Abstract
AIM
To investigate the cross-talk between oxidative stress and the epidermal growth factor receptor (EGFR)/AKT signaling pathway in retinal pigment epithelial (RPE) cells.
METHODS
Human RPE cell lines (ARPE-19 cell) were treated with different doses of epidermal growth factor (EGF) and hydrogen peroxide (H2O2). Cell viability was determined by a methyl thiazolyl tetrazolium assay. Cell proliferation was examined by a bromodeoxyuridine (BrdU) incorporation assay. EGFR/AKT signaling was detected by Western blot. EGFR localization was also detected by immunofluorescence. In addition, EGFR/AKT signaling was intervened upon by EGFR inhibitor (erlotinib), PI3K inhibitor (A66) and AKT inhibitor (MK-2206), respectively. H2O2-induced oxidative stress was blocked by antioxidant N-acetylcysteine (NAC).
RESULTS
EGF treatment increased ARPE-19 cell viability and proliferation through inducing phosphorylation of EGFR and AKT. H2O2 inhibited ARPE-19 cell viability and proliferation and also suppressed EGF-stimulated increase of RPE cell viability and proliferation by affecting the EGFR/AKT signaling pathway. EGFR inhibitor erlotinib blocked EGF-induced phosphorylation of EGFR and AKT, while A66 and MK-2206 only blocked EGF-induced phosphorylation of AKT. EGF-induced phosphorylation and endocytosis of EGFR were also affected by H2O2 treatment. In addition, antioxidant NAC attenuated H2O2-induced inhibition of ARPE-19 cell viability through alleviating reduction of EGFR, and phosphorylated and total AKT proteins.
CONCLUSION
Oxidative stress affects RPE cell viability and proliferation through interfering with the EGFR/AKT signaling pathway. The EGFR/AKT signaling pathway may be an important target in oxidative stress-induced RPE cell dysfunction.
Keywords: oxidative stress, epidermal growth factor receptor, AKT, retinal pigment epithelial cell
INTRODUCTION
Age-related macular degeneration (AMD) is a leading cause of blindness in the elderly in developed countries[1]–[3]. The retinal pigment epithelial (RPE) cells between the photoreceptors and the choroid are essential for maintaining normal physiological function of the retina[4]. Increasing evidence indicates that oxidative stress-induced RPE cell dysfunction is considered a major factor in the pathogenesis of early AMD[5]–[7]. However, the exact mechanism of oxidative stress affecting the RPE cells survival is not well understood.
Epidermal growth factor receptor (EGFR) is a tyrosine kinase receptor located at the cell membrane. EGFR plays an important role in the development and normal physiology of epithelial cells, such as stimulating cell proliferation, differentiation and migration[8]. Some evidence indicates that EGFR-mediated signaling can activate intracellular phosphorylation of AKT, which can lead to proliferation, differentiation and migration of RPE cells[9]–[11]. Recent evidence also indicates that cellular stress triggers robust trafficking and signaling of ligand-independent EGFR[12]–[14]. Wang et al[15] found that EGFR-dependent AKT activation enhanced cell survival under oxidative stress. Yang et al[16] reported that oxidant triggered activation of AKT in human RPE cells. Recent studies have also shown that activation of EGFR or AKT proteins is involved in the protection of RPE cell injury caused by oxidative stress[17]–[20]. However, the mechanism of oxidative stress affecting the EGFR/AKT signaling pathway in RPE cells is not fully known.
In the present study, our objective was to investigate the effects of epidermal growth factor (EGF) and oxidative stress induced by hydrogen peroxide (H2O2) on the EGFR/AKT signaling pathway and their potential cross-talk in RPE cells in vitro. Our data indicate that oxidative stress induced by H2O2 inhibits ARPE-19 cell survival through affecting the EGFR/AKT signaling pathway. These results suggest the EGFR/AKT signaling pathway may be an important target for preventing and treating oxidative injury in RPE cells.
MATERIALS AND METHODS
Materials
EGF was purchased from Peprotech Inc. (Rocky Hill, NJ, USA). H2O2, methyl thiazolyl tetrazolium (MTT), and N-acetylcysteine (NAC) were purchased from Sigma-Aldrich (St. Louis, MO, USA). EGFR inhibitor (erlotinib), PI3K inhibitor (A66), AKT inhibitor (MK-2206), and bromo-deoxyuridine (BrdU) were purchased from Selleck Chemicals LLC (Houston, TX, USA). Total and phosphorylated EGFR primary antibodies and secondary antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Total and phosphorylated AKT primary antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). BrdU primary antibody was purchased from Proteintech Inc. (Wuhan, Hubei Province, China). Alexa Fluor® 488- or 594-conjugated secondary antibodies were purchased from Abcam Inc. (Cambridge, MA, USA).
