Abstract
Nonclinical juvenile animal tests perform a valuable role in determining adverse drug effects during periods of organogenesis and/or functional maturation. Developmental anatomic and functional maturation time points are important to consider between juveniles and adults when regarding different organ toxicities in response to drug administration. The kidney is an example of a major organ that has differences in these time points in comparing juveniles to adults and in contrasting humans to laboratory animal species. Toxicologic pathologists, involved in juvenile studies, need to be aware of these time points which are age-related exposure periods of sensitivity to drug toxicity. Age-related developmental anatomic and functional maturation are factors which can affect the way that a drug is absorbed, distributed, metabolized, and excreted (ADME). Changes to any component of ADME may alter drug toxicity resulting in kidney abnormalities, nephrotoxicity, or maturational disorders. Juvenile animal kidneys may either be less resistant or more resistant to known adult nephrotoxic drug effects. Furthermore, drug toxicity observed in juvenile animal kidneys may not always correspond to similar toxicities in humans. Juvenile animal nonclinical toxicology studies targeting the kidneys have to be carefully planned to attain the maximum knowledge from each study.
Keywords: kidney, juvenile animals, development, maturation, abnormalities, toxicity
Introduction
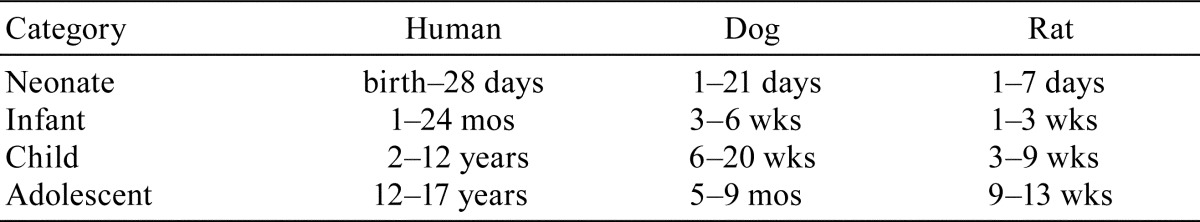
It is known that neonates, infants, or adolescents may react to drugs in ways that are difficult to predict from studies in adults. In other words, “juvenile animals are not little adults”1. Concerns over the lack of awareness of potential therapeutic drug toxicities in pediatric populations, the use of off-labeled drugs by physicians to treat children, and known medicinal adverse effects in children all contributed to the release of guidelines and recommendations for nonclinical studies evaluating drug products for pediatric populations2, 3. Listed in Table 1 are approximate equivalent ages of juveniles in humans, dogs, and rats3,4. Nonclinical juvenile studies are mainly conducted to detect toxicity or maturational defects since differences of sensitivity exist between adults and juveniles that can be chemical specific5, 6. Juvenile studies are considered “compacted” animal models serving to study changes over a relatively short period. Juvenile studies help to bridge data deficiencies between reproductive toxicology and adult toxicology studies, and aid in early hazard identification1, 4.
Table 1. Approximate Equivalent Ages of Juveniles in Humans, Dogs, and Rats (Data from Osterberg; Lewis).
The kidney is one of the best characterized organ systems in developmental biology and is regarded as a “model” organ system to study organogenesis7. Mammalian kidneys develop similarly but developmental time points vary among species8. The process of nephron development or “nephrogenesis” is important to understand since most developmental abnormalities or drug-related injury occurs during the period of nephron formation. Over the past several decades genomic technologies and knock-out mouse models have defined many of the genetic and molecular pathways associated with nephrogenesis9.
Toxicologic pathologists may have a fundamental understanding of kidney development but unaware of the histologic differences between juvenile and adult kidneys. Pathologists can refer to a recently published book chapter that illustrates the histologic appearance of the immature rat kidney to use as a guide in their studies10.
Developmental time points or “sensitive windows of exposure” are prenatal or postnatal periods of structural and functional kidney development which are vulnerable to chemical or drug administration11. For instance, depending on the age at exposure, drug administration may result in renal anomalies or malformations, nephrotoxicity, altered functional maturational, or have no effect. The range of toxicity or lack of toxicity in the juvenile animal is due to a number of factors including the competency of tubule function or the activity of enzymatic pathways in the immature animal11.
The interest and increased numbers of juvenile animal toxicity tests reflect a growing need to predict chemical hazards in pediatric populations12. Juvenile kidney studies are emphasized because of the kidney’s ongoing postnatal periods of development and maturation13. Nonclinical study protocols designed to evaluate juvenile kidneys may contain procedures, end-points, and histological requirements that are unique for each study and dependent on the drug under investigation. In other cases, newer testing models or study designs may appear as alternatives to previous standardize tests. For instance, the National Toxicology Program’s modified one generation (MOG) study which utilizes animals exposed at defined prenatal and postnatal time points can further examine subsets of F1 generation offspring for organ or systems toxicities14. The study design of the MOG study allows for the evaluation of reproductive parameters with the potential of “triggering” additional time points and examinations to look for any test article-related toxicities in juvenile animals.
The overall purpose of this brief review is to introduce and summarize key concepts in normal kidney development, abnormal development, and developmental toxicity. It is not meant to be comprehensive or inclusive of all developmental and pathogenic mechanisms involved with nephrotoxicity in the juvenile kidney.
