Abstract
AIM
To compare the potential protective effects of epi-gallocatechin gallate (EGCG) and ellagic acid (EA) in an experimental cataract model.
METHODS
Twenty-eight Spraque-Dawley rat pups were assigned into four groups. All the rats, except for those in the control group, were injected subcutaneously sodium selenite to induce experimental cataract on the postpartum ninth day, and between 10th and 14th days. Rats in the sham, EGCG, and EA groups were intraperitoneally administered 50 mg/(kg·d) saline solution, 50 mg/(kg·d) EGCG and 200 mg/(kg·d) EA, respectively. The reduced glutathione (GSH) and malondialdehyde (MDA) levels, total antioxidant status (TAS) and total oxidant status (TOS) in lens supernatants were measured.
RESULTS
The mean cataract gradings in EGCG and EA groups were found to be significantly lower than that in sham group (P<0.001). The mean GSH levels and TASs in EGCG and EA groups were significantly higher than that in sham group while mean MDA levels and TOSs in EGCG and EA groups were significantly lower than that in the sham group (P<0.001).
CONCLUSION
EGCG and EA have protective effects on cataract development via the inhibition of oxidative stress.
Keywords: sodium selenite, experimental cataract, epi-gallocatechin gallate, ellagic acid, total oxidant status, total anti-oxidant status
INTRODUCTION
Acataract is defined as an opacity in the crystalline lens; it is the leading cause of blindness across the world, affecting about 80 percent of people over 70 years old[1]. Today, surgery is the unique treatment for cataracts, which means that the number of cataract-related surgeries has tripled over the years. Meanwhile, the rising costs of cataract surgery and possible surgical complications have driven scientists to research new drugs that can prevent or delay the formation of cataracts. If the onset of cataracts can be delayed for about ten years, the number of annual cataract operations is expected to decrease by up to 45 percent[2]–[3].
Cataracts are characterised by a multifactorial pathogenesis, with the disease developing as a result of heredity, trauma, inflammation, metabolic disorders, malnutrition and age-related changes, amongst other pathways. Some risk factors, such as oxidative damage, impaired glucose metabolism, radiation damage and toxic damage to the lens, also play an important role in the pathogenesis of cataracts. One of the most common types of cataracts is that related to age. Although the exact mechanism of age-related cataract formation is unknown, the increase in free oxygen radicals and the reduction in antioxidant enzymes in the lens have been identified as possible mechanisms[4]–[7]. According to the theory of oxidative damage, free oxidant radicals lead to cataract formation by cross-linking and aggregation of lens proteins, the peroxidation of membrane lipids and by apoptosis of epithelial cells in the lens[4]–[6].
Increased amounts of oxidative substances and reduced levels of antioxidants in the lens such as glutathione were proposed to be involved in the pathogenesis of cataracts[1]–[5]. Researchers have uncovered the importance of increased oxidative substances and reduced levels of antioxidants in the pathogenesis of cataracts[6]–[7].
Glutathione is the most important antioxidant in the lens and is synthesized the lens epithelium. The reduced glutathione (GSH) exists in high concentration in the lens. GSH provides maintenance of the lens transparency by scavenging reactive oxygen species and protecting protein thiols. It has been reported that the GSH level in the lens is decreased in age-related cataract[6]–[10].
Lipid peroxidation plays an important role in pathogenesis of cataract. The degree of lipid peroxidation can be estimated by the amount of malondialdehyde (MDA). It has been reported that MDA levels are higher in lenses with cataract than in those without[11]–[14].
Epigallocatechin gallate (EGCG) is a green tea catechin with potent antioxidant effects[15]. Recent studies have shown that EGCG has antioxidant and anti-inflammatory effects and that EGCG is able to scavenge superoxide[15]–[17]. EGCG has also been reported to protect the γB-crystallin and lens epithelial cells in humans from UV or hyperglycaemia-induced damage[18]–[20].
Ellagic acid (EA) is a polyphenolic compound found in many fruits, including hazelnuts, raspberries, pomegranates, walnuts, raisins and currants. Some of the benefits derived from EA are its antioxidant and anti-inflammatory effects; its cleansing of oxygen and hydroxyl radicals; and its inhibition of lipid peroxidation[21]–[25]. A study has also demonstrated its anti-cataract effects[26].
In our study, we aimed to compare the preventive effect of EGCG and EA against cataract formation in experimental sodium selenite cataract model.
MATERIALS AND METHODS
Study Design and Ethics
This study was performed in the Eye Diseases Clinic of Firat University Medicine Faculty with the addition of the Department of Biochemistry and with the approval of Firat University Medicine Faculty Ethics Committee. It was funded by an unrestricted grant from the Firat University Scientific Research Unit. Throughout the study, the rats were maintained in the experimental research center at Firat University. The animals were housed in special wire-bottom cages and in standard conditions (12-hour daylight-dark cycle, ventilated, constant room temperature). It has been considered that solid-bottom cages are more adequate for the housing of the rodents. However, we had to house them in wire-bottom cages because our research unit has no solid-bottom cages.
All were fed only with breast milk until 21d, on which they were sacrificed. The study was carried out using one eye from each animal. All procedures were performed with strict adherence to the guidelines for animal care and experimentation as prepared by the Association for Research in Vision and Ophthalmology and Guidelines for the Housing of Rats in Scientific Institutions.
