Abstract
The involvement of angiogenesis in disease and its potential as a therapeutic target have been firmly established over recent decades. Endothelial cells (ECs) are central elements in vessel homeostasis and regulate the passage of material and cells into and out of the bloodstream. EC proliferation and migration are modified by alterations to mitochondrial biogenesis and dynamics resulting from several signals and environmental cues, such as oxygen, hemodynamics, and nutrients. As intermediary signals, mitochondrial ROS are released as important downstream modulators of the expression of angiogenesis-related genes. In this review, we discuss the physiological actions of these signals and aberrant responses during vascular disorders.
Keywords: Endothelial cells, Mitochondria, Biogenesis, Dynamics, ROS
Highlights
-
•
Mitochondria in EC act as integrators of environmental cues.
-
•
Circulating signals modify mitochondrial dynamics, altering EC phenotype.
-
•
ROS release by EC mitochondria regulates expression of vascular genes.
1. Mitochondria in endothelial cells
The entire vascular system, from the heart to the smallest capillary, is lined by endothelial cells (EC). ECs are central elements in vessel homeostasis and regulate and passage of materials and cells into and out of the bloodstream. Every metabolically active tissue requires the development of an angiogenic network, and the development of this network is associated with metabolic changes and accompanying morphological and phenotypical variations in ECs. In most cell types, the key metabolic regulators are mitochondria, which are linked to a diversity of regulatory processes that allow cell proliferation [1] or trigger apoptosis [2], and also signals to nucleolus [3]. Mitochondrial content varies between endothelial beds; for example, brain ECs contain more mitochondria than ECs in other tissues. However, mitochondrial content in all ECs is low (2–6% of cytoplasm volume) compared with other cell types for example cardiomyocytes (about 32%) [4], [5]. ECs have an absolute requirement for glucose and generate more than 80% of their ATP through glycolysis, and the low mitochondrial content in ECs is thus consistent with a role in regulating signaling responses to environmental cues rather than in energy production. Indeed, the activity of EC mitochondria is influenced by a variety of circulating factors, and these organelles provide important factors for ECs signaling (Fig. 1) [6], [7], [8].
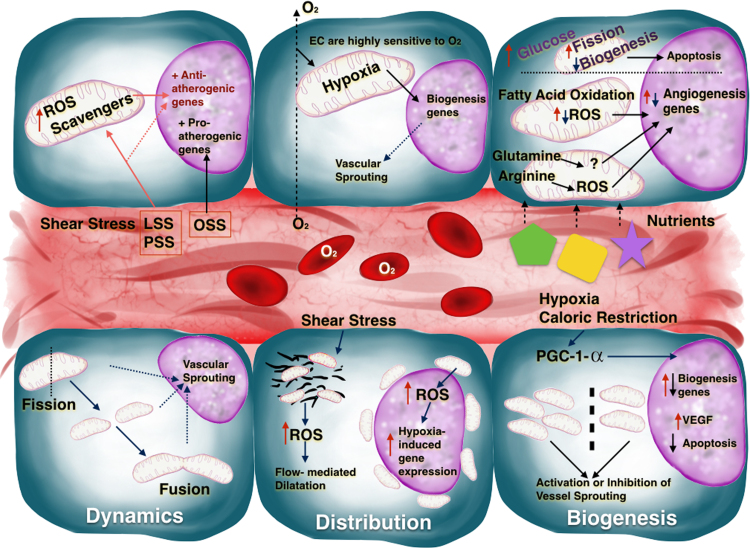
Fig. 1.
A blood vessel delimited by ECs and some of the signals which affect angiogenic behavior via mitochondria. The cells at the top represent the effect on mitochondria of nutrients, oxygen, and shear stress (LSS, laminar shear stress; PSS, pulsatile shear stress; OSS, oscillatory shear stress) and how some of the signals released affect the expression of angiogenic genes. The cells at the bottom represent mitochondrial biogenesis, distribution, and dynamics and the consequences of these processes on EC proliferation and vessel sprouting.
1.1. Mitochondrial features: distribution, biogenesis and dynamics
The communication of EC mitochondria with other organelles is determined by their distribution, biogenesis, and dynamics [9]. For example, mitochondria are anchored to the cytoskeleton in coronary arterioles isolated from human myocardium; reactive oxygen species (ROS) released from these mitochondria in response to shear stress contribute to flow-mediated dilatation [10]. In pulmonary artery ECs, hypoxia triggers a perinuclear mitochondrial redistribution, with concomitant ROS accumulation in the nucleolus and induction of hypoxia-induced genes [3].
An increase in mitochondrial mass requires the coordinated replication and expression of mitochondrial DNA (mtDNA) with the parallel expression of nuclear-encoded mitochondrial genes. A key player in this coordination is proliferator-activated receptor-γ coactivator-1α (PGC-1α), which activates nuclear respiratory factors 1 and 2 and the nuclear expression of genes necessary for mitochondrial biogenesis [11]. In parallel, PGC-1α also activates mitochondrial transcription factors A and B (TFAM and TFBM), which regulate the expression of mtDNA genes. PGC-1α is expressed in ECs and regulates mitochondrial biogenesis and the expression of several mitochondrial antioxidant enzymes, playing a dual role in the protection against oxidative stress by supplying undamaged mitochondria and enhancing ROS defenses [12], [13]. PGC-1α expression and mitochondrial biogenesis are affected by several factors, such as hypoxia and caloric restriction, in parallel with variations in vessel formation [14], [15], [16]. PGC-1α moreover regulates the expression of several genes related to lipid and glucose metabolism [14], [15], induces VEGF expression [17], and decreases apoptosis [18], resulting in a coordinated acceleration of angiogenesis as ECs increase their metabolic demands during proliferation.
