Abstract
PURPOSE: Gastric cancer studies indicated a potential correlation between circulating tumor cells (CTCs) in peripheral blood and tumor relapse/metastasis. The prevalence and significance of circulating tumor microemboli (CTM) in gastric cancer remain unknown. We investigated the prevalence and prognostic value of CTCs and CTM for progression-free survival (PFS) and overall survival (OS) in gastric cancer patients. METHODS:Eighty-one gastric cancer patients consented to provide 5 ml of peripheral blood before systematic therapy. CTCs and CTM were isolated using isolation by size of epithelial tumor cells and characterized by cytopathologists. For 41 stage IV gastric cancer patients, CTM was investigated as a potential biomarker to predict prognosis. RESULTS:CTCs were detected in 51 patients; the average count was 1.81. In clinical stage I, II, III, and IV patients, the average CTC counts were 1.40, 0.67, 1.24, and 2.71, respectively. CTM were detected in 3 of 33 clinical stage I to IIIb patients, at an average of 0.12 (0-2). CTM were detected in 13 of 53 clinical stage IIIc to IV patients, at an average of 1.26 (0-22). In stage IV patients, CTM positivity correlated with the CA125 level. PFS and OS in CTM-positive patients were significantly lower than in CTM-negative patients (P < .001). CTM positivity was an independent factor for determining the PFS (P = .016) and OS (P = .003) of stage IV patients in multivariate analysis. Using markers of the epithelial-mesenchymal transition, single CTCs were divided into three phenotypes including epithelial CTCs, biphenotypic epithelial/mesenchymal CTCs, and mesenchymal CTCs. For CTM, CK−/Vimentin+/CD45− and CK+/Vimentin+/CD45− phenotypes were observed, but the CK+/Vimentin−/CD45− CTM phenotype was not. CA125 was detected in gastric cancer cell lines BGC823 and MGC803. CONCLUSIONS: In stage IV patients, CTM positivity was correlated with serum CA125 level. CTM were an independent predictor of shorter PFS and OS in stage IV patients. Thus, CTM detection may be a useful tool to predict prognosis in stage IV patients.
Introduction
Gastric cancer is currently the fourth most commonly diagnosed cancer and the third leading cause of cancer death worldwide [1]. Metastasis and recurrence after treatment are the leading causes of cancer-related death. Currently, clinical decisions are made according to the diagnosis based on tumor-node-metastasis (TNM) factors. Tumor markers carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), carbohydrate antigen 72-4 (CA72-4), and carbohydrate antigen 125 (CA125) have been widely used in patients with gastric cancer [2]. However, it is difficult to use serum tumor markers in the early diagnosis of gastric cancer due to low sensitivity and specificity [3], [4]. Moreover, recurrence or metastasis may occur even after curative surgery in early-stage gastric cancer. Therefore, we need more accurate biomarkers to predict the prognosis of patients with gastric cancer. Recent studies on gastric cancer have indicated a potential correlation between circulating tumor cells (CTCs) in the peripheral blood and tumor relapse/metastasis [5], with the presentation of CTCs related to worse overall survival (OS) [6], [7]. CTCs survive in the bloodstream and interact with the microenvironment at distant sites, then promote the formation of metastases [8], [9]. Thus, as a noninvasive method, detection of CTCs is an alternative approach due to the invasive characteristics of surgical and/or biopsy specimens, dynamic molecular changes of tumor cells during the therapeutic process, and tumor heterogeneity [10], [11]. CTCs in peripheral blood are extremely rare, and the frequency is approximately 1 CTC per 106 to 107 peripheral blood mononuclear cells [12]. Thus, CTC research has been greatly limited by the isolation and detection of CTCs. Currently, these methods are mainly based on differences in physical and biochemical characteristic between CTCs and blood cells [12]. As a technology based on differences in the size and deformability of tumor cells and normal blood cells, isolation by size of epithelial tumor cells (ISET) isolates CTCs and circulating tumor microemboli (CTM) from whole blood with a membrane filter [13]. ISET has been used to detect CTCs in patients with prostate cancer, breast cancer, lung cancer, and melanoma [14], [15]. In patients with non–small cell lung cancer or liver cancer, CTC/CTM detection by ISET was shown to be an important tool to predict prognosis [16], [17]. CTM, also known as CTC clusters, have been detected in breast, lung, and colorectal cancer [18], [19], [20]. Although rare in the circulation compared with single CTCs, CTC clusters may possess stronger ability to transfer, greater resistance to anoikis and cytotoxic drugs, and more metastatic potential [18], [21]. Indeed, studies on the CTM formation mechanism will help develop new drug targets. Divella et al. [19] found that CTC clusters were associated with increased level of TGF-β and CXCL1 in the blood of patients with metastatic colorectal cancer, and in the study of Aceto et al. [18], RNA sequencing showed that plakoglobin expression was higher in CTC clusters than in single CTCs in patients with breast cancer.
To our knowledge, few studies have examined CTM in gastric cancer. The detection of CTM may increase the understanding of metastasis and recurrence in gastric cancer, thus facilitating diagnosis and treatment. We investigated the prevalence and number of CTCs and CTM in patients with gastric cancer and evaluated the relationship between CTC and CTM positivity and several clinical factors. Furthermore, we investigated the correlation of CTM with prognosis in patients with stage IV gastric cancer.
Patients and Methods
Case Selection
A total of 86 consecutive patients with gastric cancer from October 2014 to November 2015 at Wuhan Union Hospital were enrolled in the study. The study was approved by the clinical investigation ethics committee of Tongji Medical College, and signed informed consent to participate in the study was obtained from all patients and controls. Eighty-six gastric cancer patients, including 5 stage I cases, 15 stage II cases, 25 stage III cases, and 41 stage IV cases, were enrolled in this study. The inclusion criteria included a confirmed pathological diagnosis of gastric cancer, ≥18 years old, no prior systemic medical therapy for cancer or a minimum of a 6-month treatment-free period, and World Health Organization Performance Status (WHO PS) between 0 and 2. The exclusion criteria included any other previous malignancy and severe organ dysfunction. The controls were recruited from 30 healthy volunteers. Blood samples were obtained from 86 patients for Diff-Quick staining at baseline. From the 86 patients, 30 more blood samples were obtained for immunofluorescence from 23 patients with clinical stages III to IV after treatment. Peripheral blood was obtained before the initiation of treatment in 86 patients and at multiple time points after the initiation of treatment in 23 of the 86 patients. The first 1.5 ml of collected blood was discarded to avoid contamination with epidermal cells. The peripheral blood (5 ml) was collected in EDTA-containing tubes and processed within 2 hours for the ISET assay. Routine blood test, liver and kidney function, and serum tumor markers were performed before each cycle of chemotherapy. Computed tomographic or magnetic resonance images were used to measure tumors every 8 weeks during therapy. Contrast-enhanced computed tomography or magnetic resonance imaging is used for the diagnosis of tumor progression.
