Abstract
AIM
To evaluate the potential role of hyperreflective foci (HF) as a prognostic indicator of visual outcome in patients with macular edema (ME) due to retinal vein occlusion (RVO).
METHODS
We retrospectively reviewed 50 eyes of 50 patients with ME due to ischemic central retinal vein occlusion (CRVO), non-ischemic CRVO and branch retinal vein occlusion (BRVO) who were treated with anti-vascular endothelial growth factor (anti-VEGF) at Beijing Tongren Eye Center from January 2013 to July 2016. All patients underwent best-corrected visual acuity (BCVA), spectral domain optical coherence tomography (SD-OCT) at baseline and follow-up. Such factors were evaluated and compared among three groups as baseline and final BCVA, central retinal thickness (CRT), external limiting membrane (ELM) status and the numbers of HF in different position. Multiple linear regression analysis was employed to analyze the relationship between baseline HF and final BCVA. Changes of HF before and after treatment were evaluated too.
RESULTS
Among three groups, HF could be located in each retinal layers, as well as in vitreous cavity. The mean HF in outer retinal layer (ORL) at baseline was 5.29±8.48 in ischemic CRVO with intact ELM, 1.93±2.76 in non-ischemic CRVO, and 1.75±2.05 in BRVO. With disrupted ELM, the mean HF in ORL increased. There was statistically difference of HF in ORL between intact and disrupted ELM. The numbers of HF in ORL were associated with poor visual outcome among three groups. However, HF in inner retinal layer (IRL) and vitreous cavity were not associated with poor visual outcome. Meanwhile, the baseline HF in ORL and vitreous cavity reduced significantly in non-ischemic CRVO and BRVO after anti-VEGF treatment.
CONCLUSION
The numbers of HF in ORL are prognostic factors associated with the final BCVA in patients with ME due to RVO after anti-VEGF treatment.
Keywords: hyperreflective foci, retinal vein occlusion, treatment, optical coherence tomography
INTRODUCTION
Retinal vein occlusion (RVO) is one of the most common retinal vascular diseases, and macular edema (ME) is the most common cause of visual impairment in people with these diseases[1]–[2]. Therefore, various treatments have been used to try to reduce the ME as soon as possible, for example, grid laser photocoagulation[3]–[4], intravitreal injections anti-vascular endothelial growth factor (anti-VEGF)[5]–[6], intravitreal injections of triamcinolone acetonide[7]–[8], and pars planavitrectomy[9].Although complete resolution of ME may be achieved with all these treatment options, some patients experience poor visual outcomes, suggesting that there may be several factors that predict the final visual outcome independent to the agent used to treat the ME.
Spectral-domain optical coherence tomography (SD-OCT), a noninvasive optical imaging modality, is the standard and the most common equipment used to objectively evaluate and monitor the efficiency of treatment by quantifying the resolution of ME[10]. With increase of resolution, more details can be evaluated by SD-OCT. Bolz et al[11] observed hyperreflective foci (HF) on SD-OCT in 2009 at the first time and Hasegawa et al[12] captured hyperreflective line in 2015. Both results showed that HF and hyperreflective line were associated with final best-corrected visual acuity (BCVA). More experts expressed their interests in HF on SD-OCT. Recently, HF was defined as the presence of small discrete, well-circumscribed, dot-shaped lesions, with equal or greater reflectivity than the retinal pigment epithelium (RPE) band on SD-OCT[13]. HF was observed in patients with age-related macular degeneration (AMD), RVO, diabetic retinopathy (DR), central serous choroidoretinopathy, Stargardt disease and retinitis pigmentosa[13]–[18]. Previous studies reported that HF regressed after treatment and the presence of HF at baseline was positively correlated with final visual acuity in patients with exudative AMD after treatment with anti-VEGF[15],[19]–[20].Furthermore, other reports suggest that the number and potentially the location of HF may be a predictor of final treatment outcome in ME treated with anti-VEGF agents[14],[21]–[22].Because of what was discussed above, the purpose of this study was to evaluate the effect of HF in different position as a prognostic factor of visual outcome in patients treated with intravitreal ranibizumab for ME due to RVO.
SUBJECTS AND METHODS
Patients
We retrospectively reviewed 50 eyes of 50 patients with ME due to RVO including ischeminc central retinal vascular occlusion (CRVO), non-ischemic CRVO and branch retinal vascular occlusion (BRVO) who were treated with intravitreal ranibizumab and followed up for at least 9mo at Beijing Tongren Eye Center from January 2013 and July 2016. The study was conducted in accordance with the tenets of the Declaration of Helsinki.
Exclusion criteria included high myopia, glaucoma, media opacities due to cataract or corneal disease, vitreous hemorrhage, history of uveitis, presence of epiretinal membrane or macular hole, AMD or other ocular pathology that may affect the visual acuity, any intraocular surgery or laser treatment, including focal/grid macular photocoagulation and panretinal photocoagulation within 6mo before the treatment, and bad quality of images because of strong eye movements.
