Abstract
AIM
To investigate the altering expression profiles of efflux transporters such as breast cancer-resistance protein (BCRP), lung resistance protein (LRP), and multidrug resistance protein 1 (MDR1) at the inner blood-retinal barrier (BRB) during the development of early diabetic retinopathy (DR) and/or aging in mice.
METHODS
Relative mRNA and protein expression profiles of these three efflux transporters in the retina during the development of early DR and/or aging in mice were examined. The differing expression profiles of Zonula occludens 1 (ZO-1) and vascular endothelial growth factor-A (VEGFA) in the retina as well as the perfusion characterization of fluorescein isothiocyanate (FITC)-dextran and Evans blue were examined to evaluate the integrity of the inner BRB.
RESULTS
There were significant alterations in these three efflux transporters' expression profiles in the mRNA and protein levels of the retina during the development of diabetes mellitus and/or aging. The development of early DR was confirmed by the expression profiles of ZO-1 and VEGFA in the retina as well as the compromised integrity of the inner BRB.
CONCLUSION
The expression profiles of some efflux transporters such as BCRP, LRP, and MDR1 in mice retina during diabetic and/or aging conditions are tested, and the attenuated expression of BCRP, LRP, and MDR1 along with the breakdown of the inner BRB is found, which may be linked to the pathogenesis of early DR.
Keywords: efflux transporters, blood-retinal barrier, multidrug resistance protein 1, lung resistance protein, breast cancer-resistance protein
INTRODUCTION
Diabetic retinopathy (DR) is one of the most common microvascular complications of diabetes and is the leading cause of blindness in young adults[1]. The role of hyperglycemia in the development of DR has now been strongly affirmed[2]–[4]. Due to the widespread clinical use of fluoresce in angiography and vitreous fluorophotometry, the inner blood-retinal barrier (BRB) is now known to be compromised early in the course of DR[5]. The pathogenesis of DR have been focused on the impairment of the retina and the inner BRB by numerous studies. However, the pathogenic mechanism behind the compromising of the inner BRB during DR remains unclear.
In the retina, vascular endothelial cells outline the inner BRB as in the blood-brain barrier (BBB). Barrier function reflects the low paracellular permeability of the endothelium (tight junctions), a low rate of transcytosis, and the high expression of certain multispecific, adenosine triphosphate (ATP)-driven xenobiotic efflux pumps[6]–[7]. Members of the ATP-binding cassette (ABC) transporter superfamily are expressed in the barrier and excretory tissues where they function as ATP-driven xenobiotic efflux pumps[8], and it has already been confirmed that some ABC efflux transporters are expressed in the retina[9]–[10]. However, little is currently known about ABC efflux transporter expression profiles during aging and/or under pathological conditions such as diabetes mellitus. It has been confirmed that the tight junctions of the inner BRB, as one of the reflections of barrier function, are impaired under diabetic conditions[11]–[12]. It is also known that BBB breakdown is an early event in the aging human brain[13]. We thus suspected that the abnormal diabetic conditions present during the development of early DR and/or aging may potentially disturb the barrier function of some ABC efflux transporters at the inner BRB. Indeed, reports have revealed that the alterations in the expression of efflux pumps such as P-glycoprotein (P-gp) could lead to damage to barrier integrity in those with diabetes, although integrity could be restored by insulin therapy[14]. However, direct data concerning the effects of diabetes and/or aging on the transport expression and functions at the inner BRB are not yet available[2].
Expression and location profiles are widely regarded as important pre-factors in functional protein research[15]. In previous research, we characterized the expression profiles of some efflux transporters, including multidrug resistance protein 1 (MDR1/P-gp), breast cancer-resistance protein (BCRP, also known as ABCG2), and lung resistance protein (LRP) in the human retina[16]. In this study, the expression profiles of these major efflux transporters in mice retina was characterized and the effects of diabetes and/or aging on transporter expressions at the inner BRB was revealed.
MATERIALS AND METHODS
Animals and Diabetic Animal Model
Male C57BL/6 mice were purchased from the Beijing Pharmacology Institute (Beijing, China). The animal care and procedures were conducted according to the Principles of Laboratory Animal Care. The use of animals in this study adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and the Shandong Eye Institute Ethics Committees for animal experimentation approved all of the experiments (Approval document No 2012-6, Qingdao, Shandong Province, China). The mice underwent the induction of type 1 diabetes mellitus with an intraperitoneal (i.p.) injection of 50 mg/kg streptozotocin (STZ, Sigma, St. Louis, MO, USA) in an ice-cold citrate-citric acid buffer (pH 4.5) for 5 consecutive days, while the control mice received an equal amount of buffer. Before each day's injection, the mice were fasted for 5h, and after each day's injection, the mice were provided with 10% sucrose water to prevent sudden hypoglycemia. All serum glucose measurements were performed after a fasting time for 5h. Blood glucose levels were monitored from the tail vein using a One Touch Basic Glucometer (LifeScan, Johnson&Johnson, Milpitas, CA, USA). The mice were considered as a diabetic model with a blood glucose level of over 16.7 mmol/L by day 10 following the last STZ injection. All control mice remained normoglycemic. The mice were assessed for body weight and serum glucose level, then further assessed for the further tests after either 4, 12, or 24wk of diabetic conditions, which corresponded to animal ages of 12, 20, or 32wk, respectively. Then, some mice were anesthetized using an i.p. injection of 10% chloral hydrate (2.5 mL/kg). The retinas of mice that had experienced diabetes for 4, 12, and 24wk were collected, immediately snap-frozen in liquid nitrogen, and stored at -80°C until further analysis. The retinas of age-matched control mice (normal) were also collected. Some mice were anesthetized prior to retinal vascular leakage and inner BRB function assessment. Some mice were sacrificed, and the eyeballs of each such mouse were enucleated, immediately embedded in an optimal cutting temperature (OCT) compound and stored at -80°C for further immunofluorescent staining of the frozen section.