Study Groups
The rats were randomly assigned to four groups, with seven rats in each group: Group 1 (control group) included rats in which cataract was not induced and did not receive any treatment; Group 2 (sham group) included rats in which cataract was induced and which were treated with saline; Group 3 (EGCG group) included rats in which cataract was induced and which were treated with EGCG; Group 4 (EA group) included rats in which cataract was induced and which were treated by EA.
Methods
All newborn rats except those of the control group were injected single dose 30 nmol/g body weight subcutaneously sodium selenite (Sigma Chemical Co., St. Louis, MO, USA) on the postnatal 10th day. The rats in the control group were also not received any treatment.
Due to EGCG contained powdered pharmaceutical forms, it was prepared in 0.9% physiological saline solution and EA was prepared by dissolving in ethanol 1% solution. Between the 10th and 14th days, rats in sham, EGCG, and EA groups were intraperitoneally administered 50 mg/(kg·d) saline solution, 50 mg/(kg·d) EGCG and 200 mg/(kg·d) EA, respectively. The intraperitoneally doses of saline solution, EGCG and EA were determined according to the previous studies which studied these compounds[18]–[19],[26]–[27].
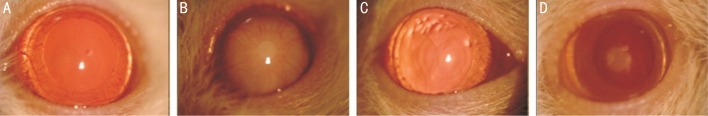
Cataract formation was evaluated weekly for 3wk by slit-lamp examination. Before slit-lamp examination for pupil dilation 0.5% tropicamide and 2.5% phenylephrine hydrochloride drop was dropped in every 30min for two hours. All lenses were evaluated and were morphologically staged for cataract development. Lens photos ×25 magnifications were taken using a camera attached to slit-lamp (Topcon, Tokyo, Japan) (Figure 1).
Figure 1. The samples for the cataract stages of the study groups.
A: Stage 0 cataract with a clear crystalline lens of one experiment from the control group; B: Stage 6 cataract with dense lens opacity of one experiment from the sham group; C: Stage 1 cataract with minimal nuclear lens opacity of one experiment from the epigallocatechin gallate group; D: Stage 2 cataract with posterior subcapsular lens opacity of one experiment from the ellagic acid group.
On day 21, after rats were sacrificed under anesthesia (for providing easy enucleation manipulation), the eyes were enucleated and the lenses were taken with their capsules. Frozen lens samples were weighed and homogenized in ice-cold phosphate buffered saline solution (0.01 mol/L and pH 7.4). Homogenization procedures were carried out using Bullet Blend tissue Homogenizer (Next Advanced Inc, Averill Park, NY, USA), according to the manufacturer's instructions at 4°C. These homogenates were centrifuged at 10 000 g for 30min at 4°C, and supernatants were obtained. Supernatants were used for the measurement of the levels of MDA, GSH, total antioxidant status (TAS) and total oxidant status (TOS).
Anesthesia Technique
The rats were injected with a combination of intramuscular ketamine hydrochloride 50 mg/kg (Ketalar, Eczacibaşi, Turkey) and xylazine hydrochloride 6 mg/kg (Rompun, Bayer, Turkey) to induce anesthesia and analgesia.
Evaluation and Staging of Cataract
The cataract formation was evaluated weekly for 3wk by slit-lamp examination. Before the cataracts were graded, the dilatation of the pupil was provided with tropicamide 0.5% and phenylephrine hydrochloride 2.5% drops. The formation and grading of cataract were evaluated and graded blindly by the single author (Ergen I) according to the slit-lamp appearance of the lenses as described at Hiraoka and Clark classification[28]: stage 0, normal clear lens; stage 1, initial sign of posterior subcapsular or minimal nuclear opacity; stage 2, slight nuclear opacity with swollen fibers or posterior subcapsular scattering foci; stage 3, cortical radiated diffuse nuclear opacity; stage 4, partial nuclear opacity; stage 5, a nuclear opacity not involving lens cortex; stage 6, mature cataract involving the entire lens.
Analysis of the Malondialdehyde and Reduced Glutathione
Levels of MDA were analyzed using an MDA kit (Immuchrom GmbH, Hessen, Germany) with high-performance liquid chromatography. Initially, protein bound malondialdehyde is hydrolyzed and converted into a fluorescent product (60min at 95°C). The fluorescent product is then cooled (2°C-8°C), centrifuged, mixed with a reaction solution and injected into the HPLC system. This fluorescent product performed by a derivatization step was added to achieve an optimum pH level. MDA-generated fluorescence was measured in the isocratic high-performance liquid chromatography system with a spectrofluorometer detector at 553 nm (emission) and 515 nm (excitation). Results were expressed as nanomoles per milliliter.
The GSH measurements were carried out using a GSH kit (ImmuchromGmbH, Hessen, Germany) with high-performance liquid chromatography. During the reaction of derivatisation glutathione is converted into a fluorescentprobe. The precipitation step removes high molecular substances. After centrifugation, the fluorescent probe is cooled (2°C-8°C) and 20 µL sample is injected into the HPLC system. Measurements were carried out on the HPLC system with a fluorescence detector at 385 nm (excitation) and 515 nm (emission). Results were expressed as micromoles per liter.
