Abstract
AIM
o identify the effects of interleukin (IL)-13 on retinal pigment epithelial (RPE) cells and the IL-13 level in aqueous humor of age-related macular degeneration (AMD) patients.
METHODS
IL-13 levels in aqueous humor specimens from AMD patients were detected with enzyme-linked immunosorbent assay (ELISA). ARPE-19 cells were treated with 10 ng/mL IL-13 for 12, 24, and 48h. The cell proliferaton was evaluated by the MTS method. The mRNA and protein levels of α-SMA and ZO-1 were evaluated with quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot respectively. The expression of tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β) and vascular endothelial growth factor (VEGF) were assessed by ELISA.
RESULTS
IL-13 levels in the aqueous humor of patients with AMD were significantly higher than those in the control (167.33±17.64 vs 27.12±5.65 pg/mL; P<0.01). In vitro, IL-13 of high concentrations (10, 15, and 20 ng/mL) inhibited ARPE-19 cell proliferation. α-SMA mRNA in ARPE-19 cell were increased (1.017±0.112 vs 1.476±0.168; P<0.001) and ZO-1 decreased (1.051±0.136 vs 0.702±0.069; P<0.001) after treated with 10 ng/mL IL-13 for 48h. The protein expression of α-SMA and ZO-1 also showed the same tendency (α-SMA: P=0.038; ZO-1: P=0.008). IL-13 significantly reduced the level of TNF-α (44.70±1.67 vs 31.79±3.53 pg/mL; P=0.005) at 48h, but the level of TGF-β2 was significantly increased from 34.44±2.92 to 57.61±6.31 pg/mL at 24h (P=0.004) and from 61.26±1.11 to 86.91±3.59 pg/mL at 48h (P<0.001). While expressions of VEGF didn't change after IL-13 treatment.
CONCLUSION
IL-13 in vitro inhibit ARPE-19 cell proliferation and expression in the aqueous may be associated with AMD.
Keywords: interleukin-13, age-related macular degeneration, retinal pigment epithelial cell
INTRODUCTION
Age-related macular degeneration (AMD) is one of the most common causes of blindness in elderly individuals in the developed world[1]. AMD is typically categorized into two forms: wet form and dry form. The pathology of neovascular (wet) AMD is related to the process of choroidal neovascularization (CNV), which leads to leak exudates and hemorrhage. The neovascular membrane in the macular region becomes fibrotic and forms a disciform scar in the involutional stage, which causes irreversible damage and explains the severe central visual loss experienced by these patients[2]. While molecular and cellular mechanisms underlying CNV are complicated and not fully elucidated, local inflammation and angiogenesis, which influence disease development and prognosis, are recognized as key factors in the progression of wet AMD.
Current clinical strategies for treating progressive AMD are primarily aimed at inhibiting vascular endothelial growth factor (VEGF), an endothelial cell mitogen shown to be a key mediator of angiogenesis and a vascular permeability-enhancing factor. Therefore, only 30% to 40% treated patients gain three lines in visual acuity, some patients continues losing visual acuity, even progressing to blindness[3]. The greater fibrotic response after ranibizumab injection during wound healing process may contribute to poor prognosis[4]–[5]. Thus, to explore cytokines related to fibrosis may provide potential novel treatments of AMD.
Interleukin (IL)-13 plays pathophysiological roles in allergic inflammation and fibrosis formation. In this study, we quantified IL-13 levels in the aqueous humor of patients with AMD to identify its roles in the pathological process of this condition and investigated its relationship with VEGF, tumor necrosis factor-α (TNF-α) and transforming growth factor-β (TGF-β)[6]. Identification of these cytokines has enabled the exploration of novel therapeutic approaches.
SUBJECTS AND METHODS
The study protocol was approved by the Ethics Committee of the institute and adhered to the guidelines of the Declaration of Helsinki. Informed consent was obtained from all patients and their families before enrollment.