Cell Culture
The human RPE cell lines (ARPE-19 cells) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). ARPE-19 cells were cultured in Dulbecco's Modified Eagle Medium/Nutrient Mixture F/12 (DMEM/F12), supplemented with 10% (v/v) fetal bovine serum, 100 units/mL of penicillin, and 100 µg/mL streptomycin in a humidified incubator with 5% carbon dioxide (CO2) atmosphere at 37°C.
Cell Viability Assay
ARPE-19 cells (1×104/well) were cultured in 96-wells culture plates and treated with different concentrations of EGF or H2O2. Cells were then incubated in a medium with MTT solution (50 µg/mL) for 4h. Next, the culture medium was removed and 150 µL of dimethylsulfoxide were added to each well. The absorbance at 570 nm was detected with a microplate reader (POLARstar Omega, BMG Labtech, Ortenberg, Germany). Cell viability was expressed as a percentage of control.
Bromodeoxyuridine Incorporation Assay
ARPE-19 cells were cultured on 0.1% gelatin-coated glass slides in 12-wells plates. Cells were divided into one control group and three treatment groups: a group treated with 300 µmol/L H2O2, a group treated with 100 ng/mL EGF, and a group treated with 100 ng/mL EGF plus 300 µmol/L of H2O2. After being treated for 24h, cells were incubated with 30 µmol/L BrdU for 4h, fixed with 4% paraformaldehyde (PFA) for 30min at room temperature, washed three times with phosphate buffered saline (PBS), and denatured with 2 mol/L hydrochloric acid in PBS containing 0.1% Tween-20 (PBST) for 30min. Cells were then blocked with 5% bovine serum albumin for 1h. After being washed three times with PBS, cells were incubated with a primary anti-BrdU antibody for 2h at room temperature. Next, cells were washed three times with PBS and incubated with a secondary antibody for 1h. Finally, after being washed three times with PBS, cells were mounted onto glass slides by FluoroShield™ with DAPI (Sigma Aldrich, MO, USA) and observed using a confocal microscope (LSM 780, Carl Zeiss, Jena, Germany).
Western Blot Analysis
ARPE-19 cells were treated with the reagent for different times. Cells were washed twice with by cold PBS and then were lysed in 1×SDS (sodium dodecyl sulfonate) loading buffer. All extracted protein samples were denatured at 100°C heat for 10min, and equal amounts of proteins were loaded on 8%-10% polyacrylamide gels to separate and be transferred to polyvinylidene difluoride (PVDF) membranes. After being blocked with 5% milk diluted in PBST, the PVDF membranes were incubated with the phosphorylated and total EGFR and AKT primary antibodies and β-actin primary antibody (1:1000 dilution in PBST) overnight at 4°C, rinsed three times with PBST, and incubated with horseradish peroxidase-conjugated secondary antibodies (1:2000 in 0.1% PBST) for 2h at room temperature. Finally, after being rinsed three times with PBST, specific protein bands were developed by addition of a chemiluminescence detection solution (Advansta Inc., Menlo Park, CA, USA) and visualized and analyzed by the Bio-Rad ChemiDoc XRS system (Bio-Rad, Hercules, CA, USA).
Immunofluorescence
ARPE-19 cells were cultured on 0.1% gelatin-coated glass coverslips for 12h. Cells were treated by different reagents, fixed with 4% PFA in PBS for 15min, and rinsed three times with PBS at room temperature. Next, cells were permeabilized with 0.1% triton-X buffer for 3min, rinsed three times with PBS, and blocked in 5% bovine serum albumin for 1h. Cells were then incubated with specified antibodies for 2h, rinsed three times with PBS at room temperature, and incubated with Alexa Fluor® 488- or 594-conjugated secondary antibodies for 1h. Finally, after being rinsed three times with PBS, cells were mounted by FluoroShield™ with DAPI and were observed by using an LSM780 confocal microscope.
Statistical Analysis
All values in the study are expressed as mean±standard error of the mean (SEM). The difference between the two groups were analyzed by Student's t-test, and differences in three or more groups were analyzed by one-way ANOVA followed by a post-hoc Tukey's test using GraphPad Prism 5 Software (GraphPad Software, LaJolla, CA, USA). P<0.05 was considered statistically significant.
RESULTS
Epidermal Growth Factor and H2O2 Affect ARPE-19 Cell Viability
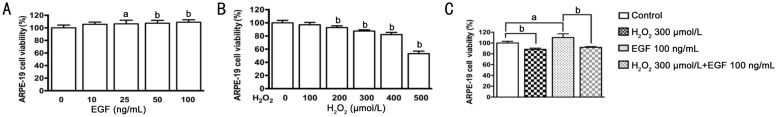
Effects of EGF and H2O2 on ARPE-19 cell viability were measured by MTT assay. EGF caused a significant increase of cell viability in a dose-dependent manner. Treatment with 10, 25, 50 and 100 ng/mL of EGF for approximately 12h increased the cell viability by 5.6%, 6.3%, 7.4% and 8.9% respectively, compared to the control (Figure 1A). H2O2 caused a significant inhibition of cell viability in a dose-dependent manner. Treatment with 100, 200, 300, 400 and 500 µmol/L of H2O2 for approximately 6h reduced the cell viability by 2.9%, 7.2%, 12.5%, 17.9% and 46.7% respectively, compared to the control (Figure 1B). In addition, EGF-induced an increase in ARPE-19 cell viability was decreased by treatment with 300 µmol/L of H2O2 (Figure 1C).
