergetic parameters in summer, relative to those with winter 25(OH)D concentrations of 50–75 nmol/L or >75 nmol/L. The absolute change in 25(OH)D was not associated with altered bioenergetics.
Conclusion
Seasonal improvements in 25(OH)D was associated with reduced systemic inflammation, PBMC bioenergetic profiles and whole body energy metabolism. These observational changes in PBMC bioenergetics were most pronounced in those who had insufficient 25(OH)D in winter. The data warrants confirmation through cause and effect study designs.
Abbreviations: 2DG, 2-deoxyglucose; BHI, bioenergetic health index; FBG, fasting blood glucose; FM, fat mass; FFM, fat free mass; IPAQ, international physical activity questionnaire; McA, McAuleys index of insulin sensitivity; PBMCs, peripheral blood mononuclear cells; PPR, proton production rate; OCR, oxygen consumption rate; RQ, respiratory quotient; RMR, resting metabolic rate
Keywords: Peripheral blood mononuclear cells, Bioenergetics, Vitamin D, Season, Inflammation, Insulin sensitivity
Graphical abstract
Highlights
-
•
Inflammation and clinical biochemistry improved in summer versus winter.
-
•
Seasonal improvements in 25(OH)D modulated the bioenergetic profile of PBMCs.
-
•
Maintaining 25(OH)D >50 nmol/L may be important for bioenergetic function.
1. Introduction
Immunology and metabolism have, until recently, existed as two predominantly separate fields of research [1]. However, immune cell activation influences metabolic function in adipose and the liver, while the microenvironment within these tissues modulates immune cell function. Low-grade, systemic inflammation (‘metaflammation’) is linked to many types of chronic disease [2] including obesity and the metabolic syndrome [3]. The cytokines most widely studied in relation to obesity are IL-6 and TNF-α [3] and are consistently elevated in serum and adipose tissue derived from obese subjects [4].
Insufficient vitamin D status, determined by serum 25-hydroxyvitamin D [25(OH)D], is commonly observed worldwide, with the greatest levels of insufficiency occurring during winter months [5], [6], [7]. The level of 25(OH)D in humans and other animals is well known to rise in summer and decline in winter in response to seasonal variation in the intensity of solar UV light. In Australia, typical seasonal variations are 10–20 nmol/L [5], [6], [7]. However, there is a lack of reliable information to indicate whether seasonal variation in 25(OH)D positively or negatively influences health, or indeed has no significant impact [8]. Interestingly, this seasonal variation coincides with seasonal variations in blood pressure [9], HbA1C [10] and circulating lipids [11], indicating a potential association between seasonality of 25(OH)D and chronic metabolic disease.
Seasonal variation in energy demands and inflammation may co-exist. There is a seasonal variation in C-reactive protein (CRP) levels, with higher values observed during winter than in summer. Elevated CRP levels can be related to an increased risk of cardiovascular events, which are more prominent during the winter months [12]. Seasonal variations in the number of white blood cells and subtypes such as neutrophils, monocytes, lymphocytes, CD4+ T cells, CD8+ T cells, CD25+ T cells, CD20+ B cells, and serum IL-6 have also been reported [13]. Elevated inflammatory cytokines can damage cell function by promoting a reduction in mitochondrial DNA integrity and protein integrity, and can thus induce redox stress which may result in bioenergetic dysfunction [14]. We are aware of only one study that has examined whether the bioenergetic profile of peripheral blood mononuclear cells (PBMCs) was related to differences in inflammatory status; those data support an association of changes in interleukin-6 and bioenergetic profile [14]. Recently, we demonstrated that 25(OH)D below 50 nmol/L was associated with increased oxidative and glycolytic bioenergetic profile responses in PBMCs obtained from adults [15]. Whether change in 25(OH)D was associated with altered bioenergetic profiles remains to be elucidated. Other research groups have proposed that baseline 25(OH)D, change in vitamin status and the final concentration achieved, may well be crucial considerations in uncovering the extra-skeletal effects of vitamin D supplementation [16], [17].
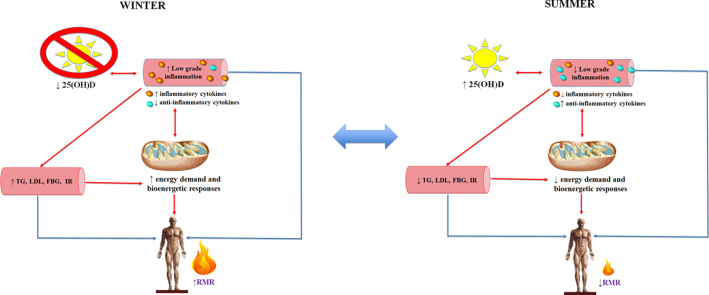
The aim of the present study was to track seasonal variations in 25(OH)D and investigate the influence on whole body energy metabolism, circulating PBMC bioenergetic profiles, and markers of systemic inflammation. To the best of our knowledge, the impact of seasonal variation in 25(OH)D on cellular energy demand and inflammation, and the potential flow through impact on whole body energy metabolism, has not been previously described. We examined the hypothesis that a lower 25(OH)D in winter may promote greater inflammation and insulin resistance; the attendant higher energetic cost would be reflected in higher resting metabolic rate (RMR) and increased PBMC bioenergetic parameters. Consequently, a seasonal increase in 25(OH)D would reverse these effects and be most evident in those who started with the lowest 25(OH)D (Fig. 1).
Fig. 1.
Seasonal change in 25(OH)D influences changes in inflammation, PBMC bioenergetics, and whole body energy metabolism. A lower 25(OH)D in winter may promote greater inflammation and insulin resistance; the attendant higher energetic cost would be reflected in higher resting metabolic rate (RMR) and increased PBMC bioenergetic parameters. Consequently, a seasonal increase in 25(OH)D would reverse these effects. Legend: 25(OH)D, 25-hydroxy vitamin D; FBG, fasting blood glucose; IR, insulin resistance; LDL, low-density lipoprotein cholesterol; RMR, resting metabolic rate; TG, triglycerides. ↓, decrease; ↑, increase.