RNA Isolation and cDNA Preparation
Total RNA was extracted from retinas using a commercial NucleoSpin RNA Kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's instructions. In brief, tissues were grinded with Trizol in the tube and then mixed with 70% ethanol. The mixture was transferred to a NucleoSpin RNA column and washed with membrane desalting buffer (MDB). Subsequently, added rDNase to the column and incubated for 15min, following washed with RA2 and RA3. RNA was eluted using 40 µL RNase-free water. cDNAs were synthesized using the PrimeScript™ First-Strand cDNA Synthesis Kit (TaKaRa, Dalian, China). The cDNA samples were aliquoted and stored at -80°C.
Reverse Transcription Quantitative-polymerase Chain Reaction
Primers for the efflux transporters, Zonula occludens 1 (ZO-1), and vascular endothelial growth factor-A (VEGFA) (Table 1) were designed using Primer 5.0 commercial software. The detail procedures of real-time polymerase chain reaction (PCR) was performed aspreviously described[16]. The data were analyzed with Sequence Detection System Software (Applied Biosystems) using GAPDH as an internal control. The relative expression of mRNA was calculated using the established 2−ΔΔCT method[17].
Table 1. Sequences of oligonucleotide primers and probes used for RT-PCR.
| Accession # | Gene | Primer Sequences | Amplicon size (bp) | Efficiency (%) |
| NM_011920.3 | BCRP | F: GGCATCTCTGGAGGAGAAAG R: AGAGGATGGAAGGGTCAGTG |
70 | 98 |
| NM_080638.3 | LRP | F: GAGAGCACTGGTAATGCCAA R: CAGAGCCTTCTCCCTCAATC |
73 | 100 |
| NM_011076.2 | MDR1 | F: GAAATGCGATGAAGAAAGCA R: CGGAAACAAGCAGCATAAGA |
88 | 98 |
| NM_009386.2 | ZO-1 | F: CCCTAAACCTGTCCCTCAGA R: ATATCTTCGGGTGGCTTCAC |
99 | 103 |
| NM_001171623.1 | VEGFA | F: GTGCCCACTGAGGAGTCCAA R: ATTTGTTGTGCTGTAGGAAGCTCAT |
100 | 99 |
| NM_001289726.1 | GAPDH | F: ACAACTTTGGCATTGTGGAA R: GATGCAGGGATGATGTTCTG |
133 | 101 |
Immunofluorescence
The expression of these three efflux transporters in the mice retinas was further examined with immunofluorescence. The antibody information is available in Table 2. Briefly, sections (7 µm) of retina from normal and diabetic mice were cut on a cryostat and fixed in 4% paraformaldehyde, then incubated with penetrating solution [0.05% Triton X-100 in phosphate buffered saline (PBS)] and blocked with 5% bovine serum albumin (BSA) in PBS. The sections were then incubated overnight with primary antibodies at 4°C in a humidity chamber and, subsequently, with fluorescein-conjugated secondary antibodies at room temperature for 1h. Finally, all sections were examined under an Eclipse TE2000-U microscope (Nikon, Tokyo, Japan) after being incubated with 4′,6-diamidino-2-phenindole (DAPI, Sigma-Aldrich, USA).
Table 2. Primary antibodies for immunofluorescent staining and Western blot.
| Primary antibody | Supplier | Code | Dilution |
|
| Western blot | Immunofluorescent | |||
| BCRP | Santa cruz | sc-130933 | 1:200 | 1:100 |
| LRP | Abcam | Ab92544 | 1:200 | 1:100 |
| MDR1 | Santa cruz | sc-59593 | 1:1000 | 1:500 |
| Alexa Fluor 488 goat anti-mouse IgG | Beyotime | A0428 | 1:1000 | |
| Alexa Fluor 488 goat anti-rabbit IgG | Beyotime | A0423 | 1:1000 | |
| Goat anti-rabbit IgG H&L (HRP) | Abcam | Ab6721 | 1:10000 | |
| Goat anti-mouse IgG H&L (HRP) | Abcam | Ab6789 | 1:10000 | |
Western Blot Analysis
Western blot was used to assess the expression of efflux transporters in mouse retinas from both the diabetic and normal experimental group. The information of antibody is listed in Table 2. The total protein was prepared from the retina using a radio immunoprecipitation assay (RIPA, Beyotime, China) buffer and then quantified by using bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of Biotechnology, China). The detail procedures of Western blot was performed as previously described[16].
Evaluation of Inner Blood-retinal Barrier Breakdown
Evans blue was used in this experiment to visualize the retinal vascular leakage. Under anesthesia, the mice were administered Evans blue (100 mg/kg) via precava injection. Immediately after injection, the animals visibly turned blue, confirming the dye uptake and distribution. After 5min, the mice were sacrificed, and their eyes were enucleated and fixed with 4% paraformaldehyde in PBS for 25min. The retinas were isolated, flat-mounted, and examined under a fluorescence microscopy (Eclipse Ni, Nikon, Tokyo, Japan) to check for Evans blue extravasation from the retinal vessels[18]–[20].
Morphology changes of retinal vessels was evaluated using fluorescein isothiocyanate (FITC) conjugated dextran (FITC-dextran, Mw=2×106 Da, Sigma-Aldrich). The perfusion of the retinal vessels with FITC-conjugated dextran was performed as described previously. Briefly, under anesthesia, a left ventricle injection of FITC-dextran dissolved in PBS (25 mg/mL) was performed. After 5min, the eyes were enucleated and fixed with 4% paraformaldehyde for 25min. The retinas were carefully isolated, then flat-mounted and examined under fluorescence microscopy (Eclipse Ni, Nikon, Tokyo, Japan)[20].
Statistical Analysis
All data are presented as mean±standard deviation (SD). The difference comparison among the diabetic or normal groups was determined with multiple comparisons in ANOVA. The differences between the diabetic and normal groups at different time points were analyzed using the independent samples t-test. SPSS 11.5 software (SPSS Inc., Chicago, IL, USA) was used for all analyses, and P<0.05 was considered significant.