Human Aqueous Humor Specimens
Aqueous humor specimens from 52 patients with a confirmed diagnosis of AMD treated with intravitreal ranibizumab injections from October 2013 to July 2014 were analyzed. Twelve eyes with clinically apparent fibrotic disciform lesion on examination were included. The study's inclusion criteria were as follows: 1) subretinal or vitreous hemorrhage; 2) absence of other evident causes of vasculopathy and exudative retinopathy, such as serious heart, lung, liver, or kidney dysfunction; 3) absence of other ocular diseases such as autoimmune uveitis, proliferative diabetic retinopathy, retinal vein branch obstruction, and pathological myopia. As controls, aqueous humor specimens, taken from the eyes of 20 patients undergoing cataract surgery, were also analyzed. There was no history of ocular operation or intravitreal ranibizumab treatment in control cases.
Sample Collection
Aqueous humor specimens (0.05-0.1 mL) were collected before each intravitreal ranibizumab injection of patients with AMD. Similarly, aqueous samples (0.05-0.15 mL) were taken in the beginning of routine cataract surgery as a control group. These aqueous samples were transferred directly into Eppendorf tubes on ice, centrifuged at a speed of 2000 rpm for 10min, aliquoted, and stored at -80°C until analyzed.
Cell Culture
Human retinal pigment epithelial (RPE)cell line ARPE-19 (ATCC, USA) was cultured using 1640 medium (Hyclone, USA) supplemented with 10% (v/v) fetal bovine serum (Hyclone, USA) and 1× solution of penicillin/streptomycin (Genview, USA). Cells were grown at 37°C, in a humidified atmosphere of 5% CO2 and 90% relative humidity.
MTS Proliferation Assay
The number of viable cells was measured using the MTS assay, which relies on the formation of a colored substrate by mitochondrial enzyme activity in viable cells. ARPE-19 cells were seeded at a density of 2×103 cells per well on a 96-well plate and incubated at 37°C overnight and another overnight in serum-free media at 37°C. The cells were incubated in 2% FBS medium with or without IL-13 (1, 5, 10, 15, 20 ng/mL; Pepro Tech), at 37°C for 12, 24 or 48h. MTS (20 µL per well) was then added for 3h. The absorbance at 490 nm was determined using a plate reader (iMark, Bio-rad, USA). Each experiment was conducted at least four replicates.
Quantitative Real-time Polymerase Chain Reaction
ARPE-19 cells were seeded in 6-well tissue culture plates in amounts of 1×105 cells for incubation of 24h and serum-starved overnight and then cultured in 2% FBS with 10 ng/mL IL-13 treatment for 12, 24, and 48h. The total RNA was extracted using RNA extraction kit (Takara, Japan) according to the manufacturer's instructions and was reverse-transcribed using PrimeScript RT reagent Kit (Takara, Japan). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed with SYBR Green RealMasterMix and specific primers for α-SMA (forward, 5′-GGGACATCAAGGAGAAACTGTGT-3′; reverse, 5′-TCTCTGGGCAGCGGAAAC-3′) and ZO-1 (forward, 5′-TTCCAGATAAAGCCCCAGTTAATG-3′; reverse, 5′-GCAGAAGATTGTGATTGAATTTAGGA-3′). GAPDH was used as an endogenous standard to estimate the mRNA levels. The PCR programme was designed as follows: the cDNA was denatured at 95°C for 30s followed by 40 cycles of 95°C for 15s, 60°C for 30s and 72°C for 30s.
Western Blot
ARPE-19 cells were seeded in 25-cm2 tissue culture flask in amounts of 4×105 cells for incubation of 24h and serum-starved overnight and then cultured in 2% FBS with 10 ng/mL IL-13 treatment for 48h. Cells were harvested in 120 µL 1.5× loading buffer. Samples were clarified by centrifugation at 12 000 rpm at 4°C for 30min. The homogenates, which contained 30 µg of protein, were then separated by SDS-PAGE and transferred into nitrocellulose membrane (Beyotime, Beijing, China). The blots were probed with the following primary antibodies: α-SMA (Abcam Cambridge, UK; 1:500), ZO-1 (Abcam Cambridge, UK; 1:1000), GAPDH (Abcam Cambridge, UK; 1:5000). Quantification of the Western blot data was performed by measuring the intensity of the hybridization signals using Image J software, and the ratios of α-SMA and ZO-1 to GAPDH were used as the relative expression level of the target protein.
Enzyme Linked Immunosorbent Assay
After experimental incubations as mentioned above, cell culture supernatants were collected and centrifuged for 10min at 1000 g (4°C) to remove cell debris, then stored at -80°C until use. The amount of secreted cytokines (TNF-α, TGF-β2 and VEGF) were examined using commercial ELISA kits (eBioscience, USA) according to the manufacturer's protocol. The plates were read at 450 nm, and cytokine levels were extrapolated from standard curves and normalized to protein concentrations.