2. Materials and methods
2.1. Participant recruitment
Participant recruitment and eligibility criteria have previously been reported [15]. Briefly, participants were Australians of European origin, aged between 20 and 70 years, not suffering from any current illness or infection requiring antibiotics, not taking medications that influenced mitochondrial function (insulin, HMG-CoA reductase inhibitors, thiazolidinedione's), or any of the following: parathyroid hormone (PTH) or its derivatives, calcitonin, hormone replacement therapy, corticosteroids, or vitamin D supplements. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Human Research Ethics Committee of Curtin University, Perth, Western Australia (Ethics approval number RDHS-13-15]. Written informed consent was obtained from all participants.
2.2. Period of observation
The data collection described below was conducted in Australian winter 2015 (July-September) and the protocol was repeated in Australian summer 2016 (February-March). Participants were instructed not to perform any strenuous activity the day before the experiment to avoid effects of physical activity on RMR. Data were collected at the Curtin University laboratory with participants in the fasted state.
2.2.1. Physical characteristics, body composition and physical activity
Weight was measured using an electronic platform balance (CW-11, Precision Balances Pty Ltd). Height was measured using a stadiometer fixed to a wall to the nearest 0.1 cm (Seca, Hamburg, Germany). Body composition was assessed by dual energy x-ray absorptiometry (Prodigy, Lunar Corp USA). Physical activity was determined from the short version of international physical activity questionnaire (IPAQ) [18].
2.2.2. Resting metabolic rate and forearm-fingertip gradient
RMR was measured by indirect calorimetry in the environmental chamber housed at the School of Public Health, Curtin University, Western Australia as per our established protocol [15]. The TrueOne system (Parvo Medics, USA) was calibrated with gas mixtures of known composition before each measurement session and regularly checked by 30 min ethanol burn tests. Mean and SD for six tests during the winter phase of the study was substrate oxidation=0.67±0.01. Mean and SD for seven tests during the summer phase of the study was substrate oxidation=0.68±0.01. Forearm-fingertip gradient (FFG), an indicator of peripheral vasoconstriction, was measured using iButtons (iButton type DS1921H-F#, Maxim Integrated Products). One iButton was placed on the ventral side of the left middle fingertip and a second iButton was located along the dorsal left forearm, midway between the elbow and wrist [19].
2.2.3. Blood collection and analysis
A fasting venous sample was obtained by an experienced phlebotomist. Blood from fasted participants was collected, processed, and serum stored at −80 °C, until analysis. Triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL) and fasting blood glucose (FBG) were determined using routine automated procedures on an Architect c16000 analyser that used specific enzyme-based colorimetric reagents (Abbott Diagnostics; CV<2%). Low-density lipoprotein cholesterol (LDL) was estimated using the Friedewald equation [20]. Fasting insulin was measured using an Architect i2000SR Analyser (Abbott Diagnostics; CV<3%). McAuley's index for insulin sensitivity (McA) was calculated from TG and insulin concentrations. Quantitative insulin sensitivity check index (QUICKI) was determined from FBG and insulin [21]. Parathyroid hormone (PTH) was determined using a Cobas e601 Analyser (Roche Diagnostics). 25(OH)D was measured by chemi-luminescent micro particle immunoassay (Architect 25-OH Vitamin D assay, Abbott Diagnostics). Inflammatory cytokines IL-6, IL-8, IL-10, IL-12p70 and TNF-α were measured using Human High Sensitivity T Cell kits and run on a MAGPIX® system (Merck Millipore, Germany). All samples were measured in duplicate, and the average of the two values was used for data analyses. CRP was measured using QuickRead Go CRP kits from Orian Diagnostica (Espoo, Finland). MCP-1 was measured by ELISA (elisakit.com, Australia).
2.2.4. Immune cell isolation, population determination, Seahorse XFe96 measurements and analysis
PBMCs were isolated and the population determined as previously described [22]. After recording the basal oxygen consumption rate, the Mito Stress Test inhibitor/activator injection strategy consisted of oligomycin (5 µM), FCCP (1.5 µM), and rotenone/antimycin A in combination (5 µM). Following the measurement of the basal proton production rate, the Glycolytic Stress Test injection strategy commenced with glucose (25 mM), then oligomycin (5 µM), followed by 200 mM 2-deoxyglucose (2DG). The bioenergetic health index (BHI) of each sample was calculated as previously described and no power function was applied to these parameters [15], [23].
2.3. Statistical analysis
Data are presented as median (IQR). Differences among seasons were compared using Wilcoxon non-parametric tests. Change variables were calculated as the summer value minus the winter value. To examine the influence of initial 25(OH)D on bioenergetics and inflammation change, we categorized our study sample into three groups based on cut offs for 25(OH)D of 50 nmol/L and 75 nmol/L. Participants with 25(OH)D <50 nmol/L formed Group 1, 50–75 nmol/L Group 2 and those with status ≥75 nmol/L were defined as Group 3. To examine the influence of final 25(OH)D achieved and absolute change in 25(OH)D on bioenergetics and inflammation change, we categorized our study sample into three tertiles. The three independent groups were compared using multivariate GLM adjusting for effects of change in fat mass (FM) (kg), fat-free mass (FFM) (kg), PTH (pmol/L), and QUICKI on all bioenergetics and inflammatory parameters. All statistical calculations were performed using SPSS for Windows version 24.0 (SPSS, Chicago, IL). Statistical significance was accepted at p<0.05.
3. Results
3.1. Demographic characteristics of participants
The participant cohort consisted of 30 adults aged between 20 and 69 years.
There were 15 males and 15 females, 14 of which were lean (BMI<25 kg/m2) and 16 were overweight or obese (BMI≥25 kg/m2).
