Abstract
AIM
To investigate the association of Manganese superoxide dismutase (MnSOD) Val16Ala polymorphism with diabetic retinopathy (DR).
METHODS
PubMed, Embase, China National Knowledge Infrastructure, and Wanfang databases were searched. The pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the strength of the association. Subgroup, sensitivity, and cumulative analyses were performed. Publication bias was also analyzed.
RESULTS
Eight studies were included in the pooled analysis. The MnSOD Val16Ala polymorphism was associated with the risk of DR under the dominant model (OR=0.66, 95%CI=0.48-0.91, P<0.0001), this result was demonstrated to be relatively stable in cumulative analysis. No significant publication bias was found. This polymorphism was also associated with the risk of DR in Caucasians under the dominant model (OR=0.64, 95%CI=0.42-0.97, P=0.04,) and in Asians under the recessive model (OR=0.31, 95%CI=0.11-0.88, P=0.03).
CONCLUSION
These findings suggest that the MnSOD Val16Ala polymorphism is a risk factor for DR, and that more attention should be paid to carriers of these susceptibility genes.
Keywords: diabetic, retinopathy, MnSOD, Val16Ala
INTRODUCTION
Diabetic retinopathy (DR) is a microvascular complication of diabetes affecting the retina and a leading cause of blindness worldwide[1]. DR is generally associated with the diabetes duration and poor glycemic control, but several studies have indicated that variations in the onset and severity of DR are not totally explained by known risk factors[2]–[3].
An important reason for DR is damage to vessel endothelia due to oxidative stress[2],[4]. It was demonstrated that oxidative stress plays a major role in the development and persistence of DR even after establishing adequate glycemic control[5]. Kowluru[6] also found that the enzymatic mechanisms involved in protection against oxidative stress were impaired in the retinas of diabetic rats. A high level of reactive oxygen species (ROS) due to oxidative stress is assumed to damage the mitochondrial DNA and influence the respiratory chain via the lipid peroxidation of nerve cells[7]–[9]. The overproduction of ROS (especially superoxide) by the mitochondrial electron-transport chain is prevented by overexpression of the manganese superoxide dismutase (MnSOD) enzyme, which catalyses the dismutation of superoxide radicals into hydrogen peroxide and hence protects retinal endothelial cells from oxidative damage[2],[10].
These results suggest that the enzyme activity of MnSOD could be related to the development of DR. Val16Ala is a single-nucleotide polymorphism in MnSOD that has been demonstrated to be functionally significant[11]. It was reported that the Val allele disrupts the alpha-helix structure of MnSOD. The protein encoded by Val was thus retained at the level of the mitochondrial inner membrane, and has been associated with lower enzyme activity, resulting in increased susceptibility to oxidative stress[11]. Therefore, Val16Ala may cause inter individual differences in the susceptibility to DR.
Many epidemiological studies performed in different countries have investigated the association between Val16Ala and susceptibility to DR during the past few decades, but these studies have yielded some controversial results, and there has been no systematic or quantitative assessment of published findings on this topic. Therefore, a Meta-analysis of case-control studies was conducted to summarize the available evidence on this issue.
MATERIALS AND METHODS
This Meta-analysis was designed according to the guidelines described in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement[12].
Data Sources and Selection of the Studies
Relevant studies were identified by manually searching the following databases: PubMed, Embase, China National Knowledge Infrastructure, and Wanfang. Articles published before August 27, 2015 were screened using the following search terms: diabetic retinopathy (diabetic microangiopathy) AND manganese superoxide dismutase (MnSOD OR SOD2 OR SOD-2) AND polymorphism (genetic OR mutation OR variation OR variant OR genotype). Only journal articles were included, and the publication languages were restricted to English and Chinese. All references cited in the included articles were also reviewed to identify additional relevant work.
Eligibility Criteria
All relevant studies were independently reviewed by two authors. The following study selection criteria were used: using a case-control design; investigating the association of the Ala16Val polymorphism with the risk of retinopathy in diabetic patients; and presenting sufficient data on the distribution of the Ala16Val polymorphism in patients with and without DR to calculate odds ratios (ORs) and 95% confidence intervals (CIs), or data necessary to calculate these parameters. When multiple publications related to the same study were available, we used the publication with the largest number of cases and the most applicable information.
Data Extraction
For each study that met the inclusion criteria, the following data were abstracted by two authors independently: first author, publication year, race(s) of study population, genotyping method, number of cases and controls with different genotypes, conformity with Hardy-Weinberg equilibrium (HWE) for controls, and study quality assessment.
Quality Assessment
Two of the authors independently assessed the quality of the included studies. The quality scoring criteria were modified from the Meta-analysis performed by Niu et al[13] that produced a scale for quality assessment used in observational studies of genetic epidemiology. The item “Source of controls” was replaced by “Definition of the DR,” because the control group in the present study comprised diabetic patients without retinopathy, and a clear definition of DR would promote the authenticity of the results and reflect the quality of publications to some extent. The scores for the modified criteria ranged from 0 points (the worst) to 9 points (the best). Studies with a score of <6 were classified as low quality, and were not included in the subsequent Meta-analysis.