Statistical Analysis
All data from quantitative assays were expressed as the mean±standard deviation (SD). At least 2-3 independent repetitions with triplicate determinates were performed for each quantitative assay. Statistical analysis was performed using one-way ANOVA (Tukey's post hoc test was used to analyze differences between parametric groups) or Student's t-test (independent sample t-test) with the aid of SPSS17.0. A significance cutoff of P<0.05 was used.
RESULTS
Clinical Characteristics of Subjects
Aqueous humor specimens from 52 patients (60 eyes; 27 men and 25 women; mean age, 62.05±7.06y) with a confirmed diagnosis of AMD treated with intravitreal ranibizumab injections from October 2013 to July 2014 were analyzed. Mean visual acuity was 0.25 preoperatively (range 0.02-0.6) and 0.37 postoperatively (4wk after injection, range 0.05-0.8). As controls, aqueous humor specimens, taken from the eyes of 20 patients (20 eyes; 10 men and 10 women; mean age, 61.63±7.58y) undergoing cataract surgery, were also analyzed. There were no significant differences in the distribution of age and gender between the two groups (P>0.05).
Interleukin-13 Level in Aqueous Humor
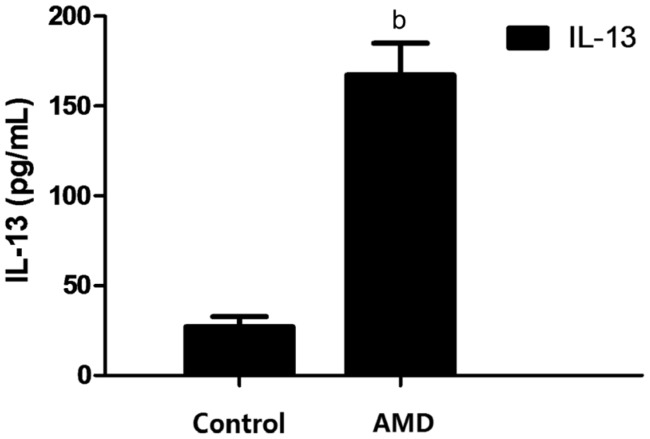
IL-13 levels in the aqueous humor of patients with AMD were significantly higher than those in the control (167.33±17.64 vs 27.12±5.65 pg/mL; P<0.01) (Figure 1).
Figure 1. Concentrations of IL-13 in the aqueous humor of patients with AMD and control group.
The concentrations of IL-13 in the aqueous humor in the AMD group and control group were determined by ELISA. bP<0.01.
MTS Proliferation Assay
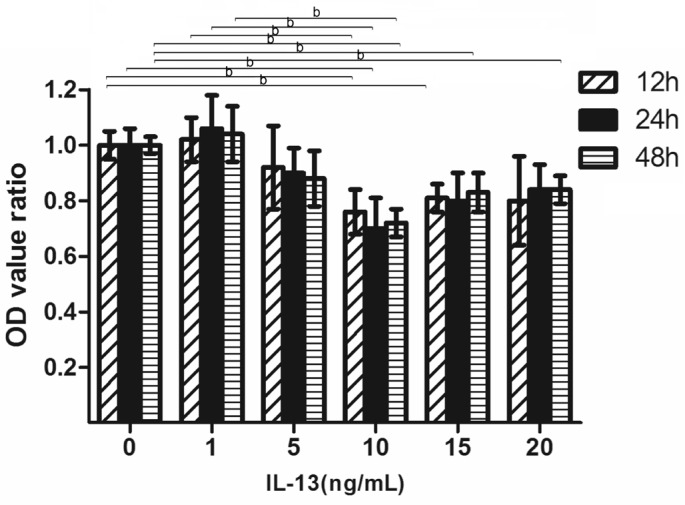
After the ARPE-19 cells were incubated with different doses of IL-13 for 48h, the optical density (OD) values, which reflect cell number, were not significantly different in the low concentration of IL-13 (1 and 5 ng/mL) treatments compared with the control group, while cellular viability decreased significantly of increasing concentration of IL-13 (10, 15, and 20 ng/mL) groups. As shown by Tukey's post hoc tests (Figure 2), relative OD values were significantly decreased in groups treated with 10 ng/mL (12h, 0.76±0.08; 24h, 0.70±0.11; 48h, 0.72±0.05; P<0.01), 15 ng/mL (12h, 0.81±0.05; 48h, 0.83±0.07; P<0.01) and 20 ng/mL (48h, 0.84±0.05; P<0.01).