3.2. Seasonal influence on 25(OH)D, body composition and physical activity
Median 25(OH)D significantly increased from winter 62.85 (30.35) nmol/L to summer 73.75 (34.05) nmol/L, p<0.001. Initial winter 25(OH)D was strongly associated with final summer status unadjusted (r=0.711, p<0.001) and adjusted for PTH, QUICKI, FFM, FM (r=0.766, p<0.001). FFM (kg) did not change between seasons [winter 53.31 (18.81) kg vs summer 53.89 (20.25) kg, p=0.299]. Similarly, FM (kg) was not different between seasons [winter 22.15 (11.20) kg vs summer 22.68 (9.10) kg, p=0.136], nor was IPAQ [winter 1870 (2982) vs summer 1780 (4155), p=0.922].
3.3. Seasonal influence on whole body and PBMC bioenergetics
There was a trend for absolute RMR to decrease (p=0.060) and RQ to increase (p=0.066) from winter to summer (Table 1). PBMC basal respiration, non-mitochondrial respiration, ATP production, proton leak, and maximal respiration were significantly lower in summer than winter. Background glycolytic activity, glucose response and glycolytic capacity were also reduced over the same period (Table 1).
Table 1.
Whole body and cellular bioenergetics in winter and summer.
| Characteristic | Winter | Summer | P value | Direction & degree of change (%) |
|---|---|---|---|---|
| Whole body energy metabolism markers | ||||
| RMR (kJ/d) | 6107 (1707) | 5915 (2661) | 0.060 | ↓ 3 |
| RQ | 0.83 (0.04) | 0.85 (0.04) | 0.066 | ↑ 2 |
| Mito stress test parameters | ||||
| Basal respiration (pmol O2/min/350,000 cells) | 47.22 (25.17) | 36.15 (16.82) | 0.003 | ↓ 23 |
| Non mitochondrial respiration (pmol O2/min/350,000 cells) | 36.10 (12.16) | 28.86 (10.33) | 0.005 | ↓ 20 |
| ATP production (pmol O2/min/350,000 cells) | 33.79 (23.80) | 29.52 (13.66) | 0.009 | ↓ 14 |
| Proton leak (pmol O2/min/350,000 cells) | 15.71 (5.59) | 11.57 (4.85) | 0.015 | ↓ 26 |
| Maximal respiration (pmol O2/min/350,000 cells) | 212.67 (139.58) | 164.71 (75.54) | 0.027 | ↓ 24 |
| Coupling efficiency (pmol O2/min/350,000 cells) | 73.22 (12.80) | 73.57 (10.98) | 0.992 | 0 |
| Reserve capacity (pmol O2/min/350,000 cells) | 159.42 (119.35) | 127.41 (65.52) | 0.072 | ↓ 20 |
| BHI | 10.55 (9.31) | 10.37 (6.04) | 0.491 | ↓ 11 |
| Glycolysis stress test parameters | ||||
| Background glycolysis (pmol H+/min/350,000 cells) | 19.65 (14.88) | 15.88 (7.46) | 0.011 | ↓ 19 |
| Glucose response (pmol H+/min/350,000 cells) | 28.36 (13.41) | 20.55 (11.43) | 0.002 | ↓ 28 |
| Glycolytic reserve (pmol H+/min/350,000 cells) | 30.79 (18.68) | 28.00 (10.28) | 0.399 | ↓ 9 |
| Glycolytic capacity (pmol H+/min/350,000 cells) | 59.78 (25.48) | 47.29 (19.81) | 0.004 | ↓ 21 |
Data are presented as median (IQR).
P value determined by Wilcoxon non-parametric tests.
ATP, adenosine triphosphate; BHI, bioenergetics health index; RMR, resting metabolic rate; RQ, respiratory quotient.
↓, decrease in summer compared to winter; ↑, increase in summer compared to winter.
3.4. Seasonal influence on inflammation and clinical biochemistry
All inflammatory markers significantly decreased from winter to summer, with the exception of CRP which did not change (Table 2). There were no significant changes in the percentages of lymphocytes (winter 66.32 (9.58)%; summer 65.91 (9.94)%; change −0.41 (12.51)%, p=0.860) or monocytes (winter 11.00 (4.21)%; summer 9.59 (3.32)%; change −1.01 (5.28)%, p=0.305). From winter to summer, FBG, TG, TC, LDL and HDL decreased, while indices of insulin sensitivity, QUICKI and McA significantly increased (Table 2). Forearm to fingertip skin temperature gradient (FFG) did not significantly change between seasons [winter −0.02 (4.84) °C vs summer −0.85 (3.92) °C, p=0.098].
Table 2.
Inflammatory markers and clinical biochemistry in winter and summer.
| Characteristic | Winter | Summer | P value | Direction & degree of change (%) |
|---|---|---|---|---|
| Inflammatory markers | ||||
| CRP (mg/L) | 1.00 (1.65) | 0.75 (1.33) | 0.565 | ↓ 25 |
| MCP-1 (pg/mL) | 190.48 (135.80) | 133.78 (58.62) | <0.001 | ↓ 30 |
| TNF-α (pg/mL) | 11.72 (4.12) | 3.31 (2.13) | <0.001 | ↓ 71 |
| IL-6 (pg/mL) | 2.39 (1.99) | 0.66 (0.80) | <0.001 | ↓ 72 |
| IL-8 (pg/mL) | 9.29 (4.47) | 2.04 (1.70) | <0.001 | ↓ 78 |
| IL-10 (pg/mL) | 21.96 (21.22) | 3.73 (6.54) | <0.001 | ↓ 83 |
| IL-12 (pg/mL) | 8.46 (5.26) | 1.84 (2.17) | <0.001 | ↓ 78 |
| Clinical biochemistry | ||||
| FBG (mmol/L) | 5.20 (0.63) | 4.50 (1) | <0.001 | ↓ 13 |
| Insulin (uU/mL) | 4.75 (3.65) | 4.95 (3.5) | 0.552 | ↑ 4 |
| QUICKI | 0.38 (0.04) | 0.40 (0.06) | 0.028 | ↑ 5 |
| McA | 9.17 (2.33) | 9.83 (3.64) | 0.019 | ↑ 7 |
| TG (mmol/L) | 0.88 (0.77) | 0.80 (0.51) | 0.015 | ↓ 9 |
| TC (mmol/L) | 4.96 (1.58) | 4.20 (1.87) | <0.001 | ↓ 15 |
| LDL (mmol/L) | 2.82 (1.58) | 2.58 (1.44) | <0.001 | ↓ 9 |
| HDL (mmol/L) | 1.49 (0.57) | 1.26 (0.33) | <0.001 | ↓ 15 |
Data are presented as median (IQR).
