Abstract
Brown adipose tissue (BAT) is a specialized tissue critical for non-shivering thermogenesis producing heat through mitochondrial uncoupling; whereas white adipose tissue (WAT) is responsible of energy storage in the form of triglycerides. Another type of fat has been described, the beige adipose tissue; this tissue emerges in existing WAT depots but with thermogenic ability, a phenomenon known as browning. Several peripheral signals relaying information about energy status act in the brain, particularly the hypothalamus, to regulate thermogenesis in BAT and browning of WAT. Different hypothalamic areas have the capacity to regulate the thermogenic process in brown and beige adipocytes through the sympathetic nervous system (SNS). This review discusses important concepts and discoveries about the central control of thermogenesis as a trip that starts in the hypothalamus, and taking the sympathetic roads to reach brown and beige fat to modulate thermogenic functions.
1. Introduction
Brown adipose tissue (BAT), and more recently beige adipose tissue, are responsible for heat production through non-shivering thermogenesis (NST). Initially, BAT was thought to exist only in small or hibernating mammals and newborn humans. However, in 2009 functional BAT was identified in adult humans through positron-emission and computed tomographic (PET-CT) scans [1], [2], [3], [4], [5]. Since then interest by the scientific community in studying the molecular mechanisms involved in the regulation of NST have expanded greatly. Another type of thermogenic fat was described, “brown-like” adipocytes present in WAT depots named the beige or brite (“brown in white”) adipose tissue, that emerges in located depots of white adipose tissue (WAT) under some stimuli and which possesses thermogenic properties [1], [2]. Thermogenesis in BAT and browning of WAT are activated with cold exposure and sympathetic stimulation [3], [4], [5]. Several central mechanisms regulating thermogenesis in brown and beige adipocytes have been described, which are the focus of this review. For this purpose, we will start a trip at the control center, namely the hypothalamus, the most studied region of the central nervous system (CNS) with respect to the control of energy balance. From the hypothalamus, we will follow the road through the autonomous nervous system (ANS), via the sympathetic fibers that connect and regulate the different fat pads, where the thermogenic pathway ends.
2. The hypothalamus: the beginning of the travel
The control of energy homeostasis is orchestrated by the CNS with the hypothalamus playing a major role. Indeed, most of the peripheral cues, such as sensory and nutritional signals and humoral factors arrive at the hypothalamus to provide information about the nutritional status of the body. The information is integrated to emit a response through regulation of food intake, energy expenditure (EE) and nutrient partitioning [6], [7], [8], [9], [10], [11], [12], [13], [14]. The hypothalamus is composed of several neural populations distributed in different hypothalamic nuclei (Fig. 1, Fig. 2) that are interconnected to each other and to other brain areas. Furthermore, peripheral energy balance is controlled by the hypothalamus through the ANS. Thermogenesis in brown and beige adipose tissues can be induced by activation of the sympathetic nervous system (SNS) [7], [8], [11], [15], [16], [17] (Fig. 1, Fig. 2). The control of ANS by the hypothalamus is complex, involving several neuronal populations and signaling pathways in various nuclei.
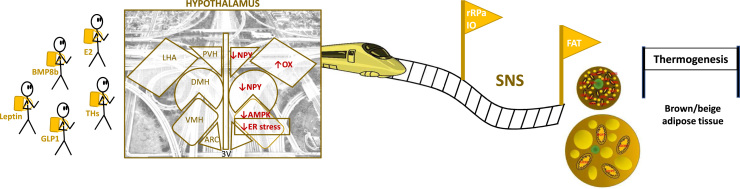
Fig. 1.
Brain adipose axes. Several peripheral signals act on the hypothalamus to inform about the nutritional status of the body. Therefore, leptin, glucagon like peptide (GLP-1), bone morphogenetic protein 8B (BMP8B), estradiol (E2) or thyroid hormones (THs) act in the ventromedial nucleus of the hypothalamus (VMH), decreasing AMP-activated protein kinase (AMPK) which activates the sympathetic nervous system (SNS) signaling to brown and beige adipose tissues. Events such as decreasing the endoplasmic reticulum (ER) stress in the VMH or increasing orexins (OX) in the lateral hypothalamic area (LHA) also activate thermogenesis through sympathetic activation. rRPa: rostral raphe nucleus, IO: inferior olive.
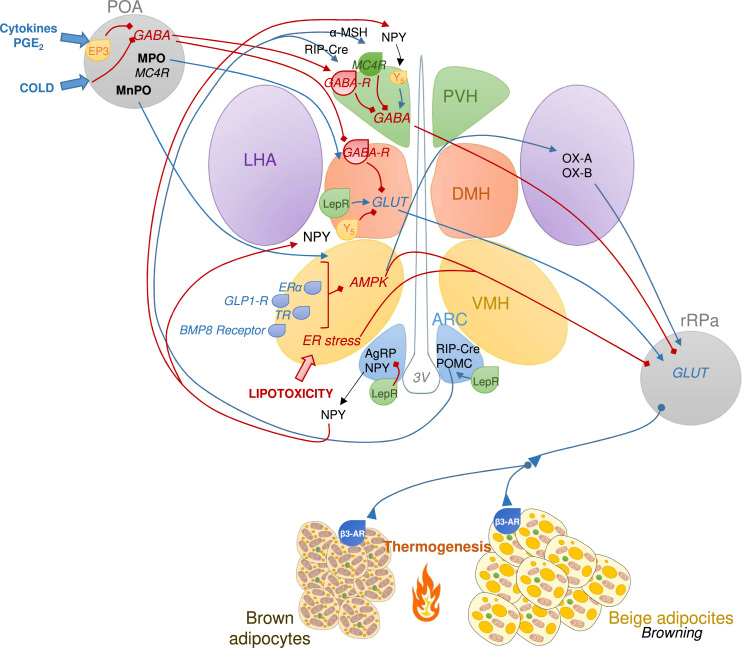
Fig. 2.
Hypothalamic circuits regulating thermogenesis of BAT and browning of WAT. The preoptic area (POA) receives peripheral information about external temperature, being activated by cold and prostaglandin E2 (PGE2), and mediate the febrile response. The POA projects to other hypothalamic nuclei such as the ventromedial nucleus of hypothalamus (VMH) leading the activation of BAT sympathetic traffic through projections to rostral raphe nucleus (rRPa). Several peripheral signals converge in the VMH to regulate thermogenesis: activation of estrogen receptor α (ERα), glucagon like peptide 1 receptor (GLP1-R), thyroid hormone receptor (TR), or bone morphogenic protein 8 receptor (BMP8R) inhibit AMPK in the VMH stimulating thermogenesis in in brown adipose tissue (BAT) and white adipose tissue (WAT), as well as lipotoxicity-induced ER stress. The dorsomedial nucleus of the hypothalamus (DMH) is also involved in the thermogenesis regulation, since it is inhibited under normothermic environment by a GABAergic tone which is disinhibited by POA stimulation inducing thermogenesis. The arcuate nucleus of hypothalamus (ARC) contain neurons that express orexigenic factors such as agouti-related protein (AgRP) along with neropeptide Y (NPY) and anorexigenic neurons expressing proopiomelanocortin (POMC). Leptin induces POMC expression increasing the release of POMC products in the second order neurons. Leptin action in the ARC also activate RIP-Cre expressing neurons, which through disinhibiton of GABAergic population act on paraventricular nucleus of the hypothalamus (PVH) to induce thermogenesis. Conversely, AgRP and NPY in the ARC act on the PVH decreasing the sympathetic activation of BAT. In the lateral nucleus of the hypothalamus (LHA), neurons expressing orexins (OX) promote BAT thermogenesis, a process that can be activated by low AMPK activity in the VMH. LepR: Leptin receptor; GABA-R: GABA receptor; MPO: medial POA; MnPO: median part of the POA; MC4R: melanocortin 4 receptor; β3-AR: beta 3 adrenoreceptor.
2.1. The preoptic area
The preoptic area (POA) is the main brain nucleus that senses temperature. It acts as a thermometer that receives the peripheral and central thermal signals through temperature-sensitive neurons to regulate body temperature according to the external environment [18], [19], [20], [21], [22]. The POA receives input from thermosensitive areas elsewhere in the body. Thus, detection of cold signals by the median POA (mnPOA) triggers hypothalamic mechanisms to induce thermogenesis in BAT [23]. More specifically, POA connects directly with the ventromedial nucleus of the hypothalamus (VMH) to activate BAT thermogenesis in response to cold, as demonstrated by the fact that destruction of the VMH abolishes the ability of external cold to stimulate BAT thermogenesis [24], [25].
The POA also controls the febrile response, a physiological mechanism to induce a hyperthermic environment needed to defend against pathogens [26]. During infections, prostaglandins (PG) are released in the vasculature and peripheral tissues and upon arrival to the POA trigger activation of the BAT thermogenic program [27], [28], [29]. Specifically, POA contains PG receptors subtype EP3, which are connected to the dorsomedial nucleus of the hypothalamus (DMH), as well as the rostral raphe pallidus (rRPA) in the brainstem that control thermogenesis in BAT to induce fever through a mechanism that involve cAMP [27], [28], [29], [30]. A population of the POA neurons expressing EP3 subtype of PGE receptor is mainly GABAergic and projects to DMH and the rRPa antagonizing some inhibitory fibers to induce fever [30]. Furthermore, neurons of the mnPOA which are enriched in melanocortin 4 receptors (MC4R) and connected to the DMH can also activate thermogenesis in brown and beige adipocytes [31], although the exact role of the POA in regulating browning remains unknown.