At initial visit (baseline examination), each patient underwent comprehensive ophthalmic examination including measurement of BCVA, intraocular pressure, slit-lamp biomicroscopy, indirect ophthalmoscopy, color fundus photography, fluorescein angiography, and SD-OCT (Spectralis HRA+OCT®; Heidelberg Engineering, Heidelberg, Germany). Specifically, for BCVA measurement, all patients were refracted by a certified examiner, and BCVA was measured using standard Early Treatment Diabetic Retinopathy Study (ETDRS) protocol at 4 m distance with a modified ETDRS distance chart.
All patients were treated with an injection of intravitreal ranibizumab (0.5 mg/0.05 mL), and BCVA, color fundus photography, and SD-OCT were evaluated at every visit. Re-treatment was permitted for persistent fluid and/or decrease in visual acuity by at least five letters.
Optical Coherence Tomography Measurement
The OCT acquisition was performed on the SD-OCT. The OCT volume scan was performed on a 20×20° cube, consisted of 49 horizontal B-scans with 20 averaged frames per B-scan centered over the fovea, each containing 1064 pixels, separated by 125 mm. The integrated follow-up mode of the device was used to ensure that the exact same retinal area was imaged at every follow-up visit. The eye-tracking system of the device was used to assure that the correct position was maintained during the scanning process. The morphologic features of ME, central retinal thickness (CRT), external limiting membrane (ELM), ellipsoid zone (EZ), interdigitation zone (IZ), and the number of HF in different position were assessed and analyzed with SD-OCT by two experienced masked graders.
The CRT defined as the retinal thickness within the central circle of the ETDRS grid (1 mm diameter) centered over the fovea was automatically calculated using the incorporated software of the Spectralis SD-OCT. The status of the ELM, EZ and IZ was classified into intact or disrupted status. Intact ELM, EZ and IZ were defined as a continuous hyperreflective line. Disrupted ELM, EZ and IZ were defined as loss or irregularity of the hyperreflective line.
The method used for evaluation of HF has been reported in several previous studies as a reliable measure of HF[11],[22]–[25]. The numbers of HF in the cube were subjectively determined in all patients at baseline and final visit by two experienced masked graders. The position of the HF was classified as 1) HF in the outer retinal layers (from the ELM to the RPE); 2) HF in the inner retinal layers [from the internal limiting membrane (ILM) to the outer nuclear layer (ONL)]; 3) HF in the whole retinal layers (from the ILM to the RPE); 4) HF in the vitreous cavity. Due to the variation of scan window location, the numbers of HF in the vitreous cavity were not counted directly. Firstly, HF numbers of the vitreous cavity on each scan window had to be counted. Meanwhile, the areas for each vitreous cavity had to be measured. Then, the ratio of HF numbers to the total areas of vitreous cavity (it's abbreviated as RATIO in the following part) can be calculated.
Statistical Analysis
All data were analyzed using SPSS for Windows, Version 22.0. Chicago, IL: SPSS Inc., and results were presented as the mean±SD. Differences were evaluated using ANOVA, followed by the Student-Newman-Keuls test for multiple comparisons. The Student's t-test was used to analyze pairwise comparisons. Then, multiple linear regression analysis with backward elimination was employed to analysis the association of BCVA with the numbers of HF in different layers. A P-value<0.05 was considered statistically significant.
RESULTS
Baseline Characteristics of Patients
The baseline characteristics of the patients in three groups are shown in Table 1. A total of 50 patients were included in this study, 14 eyes of 14 patients with ME secondary to ischemic CRVO, 21 eyes with non-ischemic CRVO, 15 eyes with BRVO were examined. There was no significant difference among ischemic CRVO, non-ischemic CRVO and BRVO with regard to age, follow-up period, and mean number of injections using ANOVA; there was no significant difference statistically between non-ischemic CRVO and BRVO with regard to CRT and BCVA; however, ischemic CRVO diversely different from non-ischemic CRVO and BRVO regarding CRT and BCVA (P<0.05). The mean BCVA at baseline was 49.36±18.11 in ischemic CRVO, 57.67±7.98 in non-ischemic CRVO, 60.13±9.68 in BRVO.
Table 1. The baseline characteristics of patients.