Statistical Analysis
STATA (version 11.0, Stata Corporation, College Station, TX, USA) was used to perform statistical analyses. The pooled OR and 95%CI values were calculated to evaluate the strength of the association between the Ala16Val polymorphism and the risk of retinopathy in diabetic patients. The Meta-analysis examined the overall association under the dominant model (Ala/Ala+Val/Ala vs Val/Val), recessive model (Ala/Ala vs Val/Ala+Val/Val), and additive model (Ala vs Val). Subgroup analyses were performed according to race and conformity with HWE in controls.
The presence of heterogeneity was assessed using Cochran's Q statistic and quantified using the I2 statistic. A P value of <0.1 or I2>40% indicates the presence of a very high degree of heterogeneity[13]–[14]. The overall pooled OR and 95% CI values were estimated using the Mantel-Haenszel method with a fixed-effects model. Otherwise, the Der Simonian and Laird method with a random-effects model was used. The significance of the pooled OR was determined by a Z test. The cutoff for statistical significance was set at P<0.05.
Cumulative Meta-analyses according to the accuracy or publication year of single studies were used to evaluate the change trends in the results. In addition, the overall robustness of the results was assessed by analyzing the influence of single studies.
Publication bias was analyzed using funnel plots. The absence of obvious publication bias was suggested when the data in a funnel plot were distributed roughly symmetrically, and vice versa[15]. Egger's linear regression was used to test the symmetry of the funnel plot, and a probability value of P<0.05 was considered suggestive of poor symmetry.
RESULTS
Study Characteristics
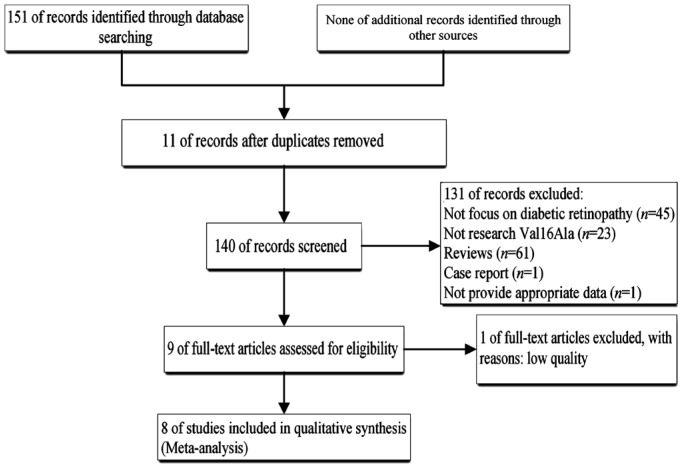
A systematic review of the literature identified 151 relevant studies. Figure 1 shows the study flow diagram (PRISMA template). Duplicate studies, studies not focusing on DR, studies not investigating the Val16Ala polymorphism, studies not providing appropriate data, and reviews involving low-quality studies were excluded (n=142), which meant that nine studies meet the inclusion criteria. Ye et al[16] investigated the association between the Val16Ala polymorphism and the risk of retinopathy in diabetic patients with sufficient data, but its quality was low, and so this study was excluded. Therefore, eight studies[2],[9],[17]–[22] were finally included in the quantitative analysis.
Figure 1. Flow chat of study selection.
The characteristics of the nine included articles (including the low-quality studies[16]) along with their quality assessment scores are listed in Table 1. Eight studies were included in the Meta-analysis of the role of the Val16Ala polymorphism in the risk of retinopathy in diabetics, and this included 1235 cases and 1051 controls. Five of the eight studies focused on Caucasian populations[2],[9],[17]–[18],[20], while the other three focused on Asian populations[19],[21]–[22]. The Val16Ala distribution in controls deviated from HWE in three studies[9],[17],[19].
Table 1. Characteristic of included studies.