2.2. The arcuate nucleus of the hypothalamus
The arcuate nucleus of the hypothalamus (ARC) is located just above the median eminence (ME) where the blood brain barrier (BBB) is permeable allowing access of peripheral signals to the brain [7], [8], [11], [15], [16], [17]. Thus, the ARC is considered as one of the gates for the brain to sense circulating factors. The ARC is heterogeneous, containing several populations of neurons, but the most studied are those expressing the orexigeneic neuropeptides, such as agouti-related peptide (AgRP) and neuropeptide Y (NPY), or anorexigenic neuropeptide precursors, such as pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) [7], [8], [11], [15], [16], [17]. In addition to the control of feeding behavior, the ARC also regulates energy expenditure [14], [32], [33]. For example, leptin action in the ARC is required for the induction of action potential and firing of the sympathetic nerves subserving BAT [34], [35]. Interestingly, activation of ARC neurons expressing NPY was found to decrease BAT thermogenesis [36], [37]. A recent study describes that this observation is sexually dimorphic, with female mice lacking corticotropin-releasing hormone receptor 1 (CRFR-1) in AgRP neurons showing decreased thermogenesis in brown and beige adipocytes in response to cold [38]. On the other hand, POMC neurons have been related to increased thermogenesis through activation of SNS to BAT [39], [40], [41]. As a consequence, mice lacking genes downstream to POMC neurons, such as the Mc4r gene display suppressed leptin-dependent augmentation of thermogenesis in BAT and WAT [42]. Conversely, absence of protein tyrosine phosphatases 1B (PTP1B) and tyrosine-protein phosphatase non-receptor type 2 (TCPTP), both of which suppress signaling of leptin and insulin receptors, promote WAT browning and energy expenditure in response to insulin and leptin, preventing the development of diet-induced obesity [43]. The capacity of leptin to induce BAT thermogenesis by through the ARC may also involve another population of neurons; the GABAergic RIP-Cre (Cre-mediated expression of rat insulin II promoter)- expressing neurons through their projections to other hypothalamic sites, such as the paraventricular nucleus (PVH) [44], [45], [46]. Overall, these findings suggest that both orexigenic and anorexigenic neural populations of the ARC can regulate thermogenesis.
The molecular mechanism mediating the effect of the ARC on thermogenesis remains unclear. There is evidence pointing to endoplasmic reticulum (ER) stress in the ARC as an important mechanism involved in the regulation of energy expenditure. Indeed, increased ER stress in POMC neurons reduced thermogenesis in BAT [47], [48], [49], while reduction of ER stress, or O-linked-β-N-acetylglucosamine transferase (OGT) expression are associated with increased energy expenditure and thermogenesis in BAT and WAT [50], [51].
2.3. The dorsomedial nucleus of the hypothalamus
It is widely reported that the DMH is involved in the central control of thermogenesis in brown and beige adipose tissues through sympathetic transmission. The DMH is involved in the febrile response, during which some DMH glutamatergic neurons activate sympathetic fibers to BAT through rRPa. This process involves two groups of neurons. Initially, the GABAergic neurons within the DMH inhibit the glutamatergic neurons. However, under prostaglandin (PG)E2 activation of the POA, stimulation of a second group of GABAergic neurons in the POA inhibit the DMH GABAergic neurons [11], [30], [52], [53], [54], [55], [56].
There are NPY-expressing neurons in the DMH that are able to regulate thermogenesis through sympathetic activation to BAT and WAT. In fact, disruption of NPY in the DMH increases BAT thermogenesis and browning of WAT [31], [57]. The existing evidence suggests that hypothalamic NPY plays an important role in the control of thermogenesis and promote energy storage in fat [58]. However, under some conditions, such as high energy demand, diet-induced obesity or upon cold exposure, NPY in the DMH increases the thermogenesis process, a phenomenon different from what happens in the ARC where NPY expression is reduced after cold exposure, and where NPY reduces BAT activity [57], [59]. Taken together, NPY in the DMH could play a role in thermogenic regulation as part of a basal inhibitory tone that can be disinhibited under some stimuli, for example the induction of the febrile response from the POA. However, such possibility remains to be tested.
Finally, there are some neurons expressing leptin receptor (LepR) in the DMH, which contribute to sympathetic activation to some fat depots in obese mice [60], [61]. Accordingly, lack of LepR in the DMH was found to decrease BAT thermogenesis promoting weight gain [60], suggesting that leptin in the DMH plays a key role in the sympathetically-mediated activation of BAT thermogenesis.
2.4. The ventromedial nucleus of the hypothalamus
The importance of the VMH in the regulation of thermogenesis is well established. The main role of this nucleus consists in the integration of various peripheral signals to coordinate the thermogenic response, particularly the sympathetic tone to BAT and WAT. This is supported by the anatomical link between the VMH and the brown and white fat pads [62], [63] through the rRPa and inferior olive (IO) in the brainstem [32], [64], [65], [66], [67], [68], [69]. Thereby, inhibitory factors, such as GABA agonists in the VMH impair the PGE2-induced thermogenesis [27], while action of excitatory neuropeptides such as glutamate, noradrenaline, serotonin and tryptophan in the VMH activate BAT thermogenesis [70], [71], [72], [73], [74], [75], [76]. A novel paradigm postulates that the VMH-dependent control of thermogenesis is mediated by circadian rhythms, since mice lacking the clock-control gen Bmal1 in the VMH show impaired thermogenesis during the night phase [77].
As mentioned above, several peripheral signals arrive at the VMH to activate thermogenesis in BAT and browning of WAT. Many of these signals such as thyroid hormones (THs) [66], [78], [79], bone morphogenetic protein 8B (BMP8B) [80], [81], leptin [82], estradiol (E2) [67], glucagon-like-peptide-1 (GLP1) analogues [83], and drugs such as nicotine [84] have been demonstrated to act through a common mechanism, involving inhibition of AMP-activated protein kinase (AMPK) which leads to the activation of sympathetic fibers to BAT and WAT [79], [81], [83]. More recent data demonstrate that BMP8B acts by decreasing AMPK in VMH neurons connected to the LHA through glutamatergic fibers that stimulate the orexin (OX) system which in turn activate the SNS promoting thermogenesis in BAT and WAT [81]. Conversely, adiponectin (ADP) and resistin (RSTN) caused a decrease in BAT thermogenesis by activation of hypothalamic AMPK [85], [86], [87], [88], [89], [90], [91], although the role of the VMH in these actions remains unproven. Additional peripheral signals that act on the hypothalamus eliciting sympathetic firing to BAT and activating thermogenesis include amylin and uroguanylin (UGN), with UGN also inducing browning of WAT [92], [93], [94]. However, it remains unknown whether the mechanism mediating the actions of these two hormones involves a decrease of AMPK activity in the VMH. The current evidence points to the energy sensor AMPK in the VMH as a canonical regulator of the brain-adipose axes through the SNS [16]. This makes hypothalamic AMPK a potential therapeutic target in the fight against obesity [16].
Hypothalamic complex lipids, such as ceramides, have been involved in the regulation of thermogenesis. Low levels of ceramides are implicated in cellular functions such as growth, differentiation, adhesion and apoptosis [95], [96], [97]. High levels of hypothalamic ceramides have been shown to be lipotoxic, by promoting ER stress in the VMH and reducing the BAT sympathetic tone which impairs the thermogenic process [98]. Overexpression of the chaperone glucose related protein 78 (GRP78) in the VMH, decreases ER stress thereby promoting BAT thermogenesis and browning of WAT causing weight loss and improvement in the metabolic phenotype of diet-induced obese rats in manner independent of feeding [99], [100]. These data suggest that modulation of hypothalamic ER stress could be a promising therapy to treatment of obesity and associated metabolic alterations.
2.5. The lateral hypothalamic area
The LHA has long been implicated in the control of thermogenesis in BAT and more recently in browning of WAT. Most of the evidences point to orexins A and B (OX-A and -B), that are orexigenic neuropeptides expressed predominantly in the LHA, as well as thermogenic inducers in BAT and WAT [81], [101], [102], [103], [104], [105], [106]. Interestingly, OXs are also required to correct development, differentiation and function of brown adipocytes [107]. In fact OXs act on both components of energy expenditure, thermogenesis and locomotor activity [104], [107], [108], [109], [110].
As discussed in the previous section, the LHA seems to receive projections from the VMH and overexpressing OXs activate sympathetic fibers innervating BAT and WAT [81], [108], [109], [110], [111], [112], [113], [114], [115]. It should be noted however, that OX null mice display normal temperature although they are unable to induce thermogenesis in response to stress induced by handling, suggesting that OX plays an important role in stress-dependent thermogenesis [116]. The involvement of OX neurons in BMP8B-induced thermogenesis has been aforementioned [81]. The possible induction of OX in the LHA to activate thermogenesis by other peripheral signals that trigger AMPK-VMH remains to be determined.
2.6. The paraventricular nucleus of the hypothalamus
The PVH regulates feeding behavior and energy homeostasis, and is widely interconnected with other hypothalamic and extra-hypothalamic areas. However, the exact role of the PVH in the control of thermogenic processes is controversial due to conflicting findings. For instance, direct stimulation of the PVH was reported to stimulate [71], inhibit [46] or have no effect [117] on BAT thermogenesis.
Some evidence suggests that the PVH activates thermogenesis during the febrile process by inducing thermogenesis in BAT and browning of WAT through sympathetic activation, while lesions in the PVH reduce fever [116], [118], [119], [120]. Furthermore there is evidence for anatomical connection between the PVH and the BAT [4], [63], [114]. The link between the PVH and BAT is further supported by the fact that administration of various excitatory substances in the PVH, such as corticotropin -releasing hormone (CRH), hydroxybutyrate, glutamate, noradrenaline, serotonin, tryptophan, cholecystokinin (CCK), brain-derived neurotrophic factor (BDNF), histamine, UCN, CART, PGE2, leptin or melanocortins leads to the activation of BAT thermogenesis [44], [71], [74], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131]. It is worth mentioning that genetic ablation of single-minded homolog 1 (SIM1; a factor necessary for the correct development of the PVH) reduces thermogenesis in BAT leading to obesity, suggesting that overall the PVH induces BAT thermogenesis [132]. There is also evidence suggesting that the PVH induces browning of white fat. For instance, CART administration into the PVH induces uncoupling 1, 2 and 3 (UCP1, UCP2 and UCP3) expression in brown and beige adipocytes [126]. Moreover, genetic ablation of liver X receptors (LXRs) leads to browning of WAT through activation of thyrotropin-releasing hormone (TRH) in the PVH [133]. Together these results demonstrate that the PVH regulates thermogenesis in both BAT and WAT.
Since stimulation or disinhibition of PVH neurons impairs thermogenesis in BAT and reverses the effects induced by cold or by N-methyl-D-aspartate receptor (NMDA; a specific agonist at the NMDA receptor mimicking the action of glutamate) administration into the rRPa led to the suggestion of the existence of a group of neurons in the PVH that project to the brainstem inhibiting the BAT sympathetic activation [46], [134]. This is supported by the demonstration that NPY administration into the PVH reduces BAT thermogenesis [37], [135]. On the other hand, the melanocortin system in the PVH regulates feeding behavior but appears not involved in the regulation of thermogenesis [136]. Finally, an interesting recent report described a negative link between oxidative stress in the PVH and thermogenesis, since inhibition of NADPH-oxidase, a reactive oxygen species (ROS) inducer, selectively in the PVH of obese mice increased BAT thermogenesis and browning of WAT through sympathetic activation protecting against diet-induced obesity [137].