P value determined by Wilcoxon non-parametric tests.
CRP, C reactive protein; FBG, fasting blood glucose; IL, interleukin; HDL, high density lipoprotein cholesterol.; LDL, low density lipoprotein cholesterol; McA, McAuleys index of insulin sensitivity; MCP, macrophage chemoattractant protein; QUICKI, quantitative insulin sensitivity check index; TC, total cholesterol; TG, triglycerides; TNF-α, tumour necrosis factor alpha.
↓, decrease in summer compared to winter; ↑, increase in summer compared to winter
3.5. The influence of baseline (winter) 25(OH)D on change in seasonal bioenergetics and inflammation
When the patient data was analysed according to baseline (winter) 25(OH)D, with adjustment for change in FM, FFM, PTH and QUCKI, significant differences in bioenergetic parameters such as basal respiration (p=0.001), non-mitochondrial respiration (p=0.021), ATP production (p=0.001), maximal respiration (p=0.022), coupling efficiency (p=0.047), reserve capacity (p=0.047), background glycolysis (p=0.002), glycolytic reserve (p=0.039) and glycolytic capacity (p=0.026) were observed across the 25(OH)D groups (Table 3). Group 1 (<50 nmol/L) had the greatest reduction in bioenergetic parameters compared to the other two groups, and no differences were found between Group 2 and Group 3 in any of the cellular bioenergetic parameters measured (Table 3). No differences in inflammatory marker change among the three 25(OH)D groups was found after adjustment for changes in FFM, FM, PTH and QUICKI (data not shown).
Table 3.
Summer change in adjusted bioenergetic measurements according to winter vitamin D status at start.
| Characteristic | <50 nmol/L at start Group 1 n=8 | 50–75 nmol/L at start Group 2 n=13 | ≥75 nmol/L at start Group 3 n=9 | P value |
|---|---|---|---|---|
| 25(OH)D (nmol/L) | 42.76 (3.78) | 62.18 (6.13) | 86.39 (13.26) | 0.416 |
| Range of final 25(OH)D achieved (nmol/L) | 37–48.3 | 51.5–72.4 | 76.8–116.5 | – |
| Whole body energy metabolism markers | ||||
| ∆RMR (kJ/d) | −291 (797) | −342 (782) | −216 (742) | 0.935 |
| ∆RQ | +0.03 (0.04)* | −0.001 (0.03) | +0.02 (0.04) | 0.161 |
| Mito stress test | ||||
| ∆Basal respiration (pmol O2/min/350,000 cells) | −42.91 (21.43)a* | −6.44 (21.03)b,c | −1.37 (19.95)c | 0.001 |
| ∆Non-mitochondrial respiration (pmol O2/min/350,000 cells) | −20.63 (14.27)a* | −5.56 (14.01) | −1.15 (13.29)b | 0.021 |
| ∆ATP production (pmol O2/min/350,000 cells) | −36.67 (19.20)a* | −4.30 (18.85)b,c | +1.91 (17.87)c | 0.001 |
| ∆Proton leak (pmol O2/min/350,000 cells) | −6.42 (5.96)* | −2.41 (5.85) | −0.95 (5.55) | 0.159 |
| ∆Maximal respiration (pmol O2/min/350,000 cells) | −139.25 (105.72)a* | −21.70 (103.76) | −1.67 (98.41)b | 0.022 |
| ∆Coupling efficiency (%) | −6.67 (12.79)a | −0.81 (12.55) | +9.13 (11.91)b* | 0.047 |
| ∆Reserve capacity (pmol O2/min/350,000 cells) | −102.99 (93.61)* | −10.92 (91.88) | +6.29 (87.14) | 0.047 |
| ∆BHI | −8.10 (11.26)* | +1.69 (11.05) | +1.62 (10.48) | 0.129 |
| Glycolysis stress test | ||||
| ∆Background glycolysis (pmol H+/min/350,000 cells) | −15.03 (9.33)a* | −3.94 (8.56)b,c | +2.00 (8.24)b,c | 0.002 |
| ∆Glucose response (pmol H+/min/350,000 cells) | −14.28 (12.27)* | −14.74 (18.03)* | −4.45 (20.30) | 0.355 |
| ∆Glycolytic reserve (pmol H+/min/350,000 cells) | −18.41 (18.62)a* | −1.74 (18.01) | +6.27 (17.08)b | 0.039 |
| ∆Glycolytic capacity (pmol H+/min/350,000 cells) | −38.05 (29.56)a* | −16.44 (28.73)* | +3.78 (27.12)b | 0.026 |
Data are presented as mean (SD) after adjustment for change in PTH, FFM, FM and QUICKI.
Identical superscripts denote no significant difference between groups. Different superscripts denote significant differences between groups.
25(OH)D, 25-hydroxy vitamin D; ATP, adenosine triphosphate; BHI, Bioenergetic Health Index; RMR, resting metabolic rate; RQ, respiratory quotient.
∆, change.
Denotes significant within group differences.