Analysis of Total Antioxidant Status and Total Oxidant Status
The TAS and TOS in lens supernatants were measured using an automated colorimetric measurement method with commercially available kits (Relassay, Gaziantep, Turkey) on an autoanalyzer (Siemens Advice 2400 Chemistry System, Japan). The principle of TAS measurement method was based on the oxidation of the 2.2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) molecule to the ABTS+ molecule in the presence of hydrogen peroxide. The rate of the reaction was calibrated with the standard method of Trolox which was a vitamin E analog, and its unit was mmol Trolox Equivalent/L. TOS method was based on the oxidation of ferrous ion-o-dianisidine complex to ferric ion by the oxidants present in the sample. The color density was correlated with the amount of oxidants in the sample. The spectrophotometric assay method calibrated with hydrogen peroxide and the results were expressed in terms of micromolar hydrogen peroxide equivalent per liter (µmol H2O2 Equiv./L).
Statistical Analysis
Statistical analyses were performed using SPSS 22.0 software package to determine the differences between groups. Results were presented as a mean ± standard deviation. Normality test was performed for each variable. ANOVA test was used for parametric data fit a normal distribution. The results between groups were compared with the Mann-Whitney U test, Kruskal-Wallis test, and One-way analysis of variance according to the characteristics of the variables. A P value less than 0.05 was considered significant.
RESULTS
The development of cataract was not detected in the control group. The mean cataract stages in sham, EGCG and EA groups were 3.50±1.41, 0.21±0.57 and 0.28±0.57, respectively. It was found that the mean cataract stage in the sham group was significantly higher than that in the control group (P<0.001). The mean cataract stage in the EGCG group and EA group was found to be significantly lower than that in the sham group (P<0.001). There was no statistically significant difference in the mean cataract stages between EA and EGCG groups (P>0.05). The mean cataract stages of the study groups are shown in Table 1.
Table 1. The mean stages of the cataract development, the levels of MDA, GSH, TOS and TAS in the study groups.
| Groups | Mean stage of cataract (±SD) |
MDA (µmol/L ±SD) |
GSH (µmol/L ±SD) |
TOS (µmol H2O2 Equiv./L±SD) |
Mean TAS (mmol Trolox Equiv./L±SD) |
| Control | 0.00±0.00 | 4±0.46 | 13±0.90 | 121±0.99 | 6.75±0.97 |
| Sham | 3.50±1.41a | 12±0.87a | 6±0.15a | 177±0.18a | 3.09±0.50a |
| EGCG | 0.21±0.57c | 6±0.20c | 12±0.90c | 128±0.59c | 7.14±0.80c |
| EA | 0.28±0.57c | 6±0.66c | 12±0.16c | 140±0.80c | 6.44±0.06c |
TAS: Total anti-oxidant status; TOS: Total oxidant status; MDA: Malondialdehyde; GSH: Reduced glutathione; EGCG: Epigallocatechin gallate group; EA: Ellagic acid group; SD: Standard deviation. aSignificant difference (P<0.05) compared with control group; cSignificant difference (P<0.05) compared with sham group.
The mean MDA levels in control, sham, EGCG and EA groups are shown in Table 1. The levels of MDA in the sham group were significantly higher than in the control group (P<0.001).
In addition, levels of MDA were found significantly lower in the EGCG group and in the EA group than in the sham group (P<0.001). There was no difference in this parameter between EA and EGCG groups (P>0.05). When the mean MDA levels in the EGCG and EA groups compared with that of the control group, there was no significant difference between the MDA levels in EA and control or EGCG and control groups (P>0.05). Additionally, there was no statistically significant difference in the mean MDA levels between EA and EGCG groups (P>0.05).
The mean GSH levels in control, sham, EGCG and EA groups are shown in Table 1. The levels of GSH in the sham group were significantly higher than in the control group (P<0.001). The levels of GSH were found significantly higher in the EGCG group and in the EA group than in the sham group (P<0.001 and P<0.01, respectively). The mean GSH levels in the EGCG and EA groups did not show significant difference than that of the control group (P>0.05). There was no significant difference in the mean GSH levels between EA and EGCG groups (P>0.05).
The mean TOSs in control, sham, EGCG and EA groups are shown in Table 1. The mean TOS was found to be significantly higher in the sham group than in the control group (P<0.001). The mean TOS was found significantly lower in the EGCG and EA groups than that of the sham group (P<0.05). There was no statistically significant difference among the control, EA and EGCG groups regarding the mean TOS (P>0.05). The mean TOSs were significantly lower in the EA and EGCG groups than in the sham group (P<0.001, P<0.001).
The mean TASs in control, sham, EGCG and EA groups are shown in Table 1. The mean TAS was significantly lower in the sham group than the control group (P<0.001). The mean TASs were found to be significantly higher in EGCG and EA groups than in the sham group (P<0.001). There was no statistically significant difference among the control, EA and EGCG groups regarding the mean TAS (P>0.05).
DISCUSSION
In most experimental cataract models, radiation, galactose, streptozocin and selenite are used to induce cataracts[28]–[30]. Selenite cataract is similar in many respects to human cataracts. Selenite, which is one of the most commonly used pharmacological agents in experimental cataract models, was first used in such a model by Ostadalova et al[30] in 1978. The basic mechanism of selenite in the formation of cataracts is that it acts like an oxidant, thereby causing damage to the lens[30]–[34].
It also causes lipid peroxidation in the crystalline lens, generates hydrogen peroxide and reduces the concentration of GSH in the lens[34].