| Baseline characteristic | CRVO |
BRVO | |
| Ischemic | Non-ischemic | ||
| No. of eyes/patients | 14 | 21 | 15 |
| Age (a) | 58.64±12.31 | 55.33±13.15 | 61.3±7.19 |
| Men/women | 10/4 | 9/12 | 10/5 |
| Study eye (n) | |||
| Right | 7 | 10 | 8 |
| Left | 7 | 11 | 7 |
| BCVA (EDTRS) | 49.36±18.11 | 57.67±7.98 | 60.13±9.68 |
| CRT (µm) | 841.14±147.09 | 659.57±136.16 | 549.07±125.11 |
| Follow-up (mo) | 24.71±11.01 | 25.67±8.40 | 27.80±9.98 |
| Times of injection | 12.14±4.87 | 11.52±3.68 | 9.27±4.82 |
| No. HF | 1/14 | 2/21 | 3/15 |
Baseline Hyperreflective Foci Situation in Retinal Layers and Vitreous Cavity
Among the three groups, HF can be located in each retinal layers, as well as in vitreous cavity, but mainly located around outer plexiform layer (OPL) within the inner retinal layer (Figures 1 and 2). The location of HF in retinal layers can be confluent and/or sparse, however, its location in vitreous cavity is sparse. The confluent location is mainly around OPL, corresponding to hard exudates on color fundus photographs. There was no obvious difference about numbers of HF in inner retinal layer, outer retinal layer and RATIO among the three groups. Meanwhile, we evaluated the situation of HF location according to the status of ELM/EZ/IZ. The results showed that numbers of HF in outer retinal layer in intact ELM were much less than those in disrupted ELM, no matter that it's CRVO or BRVO. The mean HF in outer retinal layer at baseline was 5.29±8.48 in ischemic CRVO with intact ELM, 1.93±2.76 in non-ischemic CRVO with intact ELM, 1.75±2.05 in BRVO with intact ELM. While, with disrupted ELM, the mean HF in outer retinal layer was 16.43±34.09 in ischemic CRVO, 8.29±8.88 in non-ischemic CRVO, 8.29±6.42 in BRVO. There was statistically difference of HF in outer retinal layer between intact ELM and disrupted ELM. However, there was no apparent variation of the numbers of inner retinal layers and RATIO according to the status of ELM/EZ/IZ among the three groups. More details can be referenced in Table 2.
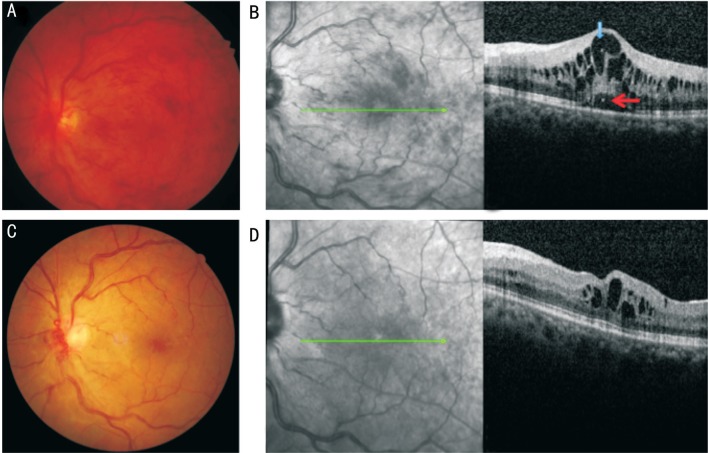
Figure 1. Representative case of CRVO with HF.
The initial BCVA was 51 letters and the final BCVA was 65 letters. A: Fundus finding at baseline shows optic vascular loop at baseline; B: SD-OCT image shows ME with serous retinal detachment, the HF in the inner (blue arrow) and outer retina (red arrow) were detected; C: Fundus finding at final visit after intravitreal injection; D: SD-OCT image shows ME decreased and HF were not detected.
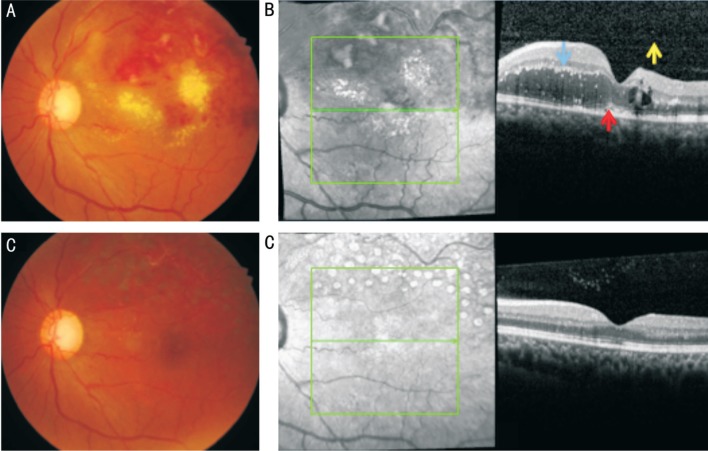
Figure 2. Representative case of BRVO with HF.
The initial BCVA was 57 letters and the final BCVA was 80 letters. A: Fundus finding at baseline shows lots of hard exudates in the unaffected area at baseline; B: SD-OCT image shows ME with HF in the inner (blue arrow) and outer retina (red arrow), and in the vitreous cavity (yellow arrow); C: Fundus finding showed hard exudates resolved at final visit after intravitreal injection; D: SD-OCT image shows ME resolved.