Kidney Development
Embryology
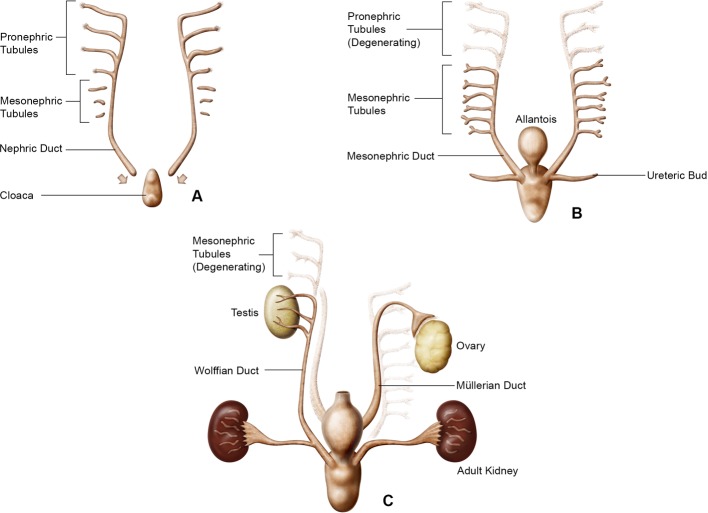
Developmentally, all mammals share a common sequential and structural process which arises from the intermediate mesoderm, one of the embryonic germ layers, and includes the appearance of three stages of kidney development; the pronephros, the mesonephros, and the metanephros or the adult kidney as depicted in Fig.1. The pronephros and mesonephros are transient structures in mammals which regress allowing the metanephros or “metanephric kidney” to differentiate into the adult kidney15, 16.
Fig. 1.
Schematic of kidney development representing the pronephros (A), mesonephros (B), and metanephros (C). Modified from Patten and Saxén (schematic courtesy of David Sabio).
The pronephros along with its duct is the first stage of kidney development and appears approximately around gestational day (GD) 22 in humans, GD 11 in the rat, and GD 8 in the mouse17, 18. It is nonfunctional but important since as the pronephros regresses the caudal portion of the pronephric duct remains and eventually becomes the Wolffian duct. One function of the Wolffian duct is to induce the mesonephros to develop.
The mesonephros is the functional kidney of fish and amphibians but is nonfunctional in adult mammals. However, during mammalian embryogenesis, mesonephric nephrons are formed but later regress. The Wolffian duct elongates growing downwards to join the urogenital sinus. The urogenital sinus eventually differentiates into the urinary bladder. During mesonephros, a diverticulum off the Wolffian duct becomes the ureteric bud (UB).
As the mesonephros regresses, the metanephric kidney develops by the outgrowth and branching of the UB into the metanephric mesenchyme (MM) initiating nephrogenesis or the formation of nephrons. The UB as it contacts the MM continues to branch, each branch representing a future nephron. In addition, the UB has an important role in controlling kidney morphogenesis as well as determining the numbers of nephrons in the kidney19. The UB also contributes to the formation of the collecting ducts, renal pelvis, and ureters.
The kidney develops in close relationship with the male and female genital tracts. The Wolffian duct induces development of the Müllerian duct (paramesonephric duct) the anlagen of the female oviducts, uterus, cervix, and the upper portion of the vagina20. In males, and under the influence of testosterone, the Wolffian duct is transformed into the male excretory ducts, the epididymis and vas deferens. Subsequently, in males, the Müllerian duct regresses due to the production of anti-Müllerian hormone secreted by the Sertoli cells of the testes21.
Nephrogenesis
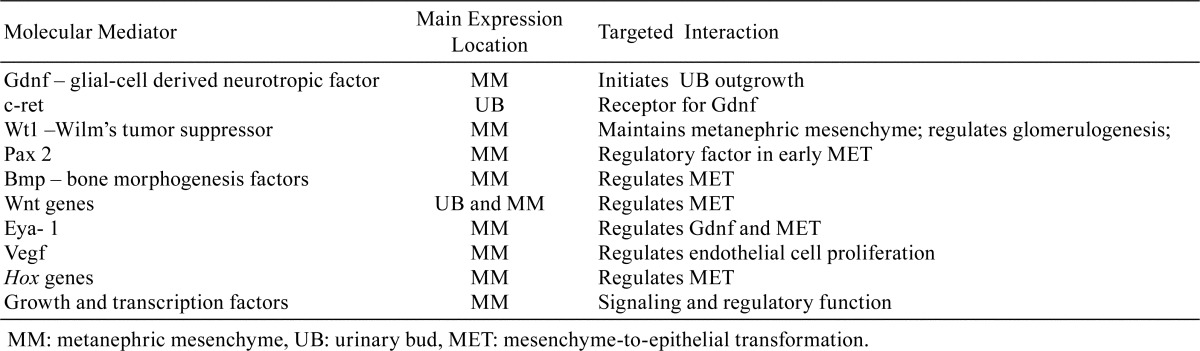
Nephrogenesis begins in the fetus and is completed in humans and mice before birth but continues postnatally in the rat as shown in Table 28, 10. The formation of nephrons involves tightly controlled genetic and molecular pathways which result in the transformation of the MM to epithelial lined structures which undergo further configurational changes to form nephrons. This entire process is termed mesenchymal-to-epithelial transformation (MET). Nephrogenesis begins with the reciprocal inductive interactions between the MM and the UB22. Reciprocal induction refers to the mutually dependent exchange of signaling and regulatory molecules between the MM and UB as schematically represented in Fig. 2. Nephrogenesis induction is initiated by glial derived neurotrophic factor (Gdnf) expressed in the MM which induces UB outgrowth. However, further MET development is dependent on UB outgrowth into the MM before the transformation of mesenchyme to epithelium can actually begin. As with any major biological process, the entire process of MET involves a large number of molecules that are encoded by different gene families and regulated by signaling, growth, transcription, extracellular matrix, and other factors23. Overall, the genetic and molecular pathways regulating MET are highly complicated and several major genes and/or factors are predominantly expressed17, 18, 24,25,26,27,28,29,30. Table 3 contains a partial list of the more important selective mediators involved in MET. The Wt1 gene, in particular, has been studied extensively since it has been identified as having a prominent role in MET and associated with the Wilm’s tumor or nephroblastoma31.