RESULTS
Measurement of Body Weight and Blood Glucose Concentration
As shown in Table 3, the results demonstrated that changes in body weight and blood glucose levels in the diabetic mice were distinct when compared with the age-matched normal control mice (nondiabetic mice). The body weight of the diabetic mice was noticeably lower than that of the age-matched normal control mice (P<0.001). Further, the blood glucose levels were elevated to the hyperglycemic range by day 10 following the last STZ injection, and this elevation was maintained throughout the whole experimental observation, while the age-matched normal control mice remained normoglycemic (P<0.001 when compared to the age-matched diabetic mice).
Table 3. Analysis of body weight and blood glucose level.
| Parameters | 4wk |
12wk |
24wk |
|||
| Normal | STZ | Normal | STZ | Normal | STZ | |
| Body weight (g) | 27.52±1.40 | 22.44±1.46a | 28.25±1.51 | 23.56±2.06a | 30.51±3.22 | 26.55±3.02a |
| Blood glucose concentration (mmol/L) | 9.97±1.43 | 27.85±4.41a | 10.2±1.54 | 28.71±2.86a | 8.96±2.33 | 25.87±4.52a |
aP<0.001 compared to normal.
n=30
Differential Gene Expression in Retinas at Different Time Points Following the Streptozotocin Induction of Diabetes
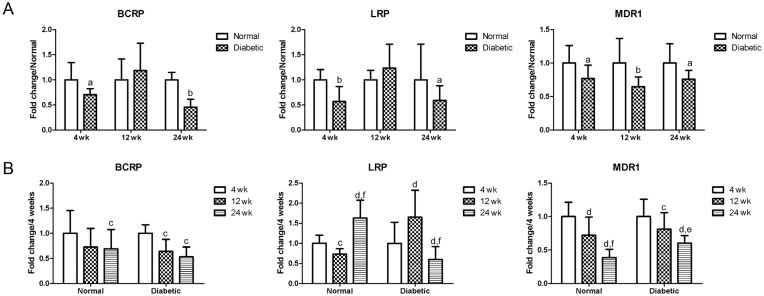
As shown in Figure 1, after 4wk of diabetes, the expression of BCRP, LRP and MDR1 significantly decreased when compared to the age-matched controls (P<0.05 or P<0.01). After 12wk of diabetes, the mRNA expression profiles of MDR1 still showed a significant decrease when compared to the age-matched controls (P<0.01). BCRP and LRP increased, but without a significant difference when compared to the age-matched controls (P>0.05). After 24wk of diabetes, the expression of BCRP, LRP and MDR1 significantly decreased when compared to the age-matched controls (P<0.05 or P<0.01).
Figure 1. Changing mRNA expression profiles of BCRP, LRP and MDR1 in the retina.
A: Comparison of changing mRNA expression profiles of efflux transporters in the retina of normal and STZ-induced diabetic mice; aP<0.05 when compared to the normal age-matched control mice (normal); bP<0.01 when compared to the normal age-matched control mice (normal); n=6. B: Changing mRNA expression profiles of efflux transporters in the retina of normal and STZ-induced diabetic mice during aging; cP<0.05 when compared to their expression at 4wk; dP<0.01 when compared to their expression at 4wk; eP<0.05 when compared to their expression at 12wk; fP<0.01 when compared to their expression at 12wk; n=6.
The effect of aging was also analyzed in both the normal and the diabetic mice (Figure 1). To normal mice, BCRP and MDR1 displayed decrease during aging at 12wk and 24wk, when compared to their expression levels at 4wk; A change of decrease at 12wk and then an increase at 24wk was observed for LRP. While, the changing expression profiles of BCRP and MDR1 in the diabetic mice were similar to those of the normal mice. However, LRP in the diabetic mice showed different change trends to the normal mice, which showed an increase at 12wk and then a decrease at 24wk.
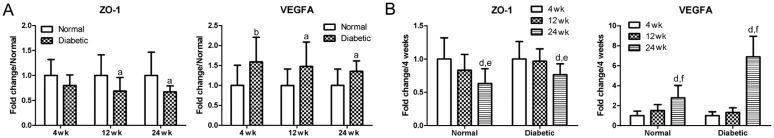
ZO-1 and VEGFA were tested in this experiment as an indication of the tight junction integrity of the inner BRB (Figure 2). The expression of ZO-1 exhibited a significant decrease when compared to the age-matched controls, while the expression of VEGFA displayed a significant increase (P<0.05 or P<0.01). As to the effect of aging, the ZO-1 continued to decrease, while the VEGFA continued to increase in both normal and diabetic mice during aging at 12wk and/or 24wk when compared to their expression at 4wk.
Figure 2. Changing mRNA expression profiles of ZO-1 and VEGFA in the retina.
A: Changing profiles of ZO-1 and VEGFA between the normal and diabetic mice; aP<0.05 when compared to the normal age-matched control mice (normal); bP<0.01 when compared to the normal age-matched control mice (normal); n=6. B: Changing profiles of ZO-1 and VEGFA between the normal and STZ-induced diabetic mice during aging; dP<0.01 when compared to their expression at 4wk; eP<0.05 when compared to their expression at 12wk; fP<0.01 when compared to their expression at 12wk; n=6.