Within the low 25(OH)D group (Group 1), significant decreases in inflammatory markers MCP (p=0.027), IL-6 (p<0.001), IL-8 (p<0.001), IL-10 (p<0.001), IL-12p70 (p<0.001), and TNF-α (p<0.001) were found in summer. In addition, oxidative phosphorylation parameters decreased in this group in summer including basal respiration (p<0.001), non-mitochondrial respiration (p=0.011), ATP production (p<0.001), proton leak (p=0.024), maximal respiration (p=0.001), reserve capacity (p=0.002), and BHI (p=0.020). Glycolytic parameters background glycolysis (p=0.004), glucose response (p=0.013), glycolytic reserve (p=0.024), and glycolytic capacity (p=0.003) also decreased. In those with 25(OH)D between 50 nmol/L and 75 nmol/L (Group 2), insulin sensitivity increased as determined by QUICKI (p=0.014) and McA (p=0.011), while bioenergetics parameter glucose response and glycolytic capacity and inflammatory markers MCP (p=0.049), IL-6 (p<0.001), IL-8 (p<0.001), IL-10 (p=0.001), and TNF-α (p<0.001) decreased in summer. In those with 25(OH)D >75 nmol/L (Group 3), inflammatory markers IL-6 (p=0.007), IL-8 (p<0.001), IL-10 (p=0.027), IL-12p70 (p=0.002), and TNF-α (p<0.001) decreased in summer but there were no significant alterations in bioenergetics.
3.6. The influence of final 25(OH)D achieved (summer) on change in seasonal bioenergetics and inflammation
After adjustment for change in FM, FFM, PTH and QUCKI, significant differences across tertiles of final 25(OH)D achieved were found in bioenergetic parameters basal respiration (p=0.016), ATP production (p=0.019), proton leak (p=0.013), and background glycolysis (p=0.023) (Table 4). Tertile 1, representing the lowest tertile of summer 25(OH)D, had the greatest reduction in bioenergetics compared to the other two groups; no differences were found between Tertile 2 and Tertile 3 in any of the cellular bioenergetic parameters (Table 4). There were no significant between group differences among inflammatory markers (data not shown).
Table 4.
Summer change in adjusted bioenergetic measurements according to tertiles of final vitamin D status achieved.
| Characteristic | Final 25(OH)D Tertile 1 n=10 | Final 25(OH)D Tertile 2 n=10 | Final 25(OH)D Tertile 3 n=10 | P value |
|---|---|---|---|---|
| Final 25(OH)D (nmol/L) | 54.31 (7.45)a | 74.16 (8.06)b | 101.36 (7.17)c | <0.001 |
| Range of final 25(OH)D achieved (nmol/L) | 40.7–63 | 63.2–91.9 | 93.3–112.40 | – |
| Whole body energy metabolism markers 3) | ||||
| ∆RMR (kJ/d) | −110 (738) | −654 (700)* | −116 (726) | 0.166 |
| ∆RQ | +0.02 (0.04) | +0.01 (0.04) | +0.01 (0.04) | 0.575 |
| Mito stress test | ||||
| ∆Basal respiration (pmol O2/min/350,000 cells) | −33.63 (24.40)a* | −16.83 (23.14)* | +2.37 (23.98)b | 0.016 |
| ∆Non-mitochondrial respiration (pmol O2/min/350,000 cells) | −13.96 (16.12)* | −9.65 (15.28) | −3.11 (15.84) | 0.361 |
| ∆ATP production (pmol O2/min/350,000 cells) | −27.60 (22.24)a* | −13.71 (21.09) | +4.24 (21.86)b | 0.019 |
| ∆Proton leak (pmol O2/min/350,000 cells) | −7.89 (5.33)a* | −1.48 (5.06)b,c | +0.43 (5.24)c | 0.013 |
| ∆Maximal respiration (pmol O2/min/350,000 cells) | −102.28 (112.15)* | −65.63 (106.35) | +15.70 (110.24) | 0.087 |
| ∆Coupling efficiency (%) | −4.33 (13.56) | −0.66 (12.86) | +7.34 (13.33) | 0.185 |
| ∆Reserve capacity (pmol O2/min/350,000 cells) | −77.54 (98.02)* | −40.54 (92.95) | +16.21 (96.35) | 0.139 |
| ∆BHI | −4.08 (11.30) | −4.49 (10.72) | +4.74 (11.11) | 0.145 |
| Glycolysis stress test | ||||
| ∆Background glycolysis (pmol H+/min/350,000 cells) | −13.00 (9.67)a* | −3.04 (9.38)b,c | +0.19 (10.03)c | 0.023 |
| ∆Glucose response (pmol H+/min/350,000 cells) | −14.46 (19.37)* | −14.64 (18.79)* | −6.51 (20.08) | 0.628 |
| ∆Glycolytic reserve (pmol H+/min/350,000 cells) | −12.06 (20.28) | 0.70 (19.40) | −0.29 (20.77) | 0.347 |
| ∆Glycolytic capacity (pmol H+/min/350,000 cells) | −29.06 (31.86)* | −6.51 (30.90) | −5.53 (33.03) | 0.323 |
Data are presented as mean (SD) after adjustment for change in PTH, FFM, FM and QUICKI.
Identical superscripts denote no significant difference between groups. Different superscripts denote significant differences between groups.
25(OH)D, 25-hydroxy vitamin D; ATP, adenosine triphosphate; BHI, Bioenergetic Health Index; RMR, resting metabolic rate; RQ, respiratory quotient.
∆, change.
Denotes significant within group differences.
The lowest tertile of summer 25(OH)D achieved (Tertile 1), demonstrated significant decreases in basal respiration, non-mitochondrial respiration, ATP production, proton leak, maximal respiration, reserve capacity, background glycolysis, glucose response, glycolytic reserve and glycolytic capacity in comparison to winter bioenergetic parameters. Decreases in RMR, basal and glucose response occurred in the middle tertile (Tertile 2). In those who achieved the greatest summer 25(OH)D (Tertile 3), no changes in bioenergetics with season were observed (Table 4). All tertiles displayed significant reductions in TNF- α, IL-6, IL-8, IL-10 and IL-12p70 in summer, with Tertile 2 also demonstrating a reduction in MCP-1 (data not shown).