Table 2. Completion of Nephrogenesis (Data from Brown et al.; Zoetis and Hurtt).
Fig. 2.
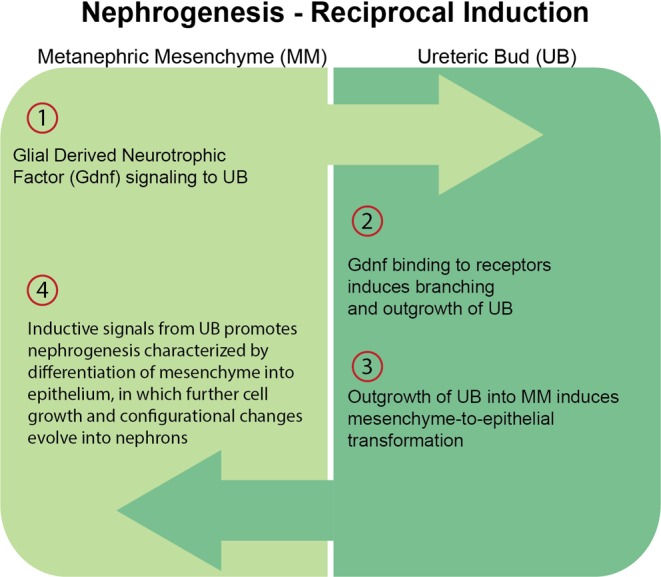
Nephrogenesis – Reciprocol Induction. The four initial steps of mesenchymal-to-epithelial transformation (MET) dependent on the close interaction of metanephric mesenchyme (MM) and the urinary bud (UB).
Table 3. Selective Molecular Mediators Expressed during Nephrogenesis. Most Molecular Mediators are Expressed and Subjected to Positive and Negative Regulation by a Variety of Other Factors (Data from Kuure et al.; Dressler; McMahon).
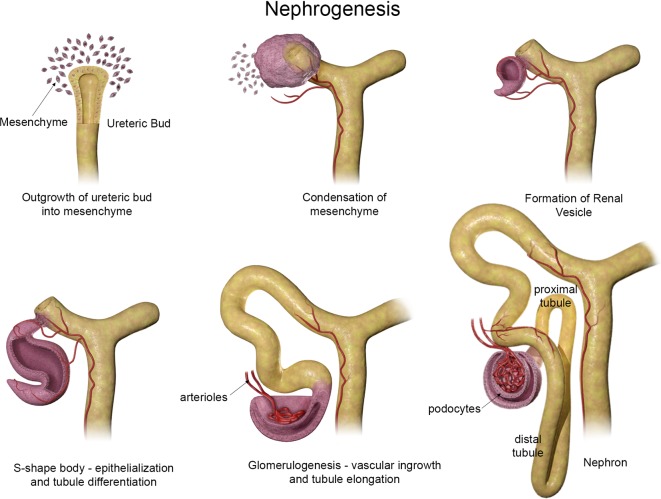
Nephrogenesis can be simply depicted as illustrated in Fig. 3. Early stages consist of a “cap” of aggregated MM around the tip of the UB. After contact with the UB, MM cells further condense and begin to epithelialize. As nephrogenesis continues, the primitive epithelium initially forms small vesicles which undergo reorganizational patterning to form comma- and subsequent S-shaped bodies. As the S-shape body elongates, vasculogenesis begins as endothelial cells migrate into the distal end of the S-shaped body. Further nephron differentiation and formation of capillary loops denotes the beginning of glomerulogenesis. Also during this stage, portions of the S-shaped body begin to express markers for proximal and distal tubules. The primitive glomeruli continue to differentiate incorporating the vascular loops and allowing endothelial cells to contact visceral epithelial cells (early podocytes) forming the filtration barrier of the mature glomerulus. Within the MM, a multipotent progenitor pool remains that does not undergo epithelialization ultimately giving rise to interstitial and vascular cells27.
Fig. 3.
Schematic representation of nephrogenesis illustrating the configurational changes as nephrons develop. Modified from Saxén, Cho and Dressler, Dressler, and McMahon (schematic courtesy of David Sabio).
Histology
The histological appearance of the rodent kidney at birth and immediate postnatal period is very different from the appearance in adults. Histologically, three densely basophilic cortical zones (nephrogenic, juxtamedullary, and intercortical) may be recognized as well as the renal medulla and the papilla10, 32. However, at this time point, tissue maturation within the kidney varies because the medulla appears immature while the papilla appears more mature33. Nephrogenic maturation occurs from the deeper cortex (juxtamedullary zone) where older, larger, and more mature glomeruli are found in contrast to the outer cortex (nephrogenic zone) which contains newer, smaller, and immature nephrons that lack full differentiation as shown in Fig. 4. Four stages of nephrogenesis have been described during the postnatal period and are summarized in Table 410, 32. The increase size of the kidney during the postnatal period is due to a combination of tubule hypertrophy and hyperplasia followed by interstitial expansion which is temporally different for each zone33. These various zones change quickly underlying the need for collecting age-matched control histological samples at multiple time points for comparative purposes.