Some studies revealed that the presence of a substantial amount of vitamins, carotenoids, caffeine, acetyl-L-carnitine, ebselen, quercetin, flavonoids, caffeic acid phenylester and curcumin exerts inhibitory effects on cataract[1],[5],[31]–[34]. However, no agent can totally block or delay the opacification of the lens.
Recent studies have indicated that the cathechins in green tea have antioxidant, anti-inflammatory, antiangiogenic and antibacterial effects[35]–[39]. Such cathechins bind reactive oxygen and nitrogen species and exert indirect antioxidant effects by stimulating the synthesis of endogenous antioxidant enzymes, such as superoxide dismutase, glutathione reductase, glutathione-S-reductase, catalase and quinonereductase. Owing to these effects, green tea can inhibit lipid peroxidation and mutation in DNA. Green tea has high concentrations of EGCG and it exhibits antioxidant activities more strongly than do vitamins C and E[37]–[41]. A recent study has shown that EGCG may effectively protect individuals from corneal surface diseases, such as dry eye, via its antioxidant and anti-inflammatory effects[42]. Emoto et al[43] indicated that green tea extract suppresses N-methyl-N-nitrosourea-induced photoreceptor apoptosis in Sprague-Dawley rats, and Cia et al[44] reported that EGCG prevents H2O2-induced oxidative stress in the retinal pigment epithelial cells of primary rat cultures. Chen et al[45] reported that eye drops with EGCG exhibit potent protective effects on ultraviolet B radiation-induced corneal oxidative damage in mice; the effects are likely due to the increase in the antioxidant activity of the defence system and the inhibition of lipid peroxidation and protein oxidation. Silva et al[46] found that green tea protects the retina against glutamate toxicity via an antioxidant mechanism. They demonstrated that treatment with green tea reduced the expression of glial fibrillary acidic protein (GFAP), oxidative retinal markers such as reactive oxygen species (ROS), and glutamine synthetase levels, whileas it increased the reduced levels of glutathione, glutamate transporter, and glutamate receptor in diabetic rats as well as in retinal cells.
Recent studies have demonstrated that EGCG also protects human γB-crystallin from UV-induced damage and cultured human lens epithelial cells from hyperglycaemia-induced damage[18]–[20]. EGCG prevents tryptophan oxidation in cataractous human lens γ-crystallin in the presence of H2O2[19]. Heo et al[47] showed that EGCG increased cell count and cell viability after the UV irradiation of cultured human lens epithelial cells, indicating that EGCG can protect lens epithelium against UV damage. No studies have thus far been conducted on the preventive effects of the EGCG in experimental cataract models. The current research demonstrated that EGCG significantly inhibits the development of cataracts.
EA is a polyphenolic compound with strong antioxidant and anti-inflammatory effects. It is an oxygen and hydroxyl radical cleaner and lipid peroxidation inhibitor[21]–[25]. EA also exerts protective effects against cataracts[26],[48]. Research showed that the mean GSH levels in an EA group was higher than that in the sham group but that the MDA level in the former was lower than that in latter[26],[48]. This supports the finding on EA's prevention of cataract development by powering antioxidant systems and inhibiting lipid peroxidation. Our results are similar to those in the literature, and we found that the development of cataracts in the EA group was strongly inhibited compared with that in the sham group. These effects of EA resemble those found by Sakthivel et al[26],[48]. In a recent study on the effect of EA and a selenite-induced cataract model, 53 percent of the rats did not suffer from lens opacification, and 47 percent of the rats exhibited only mild degrees (levels 1-2) of lens opacification[48]. In our study, we found low lens opacificationin, only 25 percent of the rats. In the previous study by Sakthivel et al[48], rats were sacrificed at postpartum 30th day while as we sacrificed our rats at postpartum 21st day. This difference may explain the variances in the percentages of cataract formation.
GSH (L-γ-glutamyl-L-cysteinyl-glycine), which is of a tripeptide structure, is an antioxidant agent that plays an important role in reducing oxidative stress and protecting cells against oxidative damage. Its intracellular level is balanced by the enzymes of the complex compounds of glutathione synthetase, glutathione peroxidase and glutathione reductase[4]–[6],[41]–[42]. GSH, which is synthesised by the lens epithelium, is equally critical in protecting the lens from oxidative damage. The many parts of glutathione in the lens are reduced form (GSH)[42]. In the prevention of cataract formation, GSH might act providing to maintain the protein thiols in the reduced state, to protect membrane -sulfhydryl groups which play important role in cation transport and permeability, and to detoxify hydrogen peroxide and other organoperoxides. The glutathione redox cycle is intimately involved in the detoxification of H2O2 which is normally present in the aqueous humor. It is well-known that the gluthathione levels are high in cataractous lenses but low in normal lenses. That of the decreasing of the intracellular GSH level leads to lipid peroxidation and damage of multitidous cellular systems by free radicals[4]–[6],[49]–[50]. In our study, we found the mean GSH levels in EGCG and EA groups were significantly higher than those in sham group. This supports that both agents have antioxidant effects against cataract formation.