ISET Assay
CTCs were enriched using the ISET assay as described in an earlier study by Vona et al. [22]. The samples were processed on an automated platform according to the manufacturer's instructions. Five milliliters of whole blood was diluted with 3-ml buffer containing 0.2% paraformaldehyde and left for 10 minutes at room temperature. Then, the 8 ml of diluted blood was filtered through a membrane with 8-μm–diameter circular pores. It took 15 minutes to process one blood sample. These enriched CTCs were stained with Diff-Quick stain, air dried, and mounted. Based on our experience and the criteria proposed by other research groups [22], [23], [24], [25], [26], cells with visible cytoplasm were considered. Then, CTCs were characterized by the presence of at least four of the following criteria: irregular nuclei, prominent nucleoli, nuclear diameter >15 μm, nuclear-cytoplasmic ratio >0.8, hyperchromatic nuclei and nonhomogeneous staining, irregular nuclear membrane, tumor cell aggregations, or CTM. CTM were defined as tumor cell clusters containing three or more distinct nuclei [27]. All candidate CTCs/CTM were reviewed and identified independently by three senior cytopathologists without knowledge of the patients' clinical status and pathologic diagnosis.
Immunofluorescence Staining
Immunofluorescence staining on slides was performed as described in a previous study by Hao Li [26]. Membranes with CTCs enriched by ISET were transferred to glass slides, which were then rinsed with Perm/Wash buffer (BD) for 3 minutes and processed with Cytofix/Cytoperm Fixation/Permeabilization solution (BD) for 20 minutes at room temperature. Processed slides were incubated with blocking buffer (10% goat serum, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 30 minutes at room temperature. The slides were then incubated overnight at 4°C in a wet chamber with anti-CD45 antibody (Santa-Cruz Biotechnology, Inc., Dallas, TX), anti-CK8/18/19 antibody (Abcam Trading [Shanghai] Company Ltd., Pudong, Shanghai, China), and anti-vimentin antibody (Abcam). Slides were rinsed in washing buffer the next day. Then, the slides were incubated with secondary antibodies in the dark for 1 hour at room temperature, including Alexa Fluor 488–conjugated goat anti-mouse (Invitrogen, Thermo Fisher Scientific, Waltham, MA), Alexa Fluor 546–conjugated goat anti-rat (Invitrogen, Thermo Fisher Scientific, Waltham, MA), Cy5-conjugated goat anti-rabbit (Invitrogen, Thermo Fisher Scientific, Waltham, MA), and Hoechst 33,342 (Sigma, St. Louis, MO) in blocking buffer. For each experiment, the white blood cells captured on the membrane were used as the CD45 positive control and CK8/18/19 negative control. The human breast cancer cell line SKBr3 was used as the CK8/18/19 positive control, and the human osteosarcoma cell line MG63 was used as the vimentin positive control.
Cell Culture
A human gastric cell line, GES-1, and the gastric cancer cell lines BGC823, MGC803, MKN45, and SGC7901 were purchased from Typical China Academy Culture Collection Commission Cell Library (Shanghai, China). The cells were cultured in an RPMI-1640 or Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and were maintained at 37°C in 5% CO2. Passages were performed to maintain monolayer growth. Cells were collected at the exponential growth phase for subsequent experiments.
SYBR-Green–Based Real-Time Quantitative Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Total mRNA was isolated with Trizol Reagent (Invitrogen, USA) and chloroform. Reverse transcription was performed with the PrimeScript RT reagent kit (TaKaRa) according to the manufacturer's instructions. The RT-PCR analysis for the detection of CA125 mRNA and GAPDH mRNA was performed using SYBR Premix Ex Taq kit (TaKaRa) in a 20-μl final volume. The primers were as follows: CA125-specific forward primer: 5′-GGCTTGGCATCTTGTCCTCATC-3′, CA125-specific reverse primer: 5′-GGAAGTCGTGGAAGGTAAGTTGG-3′, GAPDH-specific forward primer: 5′-CCACTCCTCCACCTTTGAC-3′, and GAPDH-specific reverse primer: 5′-ACCCTGTTGCTGTAGCCA-3′. PCR program: 1) 95°C for 30 seconds, 2) 95°C for 5 seconds, 3) 60°C for 30 seconds, 4) 95°C for 15 seconds, 5) 60°C for 1 minute, and 6) 95°C for 15 seconds. All samples were amplified in duplicates, and the mean was used for further analysis.
Statistical Analysis
All statistical evaluations were performed using SPSS16.0 (SPSS Inc., Chicago, IL). The χ2 analysis or Fisher exact test was used to explore any correlation between CTC positivity and patient characteristics. The Student's t test, Mann-Whitney U test, and Kruskal-Wallis test were used for continuous variables. OS was defined as the period from blood collection to death. PFS was calculated as the time from blood collection until the first objective evidence of disease progression. For survival analyses, the Kaplan-Meier method was used, and the log-rank test was used for univariate analyses. Prognostic factors were assessed in multivariate analyses using Cox proportional hazards models, and the hazard ratio (HR) was estimated. Differences were considered statistically significant when the P value was <.05.
Results
Patient Characteristics
A total of 86 patients (45 males, 41 females) with gastric cancer and a mean age of 53 years (range 25-74 years) were enrolled in this study. All patients had not received chemotherapy or radiotherapy within 6 months. The clinicopathological features of the patients are shown in Table 1. Of these patients, 41 had stage IV gastric cancer, including those newly diagnosed or relapsed after adjuvant chemotherapy, radiotherapy, or surgery. For the patients with stage IV gastric cancer, 6 patients received abdominal exploration or palliative surgery, and the remaining 35 patients did not undergo surgery. All the 45 patients without metastasis underwent surgical treatment. The number of patients with WHO PS of 0, 1, and 2 was 22 (25.58%), 59 (68.60%), and 5 (5.81%), respectively. The baseline mean erythrocyte counts, platelet levels, and ratio of neutrophils to lymphocytes were 3.79 × 1012/l, 196.49 × 109/l, and 3.51, respectively. With regard to the grade of tumor differentiation, 18 cases (20.93%) were moderately or well differentiated, and 68 cases (79.07%) were poorly differentiated. For the depth of tumor invasion, the number of patients with T1, T2, T3, and T4 was 3 (3.49%), 6 (6.98%), 27 (31.40%), and 50 (58.14%), respectively. Seventy-seven (89.53%) patients had lymph node metastasis. For the clinical stages, 5 (5.81%), 15 (17.44%), 25 (29.07%), and 41 (47.67%) cases were stage I, stage II, stage III, and stage IV, respectively. Twenty nine patients (33.72%) had lymphatic, nerve, or venous invasion, and elevated Her2 levels (+++ or ++) were detected in 8 (9.3%) gastric cancer patients.
Table 1.