Fig. 4.
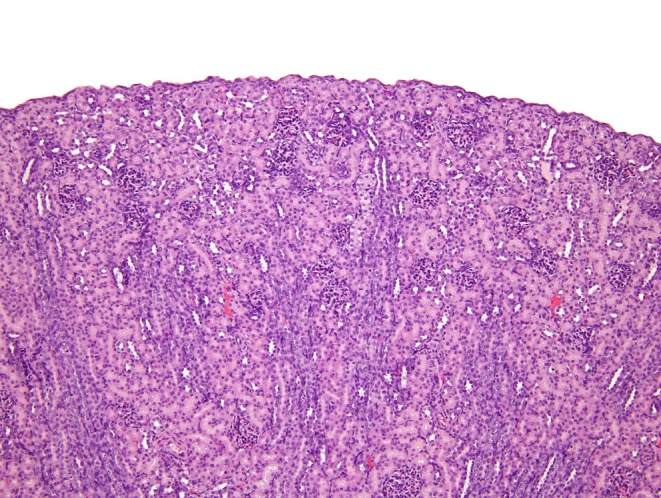
Histological appearance of postnatal day 11 rat kidney. Most glomeruli, particularly in the outer cortex, are immature appearing. Tubules are not fully mature.
Table 4. Four Histological Stages of Postnatal Nephrogenesis (Data from Brown et al.; Dodge).
Maturation
The kidneys, at birth and the postnatal period are marked by growth and physiologic functional changes that adapt to extra-uterine life and progress to adult renal function34. Juvenile animals are born functionally immature with regard to renal physiologic capacity and the acquisition of functional maturity may be just as importance as structural development when recognizing short or long term adverse drug toxicity effects. In general, immature rat kidneys quickly mature to full functional levels between birth and weanling but full functional maturation may take 2–3 years in the human and 5 months in the monkey35. Functional maturity of the kidney lags behind anatomic maturation in all species36.
Functional maturity is dependent on many factors. The reduced tubular mass and lack of tubule maturity in neonatal or juvenile kidneys are responsible for an inability to maintain kidney homeostasis36. Renal blood flow, glomerular filtration rate (GFR), tubule secretion and absorption, maintenance of acid-base equilibrium, and urine concentrating mechanisms are all reduced in the juvenile animal6, 11, 34,35,36. Furthermore, levels of organic anion transporters needed to help with drug and toxin elimination, are also reduced37. Therefore, functional immaturity exposes the juvenile kidney to more toxic agents by decreasing drug clearance or its ability to detoxify drugs or their metabolites34, 37. Paradoxically, in some cases of drug administration, the decrease in functional maturity may actually protect the juvenile kidney due to the inability of the kidney to deliver toxic agents to vulnerable tubules and the decreased ability to concentrate toxic agents in tubules to toxic levels.
Functional maturation end-points in humans and laboratory animals are generally well characterized2, 8, 11, 35, 38, 39. Studies in rats have evaluated functional maturation in neonates and weanlings while also examining how effectively juvenile animals can respond to physiological challenges in their ability to maintain kidney homeostasis40,41,42,43. Generally, as mentioned previously, these studies indicated that functional maturation, although weak at birth, accelerates rapidly.
Clinical pathology determinations are used not only to monitor drug toxicity but can help determine functional maturation. Conducting clinical pathology assessments on juvenile rodent kidneys present certain problems that need to be addressed. For instance, pups can’t be removed from dams before weanling and sampling of blood or urine volume may not yield quantities sufficient for normal testing clinical determinations. Other concerns might involve the most appropriate type of testing methodology to use and the need for age-matched historical control samples13.
Developmental Abnormalities
Developmental renal abnormalities or malformations may arise spontaneously, associated with genetic disorders, or can be induced. Table 5 contains an abbreviated list of kidney teratogens reported in humans34, 44. Renal abnormalities represent a heterogeneous group of disorders that include, but may not be limited to, agenesis, hypoplasia, dysplasia, ectopic, fused, or cystic kidneys. Although rodents generally have few spontaneous kidney malformations human kidney malformations are among the most common birth defects in humans19, 45, 46. Congenital anomalies of the kidney and urinary tract (CAKUT) account for a reported range of 20–50% of children with chronic kidney disease or abdominal masses in neonates45, 47, 48. Many of these kidney abnormalities are linked to specific gene mutations associated with a defect in kidney organogenesis48, 49. Even small developmental defects may have serious consequences. For example, positioning or branching defects of the UB or misplaced gene expressions may result in congenital renal anomalies that can lead to reflux, obstruction and hydronephrosis in children19, 26, 47. There are many genetically altered mouse models that have been developed to study the effects of specific gene deletion and renal abnormalities. For instance, Gdnf deficient mice do not develop kidneys because Gdnf is required to induce UB outgrowth initiating nephrogenesis50, 51. Interested individuals may want to consult the Genitourinary Developmental Molecular Anatomy Project (GUDMAP) an evolving database that provides research information on developmental kidney research and novel mouse strains targeting specific genes52.
Table 5. Maternal Factors and Nephroteratogens Associated with Abnormal Kidney Development (Data from Solhaug et al.; Upadhyay and Silverstein).