Table 2. Numbers of HF in retinal layers and in the vitreous cavity according to the status of ELM/EZ/IZ.
| Groups | Baseline HF in retinal layer | Baseline HF in ORL | Baseline HF in INL | Baseline RATIO | ||
| CRVO | ||||||
| Ischemic | ELM/EZ | Intact | 84.43±146.78 | 5.29±8.48 | 79.14±138.58 | 0.242±0.260 |
| Disrupted | 82.29±86.64 | 16.43±34.09 | 65.86±81.23 | 0.209±0.193 | ||
| IZ | Intact | 93±158.86 | 6±9.06 | 87±150.09 | 0.242±0.285 | |
| Disrupted | 76.13±82.09 | 14.5±32.03 | 61.63±76.15 | 0.213±0.179 | ||
| Non-ischemic | ELM | Intact | 44.36±60.27 | 1.93±2.76 | 42.50±59.31 | 0.453±0.711 |
| Disrupted | 75.57±97.21 | 8.29±8.88 | 67.29±92.12 | 0.507±0.401 | ||
| EZ | Intact | 51.0±62.89 | 1.92±2.87 | 49.17±61.78 | 0.429±0.749 | |
| Disrupted | 59.78±89.83 | 6.89±8.24 | 52.89±84.74 | 0.527±0.409 | ||
| IZ | Intact | 55.27±64.11 | 2.09±2.95 | 53.27±63.06 | 0.443±0.784 | |
| Disrupted | 54.20±86.51 | 6.20±8.07 | 48.0±81.38 | 0.502±0.394 | ||
| BRVO | ELM/EZ/IZ | Intact | 29.63±62.61 | 1.75±2.05 | 27.88±61.07 | 0.238±0.323 |
| Disrupted | 98.43±122.15 | 8.29±6.42 | 90.14±121.04 | 0.097±0.963 | ||
ORL: Outer retinal layer.
Relationship Between Baseline Hyperreflective Foci in Different Location and Final Best-corrected Visual Acuity
To evaluate the association of final BCVA with baseline HF in retinal layer and vitreous cavity, multivariate analysis was performed (Table 3). The results showed that the numbers of HF in the outer retinal layers were associated with poor visual outcome in ischemic CRVO (R=-0.527, P=0.050), non-ischemic CRVO (R=-0.609, P=0.049) and BRVO (R=-0.581, P=0.023). While, the numbers of HF in inner retinal layer and RATIO were not associated with poor visual outcome among the three groups.
Table 3. Results of multivariate regression on numbers of HF as a prognostic parameter for poor visual outcome.
| Baseline HF in different layers | CRVO |
BRVO |
||||
| Ischemic |
Non-ischemic |
|||||
| Pearson correlation coefficient | P | Pearson correlation coefficient | P | Pearson correlation coefficient | P | |
| Baseline HF in retinal layer | 0.025 | 0.931 | 0.116 | 0.618 | 0.066 | 0.817 |
| Baseline HF in ORL | -0.527 | 0.050 | -0.609 | 0.049 | -0.581 | 0.023 |
| Baseline HF in IRL | 0.143 | 0.626 | 0.108 | 0.640 | 0.101 | 0.720 |
| Baseline RATIO | -0.494 | 0.073 | 0.040 | 0.862 | 0.184 | 0.513 |
Changes of Hyperreflective Foci Before and After Anti-vascular Endothelial Growth Factor Treatment
We analyzed the changes of HF after anti-VEGF injection (Table 4). The numbers of HF in retinal layers decreased in 7 eyes (50%), was stable in 4 eyes (28.57%), and increased in 3 eyes (21.43%) in ischemic CRVO. The numbers of HF in the whole retinal layers at baseline reduced significantly from 83.36±115.80 to 42.29±64.94 at final visit in ischemic CRVO (P=0.272). The numbers of HF in outer retinal layer at baseline reduced from 10.86±24.56 to 6.57±15.52 at final visit in ischemic CRVO (P=0.611). Our results also showed that the numbers of HF in retinal layers decreased in 14 eyes (66.67%), was stable in 3 eyes (14.29%), and increased in 4 eyes (19.05%) in non-ischemic CRVO; the numbers of HF decreased in 8 eyes (53.33%), was stable in 6 eyes (40%), and increased in 1 eye (6.67%) in BRVO. The numbers of baseline HF in outer retinal layer reduced significantly in non-ischemic CRVO and BRVO, meanwhile, the RATIO also decreased significantly in non-ischemic CRVO and BRVO. There was statistically difference of numbers of HF in outer retinal layer and RATIO for non-ischemic CRVO and BRVO before and after anti-VEGF treatment. However, there was no significant difference in ischemic CRVO.