Clinical Characteristics of the Study Population
| Characteristics | N (%) (N = 86) |
|---|---|
| Age, mean (range), y | 52.98 ± 11.43 (25-74) |
| Sex | |
| Male | 45 (52.32) |
| Female | 41 (47.67) |
| PS | |
| 0 | 22 (25.58) |
| 1 | 59 (68.60) |
| 2 | 5 (5.81) |
| Distant metastasis | |
| Yes | 41 (47.67) |
| No | 45 (52.33) |
| Grade of differentiation | |
| Moderate or well differentiated | 18 (20.93) |
| Poorly differentiated | 68 (79.07) |
| Tumor depth | |
| T1 | 3 (3.49) |
| T2 | 6 (6.98) |
| T3 | 27 (31.40) |
| T4 | 50 (58.14) |
| Lymph node metastasis | |
| Yes | 77 (89.53) |
| No | 9 (10.47) |
| Clinical stage | |
| I | 5 (5.81) |
| II | 15 (17.44) |
| III | 25 (29.07) |
| IV | 41 (47.67) |
| Lymphatic, nerve or venous invasion | |
| Positive | 29 (33.72) |
| Negative | 40 (46.51) |
| Unknown | 17 (19.77) |
| Platelet, mean (range), ×109/L | 196.49 ± 69.00 (62-399) |
| N/L, mean (range) | 3.51 ± 5.40 (0.02-38.30) |
| Erythrocyte (range), ×1012/L | 3.79 ± 0.46 (2.83-4.91) |
| Her2 | |
| 0 | 31 (36.05) |
| 1 | 6 (6.98) |
| 2 | 8 (9.30) |
| Unknown | 41 (47.67) |
| CEA | |
| Positive | 15 (17.44) |
| Negative | 71 (82.56) |
| CA19-9 | |
| Positive | 22 (25.58) |
| Negative | 64 (74.42) |
| CA125 | |
| Positive | 26 (30.23) |
| Negative | 60 (69.77) |
N/L, the ratio of neutrophils to lymphocytes; Her2, human epidermal growth factor receptor 2.
Results of CTC and CTM Detection by ISET Assay
Overall, CTCs were detected in 51 patients (Figure 1); the average count was 1.81. In patients with clinical stage I, II, III, and IV disease, the average CTC count was 1.40, 0.67, 1.24, and 2.71, respectively. No significant differences were found for the CTC-positive rate and the average number of CTCs in different clinical stages. However, for the 33 patients with disease stage I to IIIB, CTCs were detected in 16 patients, and the average count was 0.97. In the remaining 53 patients with stage IIIC to IV, CTCs were detected in 35 patients, with an average CTC number of 2.40. Significant differences were found for the average number of CTCs between these two groups of patients (P = .041, Table 2). With regard to CTM, the average counts were 0 (0-0), 0.13 (0-2), 0.60 (0-6), and 1.29 (0-22) in patients with clinical stage I, II, III, and IV disease, respectively. There were no significant differences between the average CTM counts in different stages. However, in patients with clinical stage I to IIIB, CTMs were detected in 3 of 33 patients, and the average number was 0.12 (0-2). In the remaining 53 patients with clinical stages IIIC to IV, CTM were detected in 13 patients, and the average number was 1.26. Significant differences were found for the average CTM number in these two groups (P = .05, Table 3). No CTCs/CTM were detected in healthy volunteers.
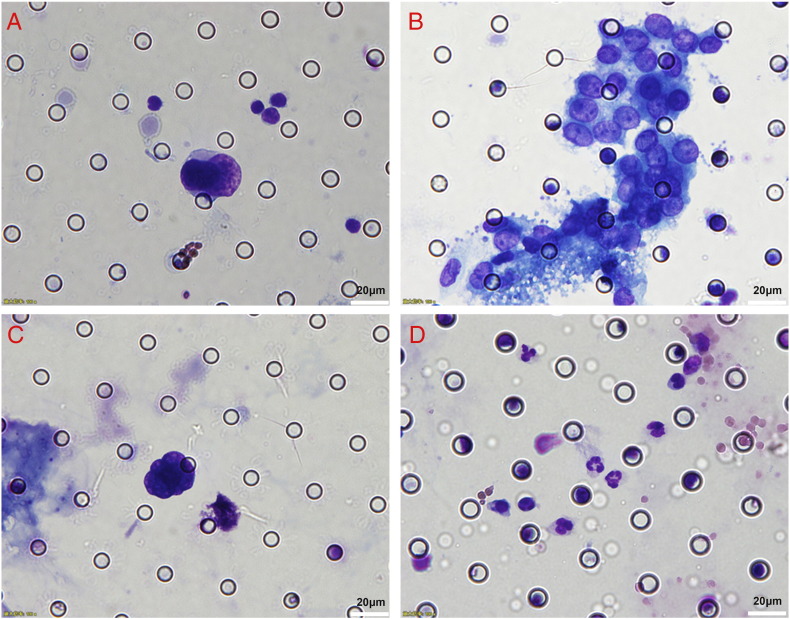
Figure 1.
Cells enriched by the ISET method in patients with gastric cancer. Diff-Quick staining. (A) CTC: irregular nucleus; nuclear diameter >15 μm; nuclear-cytoplasmic ratio >0.8; hyperchromatic nucleus and nonhomogeneous staining. (B) CTM: presence of tumor cell aggregation, prominent nucleoli seen in the nuclei. (C) Nucleus without plasma. (D) Normal blood cells isolated on the membrane. The scale bar represents 20 μm.
Table 2.
Prevalence of CTCs Based on TNM Stage
| N | Detection Rate (%) | Mean (Range) | |
|---|---|---|---|
| Total | 86 | 51/86 (59.3) | 1.81 ± 3.01 (0-21) |
| Stage I | 5 | 3/5 (60) | 1.40 ± 1.67 (0-4) |
| Stage II | 15 | 6/15 (40.0) | 0.67 ± 0.98 (0-3) |
| Stage III | 25 | 14/25 (56.0) | 1.24 ± 1.33 (0-4) |
| Stage IV | 41 | 28/41 (68.29) | 2.71 ± 4.08 (0-21) |
| P1 = .284 | P2 = .111 | ||
| Stage IA-IIIB | 33 | 16/33 (48.5) | 0.97 ± 1.21 (0-4) |
| Stage IIIC-IV | 53 | 35/53 (66.0) | 2.40 ± 3.68 (0-21) |
| P3 = .081 | P4 = .041 |
P1 = χ2 test, P2 = Kruskal-Wallis test, P3 = χ2 test, P4 = Mann-Whitney U test.
Table 3.