Figure 2. Effects of IL-13 on the proliferation of ARPE-19 cell.
OD value ratio: OD value in the group with different concentration of IL-13/OD value in the control group. bP<0.01 according to Tukey's post hoc tests; n=6.
Effect of Interleukin-13 on α-SMA and ZO-1 Expression in ARPE-19 Cells
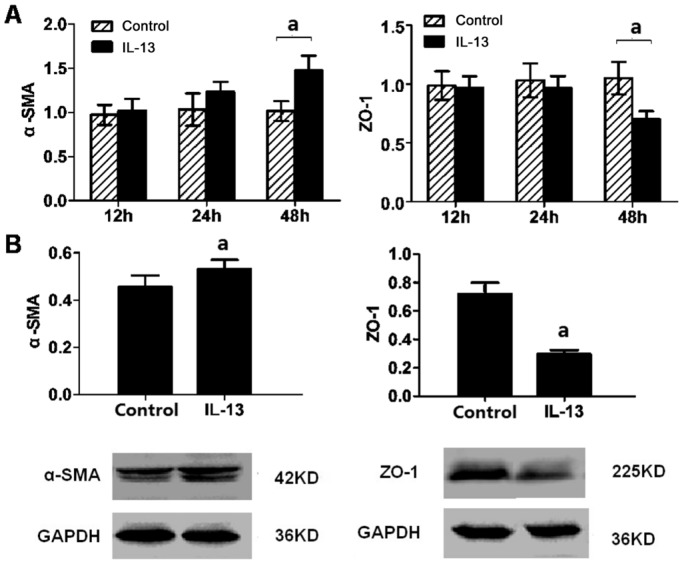
Epithelial-mesenchymal transition (EMT) is characterized by loss of epithelial makers such as ZO-1 and replacement by mesenchymal markers as α-SMA. After ARPE-19 cell were incubated with IL-13 treatment for 48h, the mRNA levels of α-SMA and ZO-1 were not affected at 12 and 24h. In contrast, α-SMA mRNA increased (1.017±0.112 vs 1.476±0.168, P<0.001) and ZO-1 decreased (1.051±0.136 vs 0.702±0.069, P<0.001) at 48h (Figure 3A). Meanwhile, the α-SMA and ZO-1 protein expression also showed the same tendency in Western blot assay (α-SMA: P=0.038; ZO-1: P=0.008) (Figure 3B).
Figure 3. Signals of EMT in ARPE-19 cell promoted by IL-13.
A: The mRNA expression of α-SMA and ZO-1 was examined by qRT-PCR after 10 ng/mL IL-13 exposure for 12, 24, and 48h; B: The protein levels of α-SMA and ZO-1 were analyzed by Western blot at 48h after IL-13 (10 ng/mL) treatment. aP<0.05 vs control group; n=3.
Effects of Cytokines Induced by Interleukin-13
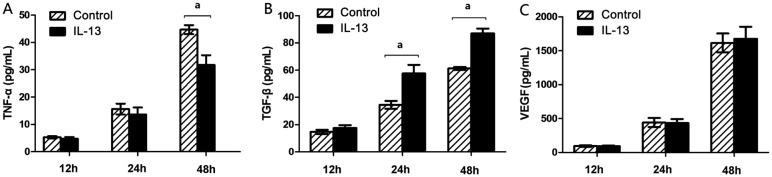
ARPE-19 cells were pretreated either with IL-13 or vehicle only and following 12, 24 and 48h of activation, conditioned media were analyzed by ELISA for levels of TNF-α, TGF-β2 and VEGF-A. IL-13 significantly reduced the level of TNF-α (44.70±1.67 vs 31.79±3.53 pg/mL; P=0.005) at 48h (Figure 4A). However, the level of TGF-β2 was significantly increased from 34.44±2.92 to 57.61±6.31 pg/mL at 24h (P=0.004) and from 61.26±1.11 to 86.91±3.59 pg/mL at 48h (P<0.001) (Figure 4B). While there was no significant difference of VEGF-A level between IL-13 group and control group (Figure 4C).