| Authors | Year | Ethnicity | Genotype method | Genotype in DR(+) |
Genotype in DR(-) |
HWEa | Quality score | ||||
| VV | VA | AA | VV | VA | AA | ||||||
| Zhao et al[22] | 2006 | Asian (Chinese) | PCR-RFLP | 80 | 5 | 2 | 64 | 16 | 3 | 0.141 | 7 |
| Jia et al[21] | 2004 | Asian (Chinese) | PCR-RFLP | 84 | 31 | 1 | 62 | 24 | 3 | 0.721 | 7 |
| Lee et al[19] | 2006 | Asian (Korean) | PCR-RFLP | 105 | 23 | 2 | 138 | 24 | 12 | <0.01 | 7 |
| Petrovic et al[20] | 2008 | Caucasian (Slovene) | PCR-RFLP | 80 | 140 | 63 | 23 | 69 | 51 | 0.967 | 8 |
| Hovnik et al[18] | 2009 | Caucasian | TaqMan | 11 | 15 | 6 | 16 | 50 | 26 | 0.825 | 7 |
| Kangas-Kontio et al[2] | 2009 | Caucasian (Finlander) | TaqMan | 30 | 56 | 40 | 29 | 49 | 18 | 0.736 | 7 |
| Vanita et al[9] | 2014 | Caucasian (North Indian) | TaqMan | 127 | 218 | 101 | 58 | 194 | 60 | <0.01 | 7 |
| Houldsworth et al[17] | 2015 | Caucasian (UK) | PCR-RFLP | 5 | 9 | 1 | 15 | 40 | 7 | 0.014 | 6 |
| Ye et al[16] | 2008 | Asian (Chinese) | PCR-RFLP | 99 | 8 | 18 | 93 | 10 | 36 | <0.01 | 5 |
aP value for HWE in control.
Meta-analysis Results
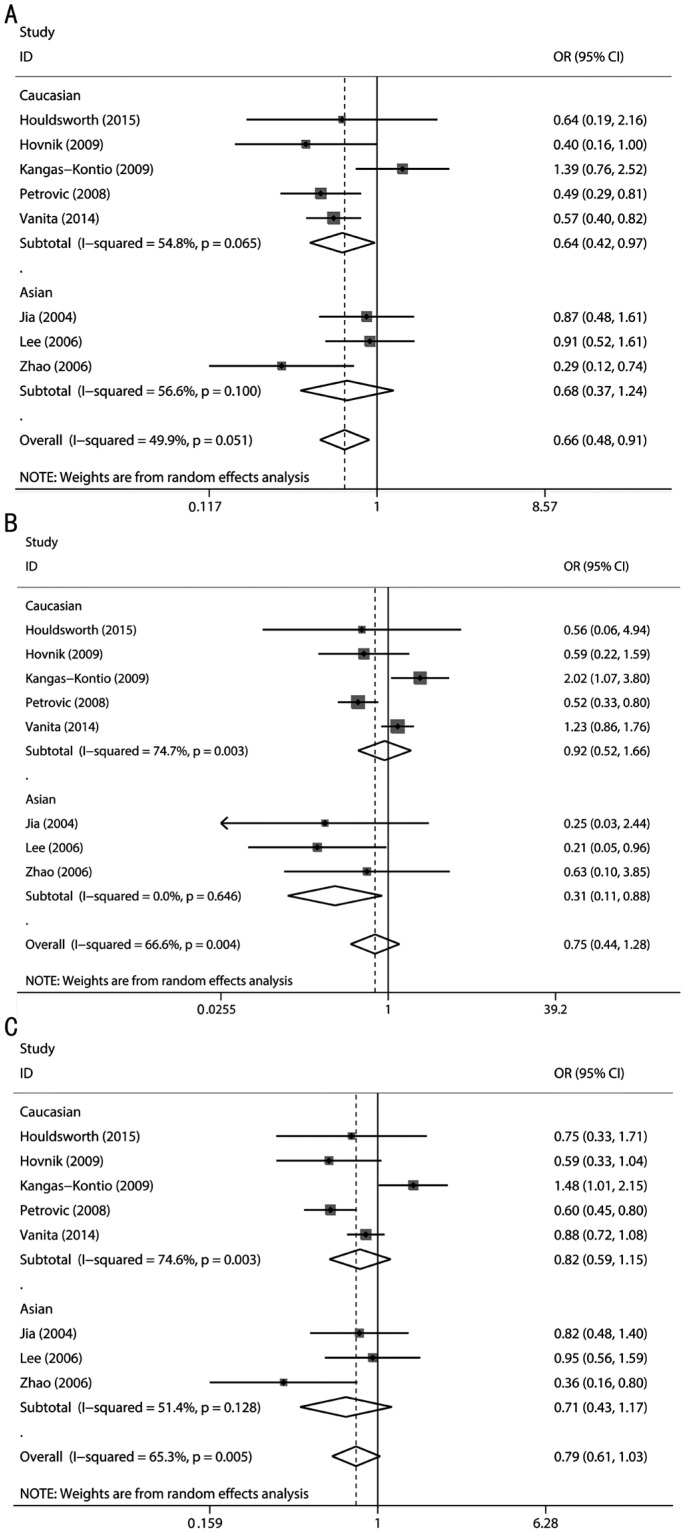
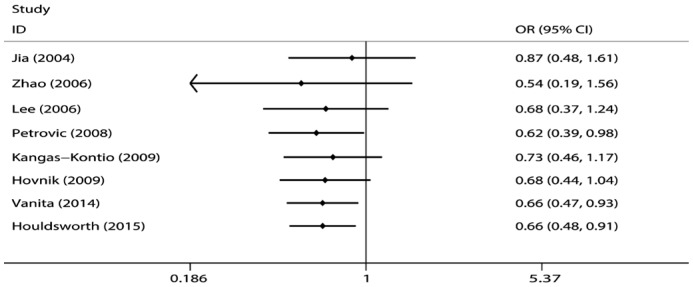
The combined analyses of the eight studies revealed that the Val16Ala polymorphism was associated with the risk of DR under the dominant model (OR=0.66, 95%CI=0.48-0.91, P<0.0001) but not under the recessive model (OR=0.75, 95%CI=0.44-1.28, P=0.29) or the additive model (OR=0.79, 95%CI=0.61-1.03, P=0.08). The level of heterogeneity in all three models was high: P=0.051 (I2=49.9%), P=0.004 (I2=66.6%), and P=0.005 (I2=65.3%) for the dominant, recessive, and addictive models, respectively; a random effect was therefore used in the above three genetic models (Figure 2).