Prevalence of CTM Based on TNM Stage.
| N | Detection Rate (%) | Mean (Range) | |
|---|---|---|---|
| Total | 86 | 16/86 (18.60) | 0.83 ± 2.76 (0-22) |
| Stage I | 5 | 0/5 (0) | 0 (0-0) |
| Stage II | 15 | 1/15 (6.67) | 0.13 ± 0.52 (0-2) |
| Stage III | 25 | 5/25 (20.0) | 0.60 ± 1.50 (0-6) |
| Stage IV | 41 | 10/41 (24.39) | 1.29 ± 3.73 (0-22) |
| P1 = .428 | P2 = .30 | ||
| Stage I-IIIB | 33 | 3/33 (9.09) | 0.12 ± 0.42 (0-2) |
| Stage IIIC-IV | 53 | 13/53 (24.53) | 1.26 ± 3.44 (0-22) |
| P3 = .074 | P4 = .05 |
P1 = Fisher exact test, P2 = Kruskal-Wallis test, P3 = χ2 test, P4 = Mann-Whitney U test.
Identification of CTCs/CTM by Immunofluorescence
To further investigate the molecular characteristics of CTCs/CTM enriched by ISET in gastric cancer, we used immunofluorescence to analyze 30 blood samples from 23 patients with clinical stages III to IV. Hoechst, CK8/18/19, Vimentin, and CD45 were used to mark nuclei, epithelial cells, mesenchymal cells, and leukocytes, respectively. CTCs were detected in 10 blood samples, and CTM were detected in 6 blood samples. Cells containing nuclei larger than 15 μm with CK(green)±/CD45−(red)/Vimentin (purple)±/Hoechst+ (blue) staining were enumerated as CTCs. Microemboli with multiple cells containing at least three distinct nuclei with CK±/CD45−/Vimentin±/Hoechst+ CTCs were classified as CTM. The images and detailed classification of CTCs are shown in Figure 2. Using markers of the epithelial-mesenchymal transition (EMT), single CTCs were divided into three phenotypes including epithelial CTCs, biphenotypic epithelial/mesenchymal CTCs, and mesenchymal CTCs. For CTM, the CK−/Vimentin+/CD45− and CK+/Vimentin+/CD45− phenotypes were observed, whereas the CK+/Vimentin−/CD45− CTM phenotype was not observed. The phenotypes of single cells in CTM were not uniform; some cells were positive for CK and Vimentin, whereas other cells were positive for only Vimentin.
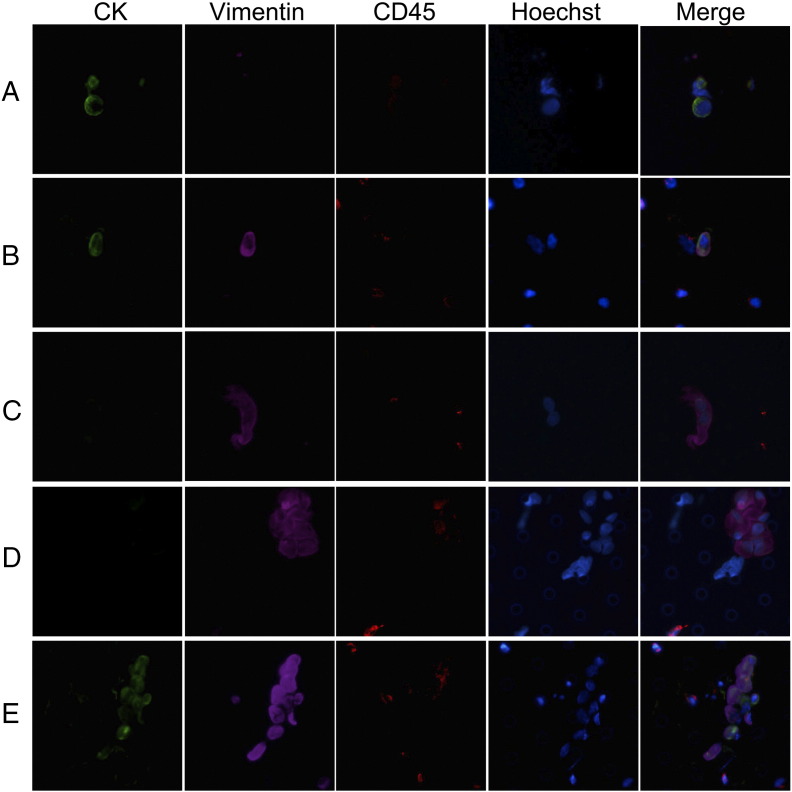
Figure 2.
Immunofluorescent staining of CTCs/CTM isolated by ISET assay. (A–D) Stained with CK8/18/19 (green fluorescence for epithelial cells), Vimentin (purple fluorescence for EMT cells), CD45 (red fluorescence for leukocytes), and Hoechst (blue fluorescence for nuclei). (A) Single CTC: CK+/Vimentin−/CD45− phenotype; (B) single CTC: CK+/Vimentin+/CD45− phenotype; (C) single CTC: CK−/Vimentin+/CD45− phenotype; (D) CTM: CK−/Vimentin+/CD45− phenotype; (E) CTM: CK+/Vimentin+/CD45− phenotype. The cells were analyzed at 40× magnification.
Correlation of CTCs and CTM Detection with Clinicopathological Features
There were no significant correlations between CTCs/CTM positivity with age and sex. Moreover, CTC/CTM positivity was not significantly correlated with pathological features such as tumor depth; lymph node metastasis; and lymphatic, venous, or nerve infiltration (Tables 4 and 5). However, CTC positivity significantly correlated with the grade of differentiation (P = .047, Table 4). We also found significant correlation between CTC positivity and CTM positivity (P = .048). Moreover, we found that CTM positivity correlated with the CA125 level in patients with stage IV gastric cancer (P = .037, Table 6).
Table 4.
Correlation between the Presence of CTCs in the Peripheral Blood of Gastric Cancer Patients and Clinical Characteristics
| Positive | Negative P | |
|---|---|---|
| Age | .735* | |
| Mean ± SD, y | 52.63 ± 12.11 | 53.49 ± 10.53 |
| Sex | .459† | |
| Male | 25 | 20 |
| Female | 26 | 15 |
| PS | .186‡ | |
| 0 | 12 | 10 |
| 1 | 34 | 25 |
| 2 | 5 | 0 |
| Distant metastasis | .105† | |
| Yes | 28 | 13 |
| No | 23 | 22 |
| Grade of differentiation | .047† | |
| Moderate or well differentiated | 7 | 11 |
| Poorly differentiated | 44 | 24 |
| Tumor depth | .974‡ | |
| T1 | 2 | 1 |
| T2 | 3 | 3 |
| T3 | 16 | 11 |
| T4 | 30 | 20 |
| Lymph node metastasis | .188† | |
| Yes | 48 | 29 |
| No | 3 | 6 |
| Lymphatic, nerve, or venous invasion | .239† | |
| Positive | 14 | 15 |
| Negative | 25 | 15 |
| Unknown | 12 | 5 |
| Platelet | .70* | |
| Mean ± SD, ×109/L | 198.88 ± 73.49 | 193.00 ± 62.74 |
| N/L | .318§ | |
| Mean ± SD | 4.19 ± 6.76 | 2.52 ± 2.02 |
| Erythrocyte | .069* | |
| Mean ± SD, ×1012/L | 3.86 ± 0.46 | 3.68 ± 0.44 |
| Her2 | .648‡ | |
| 0 | 18 | 13 |
| 1 | 2 | 4 |
| 2-3 | 5 | 3 |
| Unknown | 26 | 15 |
| CEA | .273† | |
| Positive | 7 | 8 |
| Negative | 44 | 27 |
| CA19-9 | .326† | |
| Positive | 15 | 7 |
| Negative | 36 | 28 |
| CA125 | .781† | |
| Positive | 16 | 10 |
| Negative | 35 | 25 |
| CTM | .048† | |
| Positive | 13 | 3 |
| Negative | 38 | 32 |
Student's t test.