Figure 4. Levels of TNF-α, TGF-β2 and VEGF in ARPE-19 induced by IL-13.
ARPE-19 cells were either left untreated (control) or cultured with 10 ng/mL IL-13 for 12, 24 and 48h. Levels of TNF-α (A), TGF-β2 (B), and VEGF-A (C) in conditioned media were measured by ELISA. aP<0.05 vs control group; n=6.
DISCUSSION
The advent of treatments focusing on CNV suppression has caused a major shift in the approach to disease therapy and prevention. Along with the development of molecular biology technology and cell biology, investigation into the roles of cytokines in the pathogenesis of CNV has progressed. The advent of intravitreous VEGF inhibitors has revolutionized the management of neovascular AMD[7]. Although these available treatments may limit CNV progression to a certain extent, a portion of CNV patients still experience clinical deterioration. Furthermore, the loss of VEGF neuroprotectivity can be a potential side effect[8]–[10]. Therefore, new antiangiogenic therapies for CNV are eagerly awaited. Our results support the previous viewpoints.
IL-13, a cytokine mainly produced by type 2 helper T cells and monocytes/macrophages, plays pathophysiological roles in allergic inflammation and fibrosis formation, and more recently has been shown to play a pivotal role in a number of fibrotic diseases including hepatic and pulmonary fibrosis, and nodular sclerosing Hodgkin's disease[11]–[12]. In this study, we found that IL-13 of aqueous humor was significantly upregulated during AMD development, suggesting that IL-13 could have potent effects on the pathological process of this disease. During chronic inflammation, IL-13 is induced concomitantly with activation of its downstream signaling via an IL-13 receptor, IL-13 Rα2[13]. Concentrations of IL-13 increased in vitro could inhibit ARPE-19 cell proliferation in our study. The degeneration and decrease in cell viability of RPE cell are mostly contributed to process of AMD[14]–[15]. We also found that IL-13 (10 ng/mL) can promote EMT in ARPE-19 cells. Greater fibrotic responses after IL-13 may result from an imbalance in the complex interactions between angiogenesis and pro-fibrotic signaling pathway. In the development of AMD, the formation of neovascular membrane fibrosis leads to the development of disciform scars, which indicate irreversible damage[16]. Meanwhile, there is no effective therapy for the associated symptoms once central vision decreases because of metamorphosis and scotoma caused by the scarring. IL-13 is an indispensable mediator of fibrosis[17]. Recently, it was discovered that TGF-β, which contribute to the pathogenesis of subretinal fibrogenesis and angiogenesis, promotes the differentiation of fibroblasts into myofibroblasts, and can be regulated by IL-13[18]. TGF-β is of primary importance in the development of AMD as it is a strong inducer of extracellular matrix synthesis and accumulation[19]. Moreover, TGF-β can induce the transformation of RPE cells into fibroblast-like cells in vitro[20]. TGF-β2, as a number of TGF-β family, is the main isoform of TGF-β in the eye and it is produced locally[21]–[23]. In this study, we found IL-13 could significantly upregulate the concentrations of TGF-β2 in ARPE-19 cells. Similar foundings have been shown that IL-13 induces lung fibrosis by selectively stimulating TGF-β[24]–[25]. Blockage of TGF and IL-13 receptors prevents the progression of fibrosis[26]–[28]. In this study, we also found IL-13 could inhibit TNF-α. TNF-α, as a potent proinflammatory cytokine implicated in tissue damages, mediates RPE cell dysfunction and plays an important role in the pathology of AMD[29]–[30]. TNF-α increases the expression of VEGF and promotes monocyte-RPE adhesion[31]–[32]. Accumulating evidence supports the theory that other factors, such as IL-13, also play a crucial role in the development of AMD[1],[33]. This study suggested that the possible mechanism of IL-13 in AMD: to promote the transformation from chronic inflammation to fibrogenesis by decreasing TNF-α and upregulating TGF-β. In addition, reports on the effects of IL-13 on angiogenesis have been controversial. Some studies suggest that IL-13 inhibits tube formation and endotheliocyte migration[34]. IL-13 also reportedly suppresses the expression of VEGF and attenuates vascular tube formation via the JAK2-STAT6 pathway[35]. Haas et al[36] found that IL-13 an in vivo antiangiogenic factor and provide a rationale for its use in rheumatoid arthritis to control pathologic neovascularization. Nevertheless, our study in vitro showed that IL-13 couldn't affect the accumulation of VEGF in ARPE-19 cells.