Immunofluorescence Characterization of Efflux Transporters in Retinas
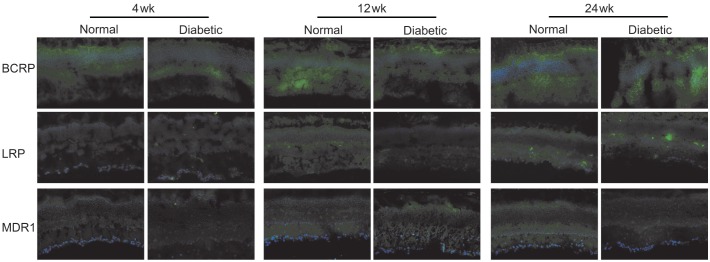
To validate the changes at the protein level, we performed an immunofluorescence analysis using antibodies against BCRP, LRP and MDR1. The results of the immunofluorescence microscopy are summarized in Figure 3. There were also some differences in the expression fluorescence intensity of the efflux transporters between the normal and diabetic mice. High BCRP expression, as well as some faint LRP and MDR1 expression, was detected throughout the whole retina. The fluorescence intensities of all three efflux transporters were somewhat lower in the diabetic retina when they were compared to the normal mice at 4, 12 and 24wk. However, no significant change was found during aging in either the normal or the diabetic mice.
Figure 3. Representative figures of the immunofluorescence characterization of efflux transporters in the retina of normal and diabetic mice.
A green result was seen in positive tissues. The nuclei were counterstained in blue with DAPI (bar=25 µm).
Western Blot Characterization of Protein Expression in Retina Tissues
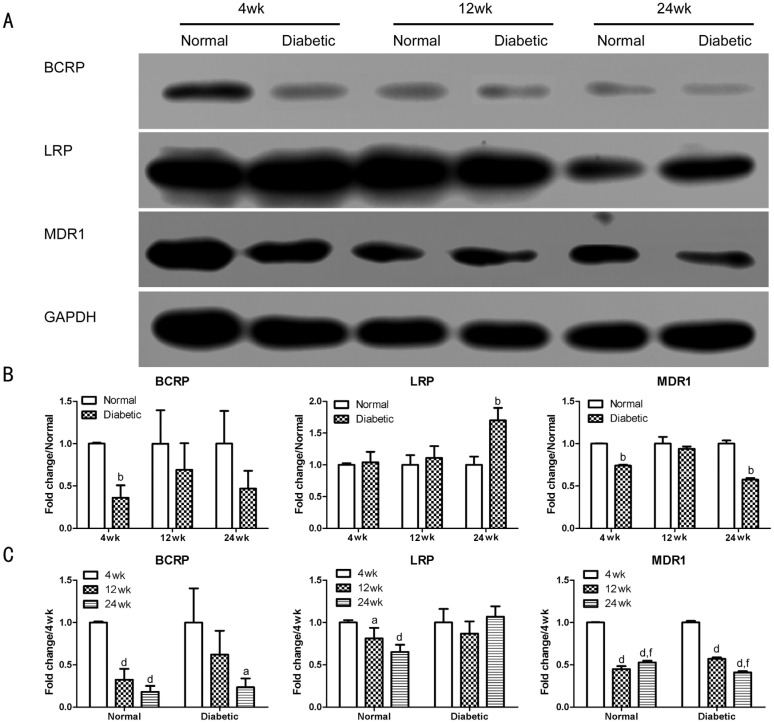
To validate the changes in the protein level, we further performed Western blot analysis using antibodies against BCRP, LRP and MDR1. Densitometric analysis revealed that all three molecules displayed different expression profiles (Figure 4). After 4wk of diabetes, the expression of BCRP and MDR1 was significantly decreased (P<0.01) when compared to the age-matched controls. LRP showed no change when compared to the age-matched controls. After 12wk of diabetes, the expression profiles of MDR1 and BCRP still showed a decrease, but without a significant difference when compared to the age-matched controls (P>0.05). LRP still showed no change when compared to the age-matched controls. After 24wk of diabetes, the expression of MDR1 was significantly decreased (P<0.01) when compared to the age-matched controls. BCRP maintained a decrease, but without a significant difference when compared to the age-matched controls (P>0.05). However, the expression of LRP displayed a significant increase (P<0.01) when compared to the age-matched controls.
Figure 4. Efflux transporter expression profiles in the retina of mice by Western blot.
A: Band images. B: Changing profiles of BCRP, LRP, and MDR1 between normal and diabetic mice; bP<0.01 when compared to the normal age-matched control mice (normal); n=6. C: Changing profiles of BCRP, LRP, and MDR1 between normal and STZ-induced diabetic mice during aging. aP<0.05 when compared to their expression at 4wk; dP<0.01 when compared to their expression at 4wk; fP<0.01 when compared to their expression at 12wk; n=6.
The effect of aging was also analyzed in the normal and diabetic mice. BCRP and MDR1 continued to decrease during aging at 12wk and/or 24wk when compared to their expression at 4wk. In the normal mice, LRP continued to decrease during aging at 12wk and 24wk, while in the diabetic mice, LRP maintained a similar level when compared to the expression at 4wk.
Evaluation of Inner Blood-retinal Barrier Breakdown
The blood vessel leakage was visualized with Evans blue in retina flat-mounts. Representative images showing Evans blue fluorescence allowed the detection of leakage sites in the retinal vessels (Figure 5). In the control retinas, Evans blue fluorescence was limited to the blood vessels at all three observation time points. In the diabetic mice, no significant changes were observed after 4 and 12wk diabetes, although some focal leakage of the dye from the capillaries and larger vessels could be detected at 24wk.
Figure 5. Fluorescence angiography using Evans blue infusion.