χ2 test.
Fisher exact test.
Mann-Whitney U test.
Table 5.
Correlation between the Presence of CTM in the Peripheral Blood of Gastric Cancer Patients and Clinical Characteristics
| Positive | Negative P | |
|---|---|---|
| Age | .676* | |
| Mean ± SD, y | 54.06 ± 9.30 | 52.73 ± 11.91 |
| Sex | .447† | |
| Male | 7 | 38 |
| Female | 9 | 32 |
| PS | .105‡ | |
| 0 | 1 | 21 |
| 1 | 14 | 45 |
| 2 | 1 | 4 |
| Distant metastasis | .188† | |
| Yes | 10 | 31 |
| No | 6 | 39 |
| Grade of differentiation | .433† | |
| Moderate or well differentiated | 5 | 13 |
| Poorly differentiated | 11 | 57 |
| Tumor depth | .513‡ | |
| T1 | 0 | 3 |
| T2 | 1 | 5 |
| T3 | 3 | 24 |
| T4 | 12 | 38 |
| Lymph node metastasis | .288† | |
| Yes | 16 | 61 |
| No | 0 | 9 |
| Platelet | .391* | |
| Mean ± SD, ×109/L | 183.06 ± 77.04 | 199.56 ± 67.24 |
| N/L | .381§ | |
| Mean ± SD | 4.57 ± 7.49 | 3.27 ± 4.84 |
| Erythrocyte | .442* | |
| Mean ± SD, ×1012/L | 3.86 ± 0.49 | 3.73 ± 0.63 |
| CEA | .564† | |
| Positive | 2 | 13 |
| Negative | 14 | 57 |
| CA19-9 | 1.00† | |
| Positive | 4 | 18 |
| Negative | 12 | 52 |
| CA125 | .108† | |
| Positive | 8 | 18 |
| Negative | 8 | 52 |
Student's t test.
χ2 test.
Fisher exact test.
Mann-Whitney U test.
Table 6.
Correlation between the Presence of CTM and Serum Tumor Markers in the Peripheral Blood of Patients with Stage IV Gastric Cancer
| Positive | Negative P | |
|---|---|---|
| CEA | .905⁎ | |
| Positive | 3 | 12 |
| Negative | 7 | 19 |
| CA19-9 | 1.000⁎ | |
| Positive | 3 | 11 |
| Negative | 7 | 20 |
| CA125 | .037⁎ | |
| Positive | 8 | 11 |
| Negative | 2 | 20 |
χ2 test
The Prognostic Value of CTM and Clinical Features
To investigate whether CTM positivity was associated with patient survival, the Kaplan-Meier method and the log-rank test were used to evaluate the effect of several clinical factors on PFS. PFS was significantly shorter for patients with CTM-positive status (Figure 3A) and CA125-positive status. Multivariate analysis using Cox proportional hazards models with variables that had a P value lower than .20 in the univariate analysis showed that tumor depth, CEA-positive status, CA125-positive status, and CTM-positive status were independent prognostic factors (Table 7).
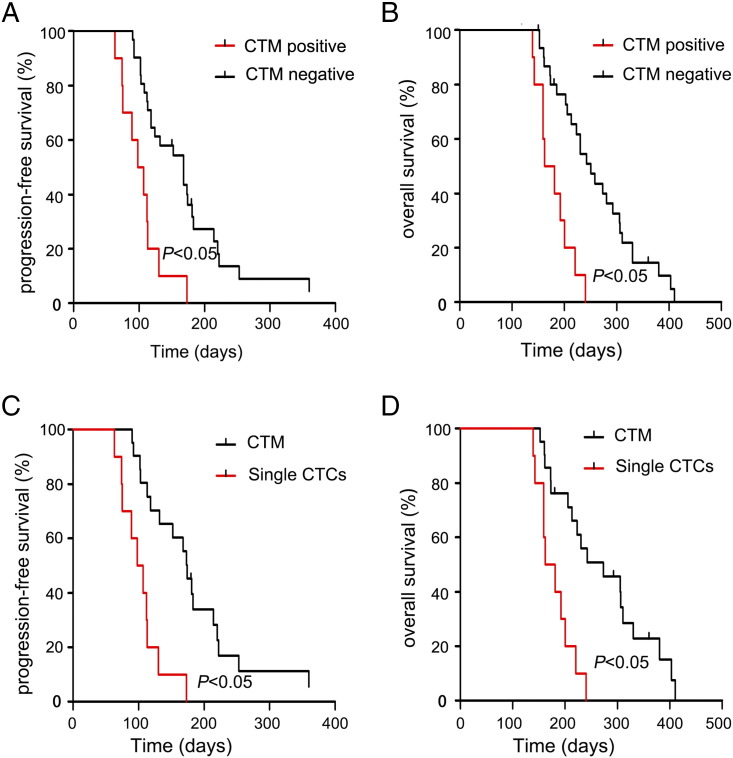
Figure 3.
(A) In 41 patients with stage IV gastric cancer, the PFS rate was significantly lower in patients with CTM than in those without CTM (P < .05). (B) In 41 patients with stage IV gastric cancer, the OS rate was significantly lower in patients with CTM than in those without CTM (P < .05). (C) In 30 patients with single CTCs or CTM, the PFS rate was significantly lower in patients with CTM than in those with only single CTCs (P < .05). (D) In 30 patients with single CTCs or CTM, the OS rate was significantly lower in patients with CTM than in those with only single CTCs (P < .05).
Table 7.
Univariate and Multivariate Analysis of Clinical Factors for PFS
| Clinical Factors | Univariate Analysis |
Multivariate Analysis |
|
|---|---|---|---|
| P Value⁎ | HR (95% CI) | P Value† | |
| Sex | .521 | - | - |
| Age (≥ 60 vs <60) | .457 | ||
| PS | .791 | - | - |
| Grade of differentiation | .401 | - | - |
| Tumor depth | .076 | 3.94 (1.38-11.27) | .010 |
| Lymph node metastasis | .601 | - | |
| CTM | <.001 | 2.87 (1.22-6.77) | .016 |
| CTC | .575 | - | - |
| CEA | .054 | 4.40 (1.85-10.51) | .001 |
| CA125 | .015 | 2.99 (1.29-6.93) | .011 |
| CA19-9 | .219 | - | - |
Log-rank test.