Taken together, it appears that PVH negatively regulates BAT thermogenesis under basal conditions and some stimuli that activate the inhibitory presynaptic GABAergic neurons hinder the inhibition of thermogenesis by the PVH. However, further investigations are needed to establish the exact role of PVH in thermogenic regulation.
3. Next stop: the brainstem
As mentioned above, the different hypothalamic areas which receive several inputs including hormones and nutritional signals integrate the information that is transmitted through efferent fibers to many other CNS areas particularly the brainstem [11], [30], [109], [110], [138]. Among the brainstem nuclei, rRPa neurons receive tonic inhibitory inputs most notably from warm-sensitive GABAergic POA neurons, and disinhibition of rRPa neurons by various thermogenic signals increases BAT sympathetic activity [30]. The rRPa sympathetic premotor neurons integrate several signals from peripheral thermoreceptors, from the hypothalamus and other brain areas implicated in the regulation of the body temperature to control sympathetic output that activates BAT. Thus several neural networks converge in the rRPa which controls the sympathetic nerves subserving BAT and WAT [11], [22]. Viral tracing evidence demonstrates that sympathetic nerves that innervate subcutaneous WAT originate from rRPa [139]. Thus the role of the rRPa in the control of BAT and WAT sympathetic tone is well established.
Substantial evidence points to the importance of the brainstem nuclei such as the rRPa in mediating the sympathetically-mediated effects of the hypothalamic nuclei on BAT thermogenesis and WAT browning. The VMH is anatomically linked to rRPa as well as the IO [17], [64], [65], [66], [67], [68], [69]. It has been also demonstrated that a morphological connection between neurons expressing OX in the LHA with the rRPa neurons exists [111], [114]. Moreover, activation of the PVH neurons attenuates the increase in BAT activity evoked by NMDA injections into the rRPa, suggesting the existence of inhibitory projections from the PVH to rRPa decreasing BAT sympathetic nerve activity [46]. Although the cellular mechanisms mediating these actions are not fully understood, it has been reported that sympathetic premotor neurons expressing vesicular glutamate transporter type 3 (vGlut3) in the rRPA activate BAT [11], [22], [140]. Neurons in the rRPA also express serotonin (5-HT) that plays a role in BAT activation [64]. Thus, cold activates 5-HT neurons in the rRPa [141], and accordingly pharmacological activation of 5-HT receptors in the rRPa affects sympathetic tone to BAT in response to cold [142], [143]. Furthermore, inhibition of 5-HT biosynthesis inhibits thermogenesis [144].
Other brainstem areas have been implicated in the control of BAT sympathetic tone. For instance, catecholaminergic neurons in the ventrolateral medulla, also located in the brainstem, inhibit rRPa BAT premotor neurons through activation of α2 adrenergic receptors probably regulating a hypothermic process [134], [145]. The lateral parabrachial nucleus, periaqueductal gray, and locus coeruleus have also been linked to the control of thermogenesis [146], [147], [148], [149], [150], their exact role remains unclear.
4. The sympathetic highway
The ANS is a major and powerful regulatory system that ensures homeostasis of the body’s functions. The metabolic organs are innervated and tightly regulated by two antagonistic branches of the ANS, the SNS and the parasympathetic nervous system (PSNS) [4], [151], [152], [153]. Generally, the SNS has been associated with emergency and threat situations and thus referred to as the “fight or flight” response. The PSNS compensates for the SNS to restore the body to a state of calm and thus thought to mediate the "rest and digest" response.
Both BAT and WAT are innervated by the SNS with a wide presence of nerve terminals and postsynaptic β3-adrenoreceptors (β3-AR) on adipocytes [3], [154]. Induction of thermogenesis in adipocytes is triggered by the arrival of the sympathetic signal, consisting in the release of noradrenaline (NA) which binds to β3-AR activating lipolysis in WAT and the thermogenesis in brown and beige adipocytes. Stimulation with adrenergic agonist leads to the activation of thermogenesis and browning in both rodents and humans [3], [4], [5], [154]. Conversely, subcutaneous administration of β3-AR antagonist inhibits the thermogenic process in both BAT and WAT [66], [67], [99].
There are many peripheral signals that act directly on BAT and WAT to induce thermogenesis. THs act directly on BAT, where TH receptors (TRs) are widely expressed: the TRα1 subtype ensures the normal adrenergic thermogenic response and the TRβ1 subtype increases UCP1 expression [155], [156], [157], [158]. Another important peripheral signal that affects adipocytes is leptin. This is supported by the demonstration that leptin- or leptin receptor null mice have atrophied BAT and reduced thermogenesis activity [159], [160]. Insulin also regulates BAT activity, therefore mice lacking insulin receptor specifically in BAT show decreased BAT mass and fatty acid synthesis in brown adipocytes [161]. There is evidence demonstrating that noradrenergic stimulation of BAT induces the release of fibroblast growth factor 21 (FGF21) [162] which promotes thermogenic activity, increasing energy expenditure and UCP1 expression in BAT and WAT [163], [164], [165], [166].
5. Final destination: thermogenesis in the adipocytes
Classically, there were two main types of fat which exerted opposite functions. On the one hand, BAT underlie NST due to its high capacity to dissipate energy as heat (Fig. 3) [8], [167], [168], [169], [170], which is essential to maintain body temperature through uncoupled mitochondrial fatty acid oxidation [15], [169], [171]. For this purpose, brown adipocytes are specialized cells characterized by multilocular lipid droplets and a wide presence of mitochondria (giving the characteristic brown color), UCP1 in the inner mitochondrial membrane. White adipocytes are larger, contain fewer mitochondria and have low oxidative rate but a higher capacity to store energy in the form of TGs in a unilocular lipid droplets (Fig. 3) [172]. Moreover, the WAT acts as an endocrine organ producing various hormones and peptides including leptin [167], [173], [174], [175]. A third type of fat, the beige or brite fat (Fig. 3), has functional and anatomical characteristics that are intermediate between brown and white adipocytes. Beige adipocytes are induced in localized depots of WAT by a process named browning, and express thermogenic markers to support their thermogenic capacity [1], [2], [168], [169], [176], [177], [178].
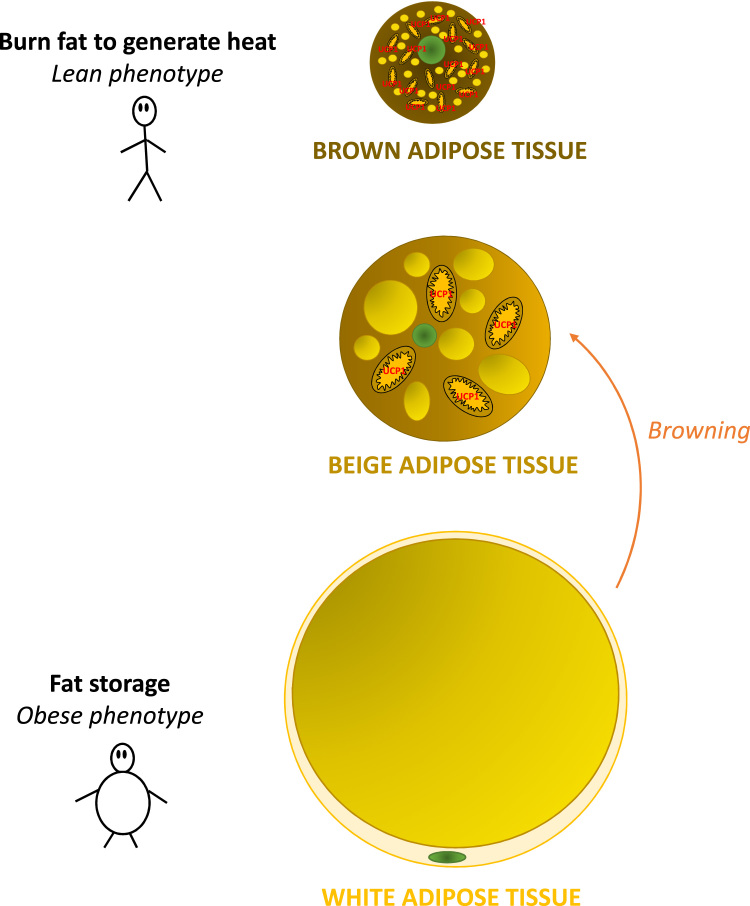
Fig. 3.
Types of adipose tissue. Brown adipose tissue (BAT, above) is characterized by the presence of small lipid droplets and many mitochondria expressing UCP1, that metabolize lipids to generate heat in a process termed thermogenesis. White adipocytes contain one lipid droplet that fills almost all of the cytoplasm acting as an energy store. Beige adipose tissue (middle) emerges in located depots of WAT and expresses some thermogenic markers. It shows anatomical and functional characteristics between BAT and WAT.
As was described in the previous sections, thermogenesis in brown and beige adipocytes is activated by increased firing rates in sympathetic nerves releasing NA which binds to β3-AR in the adipocytes [7], [11], [15], [167]. β3-AR is coupled to a stimulatory G-protein that triggers activation of adenylate cyclase (AC) which in turn catalyzes the conversion of ATP into cyclic AMP (cAMP) which leads the activation of protein kinase A (PKA). Several downstream pathways that increase thermogenesis are activated PKA in adipocytes. First, PKA activates p38 mitogen-activated protein kinase (MAPK) and extracellular signal regulated kinases (ERK)1/2, which facilitate UCP1 transcription. Moreover, PKA induces lipolysis, activating several lipases, such as adipose tissue triglyceride lipase (ATGL), hormone-sensitive lipase (HSL) and monoacylglycerol lipase (MGL), which hydrolyze triacylglycerol, diacylglycerol and monoacylglycerol, respectively, into free fatty acids (FFAs) and glycerol. Then, carnitine palmitoyltransferase 1a (CPT1a) introduces FFAs into the mitochondria, where they are oxidized through β-oxidation leading to reduction of NAD+ and FAD to NADH and FADH2, which serve as fuel of the electron transport chain. Oxidation drive protons out of the mitochondrial matrix from where they are re-introduced by UCP1, dissipating energy as heat [7], [170], [179]. This heat-producing process in brown or beige adipocytes, named thermogenesis, is the final destination of our trip.