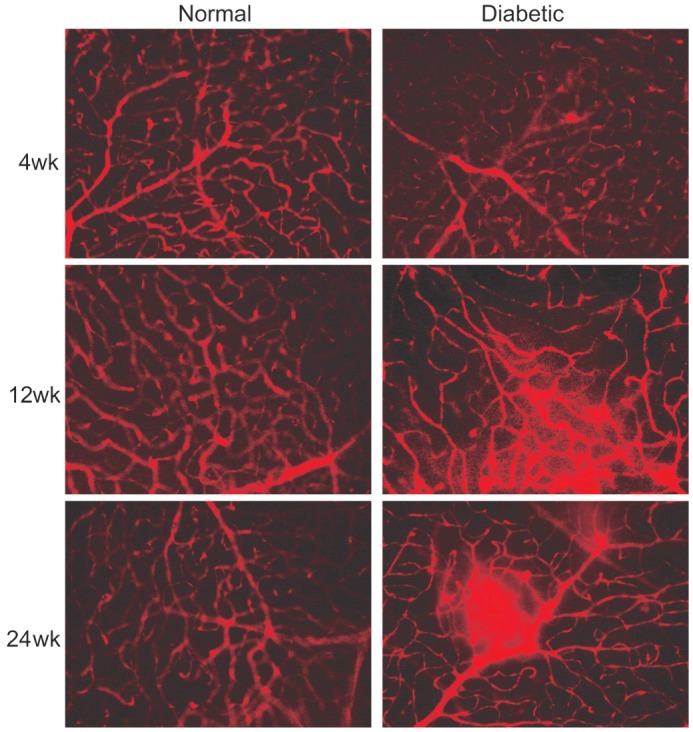
The flat-mounted retinas of mice with 4, 12 and 24wk after the induction of diabetes as compared with controls (400×).
The morphology changes of retinal vessels were evaluated using an FITC-dextran infusion (Figure 6). The retinas of normal mice had both superficial and deep vascular layers (joined by connecting vessels). Diabetes did not significantly alter the distribution volume of the dextrans in the retinas. Non-perfusion areas and marked fluorescein diffusion were not observed in either the normal or the STZ-induced diabetic mice retinas. No marked histopathological changes or vascular components were observed during aging in either the normal or the STZ-induced diabetic mice retinas. Only certain vessels showed an irregular distribution, a dilated and tortuous appearance, and minor fluorescein leakage in the retinas following 24wk of diabetes. Thus, even 24wk of uncontrolled diabetes was not a sufficient period in which to detect significant changes in retinal permeability to dextrans, which corroborates the results obtained for the retinal vessel leakage using an Evans blue assay.
Figure 6. Fluorescence angiography using FITC-dextran infusion.
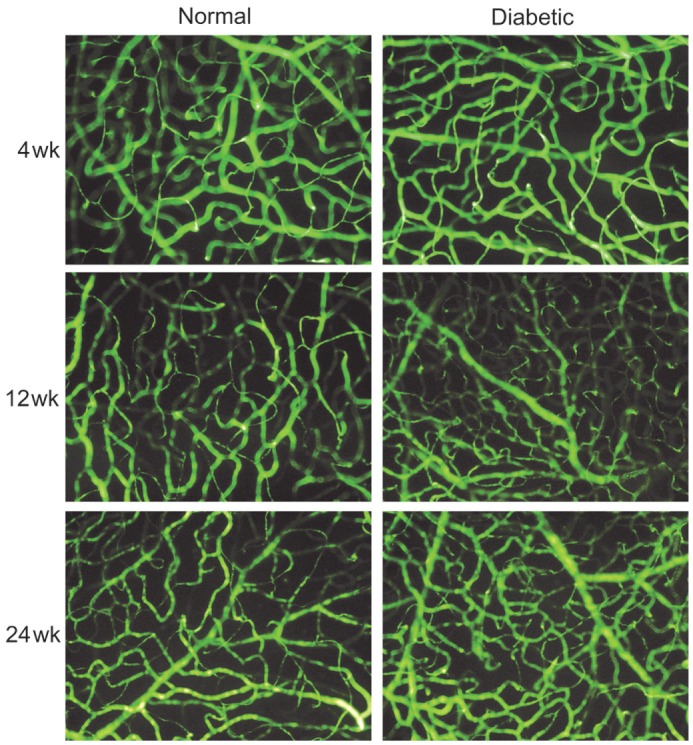
The flat-mounted retinas of mice with 4, 12 and 24wk after the induction of diabetes as compared with controls (400×).
DISCUSSION
Efflux transporters contribute to the barrier integrity of the inner BRB. Attenuated efflux transporter expression and function due to diabetes mellitus have been reported in various tissues, such as the BBB, and are linked to significant clinic complications[21]–[22]. However, whether this is also true for the inner BRB and whether it plays a role in the pathogenesis of early DR is still unclear. The effect of aging on BBB P-gp expression has been studied, and decreased expression was found with aging[23]. Despite this, relatively few papers have so far been published on the impact of advancing age on efflux transporter expression in the inner BRB. In the current study, we successfully formulated a clear description of the expression profiles of some efflux transporters in retinas during the development of diabetes and/or aging retinas. To the best of our knowledge, this is the first report concerning this issue.
In this study, STZ-induced diabetic mice were included as models of early DR since such an approach is among the best DR models for studying molecular mechanisms and drug screening[24]. As the life span of mice is relatively short, only the early stages of DR can be observed, which accorded with our research purposes. Vascular changes in C57BL/6 mice such as acellular capillaries, pericyte ghosts and vascular cell apoptosis are detectable 6mo after the induction of diabetes mellitus[24]. Furthermore, retinal neovascularization has not been observed in this model. In this experiment, we observed similar results in the visualization of the inner BRB breakdown using FITC-dextran and Evans blue (Figures 5, 6). Diabetic mice only showed some minor lesions in the retinal capillary network as well as large vessels at 24wk after the induction of diabetes mellitus when compared with the age-matched normal controls, and these observed pathological changes were in accordance with previously published reports in rats[25]–[26].
VEGFA has been identified as an important mediator of the BRB alteration in DR[27]–[31]. An increased VEGFA mRNA expression has already been observed in both STZ-induced diabetic rats and mice[32]–[33]. As the VEGFA level in a single mouse retina is too low to be detected by Western blot or ELISA, real-time PCR is usually used to detect changes in the VEGFA mRNA level[34]. Indeed, our results confirm that diabetic conditions promote an increase in the transcription of VEGFA mRNA (Figure 2). Our results further demonstrate that physiological aging also promotes an increase in the transcription of VEGFA mRNA in both normal and diabetic mice.