Table 4. Changes of baseline HF and baseline BCVA after anti-VEGF treatment.
| Main outcomes | CRVO |
BRVO |
|||||||
| Ischemic |
Non-ischemic |
||||||||
| Baseline | Final | P | Baseline | Final | P | Baseline | Final | P | |
| BCVA | 49.36±18.11 | 55.93±20.47 | 0.361 | 57.67±7.98 | 69.33±14.70 | 0.002 | 60.13±9.68 | 76.67±6.34 | 0.0001 |
| CRT | 841.14±266.21 | 308.79±147.09 | 0 | 659.57±136.19 | 302.43±122.16 | 0.0000 | 549.07±125.11 | 292.8±72.96 | 0.0001 |
| HF in retinal layer | 83.36±115.80 | 42.29±64.94 | 0.272 | 54.76±73.64 | 19.76±26.83 | 0.020 | 61.67±98.11 | 17.33±22.52 | 0.106 |
| HF in ORL | 10.86±24.56 | 6.57±15.52 | 0.611 | 4.05 ± 6.17 | 0.57 ± 1.25 | 0.015 | 4.80 ± 5.58 | 2.13 ± 2.78 | 0.027 |
| HF in IRL | 72.50±109.35 | 36.43±59.67 | 0.290 | 50.76±70.54 | 18.71±26.89 | 0.024 | 56.93±95.80 | 15.27±21.40 | 0.126 |
| RATIO | 0.225±0.221 | 0.112±0.129 | 0.105 | 0.471±0.615 | 0.051±0.041 | 0.005 | 0.248±0.064 | 0.089±0.023 | 0.035 |
We also compare the changes of BCVA and CRT before and after anti-VEGF treatment. The mean BCVA showed significant improvement from baseline to final in non-ischemic CRVO and BRVO, but there was no significant difference in ischemic CRVO. The mean CRT showed significant reduction from baseline in the three groups (P<0.05) (Table 4).
DISCUSSION
ME is a well-known cause of visual impairment in retinal vascular diseases including retinal vascular occlusion and diabetic retinal disease. In the last 2 decades, the role of OCT was stressed to analyze morphologic retinal changes and to understand physiopathologic mechanisms of ME. In 2009, Bolz et al[11] described a novel finding on SD-OCT characterized by dots scattered throughout all retinal layers by DME firstly. Recently, HF was defined as the presence of small discrete, well-circumscribed, dot-shaped lesions, with equal or greater reflectivity than the retinal pigment epithelium band on SD-OCT[13]. HF was observed in patients with AMD, RVO, DR, central serous choroidoretinopathy, Stargardt disease and retinitis pigmentosa[13]–[18].
Several studies have shown that the presence of HF in various retinal layers was positively associated with final BCVA, on the other hand, other studies showed that the presence of HF was not associated with final BCVA. Chatziralli et al[21] reported that the number and foveal location of HF are independent factors associated with the final BCVA in patients with ME due to DR and BRVO, suggesting HF as a potential biomarker of poor final visual outcome. Uji et al[23] showed the clinical relevance of HF, reporting that their presence in the outer retina layers decreases visual acuity of subjects with DME. Coscas et al[20]reported that HF were primarily detected in the outer retinal layers and near the RPE but also disseminated into all retinal layers (but less numerous) and HF resolution was associated with better final visual acuity in nAMD and polypoidal choroidal vasculopathy (PCV). However, Akagi-Kurashige et al[26] reported that the presence of HF in neurosensory retina at baseline was not correlated with final visual acuity in univariate regression analysis in 35 nAMD patients and 61 PCV patients together. The present study prompted us to focus on HF in different location, the relationship between baseline HF and final BCVA, and whether or not the numbers of HF decrease after anti-VEGF treatment in other retinal vascular diseases such as ischemic CRVO, non-ischemic CRVO and BRVO.
Our results showed that HF could be located in each retinal layers, as well as in vitreous cavity, but mainly located around outer plexiform layer within the inner retinal layer. The location of HF in retinal layers can be confluent and/or scattered, however, its location in vitreous cavity is sparse. The confluent location is mainly around OPL, corresponding to hard exudates on color fundus photographs. In our study, only 1 eye (7.14%) in ischemic CRVO, 2 eyes (9.52%) in non-ischemic CRVO and 3 eyes (20%) in BRVO at baseline had no HF detected on SD-OCT. The percentage of eyes with no HF was similar to that of a previous study by Ogino et al[18], who reported the absence of HF in 22.4% of eyes with BRVO. What's the origin of HF? The exact origin of HF is unclear, but recent studies suggested that HF could be deposition of extravasated lipoproteins (the precursor of hard exudates), lipid-laden macrophages, photoreceptor degeneration, and activated or over-phagocytosed retinal pigment epithelium cells, or retinal pigment epithelium metaplasia, microglia in an inflammatory environment[13]–[18]. In RVO, HF seems to reflect the extravasation of fluid caused by breakdown of the blood-retina barrier, which finally lead to hard exudates and ME. Ogino et al[18] reported that in BRVO, the fine scattered HF in the affected area represented leakage of blood constituents, whereas the confluent HF around the OPL in the unaffected area were associated with absorption of water and solutes. This study also showed that hard exudates or confluent HF in BRVO spared the fovea, where there are no retinal capillaries, and that confluent HF were rare in the retinal parenchyma in CRVO. In AMD, the origin of HF could reflect RPE migrating cells because there is similar expression as for the RPE layer providing the most highly reflective surface, and it was presumed that HF might reflect leukocytes, which are able to invade the extracellular spaces in inflammatory regions. In 2016, Chen et al[27] reported that HF associated with acquired vitelliform lesions were of RPE origin by light microscopy and transmission electron microscopy from a donor eye. We can speculate that the origin of HF differs in different diseases, consequently, the location of HF varies in SD-OCT.