6. The main reason for this travel: the impact of thermogenesis on obesity
Clearly, the autonomic control of thermogenic processes is critical for maintaining energy homeostasis and dysregulation in this regulatory mechanism is involved in metabolic disease and obesity. Thus, it may possible to use therapeutic strategies to reverse obesity-associated defects in autonomic control of thermogenesis. Indeed, evidence gathered in recent years suggests that activation of thermogenesis could became a promising target to curb obesity due its capacity to burn fat, especially after the recognition of the importance of BAT and browning in adult humans [180], [181], [182], [183], [184]. It is estimated that cold-induced thermogenesis in BAT in lean healthy volunteers burn as much as 25–400 kcal/day [185]. It should be noted that activation of thermogenesis not only induces weight loss, but also has a positive impact on other alterations in the metabolic syndrome such as insulin and leptin resistance, hepatic steatosis, hyperlipidemia, hyperglycemia, hypercholesterolemia and hypertriglyceridemia, likely due to the ability of the thermogenic adipocytes to uptake lipids and glucose from the circulation [15], [161], [186], [187], [188], [189], [190], [191]. Studies targeting central pathways such as hypothalamic AMPK [66], [67], [80], [81], [83], [84] or ER stress [48], [98], [99], have demonstrated the beneficial and specific effects of activating BAT thermogenesis and browning of WAT. Currently, some agents that have demonstrated action on hypothalamic targets such as AMPK including metformin, nicotine or liraglutide are used in the clinic [16].
7. Conclusions
The hypothalamus plays a major role in the regulation of systemic energy balance by regulating feeding behavior and energy expenditure. Our knowledge of the neuronal and molecular mechanisms underlying the control of thermogenesis has been expanded in recent years by the identification of many neuronal and peripheral signals that interact together to form a network that ensure tight regulation of energy balance. The new knowledge about the processes that regulate thermogenesis, especially the findings that brown fat exist in humans, has generated great enthusiasm and hope to identify new pharmacological targets against obesity and associated metabolic alterations.
The potential and serious side effects of activating a major and powerful system, such as the ANS must be taken also in consideration. In line with this, it is important to highlight that most of the studies focused on the central control of thermogenesis do not assess the possible effects of these signals on cardiovascular system, which is highly influenced by the ANS [158]. For this reason, strategies aiming to target neuronal circuits controlling the thermogenic activity of BAT and beige fat must have a high specificity in order to avoid undesired deleterious effects. This highlights the need to understand and define the different routes that travel from the CNS to peripheral organs of the body, before the way through the thermogenic pathways can be exploited safely.
Acknowledgements
The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreements no. 281408-the OBESITY53 project (RN) and no. 281854 -the ObERStress project (ML); Xunta de Galicia (RN: EM 2012/039 and 2012-CP069; ML: 2015-CP079); MINECO co-funded by the FEDER Program of EU (RN: BFU2015-70664R; CD: BFU2014-55871-P; ML: SAF2015-71026-R and BFU2015-70454-REDT/Adipoplast); Atresmedia Corporación (ML); US National Institutes of Health (KR: HL084207); American Heart Association (KR: EIA#14EIA18860041) and The University of Iowa Fraternal Order of Eagles Diabetes Research Center (KR). CC is recipient of a Sara Borrel Contract (CD14/0007); CIBER de Fisiopatología de la Obesidad y Nutrición is an initiative of ISCIII. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Cristina Contreras, Email: cristina.contreras@usc.es.
Miguel López, Email: m.lopez@usc.es.
References
- 1.Jespersen N.Z., Larsen T.J., Peijs L., Daugaard S., Homoe P., Loft A., de Jong J., Mathur N., Cannon B., Nedergaard J., Pedersen B.K., Moller K., Scheele C. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17:798–805. doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Wu J., Bostrom P., Sparks L.M., Ye L., Choi J.H., Giang A.H., Khandekar M., Virtanen K.A., Nuutila P., Schaart G., Huang K., Tu H., van Marken Lichtenbelt W.D., Hoeks J., Enerback S., Schrauwen P., Spiegelman B.M. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartness T.J., Liu Y., Shrestha Y.B., Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front. Neuroendocrinol. 2014;35:473–493. doi: 10.1016/j.yfrne.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartness T.J., Vaughan C.H., Song C.K. Sympathetic and sensory innervation of brown adipose tissue. Int J. Obes. 2010;34(Suppl. 1):S36–S42. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cypess A.M., Weiner L.S., Roberts-Toler C., Franquet Elia E., Kessler S.H., Kahn P.A., English J., Chatman K., Trauger S.A., Doria A., Kolodny G.M. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab. 2015;21:33–38. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cansell C., Luquet S. Triglyceride sensing in the reward circuitry: a new insight in feeding behaviour regulation. Biochimie. 2016;120:75–80. doi: 10.1016/j.biochi.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Contreras C., Nogueiras R., Dieguez C., Medina-Gomez G., López M. Hypothalamus and thermogenesis: heating the BAT, browning the WAT. Mol. Cell Endocrinol. 2016;438:107–115. doi: 10.1016/j.mce.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Lage R., Ferno J., Nogueiras R., Dieguez C., López M. Contribution of adaptive thermogenesis to the hypothalamic regulation of energy balance. Biochem. J. 2016;473:4063–4082. doi: 10.1042/BCJ20160012. [DOI] [PubMed] [Google Scholar]
- 9.López M., Dieguez C., Nogueiras R. Hypothalamic GLP-1: the control of BAT thermogenesis and browning of white fat. Adipocyte. 2015;4:141–145. doi: 10.4161/21623945.2014.983752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magnan C., Levin B.E., Luquet S. Brain lipid sensing and the neural control of energy balance. Mol. Cell. Endocrinol. 2015;418(Pt 1):3–8. doi: 10.1016/j.mce.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Morrison S.F., Madden C.J., Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014;19:741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez S., Claret M. Hypothalamic ER stress: a bridge between leptin resistance and obesity. FEBS Lett. 2015;589:1678–1687. doi: 10.1016/j.febslet.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Schneeberger M., Gomez-Valades A.G., Ramirez S., Gomis R., Claret M. Hypothalamic miRNAs: emerging roles in energy balance control. Front. Neurosci. 2015;9:41. doi: 10.3389/fnins.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeo G.S., Heisler L.K. Unraveling the brain regulation of appetite: lessons from genetics. Nat. Neurosci. 2012;15:1343–1349. doi: 10.1038/nn.3211. [DOI] [PubMed] [Google Scholar]
- 15.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 16.Labbe S.M., Caron A., Lanfray D., Monge-Rofarello B., Bartness T.J., Richard D. Hypothalamic control of brown adipose tissue thermogenesis. Front. Syst. Neurosci. 2015;9:150. doi: 10.3389/fnsys.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.López M., Nogueiras R., Tena-Sempere M., Dieguez C. Hypothalamic AMPK: a canonical regulator of whole-body energy balance. Nat. Rev. Endocrinol. 2016;12:421–432. doi: 10.1038/nrendo.2016.67. [DOI] [PubMed] [Google Scholar]
- 18.Boulant J.A. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin. Infect. Dis. 2000;31(Suppl. 5):S157–S161. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- 19.Fuller C.A., Horwitz B.A., Horowitz J.M. Shivering and nonshivering thermogenic responses of cold-exposed rats to hypothalamic warming. Am. J. Physiol. 1975;228:1519–1524. doi: 10.1152/ajplegacy.1975.228.5.1519. [DOI] [PubMed] [Google Scholar]
- 20.Guieu J.D., Hardy J.D. Effects of heating and cooling of the spinal cord on preoptic unit activity. J. Appl. Physiol. 1970;29:675–683. doi: 10.1152/jappl.1970.29.5.675. [DOI] [PubMed] [Google Scholar]
- 21.Imai-Matsumura K., Matsumura K., Nakayama T. Involvement of ventromedial hypothalamus in brown adipose tissue thermogenesis induced by preoptic cooling in rats. Jpn. J. Physiol. 1984;34:939–943. doi: 10.2170/jjphysiol.34.939. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura K. Central circuitries for body temperature regulation and fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R1207–R1228. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K., Morrison S.F. Preoptic mechanism for cold-defensive responses to skin cooling. J. Physiol. 2008;586:2611–2620. doi: 10.1113/jphysiol.2008.152686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preston E., Triandafillou J., Haas N. Colchicine lesions of ventromedial hypothalamus: effects on regulatory thermogenesis in the rat. Pharmacol. Biochem. Behav. 1989;32:301–307. doi: 10.1016/0091-3057(89)90247-5. [DOI] [PubMed] [Google Scholar]
- 25.Hogan S., Coscina D.V., Himms-Hagen J. Brown adipose tissue of rats with obesity-inducing ventromedial hypothalamic lesions. Am. J. Physiol. 1982;243:E338–E344. doi: 10.1152/ajpendo.1982.243.4.E338. [DOI] [PubMed] [Google Scholar]
- 26.Scammell T.E., Elmquist J.K., Griffin J.D., Saper C.B. Ventromedial preoptic prostaglandin E2 activates fever-producing autonomic pathways. J. Neurosci. 1996;16:6246–6254. doi: 10.1523/JNEUROSCI.16-19-06246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amir S., Schiavetto A. Injection of prostaglandin E2 into the anterior hypothalamic preoptic area activates brown adipose tissue thermogenesis in the rat. Brain Res. 1990;528:138–142. doi: 10.1016/0006-8993(90)90206-q. [DOI] [PubMed] [Google Scholar]
