Abstract
AIM
To see how patterns of care changed over time, and how institution type effected these decisions.
METHODS
A retrospective analysis was performed using the National Cancer Database, looking at all patients that were diagnosed with rectal cancer from 1998 to 2011. We tested differences in rates of treatment and stage migration using χ2 tests and logistic regression models.
RESULTS
A review of ninety thousand five hundred and ninety four subjects underwent multimodality therapy for cancer of the rectum. Staging and response to treatment varied greatly between centers. Forty-six percent of the time staging was missing in academic practices, vs fifty-four percent of the time in community centers (P < 0.001). As a result, twenty-percent were down-staged and eight percent up-staged in academia, whereas only fifteen percent were down-staged and 8% up-staged in community practices (P < 0.001). Forty-two percent of individuals underwent radiation before surgery in 1998. Within two years this increased to fifty-three percent. This increased to eighty-six percent by 2011 (P < 0.001). Institution specific treatment varied greatly. Fifty-one percent received therapy before surgery in academic centers in 1998. Thirty-nine percent followed this pattern in the same year in the community (P < 0.001). By 2011, ninety-one percent received radiation before their procedure in academic centers, vs eighty-four percent in the community (P < 0.001). Rates of adoption were better in academia, although an increase was seen in both center types.
CONCLUSION
From the study dates of 1998 to 2011, preoperative treatment with radiation has been on the rise. There is certainly an increased rate of use of radiation in academia, however, this trend is also seen in the community. Practice patterns have evolved over time, although rates of assigning clinical stage are grossly underreported prior to initiation of preoperative therapy.
Keywords: Neoadjuvant therapy, Community, Rectal cancer, Academic
Core tip: This paper serves to show how changes in practice patterns evolve over time. The adoption of these practice patterns differ across institution type, and the role of appropriate clinical staging is often not included. In order for proper treatments to be initiated, we not only need data substantiated by level one evidence, but we also need proper clinical staging so we can ensure appropriate therapies are delivered to these patients.
INTRODUCTION
The implementation of radiotherapy for rectal cancer has seen many adaptations over time, particularly when comparing adoption in community vs academic centers in the United States. Surgical resection with sound oncologic technique is a critical component. Various series report local regional recurrence rates anywhere between 50%-60% in patients undergoing surgery for rectal adenocarcinoma[1-3]. Histological grade, primary tumor invasion, and length of the lesion, have all been found to influence rates of local recurrence[1,2,4]. Another important correlate for local recurrence are those subset of patient found to have positive nodal disease[4]. Local recurrence rates, in addition to overall survival, were both adversely affected when any of these criteria were met.
The use of radiotherapy was initially met with skepticism, as many believed that surgery, which included a total mesorectal excision (TME), offered superior results. Heald et al[5] surmised that patients with low tumors did no worse than those with high tumors when treated by anterior resection, provided that the mesorectum is excised intact with the cancer. Karanjia et al[6] and Heald et al[7] went as far as to suggest that less margins may not increase recurrence or effect survival, as long as a good TME was performed. As surgical techniques for rectal cancer improved, innovations regarding the selective use of radiotherapy were also being explored. Despite this, many continue to argue that a technically sound TME may eliminate radiation[8,9].
The addition of radiotherapy to surgical resection has been an evolving process, and several randomized controlled trials have compared various regimens to surgery alone. Many of these trials were done in an academic institution, and although validated by randomized trials, adoption into the community initially lagged. The Colorectal Cancer Collaborative Group reviewed twenty eight randomized trials, and found a decreased risk of recurrence when preoperative therapy was given[10]. The Dutch group implemented short course radiation and TME, and found lower recurrence rates then when TME was done by itself[11]. Implementation of chemoradiotherapy (CRT) was widely adopted in the 1990’s, when two trials were completed. These compared pre and postoperative therapy.