Figure 2. Calculated OR and 95%CIs for the associations between MnSOD Val16Ala polymorphism and DR risk for ethnicity subgroup.
A: Dominant model; B: Recessive model; C: Additive model.
Subgroup Analysis
Subgroup analysis according to race showed that the Val16Ala polymorphism was associated with the risk of DR in Caucasians under the dominant model (OR=0.64, 95%CI=0.42-0.97, P=0.04) and in Asians under the recessive model (OR=0.31, 95%CI=0.11-0.88, P=0.03) (Figure 2).
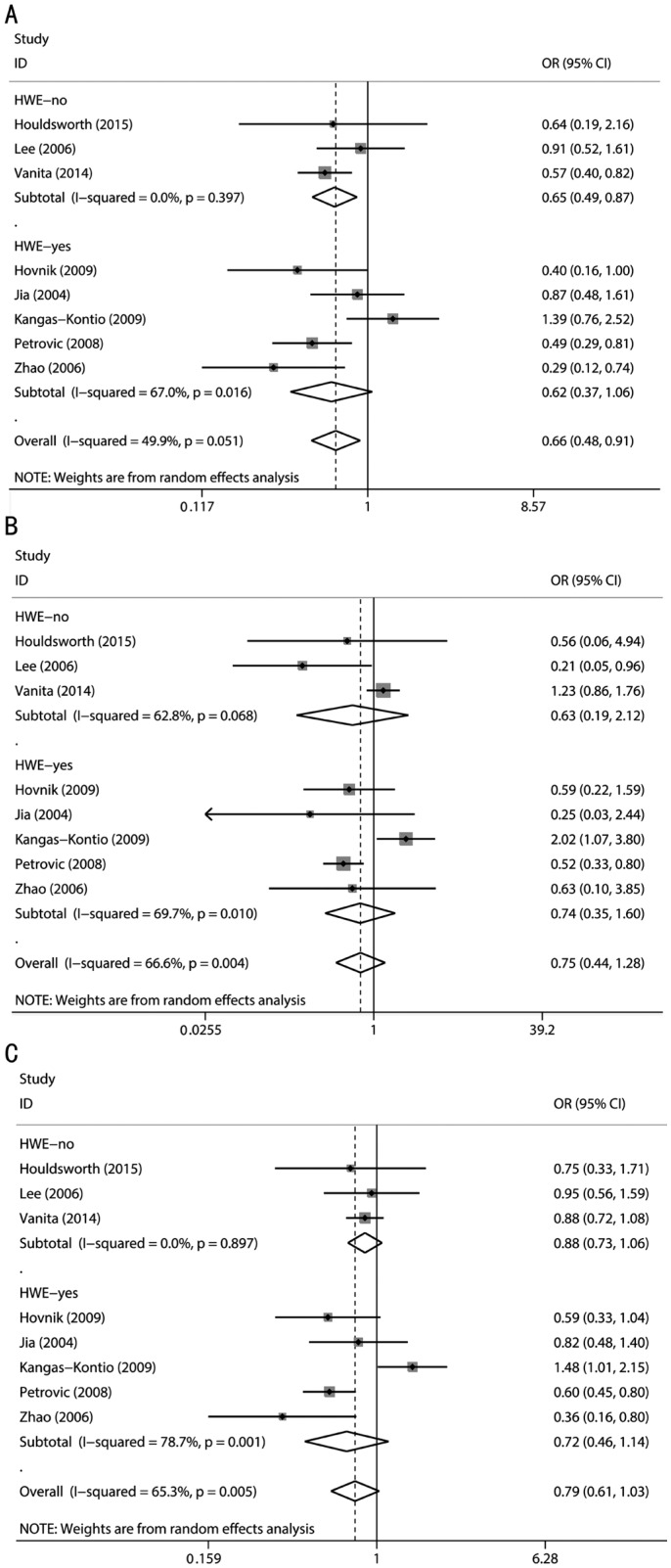
Subgroup analyses accounting for conformity with HWE in controls were also performed. A pooled analysis of three studies [9],[17],[19] in which the Val16Ala distribution in controls deviated from HWE revealed an association of the Val16Ala polymorphism with the risk of DR under the dominant model (OR=0.65, 95%CI=0.49-0.87, P=0.004). A Meta-analysis of the remaining five studies[2],[18],[20]–[22] in which the Val16Ala distribution in controls did not deviate from HWE found no association of the polymorphism with the risk of DR (Figure 3).