MDA is the main metabolite generated by oxidation of the lipids in the cell lipids and it might change the function and activity of DNA and the proteins by cross-linking them. It is considered that the tissue MDA level is an index of lipid peroxidation. The membrane phospholipids and low-density lipoprotein are the most susceptible macromolecules to the effects of free radicals. Lipid peroxidation may cause the deterioration in membrane permeability and fluidity for ions in the plasma membranes of the crystalline lens fibres and the loss of the thiol groups of the membrane bound crystallines and large protein aggregates with low solubilities in the lens[6]–[7],[12]–[14]. The previous studies have demonstrated that the MDA levels in plasma and lenses of the patients with cataract increased compared with those in the controls[11]–[14],[51]–[55]. In our study, we found the mean MDA levels in EGCG and EA groups were significantly lower than those in the sham group. This finding is compatible with literature and supports that both agents show antioxidant effects by decreasing MDA level in the lens and prevent cataract formation.
Organism is permanently exposed to oxidative stress induced by endogenous and exogenous factors and it fights to against oxidative stress by the antioxidant defence system. Serum TAS and TOS measurements have been used to evaluate the status between oxidant stress and the severity of the disease and to monitor the results of the antioxidant therapy in many studies on ocular and extraocular diseases[56]–[60]. The measurements of TAS and TOS provide more valuable information from a single measurement of antioxidants and oxidants in blood[56]–[57],[61]. The TAS and TOS in the cataractous lenses were also studied in many studies[62]–[64].
The TOS indicates the total oxidative products in the body. Serum levels of oxidative products such as ROS (reactive nitrogen species), hydrochloric acid, MDA, and lipid peroxides constitute TOS. The reaction was enhanced by using glycerol molecules, and similar absorbances per micromolar concentration of various oxidant species, namely H2O2, t-butyl hydroperoxide and cumene hydroperoxide solutions, were obtained from this method[56]–[57],[61].
As the effects of the antioxidant components in the tissue or plasma are additive, measurement of the total antioxidant response accurately reflects the redox status of the plasma. There are antioxidant defence systems that eliminate the harmful effects of ROS in the body. The plasma concentrations of antioxidant molecules can be measured separately; however, this method is very difficult and time-consuming. Thus, instead of measuring individual antioxidant components of plasma as single tests, the TAS may be more useful and practical for evaluating the antioxidant status of samples. The reaction kinetics and characteristics of some endogenous and exogenous antioxidants, namely Trolox (a water-soluble analogue of vitamin E), vitamin C, GSH, bilirubin and uric acid, were determined by this method[56]–[57],[61].
In our study, we chose to measure the TAS and TOS as the sum of antioxidant and oxidant molecules in the lens instead of those in blood because the local TAS and TOS values projects better than serum values for the evaluating of a cataractous lens. It has been known that the lower TAS beside higher TOS shows increased oxidative stress or damage. In our study, we found the mean TAS in EGCG and EA groups were higher while mean and TOS in EGCG and EA groups were significantly lower compared with the sham group. This is concordance with literature.
In conclusion, the lower MDA level and TOS, and the higher GSH level and TAS obtained in our study suggest that EGCG and EA might inhibit the cataract formation by their antioxidant effects. Our study shows that EGCG and EA are protective against cataract by their antioxidant effects. In order to reveal the potential antioxidant effects of these agents in humans are needed for further researches.
Acknowledgments
Authors' Contributions: All authors listed on the title page made significant contributions to the design, conduct, collection and management, analysis and interpretation of the data, preparation of study of the manuscript.
Foundation: Funded by an unrestricted grant from Firat University Scientific Research Unit.
Conflicts of Interest: Ergen I, None; Turgut B, None; Ilhan N, None.
REFERENCES
- 1.Prokofyeva E, Wegener A, Zrenner E. Cataract prevalence and prevention in Europe: a literature review. Acta Ophthalmol. 2013;91(5):395–405. doi: 10.1111/j.1755-3768.2012.02444.x. [DOI] [PubMed] [Google Scholar]