The present study reveals the relationship of IL-13 with AMD. Therapys targeting VEGF alone already prove inadequate. Focusing on IL-13 and other factors involved in disciform scarring, which prolongs the time for effective treatment, can bring about a major shift in the approach to disease treatment and prevention. This potential bio-target may also improve prognosis assistant with anti-VEGF treatments. However, this study did not investigate the relationship between the size or extent of disciform scarring and IL-13 levels. This needs to be clarified in further research.
In summary, our study showed that concentrations of IL-13 increased in aqueous humor of patients with AMD and in vitro inhibited ARPE-19 cell proliferation, suggesting that IL-13 may play important roles in the pathogenesis of neovascular AMD. Furthermore, the possible mechanism of IL-13 in the pathogenesis of AMD was investigated that IL-13 may promote the transformation from chronic inflammation to fibrogenesis by decreasing RPE cell viability and regulating concentrations of related cytokines. These findings may provide evidence to support the development of future AMD therapies.
Acknowledgments
Conflicts of Interest: Fu B, None; Liu ZL, None; Zhang H, None; Gu F, None.
REFERENCES
- 1.Sakurada Y, Nakamura Y, Yoneyama S, Mabuchi F, Gotoh T, Tateno Y, Sugiyama A, Kubota T, Iijima H. Aqueous humor cytokine levels in patients with polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Ophthalmic Res. 2015;53(1):2–7. doi: 10.1159/000365487. [DOI] [PubMed] [Google Scholar]
- 2.Haas P, Kubista KE, Krugluger W, Huber J, Binder S. Impact of visceral fat and pro-inflammatory factors on the pathogenesis of age-related macular degeneration. Acta Ophthalmol. 2015;93(6):533–538. doi: 10.1111/aos.12670. [DOI] [PubMed] [Google Scholar]
- 3.Stewart MW. Individualized treatment of neovascular age-related macular degeneration: what are patients gaining? or losing? J Clin Med. 2015;4(5):1079–1101. doi: 10.3390/jcm4051079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boltz A, Ruiß M, Jonas JB, Tao Y, Rensch F, Weger M, Garhofer G, Frantal S, El-Shabrawi Y, Schmetterer L. Role of vascular endothelial growth factor polymorphisms in the treatment success in patients with wet age-related macular degeneration. Ophthalmology. 2012;119(8):1615–1620. doi: 10.1016/j.ophtha.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Gillies MC, Campain A, Barthelmes D, Simpson JM, Arnold JJ, Guymer RH, McAllister IL, Essex RW, Morlet N, Hunyor AP. Long-term outcomes of treatment of neovascular age-related macular degeneration: data from an observational study. Ophthalmology. 2015;122(9):1837–1845. doi: 10.1016/j.ophtha.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Doganay S, Evereklioglu C, Er H, Turkoz Y, Sevinc A, Mehmet N, Savli H. Comparison of serum NO, TNF-alpha, IL-1beta, sIL-2R, IL-6 and IL-8 levels with grades of retinopathy in patients with diabetes mellitus. Eye (Lond) 2002;16(2):163–170. doi: 10.1038/sj.eye.6700095. [DOI] [PubMed] [Google Scholar]
- 7.Ba J, Peng RS, Xu D, Li YH, Shi H, Wang Q, Yu J. Intravitreal anti-VEGF injections for treating wet age-related macular degeneration: a systematic review and meta-analysis. Drug Des Devel Ther. 2015;9:5397–5405. doi: 10.2147/DDDT.S86269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazzeri S, Ripandelli G, Sartini MS, Parravano M, Varano M, Nardi M, Di Desidero T, Orlandi P, Bocci G. Aflibercept administration in neovascular age-related macular degeneration refractory to previous anti-vascular endothelial growth factor drugs: a critical review and new possible approaches to move forward. Angiogenesis. 2015;18(4):397–432. doi: 10.1007/s10456-015-9483-4. [DOI] [PubMed] [Google Scholar]