Figure 3. Calculated OR and 95% CIs for the associations between MnSOD Val16Ala polymorphism and DR risk for HWE status of controls subgroup.
A: Dominant model; B: Recessive model; C: Additive model.
Analyzing the Influence of Single Studies
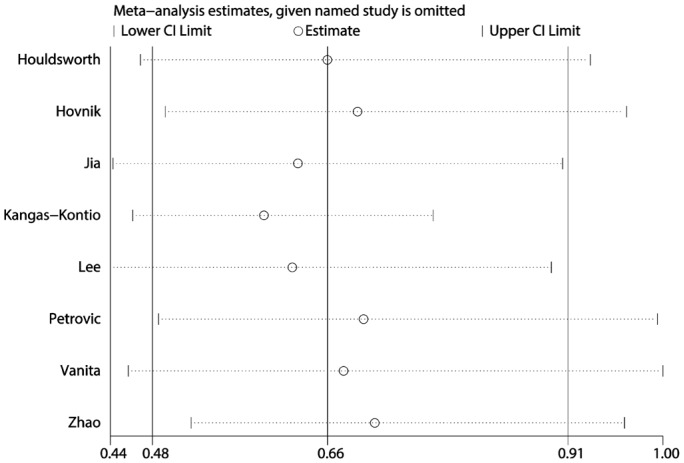
A sensitivity analysis of the dominant model was performed by analyzing the influence of single studies individually (Figure 4). We found that including the study of Vanita[9] greatly impacted the total pooled effect size. Excluding this study led to a change in the pooled effect size from 0.66 (0.48-0.91) to 0.68 (0.46-1.004), and a loss of the significant association between the Val16Ala polymorphism and the risk of retinopathy in diabetics. Also, for the recessive model and the allele contrast model, the study of Kangas-Kontio et al[2] greatly impacted the total pooled effect size.
Figure 4. Sensitivity analysis via deletion of each individual study reflects the relative influence of each individual dataset on the pooled ORs under the dominant model of MnSOD Val16Ala polymorphism.
Cumulative Analysis
Cumulative analysis by publication date demonstrated that the risk of DR decreased gradually and became positive, which is in accordance with the findings of Vanita[9] (Figure 5 for the dominant model). Under the recessive and additive models, the risk of DR showed a stable trend with publication date.
Figure 5. Cumulative Meta-analyses according to publication year under the dominant model of MnSOD Val16Ala polymorphism.
Publication Bias
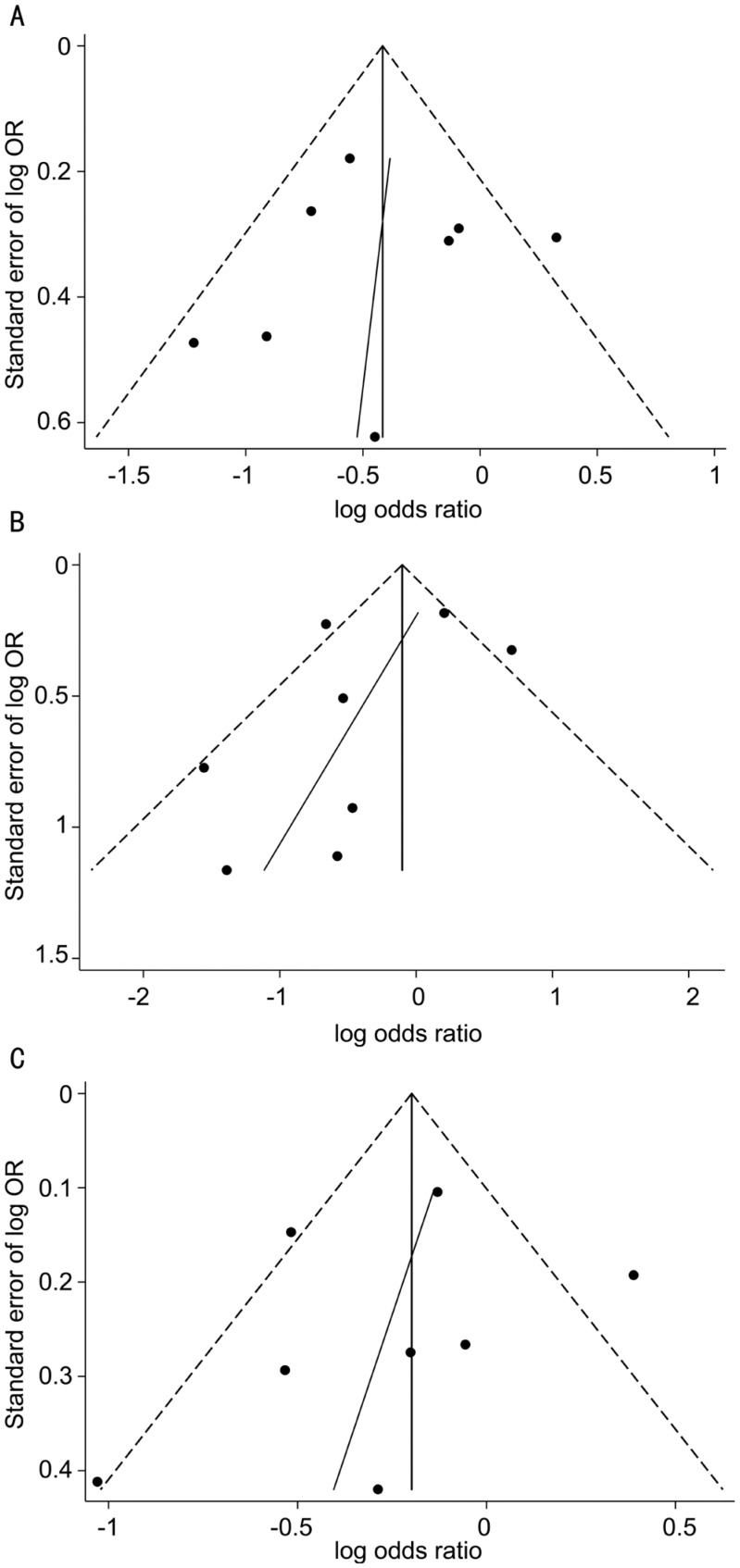
The evaluation of publication bias using funnel plots found no evidence of obvious asymmetry (Figure 6). The results were further verified by Egger's test: P=0.845, 0.32, and 0.577 for the dominant, recessive, and additive models, respectively. All of these suggested there was no obvious publication bias for the Meta-analysis under the three genetic models.
Figure 6. Funnel plot analysis with Egger regression line to detect publication bias.
A: Dominant model; B: Recessive model; C: Additive model.
DISCUSSION
The results from this comprehensive Meta-analysis of case-control studies suggest that the MnSOD Val16Ala polymorphism is a risk factor for retinopathy in diabetics under a dominant model. Subgroup analysis according to race also showed significant associations under different genetic models: in Caucasians under the dominant model and in Asians under the recessive model. The main results of this Meta-analysis were stable according to verification performed in a cumulative analysis, and moreover no obvious publication was found.