3.7. The influence of absolute change in 25(OH)D on change in seasonal bioenergetics and inflammation
After adjustment for change in FM, FFM, PTH and QUCKI, no significant differences across tertiles of change in 25(OH)D were found in any bioenergetic parameters (data not shown). A smaller reduction in IL-10 in those with the greatest change in 25(OH)D (Tertile 3) compared to the other two groups occurred [Tertile 3+∆25(OH)D (nmol/L) +30.89 (8.95), ∆IL-10 (pg/mL) −7.02 (12.95) vs Tertile 1 ∆25(OH)D (nmol/L) −1.63 (8.06), ∆IL-10 (pg/mL) −19.25 (11.66) (p=0.049); Tertile 3+∆25(OH)D (nmol/L) +30.89 (8.95), ∆IL-10 (pg/mL) −7.02 (12.95); (p=0.064 vs Tertile 2+∆25(OH)D (nmol/L) +12.14 (8.23); ∆IL-10 (pg/mL) −21.35 (11.91 (p=0.026)]. All tertiles displayed significant reductions in TNF- α, IL-6, IL-8, IL-10, and IL-12p70, with the exception of Tertile 3 where no significant reduction in IL-10 was observed. Tertile 3 also demonstrated a reduction in MCP-1 (data not shown).
3.8. Correlations between inflammation, bioenergetics, insulin sensitivity and 25(OH)D
The seasonal increase in 25(OH)D was positively associated with IL-10 (r=0.389, p=0.033). As RQ decreased, McA increased (r=−0.367, p=0.046), as did IL-10 (r=−0.611, p<0.001). RMR was positively associated with CRP (r=0.379, p=0.039). Non-mitochondrial respiration was positively associated with CRP (r=0.570, p=0.001) and TNF-α (r=0.411, p=0.024). After adjustment for FM, FFM, PTH, and QUICKI, RMR was positively associated with TNF-α (r=0.540, p=0.006) and CRP (r=0.462, p=0.023). Non-mitochondrial respiration was positively associated with CRP (r=0.668, p<0.001) and TNF- α (r=0.452, p=0.027).
4. Discussion
Recently, we demonstrated that in vivo circulating 25(OH)D, a proxy for vitamin D status, was associated with ex-vivo PBMC cell bioenergetic capacity and activity. Specifically, 25(OH)D below 50 nmol/L was associated with increased oxidative and glycolytic bioenergetic profile responses in PBMCs obtained from adults and we speculated that this was due to inflammatory activation of these cells [15]. The present study investigated whether seasonal change in 25(OH)D was associated with altered bioenergetic and inflammatory profiles. We also investigated whether baseline (winter) 25(OH)D, the final 25(OH)D achieved in summer, and absolute change in vitamin status mediated the associations between 25(OH)D, bioenergetics and inflammation.
The winter to summer increase in 25(OH)D observed was typical of Australia [5], [6], [7] and we document that seasonal variation in circulating 25(OH)D, was associated with a change in ex vivo PBMC bioenergetic profiles. A reduction in non-mitochondrial respiration was associated with a reduction in CRP and TNF-α. This reduction in non-mitochondrial respiration may represent a reduction in reactive oxygen species generation or reduced demand for use in cytochrome P-450 monooxygenases, cyclooxygenases, and pro-inflammatory NADPH oxidases. The concurrent observation of lowered bioenergetic parameters and reduced inflammation in summer as compared to winter supports our hypothesis that a lower 25(OH)D in winter may result in greater inflammation which has an energetic cost that could account for part of the higher RMR in winter (Fig. 1). It is clear that those who had an initial low 25(OH)D (<50 nmol/L) were associated with decreased bioenergetic parameters from winter to summer, while individuals with higher initial status (>50 nmol/L) did not. In contrast, multiple systemic inflammatory markers decreased within each group suggesting higher levels of 25(OH)D may be required to dampen inflammation. Taken together, these results may suggest that the optimal 25(OH)D depends on the target variable.
Those who started with lower 25(OH)D in winter were likely to demonstrate a lower status in summer compared to those who started with greater 25(OH)D. We found that those who began the study with a low 25(OH)D in winter (<50 nmol/L), were associated with reduced bioenergetic parameters (to a greater extent) in summer compared to the other two groups (50–75 nmol/L and >75 nmol/L). We also found that in summer, those who were in the lowest tertile of final 25(OH)D achieved a reduced bioenergetic profile, while those in the middle quartile reduced only some bioenergetic parameters, and those in the upper quartile demonstrated no significant change in bioenergetics with season. Taken together, these results suggest that there is a 25(OH)D level which is associated with change in bioenergetic parameters and above this, bioenergetics do not change with season. To further these findings, we also investigated whether absolute change in 25(OH)D may be important in the association between bioenergetics, inflammation and 25(OH)D. Although we found no relationship between these variables, the results are none-the-less interesting and warrant further investigation through cause-and-effect study designs.
Although three of the four bioenergetic parameters used to calculate BHI were associated with significant reductions from winter to summer, BHI remained similar between seasons. Our observations of a significantly decreased ATP production and non-significant decreased reserve capacity [numerator term] were cancelled out by the decreased proton leak and non-mitochondrial respiration [denominator term], such that no significant effect across seasons was observed. It is unlikely that the lack of significant seasonal variation in BHI is due to sample size, since a very minimal 1.71% difference between winter and summer values was found. It is possible that a more refined BHI calculation which used exponents to modify the relative weighting of the respiratory parameters may be more appropriate to reflect seasonal difference [24]. We [19] and others [20] have not adopted the use of power functions, as there is insufficient data in the field to be confident that such power functions actually apply to the individuals under study. We also acknowledge that there may be several possible variants for a BHI calculation, however, developing such an equation was outside the scope of this manuscript.
As reductions in PBMC bioenergetic parameters were apparent with season, it is not surprising that a change in the same direction occurred in whole body energy metabolism. The trend towards a lower RMR in summer, reflected other longitudinal studies where a greater RMR was observed in winter compared to summer [4], [5], [6], [7]. A seasonal change in RMR may be explained by several mechanisms including indoor temperature at measurement, outside ambient temperature, and level of thyroid hormones. RMR, is influenced by environmental temperature, specifically room temperature, wherein RMR is higher when measured in a cold environment [24]. Our results are not an artefact of indoor temperature during RMR measurement as the indoor temperature was set to 25 °C on both measurement occasions and patients were allowed to equilibrate prior to measurement. There was, however, an average difference in ambient temperatures between winter and summer in Perth, WA, of ~27 °C. The existence of other factors such as thyroid hormone levels may control modifications of RMR in the normal population throughout the year [25]. Serum levels of thyroid hormones T3 and T4 have been shown to decrease in summer compared to winter [26]. It is likely that a complex interplay of mechanisms are responsible for the change in RMR observed with season.