Cox proportional hazards models.
Univariate and multivariate analyses were also used to evaluate the effect of clinical factors on OS. CTM-positive status was associated with shorter OS (Figure 3B), as were tumor depth and CA125-positive status. Multivariate analyses showed that tumor depth, CEA-positive status, and CTM-positive status were independent prognostic factors (Table 8).
Table 8.
Univariate and Multivariate Analysis of Clinical Factors for OS
| Clinical Factors | Univariate Analysis |
Multivariate Analysis |
|
|---|---|---|---|
| P Value⁎ | HR (95% CI) | P Value† | |
| Sex | .738 | - | - |
| Age (≥ 60 vs <60) | .788 | ||
| PS | .279 | - | - |
| Grade of differentiation | .999 | - | - |
| Tumor depth | .025 | 2.96 (1.25-7.04) | .014 |
| Lymph node metastasis | .455 | - | - |
| CTM | <.001 | 4.49 (1.67-12.03) | .003 |
| CTC | .879 | - | - |
| CEA | .125 | 3.40 (1.52-7.61) | .003 |
| CA125 | .021 | 2.03 (0.89-4.66) | .093 |
| CA19-9 | .375 | - | - |
Log-rank test.
Cox proportional hazards models.
We next investigated the different impact of single CTCs and CTM on the prognosis of patients with stage IV gastric cancer. Single CTCs were detected in 28 patients with stage IV gastric cancer, and CTM findings were positive in 8 of the 28 patients. Single CTCs were not detected in two other CTM-positive patients. We analyzed the different prognostic values of CTM and single CTCs in 10 patients with CTM and 18 patients with single CTCs but without CTM. The results showed that CTM correlated with shorter PFS and OS than single CTCs (P < .05, Figure 3, C and D).
CA125 Expression in Human Gastric Cancer Cell Lines
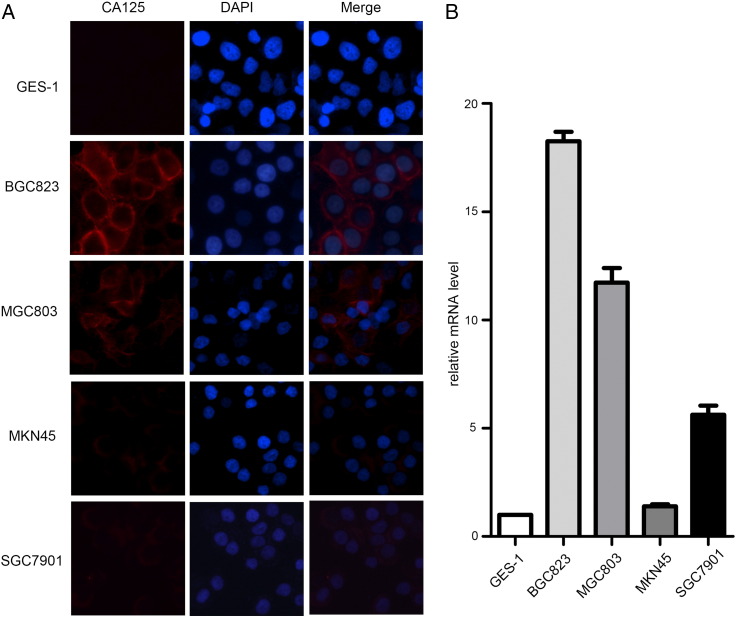
In our study, we found that CTM positivity correlated with the CA125 level in patients with stage IV gastric cancer. To further investigate the role of CA125 in gastric cancer, expression of CA125 in human gastric cancer cell lines was analyzed by immunofluorescence staining and quantitative RT-PCR. Staining of CA125 was not observed in human gastric epithelial cell line GES-1 (Figure 4A). Staining of CA125 was markedly stronger in human gastric cancer cell lines BGC823 and MGC803 than in MKN45 and SGC7901 (Figure 4A). Expression of CA125 mRNA in human gastric cancer cell lines was analyzed by quantitative RT-PCR. CA125 mRNA expression was observed in human gastric cancer cell lines BGC823 and MGC803 (Figure 4B).
Figure 4.
Detection of CA125 expression in human gastric epithelial cell line GES-1 and human gastric cancer cell lines. (A) Immunofluorescent staining of GES-1, BGC823, MGC803, MKN45, and SGC7901 cell lines with CA125 (red fluorescence for CA125) and DAPI (blue fluorescence for nuclei). (B) Quantitative RT-PCR was carried out to detect relative CA125 mRNA expression in GES-1, BGC823, MGC803, MKN45, and SGC7901 cell lines. The cells were analyzed at 40× magnification.
Discussion
CTCs are critical for tumor metastasis [28]. As the so-called “liquid biopsy,” CTCs play an important role in diagnosis, therapeutic monitoring, and prognosis [5], [29], [30]. However, it remains difficult to detect extraordinarily rare CTCs in the peripheral blood. Current technologies for CTC detection include tumor marker–dependent and –independent detection. RT-PCR is most frequently used to investigate CTCs in patients with gastric cancer, although this approach cannot validate the characteristics of single CTCs or CTM [31], [32], [33]. CTM exhibit more metastatic properties than single CTCs and can be isolated by ISET [18], [22]. However, there is little research about the prevalence and clinical value of CTM in patients with gastric cancer. In this study, we investigated the prognostic value of CTM in patients with stage IV gastric cancer and correlated CTM detection with clinicopathological features.
In previous studies, the uses of different markers with different sensitivities led to significant variations in the CTC detection rates among patients with gastric cancer (3.2%-73.3% for stage I-II, 12.5%-100% for stage III-IV). Indeed, there is no unified standard to detect CTCs in gastric cancer, and more research is needed to validate the clinical value of CTCs. Detection platforms with good specificity and sensitivity are imperative. ISET has been used to detect CTCs in lung cancer, liver cancer, breast cancer, and esophageal cancer [22], [23], [26], [34]. Until now, there has been no report of CTCs/CTM detection by ISET in patients with gastric cancer. In this study, the CTC positive rates detected by ISET were 45.0% and 63.6% in stage I-II and III-IV patients, respectively, indicating that invasion and entry into the blood circulation occur in the early stage of tumorigenesis. The reason why no CTCs can be detected in some patients with advanced gastric cancer requires additional investigation. In previous studies of CTC detection in gastric cancer using the CellSearch system, Uenosono et al. reported that the positive rates were 2.2% and 47.2% in stage I-II and III-IV patients, respectively, whereas H. Okabe reported positive rates of 10.9% and 22.2% in stage I-II and III-IV patients, respectively [6], [7]. The positive rates of CTC detection by ISET were higher than those reported with the CellSearch system. This difference may be due to the different specificities and sensitivities of the two methods. In addition, CTM were detected in 16 patients, including 1 patient with stage II disease and 15 patients with stage III-IV disease. CTM are defined as CTC clusters containing three or more distinct nuclei. Compared with single CTCs, CTM have a survival advantage and greater metastatic potential. CTM were detected in patients with gastric cancer using ISET but not the CellSearch system [6], [7].