The risk factors for DR have been studied widely. Ting et al[23] concluded that the modifiable risk factors include hyperglycemia, hypertension, hyperlipidemia, obesity, duration of diabetes, puberty, pregnancy, cataract surgery, inflammatory biomarkers, and genetic risk factors. The severity and rapidity of DR onset have previously been associated with several genetic factors[24], including the ALR2 (aldose reductase gene), RAGE (receptor for advance glycation end products) gene, TGF-beta1 (transforming growth factor beta 1) gene, VEGF (vascular endothelial growth factor) gene, eNOS (endothelial nitric oxide synthase) gene, and IGF-I (insulin-like growth factor 1) gene[23]. MnSOD is also a widely studied gene[2],[9],[16]–[22]. However, these associations were found to be weak, inconsistent, and with a lack of standardization of the DR phenotype across different populations. It is difficult to draw any conclusions from these studies also because the individual studies often included small samples. The present study applied a Meta-analysis involving 1235 cases and 1051 controls, and found that the MnSOD polymorphism was associated with the risk of DR, thereby verifying the role of the MnSOD gene in the development of DR. Moreover, the statistical power of the Meta-analysis was stronger than that in any of the included studies.
The heterogeneity in this Meta-analysis was a little high, and was still present even after subgroup analysis according to race and conformity with HWE in controls. The sources of heterogeneity can generally be found by Meta-regression, but this method could not be performed due to the limitations associated with the small number of included studies. Therefore, random-effects models were used, which assume different underlying effects, considering both within- and between-study variations, offering an advantage that this accommodates diversity between studies and provides more conservative estimates of the assessed effects[25].
Subgroup analysis by race revealed that the Val16Ala polymorphism influenced the risk of DR in Caucasians and Asians under the dominant and recessive models, respectively. The disparity between races may reflect the existence of a true population-specific disease variant, but may also be attributable to differences in genomic structure at these loci between populations. In additional, the number of included studies, especially for Asians, was too small to draw stable conclusions.
The study of Vanita[9] was identified as an outlier for the dominant model in the sensitivity analysis. There was no obvious difference in study design between that study and the other included studies, and its outlier nature was attributed to the race of the included population. Cumulative analysis showed that the number of studies on the same topic increased as time passed, causing the association between the Val16Ala polymorphism and the risk of DR to become more significant under the dominant model. However, the significant association only appeared for the cumulative data at 2014, which suggested that more similar studies are still needed to verify the stability of the association.