Figure 1. Effects of EGF and H2O2 on ARPE-19 cells viability.
A: ARPE-19 cells were treated with 0, 10, 25, 50, 100 ng/mL EGF for 12h (n=12); B: ARPE-19 cells were treated with 0, 100, 200, 300, 400 and 500 µmol/L H2O2 for 6h (n=8); C: ARPE-19 cells were untreated or treated with H2O2 (300 µmol/L) in the absence or presence of EGF (100 ng/mL) for 12h (n=4). Cell viability was measured by MTT assay, aP<0.05, bP<0.01.
H2O2-induced Oxidative Stress Inhibits Epidermal Growth Factor-dependent Proliferation of ARPE-19 Cells
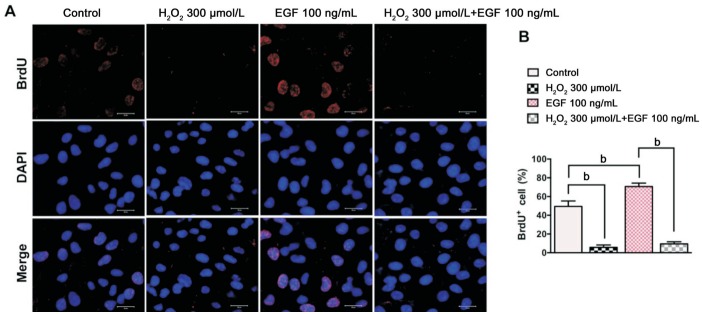
Effects of EGF and H2O2 on ARPE-19 cell proliferation were detected by a BrdU incorporation assay. The number of BrdU+ ARPE-19 cells is about 49.5% in control group. The number of BrdU+ ARPE-19 cells in the H2O2 (300 µmol/L) treatment group was reduced by about 43.8% compared with the control group, while the number of BrdU+ ARPE-19 cells in the EGF (100 ng/mL)treatment group increased by about 21% compared with the control group. Furthermore, the number of BrdU+ cells in the combined treatment group (EGF+H2O2) was reduced by about 61% compared only with the EGF treatment group (Figure 2A, 2B).
Figure 2. Oxidative stress induced by H2O2 inhibits EGF-dependent proliferation of ARPE-19 cells.
A: Immunofluorescence microphotographs of proliferating ARPE-19 cells with BrdU (red) and DAPI (blue) staining were taken after ARPE-19 cells were left untreated or treated with H2O2 (300 µmol/L) in the absence or presence of EGF (100 ng/mL) for 24h and then subjected to BrdU labeling for 4h, followed by immunostaining with anti-BrdU antibody and DAPI; B: Quantitative data of BrdU+ cell are shown in panel A (n=3, bP<0.01). Scale bars=20 µm.
Epidermal Growth Factor-induced Epidermal Growth Factor Receptor/AKT Signaling in ARPE-19 Cells
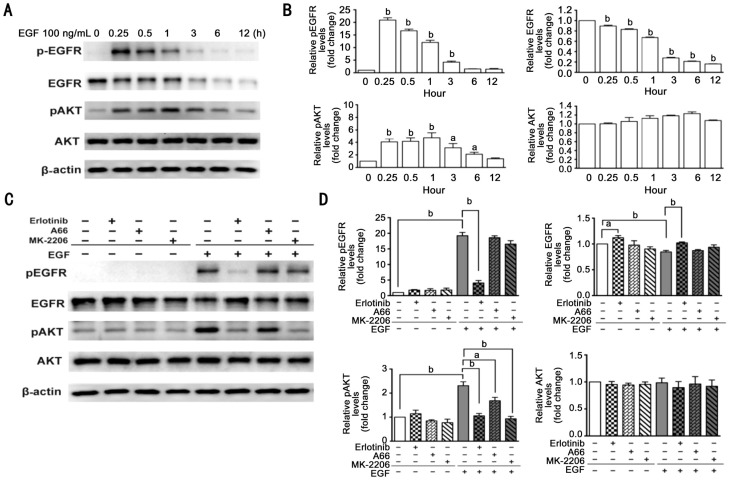
EGF-induced EGFR/AKT signaling in ARPE-19 cells was detected by Western blot. As shown in Figure 3A and 3B, after ARPE-19 cells were treated by 100 ng/mL EGF, phosphorylated EGFR levels increased to a maximum within 15min and subsequently slowly declined. Meantime, EGF treatment slowly declined total EGFR levels in a time-dependent manner. Similarly, phosphorylated AKT levels increased to maximum within one hour and subsequently slowly declined. Total AKT levels were not greatly affected by this EGF treatment. In addition, we detected effects of erlotinib, A66, and MK-2206 on EGFR/AKT signaling pathway. As shown in Figure 3C and 3D, erlotinib blocked EGF-induced phosphorylation of EGFR and AKT, and attenuated degradation of total EGFR caused by EGF. However, A66 and MK-2206 only blocked EGF-induced phosphorylation of AKT.