Despite prospective data showing the success of radiation, its adoption within the community seems limited, and could partially be a result of inaccurate initial staging. Using the National Cancer Database (NCDB) we looked to see how patterns of care changed over time, and how institution type effected these decisions. We also looked to see if clinical staging was lacking, and if so, how this effected the adoption of neoadjuvant therapies.
MATERIALS AND METHODS
A retrospective review was performed using the NCDB. All patients diagnosed with rectal cancer from 1998 to 2011 were included. Patients were stratified by those who underwent surgery as initial treatment, vs those who underwent neoadjuvant radiotherapy. Of these patients, clinical staging was reviewed, and compared between academic and community centers. Clinical stage was further divided into node positive and node negative disease, and tumor response by induction therapy was determined by final pathological stage. Differences in rates of treatment and stage migration were tested using χ2 tests and Cochran-Armitage tests for trend.
RESULTS
A review of ninety-thousand five hundred and ninety four subjects underwent multimodality therapy for cancer of the rectum. The total cohort included 62% males and 38% female. Fifty-four percent of patients were between the ages of 51-70. The overwhelming majority of patients were Caucasian, at 88%. Patient’s insurance status was 50% privately insured, and 43% with Medicare/Medicaid (Table 1).
Table 1.
Patient demographics
| Demographics (%) | |
| Gender | |
| Male | 62 |
| Female | 38 |
| Age | |
| < 50 | 18 |
| 51-70 | 54 |
| > 70 | 28 |
| Race | |
| Caucasian | 88 |
| African American | 8 |
| Other | 4 |
| Insurance | 50 |
| Private | 43 |
| Medicare/Medicaid | |
| None | 4 |
| Other | 3 |
Forty-two percent of individuals underwent radiation before surgery in 1998. Within five years, this proportion had increased to 64%, and over the course of the study period we saw a 33% increase in adoption of radiotherapy. By 2011, 86% received induction radiotherapy prior to surgery (P < 0.001). In 1998, 51% of patients underwent induction radiotherapy when seen in an academic center vs 39% when seen in the community. Within five years there was a rise in the routine application of radiotherapy at 74% and 61%, respectively. By 2011, 91% of academic centers, and 84% of community centers routinely used induction radiotherapy in the treatment of rectal cancers (P < 0.001). Adoption was better in academia overall, but an increase was seen in both center types (Figure 1).
Figure 1.
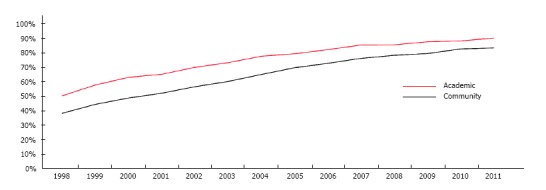
Trends in the adoption of neoadjuvant therapy. Graphical interpretation of the adoption of neoadjuvant therapy over time when comparing academic and community institutions.
Across the cohort of patients who received neoadjuvant radiotherapy, 21% did not have a clinical stage recorded, 25% had no pathological stage, and 6% had neither recorded. When assessing staging differences between academic and community centers, clinical stage was unknown in 17% vs 23%, respectively (P < 0.001). Pathological staging was not recorded 24% of the time in academic centers, and 26% of the time in the community (P < 0.001). Neither stage was recorded in 5% and 6% of the time in academic vs community centers, respectively (P < 0.001). Overall, staging was incomplete 46% of the time in academic centers, and 55% of the time within the community (P < 0.001) (Table 2).
Table 2.