The developing kidney in humans exposed to poor maternal intrauterine conditions such as malnutrition, disease, stress, or poverty seem to develop kidney deficits that have been linked to disease conditions appearing later in adult life53, 54. Barker cited epidemiological studies which found that individuals born from women with clinical histories of fetal intrauterine growth retardation (IUGR) or individuals born with significant lower birth weights were at risk to develop ischemic cardiovascular disease, pulmonary obstructive disease, or hypertension later in adult life53. The assumption arising from these observations was postulated as “fetal programming of adult disease” and hypothesized that adverse events occurring in fetal life could affect adult life55. More fascinating are human studies that demonstrated the direct relationship of lower nephron numbers or deficits to adult hypertension56,57,58,59. Animal models using various methods to recreate IUGR such as low protein diets or uterine artery ligation models in rat studies also resulted in reduced populations of nephrons and elevated blood pressure60, 61. Another uterine ligation model using rabbits also resulted in kidney growth retardation and nephron deficits62. Furthermore, the relationship between the number of nephrons and hypertension was investigated in the spontaneously hypertensive rat (SHR). Glomerular counts, indicative of actual nephron numbers, were lower in the SHR when compared to normal rats63.
Drug Toxicity
Juvenile renal studies are usually recommended or required when the drug under investigation is targeted for a pediatric population in which drug toxicity was observed in adult animal studies. Therefore, in designing nonclinical juvenile animal kidney studies it is necessary to consider the class of drug, anticipated renal effects, fetal or postnatal age at dosing, the period of dosing, histopathology, clinical pathology requirements, and any ancillary tests in order obtain the maximum information possible. Therefore, juvenile kidney studies are more likely to be designed “case by case”.
As previously discussed, the outcome of renal drug toxicity in the juvenile kidney depends primarily on the age of structural development and the stage of functional maturity. Pharmacokinetic factors that determine the ADME of drugs and the interrelationship between these factors and drug toxicity depends largely on maintaining a fine balance between metabolism and drug clearance mechanisms through the kidneys64. Any disturbances in the expression of genetic factors, altering blood flow and expression of tubule transporters, or by causing maturational delays may influence toxicity65, 66.
Conversely, in some cases, the developing kidney is more resistant to toxicity than the adult11. For instance, the relative tolerance of the developing kidney to some drugs may be explained by the immaturity of pharmacokinetic enzyme systems or by the immaturity and distribution of the renal blood flow which spares certain tubules from receiving toxic concentrations of a drug35, 67.
Unfortunately, it was apparent that inadvertent drug administration to pregnant women resulted in abnormal fetal kidney development and birth defects. Drugs recognized as nephroteratogens include aminoglycosides, cyclosporines, angiotension converting enzyme (ACE) inhibitors, prostaglandin synthetase inhibitors, steroids, furosemide, thalidomide and antiepileptic drugs and are not recommended during pregnancy65, 68. Of recent concern, as the number of older pregnant women become pregnant, is the potential threat of increased fetal exposures to nephroteratogenic drugs69.
An example that is often cited as an age-dependent teratogen is the administration of ACE inhibitors during kidney development. The renin-angiotensin aldosterone system is expressed in the developing kidney and has a role in several normal body functions including regulating blood pressure and extracellular fluid volume11. Interruption of angiotensin-mediated receptor processes by ACE inhibitors resulted in renal dysplasia and other renal abnormalities in infants from mothers given these drugs during pregnancy to treat hypertension70. ACE inhibitors given to pregnant monkeys also resulted in similar renal anomalies35. No renal abnormalities were seen when ACE inhibitors were given postnatally after completion of nephrogenesis. Exposure with ACE inhibitors to newborn rats, with ongoing postnatal kidney development, induced renal abnormalities similar to those observed in human infants and included papillary atrophy, thickening of cortical arteries, tubule atrophy, tubule dilatation, fibrosis, and inflammatory cell infiltrates71.
Summary
Kidney development is basically similar for all mammals. During renal organogenesis, 3 sequential stages arise from embryonic mesoderm terminating in the metanephros or adult kidney. Nephrogenesis, or the formation of nephrons, is a process that uniquely transforms metanephric mesenchyme to epithelium and involves a complex and coordinated array of genetic and molecular pathways.
The kidney, like other major organs, has “sensitive windows of exposure”. Exposure to a wide variety of chemicals or drugs at crucial periods may result in malformations, nephrotoxicity, or functional maturation defects depending on the developmental time point of exposure. Although there are species differences “sensitive windows of exposure” time points are generally well known for humans and laboratory animal species.
Congenital anomalies of the kidney or malformations are common in humans but less common in laboratory animals. Genetic mutations associated with specific kidney anomalies are known in both humans and animals. Genetically altered mice are used routinely to investigate the role of genes during kidney development and have assisted in the investigations of genetic mutations and malformations. In addition, kidney malformations may arise from fetal exposure to diverse types of nephroteratogens.
Growth and development of the kidney may be compromised during fetal life and subsequently associated with clinical disease later in adult life. Epidemiological, human, and animal investigations provide strong evidence that the theory “fetal programming of adult disease”, as a cause of some cases of adult hypertension, is a real entity.
The pathology of renal toxicology is essentially similar between juveniles and adults. However, the extent and severity of either direct or indirect drug-related toxicity is strongly influenced by the stage of renal development at the time of drug exposure.
Toxicologic pathologists need to carefully evaluate juvenile kidneys keeping in mind the unique structural and physiological differences between juveniles and adults. Harmonized guidelines for recommending specific age-related renal clinical pathology, biomarker, or histopathology end-points need to be proposed to standardize historical control data among laboratories and to ensure time relevant data collection. Extrapolation of nonclinical juvenile kidney studies to human risk assessment needs to account for species specific differences in structural and functional maturation.