- 28.Monda M., Sullo A., De Luca V., Viggiano A., Pellicano M.P. Acute lesions of the ventromedial hypothalamus reduce sympathetic activation and thermogenic changes induced by PGE1. J. Physiol. Paris. 1997;91:285–290. doi: 10.1016/s0928-4257(97)82408-4. [DOI] [PubMed] [Google Scholar]
- 29.Steiner A.A., Antunes-Rodrigues J., Branco L.G. Role of preoptic second messenger systems (cAMP and cGMP) in the febrile response. Brain Res. 2002;944:135–145. doi: 10.1016/s0006-8993(02)02738-5. [DOI] [PubMed] [Google Scholar]
- 30.Cao W.H., Fan W., Morrison S.F. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126:229–240. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Monge-Roffarello B., Labbe S.M., Lenglos C., Caron A., Lanfray D., Samson P., Richard D. The medial preoptic nucleus as a site of the thermogenic and metabolic actions of melanotan II in male rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:R158–R166. doi: 10.1152/ajpregu.00059.2014. [DOI] [PubMed] [Google Scholar]
- 32.López M. Hypothalamic leptin resistance: from BBB to BBSome. PLoS Genet. 2016;12:e1005980. doi: 10.1371/journal.pgen.1005980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waterson M.J., Horvath T.L. Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell Metab. 2015;22:962–970. doi: 10.1016/j.cmet.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 34.Rahmouni K., Morgan D.A. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49:647–652. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 35.Harlan S.M., Morgan D.A., Agassandian K., Guo D.F., Cassell M.D., Sigmund C.D., Mark A.L., Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ. Res. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bewick G.A., Gardiner J.V., Dhillo W.S., Kent A.S., White N.E., Webster Z., Ghatei M.A., Bloom S.R. Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J. 2005;19:1680–1682. doi: 10.1096/fj.04-3434fje. [DOI] [PubMed] [Google Scholar]
- 37.Shi Y.C., Lau J., Lin Z., Zhang H., Zhai L., Sperk G., Heilbronn R., Mietzsch M., Weger S., Huang X.F., Enriquez R.F., Baldock P.A., Zhang L., Sainsbury A., Herzog H., Lin S. Arcuate NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN. Cell Metab. 2013;17:236–248. doi: 10.1016/j.cmet.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Kuperman Y., Weiss M., Dine J., Staikin K., Golani O., Ramot A., Nahum T., Kuhne C., Shemesh Y., Wurst W., Harmelin A., Deussing J.M., Eder M., Chen A. CRFR1 in AgRP neurons modulates sympathetic nervous system activity to adapt to cold stress and fasting. Cell Metab. 2016;23:1185–1199. doi: 10.1016/j.cmet.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler A.A., Cone R.D. The melanocortin receptors: lessons from knockout models. Neuropeptides. 2002;36:77–84. doi: 10.1054/npep.2002.0890. [DOI] [PubMed] [Google Scholar]
- 40.Farooqi S., O'Rahilly S. Genetics of obesity in humans. Endocr. Rev. 2006;27:710–718. doi: 10.1210/er.2006-0040. [DOI] [PubMed] [Google Scholar]
- 41.Yasuda T., Masaki T., Kakuma T., Yoshimatsu H. Hypothalamic melanocortin system regulates sympathetic nerve activity in brown adipose tissue. Exp. Biol. Med. 2004;229:235–239. doi: 10.1177/153537020422900303. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y., Kilroy G.E., Henagan T.M., Prpic-Uhing V., Richards W.G., Bannon A.W., Mynatt R.L., Gettys T.W. Targeted deletion of melanocortin receptor subtypes 3 and 4, but not CART, alters nutrient partitioning and compromises behavioral and metabolic responses to leptin. FASEB J. 2005;19:1482–1491. doi: 10.1096/fj.05-3851com. [DOI] [PubMed] [Google Scholar]
- 43.Dodd G., Descherf S., Loh K., Simonds S.E., Wiede F., Balland E., Merry T.L., Münzberg H., Zhang Z.Y., Kahn B.B., Neel B.G., Bence K.K., Andrews Z.B., Cowley M.A., Tiganis T. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160:88–104. doi: 10.1016/j.cell.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kong W., Stanley S., Gardiner J., Abbott C., Murphy K., Seth A., Connoley I., Ghatei M., Stephens D., Bloom S. A role for arcuate cocaine and amphetamine-regulated transcript in hyperphagia, thermogenesis, and cold adaptation. FASEB J. 2003;17:1688–1690. doi: 10.1096/fj.02-0805fje. [DOI] [PubMed] [Google Scholar]
- 45.Kong D., Tong Q., Ye C., Koda S., Fuller P.M., Krashes M.J., Vong L., Ray R.S., Olson D.P., Lowell B.B. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell. 2012;151:645–657. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madden C.J., Morrison S.F. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R831–R843. doi: 10.1152/ajpregu.91007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X., Zhang G., Zhang H., Karin M., Bai H., Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneeberger M., Dietrich M.O., Sebastian D., Imbernon M., Castano C., Garcia A., Esteban Y., Gonzalez-Franquesa A., Rodriguez I.C., Bortolozzi A., Garcia-Roves P.M., Gomis R., Nogueiras R., Horvath T.L., Zorzano A., Claret M. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell. 2013;155:172–187. doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneeberger M., Gómez-Valadés Alicia G., Altirriba J., Sebastián D., Ramírez S., Garcia A., Esteban Y., Drougard A., Ferrés-Coy A., Bortolozzi A., Garcia-Roves Pablo M., Jones John G., Manadas B., Zorzano A., Gomis R., Claret M. Reduced α-MSH underlies hypothalamic ER-stress-induced hepatic gluconeogenesis. Cell Rep. 2015;12:361–370. doi: 10.1016/j.celrep.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 50.Williams K.W., Liu T., Kong X., Fukuda M., Deng Y., Berglund E.D., Deng Z., Gao Y., Liu T., Sohn J.W., Jia L., Fujikawa T., Kohno D., Scott M.M., Lee S., Lee C.E., Sun K., Chang Y., Scherer P.E., Elmquist J.K. Xbp1s in Pomc neurons connects ER stress with energy balance and glucose homeostasis. Cell Metab. 2014;20:471–482. doi: 10.1016/j.cmet.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruan H.B., Dietrich M.O., Liu Z.W., Zimmer M.R., Li M.D., Singh J.P., Zhang K., Yin R., Wu J., Horvath T.L., Yang X. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell. 2014;159:306–317. doi: 10.1016/j.cell.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao W.H., Morrison S.F. Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacology. 2006;51:426–437. doi: 10.1016/j.neuropharm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 53.Dimicco J.A., Zaretsky D.V. The dorsomedial hypothalamus: a new player in thermoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R47–R63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- 54.Okamura H., Abitbol M., Julien J.F., Dumas S., Berod A., Geffard M., Kitahama K., Bobillier P., Mallet J., Wiklund L. Neurons containing messenger RNA encoding glutamate decarboxylase in rat hypothalamus demonstrated by in situ hybridization, with special emphasis on cell groups in medial preoptic area, anterior hypothalamic area and dorsomedial hypothalamic nucleus. Neuroscience. 1990;39:675–699. doi: 10.1016/0306-4522(90)90252-y. [DOI] [PubMed] [Google Scholar]
- 55.Osaka T. Blockade of prostaglandin E2-induced thermogenesis by unilateral microinjection of GABAA receptor antagonist into the preoptic area. Brain Res. 2008;1230:107–114. doi: 10.1016/j.brainres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 56.Zaretskaia M.V., Zaretsky D.V., Shekhar A., DiMicco J.A. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]
- 57.Chao P.T., Yang L., Aja S., Moran T.H., Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab. 2011;13:573–583. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang W., Cline M.A., Gilbert E.R. Hypothalamus-adipose tissue crosstalk: neuropeptide Y and the regulation of energy metabolism. Nutr. Metab. 2014;11:27. doi: 10.1186/1743-7075-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang L., Scott K.A., Hyun J., Tamashiro K.L., Tray N., Moran T.H., Bi S. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. J. Neurosci. 2009;29:179–190. doi: 10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rezai-Zadeh K., Yu S., Jiang Y., Laque A., Schwartzenburg C., Morrison C.D., Derbenev A.V., Zsombok A., Munzberg H. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol. Metab. 2014;3:681–693. doi: 10.1016/j.molmet.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Enriori P.J., Sinnayah P., Simonds S.E., Garcia Rudaz C., Cowley M.A. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J. Neurosci. 2011;31:12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bamshad M., Aoki V.T., Adkison M.G., Warren W.S., Bartness T.J. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am. J. Physiol. 1998;275:R291–R299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- 63.Bamshad M., Song C.K., Bartness T.J. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am. J. Physiol. 1999;276:R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- 64.Cano G., Passerin A.M., Schiltz J.C., Card J.P., Morrison S.F., Sved A.F. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J. Comp. Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- 65.Lindberg D., Chen P., Li C. Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to autonomic centers of the hypothalamus and hindbrain. J. Comp. Neurol. 2013;521:3167–3190. doi: 10.1002/cne.23338. [DOI] [PubMed] [Google Scholar]
- 66.López M., Varela L., Vazquez M.J., Rodriguez-Cuenca S., Gonzalez C.R., Velagapudi V.R., Morgan D.A., Schoenmakers E., Agassandian K., Lage R., Martinez de Morentin P.B., Tovar S., Nogueiras R., Carling D., Lelliott C., Gallego R., Oresic M., Chatterjee K., Saha A.K., Rahmouni K., Dieguez C., Vidal-Puig A. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat. Med. 2010;16:1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinez de Morentin P.B., Gonzalez-Garcia I., Martins L., Lage R., Fernandez-Mallo D., Martinez-Sanchez N., Ruiz-Pino F., Liu J., Morgan D.A., Pinilla L., Gallego R., Saha A.K., Kalsbeek A., Fliers E., Bisschop P.H., Dieguez C., Nogueiras R., Rahmouni K., Tena-Sempere M., López M. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20:41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morrison S.F. RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. Am. J. Physiol. 1999;276:R962–R973. doi: 10.1152/ajpregu.1999.276.4.R962. [DOI] [PubMed] [Google Scholar]
