Abstract
Context:
Oral squamous cell carcinoma (OSCC) is a malignant neoplasm of epithelial tissue origin. OSCC is traditionally graded into well, moderate and poorly differentiated. The assessment of cellular and nuclear features is very important in the histological grading of OSCC.
Aims:
To establish morphometric study as an important prognostic indicator of OSCC.
Settings and Design:
The present study is undertaken for computer-assisted morphometric evaluation of the following parameters: changes in cell perimeter, nuclear perimeter (NP), cell area (CA), nuclear area (NA) and nuclear-cytoplasmic ratio (N:C) in twenty cases of OSCC and ten cases of normal oral mucosa.
Subjects and Methods:
The hematoxylin- and eosin-stained sections were observed under microscope for dysplastic features. For morphometric analysis, images were captured with a 2MP camera attached to a NLCD 307 microscope (Lawrence and Mayo India Pvt., Ltd.,) with a ×40 objective used for morphometric analysis. The images were classified, transferred and stored in the computer for image analysis.
Statistical Analysis Used:
One-way analysis of variance is used for comparing the parameter for multiple groups followed by Mann-Whitney U-test for pair-wise comparisons.
Results:
Highly significant difference was seen between cases and controls with respect to CA, NP and NA. Highly significant difference is seen in N:C ratio between the means of cases and control groups.
Conclusions:
Techniques of image analysis offer an opportunity to quantify the nuclear and cell changes associated with malignancy and provide an objective basis for grading dysplasia and tumors.
Keywords: Cell perimeter, morphometry, nuclear perimeter, nuclear-cytoplasmic ratio
INTRODUCTION
Squamous cell carcinoma (SCC) is a malignant neoplasm of epithelial tissue origin; exhibiting varying degree of squamous differentiation as characterized by the formation of keratin and a propensity to early and extensive lymph node metastasis.[1,2]
The assessment of cellular and nuclear features is very important in the histological grading of oral SCC (OSCC).
The various morphometric parameters studied for epithelial tumors are nuclear area (NA), nuclear perimeter (NP), nuclear shape factor, smallest and longest axes, nuclear size along with nuclear-cytoplasmic ratio (N:C) and nucleolar-nuclear ratio.[3,4]
The morphometry of epithelial tumor cells has been studied to examine its correlation with the prognosis of various tumors of the breast, cervix, ovary and bladder.[3,5]
The present study is undertaken for computer-assisted microscopic evaluation of the following parameters: Changes in cell perimeter (CP), NP, cell area (CA), NA and N:C ratio in twenty cases of OSCC and ten cases of normal oral mucosa, to explore the correlation between morphometric study and gradation of OSCC as well as to establish morphometric study as an important prognostic indicator of OSCC.
SUBJECTS AND METHODS
A morphometric study on tissue sections of OSCC was conducted in our department.
Tissues were obtained from the biopsy specimens received in the Department of Oral Pathology and Microbiology, Buddha Institute of Dental Sciences and Hospital, Patna from internal and external sources, during the period between January 2012 and December 2013.
After obtaining the ethical clearance, thirty cases (ten of normal oral buccal mucosa and twenty of OSCC showing various grades) were included in the study.
The control group comprised normal buccal mucosa from healthy adult individuals irrespective of age and sex.
The hematoxylin- and eosin-stained sections were observed under microscope for dysplastic features. Histologically, early invasive, well–differentiated and moderately differentiated SCC was included in the study. Poorly differentiated SCC was excluded from the study.
After reviewing, the sections were further subjected to morphometric analysis. For morphometric analysis, images were captured with a 2MP camera attached to a NLCD 307 microscope (Lawrence and Mayo India Pvt., Ltd.,) with a ×40 objective used for morphometric analysis. The images were classified, transferred and stored in the computer.
Microscopic fields were selected randomly, 5 fields were selected from each section. From each selected field, five spinous cells were selected with clear cellular and nuclear outline [Figures 1 and 2].
Figure 1.
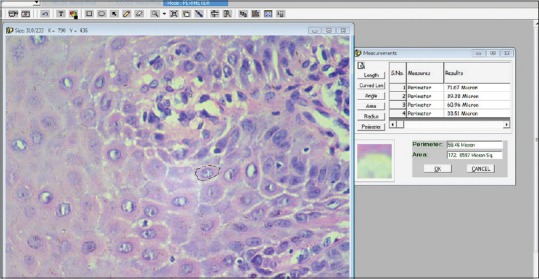
Calculation images (Cellular outline)
Figure 2.
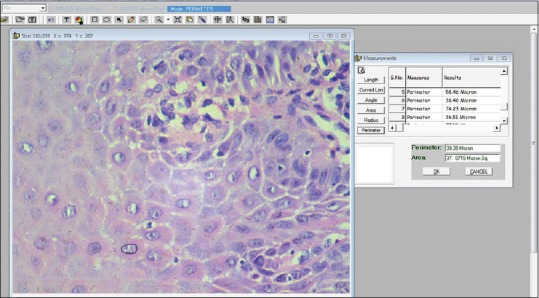
Calculation images (Nuclear outline)
Microscopic image which was input directly into the system was viewed on a monitor. The actual measurements were done using Image Analysis software (Lynx BIOLUX AUTO Series), after accurate calibration using a stage micrometer.
All measurements were measured in microns. For measurement, the cell outline was traced, and the software automatically calculated the CP (number of boundary pixels detected, converted to microns) and the CA (number of pixels detected, converted to micrometer).[3]
The data collected in this study were analyzed statistically by computing descriptive statistics, namely, percentage, mean, standard deviation, standard error of mean and 95% confidence interval for mean. One-way analysis of variance is used for comparing the parameter for multiple groups followed by Mann-Whitney U-test for pair-wise comparisons.
RESULTS
In the present study, out of twenty cases of OSCC, 13 were males (65%) and seven were females (35%). The cases were found from the third decade to ninth decade. The mean age is found 56.2. Eight cases were found in the sixth decade (40%), four cases seen in the seventh decade (20%), two cases in the 4th decade (10%), three cases in the fifth decade (15%) and finally one case is found in 3rd, 8th and 9th (5% each) decade of life.
As per histopathological diagnosis, eight cases were diagnosed as well-differentiated SCC (26.7%), eight were of moderately differentiated SCC (26.7%), four were of early invasive SCC (13.3%) and ten were normal buccal mucosa (33.3%).
Taking the mean of calculated results, the gender-wise comparison showed the mild difference between male and female cases but which was not statistically significant (P = 0.157, 0.081, 0.135 and 0.938) [Table 1 and Figure 3].
Table 1.
Gender wise comparison in cases