Institutional staging
| Unknown staging | % |
| Overall unknown | |
| Path stage | 25 |
| Clinical stage | 21 |
| Both stages | 5 |
| Academic unknown | |
| Path stage | 24 |
| Clinical stage | 17 |
| Both stages | 5 |
| Community unknown | |
| Path stage | 26 |
| Clinical stage | 23 |
| Both stages | 6 |
Overall response to treatment showed that seventeen percent were down-staged, eight percent up-staged, and twenty-four percent had no change. Within academic centers, twenty percent were down-staged, eight percent up-staged, and twenty-six percent had no changes. Down-staging in the community occurred fifteen percent of the time, up-staging eight percent, and no changes in twenty-three percent. Patients at academic centers were down-staged more often after neoadjuvant therapy than when in the community (P < 0.001) (Table 3). Patients were also stratified by T-stage and nodal status. Fifty-four percent with clinically negative nodes had node negative disease on final pathology. Twenty-two percent of patients without palpable nodes were found to be node positive. Thirty-seven percent were down-staged to node negative status.
Table 3.
Trends in staging
| Unknown staging | % |
| Overall | |
| Up-stage | 8 |
| Down-stage | 17 |
| No change | 24 |
| Academic | |
| Up-stage | 8 |
| Down-stage | 20 |
| No change | 26 |
| Community | |
| Up-stage | 8 |
| Down-stage | 15 |
| No change | 23 |
DISSCUSION
The use of neoadjuvant radiation has increased over time. Unfortunately evidence-based medicine remains difficult to enforce[12]. In our review, adoption of these practices seems to be initially lower within the community compared to academics; however, trends suggest a steady increase in its implementation. One explanation for this is the non-uniform anatomic definition of rectal cancer, and as a result, the lack of appropriate clinical staging done. In a systematic review searching for national and international guidelines, no consensus concerning a definition was found[13]. Four guidelines used fifteen centimeters from the anus as the anatomic rectum, and two used twelve centimeters[13]. In addition to this, how measurements were made varied between consensus guidelines; some used proctoscopy, others flexible endoscopy, and some MRI. The lack of a universal definition could be attributing to the lack of compliance in undergoing appropriate staging studies and thus assigning clinical stage, and subsequent delivery of care.
Staging modalities
Standardized treatment would not be possible without appropriate staging modalities. Proper disease staging will determine whether or not induction therapy would be of value. Imaging options include endorectal ultrasound, computerized tomography, and magnetic resonance imaging[14]. We found that 21% of patients that underwent neoadjuvant therapy had no clinical stage recorded. Although clinical staging seems to occur less within the community, it is difficult to tell if this is a result of improper data collection, or reflective of the institution itself. Similarly, pathological staging was unavailable more often within community centers than in academic places. Charlton et al[15] demonstrated that fellowship trained surgeons more often ordered endorectal ultrasounds and MRI’s. They were also more likely to refer for neoadjuvant treatments[15]. Although not certain, this could be suggestive that this trend would hold in academic centers, as opposed to the community based practices. In our review, in patients with data available for staging, it seemed as though academic institutions had improved rates of down staging tumors, when compared to the community. This could be correlated to the difference in clinical stage recorded amongst these centers. However, a flaw in our work is that we do not know whether clinical staging was done or simply not recorded.
TME
The use of TME challenged implementation of radiotherapy in the treatment algorithm. Since its inception, reductions in local recurrence, improved survival, and sphincter preservation have been noted. The main issue with this surgical approach is that it is operator dependent. Whether or not the surgeon has been properly instructed in the technique ultimately plays a role in recurrence patterns. Unfortunately, whether or not a TME was implemented at the time of surgical resection in our study is not known. One could argue that surgeons practicing in academic centers have had extra training in TME’s, and this again supports the lack of adoption of evidence-based practices within the community. When properly performed, a TME provides excellent local control. Heald et al[5] found a recurrence rate of 7.2%. Several years later this was 3.5%[16]. Macfarlane et al[17] confirmed recurrence rates of 5% with TME, 25% with conventional surgery and radiotherapy, and 13.5% with conventional surgery and CRT. Enker et al[9] reports recurrence in 7.3%. Nodal involvement and perineural invasion were statistically significant risk factors.