DR and diabetic nephropathy are the two common forms of diabetic microangiopathy[26]. The retina is particularly susceptible to oxidative stress because of its high consumption of oxygen, high proportion of polyunsaturated fatty acids, and exposure to visible light[20]. The present study found an effect of MnSOD-an enzyme associated with oxidative stress-on DR susceptibility. Oxidative stress also contributes to the development of diabetic nephropathy[27], and Mollsten et al[28] found that the Val/Val genotype combined with smoking is associated with diabetic nephropathy in type 1 diabetes. However, few published studies have focused on diabetic nephropathy and MnSOD polymorphism, thus limiting the ability to perform a Meta-analysis to obtain more reliable conclusions.
The results presented herein increase the knowledge about genetic risk factors underlying the development of retinopathy in diabetes. We found that the frequency of the Val/Val genotype was higher in retinopathy cases than in diabetes without retinopathy, which suggests that diabetic patients carrying the Val/Val genotype have a greater risk of developing retinopathy. For such diabetic patients, early prevention is very important.
The results of this Meta-analysis should be interpreted with caution due to some limitations in our analysis. Firstly, only a small number of studies were included in the Meta-analyses. Secondly, selection bias was a possible major source of heterogeneity from uncontrolled confounders and bias inherent in the study design, and the direction and magnitude of this bias are uncertain. For example, identical criteria were not clearly applied to the cases and controls in most of the included studies. Thirdly, there is a possibility of interactions between the MnSOD Val16Ala polymorphism and other environmental factors, such as the glucose level and smoking status, and these were not taken into account.
In summary, the present Meta-analysis supports that the MnSOD Val16Ala polymorphism is positively associated with the risk of retinopathy in diabetes. Future studies are required that involve larger samples and incorporate both the effects of environmental agents and genotypes of enzymes related to oxidative stress.
Acknowledgments
Foundation: Supported by the Natural Science Foundation of Shaanxi Province (No.2015JM8415).
Conflicts of Interest: Huang L, None; Lyu J, None; Liu QP, None; Chen C, None; Wang T, None.
REFERENCES
- 1.See JL, Wong TY, Yeo KT. Trends in the pattern of blindness and major ocular diseases in Singapore and Asia. Ann Acad Med Singapore. 1998;27(4):540–546. [PubMed] [Google Scholar]
- 2.Kangas-Kontio T, Vavuli S, Kakko SJ, Penna J, Savolainen ER, Savolainen MJ, Liinamaa MJ. Polymorphism of the manganese superoxide dismutase gene but not of vascular endothelial growth factor gene is a risk factor for diabetic retinopathy. Br J Ophthalmol. 2009;93(10):1401–1406. doi: 10.1136/bjo.2009.159012. [DOI] [PubMed] [Google Scholar]
- 3.Uhlmann K, Kovacs P, Boettcher Y, Hammes HP, Paschke R. Genetics of diabetic retinopathy. Exp Clin Endocrinol Diabetes. 2006;114(6):275–294. doi: 10.1055/s-2006-924260. [DOI] [PubMed] [Google Scholar]
- 4.Naudi A, Jove M, Ayala V, Cassanye A, Serrano J, Gonzalo H, Boada J, Prat J, Portero-Otin M, Pamplona R. Cellular dysfunction in diabetes as maladaptive response to mitochondrial oxidative stress. Exp Diabetes Res. 2012;2012:696215. doi: 10.1155/2012/696215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowluru RA, Kern TS, Engerman RL. Abnormalities of retinal metabolism in diabetes or experimental galactosemia. IV. Antioxidant defense system. Free Radic Biol Med. 1997;22(4):587–592. doi: 10.1016/s0891-5849(96)00347-4. [DOI] [PubMed] [Google Scholar]
- 6.Kowluru RA. Effect of reinstitution of good glycemic control on retinal oxidative stress and nitrative stress in diabetic rats. Diabetes. 2003;52(3):818–823. doi: 10.2337/diabetes.52.3.818. [DOI] [PubMed] [Google Scholar]
- 7.Guidot DM, McCord JM, Wright RM, Repine JE. Absence of electron transport (Rho 0 state) restores growth of a manganese-superoxide dismutase-deficient Saccharomyces cerevisiae in hyperoxia. Evidence for electron transport as a major source of superoxide generation in vivo. J Biol Chem. 1993;268(35):26699–26703. [PubMed] [Google Scholar]
- 8.Low PA, Nickander KK, Tritschler HJ. The roles of oxidative stress and antioxidant treatment in experimental diabetic neuropathy. Diabetes. 1997;46(Suppl 2):S38–S42. doi: 10.2337/diab.46.2.s38. [DOI] [PubMed] [Google Scholar]