Currently, clinical decision making for gastric cancer is based on the diagnosis according to the TNM classification. Even after radical resection, recurrence and metastasis may occur, and there is no accurate predictor of this occurrence. Therefore, identifying additional risk factors is necessary. In multiple cancers, including breast, lung, colorectal, and prostate cancer, CTCs have been associated with poor prognosis [35], [36], [37], [38], and CTM possess greater metastatic potential than single CTCs. In our study, the mean number of CTCs in stage IIIC-IV patients was significantly higher than that in stage I-IIIB patients (P = .041). In a previous study, high TNM stages predict worse survival of patients [39]. In our study, we found that CTM positivity correlated with worse PFS and OS, and CTM represented an independent prognostic factor. We also found that CTM correlated with more unfavorable outcomes than single CTCs. However, the expression of single CTCs was not significantly correlated with worse survival, which may be because CTM and single CTCs did not appear together in peripheral blood. More clinical studies are necessary to evaluate the significance of CTM in patients with gastric cancer. However, CTCs/CTM may serve as supplementary markers for TNM staging to predict the survival of patients with gastric cancer, and our finding is consistent with study showing that an elevated CTM number was associated with a worse prognosis [18].
Upon investigating the correlation between CTM positivity and clinical features, we found that CTM positivity was not significantly correlated with clinicopathological features. However, in patients with stage IV gastric cancer, CTM positivity significantly correlated with the serum CA125 level. In a previous study, serum CA125 is identified as an independent prognostic factor in patients with gastric cancer [40]. CA125 is also known as the mucin MUC16. In previous study, mesothelin-MUC16 binding is associated with adhesion between MUC16-positive cancer cells and mesothelin-positive cells [41], [42], and both CA125 and mesothelin have been detected in gastric cancer tissues [43], [44]. Meanwhile, MUC16 promotes the formation of multicellular aggregates by inhibiting β-catenin degradation in ovarian cancer [45]. To further investigate the role of CA125 in gastric cancer, we found that CA125 expression was positive in gastric cancer cell lines BGC823 and MGC803. Few studies have investigated the role of CA125 in gastric cancer. It still needs more research about the role of CA125 expression in gastric cancer.
In our study, CTM positivity was correlated with single CTC positivity. However, CTM were negative in most patients with single CTCs in peripheral blood. This finding may be due to different formation mechanisms for CTM and single CTCs. In addition to single cell migration, the second principal mode of cell movement is collective cell migration. Collective invasion is displayed in histopathological sections of most epithelial cancers and in vitro experiments [46]. In a previous study of breast cancer [18], plakoglobin, also known as γ-catenin, was increased in CTM compared with single CTCs. Thus, clinical studies of CTCs should not be limited by their low numbers, and further validation of the associated molecular mechanisms should provide more information about disease development and new therapeutic targets.
Tumor cells obtain invasive and migratory capabilities through EMT. During EMT, epithelial markers are decreased, while mesenchymal markers are elevated [47]. As a result, EpCAM-based enrichment methods cannot detect CTCs that have undergone EMT [48]. However, the ISET methodology is tumor marker-independent, and cells that have undergone EMT can be isolated. We used immunofluorescence to identify CTCs/CTM enriched by ISET in 30 blood samples; CTCs were detected in 10, and CTM were detected in 6 samples. CTCs were divided into three phenotypes, including epithelial CTCs, biphenotypic epithelial/mesenchymal CTCs, and mesenchymal CTCs, which are consistent with previous reports [49]. At the same time, CTM were classified as mesenchymal CTM or epithelial/mesenchymal CTM. We identified no epithelial CTM, and the cells constituting CTM were heterogeneous. These findings indicate that CTM express mesenchymal markers and may be partially mesenchymal. Partial EMT contributes to residual cell-cell adhesion which enables cells to migrate as clusters. CTCs can display as single CTCs or CTM depending on whether they have undergone complete EMT or partial EMT [50]. In a previous study, CTM coexpressing mesenchymal markers and epithelial markers are found to be apoptosis resistant and correlate with poor prognosis in patients [51]. In the study of lung cancer, EMT is not required for metastasis and it could contribute to the formation of chemoresistant metastasis [52]. The implications of partial EMT for tumor invasion, metastasis, and chemoresistance need more investigations. During cancer progression, epithelial and mesenchymal tumor cells in peripheral blood exhibited dynamic changes [11]. Thus, the dynamic detection of CTCs/CTM with EMT markers could provide greater understanding of metastasis, progression, and relapse in gastric cancer.
In conclusion, ISET can be used to efficiently detect CTCs/CTM in patients with gastric cancer. CTM positivity was associated with worse survival in patients with stage IV gastric cancer. The mean number of CTCs/CTM correlated with clinical stage, and CTM positivity was correlated with the serum CA125 level. Further investigation on the correlation between CA125 and the expression of CTM is necessary. Furthermore, our results showed that CTCs could be divided into epithelial CTCs, biphenotypic epithelial/mesenchymal CTCs, and mesenchymal CTCs, whereas CTM could be divided into two subpopulations, including mesenchymal CTM and partially mesenchymal (epithelial/mesenchymal) CTM. Further investigation of CTM in gastric cancer should help improve our understanding of disease development and identify new therapeutic targets.
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
All authors declare no conflict of interest.
Acknowledgements
The authors thank Ting Ye, Xinsen Ruan, Yuan Huang, Liang Wei, Shili Han, and Hao Zhang for technical assistance. The authors thank Wuhan YZY Medical Science & Technology Co., Ltd., for excellent technical support.
Footnotes
Founding sources: The research was supported by the National Natural Science Foundation of China (no. 81172152) and the Wuhan Planning Project of Science and Technology (no. 2014060101010047).