Mitochondria are highly dynamic organelles that undergo cycles of fusion and fission that maintain mitochondrial integrity and intersect with apoptosis pathways [19]. Key regulators of this process include mitofusin-1 and 2 (MFN-1 and 2) and optic atrophy protein 1 (OPA-1). These proteins regulate fission-1 (FIS-1), which recruit DRP-1 (dynamin-related protein-1) to initiate the fission process. Fusion and fission are physiologically balanced processes. Fusion allows a better distribution of proteins, mtDNA, and metabolites to maintain electrical and biochemical connectivity [11]. Fusion is a determining factor in angiogenesis that can accelerate responses to VEGF and the activation of eNOS [20]. Fission, on the other hand, is a key factor in vascular sprouting. In pulmonary ECs, DRP-1 activation, which induces mitochondrial fission, stimulates angiogenesis by promoting proliferation, migration, and inhibition of apoptosis in a mitochondrial Ca2+-dependent manner [19], [21]. A defective mitochondrial network is observed in several pathological settings, such as high glucose and ischemia-reperfusion, and also during EC aging, highlighting the importance of the fusion/fission cycle during disease development [22], [23], [24], [25]. Accumulated mitochondrial damage leads to a rearrangement of these networks, and organelles with normal membrane potential and functional components are reincorporated into mitochondrial networks after fission. In contrast, dysfunctional mitochondria cannot fuse with the network and become targets for elimination by mitophagy, the destruction of damaged mitochondria by autophagy degradation. Mitophagy enables ECs to purge defective organelles and thus avoid dysfunctional signaling to prevents apoptosis. In parallel with mitophagy, biogenetic mechanisms act during homeostasis to replace the loss of mitochondrial mass, providing a mechanism to control mitochondria quality [11], [26], [27]. Mitophagy during homeostasis is crucially regulated by mitochondrial membrane depolarization. The PTEN-induced putative kinase 1(PINK1) is normally recruited and degraded at the inner mitochondrial membrane. When mitochondria are damaged, membrane potential loss prevents PINK1 degradation, and its accumulation induce mitophagy. This process is led by the E3 ubiquitin ligase Parkin, that ubiquitinate mitochondrial proteins, promoting the organelle recognition and recruitment by the autophagosome [28], [29], [30]. Stimuli such as metabolic stress trigger mitophagy to protect EC mitochondria from damage [31]. Deregulation of mitophagy results in ROS production, inflammation, and cell death, ultimately leading to neurodegeneration [30], cardiovascular disease [32], and endothelial dysfunction during aging [33]. Mitophagy is activated in ECs by mitochondrial depolarization triggered by exposure to hydrogen peroxide, irradiation, or promoters of lipid peroxidation [27], [34], [35]. Recent reports demonstrate that PINK and Parkin are unregulated in diabetic and obese mice, and knock down of these proteins in the vascular wall resulted in deregulation of mitophagy, with a concomitant accumulation of damaged mitochondria and ROS, resulting in apoptosis. Administration of low doses of palmitic acid stimulates PINK-Parkin pathway to eliminate damaged EC mitochondria; however, under severe stress this mechanism of protection is shut down and the accumulated defective mitochondria trigger cell death [36]. Thus, the PINK-Parkin pathway, by promoting mitophagy, protects ECs from metabolic stress and has potential as a target for the prevention of cardiovascular disorders triggered by oxidative stress in the endothelium [11], [36], [37].
2. Mitochondria in endothelial cells: responses to environmental cues
2.1. The role of oxygen in EC mitochondrial behavior
Oxygen carried in from circulating blood transfers to perivascular tissues through ECs. Oxygen consumption by ECs is relatively low compared with other cell types, contributing to just 15% of EC ATP generation [7]. This low consumption allows ECs to transfer most of the oxygen to perivascular tissues [38]. A sharp drop in oxygen levels across the arteriolar vessel wall is proposed to reflect fast diffusion through the endothelium [39], [40]; however, these observations are still debated due to discrepant reports. The vessel wall has been proposed to act as an “oxygen sink”, preventing potential harmful effects due to oxidative stress in perivascular tissues [41]. Despite their normally low oxygen utilization, EC mitochondria are able to increase respiration substantially in response stress stimuli such as glucose deprivation or oxidative stress [42], [43].
ECs are quite sensitive to hypoxic environments, and their mitochondria participate in the response to hypoxia by initiating the angiogenic cascade. A hypoxic environment increases VEGF levels, which enhance mitochondrial biogenesis via AKT-dependent signaling, resulting in increased vascular branching [44]. Conversely, vessel branching is reduced upon inhibition of EC mitochondrial biogenesis by silencing sirtuin 1 (SIRT1) [45]. The response of ECs to oxygen involves the inner mitochondrial membrane protein UCP-2 (uncoupling protein 2). In a model of pulmonary hypertension induced by intermittent hypoxia, UCP-2 promotes mitophagy and inadequate mitochondrial biogenesis and increases apoptosis in the lung endothelium [46]. During development, the vascular system shifts to a more oxygenated environment by increasing postnatal mitochondrial biogenesis through a decrease in HIF expression [47].
2.2. The role of dietary nutrients in EC behavior via mitochondria
ECs follow a very strict metabolic program in accordance to the required phenotype. For example, in a non-growing tissue, quiescent EC show a simultaneous reduction of mitochondrial respiration and glycolysis [48] whereas in sprouted vessels, tip ECs rely more in anaerobic glycolysis than in mitochondria (glucose can diffuse faster than oxygen allowing migration of EC to the new vessel). However stalk ECs use fatty acid oxidation and its ablation impairs proliferation [49], [50]. On the other hand, EC mitochondria are sensitive to variations in nutrient availability, which has an impact on its metabolic reprograming and further vascular properties. One of the best-studied nutritional effects on EC mitochondria is the impact of chronic elevated glucose, as occurs in diabetes. Elevated blood glucose causes EC injury and increased apoptosis, resulting in impaired endothelial integrity and blood vessel function [51]. Glucose-induced endothelial injury alters mitochondrial dynamics, with an increase in organelle fission leading to mitochondrial fragmentation, elevated ROS production, and EC dysfunction and apoptosis [52]. High glucose also modifies mitochondrial morphology and ultrastructure (increasing vacuolation and disrupting cristae) via activation of metalloproteinase 9, resulting in altered mitochondrial membrane potential [53]. High glucose also downregulates mitochondrial biogenesis via a significant decrease in PGC-1α expression [54]. PGC-1α downregulation by high glucose is paralleled in HUVECs by increased expression of VDAC1 (voltage-dependent anion channel 1), which may underlie glucose-induced EC apoptosis [55]. In contrast, in vivo models of diabetes type 2 and human samples reveal that, PGC-1α expression increase in parallel to a defective angiogenesis, while PGC-1α loss of function improves vasculature [56]. However in other models of vascular damage and hypertension PGC-1α up-regulation exert a protective role in angiogenesis, reflecting a contribution of PGC-1α to endothelial phenotype in a context-dependent manner [57].
EC function is also modulated by lipids. Fatty acid oxidation, especially when cells are supplied with L-carnitine, may be critical for mitochondrial angiogenic signaling [58], [59], [60], [61]. In a model of congenital heart disease, L-carnitine supplementation of cultured lung ECs reduces oxidative stress, NO signaling, and mitochondrial function. Little is known about the direct participation of lipids other than fatty acids in angiogenic mitochondrial signaling; however, there is evidence for indirect action. For example, efficient cholesterol efflux from ECs affects VEGF-induced angiogenesis, influencing oxidative stress and, indirectly, mitochondrial biogenesis [44], [62].
Clinical studies show a close relation between dietary protein intake and endothelial function or dysfunction [63]. Several amino acids metabolized by ECs have the potential to modulate angiogenesis [64], [65], [66], [67], [68]. Glutamine not only serves as a fuel source in ECs; it also affects vessel branching and EC proliferation via unknown mechanisms [69], [70]. Arginine affects EC ROS production and thus angiogenesis [71], [72].