Figure 3.

Bar graph showing gender-wise comparison in cases
Comparison according to histopathological diagnosis showed no significant difference with respect to CP, CA, NP and NA in various grades of OSCC [Table 2 and Figure 4].
Table 2.
Statistical analysis and case-wise comparison
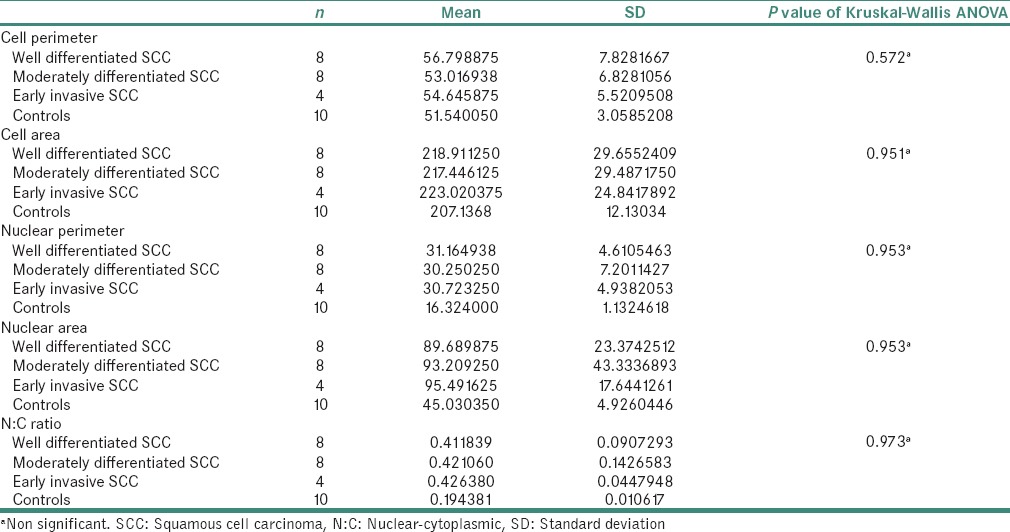
Figure 4.
Bar graph showing statistical analysis and case-wise comparison
Statistically highly significant difference was seen between cases and controls with respect to CA, NP and NA [Table 3 and Figure 5].
Table 3.
Statistical analysis between cases and controls
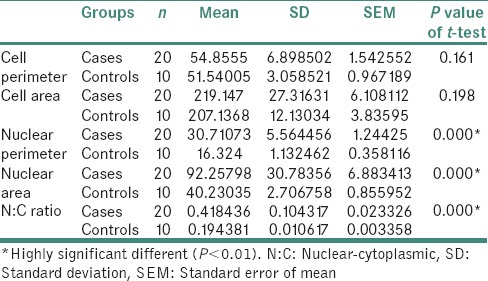
Figure 5.