An important component of BRB is the endothelial tight junction complex. The transmembrane proteins occludin, tricellulin, the claudin family, and the junction adhesion molecules, along with the scaffolding ZO-1, -2, -3, play major roles in the formation and regulation of the tight junction barrier[30],[35]–[36]. Indeed, our results confirm that hyperglycemic conditions promote a decrease in the transcription of ZO-1 mRNA (Figure 2). Physiological aging also promotes a decrease in the transcription of ZO-1 mRNA in both normal and diabetic mice. The altered trends of VEGFA and ZO-1 obtained in this study agree with those reported previously[35],[37]. Combined with the results of the inner BRB breakdown evaluation, we could conclude that early DR was successfully established in this experiment.
Human ABC transporters are membrane transporters that use energy from ATP hydrolysis to transport a wide variety of substrates across the cellular membrane. Prominent efflux transporters previously identified in tissues belong to the ABC superfamily[16]. Of these, MDR1 is the most extensively researched transporter[38]–[39], while BCRP is a well-recognized ABC half-transporter[40]. LRP is not an ABC transporter, although it is a major vault protein (MVP)[41]. As it is thought to drive drugs away from the nucleus, LRP has also been reported as an efflux transporter[16],[41]. So, we chose MDR1, LRP, and BCRP as representatives of efflux transporters in this present study. Efflux transporter expression profiles in the mRNA (Figure 1) and protein levels (Figures 3, 4) were elucidated. The general trend of these efflux transporters with regard to mRNA expression in diabetic mice retinas was decline when compared to age-matched normal mice, although different efflux transporters had different expression profiles.
From the western blot analysis (Figure 4), we could see that the expressions of MDR1 and BCRP were in decline in the diabetic mice retinas when compared to the normal mice retinas. A declining trend was also observed in both the normal and diabetic mice retina during aging, and these expression profiles were similar to those of the mRNA level. However, the expression of LRP was somewhat different between MDR1 and BCRP. The expression level showed no change at the 4 wk and 12wk time points between the normal and diabetic mice retina, although a significant increase in expression was observed in the diabetic mice retina when compared to the normal mice retinas at the 24wk time point. The expression of LRP was in decline in the normal mice, but maintained the same level in the diabetic mice during aging, which could also be explained that up-regulation might act as a compensatory mechanism, and a longer observation period should be needed to fully elucidate its change profile in the diabetic mice during aging. The immunofluorescence test results confirmed these three efflux transporters' expression levels in both normal and diabetic mice retinas. Yet, the changing expression profiles were difficult to read.
Extensive research has determined that DR is not only a vascular disease but also a neurodegenerative one[42]–[43]. Several reports have revealed that efflux transporters such as P-gp the BBB display a significant decline in expression rates and function with aging, and so strongly support the notion that age-dependent changes might predispose sufferers to neurodegenerative diseases[23],[44]–[45]. In this study, we also obtained similar results concerning the changing expression profiles of the efflux transporters in the retina during aging in both normal and diabetic mice (Figure 1), and these declines in expression might also be attributed to the development of early DR.
From the analysis of the altered expression profiles of efflux transporters at the gene and protein levels, we could conclude that a significant alteration of efflux transporters in the retina was coupled with the development of early DR and/or aging in mice, although we could not confirm the causal relationship between the altered expression profiles of the efflux transporters and the development of DR in the present study. Some similar reports about the efflux transporters in posterior segment diseases were also consistent with our findings. For example, retinal expression of efflux transporters ABC transporter A1 (ABCA1) and ABC transporter G1 (ABCG1) was found to be down-regulated and linked to age-related macular degeneration (AMD) in mice model[46]. Sene A and colleagues revealed that age-associated reduction in expression of ABC transporters impaired the efflux capacity of macrophages and resulted in increased cholesterol accumulation, which promoted AMD[47].
Since the expression and regulation of efflux transporters are somewhat similar in mice and humans, data from this study suggests that humans who have early DR may also have altered expression of certain transporters[21]. We could thus speculate that these efflux transporters in the inner BRB might be new targets for retarding or treating the development of early DR. However, further studies are necessary to evaluate the functional regulation of these efflux transporters during the development of early DR and/or aging.
This article detailed the expression profiles of some efflux transporters in mice retina during diabetic and/or aging conditions, and the attenuated expression of some efflux transporters along with the breakdown of the inner BRB was found, which might be linked to the pathogenesis of early DR.
Acknowledgments
We are grateful for the persons who participated in this research.
Foundations: Supported by the National Natural Science Foundation of China (No.81271036; No.81500751); the Key Research Project of Shandong Province, China (No.2015GSF118121); the Natural Science Foundation of Shandong Province, China (No.ZR2015PH062).
Conflicts of Interest: Li MS, None; Xin M, None; Guo CL, None; Lin GM, None; Li J, None; Wu XG, None.