2.3. The role of hemodynamics
The vascular endothelium is constantly exposed to blood fluid hemodynamic forces (mainly shear stress), and this is both a key determinant of EC function and a critical step toward the development of coronary atherosclerosis [73], [74]. EC dysfunction is the first event in atherosclerosis, triggering a series of changes that lead to the development of cardiovascular diseases. Local hemodynamic forces contribute to defective EC function through mitochondrial oxidative stress. Blood flow occurs in three forms: steady, when the fluid velocity does not change (laminar shear stress; LSS); pulsatile, when there is unidirectional flow with a periodic variation in magnitude (pulsatile shear stress; PSS); and oscillatory, when flow is bidirectional with a periodically varying magnitude (oscillatory shear stress; OSS). LSS, PSS, and OSS modulate EC function in different ways; for example, exposure to LSS or PSS, but not OSS, induces vasodilation by activating eNOS-mediated NO production [75], [76], [77], [78]. LSS and PSS also upregulate mRNA expression of the mitochondrial ROS scavenger MnSOD, thereby preventing oxidative-stress-induced damage [79]. In contrast, OSS increases NADPH oxidase expression, thereby increasing free radical production [80]. Overall, OSS upregulates proatherogenic genes and suppresses atheroprotective genes, promoting disease initiation, whereas LSS and PSS have the opposite effect [81]. Moreover, LSS can inhibit electron transport chain (ETC), reducing ROS production in proportion to the degree of flow exposure [82]. The effects of LSS and PSS on EC mitochondria and ROS production can be summarized as follows. Shear stress onset transiently increases intercellular calcium, which can induce ROS formation by enhancing tricarboxylic acid cycle activity and enhancing electron flow to the ETC. The resulting activation of eNOS increases NO production, inducing ROS formation but also activating mitochondrial ROS scavenging. Mitochondrial membrane polarization is maintained during LSS but is hyperpolarized during PSS, explaining the elevated mitochondrial ROS production in this situation. Despite these ROS increases, mitochondria remain healthy due to NO-mediated caspase inhibition, maintenance of membrane polarity, mitochondrial biogenesis, and upregulation of antioxidant genes [81].
Shear stress also influences mitochondrial dynamics. For example, LSS activates signaling pathways that modulate mitochondrial fission by recruiting cytosolic Drp1. These signals lead to changes in respiration rate that increase mitochondrial membrane potential and ROS generation, triggering subsequent activation of PRX3 an antioxidant enzyme [83]. Moreover, in a cellular model of ischemia/reperfusion, ECs increase production of mitochondrial ROS and NO, and during reperfusion Drp1 activation is accompanied by increased mitochondrial fission; these events are not induced by LSS alone, suggesting that LSS and PSS together modulate oxidative stress in ECs during reperfusion, regulating mitochondrial fission and promoting apoptosis [81]. In line with these findings, exposure to LSS and PSS activates AMPK and JNK, altering cell redox status and modifying autophagosome formation in ECs. Interestingly, LSS has been shown to activate autophagy in cultured ECs or isolated rabbit carotid arteries [84], [85], [86].
3. Mitochondrial ROS: one signal, diverse endothelial actions
The past 10 years have seen a growing interest in the role of mitochondrial ROS in ECs. Mitochondrial ROS come from several sources, and have important signaling functions in ECs directly linked to health and disease processes. The principal ROS producers are complexes I and III in the OXPHOS system; however, their specific contribution to total mitochondrial ROS production in ECs remains unclear due to the existence of other sources of ROS [87]. One of the main enzymes linked to mitochondrial ROS production is the ECs highly expressed nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4) [88]. NOX4-mediated regulation of mitochondrial ROS signaling influences endothelial-dependent relaxation and therefore blood pressure [89]. In addition, NOX activity in ECs is increased by cholesterol, particularly LDL, resulting in altered ROS levels and promoting cell senescence [90]. In addition, NOX can affect ROS production and signaling during cell migration, angiogenesis, adaptive responses to hypoxia, oxidative stress [91], and stroke [92].
Another potential source of mitochondrial ROS is the growth factor adaptor protein p66Shc. Although its precise function remains undefined, the oxidation of cytochrome c by p66Shc generates hydrogen peroxide. In the presence of specific signals, for example high glucose or apoptosis inducers, p66Shc translocate to the mitochondrial intermembrane space, where they initiate ROS-mediated signals that might trigger endothelial dysfunction or apoptosis [93], [94], [95], [96], [97], [98]. p66Shc thus appears to transduce environmental signals into mitochondrial ROS that trigger downstream responses in ECs, with strong implications for vascular biology.
Other contributors to mitochondrial ROS production include the monoamine oxidases (MAO), an enzyme family mainly localized to the outer mitochondrial membrane. Although ECs express MAO, its relevance to EC function is poorly understood [99]. However, MAO-derived ROS are associated with cardiac failure and adverse remodeling in mice as a response to pressure overload [100], [101]. Other implicated signals include those already discussed, such as oxygen, nutrients, and hemodynamics and also ROS themselves, derived either from mitochondria or from other sources. Non-mitochondrial ROS can initiate mitochondrial ROS production through a process called ROS-induced ROS release [102]. After exposure to angiotensin II, ROS induced by NOX for example, stimulate ROS production at mitochondria through mitoKATP channel opening, mitochondrial membrane depolarization and permeability transition pore, protecting ECs from excessive NO production [103], [104]. It is proposed that ROS signals are amplified under physiological conditions by ROS-induced ROS release mechanism contributing to EC dysfunction and disease progression [102].
3.1. Mitochondrial ROS in EC physiology and pathophysiology
ROS have traditionally been considered toxic metabolites that adversely affect vascular function through direct damage to several EC processes. However, more recent research identifies the participation of ROS as signaling molecules in EC regulatory cascades [17]. In this view, mitochondrial ROS are tightly regulated signals in ECs with an important role in physiological and pathophysiological settings. Mitochondrial ROS have well-documented effects on several EC processes related to normal vascular biology, such as shear-stress-induced vasodilation, hypoxia signaling, autophagy, and proinflammatory activation. Another example of ROS signaling is flow-mediated dilatation of arterioles from human myocardium [10]. Moreover, the use of mitochondrial ROS scavengers provides indirect evidence of the physiological actions of mitochondrial ROS; for example, oral antioxidant drugs blunt flow-mediated dilatation of the brachial artery, and the lack of benefit of high doses of nonselective antioxidants in cardiovascular diseases hints at the participation of mitochondrial ROS in normal EC function [105], [106].
Several pathological factors affect ROS production by ECs, and excessive mitochondrial ROS accumulation in ECs has been linked to the development of vascular diseases. For example, mitochondrial ROS are important intermediaries in the oxidation of lipids implicated in LDL-mediated initiation of cardiovascular disease associated with dyslipidemia; however, oxidized lipid production can also increase antioxidant gene expression, thus triggering a mechanism for attenuating excessive oxidative stress [106], [107], [108], [109]. Another example is hypertension, which is linked to elevated mitochondrial superoxide in vascular cells, and can be reduced with superoxide scavengers in animal models [110], [111], [112], [113], [114]. Further evidence for a link between mitochondrial ROS and hypertension is provided by the NOX family of ROS-producing enzymes, which together with mitochondria generate excessive ROS in angiotensin-II-related models [115], [116], [117]. In contrast, ROS production and angiotensin-II-induced hypertension are suppressed in transgenic mice overexpressing mitochondrial matrix-based thioredoxin-2 [117]. Age-related hypertensive responses in rats are also attenuated by supplementation with the mitochondrial antioxidant MitoQ [118].