Use of radiotherapy
In terms of neoadjuvant radiotherapy, the German group looked to challenge the recommended standard therapy of postoperative CRT. After randomization, 6% recurred locally in the preoperative group, vs 13% in the postoperative arm[18]. Despite strong evidence, there remains a subset of clinicians that challenge this, and advocate a selective approach to induction therapy. In a single center, retrospective cohort study, Williamson and colleagues supported an individual approach to when CRT was used. The mention a 5-year recurrence rate of 6.5% in the treatment group, vs 0% when surgery was done by itself[19]. Patients receiving treatment were selected on the basis of an involved circumferential margin. This explains the variation in recurrence between these arms. However, this represents a prime example of how treatments patterns differ across institutions. To elaborate on this further, the PROSPECT trial initially evaluated patients who were candidates for a low anterior resection with TME, and were given six cycles of FOLFOX[20]. If disease was stable or progressed, then they would proceed to preoperative CRT, if they were responders, then they would go straight to surgery. The pilot study by Schrag et al[20] demonstrated that those who had chemotherapy had complete pathologic response rates of 25%, and a 0% four-year local recurrence rate.
SEER data by Fitzgerald et al[12], the use of radiotherapy was 17% in 1998, which increased to 51% in 2007. In our review, 42% of patients received induction radiotherapy, which increased to 64% in five years. By 2011, 85% of patients seen received neoadjuvant radiotherapy for rectal cancer (P < 0.001). Similar trends were noted by Jobsen et al[21], finding a steady increase in the utilization of neoadjuvant radiotherapy from 1997-2008. It remains evident that a trend for the routine use of neoadjuvant radiotherapy is there. However, factors such as volume and facility type certainly play a role[22,23]. Stewart et al[22] surmised that hospitals where teaching was a priority, increased the likelihood of receiving neoadjuvant treatments (P < 0.0001). In our review, fifty-one percent of those treated in academia underwent preoperative therapy vs 39% when seen in the community. By 2011, 91% of academic centers, and 84% of community centers, routinely used radiotherapy (P < 0.001).
Caring for those with of locally advanced rectal cancer has evolved over the decades. Advances in surgical technique with TME revolutionized the field of rectal surgery, and offered patients superior local control than when compared to conventional surgery alone. Several studies have suggested this benefit, attributing higher local recurrence rates to inadequate TME’s[24-26]. As clinical trial accrual escalated, the implementation of radiotherapy to the treatment algorithm was the next logical step. The Dutch group found that preoperative therapy was safe in patients, even if they were to undergo surgery[27]. Despite this, adoption of the routine use of neoadjuvant radiotherapy was a difficult undertaking. The data shows a trend favoring the influence of evidence-based medicine, which in turn affects the way in which we practice medicine. In order for this to continue, we must work on improving recording of clinical stage, so that patients are not only eligible for potential clinical trials, but receive the current standard of care. Although smaller discrepancies continue to exist between academic and community centers in terms of its usage of neoadjuvant therapy, the overall trends are on the rise.
COMMENTS
Background
The implementation of radiotherapy for rectal cancer has seen many adaptations over time, particularly when comparing adoption in community vs academic centers in the United State. Surgical resection with total mesorectal excision is a critical component. Various series report local regional recurrence rates anywhere between 50%-60% in patients undergoing surgery for rectal adenocarcinoma. The addition of radiotherapy to surgical resection has been an evolving process, and several randomized controlled trials have compared various regimens to surgery alone. Many of these trials were done in an academic institution, and although validated by randomized trials, adoption into the community initially lagged.
Research frontiers
The adoption of radiotherapy for this disease has altered the way in which we treat this disease. As with any new therapy, there are always experiments being conducted to see if the authors again can change their practicing treatment plan.
Innovations and breakthroughs
In this study the authors looked to see how patterns of treatment changed over time. Differences between the types of facility administering care were reviewed, and whether appropriate clinical staging was assigned was critiqued.
Applications
This study suggests that radiotherapy had slow adoption into mainstream practice, but over time, practice patterns changed.