- 69.Uno T., Shibata M. Role of inferior olive and thoracic IML neurons in nonshivering thermogenesis in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R536–R546. doi: 10.1152/ajpregu.2001.280.2.R536. [DOI] [PubMed] [Google Scholar]
- 70.Amir S. Intra-ventromedial hypothalamic injection of glutamate stimulates brown adipose tissue thermogenesis in the rat. Brain Res. 1990;511:341–344. doi: 10.1016/0006-8993(90)90181-a. [DOI] [PubMed] [Google Scholar]
- 71.Amir S. Stimulation of the paraventricular nucleus with glutamate activates interscapular brown adipose tissue thermogenesis in rats. Brain Res. 1990;508:152–155. doi: 10.1016/0006-8993(90)91129-5. [DOI] [PubMed] [Google Scholar]
- 72.Amir S., Schiavetto A., Pollock R. Insulin co-injection suppresses the thermogenic response to glutamate microinjection into the VMH in rats. Brain Res. 1990;527:326–329. doi: 10.1016/0006-8993(90)91153-8. [DOI] [PubMed] [Google Scholar]
- 73.Hugie T., Halvorson I., Thornhill J. Brown adipose tissue temperature responses following electrical stimulation of ventromedial hypothalamic and lateral preoptic areas or after norepinephrine infusion to Long Evans or Sprague-Dawley rats. Brain Res. 1992;575:57–62. doi: 10.1016/0006-8993(92)90422-6. [DOI] [PubMed] [Google Scholar]
- 74.Sakaguchi T., Arase K., Bray G.A. Effect of intrahypothalamic hydroxybutyrate on sympathetic firing rate. Metabolism. 1988;37:732–735. doi: 10.1016/0026-0495(88)90006-6. [DOI] [PubMed] [Google Scholar]
- 75.Sakaguchi T., Bray G.A. Effect of norepinephrine, serotonin and tryptophan on the firing rate of sympathetic nerves. Brain Res. 1989;492:271–280. doi: 10.1016/0006-8993(89)90910-4. [DOI] [PubMed] [Google Scholar]
- 76.Yoshimatsu H., Egawa M., Bray G.A. Sympathetic nerve activity after discrete hypothalamic injections of L-glutamate. Brain Res. 1993;601:121–128. doi: 10.1016/0006-8993(93)91702-t. [DOI] [PubMed] [Google Scholar]
- 77.Orozco-Solis R., Aguilar-Arnal L., Murakami M., Peruquetti R., Ramadori G., Coppari R., Sassone-Corsi P. The circadian clock in the ventromedial hypothalamus controls cyclic energy expenditure. Cell Metab. 2016;23:467–478. doi: 10.1016/j.cmet.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alvarez-Crespo M., Csikasz R.I., Martinez-Sanchez N., Dieguez C., Cannon B., Nedergaard J., López M. Essential role of UCP1 modulating the central effects of thyroid hormones on energy balance. Mol. Metab. 2016;5:271–282. doi: 10.1016/j.molmet.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martinez-Sanchez N., Moreno-Navarrete J.M., Contreras C., Rial-Pensado E., Ferno J., Nogueiras R., Dieguez C., Fernandez-Real J.M., López M. Thyroid hormones induce browning of white fat. J. Endocrinol. 2017;232:351–362. doi: 10.1530/JOE-16-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whittle A.J., Carobbio S., Martins L., Slawik M., Hondares E., Vazquez M.J., Morgan D., Csikasz R.I., Gallego R., Rodriguez-Cuenca S., Dale M., Virtue S., Villarroya F., Cannon B., Rahmouni K., López M., Vidal-Puig A. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martins L., Seoane-Collazo P., Contreras C., Gonzalez-Garcia I., Martinez-Sanchez N., Gonzalez F., Zalvide J., Gallego R., Dieguez C., Nogueiras R., Tena-Sempere M., López M. A Functional Link between AMPK and Orexin Mediates the Effect of BMP8B on Energy Balance. Cell Rep. 2016;16:2231–2242. doi: 10.1016/j.celrep.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tanida M., Yamamoto N., Shibamoto T., Rahmouni K. Involvement of hypothalamic AMP-activated protein kinase in leptin-induced sympathetic nerve activation. PLoS One. 2013;8:e56660. doi: 10.1371/journal.pone.0056660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beiroa D., Imbernon M., Gallego R., Senra A., Herranz D., Villaroya F., Serrano M., Ferno J., Salvador J., Escalada J., Dieguez C., López M., Fruhbeck G., Nogueiras R. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63:3346–3358. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 84.Seoane-Collazo P., Martinez de Morentin P.B., Ferno J., Dieguez C., Nogueiras R., López M. Nicotine improves obesity and hepatic steatosis and ER stress in diet-induced obese male rats. Endocrinology. 2014;155:1679–1689. doi: 10.1210/en.2013-1839. [DOI] [PubMed] [Google Scholar]
- 85.Guillod-Maximin E., Roy A.F., Vacher C.M., Aubourg A., Bailleux V., Lorsignol A., Penicaud L. M. Parquet,M. Taouis, Adiponectin receptors are expressed in hypothalamus and colocalized with proopiomelanocortin and neuropeptide Y in rodent arcuate neurons. J. Endocrinol. 2009;200:93–105. doi: 10.1677/JOE-08-0348. [DOI] [PubMed] [Google Scholar]
- 86.Koch C.E., Lowe C., Legler K., Benzler J., Boucsein A., Bottiger G., Grattan D.R., Williams L.M., Tups A. Central adiponectin acutely improves glucose tolerance in male mice. Endocrinology. 2014;155:1806–1816. doi: 10.1210/en.2013-1734. [DOI] [PubMed] [Google Scholar]
- 87.Kosari S., Camera D.M., Hawley J.A., Stebbing M., Badoer E. ERK1/2 in the brain mediates the effects of central resistin on reducing thermogenesis in brown adipose tissue. Int. J. Physiol. Pathophysiol. Pharmacol. 2013;5:184–189. [PMC free article] [PubMed] [Google Scholar]
- 88.Kosari S., Rathner J.A., Badoer E. Central resistin enhances renal sympathetic nerve activity via phosphatidylinositol 3-kinase but reduces the activity to brown adipose tissue via extracellular signal-regulated kinase 1/2. J. Neuroendocrinol. 2012;24:1432–1439. doi: 10.1111/j.1365-2826.2012.02352.x. [DOI] [PubMed] [Google Scholar]
- 89.Kubota N., Yano W., Kubota T., Yamauchi T., Itoh S., Kumagai H., Kozono H., Takamoto I., Okamoto S., Shiuchi T., Suzuki R., Satoh H., Tsuchida A., Moroi M., Sugi K., Noda T., Ebinuma H., Ueta Y., Kondo T., Araki E., Ezaki O., Nagai R., Tobe K., Terauchi Y., Ueki K., Minokoshi Y., Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 90.Vazquez M.J., Gonzalez C.R., Varela L., Lage R., Tovar S., Sangiao-Alvarellos S., Williams L.M., Vidal-Puig A., Nogueiras R., López M., Dieguez C. Central resistin regulates hypothalamic and peripheral lipid metabolism in a nutritional-dependent fashion. Endocrinology. 2008;149:4534–4543. doi: 10.1210/en.2007-1708. [DOI] [PubMed] [Google Scholar]
- 91.Wen J.P., Liu C.E., Hu Y.T., Chen G., Lin L.X. Globular adiponectin regulates energy homeostasis through AMP-activated protein kinase-acetyl-CoA carboxylase (AMPK/ACC) pathway in the hypothalamus. Mol. Cell. Biochem. 2010;344:109–115. doi: 10.1007/s11010-010-0534-2. [DOI] [PubMed] [Google Scholar]
- 92.Fernandes-Santos C., Zhang Z., Morgan D.A., Guo D.F., Russo A.F., Rahmouni K. Amylin acts in the central nervous system to increase sympathetic nerve activity. Endocrinology. 2013;154:2481–2488. doi: 10.1210/en.2012-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Folgueira C., Beiroa D., Callon A., Al-Massadi O., Barja-Fernandez S., Senra A., Fernø J., López M., Dieguez C., Casanueva F.F., Rohner-Jeanrenaud F., Seoane L.M., Nogueiras R. Uroguanylin action in the brain reduces weight gain in obese mice via different efferent autonomic pathways. Diabetes. 2016;65:421–432. doi: 10.2337/db15-0889. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Z., Liu X., Morgan D.A., Kuburas A., Thedens D.R., Russo A.F., Rahmouni K. Neuronal receptor activity-modifying protein 1 promotes energy expenditure in mice. Diabetes. 2011;60:1063–1071. doi: 10.2337/db10-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cowart L.A. Sphingolipids: players in the pathology of metabolic disease. Trends Endocrinol. Metab. 2009;20:34–42. doi: 10.1016/j.tem.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 96.Hannun Y.A., Obeid L.M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 97.Holland W.L., Summers S.A. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr. Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Contreras C., Gonzalez-Garcia I., Martinez-Sanchez N., Seoane-Collazo P., Jacas J., Morgan D.A., Serra D., Gallego R., Gonzalez F., Casals N., Nogueiras R., Rahmouni K., Dieguez C., López M. Central ceramide-induced hypothalamic lipotoxicity and ER stress regulate energy balance. Cell Rep. 2014;9:366–377. doi: 10.1016/j.celrep.2014.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Contreras C., Gonzalez-Garcia I., Seoane-Collazo P., Martinez-Sanchez N., Linares-Pose L., Rial-Pensado E., Ferno J., Tena-Sempere M., Casals N., Dieguez C., Nogueiras R., López M. Reduction of hypothalamic endoplasmic reticulum stress activates browning of white fat and ameliorates obesity. Diabetes. 2017;66:87–99. doi: 10.2337/db15-1547. [DOI] [PubMed] [Google Scholar]
- 100.Contreras C., López M. Ceramide sensing in the hippocampus: the lipostatic theory and Ockham's razor. Mol. Metab. 2014;3:90–91. doi: 10.1016/j.molmet.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hara J., Beuckmann C.T., Nambu T., Willie J.T., Chemelli R.M., Sinton C.M., Sugiyama F., Yagami K., Goto K., Yanagisawa M., Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 102.Teske J.A., Levine A.S., Kuskowski M., Levine J.A., Kotz C.M. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R889–R899. doi: 10.1152/ajpregu.00536.2005. [DOI] [PubMed] [Google Scholar]
- 103.Lubkin M., Stricker-Krongrad A. Independent Feeding and Metabolic Actions of Orexins in Mice. Biochem. Biophys. Res. Commun. 1998;253:241–245. doi: 10.1006/bbrc.1998.9750. [DOI] [PubMed] [Google Scholar]
- 104.Sellayah D., Sikder D. Orexin receptor-1 mediates brown fat developmental differentiation. Adipocyte. 2012;1:58–63. doi: 10.4161/adip.18965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang J., Osaka T., Inoue S. Energy expenditure by intracerebroventricular administration of orexin to anesthetized rats. Neurosci. Lett. 2001;315:49–52. doi: 10.1016/s0304-3940(01)02322-9. [DOI] [PubMed] [Google Scholar]