There are several limitations of study. We acknowledge that our study design does not allow demonstration of a causal effect of 25(OH)D on bioenergetics and inflammation. Also, we cannot rule out potential changes to energy intake and food intake that are known to influence systemic inflammation. However, given the lack of difference in body composition, we are confident that participants were in energy balance. The samples used contained a heterogeneous population of immune cells, with each subpopulation known to have a unique bioenergetic profile [27], [28]. The use of a mixed population is likely to reflect the metabolic state of the human system as immune cells interact with one another in vivo. Moreover our method of isolation results in minimal platelet contamination with no measurable oxygen consumption [15]. However, we accept the value of future studies examining the influence of 25(OH)D on bioenergetic parameters in isolated and purified populations of immune cells. Furthermore, different cell types and the subsets within each immune cell type display a unique seasonal variation [29], [30]. As it has been demonstrated, that CD4+ and CD8+ T cells reveal a reduced capacity to produce pro-inflammatory cytokines in summer [31], whether the seasonal variation in 25(OH)D in vivo throughout the year is associated with changes in bioenergetics through changes in T cell compartment or other immune cell subsets remains to be elucidated. Knowledge of metabolic control of immunity is primarily derived from cultured cells exposed to potent stimuli, such as LPS, rather than stimulating them with physiologic ligands that might activate cells in vivo. Therefore, our understanding of immune cell metabolism is still in its infancy and merits further investigation. Future intervention trials should investigate our hypothesis that 25(OH)D should remain above 50 nmol/L throughout the year, and whether very high 25(OH)D has deleterious health effects as a U-shaped relationship is not uncommon between health indicators and dietary/nutritional components. We also recommend that future studies of larger sample size investigate whether changes in immune cell subsets explains the relationships between 25(OH)D, inflammation and bioenergetics that we have observed.
In conclusion, this study extends our previous cross sectional demonstration that PBMC bioenergetics varies with 25(OH)D (15). Seasonal increases in 25(OH)D significantly reduce bioenergetics such that greater improvement was seen in those who normalized their status to ~ 50 nmol/L, while those who achieved values >50 nmol/L showed no further improvements. Future intervention trials are required to establish a potential cause-and-effect relationship between 25(OH)D and altered cellular bioenergetic parameters.
Author contributorship
The present work was designed by KNK, MJS, PN and EKC. Initial manuscript preparation and draft was undertaken by EKC and revised by KNK, MJS, PN, JR and RR. Patients were recruited by EKC and body composition was assessed by EKC. PBMC isolation was performed by JR, KNK, RR and EKC. Bioenergetics parameters were measured by EKC. Data analysis and statistical analysis were made by EKC, KNK, and MJS. Figure preparation was made by EKC. Supervision of the manuscript preparation was the responsibility of PN and MJS. All authors approved the final version of the paper.
Funding
The present work was supported by competitive research funds provided internally by the Schools of Biomedical Sciences and Public Health in the Faculty of Health Sciences, Curtin University.
Acknowledgments
The authors thank the School of Biomedical Sciences and School of Public Health in the Faculty of Health Sciences at Curtin University for research support and CHIRI for access to technology platforms and research equipment. EKC is the recipient of an Australian postgraduate scholarship award (APA).
References
- 1.Curi R., de Siquerira Mendes R., de Campos Crispin L.A., Norata G.D., Coccuzzo Sampaio S., Newsholme P. A past and present overview of macrophage metabolism and functional outcomes. Clin. Sci. 2017:1–14. doi: 10.1042/CS20170220. (in press) [DOI] [PubMed] [Google Scholar]
- 2.Egger G., Dixon J. Non-nutrient causes of low-grade, systemic inflammation: support for a 'canary in the mineshaft' view of obesity in chronic disease. Obes. Rev. 2011;12(5):339–345. doi: 10.1111/j.1467-789X.2010.00795.x. [DOI] [PubMed] [Google Scholar]
- 3.Alam I., Lewis K., Stephens J.W., Baxter J.N. Obesity, metabolic syndrome and sleep apnoea: all pro-inflammatory states. Obes. Rev. 2007;8(2):119–127. doi: 10.1111/j.1467-789X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 4.Cottam D.R., Mattar S.G., Barinas-Mitchell E., Eid G., Kuller L., Kelley D.E., Schauer P.R. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes. Surg. 2004;14(5):589–600. doi: 10.1381/096089204323093345. [DOI] [PubMed] [Google Scholar]
- 5.Gill T.K., Hill C.L., Shanahan E.M., Taylor A.W., Appleton S.L., Grant J.F., Shi Z., Grande E.D., Price K., Adams R.J. Vitamin D levels in an Australian population. BMC Public Health. 2014;14:1001. doi: 10.1186/1471-2458-14-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pittaway J.K., Ahuja K.D.K., Beckett J.M., Bird M.-L., Robertson I.K., Ball M.J. Make vitamin D while the sun shines, take supplements when it doesn′t: a longitudinal, observational study of older adults in Tasmania, Australia. PLoS One. 2013;8(3):e59063. doi: 10.1371/journal.pone.0059063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowson C.A., McGrath J.J., Ebeling P.R., Haikerwal A., Daly R.M., Sanders K.M., Seibel M.J., Mason R.S. Vitamin D and health in adults in Australia and New Zealand: a position statement. Med. J. Aust. 2012;196(11):686–687. doi: 10.5694/mja11.10301. [DOI] [PubMed] [Google Scholar]