Figure 3. EGF-induced EGFR/AKT signaling in ARPE-19 cells.
A: ARPE-19 cells were treated with 100 ng/mL EGF for 0, 0.25, 0.5, 1, 3, 6, 12h in a serum-free medium; cell lysates were subjected to Western blot analysis for total and phosphorylated EGFR and AKT; β-actin was used as a loading control. B: Quantitative data of Western blot are shown in panel A; the levels of protein in the treatment groups were compared with the control, aP<0.05, bP< 0.01. C: ARPE-19 cells were pretreated with 0.5 µmol/L erlotinib, 10 µmol/L A66 or 10 µmol/L MK-2206 for 12h and treated further with EGF for another 15min in the serum-free medium; cell lysates were subjected to Western bolt analysis with total and phosphorylated EGFR and AKT antibody; β-actin was used as a loading control. D: Quantitative data of Western blot are shown in panel C; n=3, aP<0.05, bP<0.01.
H2O2 Affects Expression Levels of Epidermal Growth Factor Receptor and AKT Protein in ARPE-19 Cells
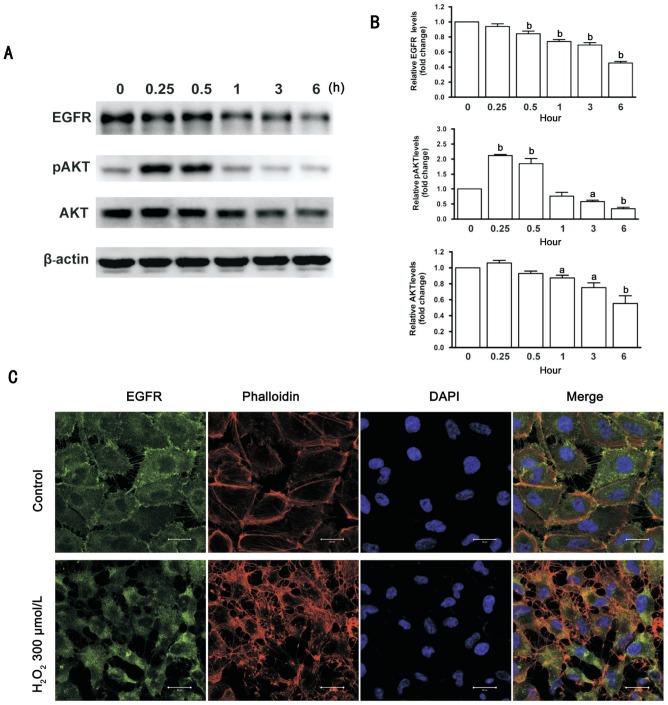
Western blot results showed that 300 µmol/L H2O2 induced a significant down-regulation of total EGFR levels in a time-dependent manner. Treatment with 300 µmol/L H2O2 for less than 1h induced a significant increase in the expression levels of phosphorylated AKT, whereas treatment with 300 µmol/L H2O2 more than 3h induced a significant reduction of phosphorylated and AKT. Additionally, the total AKT levels declined after treatment with 300 µmol/L H2O2 for 1h and longer (Figure 4A, 4B). Immunofluorescence evaluation showed that treatment with 300 µmol/L of H2O2 for 6h caused translocation of EGFR from the cell membrane to the cytoplasm of ARPE-19 cells. Cell body shrinkage and cytoskeleton destruction were observed by a fluorescent phalloidin probe staining, and cellular nuclei shrinkage was observed by DAPI staining in ARPE-19 cells (Figure 4C).
Figure 4. H2O2 affects expression of EGFR and AKT protein in ARPE-19 cells.
A: ARPE-19 cells were treated with 300 µmol/L H2O2 for 0, 0.25, 0.5, 1, 3 and 6h. Total EGFR, and phosphorylated and total AKT were detected by Western blot; β-actin was used as a loading control. B: Quantitative data of Western blot are shown in panel A; the levels of protein in the treatment groups were compared with the control; n=3, aP<0.05, bP< 0.01. C: Immunofluorescence microphotographs of EGFR (green) with phalloidin (red) and DAPI (blue) after ARPE-19 cells were treated with 300 µmol/L H2O2 for 6h. Scale bars=20 µm.