- 2.Richter GM, Torres M, Choudhury F, Azen SP, Varma R, Los Angeles Latino Eye Study Group Risk factors for cortical, nuclear, posterior subcapsular, and mixed lens opacities: the Los Angeles Latino Eye Study. Ophthalmology. 2012;119(3):547–554. doi: 10.1016/j.ophtha.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang JS, Xu L, Wang YX, You QS, Wang JD, Jonas JB. Five-year incidence of age-related cataract and cataract surgery in the adult population of greater Beijing: the Beijing Eye Study. Ophthalmology. 2011;118(4):711–718. doi: 10.1016/j.ophtha.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Oduntan OA, Mashige KP. A review of the role of oxidative stress in the pathogenesis of eye diseases. South African Optometrist. 2011;70(4):191–199. [Google Scholar]
- 5.Gupta SK, Selvan VK, Agrawal SS, Saxena R. Advances in pharmacological strategies for the prevention of cataract development. Indian J Ophthalmol. 2009;57(3):175–183. doi: 10.4103/0301-4738.49390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kisic B, Miric D, Zoric L, Ilic A. Catala A, editor. Role of lipid peroxidation in the pathogenesis of age-related cataract. Lipid Peroxidation. 2012:457–482. [Google Scholar]
- 7.Michael R, Bron AJ. The ageing lens and cataract: a model of normal and pathological ageing. Philos Trans R Soc Lond B Biol Sci. 2011;366(1568):1278–1292. doi: 10.1098/rstb.2010.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fris M, Tessem MB, Saether O, Midelfart A. Biochemical changes in selenite cataract model measured by high-resolution MAS H NMR spectroscopy. Acta Ophthalmol Scand. 2006;84(5):684–692. doi: 10.1111/j.1600-0420.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 9.Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80(5):709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Giblin FJ. Glutathione: a vital lens antioxidant. J Ocul Pharmacol Ther. 2000;16(2):121–135. doi: 10.1089/jop.2000.16.121. [DOI] [PubMed] [Google Scholar]
- 11.Berthoud VM, Beyer EC. Oxidative stress, lens gap junctions, and cataracts. Antioxid Redox Signal. 2009;11(2):339–353. doi: 10.1089/ars.2008.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slatter DA, Bolton CH, Bailey AJ. The importance of lipid-derived malondialdehyde in diabetes mellitus. Diabetologia. 2000;43(5):550–557. doi: 10.1007/s001250051342. [DOI] [PubMed] [Google Scholar]
- 13.Bhuyan KC, Bhuyan DK. Molecular mechanism of cataractogenesis: III. Toxic metabolites of oxygen as initiators of lipid peroxidation and cataract. Curr Eye Res. 1984;3(1):67–81. doi: 10.3109/02713688408997188. [DOI] [PubMed] [Google Scholar]
- 14.Micelli-Ferrari T, Vendemiale G, Grattagliano I, Boscia F, Arnese L, Altomare E, Cardia L. Role of lipid peroxidation in the pathogenesis of myopic and senile cataract. Br J Ophthalmol. 1996;80(9):840–843. doi: 10.1136/bjo.80.9.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nanjo F, Mori M, Goto K, Hara Y. Radical scavenging activity of tea catechins and their related compounds. Biosci Biotechnol Biochem. 1999;63(9):1621–1623. doi: 10.1271/bbb.63.1621. [DOI] [PubMed] [Google Scholar]
- 16.Taş S, Sarandöl E, Ziyanok S, Aslan K, Dirican M. Effects of green tea on serum paraoxonase/arylesterase activities in streptozotocin-induced diabetic rats. Nutr Res. 2005;25(12):1061–1074. [Google Scholar]
- 17.Weinreb O, Mandel S, Amit T, Youdim MB. Neurological mechanisms of green tea polyphenols in Alzheimer's and Parkinson's diseases. J Nutr Biochem. 2004;15(9):506–516. doi: 10.1016/j.jnutbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhury S, Bag S, Bose M, Das AK, Ghosh AK, Dasgupta S. Protection of human γB-crystallin from UV-induced damage by epigallocatechin gallate: spectroscopic and docking studies. Mol Biosyst. 2016;12(9):2901–2909. doi: 10.1039/c6mb00256k. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhury S, Ghosh I, Saha G, Dasgupta S. EGCG prevents tryptophan oxidation of cataractous ocular lens human γ-crystallin in presence of H2O2. Int J Biol Macromol. 2015;77:287–292. doi: 10.1016/j.ijbiomac.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 20.Ye P, Lin K, Li Z, Liu J, Yao K, Xu W. Epigallocatechin gallate regulates expression of apoptotic genes and protects cultured human lens epithelial cells under hyperglycemia. Mol Biol (Mosk) 2013;47(2):251–257. doi: 10.7868/s0026898413020109. [DOI] [PubMed] [Google Scholar]
- 21.Priyadarsini KI, Khopde SM, Kumar SS, Mohan H. Free radical studies of ellagic acid, a natural phenolic antioxidant. J Agric Food Chem. 2002;50(7):2200–2206. doi: 10.1021/jf011275g. [DOI] [PubMed] [Google Scholar]
- 22.Kaur S, Grover IS, Kumar S. Antimutagenic potential of ellagic acid isolated from Terminalia arjuna. Indian J Exp Biol. 1997;35(5):478–482. [PubMed] [Google Scholar]
- 23.Ihantola-Vormisto A, Summanen J, Kankaanranta H, Vuorela H, Asmawi ZM, Moilanen E. Anti-inflammatory activity of extracts from leaves of phyllanthus emblica. Planta Med. 1997;63(6):518–524. doi: 10.1055/s-2006-957754. [DOI] [PubMed] [Google Scholar]