Our results showed that the baseline numbers of HF in the outer retinal layers were associated with poor visual outcome in ischemic CRVO (R=-0.527, P=0.050), non-ischemic CRVO (R=-0.609, P=0.049) and BRVO (R=-0.581, P=0.023). While, the baseline numbers of HF in inner retinal layer and RATIO were not associated with poor visual outcome among the three groups. It was similar with the previous study by Ogino et al[18] and Chatziralli et al[21]. However, Lee et al[15] reported that subretinal HF could be considered as a new reliable visual prognostic factor in nAMD and PCV. Why is the numbers of HF in outer retinal layer relevant to final visual outcome in RVO? Murakami et al[28] reported that HF in the outer retinal layers were associated with either a disrupted ELM or EZ and poor prognoses in diabetic ME. Chatziralli et al[21] also reported that poor final visual acuity was correlated with poor initial visual acuity and photoreceptor disruption. It had already been verified by our results. The results showed that numbers of HF in outer retinal layer in intact ELM were much less than those in disrupted ELM, no matter that it's CRVO or BRVO. There was statistically difference of HF in outer retinal layer between intact ELM and disrupted ELM. However, there was no apparent variation of the numbers of inner retinal layers and RATIO according to the status of ELM/EZ/IZ among the three groups. A disrupted ELM cannot block the migration of extravasated lipoproteins in the inner retinal layers to the outer retinal layers of the ELM, and makes it possible to pass these extravasated blood constituents through the outer retinal layer[25]. These materials are deposited to the outer retinal layers including the photoreceptors. For this reason, we could find that the eyes with HF in the outer retinal layers had significantly more ELM disruption and EZ disruption at final visit. HF position, which was positively associated with final visual outcome, varied in different diseases depending on various pathologic mechanisms.
The mean number of baseline HF in outer retinal layer reduced significantly in non-ischemic CRVO and BRVO, meanwhile, the RATIO also decreased significantly in non-ischemic CRVO and BRVO after anti-VEGF treatment. There was statistically difference of numbers of HF in outer retinal layer and RATIO for non-ischemic CRVO and BRVO before and after anti-VEGF treatment. Chatziralli et al[21] and Kang et al[25] reported that there was a significant reduction in the number of HF and retinal tissue integrity was enhanced and leakage was reduced after anti-VEGF treatment as in ME patients of BRVO and DR. Our result was consistent with previous studies. However, there was no significant difference of numbers of HF in outer retinal layer in ischemic CRVO before and after anti-VEGF treatment. Also, the mean BCVA showed no significant improvement from baseline to final in ischemic CRVO. Because of severe ischemia, inner retinal ischemia cannot be improved and blood-retinal barrier function would not be improved in ischemic CRVO after anti-VEGF treatment; for ischemic CRVO, neither final BCVA showed significant improvement, nor the numbers of HF in outer retinal layer was reduced obviously.
In 2016, Korot et al[29] showed that higher vitreous HF scores existed in patients with DME compared with controls and diabetic patients without retinopathy. In that paper, the analysis against the relationship between HF in vitreous cavity and final visual outcome was not done, nor the analysis against the changes of vitreous HF after treatment. In this study, it showed that baseline ratio of HF in vitreous cavity to the total areas of vitreous cavity was not associated with poor visual outcome, there was a significant reduction in both non-ischemic CRVO and BRVO after anti-VEGF treatment. It is the first time that this result is reported. Some investigators had noticed the presence of HF in OCT scans of the vitreous of patients with inflammatory conditions, including uveitis[30]–[31]. If these vitreous HF represent inflammatory cells, additional questions are raised regarding the contribution of vitreous inflammatory cells in the pathogenesis and progression of ME in RVO that warrant further investigation. Our results encouraged us to hypothesize that the vitreous HF possibly represent cell debris or other factors except inflammatory cells, and extravasated blood constituents might diffuse into the vitreous cavity in RVO. Extravasated proteins or lipids might increase the osmotic pressure in the vitreous, resulting in the development of serous retinal detachment (SRD). In this study, there was no statistically significant difference in the ratio of HF in vitreous cavity to the total areas of vitreous cavity between CRVO and BRVO. However, Ogino et al[18] and Tsujikawa et al[32] stated that the hyperreflective foci in the vitreous were often observed in CRVO, instead of the deposition of foci on the ELM or the subfoveal hard exudates, suggesting the flow of blood constituents into the vitreous. They did not provide the method of measuring vitreous HF. The vitreous is a fluid structure with likely nonhomogeneous distribution of vitreous HF, and our scan window is restricted to the posterior portion; due to the variation of scan window location, the numbers of HF were not counted directly. So, the ratio of HF numbers to the total areas of vitreous cavity can be calculated. It is also the first time that this method is used for calculation, and it still need to be verified in the future.