H2O2 Affects Epidermal Growth Factor-induced Epidermal Growth Factor Receptor/AKT Signaling in ARPE-19 Cells
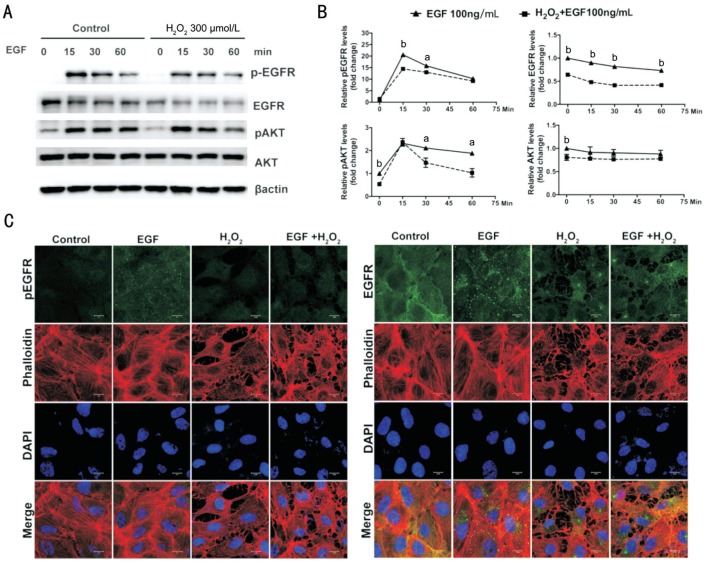
To investigate the molecular mechanism of H2O2 inhibiting EGF-stimulated cell viability and proliferation, the effect of H2O2 on EGF-induced EGFR/AKT signaling was detected. As shown in Figure 5A and 5B, significant phosphorylation of EGFR and AKT and reduction of the total EGFR were induced by treatment with 100 ng/mL EGF for 15, 30 and 60min. In contrast, pretreatment with 300 µmol/L H2O2 for 3h caused a significant decrease in levels of total EGFR and phosphorylated and total AKT. In addition, the phosphorylation of EGFR induced by treatment with EGF for 15 and 30min and the phosphorylation of AKT induced by treatment with EGF for 30 and 60min were also inhibited significantly by pretreatment with 300 µmol/L H2O2 for 3h.
Figure 5. H2O2 affects EGF-induced EGFR/AKT signaling in ARPE-19 cells.
A: ARPE-19 cells were pretreated with or without H2O2 (300 µmol/L) for 3h, and then were further stimulated by 100 ng/mL EGF for 0, 15, 30 and 60min in the serum-free medium; phosphorylated and total EGFR and AKT were detected by Western blot with respective antibodies; β-actin was used as a loading control. B: Quantitative data of Western blot are shown in panel A; comparison was between the control group and H2O2 pretreatment group at the same time point; n=3; aP<0.05, bP< 0.01. C: Immunofluorescence microphotographs of phosphorylated and total EGFR (green), with phalloidin (red) and DAPI (blue), after ARPE-19 cells were pretreated with or without H2O2 (300 µmol/L) for 3h, and then were stimulated further by 100 ng/mL EGF for 0 or 15min in the serum-free medium. Scale bars=10 µm.
Immunofluorescence evaluation showed that treatment with EGF for 15min induced a significant expression of phosphorylated EGFR and induced EGFR endocytosis from the cell membrane to the cytoplasm. In contrast, phosphorylation and endocytosis of EGFR induced by EGF treatment for 15min were suppressed by pretreatment with 300 µmol/L H2O2 for 3h. F-actin fiber staining with a fluorescent phalloidin showed that pretreatment with H2O2 caused cell body shrinkage and F-actin fiber destabilization in ARPE-19 cells (Figure 5C).
N-acetylcysteine Attenuates Inhibition of ARPE-19 Cell Viability Induced by H2O2
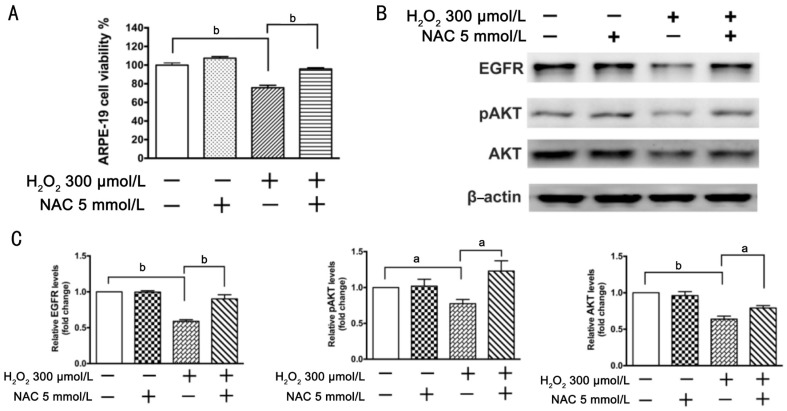
The effect of antioxidant NAC on H2O2-induced oxidative stress in ARPE-19 cells was also detected. Cell viability assay showed that NAC significantly blocked H2O2-caused inhibition of cell viability (Figure 6A). Western blot results showed that treatment with 300 µmol/L H2O2 for 6h reduced expression levels of total EGFR and phosphorylated and total AKT, while NAC significantly blocked down-regulation of total EGFR and phosphorylated and total AKT induced by H2O2 (Figure 6B, 6C).