Two well-documented examples of the role of mitochondrial ROS in vascular signaling are linked to diabetes and atherosclerosis. In diabetes, elevated circulating glucose and free fatty acids induce EC mitochondrial fission, with concomitantly high levels of ROS that lead to blunted cell growth and altered cell-adhesion molecule expression on the cell surface [119], [120], [121]. Excessive mitochondrial ROS production in diabetes has been described as a master switch for the activation of subsequent events linked to endothelial dysfunction, such as activation of protein kinase C, increased polyol and hexosamine pathway flux, and increased age-related glycation end-product formation [122]. The influence of excessive ROS production on atherosclerosis is supported by multiple lines of evidence, including oxidative damage to mtDNA by ROS-induced ROS that is directly linked to the degree of arterial damage [17], [123]. Moreover, mitochondrial membrane hyperpolarization triggers further ROS generation and accelerates atherosclerosis progression [17].
4. Conclusions
ECs regulate the passage of materials and cells between the blood stream and tissues. Within this process, EC mitochondria integrate environmental cues such as oxygen, hemodynamics, and nutrients by modifying their biogenesis, dynamics, and programmed degradation. These processes determine the EC phenotype in physiological or pathological conditions. EC mitochondria additionally release ROS as signals with important downstream actions that include the expression of angiogenesis-related genes in physiological conditions and also in aberrant responses during vascular disease.
Acknowledgments
We thank M. M. Muñoz-Hernandez and Dr Concepción Jimenez for technical assistance, Keren López Fernández for the artwork in Fig. 1 and Simon Bartlett for English editing. This study was supported by MINECO: SAF2015-65633-R MSCA-COFUND-DP Doctoral programmes 2014. The CNIC is supported by MINECO and Pro-CNIC Foundation, and is a SO-MINECO (award SEV-2015-0505).
References
- 1.Mitra D., Fisher D.E. Transcriptional regulation in melanoma. Hematol. Oncol. Clin. North Am. 2009;23:447–465. doi: 10.1016/j.hoc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Kroemer G., Galluzzi L., Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 3.Al-Mehdi A.B., Pastukh V.M., Swiger B.M., Reed D.J., Patel M.R., Bardwell G.C., Pastukh V.V., Alexeyev M.F., Gillespie M.N. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci. Signal. 2012;(5 () doi: 10.1126/scisignal.2002712. (ra47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barth E., Stammler G., Speiser B., Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J. Mol. Cell. Cardiol. 1992;24:669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- 5.Oldendorf W.H., Cornford M.E., Brown W.J. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann. Neurol. 1977;1:409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- 6.Culic O., Gruwel M.L., Schrader J. Energy turnover of vascular endothelial cells. Am. J. Physiol. 1997;273:C205–C213. doi: 10.1152/ajpcell.1997.273.1.C205. [DOI] [PubMed] [Google Scholar]
- 7.De Bock K., Georgiadou M., Carmeliet P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 2013;18:634–647. doi: 10.1016/j.cmet.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Krutzfeldt A., Spahr R., Mertens S., Siegmund B., Piper H.M. Metabolism of exogenous substrates by coronary endothelial cells in culture. J. Mol. Cell. Cardiol. 1990;22:1393–1404. doi: 10.1016/0022-2828(90)90984-a. [DOI] [PubMed] [Google Scholar]
- 9.Park J., Lee J., Choi C. Mitochondrial network determines intracellular ROS dynamics and sensitivity to oxidative stress through switching inter-mitochondrial messengers. PLoS One. 2011;6:e23211. doi: 10.1371/journal.pone.0023211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Li H., Bubolz A.H., Zhang D.X., Gutterman D.D. Endothelial cytoskeletal elements are critical for flow-mediated dilation in human coronary arterioles. Med. Biol. Eng. Comput. 2008;46:469–478. doi: 10.1007/s11517-008-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kluge M.A., Fetterman J.L., Vita J.A. Mitochondria and endothelial function. Circ. Res. 2013;112:1171–1188. doi: 10.1161/CIRCRESAHA.111.300233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valle I., Alvarez-Barrientos A., Arza E., Lamas S., Monsalve M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 2005;66:562–573. doi: 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Afolayan A.J., Eis A., Alexander M., Michalkiewicz T., Teng R.J., Lakshminrusimha S., Konduri G.G. Decreased endothelial NOS expression and function contributes to Impaired mitochondrial biogenesis and oxidative stress in fetal lambs with PPHN. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015 doi: 10.1152/ajplung.00392.2014. (ajplung00392 02014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leone T.C., Kelly D.P. Transcriptional control of cardiac fuel metabolism and mitochondrial function. Cold Spring Harb. Symp. Quant. Biol. 2011;76:175–182. doi: 10.1101/sqb.2011.76.011965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patten I.S., Arany Z. PGC-1 coactivators in the cardiovascular system. Trends Endocrinol. Metab. 2012;23:90–97. doi: 10.1016/j.tem.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Kim J.S., Kim B., Lee H., Thakkar S., Babbitt D.M., Eguchi S., Brown M.D., Park J.Y. Shear stress-induced mitochondrial biogenesis decreases the release of microparticles from endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H425–H433. doi: 10.1152/ajpheart.00438.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Widlansky M.E., Gutterman D.D. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid. Redox Signal. 2011;15:1517–1530. doi: 10.1089/ars.2010.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J., Zhang Y., Liu Y., Shen T., Zhang H., Xing Y., Zhu D. PGC-1alpha plays a major role in the anti-apoptotic effect of 15-HETE in pulmonary artery endothelial cells. Respir. Physiol. Neurobiol. 2015;205:84–91. doi: 10.1016/j.resp.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Suen D.F., Norris K.L., Youle R.J. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lugus J.J., Ngoh G.A., Bachschmid M.M., Walsh K. Mitofusins are required for angiogenic function and modulate different signaling pathways in cultured endothelial cells. J. Mol. Cell. Cardiol. 2011;51:885–893. doi: 10.1016/j.yjmcc.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen T., Wang N., Yu X., Shi J., Li Q., Zhang C., Fu L., Wang S., Xing Y., Zheng X., Yu L., Zhu D. The Critical Role of Dynamin-Related Protein 1 in Hypoxia-Induced Pulmonary Vascular Angiogenesis. J. Cell. Biochem. 2015;116:1993–2007. doi: 10.1002/jcb.25154. [DOI] [PubMed] [Google Scholar]