In summary, and to emphasize the serious relationship between kidney development and drug administration, Schreuder and his colleagues remarked that “No drug, in their experience, has been proven safe to use during renal development”65.
Acknowledgments
The author would like to thank my following Experimental Pathology Laboratory, Inc. co-workers for their assistance: Mr. David Sabio for his technical illustrations; Ms. Michelle McCrimmon and Mr. Dean Woody for obtaining most of the references cited, Ms. Emily Singletary for image support, and Dr. Jerry F. Hardisty for manuscript review.
Footnotes
Disclosure of Potential Conflict of Interest: None
References
- 1.Beck MJ, Padgett EL, Bowman CJ, Wilson DT, Kaufman LE, Varsho BJ, Stump DG, Nemec MD, and Holson JF. Nonclinical juvenile toxicity testing. In: Developmental and Reproductive Toxicology, 2nd ed. RD Hood (ed). Taylor and Francis, Boca Raton. 263–327. 2006. [Google Scholar]
- 2.Food and Drug Administration (FDA) Guidance for Industry Nonclinical Safety Evaluation of Pediatric Drug Products. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER); Rockville. 2006. [Google Scholar]
- 3.Osterberg RE. FDA Approach to pediatric testing. In: Pediatric Non-clinical Drug Testing: Principles, Requirements, and Practices. AM Hoberman, and EM Lewis (eds). John Wiley & Sons, Inc., Hoboken. 59–77. 2012. [Google Scholar]
- 4.Lewis EM, De Schaepdrijver LM, and Coogan TP. Introduction. In: Pediatric Non-clinical Drug Testing: Principles, Requirements, and Practices. AM Hoberman, and EM Lewis (eds). John Wiley & Sons, Inc., Hoboken. 1–27. 2012. [Google Scholar]
- 5.Bruckner JV. Differences in sensitivity of children and adults to chemical toxicity: the NAS panel report. Regul Toxicol Pharmacol. 31: 280–285. 2000. [DOI] [PubMed] [Google Scholar]
- 6.Scheuplein R, Charnley G, and Dourson M. Differential sensitivity of children and adults to chemical toxicity. I. Biological basis. Regul Toxicol Pharmacol. 35: 429–447. 2002. [DOI] [PubMed] [Google Scholar]
- 7.Lipschutz JH. Molecular development of the kidney: a review of the results of gene disruption studies. Am J Kidney Dis. 31: 383–397. 1998. [DOI] [PubMed] [Google Scholar]
- 8.Zoetis T, and Hurtt ME. Species comparison of anatomical and functional renal development. Birth Defects Res B Dev Reprod Toxicol. 68: 111–120. 2003. [DOI] [PubMed] [Google Scholar]
- 9.Rumballe B, Georgas K, Wilkinson L, and Little M. Molecular anatomy of the kidney: what have we learned from gene expression and functional genomics? Pediatr Nephrol. 25: 1005–1016. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown DL, Walling BE, and Mattix ME. Urinary System. In: Atlas of Histology of the Juvenile Rat. GA Parker, and CA Picut (eds). Elsevier, Amsterdam. 395–421. 2016. [Google Scholar]
- 11.Cappon GD, and Hurtt ME. Developmental toxicity testing of the kidney. In: Reproductive Toxicology, 3rd ed. RW Kapp, and RW Tyl (eds). Informa Healthcare, New York. 193–204. 2010. [Google Scholar]
- 12.Remick AK, Catlin NR, Quist EM, Steinbach TJ, and Dixon D. Juvenile toxicology: relevance and challenges for toxicologists and pathologists. Toxicol Pathol. 43: 1166–1171. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halpern WG, Ameri M, Bowman CJ, Elwell MR, Mirsky ML, Oliver J, Regan KS, Remick AK, Sutherland VL, Thompson KE, Tremblay C, Yoshida M, and Tomlinson L. Scientific and regulatory committee points to consider review: inclusion of reproductive and pathology end points for assessment of reproductive and developmental toxicity in pharmaceutical drug development. Toxicol Pathol. 44: 789–809. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster PMD. Regulatory Forum opinion piece: new testing paradigm for reproductive and developmental toxicity – the NTP modified one generation study and OECD 443. Toxicol Pathol. 42: 1165–1167. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patten BM. Human Embryology. McGraw-Hill, New York, NY. 1968. [Google Scholar]
- 16.Saxén L. Organogenesis of the kidney. Cambridge University Press, Cambridge, UK. 1987. [Google Scholar]
- 17.Kuure S, Vuolteenaho R, and Vainio S. Kidney morphogenesis: cellular and molecular regulation. Mech Dev. 92: 31–45. 2000. [DOI] [PubMed] [Google Scholar]
- 18.Davidson AJ. Mouse kidney development. Stembook, ed. The Stem Cell Research Community, Stembook, doi/10.3824/stembook.1.34.1. 2008. [Google Scholar]
- 19.Michos O. Kidney development: from ureteric bud formation to branching morphogenesis. Curr Opin Genet Dev. 19: 484–490. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman JM, and Cooper RL. Normal development of the female reproductive system. In: Reproductive Toxicology, 3rd ed. RW Kapp, and RW Tyl (eds). Informa Healthcare, New York. 36–50. 2010. [Google Scholar]