Terminology
Radiation: This is the emission or transmission of energy in the form of waves or particles. The use of radiation in clinical practice has greatly changed the way the authors treat disease in the modern era.
Peer-review
The paper is interesting.
Footnotes
Institutional review board statement: This study was reviewed and approved by the institution.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that was obtained from a national cancer database.
Conflict-of-interest statement: The authors have no financial relationships to disclose.
Data sharing statement: There is a potential risk of confidentiality loss during the study. The following steps will be taken to protect the confidentiality of the information obtained throughout the study: All protected information will be de-identified at the start of the research project; all original documents will be maintained on a computerized system in their original format. Data points will be maintained on a locked file in the office of Beth Handorf; only the investigators will have access to the electronic medical records; all investigators are HIPAA and NIH trained with regard to the protection of human research participants.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: October 10, 2016
First decision: November 10, 2016
Article in press: February 20, 2017
P- Reviewer: da Rocha JJR, Seo A, Wilkins S S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
References
- 1.Cass AW, Million RR, Pfaff WW. Patterns of recurrence following surgery alone for adenocarcinoma of the colon and rectum. Cancer. 1976;37:2861–2865. doi: 10.1002/1097-0142(197606)37:6<2861::aid-cncr2820370643>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Mendenhall WM, Million RR, Pfaff WW. Patterns of recurrence in adenocarcinoma of the rectum and rectosigmoid treated with surgery alone: implications in treatment planning with adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 1983;9:977–985. doi: 10.1016/0360-3016(83)90384-x. [DOI] [PubMed] [Google Scholar]
- 3.Hoffe SE, Shridhar R, Biagioli MC. Radiation therapy for rectal cancer: current status and future directions. Cancer Control. 2010;17:25–34. doi: 10.1177/107327481001700104. [DOI] [PubMed] [Google Scholar]
- 4.Minsky BD, Mies C, Recht A, Rich TA, Chaffey JT. Resectable adenocarcinoma of the rectosigmoid and rectum. I. Patterns of failure and survival. Cancer. 1988;61:1408–1416. doi: 10.1002/1097-0142(19880401)61:7<1408::aid-cncr2820610722>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–1482. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 6.Karanjia ND, Schache DJ, North WR, Heald RJ. ‘Close shave’ in anterior resection. Br J Surg. 1990;77:510–512. doi: 10.1002/bjs.1800770512. [DOI] [PubMed] [Google Scholar]
- 7.Heald RJ, Karanjia ND. Results of radical surgery for rectal cancer. World J Surg. 1992;16:848–857. doi: 10.1007/BF02066981. [DOI] [PubMed] [Google Scholar]
- 8.Merchant NB, Guillem JG, Paty PB, Enker WE, Minsky BD, Quan SH, Wong D, Cohen AM. T3N0 rectal cancer: results following sharp mesorectal excision and no adjuvant therapy. J Gastrointest Surg. 1999;3:642–647. doi: 10.1016/s1091-255x(99)80087-0. [DOI] [PubMed] [Google Scholar]
- 9.Enker WE, Thaler HT, Cranor ML, Polyak T. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg. 1995;181:335–346. [PubMed] [Google Scholar]
- 10.Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet. 2001;358:1291–1304. doi: 10.1016/S0140-6736(01)06409-1. [DOI] [PubMed] [Google Scholar]
- 11.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald TL, Biswas T, O’Brien K, Zervos EE, Wong JH. Neoadjuvant radiotherapy for rectal cancer: adherence to evidence-based guidelines in clinical practice. World J Surg. 2013;37:639–645. doi: 10.1007/s00268-012-1862-z. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen LB, Wille-Jørgensen P. National and international guidelines for rectal cancer. Colorectal Dis. 2014;16:854–865. doi: 10.1111/codi.12678. [DOI] [PubMed] [Google Scholar]