- 22.Giedt R.J., Yang C., Zweier J.L., Matzavinos A., Alevriadou B.R. Mitochondrial fission in endothelial cells after simulated ischemia/reperfusion: role of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. 2012;52:348–356. doi: 10.1016/j.freeradbiomed.2011.10.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J.R., Shen W.L., Yan C., Gao P.J. Downregulation of dynamin-related protein 1 contributes to impaired autophagic flux and angiogenic function in senescent endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2015;35:1413–1422. doi: 10.1161/ATVBAHA.115.305706. [DOI] [PubMed] [Google Scholar]
- 24.Paltauf-Doburzynska J., Malli R., Graier W.F. Hyperglycemic conditions affect shape and Ca2+ homeostasis of mitochondria in endothelial cells. J. Cardiovasc. Pharmacol. 2004;44:423–436. doi: 10.1097/01.fjc.0000139449.64337.1b. [DOI] [PubMed] [Google Scholar]
- 25.Shenouda S.M., Widlansky M.E., Chen K., Xu G., Holbrook M., Tabit C.E., Hamburg N.M., Frame A.A., Caiano T.L., Kluge M.A., Duess M.A., Levit A., Kim B., Hartman M.L., Joseph L., Shirihai O.S., Vita J.A. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green D.R., Galluzzi L., Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333:1109–1112. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mai S., Muster B., Bereiter-Hahn J., Jendrach M. Autophagy proteins LC3B, ATG5 and ATG12 participate in quality control after mitochondrial damage and influence lifespan. Autophagy. 2012;8:47–62. doi: 10.4161/auto.8.1.18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geisler S., Holmstrom K.M., Skujat D., Fiesel F.C., Rothfuss O.C., Kahle P.J., Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 29.Vives-Bauza C., Zhou C., Huang Y., Cui M., de Vries R.L., Kim J., May J., Tocilescu M.A., Liu W., Ko H.S., Magrane J., Moore D.J., Dawson V.L., Grailhe R., Dawson T.M., Li C., Tieu K., Przedborski S. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl. Acad. Sci. USA. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Youle R.J., Narendra D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green D.R., Galluzzi L., Kroemer G. Cell biology. Metabolic control of cell death. Science. 2014;345:1250256. doi: 10.1126/science.1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madamanchi N.R., Runge M.S. Mitochondrial dysfunction in atherosclerosis. Circ. Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 33.Mai S., Klinkenberg M., Auburger G., Bereiter-Hahn J., Jendrach M. Decreased expression of Drp1 and Fis1 mediates mitochondrial elongation in senescent cells and enhances resistance to oxidative stress through PINK1. J. Cell Sci. 2010;123:917–926. doi: 10.1242/jcs.059246. [DOI] [PubMed] [Google Scholar]
- 34.Higdon A.N., Benavides G.A., Chacko B.K., Ouyang X., Johnson M.S., Landar A., Zhang J., Darley-Usmar V.M. Hemin causes mitochondrial dysfunction in endothelial cells through promoting lipid peroxidation: the protective role of autophagy. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H1394–H1409. doi: 10.1152/ajpheart.00584.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q., Liang B., Shirwany N.A., Zou M.H. 2-Deoxy-d-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase. PLoS One. 2011;6:e17234. doi: 10.1371/journal.pone.0017234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu W., Xu H., Wang Z., Mao Y., Yuan L., Luo W., Cui Z., Cui T., Wang X.L., Shen Y.H. PINK1-Parkin-Mediated Mitophagy Protects Mitochondrial Integrity and Prevents Metabolic Stress-Induced Endothelial Injury. PLoS One. 2015;10:e0132499. doi: 10.1371/journal.pone.0132499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madeo F., Tavernarakis N., Kroemer G. Can autophagy promote longevity? Nat. Cell Biol. 2010;12:842–846. doi: 10.1038/ncb0910-842. [DOI] [PubMed] [Google Scholar]
- 38.Helmlinger G., Endo M., Ferrara N., Hlatky L., Jain R.K. Formation of endothelial cell networks. Nature. 2000;405:139–141. doi: 10.1038/35012132. [DOI] [PubMed] [Google Scholar]
- 39.Tsai A.G., Friesenecker B., Mazzoni M.C., Kerger H., Buerk D.G., Johnson P.C., Intaglietta M. Microvascular and tissue oxygen gradients in the rat mesentery. Proc. Natl. Acad. Sci. USA. 1998;95:6590–6595. doi: 10.1073/pnas.95.12.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai A.G., Johnson P.C., Intaglietta M. Oxygen gradients in the microcirculation. Physiol. Rev. 2003;83:933–963. doi: 10.1152/physrev.00034.2002. [DOI] [PubMed] [Google Scholar]
- 41.Golub A.S., Song B.K., Pittman R.N. The rate of O(2) loss from mesenteric arterioles is not unusually high. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H737–H745. doi: 10.1152/ajpheart.00353.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dranka B.P., Hill B.G., Darley-Usmar V.M. Mitochondrial reserve capacity in endothelial cells: the impact of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. 2010;48:905–914. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mertens S., Noll T., Spahr R., Krutzfeldt A., Piper H.M. Energetic response of coronary endothelial cells to hypoxia. Am. J. Physiol. 1990;258:H689–H694. doi: 10.1152/ajpheart.1990.258.3.H689. [DOI] [PubMed] [Google Scholar]
- 44.Wright G.L., Maroulakou I.G., Eldridge J., Liby T.L., Sridharan V., Tsichlis P.N., Muise-Helmericks R.C. VEGF stimulation of mitochondrial biogenesis: requirement of AKT3 kinase. FASEB J. 2008;22:3264–3275. doi: 10.1096/fj.08-106468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Csiszar A., Labinskyy N., Pinto J.T., Ballabh P., Zhang H., Losonczy G., Pearson K., de Cabo R., Pacher P., Zhang C., Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haslip M., Dostanic I., Huang Y., Zhang Y., Russell K.S., Jurczak M.J., Mannam P., Giordano F., Erzurum S.C., Lee P.J. Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arterioscler. Thromb. Vasc. Biol. 2015;35:1166–1178. doi: 10.1161/ATVBAHA.114.304865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neary M.T., Ng K.E., Ludtmann M.H., Hall A.R., Piotrowska I., Ong S.B., Hausenloy D.J., Mohun T.J., Abramov A.Y., Breckenridge R.A. Hypoxia signaling controls postnatal changes in cardiac mitochondrial morphology and function. J. Mol. Cell. Cardiol. 