Figure 6. NAC attenuates inhibition of ARPE-19 cells viability induced by H2O2.
ARPE-19 cells were divided into one control group and three treatment groups: a group treated with 5 mmol/L NAC, a group treated with 300 µmol/L H2O2, and a group treated with 5 mmol/L NAC plus 300 µmol/L of H2O2 for 6h. A: Cell viability was measured by MTT assay; the cell viability is expressed as a percentage of the control (n=4); B: Total EGFR and phosphorylated and total AKT were detected by Western blot; β-actin was used as a loading control. C: Quantitative data of Western blot are shown in panel B; n=3, aP<0.05, bP<0.01.
DISCUSSION
RPE, a monolayer of pigmented cells located between photoreceptor and vessels of the choriocapillaris, forms a part of the blood/retina barrier[4]. An increasing number of studies indicate that the oxidative stress-induced RPE cells damage contributes the progress of AMD[21]–[22]. Environmental factors (e.g. intense light exposure[23] or cigarette smoke[24]) could arouse the oxidative stress and increase the risk of RPE injury. Some evidence has shown that oxidative stress inhibits RPE cells viability and proliferation and induces RPE cells apoptosis[25]–[27]. However, the exact mechanism of oxidative stress-induced the RPE cells damage is not fully understood.
EGFR, an important tyrosine kinase receptor located in cell membranes, plays a vital role in eye development and photoreceptor differentiation[28]–[29]. A series of studies reported that the EGF-induced EGFR signaling pathway is involved in proliferation, migration and survival of human RPE cells[9],[11],[30]. A recent study has indicated that activation of EGFR/AKT signaling is involved in protecting RPE cells against oxidative injury[17]. In the present study, our results showed that EGF stimulated cell viability and proliferation in ARPE-19 cells through inducing phosphorylation of EGFR and degradation of total EGFR. We also found that H2O2-induced oxidative stress inhibited ARPE-19 cell viability and caused a significant reduction of the expression level of total EGFR in a time-dependent manner. These results suggest that the EGF-induced EGFR signaling pathway might play an important role in RPE cell growth, and oxidative stress could affect RPE cell viability through reduction of the EGFR signaling pathway.
A recent study showed that oxidative stress from H2O2 treatment inhibited RPE cells proliferation and induced cellular senescence[31]. Filosto et al[12] have reported that oxidative stress caused abnormal EGFR phosphorylation and aberrant EGFR conformation. In the present study, our data showed that H2O2-induced oxidative stress affected EGF-stimulated RPE cells proliferation. These data hinted that there might be a potential cross-talking between oxidative stress and the EGF-induced EGFR signaling pathway. To confirm the cross-talking between oxidative stress and the EGF-induced EGFR signaling pathway in RPE cells, we further detected the effect of oxidative stress on the EGF-induced EGFR signaling pathway. Interestingly, our results showed that H2O2-induced oxidative stress not only significantly reduced expression levels of total EGFR but also inhibited EGF-induced phosphorylation of EGFR (Figure 5A, 5B). In addition, EGF-induced EGFR endocytosis was also suppressed by pretreatment with H2O2 (Figure 5C). These results supported our initial hypothesis that oxidative stress might suppress RPE cell growth by interfering with the EGF/EGFR signaling pathway.
AKT, also known as protein kinase B (PKB), plays a key role in multiple cellular functions, such as nutrient metabolism, cell survival, proliferation, transcription, and cell migration[32]. On one hand, phosphorylation of AKT was involved in EGF-induced RPE cell survival[9]. On the other hand, phosphorylation and the activation of AKT induced by H2O2 could protect RPE cells from oxidant-induced cell death under normal circumstances and in disease states such as AMD[16]. Our results showed that EGF could induce persistent phosphorylation of AKT (Figure 3A, 3B). In contrast, although treatment with 300 µmol/L of H2O2 within 3h resulted in an increase of phosphorylation of AKT, treatment with H2O2 for more than 3h reduced expression levels of phosphorylated AKT (Figure 4A, 4B). In addition, EGF-induced phosphorylation of AKT was affected by H2O2-caused oxidative stress (Figure 5A, 5B). These results suggest that a durative oxidative stress-induced decline of phosphorylated AKT might contribute to RPE cell dysfunction.
Evidence from a number of studies has shown that antioxidants could protect the RPE against oxidative stress[33]–[35]. NAC, a derivative of cysteine and glutathione precursor, is a thiol-containing compound that could directly scavenge free radicals[36]–[37]. Some studies have shown that NAC may attenuate blue light-induced RPE cell apoptosis[38] and protect RPE against hypoxia-induced apoptosis[39]–[40]. Our results showed that NAC attenuated H2O2-induced inhibition of ARPE-19 cell viability through alleviating reduction of EGFR and phosphorylated and total AKT proteins. These data suggest that the EGFR/AKT signaling pathway might be a target for preventing and treating oxidative stress-induced RPE cell injury.