Our study has some weakness that should be mentioned. First, it was a retrospective nature and relatively small sample size. Second, we may have missed HF in eyes that were considered as the absence of HF, because the amount, size, and exact location of HF appeared to be highly variable in our study. These HF were round or oval shapes, and of different size regardless of the location of HF. Prospective, randomized clinical study with a specific demarcation and calculation of HF will be required to reveal the precise pathogenesis and predictive role of these HF in the patients with RVO in the near future.
In conclusion, this study demonstrated that the numbers of baseline HF in outer retinal layer were associated with the final BCVA in patients with ME due to CRVO and BRVO, suggesting HF as a biomarker of poor final visual outcome. However, the numbers of HF in the inner retinal layer and in the vitreous cavity were not associated with final BCVA. The numbers of HF in outer retinal layer reduced significantly in non-ischemic CRVO and BRVO after anti-VEGF treatment, but in ischemic CRVO, they were not reduced significantly.
Acknowledgments
Conflicts of Interest: Mo B, None; Zhou HY, None; Jiao X, None; Zhang F, None.
REFERENCES
- 1.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers S, Mclntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, Kowalski JW, Nguyen H, Wong TY, International ye Disease Consortium The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117(2):313–319. doi: 10.1016/j.ophtha.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esrick E, Subramanian ML, Heier JS, Devaiah AK, Topping TM, Frederick AR, Morley MG. Multiple laser treatments for macular edema attributable to branch retinal vein occlusion. Am J Ophthalmol. 2005;139(4):653–657. doi: 10.1016/j.ajo.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Arnarsson A, Stefánsson E. Laser treatment and the mechanism of edema reduction in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 2000;41(3):877–879. [PubMed] [Google Scholar]
- 5.Cekiç O, Chang S, Tseng JJ, Barile GR, Del Priore LV, Weissman H, Schiff WM, Ober MD. Intravitreal triamcinolone injection for treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2005;25(7):851–855. doi: 10.1097/00006982-200510000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, Murahashi WY, Rubio RG. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1102–1112. doi: 10.1016/j.ophtha.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Chen SD, Sundaram V, Lochhead J, Patel CK. Intravitreal triamcinolone for the treatment of ischemic macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2006;141(5):876–883. doi: 10.1016/j.ajo.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Scott IU, Ip MS, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, Chan CK, Gonzalez VH, Singerman LJ, Tolentino M. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the standard care vs corticosteroid for retinal vein occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127(9):1115–1128. doi: 10.1001/archophthalmol.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandelcorn MS, Nrusimhadevara RK. Internal limiting membrane peeling for decompression of macular edema in retinal vein occlusion: a report of 14 cases. Retina. 2004;24(3):348–355. doi: 10.1097/00006982-200406000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Wolf S, Wolf-Schnurrbusch U. Spectral-domain optical coherence tomography use in macular diseases: a review. Ophthalmologica. 2010;224(6):333–340. doi: 10.1159/000313814. [DOI] [PubMed] [Google Scholar]
- 11.Bolz M, Schmidt-Erfurth U, Deak G, Mylonas G, Kriechbaum K, Scholda C. Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology. 2009;116(5):914–920. doi: 10.1016/j.ophtha.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa T, Masuda N, Ogata N. Highly reflective line in optical coherence tomography images of eyes with macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2015;159(5):925–933. doi: 10.1016/j.ajo.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Lee H, Lee J, Chung H, Kim HC. Baseline special domain optical coherence tomographic hyperreflective foci as a predictor of visual outcome and recurrence for central serous choroidoretinopathy. Retina. 2016;36(7):1372–1380. doi: 10.1097/IAE.0000000000000929. [DOI] [PubMed] [Google Scholar]
- 14.Nishijima K, Murakami T, Hirashima T, Uji A, Akagi T, Horii T, Ueda-Arakawa N, Muraoka Y, Yoshimura N. Hyperreflective foci in outer retina predictive of photoreceptor damage and poor vision after vitrectomy for diabetic macular edema. Retina. 2014;34(4):732–740. doi: 10.1097/IAE.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 15.Lee H, Ji B, Chung H, Kim HC. Correlation between optical coherence tomographic hyperreflective foci and visual outcomes after anti-VEGF treatment in neovascular age-related macular degeneration and polypoidalchoroidal vasculopathy. Retina. 2016;36(3):465–475. doi: 10.1097/IAE.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 16.Piri N, Nesmith BL, Schaal S. Choroidal hyperreflective foci in Stargardt disease shown by spectral-domain optical coherence tomography imaging: correlation with disease severity. JAMA Ophthalmol. 2015;133(4):398–405. doi: 10.1001/jamaophthalmol.2014.5604. [DOI] [PubMed] [Google Scholar]