2014;74:340–352. doi: 10.1016/j.yjmcc.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilhelm K., Happel K., Eelen G., Schoors S., Oellerich M.F., Lim R., Zimmermann B., Aspalter I.M., Franco C.A., Boettger T., Braun T., Fruttiger M., Rajewsky K., Keller C., Bruning J.C., Gerhardt H., Carmeliet P., Potente M. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature. 2016;529:216–220. doi: 10.1038/nature16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Bock K., Georgiadou M., Schoors S., Kuchnio A., Wong B.W., Cantelmo A.R., Quaegebeur A., Ghesquiere B., Cauwenberghs S., Eelen G., Phng L.K., Betz I., Tembuyser B., Brepoels K., Welti J., Geudens I., Segura I., Cruys B., Bifari F., Decimo I., Blanco R., Wyns S., Vangindertael J., Rocha S., Collins R.T., Munck S., Daelemans D., Imamura H., Devlieger R., Rider M., Van Veldhoven P.P., Schuit F., Bartrons R., Hofkens J., Fraisl P., Telang S., Deberardinis R.J., Schoonjans L., Vinckier S., Chesney J., Gerhardt H., Dewerchin M., Carmeliet P. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 50.Schoors S., Bruning U., Missiaen R., Queiroz K.C., Borgers G., Elia I., Zecchin A., Cantelmo A.R., Christen S., Goveia J., Heggermont W., Godde L., Vinckier S., Van Veldhoven P.P., Eelen G., Schoonjans L., Gerhardt H., Dewerchin M., Baes M., De Bock K., Ghesquiere B., Lunt S.Y., Fendt S.M., Carmeliet P. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520:192–197. doi: 10.1038/nature14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Triggle C.R., Samuel S.M., Ravishankar S., Marei I., Arunachalam G., Ding H. The endothelium: influencing vascular smooth muscle in many ways. Can. J. Physiol. Pharmacol. 2012;90:713–738. doi: 10.1139/y2012-073. [DOI] [PubMed] [Google Scholar]
- 52.Pangare M., Makino A. Mitochondrial function in vascular endothelial cell in diabetes. J. Smooth Muscle Res. 2012;48:1–26. doi: 10.1540/jsmr.48.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mishiro K., Imai T., Sugitani S., Kitashoji A., Suzuki Y., Takagi T., Chen H., Oumi Y., Tsuruma K., Shimazawa M., Hara H. Diabetes mellitus aggravates hemorrhagic transformation after ischemic stroke via mitochondrial defects leading to endothelial apoptosis. PLoS One. 2014;9:e103818. doi: 10.1371/journal.pone.0103818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu L., Sun G., Zhang H., Zhang Y., Chen X., Jiang X., Jiang X., Krauss S., Zhang J., Xiang Y., Zhang C.Y. PGC-1alpha is a key regulator of glucose-induced proliferation and migration in vascular smooth muscle cells. PLoS One. 2009;4:e4182. doi: 10.1371/journal.pone.0004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng H., Zhong W., Zhao H., Chen L., Zhou X., Li F., Zhu W., Li G. Lack of PGC-1alpha exacerbates high glucose-induced apoptosis in human umbilical vein endothelial cells through activation of VADC1. Int. J. Clin. Exp. Pathol. 2015;8:4639–4650. [PMC free article] [PubMed] [Google Scholar]
- 56.Sawada N., Jiang A., Takizawa F., Safdar A., Manika A., Tesmenitsky Y., Kang K.T., Bischoff J., Kalwa H., Sartoretto J.L., Kamei Y., Benjamin L.E., Watada H., Ogawa Y., Higashikuni Y., Kessinger C.W., Jaffer F.A., Michel T., Sata M., Croce K., Tanaka R., Arany Z. Endothelial PGC-1alpha mediates vascular dysfunction in diabetes. Cell Metab. 2014;19:246–258. doi: 10.1016/j.cmet.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Craige S.M., Kroller-Schon S., Li C., Kant S., Cai S., Chen K., Contractor M.M., Pei Y., Schulz E., Keaney J.F., Jr. PGC-1alpha dictates endothelial function through regulation of eNOS expression. Sci. Rep. 2016;6:38210. doi: 10.1038/srep38210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dagher Z., Ruderman N., Tornheim K., Ido Y. The effect of AMP-activated protein kinase and its activator AICAR on the metabolism of human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 1999;265:112–115. doi: 10.1006/bbrc.1999.1635. [DOI] [PubMed] [Google Scholar]
- 59.Dagher Z., Ruderman N., Tornheim K., Ido Y. Acute regulation of fatty acid oxidation and amp-activated protein kinase in human umbilical vein endothelial cells. Circ. Res. 2001;88:1276–1282. doi: 10.1161/hh1201.092998. [DOI] [PubMed] [Google Scholar]
- 60.Hulsmann W.C., Dubelaar M.L. Aspects of fatty acid metabolism in vascular endothelial cells. Biochimie. 1988;70:681–686. doi: 10.1016/0300-9084(88)90253-2. [DOI] [PubMed] [Google Scholar]
- 61.Hulsmann W.C., Dubelaar M.L. Carnitine requirement of vascular endothelial and smooth muscle cells in imminent ischemia. Mol. Cell. Biochem. 1992;116:125–129. doi: 10.1007/BF01270579. [DOI] [PubMed] [Google Scholar]
- 62.Fang L., Choi S.H., Baek J.S., Liu C., Almazan F., Ulrich F., Wiesner P., Taleb A., Deer E., Pattison J., Torres-Vazquez J., Li A.C., Miller Y.I. Control of angiogenesis by AIBP-mediated cholesterol efflux. Nature. 2013;498:118–122. doi: 10.1038/nature12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teunissen-Beekman K.F., Dopheide J., Geleijnse J.M., Bakker S.J., Brink E.J., de Leeuw P.W., Schalkwijk C.G., van Baak M.A. Dietary proteins improve endothelial function under fasting conditions but not in the postprandial state, with no effects on markers of low-grade inflammation. Br. J. Nutr. 2015;114:1819–1828. doi: 10.1017/S0007114515003530. [DOI] [PubMed] [Google Scholar]
- 64.Dang C.V. Links between metabolism and cancer. Genes Dev. 2012;26:877–890. doi: 10.1101/gad.189365.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeBerardinis R.J., Thompson C.B. Cellular metabolism and disease: what do metabolic outliers teach us? Cell. 2012;148:1132–1144. doi: 10.1016/j.cell.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalhan S.C., Hanson R.W. Resurgence of serine: an often neglected but indispensable amino Acid. J. Biol. Chem. 2012;287:19786–19791. doi: 10.1074/jbc.R112.357194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phang J.M., Liu W. Proline metabolism and cancer. Front. Biosci. (Landmark Ed.) 2012;17:1835–1845. doi: 10.2741/4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shyh-Chang N., Locasale J.W., Lyssiotis C.A., Zheng Y., Teo R.Y., Ratanasirintrawoot S., Zhang J., Onder T., Unternaehrer J.J., Zhu H., Asara J.M., Daley G.Q., Cantley L.C. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science. 2013;339:222–226. doi: 10.1126/science.1226603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lohmann R., Souba W.W., Bode B.P. Rat liver endothelial cell glutamine transporter and glutaminase expression contrast with parenchymal cells. Am. J. Physiol. 1999;276:G743–G750. doi: 10.1152/ajpgi.1999.276.3.G743. [DOI] [PubMed] [Google Scholar]