In conclusion, our data indicate that oxidative stress inhibits ARPE-19 cells survival through affecting EGFR/AKT signaling pathway. The EGFR/AKT signaling pathway might be an important target for prevention and treatment of RPE cell oxidative injury in some diseases such as AMD.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81570875; No.31170685); the China Postdoctoral Science Foundation Funded Project (No.2015M582044); the Health Systems Young Personnel Training Projects Foundation of Fujian Province, China (No.2013-ZQN-JC-37); the Science and Technology Program Foundation of Xiamen City in China (No.3502720144044); the Scientific Research Foundation of the State Human Resource Ministry and the Scientific Research Staring Foundation for the Returned Overseas Chinese Scholars, Ministry of Education of China.
Conflicts of Interest: Chen XD, None; Su MY, None; Chen TT, None; Hong HY, None; Han AD, None; Li WS, None.
REFERENCES
- 1.Congdon N, O'Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P, Eye Diseases Prevalence Research Group Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Prokofyeva E, Zrenner E. Epidemiology of major eye diseases leading to blindness in Europe: a literature review. Ophthalmic Res. 2012;47(4):171–188. doi: 10.1159/000329603. [DOI] [PubMed] [Google Scholar]
- 3.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 4.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85(3):845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 5.Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19(2):205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 6.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45(2):115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 7.Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76(4):397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 8.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Defoe DM, Grindstaff RD. Epidermal growth factor stimulation of RPE cell survival: contribution of phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways. Exp Eye Res. 2004;79(1):51–59. doi: 10.1016/j.exer.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Xu KP, Yu FS. Cross talk between c-Met and epidermal growth factor receptor during retinal pigment epithelial wound healing. Invest Ophthalmol Vis Sci. 2007;48(5):2242–2248. doi: 10.1167/iovs.06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Wang F, Jiang Y, Xu S, Lu F, Wang W, Sun X, Sun X. Migration of retinal pigment epithelial cells is EGFR/PI3K/AKT dependent. Front Biosci (Schol Ed) 2013;5:661–671. doi: 10.2741/s398. [DOI] [PubMed] [Google Scholar]
- 12.Filosto S, Khan EM, Tognon E, Becker C, Ashfaq M, Ravid T, Goldkorn T. EGF receptor exposed to oxidative stress acquires abnormal phosphorylation and aberrant activated conformation that impairs canonical dimerization. PLoS One. 2011;6(8):e23240. doi: 10.1371/journal.pone.0023240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan X, Lambert PF, Rapraeger AC, Anderson RA. Stress-induced EGFR trafficking: mechanisms, functions, and therapeutic implications. Trends Cell Biol. 2016;26(5):352–366. doi: 10.1016/j.tcb.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heppner DE, van der Vliet A. Redox-dependent regulation of epidermal growth factor receptor signaling. Redox Biol. 2016;8:24–27. doi: 10.1016/j.redox.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275(19):14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- 16.Yang P, Peairs JJ, Tano R, Jaffe GJ. Oxidant-mediated Akt activation in human RPE cells. Invest Ophthalmol Vis Sci. 2006;47(10):4598–4606. doi: 10.1167/iovs.06-0140. [DOI] [PubMed] [Google Scholar]
- 17.Cheng LB, Chen CM, Zhong H, Zhu LJ. Squamosamide derivative FLZ protects retinal pigment epithelium cells from oxidative stress through activation of epidermal growth factor receptor (EGFR)-AKT signaling. Int J Mol Sci. 2014;15(10):18762–18775. doi: 10.3390/ijms151018762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zha X, Wu G, Zhao X, Zhou L, Zhang H, Li J, Ma L, Zhang Y. PRDX6 protects ARPE-19 cells from oxidative damage via PI3K/AKT signaling. Cell Physiol Biochem. 2015;36(6):2217–2228. doi: 10.1159/000430186. [DOI] [PubMed] [Google Scholar]
- 19.Wang K, Jiang Y, Wang W, Ma J, Chen M. Escin activates AKT-Nrf2 signaling to protect retinal pigment epithelium cells from oxidative stress. Biochem Biophys Res Commun. 2015;468(4):541–547. doi: 10.1016/j.bbrc.2015.10.117. [DOI] [PubMed] [Google Scholar]
- 20.Baek SM, Yu SY, Son Y, Hong HS. Substance P promotes the recovery of oxidative stress-damaged retinal pigmented epithelial cells by modulating Akt/GSK-3β signaling. Mol Vis. 2016;22:1015–1023. [PMC free article] [PubMed] [Google Scholar]