REFERENCES
- 1.Ramin S, Gharebaghi R, Heidary F. Scientometric Analysis and Mapping of Scientific Articles on Diabetic Retinopathy. Med Hypothesis Discov Innov Ophthalmol. 2015;4(3):81–100. [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Li C, Sun X, Kuang X, Ruan X. High glucose decreases expression and activity of p-glycoprotein in cultured human retinal pigment epithelium possibly through iNOS induction. PLoS One. 2012;7(2):e31631. doi: 10.1371/journal.pone.0031631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang C, Wang H, Nie J, Wang F. Protective factors in diabetic retinopathy: focus on blood-retinal barrier. Discov Med. 2014;18(98):105–112. [PubMed] [Google Scholar]
- 4.Wu Y, Chakrabarti S. ERK5 mediated signalling in diabetic retinopathy. Med Hypothesis Discov Innov Ophthalmol. 2015;4(1):17–26. [PMC free article] [PubMed] [Google Scholar]
- 5.Cunha-Vaz J, Lobo C, Sousa JC, Oliveiros B, Leite E, de Abreu JR. Progression of retinopathy and alteration of the blood-retinal barrier in patients with type 2 diabetes: a 7-year prospective follow-up study. Graefes Arch Clin Exp Ophthalmol. 1998;236(4):264–268. doi: 10.1007/s004170050075. [DOI] [PubMed] [Google Scholar]
- 6.Bauer B, Hartz AM, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol. 2007;71(3):667–675. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- 7.Chan GN, Saldivia V, Yang Y, Pang H, de Lannoy I, Bendayan R. In vivo induction of P-glycoprotein expression at the mouse blood-brain barrier: an intracerebral microdialysis study. J Neurochem. 2013;127(3):342–352. doi: 10.1111/jnc.12344. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Hawkins BT, Miller DS. Aryl hydrocarbon receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. Faseb J. 2011;25(2):644–652. doi: 10.1096/fj.10-169227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan J, Ruiz-Moreno JM. Advances in the understanding of retinal drug disposition and the role of blood-ocular barrier transporters. Expert Opin Drug Metab Toxicol. 2013;9(9):1181–1192. doi: 10.1517/17425255.2013.796928. [DOI] [PubMed] [Google Scholar]
- 10.Tagami M, Kusuhara S, Honda S, Tsukahara Y, Negi A. Expression of ATP-binding cassette transporters at the inner blood-retinal barrier in a neonatal mouse model of oxygen-induced retinopathy. Brain Res. 2009;1283:186–193. doi: 10.1016/j.brainres.2009.05.095. [DOI] [PubMed] [Google Scholar]
- 11.Saker S, Stewart EA, Browning AC, Allen CL, Amoaku WM. The effect of hyperglycaemia on permeability and the expression of junctional complex molecules in human retinal and choroidal endothelial cells. Exp Eye Res. 2014;121:161–167. doi: 10.1016/j.exer.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Song HB, Jun HO, Kim JH, Yu YS, Kim KW, Kim JH. Suppression of protein kinase C-zeta attenuates vascular leakage via prevention of tight junction protein decrease in diabetic retinopathy. Biochem Biophys Res Commun. 2014;444(1):63–68. doi: 10.1016/j.bbrc.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Liu H, Yang J, Liu X, Lu S, Wen T, Xie L, Wang G. Increased amyloid beta-peptide (1-40) level in brain of streptozotocin-induced diabetic rats. Neuroscience. 2008;153(3):796–802. doi: 10.1016/j.neuroscience.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Dallas S, Miller DS, Bendayan R. Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev. 2006;58(2):140–161. doi: 10.1124/pr.58.2.3. [DOI] [PubMed] [Google Scholar]
- 16.Chen P, Chen H, Zang X, Chen M, Jiang H, Han S, Wu X. Expression of efflux transporters in human ocular tissues. Drug Metab Dispos. 2013;41(11):1934–1948. doi: 10.1124/dmd.113.052704. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C(T)] Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Leal EC, Martins J, Voabil P, Liberal J, Chiavaroli C, Bauer J, Cunha-Vaz J, Ambrosio AF. Calcium dobesilate inhibits the alterations in tight junction proteins and leukocyte adhesion to retinal endothelial cells induced by diabetes. Diabetes. 2010;59(10):2637–2645. doi: 10.2337/db09-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez DC, Sande PH, Chianelli MS, Aldana Marcos HJ, Rosenstein RE. Induction of ischemic tolerance protects the retina from diabetic retinopathy. Am J Pathol. 2011;178(5):2264–2274. doi: 10.1016/j.ajpath.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin JY, Sohn J, Park KH. Chlorogenic acid decreases retinal vascular hyperpermeability in diabetic rat model. J Korean Med Sci. 2013;28(4):608–613. doi: 10.3346/jkms.2013.28.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.More VR, Slitt AL. Alteration of hepatic but not renal transporter expression in diet-induced obese mice. Drug Metab Dispos. 2011;39(6):992–999. doi: 10.1124/dmd.110.037507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nawa A, Fujita-Hamabe W, Tokuyama S. Altered intestinal P-glycoprotein expression levels in a monosodium glutamate-induced obese mouse model. Life Sci. 2011;89(23-24):834–838. doi: 10.1016/j.lfs.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Bartels AL, Kortekaas R, Bart J, Willemsen AT, de Klerk OL, de Vries JJ, van Oostrom JC, Leenders KL. Blood-brain barrier P-glycoprotein function decreases in specific brain regions with aging: a possible role in progressive neurodegeneration. Neurobiol Aging. 2009;30(11):1818–1824. doi: 10.1016/j.neurobiolaging.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Jiang X, Yang L, Luo Y. Animal models of diabetic retinopathy. Curr Eye Res. 2015;40(8):761–771. doi: 10.3109/02713683.2014.964415. [DOI] [PubMed] [Google Scholar]
- 25.Mooradian AD, Haas MJ, Batejko O, Hovsepyan M, Feman SS. Statins ameliorate endothelial barrier permeability changes in the cerebral tissue of streptozotocin-induced diabetic rats. Diabetes. 2005;54(10):2977–2982. doi: 10.2337/diabetes.54.10.2977. [DOI] [PubMed] [Google Scholar]