- 9.Moon da RC, Lee DK, Kim SH, You YS, Kwon OW. Aflibercept treatment for neovascular age-related macular degeneration and polypoidal choroidal vasculopathy refractory to anti-vascular endothelial growth factor. Korean J Ophthalmol. 2015;29(4):226–232. doi: 10.3341/kjo.2015.29.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Do DV, Greenwald L, Ibrahim M, Sepah Y, Nguyen QD. Choroidal neovascularization regression on fluorescein angiography after VEGF blockade. Case Rep Ophthalmol. 2012;3(3):384–388. doi: 10.1159/000338969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchel JA, Antoniak S, Lee JH, Kim SH, McGill M, Kasahara DI, Randell SH, Israel E, Shore SA, Mackman N, Park JA. IL-13 augments compressive stress-induced tissue factor expression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2016;54(4):524–531. doi: 10.1165/rcmb.2015-0252OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuschiotti P, Medsger TA, Jr, Morel PA. Effector CD8+ T cells in systemic sclerosis patients produce abnormally high levels of interleukin-13 associated with increased skin fibrosis. Arthritis Rheum. 2009;60(4):1119–1128. doi: 10.1002/art.24432. [DOI] [PubMed] [Google Scholar]
- 13.Sinha S, Singh J, Jindal SK, Birbian N. Association of IL13R alpha 1 +1398A/G polymorphism in a North Indian population with asthma: A case-control study. Allergy Rhinol (Providence) 2015;6(2):111–117. doi: 10.2500/ar.2015.6.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Bergen T, Spangler R, Marshall D, Hollanders K, Van de Veire S, Vandewalle E, Moons L, Herman J, Smith V, Stalmans I. The role of LOX and LOXL2 in the pathogenesis of an experimental model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2015;56(9):5280–5289. doi: 10.1167/iovs.14-15513. [DOI] [PubMed] [Google Scholar]
- 15.Dikmetas O, Kadayifcilar S, Eldem B. The effect of CFH polymorphisms on the response to the treatment of age-related macular degeneration (AMD) with intravitreal ranibizumab. Mol Vis. 2013;19:2571–2578. [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura K, Orita T, Liu Y, Yang Y, Tokuda K, Kurakazu T, Noda T, Yanai R, Morishige N, Takeda A, Ishibashi T, Sonoda KH. Attenuation of EMT in RPE cells and subretinal fibrosis by an RAR-γ agonist. J Mol Med (Berl) 2015;93(7):749–758. doi: 10.1007/s00109-015-1289-8. [DOI] [PubMed] [Google Scholar]
- 17.Lafyatis R, York M. Innate immunity and inflammation in systemic sclerosis. Curr Opin Rheumatol. 2009;21(6):617–622. doi: 10.1097/BOR.0b013e32832fd69e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baraut J, Farge D, Jean-Louis F, Masse I, Grigore EI, Arruda LC, Lamartine J, Verrecchia F, Michel L. Transforming growth factor-β increases interleukin-13 synthesis via GATA-3 transcription factor in T-lymphocytes from patients with systemic sclerosis. Arthritis Res Ther. 2015;17:196. doi: 10.1186/s13075-015-0708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedrich U, Datta S, Schubert T, Plossl K, Schneider M, Grassmann F, Fuchshofer R, Tiefenbach KJ, Langst G, Weber BH. Synonymous variants in HTRA1 implicated in AMD susceptibility impair its capacity to regulate TGF-β signaling. Hum Mol Genet. 2015;24(22):6361–6373. doi: 10.1093/hmg/ddv346. [DOI] [PubMed] [Google Scholar]
- 20.Feng Z, Li R, Shi H, Bi W, Hou W, Zhang X. Combined silencing of TGF-β2 and Snail genes inhibit epithelial-mesenchymal transition of retinal pigment epithelial cells under hypoxia. Graefes Arch Clin Exp Ophthalmol. 2015;253(6):875–884. doi: 10.1007/s00417-014-2922-x. [DOI] [PubMed] [Google Scholar]
- 21.Jobling AI, Nguyen M, Gentle A, McBrien NA. Isoform-specific changes in scleral transforming growth factor-beta expression and the regulation of collagen synthesis during myopia progression. J Biol Chem. 2004;279(18):18121–18126. doi: 10.1074/jbc.M400381200. [DOI] [PubMed] [Google Scholar]