- 8.Lucas R., Neale R. What is the optimal level of vitamin D? – separating the evidence from the rhetoric. Aust. Fam. Physician. 2014;43(3):119–122. [PubMed] [Google Scholar]
- 9.Rostand S.G. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30(2):150–156. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 10.Tseng C.-L., Brimacombe M., Xie M., Rajan M., Wang H., Kolassa J., Crystal S., Chen T.-C., Pogach L., Safford M. Seasonal patterns in monthly hemoglobin A1c values. Am. J. Epidemiol. 2005;161(6):565–574. doi: 10.1093/aje/kwi071. [DOI] [PubMed] [Google Scholar]
- 11.Ockene I.S., Chiriboga D.E., Stanek E.J., 3rd, Harmatz M.G., Nicolosi R., Saperia G., Well A.D., Freedson P., Merriam P.A., Reed G. Seasonal variation in serum cholesterol levels: treatment implications and possible mechanisms. Arch. Intern. Med. 2004;164(8):863–870. doi: 10.1001/archinte.164.8.863. [DOI] [PubMed] [Google Scholar]
- 12.Sung K.C. Seasonal variation of C-reactive protein in apparently healthy Koreans. Int. J. Cardiol. 2006;107(3):338–342. doi: 10.1016/j.ijcard.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 13.Maes M., Stevens W., Scharpe S., Bosmans E., De Meyer F., D'Hondt P., Peeters D., Thompson P., Cosyns P., De Clerck L. Seasonal variation in peripheral blood leukocyte subsets and in serum interleukin-6, and soluble interleukin-2 and -6 receptor concentrations in normal volunteers. Experientia. 1994;50(9):821–829. doi: 10.1007/BF01956463. [DOI] [PubMed] [Google Scholar]
- 14.Tyrrell D.J., Bharadwaj M.S., Van Horn C.G., Marsh A.P., Nicklas B.J., Molina A.J. Blood-cell bioenergetics are associated with physical function and inflammation in overweight/obese older adults. Exp. Gerontol. 2015;70:84–91. doi: 10.1016/j.exger.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calton E.K., Keane K.N., Soares M.J., Rowlands J., Newsholme P. Prevailing vitamin D status influences mitochondrial and glycolytic bioenergetics in peripheral blood mononuclear cells obtained from adults. Redox Biol. 2016;10:243–250. doi: 10.1016/j.redox.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannell J.J., Grant W.B., Holick M.F. Vitamin D and inflammation. Dermatol. Endocrinol. 2014;6(1):e983401. doi: 10.4161/19381980.2014.983401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calder P.C., Ahluwalia N., Brouns F., Buetler T., Clement K., Cunningham K., Esposito K., Jonsson L.S., Kolb H., Lansink M. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011;106(Suppl 3):S5–S78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- 18.Lee P.H., Macfarlane D.J., Lam T.H., Stewart S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int. J. Behav. Nutr. Phys. Act. 2011;8:115. doi: 10.1186/1479-5868-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calton E.K., Soares M.J., James A.P., Woodman R.J. The potential role of irisin in the thermoregulatory responses to mild cold exposure in adults. Am. J. Hum. Biol. 2016 doi: 10.1002/ajhb.22853. [DOI] [PubMed] [Google Scholar]
- 20.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 21.Lorenzo C., Haffner S.M., Stancakova A., Laakso M. Relation of direct and surrogate measures of insulin resistance to cardiovascular risk factors in nondiabetic finnish offspring of type 2 diabetic individuals. J. Clin. Endocrinol. Metab. 2010;95(11):5082–5090. doi: 10.1210/jc.2010-1144. [DOI] [PubMed] [Google Scholar]
- 22.Keane K.N., Calton E.K., Cruzat V.F., Soares M.J., Newsholme P. The impact of cryopreservation on human peripheral blood leucocyte bioenergetics. Clin. Sci. 2015;128(10):723–733. doi: 10.1042/CS20140725. [DOI] [PubMed] [Google Scholar]
- 23.Chacko B.K., Zhi D., Darley-Usmar V.M., Mitchell T. The Bioenergetic Health Index is a sensitive measure of oxidative stress in human monocytes. Redox Biol. 2016;8:43–50. doi: 10.1016/j.redox.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pathak K., James A.P., Soares M.J. thermogenic responses to mild cold exposure in overweight and obese individuals. J. Nutr. Intermed. Metab. 2014;1:52–53. [Google Scholar]
- 25.Kashiwazaki H. Seasonal fluctuation of BMR in populations not exposed to limitations in food availability: reality or illusion? Eur. J. Clin. Nutr. 1990;44(Suppl 1):85–93. [PubMed] [Google Scholar]
- 26.Smals A.G., Ross H.A., Kloppenborg P.W. Seasonal variation in serum T3 and T4 levels in man. J. Clin. Endocrinol. Metab. 1977;44(5):998–1001. doi: 10.1210/jcem-44-5-998. [DOI] [PubMed] [Google Scholar]
- 27.Chacko B.K., Kramer P.A., Ravi S., Johnson M.S., Hardy R.W., Ballinger S.W., Darley-Usmar V.M. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab. Investig. 2013;93(6):690–700. doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramer P.A., Ravi S., Chacko B., Johnson M.S., Darley-Usmar V.M. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol. 2014;2:206–210. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu B., Taioli E. Seasonal variations of complete blood count and inflammatory biomarkers in the us population – analysis of NHANES data. PLoS One. 2015;10(11):e0142382. doi: 10.1371/journal.pone.0142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paglieroni T.G., Holland P.V. Circannual variation in lymphocyte subsets, revisited. Transfusion. 1994;34(6):512–516. doi: 10.1046/j.1537-2995.1994.34694295067.x. [DOI] [PubMed] [Google Scholar]
- 31.Khoo A.-L., HJPM Koenen, LYA Chai, FCGJ Sweep, Netea M.G., van der Ven A.J.A.M., Joosten I. Seasonal variation in vitamin D3 levels Is paralleled by changes in the peripheral blood human T cell compartment. Plos One. 2012;7(1):e29250. doi: 10.1371/journal.pone.0029250. [DOI] [PMC free article] [PubMed] [Google Scholar]