- 9.Vanita V. Association of RAGE (p.Gly82Ser) and MnSOD (p.Val16Ala) polymorphisms with diabetic retinopathy in T2DM patients from north India. Diabetes Res Clin Pract. 2014;104(1):155–162. doi: 10.1016/j.diabres.2013.12.059. [DOI] [PubMed] [Google Scholar]
- 10.Kowluru RA, Atasi L, Ho YS. Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2006;47(4):1594–1599. doi: 10.1167/iovs.05-1276. [DOI] [PubMed] [Google Scholar]
- 11.Sutton A, Imbert A, Igoudjil A, Descatoire V, Cazanave S, Pessayre D, Degoul F. The manganese superoxide dismutase Ala16Val dimorphism modulates both mitochondrial import and mRNA stability. Pharmacogenet Genomics. 2005;15(5):311–319. doi: 10.1097/01213011-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu YM, Du XY, Cai HX, Zhang C, Yuan RX, Zeng XT, Luo J. Increased risks between Interleukin-10 gene polymorphisms and haplotype and head and neck cancer: a meta-analysis. Sci Rep. 2015;5:17149. doi: 10.1038/srep17149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.He H, He G, Wang T, Cai J, Wang Y, Zheng X, Dong Y, Lu J. Methylenetetrahydrofolate reductase gene polymorphisms contribute to acute myeloid leukemia and chronic myeloid leukemia susceptibilities: evidence from meta-analyses. Cancer Epidemiol. 2014;38(5):471–478. doi: 10.1016/j.canep.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Ye LX, Yang MP, Qiu H, Guo KQ, Yan JS. Association of the polymorphism in manganese superoxide dismutase gene with diabetic retinopathy in Chinese type 2 diabetic patients. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2008;25(4):452–454. [PubMed] [Google Scholar]
- 17.Houldsworth A, Hodgkinson A, Shaw S, Millward A, Demaine AG. Polymorphic differences in the SOD-2 gene may affect the pathogenesis of nephropathy in patients with diabetes and diabetic complications. Gene. 2015;569(1):41–45. doi: 10.1016/j.gene.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Hovnik T, Dolzan V, Bratina NU, Podkrajsek KT, Battelino T. Genetic polymorphisms in genes encoding antioxidant enzymes are associated with diabetic retinopathy in type 1 diabetes. Diabetes Care. 2009;32(12):2258–2262. doi: 10.2337/dc09-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SJ, Choi MG. Association of manganese superoxide dismutase gene polymorphism (V16A) with diabetic macular edema in Korean type 2 diabetic patients. Metabolism. 2006;55(12):1681–1688. doi: 10.1016/j.metabol.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Petrovic MG, Cilensek I, Petrovic D. Manganese superoxide dismutase gene polymorphism (V16A) is associated with diabetic retinopathy in Slovene (Caucasians) type 2 diabetes patients. Dis Markers. 2008;24(1):59–64. doi: 10.1155/2008/940703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia YK. Tianjin Medical University; 2004. Study on the relationship between polymorphism of manganese superoide dismutase gene and diabetic microvascular complications in type 2 diabetes mellitus. [Google Scholar]
- 22.Zhao Z, Liu LM, Zheng TS. Correlation of gene polymorphism of manganese superoxide dismutase and type 2 diabetic retinopathy. Journal of Shanghai Jiaotong University (Medical Science) 2006;26(9):965–967. [Google Scholar]
- 23.Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44(4):260–277. doi: 10.1111/ceo.12696. [DOI] [PubMed] [Google Scholar]
- 24.Kumaramanickavel G, Sripriya S, Ramprasad VL, Upadyay NK, Paul PG, Sharma T. Z-2 aldose reductase allele and diabetic retinopathy in India. Ophthalmic Genet. 2003;24(1):41–48. doi: 10.1076/opge.24.1.41.13889. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Lopez E, Martin-Guerrero I, Ballesteros J, Garcia-Orad A. A systematic review and meta-analysis of MTHFR polymorphisms in methotrexate toxicity prediction in pediatric acute lymphoblastic leukemia. Pharmacogenomics J. 2013;13(6):498–506. doi: 10.1038/tpj.2012.44. [DOI] [PubMed] [Google Scholar]
- 26.Henzen C. Diabetic Retinopathy and Neuropathy: New in 2015. Praxis (Bern 1994) 2015;104(12):631–634. doi: 10.1024/1661-8157/a002024. [DOI] [PubMed] [Google Scholar]
- 27.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57(6):1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 28.Mollsten A, Marklund SL, Wessman M, Svensson M, Forsblom C, Parkkonen M, Brismar K, Groop PH, Dahlquist G. A functional polymorphism in the manganese superoxide dismutase gene and diabetic nephropathy. Diabetes. 2007;56(1):265–269. doi: 10.2337/db06-0698. [DOI] [PubMed] [Google Scholar]