- 21.Rasoulpour RJ, Marty MS, Johnson KJ, and Carney EW. Normal development of the male reproductive system. In: Reproductive Toxicology, 3rd ed. RW Kapp, and RW Tyl (eds). Informa Healthcare, New York. 14- 35. 2010. [Google Scholar]
- 22.Cho EA, and Dressler GR. Formation and development of nephrons. In: The Kidney. PD Vize, AS Woolf, and JBL Bard (eds). Academic Press, Cambridge. 195–210. 2003. [Google Scholar]
- 23.Horster MF, Braun GS, and Huber SM. Embryonic renal epithelia: induction, nephrogenesis, and cell differentiation. Physiol Rev. 79: 1157–1191. 1999. [DOI] [PubMed] [Google Scholar]
- 24.Little MH. Renal organogenesis: what can it tell us about renal repair and regeneration? Organogenesis. 7: 229–241. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wellik DM. Hox genes and kidney development. Pediatr Nephrol. 26: 1559–1565. 2011. [DOI] [PubMed] [Google Scholar]
- 26.Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 22: 509–529. 2006. [DOI] [PubMed] [Google Scholar]
- 27.McMahon AP. Development of the mammalian kidney. Curr Top Dev Biol. 117: 31–64. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kispert A, Vainio S, and McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 125: 4225–4234. 1998. [DOI] [PubMed] [Google Scholar]
- 29.Fanni D, Fanos V, Monga G, Gerosa C, Locci A, Nemolato S, Van Eyken P, and Faa G. Expression of WT1 during normal human kidney development. J Matern Fetal Neonatal Med. 24(Suppl 2): 44–47. 2011. [DOI] [PubMed] [Google Scholar]
- 30.Rothenpieler UW, and Dressler GR. Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development. 119: 711–720. 1993. [DOI] [PubMed] [Google Scholar]
- 31.Lee SB, and Haber DA. Wilms tumor and the WT1 gene. Exp Cell Res. 264: 74–99. 2001. [DOI] [PubMed] [Google Scholar]
- 32.Dodge AH. Review of microscopic studies on the fetal and neonatal kidney. Microsc Res Tech. 39: 205–210. 1997. [DOI] [PubMed] [Google Scholar]
- 33.Márquez MG, Cabrera I, Serrano DJ, and Sterin-Speziale N. Cell proliferation and morphometric changes in the rat kidney during postnatal development. Anat Embryol (Berl). 205: 431–440. 2002. [DOI] [PubMed] [Google Scholar]
- 34.Solhaug MJ, Bolger PM, and Jose PA. The developing kidney and environmental toxins. Pediatrics. 113(4 Suppl): 1084–1091. 2004. [PubMed] [Google Scholar]
- 35.Frazier KS. Renal juvenile toxicology and species comparison of renal development. Birth Defects Res. (In press). [Google Scholar]
- 36.Satlin LM, Woda CB, and Schwartz GJ. Development of function in the metanephric kidney. In: The Kidney. PD Vize, AS Woolf, and JBL Bard (eds). Academic Press, Cambridge. 267–325. 2003. [Google Scholar]
- 37.Sekine T, and Endou H. Children’s toxicology from bench to bed--Drug-induced renal injury (3): Drug transporters and toxic nephropathy in childhood. J Toxicol Sci. 34(Suppl 2): SP259–SP265. 2009. [DOI] [PubMed] [Google Scholar]
- 38.Gomez RA, Sequeira Lopez ML, Fernandez L, Cherñavvsky DR, and Norwood VF. The maturing kidney: development and susceptibility. Ren Fail. 21: 283–291. 1999. [DOI] [PubMed] [Google Scholar]
- 39.Gubhaju L, Sutherland MR, Horne RSC, Medhurst A, Kent AL, Ramsden A, Moore L, Singh G, Hoy WE, and Black MJ. Assessment of renal functional maturation and injury in preterm neonates during the first month of life. Am J Physiol Renal Physiol. 307: F149–F158. 2014. [DOI] [PubMed] [Google Scholar]
- 40.Falk G. Maturation of renal function in infant rats. Am J Physiol. 181: 157–170. 1955. [DOI] [PubMed] [Google Scholar]
- 41.Kavlock RJ, and Gray JA. Evaluation of renal function in neonatal rats. Biol Neonate. 41: 279–288. 1982. [DOI] [PubMed] [Google Scholar]
- 42.Rane S, Aperia A, Eneroth P, and Lundin S. Development of urinary concentrating capacity in weaning rats. Pediatr Res. 19: 472–475. 1985. [DOI] [PubMed] [Google Scholar]
- 43.Mallie JP, and Boudzoumou P. Functional renal maturation in rat neonates after prenatal exposure to furosemide. Pediatr Nephrol. 10: 458–460. 1996. [DOI] [PubMed] [Google Scholar]
- 44.Upadhyay KK, and Silverstein DM. Renal development: a complex process dependent on inductive interaction. Curr Pediatr Rev. 10: 107–114. 2014. [DOI] [PubMed] [Google Scholar]
- 45.Schedl A. Renal abnormalities and their developmental origin. Nat Rev Genet. 8: 791–802. 2007. [DOI] [PubMed] [Google Scholar]
- 46.Frazier KS, and Seely JC. Urinary system. In: Toxicologic Pathology: Nonclinical Safety Assessment. PS Sahota, JA Popp, JF Hardisty, and C Gopinath (eds). Taylor and Francis, Abingdon. 422–484. 2013. [Google Scholar]