- 17.Kuroda M, Hirami Y, Hata M, Mandai M, Takahashi M, Kurimoto Y. Intraretinal hyperreflective foci on spectral-domain optical coherence tomographic images of patients with retinitis pigmentosa. Clinical Ophthalmology. 2014;8:435–440. doi: 10.2147/OPTH.S58164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogino K, Murakami T, Tsujikawa A, Miyamoto K, Sakamoto A, Ota M, Yoshimura N. Characteristics of optical coherence tomographic hyperreflective foci in retinal vein occlusion. Retina. 2012;32(1):77–85. doi: 10.1097/IAE.0b013e318217ffc7. [DOI] [PubMed] [Google Scholar]
- 19.Christenbury JG, Folgar FA, O Connell RV, Chiu SJ, Farsiu S, Toth CA. Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology. 2013;120(5):1038–1045. doi: 10.1016/j.ophtha.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coscas G, De Benedetto U, Coscas F, Li Calzi CI, Vismara S, Roudot-Thoraval F, Bandello F, Souied E. Hyperreflective dots: a new spectral-domain optical coherence tomography entity for follow-up and prognosis in exudative age-related macular degeneration. Ophthalmologica. 2013;229:32–37. doi: 10.1159/000342159. [DOI] [PubMed] [Google Scholar]
- 21.Chatziralli IP, Sergentanis TN, Sivaprasad S. Hyperreflective foci as an independent visual outcome predictor in macular edema due to retinal vascular diseases treated with intravitreal dexamethasone or ranibizumab. Retina. 2016;36(12):2319–2328. doi: 10.1097/IAE.0000000000001070. [DOI] [PubMed] [Google Scholar]
- 22.De Benedetto U, Sacconi R, Pierro L, Lattanzio R, Bandello F. Optical coherence tomographic hyperreflective foci in early stages of diabetic retinopathy. Retina. 2015;35(3):449–453. doi: 10.1097/IAE.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 23.Uji A, Murakami T, Nishijima K, Akagi T, Horii T, Arakawa N, Muraoka Y, Ellabban AA, Yoshimura N. Association between hyperreflective foci in the outer retina, status of photoreceptor layer, and visual acuity in diabetic macular edema. Am J Ophthalmol. 2012;153(4):710–717. doi: 10.1016/j.ajo.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 24.Framme C, Schweizer P, Imesch M, Wolf S, Wolf-Schnurrbusch U. Behavior of SD-OCT-detected hyperreflective foci in the retina of anti-VEGF-treated patients with diabetic macular edema. Invest Ophthalmol Vis Sci. 2012;53(9):5814–5818. doi: 10.1167/iovs.12-9950. [DOI] [PubMed] [Google Scholar]
- 25.Kang JW, Lee H, Chung H, Kim HC. Correlation between optical coherence tomographic hyperreflective foci and visual outcomes after intravitrealbevacizumab for macular edema in branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2014;252(9):1413–1421. doi: 10.1007/s00417-014-2595-5. [DOI] [PubMed] [Google Scholar]
- 26.Akagi-Kurashige Y, Tsujikawa A, Oishi A, Ooto S, Yamashiro K, Tamura H, Nakata I, Ueda-Arakawa N, Yoshimura N. Relationship between retinal morphological findings and visual function in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2012;250(8):1129–1136. doi: 10.1007/s00417-012-1928-5. [DOI] [PubMed] [Google Scholar]
- 27.Chen KC, Jung JJ, Curcio CA, Balaratnasingam C, Gallego-Pinazo R, Dolz-Marco R, Freund KB, Yannuzzi LA. Intraretinal hyperreflective foci in acquired vitelliform lesions of the macula: clinical and histologic study. Am J Ophthalmol. 2016;164(4):89–98. doi: 10.1016/j.ajo.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Murakami T, Uji A, Ogino K, Unoki N, Yoshitake S, Dodo Y, Horii T, Nishijima K, Yoshimura N. Macular morphologic findings on optical coherence tomography after microincision vitrectomy for proliferative diabetic retinopathy. Jpn J Ophthalmol. 2015;59(4):236–243. doi: 10.1007/s10384-015-0382-4. [DOI] [PubMed] [Google Scholar]
- 29.Korot E, Comer G, Steffens T, Antonetti DA. Algorithm for the measure of vitreous hyperreflective foci in optical coherence tomographic scans of patients with diabetic macular edema. JAMA Ophthalmol. 2016;134(1):15–20. doi: 10.1001/jamaophthalmol.2015.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pakzad-Vaezi K, Or C, Yeh S, Forooghian F. Optical coherence tomography in the diagnosis and management of uveitis. Can J Ophthalmol. 2014;49(1):18–29. doi: 10.1016/j.jcjo.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Saito M, Barbazetto IA, Spaide RF. Intravitreal cellular infiltrate imaged as punctate spots by spectral-domain optical coherence tomography in eyes with posterior segment inflammatory disease. Retina. 2013;33(3):559–565. doi: 10.1097/IAE.0b013e31826710ea. [DOI] [PubMed] [Google Scholar]
- 32.Tsujikawa A, Sakamoto A, Ota M, Kotera Y, Oh H, Miyamoto K, Kita M, Yoshimura N. Serous retinal detachment associated with retinal vein occlusion. Am J Ophthalmol. 2010;149(2):291–301. doi: 10.1016/j.ajo.2009.09.007. [DOI] [PubMed] [Google Scholar]