- 24.Cozzi R, Ricordy R, Bartolini F, Ramadori L, Perticone P, De Salvia R. Taurine and ellagic acid: two differently-acting natural antioxidants. Environ Mol Mutagen. 1995;26(3):248–254. doi: 10.1002/em.2850260310. [DOI] [PubMed] [Google Scholar]
- 25.Takagi A, Sai K, Umemura T, Hasegawa R, Kurokawa Y. Inhibitory effects of vitamin E and ellagic acid on 8-hydroxydeoxyguanosine formation in liver nuclear DNA of rats treated with 2-nitropropane. Cancer Lett. 1995;91(1):139–144. doi: 10.1016/0304-3835(95)03734-e. [DOI] [PubMed] [Google Scholar]
- 26.Sakthivel M, Elanchezhian R, Ramesh E, Isai M, Jesudasan CN, Thomas PA, Geraldine P. Prevention of selenite-induced cataractogenesis in Wistar rats by the polyphenol, ellagic acid. Exp Eye Res. 2008;86(2):251–259. doi: 10.1016/j.exer.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Yildirim AE, Dalgic A, Divanlioglu D, Akdag R, Cetinalp NE, Alagoz F, Helvacioglu F, Take G, Guvenc Y, Koksal I, Belen AD. Biochemical and histopathological effects of catechin on experimental peripheral nerve injuries. Turk Neurosurg. 2015;25(3):453–460. doi: 10.5137/1019-5149.JTN.12852-14.2. [DOI] [PubMed] [Google Scholar]
- 28.Hiraoka T, Clark JI. Inhibition of lens opacification during the early stages of cataract formation. Invest Ophthalmol Vis Sci. 1995;36(12):2550–2555. [PubMed] [Google Scholar]
- 29.Chitkara DK. Cataract formation mechanisms. In: Yanoff M, Duker JS, editors. Ophthalmology. Mosby: St Louis, MO; 1999. pp. 481–488. [Google Scholar]
- 30.Ostadalova I, Babicky A, Obenberger J. Cataract induced by administration of a single dose of sodium selenite to suckling rats. Experientia. 1978;34(2):222–223. doi: 10.1007/BF01944690. [DOI] [PubMed] [Google Scholar]
- 31.Dherani M, Murthy GV, Gupta SK, Young IS, Maraini G, Camparini M, Price GM, John N, Chakravarthy U, Fletcher AE. Blood levels of vitamin C, carotenoids and retinol are inversely associated with cataract in a North Indian population. Invest Ophthalmol Vis Sci. 2008;49(8):3328–3335. doi: 10.1167/iovs.07-1202. [DOI] [PubMed] [Google Scholar]
- 32.Christen WG, Liu S, Glynn RJ. Dietary carotenoids, vitamins C and E, and risk of cataract in women: a prospective study. Arch Ophthalmol. 2008;126(1):102–109. doi: 10.1001/archopht.126.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aydemir O, Güler M, Kaya M. Protective effects of ebselen on selenite-induced experimental cataract in rats. J Cataract Refract Surg. 2012;38(12):2160–2166. doi: 10.1016/j.jcrs.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 34.Manikandan R, Thiagarajan R, Beulaja S, Sudhandiran G, Arumugam M. Effect of curcumin on selenite-induced cataractogenesis in Wistar rat pups. Curr Eye Res. 2010;35(2):122–129. doi: 10.3109/02713680903447884. [DOI] [PubMed] [Google Scholar]
- 35.Koo MW, Cho CH. Pharmacological effects of green tea on the gastrointestinal system. Eur J Pharmacol. 2004;500(1-3):177–185. doi: 10.1016/j.ejphar.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 36.Singh AK, Seth P, Anthony P, Husain MM, Madhavan S, Mukhtar H, Maheshwari RK. Green tea constituent epigallocatechin-3-gallate inhibits angiogenic differentiation of human endothelial cells. Arch Biochem Biophys. 2002;401(1):29–37. doi: 10.1016/S0003-9861(02)00013-9. [DOI] [PubMed] [Google Scholar]
- 37.Yang CS, Chung JY, Yang G, Chhabra SK, Lee MJ. Tea and tea polyphenols in cancer prevention. J Nutr. 2000;130(2S Suppl):472S–478S. doi: 10.1093/jn/130.2.472S. [DOI] [PubMed] [Google Scholar]
- 38.Miura Y, Chiba T, Tomita I, Koizumi H, Miura S, Umegaki K, Hara Y, Ikeda M, Tomita T. Tea catechins prevent the development of atherosclerosis in apoprotein E-deficient mice. J Nutr. 2001;131(1):27–31. doi: 10.1093/jn/131.1.27. [DOI] [PubMed] [Google Scholar]
- 39.Murase T, Nagasawa A, Suzuki J, Hase T, Tokimitsu I. Beneficial effects of tea catechins on diet-induced obesity: Stimulation of lipid catabolism in the liver. Int J Obes Relat Metab Disord. 2002;26(11):1459–1464. doi: 10.1038/sj.ijo.0802141. [DOI] [PubMed] [Google Scholar]
- 40.Crespy V, Williamson G. A review of the health effects of green tea catechins in in vivo animal models. J Nutr. 2004;134(12 Suppl):3431S–3440S. doi: 10.1093/jn/134.12.3431S. [DOI] [PubMed] [Google Scholar]
- 41.Wu LY, Juan CC, Ho LT, Hsu YP, Hwang LS. Effect of green tea supplementation on insulin sensitivity in Sprague-Dawley rats. J Agric Food Chem. 2004;52(3):643–648. doi: 10.1021/jf030365d. [DOI] [PubMed] [Google Scholar]
- 42.Cavet ME, Harrington KL, Vollmer TR, Ward KW, Zhang JZ. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol Vis. 2011;17:533–542. [PMC free article] [PubMed] [Google Scholar]
- 43.Emoto Y, Yoshizawa K, Kinoshita Y, Yuri T, Yuki M, Sayama K, Shikata N, Tsubura A. Green tea extract suppresses N-methyl-N-nitrosourea-induced photoreceptor apoptosis in Sprague-Dawley rats. Graefes Arch Clin Exp Ophthalmol. 2014;252(9):1377–1384. doi: 10.1007/s00417-014-2702-7. [DOI] [PubMed] [Google Scholar]