- 14.Badger SA, Devlin PB, Neilly PJ, Gilliland R. Preoperative staging of rectal carcinoma by endorectal ultrasound: is there a learning curve? Int J Colorectal Dis. 2007;22:1261–1268. doi: 10.1007/s00384-007-0273-3. [DOI] [PubMed] [Google Scholar]
- 15.Charlton ME, Mattingly-Wells LR, Marcet JE, McMahon Waldschmidt BC, Cromwell JW. Association between surgeon characteristics and their preferences for guideline-concordant staging and treatment for rectal cancer. Am J Surg. 2014;208:817–823. doi: 10.1016/j.amjsurg.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAnena OJ, Heald RJ, Lockhart-Mummery HE. Operative and functional results of total mesorectal excision with ultra-low anterior resection in the management of carcinoma of the lower one-third of the rectum. Surg Gynecol Obstet. 1990;170:517–521. [PubMed] [Google Scholar]
- 17.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457–460. doi: 10.1016/0140-6736(93)90207-w. [DOI] [PubMed] [Google Scholar]
- 18.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 19.Williamson JS, Jones HG, Davies M, Evans MD, Hatcher O, Beynon J, Harris DA. Outcomes in locally advanced rectal cancer with highly selective preoperative chemoradiotherapy. Br J Surg. 2014;101:1290–1298. doi: 10.1002/bjs.9570. [DOI] [PubMed] [Google Scholar]
- 20.Schrag D, Weiser MR, Goodman KA, Gonen M, Hollywood E, Cercek A, Reidy-Lagunes DL, Gollub MJ, Shia J, Guillem JG, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32:513–518. doi: 10.1200/JCO.2013.51.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jobsen JJ, Aarts MJ, Siesling S, Klaase J, Louwman WJ, Poortmans PM, Lybeert ML, Koning CC, Struikmans H, Coebergh JW. Use of primary radiotherapy for rectal cancer in the Netherlands between 1997 and 2008: a population-based study. Clin Oncol (R Coll Radiol) 2012;24:e1–e8. doi: 10.1016/j.clon.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Stewart DB, Hollenbeak C, Desharnais S, Camacho F, Gladowski P, Goff VL, Wang L. Rectal cancer and teaching hospitals: hospital teaching status affects use of neoadjuvant radiation and survival for rectal cancer patients. Ann Surg Oncol. 2013;20:1156–1163. doi: 10.1245/s10434-012-2769-5. [DOI] [PubMed] [Google Scholar]
- 23.Schrag D, Panageas KS, Riedel E, Cramer LD, Guillem JG, Bach PB, Begg CB. Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Ann Surg. 2002;236:583–592. doi: 10.1097/00000658-200211000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott N, Jackson P, al-Jaberi T, Dixon MF, Quirke P, Finan PJ. Total mesorectal excision and local recurrence: a study of tumour spread in the mesorectum distal to rectal cancer. Br J Surg. 1995;82:1031–1033. doi: 10.1002/bjs.1800820808. [DOI] [PubMed] [Google Scholar]
- 25.Leong AF. Total mesorectal excision (TME)--twenty years on. Ann Acad Med Singapore. 2003;32:159–162. [PubMed] [Google Scholar]
- 26.Goldberg S, Klas JV. Total mesorectal excision in the treatment of rectal cancer: a view from the USA. Semin Surg Oncol. 1998;15:87–90. doi: 10.1002/(sici)1098-2388(199809)15:2<87::aid-ssu5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Kapiteijn E, Kranenbarg EK, Steup WH, Taat CW, Rutten HJ, Wiggers T, van Krieken JH, Hermans J, Leer JW, van de Velde CJ. Total mesorectal excision (TME) with or without preoperative radiotherapy in the treatment of primary rectal cancer. Prospective randomised trial with standard operative and histopathological techniques. Dutch ColoRectal Cancer Group. Eur J Surg. 1999;165:410–420. doi: 10.1080/110241599750006613. [DOI] [PubMed] [Google Scholar]