- 70.Unterluggauer H., Mazurek S., Lener B., Hutter E., Eigenbrodt E., Zwerschke W., Jansen-Durr P. Premature senescence of human endothelial cells induced by inhibition of glutaminase. Biogerontology. 2008;9:247–259. doi: 10.1007/s10522-008-9134-x. [DOI] [PubMed] [Google Scholar]
- 71.Park I.S., Kang S.W., Shin Y.J., Chae K.Y., Park M.O., Kim M.Y., Wheatley D.N., Min B.H. Arginine deiminase: a potential inhibitor of angiogenesis and tumour growth. Br. J. Cancer. 2003;89:907–914. doi: 10.1038/sj.bjc.6601181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhuo W., Song X., Zhou H., Luo Y. Arginine deiminase modulates endothelial tip cells via excessive synthesis of reactive oxygen species. Biochem. Soc. Trans. 2011;39:1376–1381. doi: 10.1042/BST0391376. (suppl 1372 p following 1382) [DOI] [PubMed] [Google Scholar]
- 73.Chatzizisis Y.S., Coskun A.U., Jonas M., Edelman E.R., Feldman C.L., Stone P.H. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 74.Hsieh H.J., Liu C.A., Huang B., Tseng A.H., Wang D.L. Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J. Biomed. Sci. 2014;21:3. doi: 10.1186/1423-0127-21-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huddleson J.P., Ahmad N., Srinivasan S., Lingrel J.B. Induction of KLF2 by fluid shear stress requires a novel promoter element activated by a phosphatidylinositol 3-kinase-dependent chromatin-remodeling pathway. J. Biol. Chem. 2005;280:23371–23379. doi: 10.1074/jbc.M413839200. [DOI] [PubMed] [Google Scholar]
- 76.Jin Z.G., Ueba H., Tanimoto T., Lungu A.O., Frame M.D., Berk B.C. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ. Res. 2003;93:354–363. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- 77.Kuchan M.J., Frangos J.A. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am. J. Physiol. 1994;266:C628–C636. doi: 10.1152/ajpcell.1994.266.3.C628. [DOI] [PubMed] [Google Scholar]
- 78.Silacci P., Formentin K., Bouzourene K., Daniel F., Brunner H.R., Hayoz D. Unidirectional and oscillatory shear stress differentially modulate NOS III gene expression. Nitric Oxide. 2000;4:47–56. doi: 10.1006/niox.2000.0271. [DOI] [PubMed] [Google Scholar]
- 79.Ai L., Rouhanizadeh M., Wu J.C., Takabe W., Yu H., Alavi M., Li R., Chu Y., Miller J., Heistad D.D., Hsiai T.K. Shear stress influences spatial variations in vascular Mn-SOD expression: implication for LDL nitration. Am. J. Physiol. Cell. Physiol. 2008;294:C1576–C1585. doi: 10.1152/ajpcell.00518.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sorescu G.P., Song H., Tressel S.L., Hwang J., Dikalov S., Smith D.A., Boyd N.L., Platt M.O., Lassegue B., Griendling K.K., Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ. Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 81.Scheitlin C.G., Nair D.M., Crestanello J.A., Zweier J.L., Alevriadou B.R. Fluid Mechanical Forces and Endothelial Mitochondria: a Bioengineering Perspective. Cell. Mol. Bioeng. 2014;7:483–496. doi: 10.1007/s12195-014-0357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han Z., Chen Y.R., Jones C.I., 3rd, Meenakshisundaram G., Zweier J.L., Alevriadou B.R. Shear-induced reactive nitrogen species inhibit mitochondrial respiratory complex activities in cultured vascular endothelial cells. Am. J. Physiol. Cell. Physiol. 2007;292:C1103–C1112. doi: 10.1152/ajpcell.00389.2006. [DOI] [PubMed] [Google Scholar]
- 83.Breton-Romero R., Acin-Perez R., Rodriguez-Pascual F., Martinez-Molledo M., Brandes R.P., Rial E., Enriquez J.A., Lamas S. Laminar shear stress regulates mitochondrial dynamics, bioenergetics responses and PRX3 activation in endothelial cells. Biochim. Biophys. Acta. 2014;1843:2403–2413. doi: 10.1016/j.bbamcr.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 84.Guo F., Li X., Peng J., Tang Y., Yang Q., Liu L., Wang Z., Jiang Z., Xiao M., Ni C., Chen R., Wei D., Wang G.X. Autophagy regulates vascular endothelial cell eNOS and ET-1 expression induced by laminar shear stress in an ex vivo perfused system. Ann. Biomed. Eng. 2014;42:1978–1988. doi: 10.1007/s10439-014-1033-5. [DOI] [PubMed] [Google Scholar]
- 85.Takabe W., Jen N., Ai L., Hamilton R., Wang S., Holmes K., Dharbandi F., Khalsa B., Bressler S., Barr M.L., Li R., Hsiai T.K. Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling. Antioxid. Redox Signal. 2011;15:1379–1388. doi: 10.1089/ars.2010.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Young A., Wu W., Sun W., Benjamin Larman H., Wang N., Li Y.S., Shyy J.Y., Chien S., Garcia-Cardena G. Flow activation of AMP-activated protein kinase in vascular endothelium leads to Kruppel-like factor 2 expression. Arterioscler. Thromb. Vasc. Biol. 2009;29:1902–1908. doi: 10.1161/ATVBAHA.109.193540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen F., Haigh S., Barman S., Fulton D.J. From form to function: the role of Nox4 in the cardiovascular system. Front. Physiol. 2012;3:412. doi: 10.3389/fphys.2012.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Santillo M., Colantuoni A., Mondola P., Guida B., Damiano S. NOX signaling in molecular cardiovascular mechanisms involved in the blood pressure homeostasis. Front. Physiol. 2015;6:194. doi: 10.3389/fphys.2015.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li T.B., Zhang J.J., Liu B., Liu W.Q., Wu Y., Xiong X.M., Luo X.J., Ma Q.L., Peng J. Involvement of NADPH oxidases and non-muscle myosin light chain in senescence of endothelial progenitor cells in hyperlipidemia. Naunyn Schmiede. Arch. Pharmacol. 2015 doi: 10.1007/s00210-015-1198-y. [DOI] [PubMed] [Google Scholar]
- 91.A.M. Amanso, K.K. Griendling, Differential roles of NADPH oxidases in vascular physiology and pathophysiology, Front. Biosci. (Schol Ed), 4 (2012) pp. 1044–1064. [DOI] [PMC free article] [PubMed]
- 92.Kleikers P.W., Dao V.T., Gob E., Hooijmans C., Debets J., van Essen H., Kleinschnitz C., Schmidt H.H. SFRR-E Young Investigator AwardeeNOXing out stroke: identification of NOX4 and 5as targets in blood-brain-barrier stabilisation and neuroprotection. Free Radic. Biol. Med. 2014;75(Suppl 1):S16. doi: 10.1016/j.freeradbiomed.2014.10.593. [DOI] [PubMed] [Google Scholar]
- 93.Paneni F., Cosentino F. p66 Shc as the engine of vascular aging. Curr. Vasc. Pharmacol. 2012;10:697–699. doi: 10.2174/157016112803520747. [DOI] [PubMed] [Google Scholar]