- 44.Cia D, Vergnaud-Gauduchon J, Jacquemot N, Doly M. Epigall-ocatechin gallate (EGCG) prevents H2O2-induced oxidative stress in primary rat retinal pigment epithelial cells. Curr Eye Res. 2014;39(9):944–952. doi: 10.3109/02713683.2014.885532. [DOI] [PubMed] [Google Scholar]
- 45.Chen MH, Tsai CF, Hsu YW, Lu FJ. Epigallocatechin gallate eye drops protect against ultraviolet B-induced corneal oxidative damage in mice. Mol Vis. 2014;20:153–162. [PMC free article] [PubMed] [Google Scholar]
- 46.Silva KC, Rosales MA, Hamassaki DE, Saito KC, Faria AM, Ribeiro PA, Faria JB, Faria JM. Green tea is neuroprotective in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54(2):1325–1336. doi: 10.1167/iovs.12-10647. [DOI] [PubMed] [Google Scholar]
- 47.Heo J, Lee BR, Koh JW. Protective effects of epigallocatechin gallate after UV irradiation of cultured human lens epithelial cells. Korean J Ophthalmol. 2008;22(3):183–186. doi: 10.3341/kjo.2008.22.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakthivel M, Geraldine P, Thomas PA. Alterations in the lenticular protein profile in experimental selenite-induced cataractogenesis and prevention by ellagic acid. Graefes Arch Clin Exp Ophthalmol. 2011;249(8):1201–1210. doi: 10.1007/s00417-011-1644-6. [DOI] [PubMed] [Google Scholar]
- 49.Cnubben NH, Rietjens IM, Wortelboer H, van Zanden J, van Bladeren PJ. The interplay of glutathione-related processes in antioxidant defense. Environ Toxicol Pharmacol. 2001;10(4):141–152. doi: 10.1016/s1382-6689(01)00077-1. [DOI] [PubMed] [Google Scholar]
- 50.Reddy VN, Giblin FJ. Metabolism and function of glutathione in the lens. Ciba Found Symp. 1984;106:65–87. doi: 10.1002/9780470720875.ch5. [DOI] [PubMed] [Google Scholar]
- 51.Robertson RP, Harmon JS. Diabetes, glucose toxicity and oxidative stress: A case of double jeopardy for the pancreatic islet cell. Free Radical Biologyand Medicine. 2006;41(2):177–184. doi: 10.1016/j.freeradbiomed.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 52.Garg R, Verma M, Mathur SP, Murthy PS. Blood lipid peroxidation products and antioxidants in senile cataract. Ind J Clin Biochem. 1996;11(2):182–186. [Google Scholar]
- 53.Manjunatha Goud BK, Nandini M, Kamath Asha, Nayal Sudhir, Bhavna Oxidative stress and calcium levels in senile and type 2 diabetic cataract patients. Int J Pharm Bio Sci. 2011;2(1):109–116. [Google Scholar]
- 54.Vasavada AR, Thampi P, Yadav S, Rawal UM. Acid phosphatase and lipid peroxidation in humancataractous lens epithelium. Indian J Ophthalmol. 1993;41(4):173–175. [PubMed] [Google Scholar]
- 55.Lian H, Li S, Cao X, Pan S, Liang S. Malonaldehyde, superoxide dismutase and human cataract. Yan Ke Xue Bao. 1993;9(4):186–189. [PubMed] [Google Scholar]
- 56.Koksal H, Kurban S. Total oxidant status, total antioxidant status, and paraoxonase and arylesterase activities during laparoscopic cholecystectomy. Clinics (Sao Paulo) 2010;65(3):285–290. doi: 10.1590/S1807-59322010000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Motor S, Ozturk S, Ozcan O, Gurpinar AB, Can Y, Yuksel R, Yenin JZ, Seraslan G, Ozturk OH. Evaluation of total antioxidant status, total oxidant status and oxidative stress index in patients with alopecia areata. Int J Clin Exp Med. 2014;7(4):1089–1093. [PMC free article] [PubMed] [Google Scholar]
- 58.Donma O, Yorulmaz EO, Pekel H, Suyugül N. Blood and lens lipid peroxidation and antioxidant status in normal individuals, senile and diabetic cataractous patients. Cur Eye Res. 2002;25(1):9–16. doi: 10.1076/ceyr.25.1.9.9960. [DOI] [PubMed] [Google Scholar]
- 59.Babizhayev MA, Deyev AI. Lens opacity induced by lipid peroxidation products as a model of cataract associated with retinal disease. Biochim Biophys Acta. 1989;1004(1):124–133. doi: 10.1016/0005-2760(89)90222-1. [DOI] [PubMed] [Google Scholar]
- 60.Beyazyıldız E, Çankaya AB, Ergan E, Anayol MA, Özdamar Y, Sezer S, Tırhış MH, Yılmazbaş P, Öztürk F. Changes of total antioxidant capacity and total oxidant status of aqueous humor in diabetes patients and correlations with diabetic retinopathy. Int J Ophthalmol. 2013;6(4):531–536. doi: 10.3980/j.issn.2222-3959.2013.04.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 62.Uğurlu N, Yülek F, Ergun ŞB, Aşik MD, İşikoğlu S, Akçay E. Investigation of oxidative and antioxidative status in patients with diabetic cataracts. Turk J Med Sci. 2013;43:678–683. [Google Scholar]
- 63.Saygili EI, Aksoy SN, Gurler B, Aksoy A, Erel O, Ozaslan M. Oxidant/antioxidant status of patients with diabetic and senile cataract. Biotechnol & Biotechnol Eq. 2010;24(1):1648–1652. [Google Scholar]
- 64.Katta AV, Katkam RV, Geetha H. Lipid peroxidation and the total antioxidant status in the pathogenesis of age relatedand diabetic cataracts: a study on the lens and blood. J Clin Diagn Res. 2013;7(6):978–981. doi: 10.7860/JCDR/2013/4937.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]