- 106.Yoshimichi G., Yoshimatsu H., Masaki T., Sakata T. Orexin-A regulates body temperature in coordination with arousal status. Exp. Biol. Med. 2001;226:468–476. doi: 10.1177/153537020122600513. [DOI] [PubMed] [Google Scholar]
- 107.Sellayah D., Bharaj P., Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 2011;14:478–490. doi: 10.1016/j.cmet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 108.Fernø J., Señarís R., Diéguez C., Tena-Sempere M., López M. Orexins (hypocretins) and energy balance: more than feeding. Mol. Cell. Endocrinol. 2015;418(Part 1):17–26. doi: 10.1016/j.mce.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 109.Morrison S.F., Madden C.J., Tupone D. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. Adipocyte. 2012;1:116–120. doi: 10.4161/adip.19736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tupone D., Madden C.J., Cano G., Morrison S.F. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J. Neurosci. 2011;31:15944–15955. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Berthoud H.R., Patterson L.M., Sutton G.M., Morrison C., Zheng H. Orexin inputs to caudal raphe neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem. Cell Biol. 2005;123:147–156. doi: 10.1007/s00418-005-0761-x. [DOI] [PubMed] [Google Scholar]
- 112.Cerri M., Morrison S.F. Activation of lateral hypothalamic neurons stimulates brown adipose tissue thermogenesis. Neuroscience. 2005;135:627–638. doi: 10.1016/j.neuroscience.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 113.Monda M., Viggiano A., Viggiano A., Fuccio F., De Luca V. Injection of orexin A into the diagonal band of Broca induces sympathetic and hyperthermic reactions. Brain Res. 2004;1018:265–271. doi: 10.1016/j.brainres.2004.05.084. [DOI] [PubMed] [Google Scholar]
- 114.Oldfield B.J., Giles M.E., Watson A., Anderson C., Colvill L.M., McKinley M.J. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110:515–526. doi: 10.1016/s0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- 115.Russell S.H., Small C.J., Sunter D., Morgan I., Dakin C.L., Cohen M.A., Bloom S.R. Chronic intraparaventricular nuclear administration of orexin A in male rats does not alter thyroid axis or uncoupling protein-1 in brown adipose tissue. Regul. Pept. 2002;104:61–68. doi: 10.1016/s0167-0115(01)00349-4. [DOI] [PubMed] [Google Scholar]
- 116.Zhang W., Sunanaga J., Takahashi Y., Mori T., Sakurai T., Kanmura Y., Kuwaki T. Orexin neurons are indispensable for stress-induced thermogenesis in mice. J. Physiol. 2010;588:4117–4129. doi: 10.1113/jphysiol.2010.195099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Holt S.J., Wheal H.V., York D.A. Response of brown adipose tissue to electrical stimulation of hypothalamic centres in intact and adrenalectomized Zucker rats. Neurosci. Lett. 1988;84:63–67. doi: 10.1016/0304-3940(88)90338-2. [DOI] [PubMed] [Google Scholar]
- 118.Caldeira J.C., Franci C.R., Pela I.R. Bilateral lesion of hypothalamic paraventricular nucleus abolishes fever induced by endotoxin and bradykinin in rats. Ann. N. Y. Acad. Sci. 1998;856:294–297. doi: 10.1111/j.1749-6632.1998.tb08342.x. [DOI] [PubMed] [Google Scholar]
- 119.Horn T., Wilkinson M.F., Landgraf R., Pittman Q.J. Reduced febrile responses to pyrogens after lesions of the hypothalamic paraventricular nucleus. Am. J. Physiol. 1994;267:R323–R328. doi: 10.1152/ajpregu.1994.267.1.R323. [DOI] [PubMed] [Google Scholar]
- 120.Lu J., Zhang Y.H., Chou T.C., Gaus S.E., Elmquist J.K., Shiromani P., Saper C.B. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J. Neurosci. 2001;21:4864–4874. doi: 10.1523/JNEUROSCI.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.LeFeuvre R.A., Rothwell N.J., Stock M.J. Activation of brown fat thermogenesis in response to central injection of corticotropin releasing hormone in the rat. Neuropharmacology. 1987;26:1217–1221. doi: 10.1016/0028-3908(87)90272-3. [DOI] [PubMed] [Google Scholar]
- 122.Yoshimatsu H., Egawa M., Bray G.A. Effects of cholecystokinin on sympathetic activity to interscapular brown adipose tissue. Brain Res. 1992;597:298–303. doi: 10.1016/0006-8993(92)91486-x. [DOI] [PubMed] [Google Scholar]
- 123.Wang C., Bomberg E., Billington C., Levine A., Kotz C.M. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R992–R1002. doi: 10.1152/ajpregu.00516.2006. [DOI] [PubMed] [Google Scholar]
- 124.Yasuda T., Masaki T., Sakata T., Yoshimatsu H. Hypothalamic neuronal histamine regulates sympathetic nerve activity and expression of uncoupling protein 1 mRNA in brown adipose tissue in rats. Neuroscience. 2004;125:535–540. doi: 10.1016/j.neuroscience.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 125.Kotz C.M., Wang C., Levine A.S., Billington C.J. Urocortin in the hypothalamic PVN increases leptin and affects uncoupling proteins-1 and -3 in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R546–R551. doi: 10.1152/ajpregu.00436.2001. [DOI] [PubMed] [Google Scholar]
- 126.Wang C., Billington C.J., Levine A.S., Kotz C.M. Effect of CART in the hypothalamic paraventricular nucleus on feeding and uncoupling protein gene expression. NeuroReport. 2000;11:3251–3255. doi: 10.1097/00001756-200009280-00040. [DOI] [PubMed] [Google Scholar]
- 127.Bhatnagar S., Meaney M.J., Amir S. The effects of prostaglandin E2 injected into the paraventricular nucleus of the hypothalamus on brown adipose tissue thermogenesis in spontaneously hypertensive rats. Brain Res. 1993;613:285–287. doi: 10.1016/0006-8993(93)90911-6. [DOI] [PubMed] [Google Scholar]
- 128.Bagnasco M., Dube M.G., Katz A., Kalra P.S., Kalra S.P. Leptin expression in hypothalamic PVN reverses dietary obesity and hyperinsulinemia but stimulates ghrelin. Obes. Res. 2003;11:1463–1470. doi: 10.1038/oby.2003.196. [DOI] [PubMed] [Google Scholar]
- 129.Jiang Y., Munzberg H., Derbenev A., Zsombok A. Leptin regulates synaptic activity of Brown adipose tissue-related pre-sympathetic neurons in the paraventricular nucleus of the mice. FASEB J. 2015;29 [Google Scholar]
- 130.Skibicka K.P., Grill H.J. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology. 2009;150:5351–5361. doi: 10.1210/en.2009-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Song C.K., Vaughan C.H., Keen-Rhinehart E., Harris R.B., Richard D., Bartness T.J. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R417–R428. doi: 10.1152/ajpregu.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xi D., Gandhi N., Lai M., Kublaoui B.M. Ablation of Sim1 neurons causes obesity through hyperphagia and reduced energy expenditure. PLoS One. 2012;7:e36453. doi: 10.1371/journal.pone.0036453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miao Y., Wu W., Dai Y., Maneix L., Huang B., Warner M., Gustafsson J.A. Liver X receptor beta controls thyroid hormone feedback in the brain and regulates browning of subcutaneous white adipose tissue. Proc. Natl. Acad. Sci. USA. 2015;112:14006–14011. doi: 10.1073/pnas.1519358112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Madden C.J., Tupone D., Cano G., Morrison S.F. alpha2 Adrenergic receptor-mediated inhibition of thermogenesis. J. Neurosci. 2013;33:2017–2028. doi: 10.1523/JNEUROSCI.4701-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Egawa M., Yoshimatsu H., Bray G.A. Neuropeptide Y suppresses sympathetic activity to interscapular brown adipose tissue in rats. Am. J. Physiol. 1991;260:R328–R334. doi: 10.1152/ajpregu.1991.260.2.R328. [DOI] [PubMed] [Google Scholar]
- 136.Balthasar N., Dalgaard L.T., Lee C.E., Yu J., Funahashi H., Williams T., Ferreira M., Tang V., McGovern R.A., Kenny C.D., Christiansen L.M., Edelstein E., Choi B., Boss O., Aschkenasi C., Zhang C.Y., Mountjoy K., Kishi T., Elmquist J.K., Lowell B.B. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 137.Lob H.E., Song J., Hurr C., Chung A., Young C.N., Mark A.L., Davisson R.L. Deletion of p22phox-dependent oxidative stress in the hypothalamus protects against obesity by modulating beta3-adrenergic mechanisms. JCI Insight. 2017;2:e87094. doi: 10.1172/jci.insight.87094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fan W., Morrison S.F., Cao W.H., Yu P. Thermogenesis activated by central melanocortin signaling is dependent on neurons in the rostral raphe pallidus (rRPa) area. Brain Res. 2007;1179:61–69. doi: 10.1016/j.brainres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 139.Nguyen N.L., Randall J., Banfield B.W., Bartness T.J. Central sympathetic innervations to visceral and subcutaneous white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;306:R375–R386. doi: 10.1152/ajpregu.00552.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nakamura K., Matsumura K., Hubschle T., Nakamura Y., Hioki H., Fujiyama F., Boldogkoi Z., Konig M., Thiel H.J., Gerstberger R., Kobayashi S., Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J. Neurosci. 2004;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nason M.W., Jr, Mason P. Medullary raphe neurons facilitate brown adipose tissue activation. J. Neurosci. 2006;26:1190–1198. doi: 10.1523/JNEUROSCI.4707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Madden C.J., Morrison S.F. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J. Physiol. 2006;577:525–537. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nakamura K., Morrison S.F. Central efferent pathways for cold-defensive and febrile shivering. J. Physiol. 2011;589:3641–3658. doi: 10.1113/jphysiol.2011.210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fuller N.J., Stirling D.M., Dunnett S., Reynolds G.P., Ashwell M. Decreased brown adipose tissue thermogenic activity following a reduction in brain serotonin by intraventricular p-chlorophenylalanine. Biosci. Rep. 1987;7:121–127. doi: 10.1007/BF01121875. [DOI] [PubMed] [Google Scholar]