- 47.Vivante A, Kohl S, Hwang D-Y, Dworschak GC, and Hildebrandt F. Single-gene causes of congenital anomalies of the kidney and urinary tract (CAKUT) in humans. Pediatr Nephrol. 29: 695–704. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agati VD, Jennette JC, and Silva FG. Non-neoplastic kidney disease. Atlas of nontumor pathology. ARP Press, Silver Spring. 19–51. 2005. [Google Scholar]
- 49.Woolf AS, Winyard PJD, Hewrmanns MM, and Welham SJM. Maldevelopment of the human kidney and lower urinary tract. In: The Kidney. PD Vize, AS Woolf, and JBL Bard (eds). Academic Press, Cambridge. 377–393. 2003. [Google Scholar]
- 50.Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, and Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 382: 73–76. 1996. [DOI] [PubMed] [Google Scholar]
- 51.Moore MW, Klein RD, Fariñas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, and Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 382: 76–79. 1996. [DOI] [PubMed] [Google Scholar]
- 52.McMahon AP, Aronow BJ, Davidson DR, Davies JA, Gaido KW, Grimmond S, Lessard JL, Little MH, Potter SS, Wilder EL, Zhang P. GUDMAP Project. GUDMAP: the genitourinary developmental molecular anatomy project. J Am Soc Nephrol. 19: 667–671. 2008. [DOI] [PubMed] [Google Scholar]
- 53.Barker DJ. The fetal and infant origins of adult disease. BMJ. 301: 1111 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moritz KM, Dodic M, and Wintour EM. Kidney development and the fetal programming of adult disease. Bioessays. 25: 212–220. 2003. [DOI] [PubMed] [Google Scholar]
- 55.Moritz KM, and Cullen-McEwen LA. Kidney development and fetal programming. In: Early Life Origins of Health and Disease. EM Wintour, and JA Owens (eds). Springer Science + Business Media, Berlin. 130–144. 2006. [Google Scholar]
- 56.Bagby SP. Developmental origins of renal disease: should nephron protection begin at birth? Clin J Am Soc Nephrol. 4: 10–13. 2009. [DOI] [PubMed] [Google Scholar]
- 57.Baum M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am J Physiol Renal Physiol. 298: F235–F247. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keller G, Zimmer G, Mall G, Ritz E, and Amann K. Nephron number in patients with primary hypertension. N Engl J Med. 348: 101–108. 2003. [DOI] [PubMed] [Google Scholar]
- 59.Kett MM, and Bertram JF. Nephron endowment and blood pressure: what do we really know? Curr Hypertens Rep. 6: 133–139. 2004. [DOI] [PubMed] [Google Scholar]
- 60.Merlet-Bénichour C, Gilbert T, Muffat-Joly M, Lelievre-Pégorier M, and Leroy B. Intrauterine growth retardation leads to a permanent nephron deficit in the rat. Pediatr Nephrol. 8: 175–180. 1994. [DOI] [PubMed] [Google Scholar]
- 61.Vehaskari VM, Aviles DH, and Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int. 59: 238–245. 2001. [DOI] [PubMed] [Google Scholar]
- 62.Bassan H, Trejo LL, Kariv N, Bassan M, Berger E, Fattal A, Gozes I, and Harel S. Experimental intrauterine growth retardation alters renal development. Pediatr Nephrol. 15: 192–195. 2000. [DOI] [PubMed] [Google Scholar]
- 63.Skov K, Nyengaard JR, Korsgaard N, and Mulvany MJ. Number and size of renal glomeruli in spontaneously hypertensive rats. J Hypertens. 12: 1373–1376. 1994. [PubMed] [Google Scholar]
- 64.Warner A. Drug use in the neonate: interrelationships of pharmacokinetics, toxicity, and biochemical maturity. Clin Chem. 32: 721–727. 1986. [PubMed] [Google Scholar]
- 65.Schreuder MF, Bueters RR, Huigen MC, Russel FG, Masereeuw R, and van den Heuvel LP. Effect of drugs on renal development. Clin J Am Soc Nephrol. 6: 212–217. 2011. [DOI] [PubMed] [Google Scholar]
- 66.Daston GP, Rehnberg BF, Carver B, Rogers EH, and Kavlock RJ. Functional teratogens of the rat kidney. I. Colchicine, dinoseb, and methyl salicylate. Fundam Appl Toxicol. 11: 381–400. 1988. [DOI] [PubMed] [Google Scholar]
- 67.Cowan RH, Jukkola AF, and Arant BS, Jr . Pathophysiologic evidence of gentamicin nephrotoxicity in neonatal puppies. Pediatr Res. 14: 1204–1211. 1980. [DOI] [PubMed] [Google Scholar]
- 68.Gilbert-Barness E. Teratogenic causes of malformations. Ann Clin Lab Sci. 40: 99–114. 2010. [PubMed] [Google Scholar]
- 69.Morgan TM, Jones DP, and Cooper WO. Renal teratogens. Clin Perinatol. 41: 619–632. 2014. [DOI] [PubMed] [Google Scholar]
- 70.Hanssens M, Keirse MJNC, Vankelecom F, and Van Assche FA. Fetal and neonatal effects of treatment with angiotensin-converting enzyme inhibitors in pregnancy. Obstet Gynecol. 78: 128–135. 1991. [PubMed] [Google Scholar]
- 71.Guron G, and Friberg P. An intact renin-angiotensin system is a prerequisite for normal renal development. J Hypertens. 18: 123–137. 2000. [DOI] [PubMed] [Google Scholar]