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Sun Z, Zhang N. Clinical evaluation of CEA, CA19-9, CA72-4 and CA125 in gastric cancer patients with neoadjuvant chemotherapy. World J Surg Oncol. 2014;12:397. doi: 10.1186/1477-7819-12-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17:26–33. doi: 10.1007/s10120-013-0259-5. [DOI] [PubMed] [Google Scholar]
- 4.Yang A-P, Liu J, Lei H-Y, Zhang Q-W, Zhao L, Yang G-H. CA72-4 combined with CEA, CA125 and CAl9-9 improves the sensitivity for the early diagnosis of gastric cancer. Clin Chim Acta. 2014;437:183–186. doi: 10.1016/j.cca.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 5.Huang X. Clinicopathological and prognostic significance of circulating tumor cells in patients with gastric cancer: a meta-analysis. Int J Cancer. 2015;136:21–33. doi: 10.1002/ijc.28954. [DOI] [PubMed] [Google Scholar]
- 6.Okabe H, Tsunoda S, Hosogi H, Hisamori S, Tanaka E, Tanaka S, Sakai Y. Circulating tumor cells as an independent predictor of survival in advanced gastric cancer. Ann Surg Oncol. 2015;22:3954–3961. doi: 10.1245/s10434-015-4483-6. [DOI] [PubMed] [Google Scholar]
- 7.Uenosono Y. Clinical significance of circulating tumor cells in peripheral blood from patients with gastric cancer. Cancer. 2013;119:3984–3991. doi: 10.1002/cncr.28309. [DOI] [PubMed] [Google Scholar]
- 8.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 9.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Gerlinger M. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu M. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torino F. Circulating tumor cells in colorectal cancer patients. Cancer Treat Rev. 2013;39:759–772. doi: 10.1016/j.ctrv.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Krebs MG. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker–dependent and –independent approaches. J Thorac Oncol. 2012;7:306–315. doi: 10.1097/JTO.0b013e31823c5c16. [DOI] [PubMed] [Google Scholar]
- 14.De Giorgi V. Application of a filtration- and isolation-by-size technique for the detection of circulating tumor cells in cutaneous melanoma. J Invest Dermatol. 2010;130:2440–2447. doi: 10.1038/jid.2010.141. [DOI] [PubMed] [Google Scholar]
- 15.Farace F. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br J Cancer. 2011;105:847–853. doi: 10.1038/bjc.2011.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofman V. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non–small-cell lung carcinoma: comparison of the efficacy of the CellSearch Assay and the isolation by size of epithelial tumor cell method. Int J Cancer. 2011;129:1651–1660. doi: 10.1002/ijc.25819. [DOI] [PubMed] [Google Scholar]
- 17.Vona G. Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology. 2004;39:792–797. doi: 10.1002/hep.20091. [DOI] [PubMed] [Google Scholar]
- 18.Aceto N. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Divella R. The presence of clustered circulating tumor cells (CTCs) and circulating cytokines define an aggressive phenotype in metastatic colorectal cancer. Cancer Causes Control. 2014;25:1531–1541. doi: 10.1007/s10552-014-0457-4. [DOI] [PubMed] [Google Scholar]
- 20.Reddy RM. Pulmonary venous blood sampling significantly increases the yield of circulating tumor cells in early-stage lung cancer. J Thorac Cardiovasc Surg. 2016;151:852–857. doi: 10.1016/j.jtcvs.2015.09.126. [DOI] [PubMed] [Google Scholar]
- 21.Hou JM. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol. 2012;30:525–532. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- 22.Vona G. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol. 2000;156:57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofman V. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res. 2011;17:827–835. doi: 10.1158/1078-0432.CCR-10-0445. [DOI] [PubMed] [Google Scholar]
- 24.Hofman VJ. Cytopathologic detection of circulating tumor cells using the isolation by size of epithelial tumor cell method: promises and pitfalls. Am J Clin Pathol. 2011;135:146–156. doi: 10.1309/AJCP9X8OZBEIQVVI. [DOI] [PubMed] [Google Scholar]
- 25.Kolostova K. Detection and cultivation of circulating tumor cells in gastric cancer. Cytotechnology. 2015;68:1095–1102. doi: 10.1007/s10616-015-9866-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H. Circulating tumor cell analyses in patients with esophageal squamous cell carcinoma using epithelial marker–dependent and –independent approaches. Medicine. 2015;94 doi: 10.1097/MD.0000000000001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou JM. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol. 2011;178:989–996. doi: 10.1016/j.ajpath.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 29.Heitzer E. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res. 2013;73:2965–2975. doi: 10.1158/0008-5472.CAN-12-4140. [DOI] [PubMed] [Google Scholar]
- 30.Ni X. Reproducible copy number variation patterns among single circulating tumor cells of lung cancer patients. Proc Natl Acad Sci U S A. 2013;110:21083–21088. doi: 10.1073/pnas.1320659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noh YH. Detection of circulating tumor cells in patients with gastrointestinal tract cancer using RT-PCR and its clinical implications. Exp Mol Med. 2001;33:8–14. doi: 10.1038/emm.2001.2. [DOI] [PubMed] [Google Scholar]
- 32.Wu C. Molecular detection of disseminated tumor cells in the peripheral blood of patients with gastric cancer: evaluation of their prognostic significance. Dis Markers. 2006;22:103–109. doi: 10.1155/2006/281315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang ZY, Ge HY. Micrometastasis in gastric cancer. Cancer Lett. 2013;336:34–45. doi: 10.1016/j.canlet.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Pinzani P. Isolation by size of epithelial tumor cells in peripheral blood of patients with breast cancer: correlation with real-time reverse transcriptase-polymerase chain reaction results and feasibility of molecular analysis by laser microdissection. Hum Pathol. 2006;37:711–718. doi: 10.1016/j.humpath.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 35.Cohen SJ. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 36.Cristofanilli M. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 37.de Bono JS. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 38.Krebs MG. Evaluation and prognostic significance of circulating tumor cells in patients with non–small-cell lung cancer. J Clin Oncol. 2011;29:1556–1563. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 39.Pan QX, Su ZJ, Zhang JH, Wang CR, Ke SY. A comparison of the prognostic value of preoperative inflammation-based scores and TNM stage in patients with gastric cancer. Onco Targets Ther. 2015;8:1375–1385. doi: 10.2147/OTT.S82437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang W. Prognostic significance of preoperative serum CA125, CA19-9 and CEA in gastric carcinoma. Oncotarget. 2016 doi: 10.18632/oncotarget.8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gubbels JA. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, Miyajima A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 43.Baba K. Mesothelin expression correlates with prolonged patient survival in gastric cancer. J Surg Oncol. 2012;105:195–199. doi: 10.1002/jso.22024. [DOI] [PubMed] [Google Scholar]
- 44.Streppel MM. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012;43:1755–1763. doi: 10.1016/j.humpath.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giannakouros P, Comamala M, Matte I, Rancourt C, Piche A. MUC16 mucin (CA125) regulates the formation of multicellular aggregates by altering beta-catenin signaling. Am J Cancer Res. 2015;5:219–230. [PMC free article] [PubMed] [Google Scholar]
- 46.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 47.May CD, Sphyris N, Evans KW, Werden SJ, Guo W, Mani SA. Epithelial-mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progression. Breast Cancer Res. 2011;13:202. doi: 10.1186/bcr2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gorges TM, Tinhofer I, Drosch M, Rose L, Zollner TM, Krahn T, von Ahsen O. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012;12:178. doi: 10.1186/1471-2407-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu S. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boareto M. Notch-Jagged signalling can give rise to clusters of cells exhibiting a hybrid epithelial/mesenchymal phenotype. J R Soc Interface. 2016:13. doi: 10.1098/rsif.2015.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jolly MK. Implications of the hybrid epithelial/mesenchymal phenotype in metastasis. Front Oncol. 2015;5:155. doi: 10.3389/fonc.2015.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fischer KR. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]