- 94.Paneni F., Mocharla P., Akhmedov A., Costantino S., Osto E., Volpe M., Luscher T.F., Cosentino F. Gene silencing of the mitochondrial adaptor p66(Shc) suppresses vascular hyperglycemic memory in diabetes. Circ. Res. 2012;111:278–289. doi: 10.1161/CIRCRESAHA.112.266593. [DOI] [PubMed] [Google Scholar]
- 95.Andreyev A.Y., Kushnareva Y.E., Murphy A.N., Starkov A.A. Mitochondrial ROS metabolism: 10 years later. Biochemistry (Mosc.) 2015;80:517–531. doi: 10.1134/S0006297915050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Camici G.G., Schiavoni M., Francia P., Bachschmid M., Martin-Padura I., Hersberger M., Tanner F.C., Pelicci P., Volpe M., Anversa P., Luscher T.F., Cosentino F. Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc. Natl. Acad. Sci. USA. 2007;104:5217–5222. doi: 10.1073/pnas.0609656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Giorgio M., Migliaccio E., Orsini F., Paolucci D., Moroni M., Contursi C., Pelliccia G., Luzi L., Minucci S., Marcaccio M., Pinton P., Rizzuto R., Bernardi P., Paolucci F., Pelicci P.G. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 98.Trinei M., Berniakovich I., Beltrami E., Migliaccio E., Fassina A., Pelicci P., Giorgio M. P66Shc signals to age. Aging (Albany NY) 2009;1:503–510. doi: 10.18632/aging.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meresse S., Dehouck M.P., Delorme P., Bensaid M., Tauber J.P., Delbart C., Fruchart J.C., Cecchelli R. Bovine brain endothelial cells express tight junctions and monoamine oxidase activity in long-term culture. J. Neurochem. 1989;53:1363–1371. doi: 10.1111/j.1471-4159.1989.tb08526.x. [DOI] [PubMed] [Google Scholar]
- 100.Kaludercic N., Takimoto E., Nagayama T., Feng N., Lai E.W., Bedja D., Chen K., Gabrielson K.L., Blakely R.D., Shih J.C., Pacak K., Kass D.A., Di Lisa F., Paolocci N. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ. Res. 2010;106:193–202. doi: 10.1161/CIRCRESAHA.109.198366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poon C.C., Seto S.W., Au A.L., Zhang Q., Li R.W., Lee W.Y., Leung G.P., Kong S.K., Yeung J.H., Ngai S.M., Ho H.P., Lee S.M., Chan S.W., Kwan Y.W. Mitochondrial monoamine oxidase-A-mediated hydrogen peroxide generation enhances 5-hydroxytryptamine-induced contraction of rat basilar artery. Br. J. Pharmacol. 2010;161:1086–1098. doi: 10.1111/j.1476-5381.2010.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 104.Doughan A.K., Harrison D.G., Dikalov S.I. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 105.Richardson R.S., Donato A.J., Uberoi A., Wray D.W., Lawrenson L., Nishiyama S., Bailey D.M. Exercise-induced brachial artery vasodilation: role of free radicals. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1516–H1522. doi: 10.1152/ajpheart.01045.2006. [DOI] [PubMed] [Google Scholar]
- 106.Stocker R., Keaney J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 107.Ceaser E.K., Ramachandran A., Levonen A.L., Darley-Usmar V.M. Oxidized low-density lipoprotein and 15-deoxy-delta 12,14-PGJ2 increase mitochondrial complex I activity in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2298–H2308. doi: 10.1152/ajpheart.00508.2003. [DOI] [PubMed] [Google Scholar]
- 108.Mabile L., Meilhac O., Escargueil-Blanc I., Troly M., Pieraggi M.T., Salvayre R., Negre-Salvayre A. Mitochondrial function is involved in LDL oxidation mediated by human cultured endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1997;17:1575–1582. doi: 10.1161/01.atv.17.8.1575. [DOI] [PubMed] [Google Scholar]
- 109.Zhao R., Shen G.X. Functional modulation of antioxidant enzymes in vascular endothelial cells by glycated LDL. Atherosclerosis. 2005;179:277–284. doi: 10.1016/j.atherosclerosis.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 110.Harrison D.G., Gongora M.C. Oxidative stress and hypertension. Med. Clin. North Am. 2009;93:621–635. doi: 10.1016/j.mcna.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 111.Laursen J.B., Rajagopalan S., Galis Z., Tarpey M., Freeman B.A., Harrison D.G. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 112.Miyagawa K., Ohashi M., Yamashita S., Kojima M., Sato K., Ueda R., Dohi Y. Increased oxidative stress impairs endothelial modulation of contractions in arteries from spontaneously hypertensive rats. J. Hypertens. 2007;25:415–421. doi: 10.1097/HJH.0b013e3280115b96. [DOI] [PubMed] [Google Scholar]
- 113.Nakazono K., Watanabe N., Matsuno K., Sasaki J., Sato T., Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc. Natl. Acad. Sci. USA. 1991;88:10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schnackenberg C.G., Welch W.J., Wilcox C.S. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension. 1998;32:59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]
- 115.Hoch N.E., Guzik T.J., Chen W., Deans T., Maalouf S.A., Gratze P., Weyand C., Harrison D.G. Regulation of T-cell function by endogenously produced angiotensin II. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R208–R216. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pueyo M.E., Gonzalez W., Nicoletti A., Savoie F., Arnal J.F., Michel J.B. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2000;20:645–651. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- 117.Widder J.D., Fraccarollo D., Galuppo P., Hansen J.M., Jones D.P., Ertl G., Bauersachs J. Attenuation of angiotensin II-induced vascular dysfunction and hypertension by overexpression of Thioredoxin 2. Hypertension. 2009;54:338–344. doi: 10.1161/HYPERTENSIONAHA.108.127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Graham D., Huynh N.N., Hamilton C.A., Beattie E., Smith R.A., Cocheme H.M., Murphy M.P., Dominiczak A.F. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 119.Basta G., Lazzerini G., Del Turco S., Ratto G.M., Schmidt A.M., Caterina R. De. At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler. Thromb. Vasc. Biol. 2005;25:1401–1407. doi: 10.1161/01.ATV.0000167522.48370.5e. [DOI] [PubMed] [Google Scholar]
- 120.Srinivasan S., Yeh M., Danziger E.C., Hatley M.E., Riggan A.E., Leitinger N., Berliner J.A., Hedrick C.C. Glucose regulates monocyte adhesion through endothelial production of interleukin-8. Circ. Res. 2003;92:371–377. doi: 10.1161/01.RES.0000061714.74668.5C. [DOI] [PubMed] [Google Scholar]
- 121.Yu T., Robotham J.L., Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 123.Nijtmans L.G., Henderson N.S., Attardi G., Holt I.J. Impaired ATP synthase assembly associated with a mutation in the human ATP synthase subunit 6 gene. J. Biol. Chem. 2001;276:6755–6762. doi: 10.1074/jbc.M008114200. [DOI] [PubMed] [Google Scholar]