- 145.Cao W.H., Madden C.J., Morrison S.F. Inhibition of brown adipose tissue thermogenesis by neurons in the ventrolateral medulla and in the nucleus tractus solitarius. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R277–R290. doi: 10.1152/ajpregu.00039.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Almeida M.C., Steiner A.A., Coimbra N.C., Branco L.G. Thermoeffector neuronal pathways in fever: a study in rats showing a new role of the locus coeruleus. J. Physiol. 2004;558:283–294. doi: 10.1113/jphysiol.2004.066654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen X.M., Nishi M., Taniguchi A., Nagashima K., Shibata M., Kanosue K. The caudal periaqueductal gray participates in the activation of brown adipose tissue in rats. Neurosci. Lett. 2002;331:17–20. doi: 10.1016/s0304-3940(02)00757-7. [DOI] [PubMed] [Google Scholar]
- 148.Nakamura K., Morrison S.F. A thermosensory pathway that controls body temperature. Nat. Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rathner J.A., Morrison S.F. Rostral ventromedial periaqueductal gray: a source of inhibition of the sympathetic outflow to brown adipose tissue. Brain Res. 2006;1077:99–107. doi: 10.1016/j.brainres.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 150.Yoshida K., Konishi M., Nagashima K., Saper C.B., Kanosue K. Fos activation in hypothalamic neurons during cold or warm exposure: projections to periaqueductal gray matter. Neuroscience. 2005;133:1039–1046. doi: 10.1016/j.neuroscience.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 151.Harlan S.M., Rahmouni K. PI3K signaling: a key pathway in the control of sympathetic traffic and arterial pressure by leptin. Mol. Metab. 2013;2:69–73. doi: 10.1016/j.molmet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Buijs R.M. The autonomic nervous system: a balancing act. Handb. Clin. Neurol. 2013;117:1–11. doi: 10.1016/B978-0-444-53491-0.00001-8. [DOI] [PubMed] [Google Scholar]
- 153.Seoane-Collazo P., Ferno J., Gonzalez F., Dieguez C., Leis R., Nogueiras R., López M. Hypothalamic-autonomic control of energy homeostasis. Endocrine. 2015;50:276–291. doi: 10.1007/s12020-015-0658-y. [DOI] [PubMed] [Google Scholar]
- 154.Zeng W., Pirzgalska R.M., Pereira M.M., Kubasova N., Barateiro A., Seixas E., Lu Y.H., Kozlova A., Voss H., Martins G.G., Friedman J.M., Domingos A.I. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell. 2015;163:84–94. doi: 10.1016/j.cell.2015.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Silva J.E. Thermogenic mechanisms and their hormonal regulation. Physiol. Rev. 2006;86:435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 156.Bianco A.C., Sheng X.Y., Silva J.E. Triiodothyronine amplifies norepinephrine stimulation of uncoupling protein gene transcription by a mechanism not requiring protein synthesis. J. Biol. Chem. 1988;263:18168–18175. [PubMed] [Google Scholar]
- 157.Martinez de Mena R., Scanlan T.S., Obregon M.J. The T3 receptor beta1 isoform regulates UCP1 and D2 deiodinase in rat brown adipocytes. Endocrinology. 2010;151:5074–5083. doi: 10.1210/en.2010-0533. [DOI] [PubMed] [Google Scholar]
- 158.Ribeiro M.O., Bianco S.D., Kaneshige M., Schultz J.J., Cheng S.Y., Bianco A.C., Brent G.A. Expression of uncoupling protein 1 in mouse brown adipose tissue is thyroid hormone receptor-beta isoform specific and required for adaptive thermogenesis. Endocrinology. 2010;151:432–440. doi: 10.1210/en.2009-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ueno N., Oh-ishi S., Segawa M., Nishida M., Fukuwatari Y., Kizaki T., Ookawara T., Ohno H. Effect of age on brown adipose tissue activity in the obese (ob/ob) mouse. Mech. Ageing Dev. 1998;100:67–76. doi: 10.1016/s0047-6374(97)00123-1. [DOI] [PubMed] [Google Scholar]
- 160.Commins S.P., Watson P.M., Levin N., Beiler R.J., Gettys T.W. Central leptin regulates the UCP1 and ob genes in brown and white adipose tissue via different beta-adrenoceptor subtypes. J. Biol. Chem. 2000;275:33059–33067. doi: 10.1074/jbc.M006328200. [DOI] [PubMed] [Google Scholar]
- 161.Guerra C., Navarro P., Valverde A.M., Arribas M., Bruning J., Kozak L.P., Kahn C.R., Benito M. Brown adipose tissue-specific insulin receptor knockout shows diabetic phenotype without insulin resistance. J. Clin. Investig. 2001;108:1205–1213. doi: 10.1172/JCI13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hondares E., Iglesias R., Giralt A., Gonzalez F.J., Giralt M., Mampel T., Villarroya F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem. 2011;286:12983–12990. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hondares E., Rosell M., Gonzalez F.J., Giralt M., Iglesias R., Villarroya F. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010;11:206–212. doi: 10.1016/j.cmet.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fisher F.M., Kleiner S., Douris N., Fox E.C., Mepani R.J., Verdeguer F., Wu J., Kharitonenkov A., Flier J.S., Maratos-Flier E., Spiegelman B.M. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pols T.W., Noriega L.G., Nomura M., Auwerx J., Schoonjans K. The bile acid membrane receptor TGR5: a valuable metabolic target. Dig Dis. 2011;29:37–44. doi: 10.1159/000324126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Angelin B., Larsson T.E., Rudling M. Circulating fibroblast growth factors as metabolic regulators–a critical appraisal. Cell Metab. 2012;16:693–705. doi: 10.1016/j.cmet.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 167.Contreras C., Gonzalez F., Ferno J., Dieguez C., Rahmouni K., Nogueiras R., López M. The brain and brown fat. Ann. Med. 2015;47:150–168. doi: 10.3109/07853890.2014.919727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nedergaard J., Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 169.Wu J., Cohen P., Spiegelman B.M. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cohen P., Spiegelman B.M. Brown and beige fat: molecular parts of a thermogenic machine. Diabetes. 2015;64:2346–2351. doi: 10.2337/db15-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nedergaard J., Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 2010;11:268–272. doi: 10.1016/j.cmet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 172.Jeanson Y., Carriere A., Casteilla L., New Role A. for browning as a redox and stress Adaptive mechanism? Front. Endocrinol. 2015;6:158. doi: 10.3389/fendo.2015.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ahima R.S., Qi Y., Singhal N.S., Jackson M.B., Scherer P.E. Brain adipocytokine action and metabolic regulation. Diabetes. 2006;55(Suppl. 2):S145–S154. doi: 10.2337/db06-s018. [DOI] [PubMed] [Google Scholar]
- 174.Rodriguez A., Ezquerro S., Mendez-Gimenez L., Becerril S., Fruhbeck G. Revisiting the adipocyte: a model for integration of cytokine signaling in the regulation of energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2015;309:E691–E714. doi: 10.1152/ajpendo.00297.2015. [DOI] [PubMed] [Google Scholar]
- 175.Villarroya F., Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell Metab. 2013;17:638–643. doi: 10.1016/j.cmet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 176.Lee P., Werner C.D., Kebebew E., Celi F.S. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int. J. Obes. 2014;38:170–176. doi: 10.1038/ijo.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lidell M.E., Betz M.J., Dahlqvist Leinhard O., Heglind M., Elander L., Slawik M., Mussack T., Nilsson D., Romu T., Nuutila P., Virtanen K.A., Beuschlein F., Persson A., Borga M., Enerback S. Evidence for two types of brown adipose tissue in humans. Nat. Med. 2013;19:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- 178.Sharp L.Z., Shinoda K., Ohno H., Scheel D.W., Tomoda E., Ruiz L., Hu H., Wang L., Pavlova Z., Gilsanz V., Kajimura S. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Whittle A.J., López M., Vidal-Puig A. Using brown adipose tissue to treat obesity - the central issue. Trends Mol. Med. 2011;17:405–411. doi: 10.1016/j.molmed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 180.van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M., Kemerink G.J., Bouvy N.D., Schrauwen P., Teule G.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 181.Zingaretti M.C., Crosta F., Vitali A., Guerrieri M., Frontini A., Cannon B., Nedergaard J., Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 182.Nedergaard J., Bengtsson T., Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 183.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B., Kuo F.C., Palmer E.L., Tseng Y.H., Doria A., Kolodny G.M., Kahn C.R. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Virtanen K.A., Lidell M.E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N.J., Enerback S., Nuutila P. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 185.Cypess A.M., Haft C.R., Laughlin M.R., Hu H.H. Brown fat in humans: consensus points and experimental guidelines. Cell Metab. 2014;20:408–415. doi: 10.1016/j.cmet.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bartelt A., Bruns O.T., Reimer R., Hohenberg H., Ittrich H., Peldschus K., Kaul M.G., Tromsdorf U.I., Weller H., Waurisch C., Eychmuller A., Gordts P.L., Rinninger F., Bruegelmann K., Freund B., Nielsen P., Merkel M., Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 187.Gunawardana S.C., Piston D.W. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes. 2012;61:674–682. doi: 10.2337/db11-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Meyer C.W., Willershauser M., Jastroch M., Rourke B.C., Fromme T., Oelkrug R., Heldmaier G., Klingenspor M. Adaptive thermogenesis and thermal conductance in wild-type and UCP1-KO mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R1396–R1406. doi: 10.1152/ajpregu.00021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chen K.Y., Brychta R.J., Linderman J.D., Smith S., Courville A., Dieckmann W., Herscovitch P., Millo C.M., Remaley A., Lee P., Celi F.S. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. J. Clin. Endocrinol. Metab. 2013;98:E1218–E1223. doi: 10.1210/jc.2012-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chondronikola M., Volpi E., Børsheim E., Porter C., Annamalai P., Enerbäck S., Lidell M.E., Saraf M.K., Labbe S.M., Hurren N.M., Yfanti C., Chao T., Andersen C.R., Cesani F., Hawkins H., Sidossis L.S. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.T. Yoneshiro, Recruited brown adipose tissue as an antiobesity agent in humans, 123, 2013, pp. 3404–3408. [DOI] [PMC free article] [PubMed]