- 26.Sasase T, Ohta T, Ogawa N, Miyajima K, Ito M, Yamamoto H, Morinaga H, Matsushita M. Preventive effects of glycaemic control on ocular complications of Spontaneously Diabetic Torii rat. Diabetes Obes Metab. 2006;8(5):501–507. doi: 10.1111/j.1463-1326.2005.00535.x. [DOI] [PubMed] [Google Scholar]
- 27.Stefanini FR, Badaro E, Falabella P, Koss M, Farah ME, Maia M. Anti-VEGF for the management of diabetic macular edema. J Immunol Res. 2014;2014:632307. doi: 10.1155/2014/632307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Wang JJ, Yu Q, Chen K, Mahadev K, Zhang SX. Inhibition of reactive oxygen species by Lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: role of NADPH oxidase 4. Diabetes. 2010;59(6):1528–1538. doi: 10.2337/db09-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rangasamy S, Srinivasan R, Maestas J, McGuire PG, Das A. A potential role for angiopoietin 2 in the regulation of the blood-retinal barrier in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52(6):3784–3791. doi: 10.1167/iovs.10-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murakami T, Frey T, Lin C, Antonetti DA. Protein kinase cbeta phosphorylates occludin regulating tight junction trafficking in vascular endothelial growth factor-induced permeability in vivo. Diabetes. 2012;61(6):1573–1583. doi: 10.2337/db11-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dennis MD, Kimball SR, Fort PE, Jefferson LS. Regulated in development and DNA damage 1 is necessary for hyperglycemia-induced vascular endothelial growth factor expression in the retina of diabetic rodents. J Biol Chem. 2015;290(6):3865–3874. doi: 10.1074/jbc.M114.623058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, Bozack SN, Yan Y, Boulton ME, Grant MB, Busik JV. Regulation of retinal inflammation by rhythmic expression of MiR-146a in diabetic retina. Invest Ophthalmol Vis Sci. 2014;55(6):3986–3994. doi: 10.1167/iovs.13-13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan HT, Su GF. Expression and significance of HIF-1 alpha and VEGF in rats with diabetic retinopathy. Asian Pac J Trop Med. 2014;7(3):237–240. doi: 10.1016/S1995-7645(14)60028-6. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Shi H, Zhang J, Wang G, Zhang J, Jiang F, Xiao Q. Toll-like receptor 4 in bone marrow-derived cells contributes to the progression of diabetic retinopathy. Mediators Inflamm. 2014;2014:858763. doi: 10.1155/2014/858763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He J, Wang H, Liu Y, Li W, Kim D, Huang H. Blockade of vascular endothelial growth factor receptor 1 prevents inflammation and vascular leakage in diabetic retinopathy. J Ophthalmol. 2015;2015:605946. doi: 10.1155/2015/605946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardner TW. Histamine, ZO-1 and increased blood-retinal barrier permeability in diabetic retinopathy. Trans Am Ophthalmol Soc. 1995;93:583–621. [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H, He J, Johnson D, Wei Y, Liu Y, Wang S, Lutty GA, Duh EJ, Semba RD. Deletion of placental growth factor prevents diabetic retinopathy and is associated with Akt activation and HIF1alpha-VEGF pathway inhibition. Diabetes. 2015;64(1):200–212. doi: 10.2337/db14-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwan WS, Janneh O, Hartkoorn R, Chandler B, Khoo S, Back D, Owen A. Intracellular ‘boosting’ of darunavir using known transport inhibitors in primary PBMC. Br J Clin Pharmacol. 2009;68(3):375–380. doi: 10.1111/j.1365-2125.2009.03462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ford J, Khoo SH, Back DJ. The intracellular pharmacology of antiretroviral protease inhibitors. J Antimicrob Chemother. 2004;54(6):982–990. doi: 10.1093/jac/dkh487. [DOI] [PubMed] [Google Scholar]
- 40.Sugiyama T, Shuto T, Suzuki S, Sato T, Koga T, Suico MA, Kusuhara H, Sugiyama Y, Cyr DM, Kai H. Posttranslational negative regulation of glycosylated and non-glycosylated BCRP expression by Derlin-1. Biochem Biophys Res Commun. 2011;404(3):853–858. doi: 10.1016/j.bbrc.2010.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouhamyia L, Chantot-Bastaraud S, Zaidi S, Roynard P, Prengel C, Bernaudin JF, Fleury-Feith J. Immunolocalization and cell expression of lung resistance-related protein (LRP) in normal and tumoral human respiratory cells. J Histochem Cytochem. 2007;55(8):773–782. doi: 10.1369/jhc.7A7176.2007. [DOI] [PubMed] [Google Scholar]
- 42.Barber AJ. Diabetic retinopathy: recent advances towards understanding neurodegeneration and vision loss. Sci China Life Sci. 2015;58(6):541–549. doi: 10.1007/s11427-015-4856-x. [DOI] [PubMed] [Google Scholar]
- 43.Ola MS, Alhomida AS. Neurodegeneration in diabetic retina and its potential drug targets. Curr Neuropharmacol. 2014;12(4):380–386. doi: 10.2174/1570159X12666140619205024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pekcec A, Schneider EL, Baumgartner W, Stein VM, Tipold A, Potschka H. Age-dependent decline of blood-brain barrier P-glycoprotein expression in the canine brain. Neurobiol Aging. 2011;32(8):1477–1485. doi: 10.1016/j.neurobiolaging.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Silverberg GD, Messier AA, Miller MC, Machan JT, Majmudar SS, Stopa EG, Donahue JE, Johanson CE. Amyloid efflux transporter expression at the blood-brain barrier declines in normal aging. J Neuropathol Exp Neurol. 2010;69(10):1034–1043. doi: 10.1097/NEN.0b013e3181f46e25. [DOI] [PubMed] [Google Scholar]
- 46.Ananth S, Gnana-Prakasam JP, Bhutia YD, Veeranan-Karmegam R, Martin PM, Smith SB, Ganapathy V. Regulation of the cholesterol efflux transporters ABCA1 and ABCG1 in retina in hemochromatosis and by the endogenous siderophore 2,5-dihydroxybenzoic acid. Biochim Biophys Acta. 2014;1842(4):603–612. doi: 10.1016/j.bbadis.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sene A, Khan AA, Cox D, Nakamura RE, Santeford A, Kim BM, Sidhu R, Onken MD, Harbour JW, Hagbi-Levi S, Chowers I, Edwards PA, Baldan A, Parks JS, Ory DS, Apte RS. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab. 2013;17(4):549–561. doi: 10.1016/j.cmet.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]