- 22.Roberts AB, Sporn MB. Differential expression of the TGF-beta isoforms in embryogenesis suggests specific roles in developing and adult tissues. Mol Reprod Dev. 1992;32(2):91–98. doi: 10.1002/mrd.1080320203. [DOI] [PubMed] [Google Scholar]
- 23.Jampel HD, Roche N, Stark WJ, Roberts AB. Transforming growth factor-beta in human aqueous humor. Curr Eye Res. 1990;9(10):963–969. doi: 10.3109/02713689009069932. [DOI] [PubMed] [Google Scholar]
- 24.Guo J, Yao H, Lin X, Xu H, Dean D, Zhu Z, Liu G, Sime P. IL-13 induces YY1 through the AKT pathway in lung fibroblasts. PLoS One. 2015;10(3):e119039. doi: 10.1371/journal.pone.0119039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Liu ZL. Transforming growth factor-β neutralizing antibodies inhibit subretinal fibrosis in a mouse model. Int J Ophthalmol. 2012;5(3):307–311. doi: 10.3980/j.issn.2222-3959.2012.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou CQ, Wang YY, Wang XL. The role of transforming growth factor beta1 R II and interleukin 13 Ralpha2 in schistosomiasis-induced hepatic fibrosis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2013;31(5):400–405. [PubMed] [Google Scholar]
- 27.Yao Y, Zhou C, Chu D. The effect of recombinant sTGFβ1RII and sIL13Rα2 receptor proteins on schistosomiasis japonica, hepatic fibrosis and signal transduction in a mouse model of schistosome disease. Exp Parasitol. 2014;142:17–26. doi: 10.1016/j.exppara.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Radeke MJ, Radeke CM, Shih YH, Hu J, Bok D, Johnson LV, Coffey PJ. Restoration of mesenchymal retinal pigmented epithelial cells by TGFβ pathway inhibitors: implications for age-related macular degeneration. Genome Med. 2015;7(1):58. doi: 10.1186/s13073-015-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zech JC, Pouvreau I, Cotinet A, Goureau O, Le Varlet B, de Kozak Y. Effect of cytokines and nitric oxide on tight junctions in cultured rat retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1998;39(9):1600–1608. [PubMed] [Google Scholar]
- 30.Peng S, Gan G, Rao VS, Adelman RA, Rizzolo LJ. Effects of proinflammatory cytokines on the claudin-19 rich tight junctions of human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2012;53(8):5016–5028. doi: 10.1167/iovs.11-8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thichanpiang P, Harper SJ, Wongprasert K, Bates DO. TNF-α-induced ICAM-1 expression and monocyte adhesion in human RPE cells is mediated in part through autocrine VEGF stimulation. Mol Vis. 2014;20:781–789. [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Han X, Wittchen ES, Hartnett ME. TNF-α mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent β-catenin activation. Mol Vis. 2016;22:116–128. [PMC free article] [PubMed] [Google Scholar]
- 33.Nassar K, Grisanti S, Elfar E, Luke J, Luke M, Grisanti S. Serum cytokines as biomarkers for age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2015;253(5):699–704. doi: 10.1007/s00417-014-2738-8. [DOI] [PubMed] [Google Scholar]
- 34.Savetsky IL, Ghanta S, Gardenier JC, Torrisi JS, García Nores GD, Hespe GE, Nitti MD, Kataru RP, Mehrara BJ. Th2 cytokines inhibit lymphangiogenesis. PLoS One. 2015;10(6):e0126908. doi: 10.1371/journal.pone.0126908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishimura Y, Nitto T, Inoue T, Node K. IL-13 attenuates vascular tube formation via JAK2-STAT6 pathway. Circ J. 2008;72(3):469–475. doi: 10.1253/circj.72.469. [DOI] [PubMed] [Google Scholar]
- 36.Haas CS, Amin MA, Ruth JH, Allen BL, Ahmed S, Pakozdi A, Woods JM, Shahrara S, Koch AE. In vivo inhibition of angiogenesis by interleukin-13 gene therapy in a rat model of rheumatoid arthritis. Arthritis Rheum. 2007;56(8):2535–2548. doi: 10.1002/art.22823. [DOI] [PubMed] [Google Scholar]