- 21.Hanus J, Anderson C, Wang S. RPE necroptosis in response to oxidative stress and in AMD. Ageing Res Rev. 2015;24(Pt B):286–298. doi: 10.1016/j.arr.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw PX, Stiles T, Douglas C, Ho D, Fan W, Du H, Xiao X. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol Sci. 2016;3(2):196–221. doi: 10.3934/molsci.2016.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakanishi-Ueda T, Majima HJ, Watanabe K, Ueda T, Indo HP, Suenaga S, Hisamitsu T, Ozawa T, Yasuhara H, Koide R. Blue LED light exposure develops intracellular reactive oxygen species, lipid peroxidation, and subsequent cellular injuries in cultured bovine retinal pigment epithelial cells. Free Radic Res. 2013;47(10):774–780. doi: 10.3109/10715762.2013.829570. [DOI] [PubMed] [Google Scholar]
- 24.Yu AL, Birke K, Burger J, Welge-Lussen U. Biological effects of cigarette smoke in cultured human retinal pigment epithelial cells. PLoS One. 2012;7(11):e48501. doi: 10.1371/journal.pone.0048501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu L, Hackett SF, Mincey A, Lai H, Campochiaro PA. Effects of different types of oxidative stress in RPE cells. J Cell Physiol. 2006;206(1):119–125. doi: 10.1002/jcp.20439. [DOI] [PubMed] [Google Scholar]
- 26.Eichler W, Reiche A, Yafai Y, Lange J, Wiedemann P. Growth-related effects of oxidant-induced stress on cultured RPE and choroidal endothelial cells. Exp Eye Res. 2008;87(4):342–348. doi: 10.1016/j.exer.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Saenz-de-Viteri M, Fernández-Robredo P, Hernández M, Bezunartea J, Reiter N, Recalde S, García-Layana A. Single- and repeated-dose toxicity study of bevacizumab, ranibizumab, and aflibercept in ARPE-19 cells under normal and oxidative stress conditions. Biochem Pharmacol. 2016;103:129–139. doi: 10.1016/j.bcp.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Shilo BZ. Regulating the dynamics of EGF receptor signaling in space and time. Development. 2005;132(18):4017–4027. doi: 10.1242/dev.02006. [DOI] [PubMed] [Google Scholar]
- 29.Zhang T, Du W. Groucho restricts rhomboid expression and couples EGFR activation with R8 selection during Drosophila photoreceptor differentiation. Dev Biol. 2015;407(2):246–255. doi: 10.1016/j.ydbio.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan F, Hui YN, Li YJ, Guo CM, Meng H. Epidermal growth factor receptor in cultured human retinal pigment epithelial cells. Ophthalmologica. 2007;221(4):244–250. doi: 10.1159/000101926. [DOI] [PubMed] [Google Scholar]
- 31.Aryan N, Betts-Obregon BS, Perry G, Tsin AT. Oxidative stress induces senescence in cultured RPE cells. Open Neurol J. 2016;10:83–87. doi: 10.2174/1874205X01610010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9(1):59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kagan DB, Liu H, Hutnik CM. Efficacy of various antioxidants in the protection of the retinal pigment epithelium from oxidative stress. Clin Ophthalmol. 2012;6:1471–1476. doi: 10.2147/OPTH.S35139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanus J, Zhao F, Wang S. Current therapeutic developments in atrophic age-related macular degeneration. Br J Ophthalmol. 2016;100(1):122–127. doi: 10.1136/bjophthalmol-2015-306972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carver KA, Yang D. N-acetylcysteine amide protects against oxidative stress-induced microparticle release from human retinal pigment epithelial Cells. Invest Ophthalmol Vis Sci. 2016;57(2):360–371. doi: 10.1167/iovs.15-17117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 2013;1830(8):4117–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 37.Elbini Dhouib I, Jallouli M, Annabi A, Gharbi N, Elfazaa S, Lasram MM. A mini review on N-acetylcysteine: an old drug with new approaches. Life Sci. 2016;151:359–363. doi: 10.1016/j.lfs.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Seko Y, Pang J, Tokoro T, Ichinose S, Mochizuki M. Blue light-induced apoptosis in cultured retinal pigment epithelium cells of the rat. Graefes Arch Clin Exp Ophthalmol. 2001;239(1):47–52. doi: 10.1007/s004170000220. [DOI] [PubMed] [Google Scholar]
- 39.Castillo M, Bellot JL, García-Cabanes C, Miquel J, Orts A, Palmero M. Effects of hypoxia on retinal pigmented epithelium cells: protection by antioxidants. Ophthalmic Res. 2002;34(6):338–342. doi: 10.1159/000067050. [DOI] [PubMed] [Google Scholar]
- 40.Gerona G, López D, Palmero M, Maneu V. Antioxidant N-acetyl-cysteine protects retinal pigmented epithelial cells from long-term hypoxia changes in gene expression. J Ocul Pharmacol Ther. 2010;26(4):309–314. doi: 10.1089/jop.2009.0101. [DOI] [PubMed] [Google Scholar]