Bar graph showing statistical analysis between cases and controls
Nonsignificant difference was found when N:C ratio was compared between male and female patients of cases. No difference was seen when N:C ratio was compared with the age groups of cases [Table 4 and Figure 6].
Table 4.
Nuclear-cytoplasmic ratio with age groups of cases
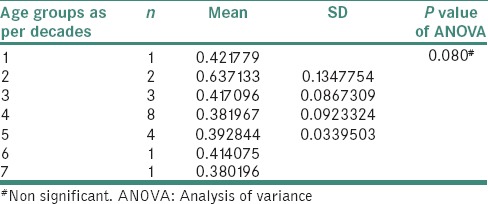
Figure 6.

Bar graph showing nuclear-cytoplasmic ratio with age groups of cases
No significant difference was seen when N:C ratio was compared with histopathological grades of OSCC [Table 2]. There is a highly significant difference is seen in N:C ratio between the means of cases and control groups [Table 5 and Figure 7].
Table 5.
Final nuclear-cytoplasmic ratio between cases and controls

Figure 7.
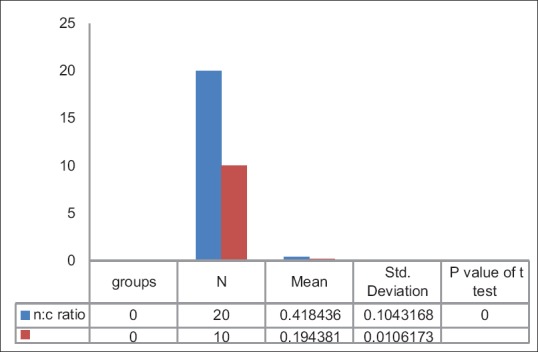
Bar graph showing final nuclear-cytoplasmic ratio between cases and controls
DISCUSSION
OSCC is the most common malignant neoplasm arising from the mucosal epithelium of the oral cavity. In the Indian subcontinent and in other parts of Asia, it remains one of the most common forms of cancer. Still now, histopathological examination with H and E stain is the gold standard for diagnosis and gradation of OSCC. There is a substantial need for in-depth histologic assessment of OSCC. Of late, interest has turned toward applying the sophisticated technique of computer-assisted morphometry to investigate the cellular and nuclear changes in correlation with the histological behavior of the lesions. It is a method of assessing the computerized images of histologically stained sections. The results have been more reliable, objective and reproducible.[1,3,6,7]
In the present study, we found that the mean NA of spinous cells was higher in OSCC lesions when compared with those from the normal oral mucosa. Malignant cells display a significant increase in mean NA. The increase in the nuclear size is related to an increase in the nuclear contents required for replication (Frost, 1997).[8,9]
Increase in the cellular perimeter and increase in the nuclear size are two significant changes that occur in actively proliferating cell (Cançado et al., 2004). Accordingly, we observed that with the progression from normal to malignancy, the nuclear and cellular dimensions increased.
Eveson and MacDonald (1981) observed that after the application of carcinogens to hamster cheek pouch epithelium, there was a progressive increase both in size (represented by mean CA) and number of progenitor cells. These findings support our observation of increase in cellular area in the spinous cells.[1,6,10]
Shabana et al. studied the size and shape of the cells in the basal cell layer of the oral epithelium in 100 specimens. The results showed a progressive increase in the dimensions (area, perimeter and maximum diameter) of the nuclei from normal mucosa through traumatic keratosis, lichen planus, leukoplakia and the “risk group” to carcinoma, with considerable differences. The nucleus in SCC was twice as large as in normal mucosa. A substantial increase in the dimensions of both the cell and the nucleus was found in the “risk group.”[11,12]
Cowpe (1984) found that tissues undergoing malignant transformation typically showed a reduction in CA before the reduction in NA, using semiautomatic image analysis techniques.[13]
Ramaesh et al. used cytomorphometric techniques to assess nuclear diameter (ND) and cell diameter (CD) in normal oral mucosa, in dysplastic lesions and in SCCs. They found that CD was highest in normal mucosa, lower in dysplastic lesions and lowest in SCCs. By contrast, ND was the lowest in normal mucosa, higher in dysplastic lesions and highest in SCCs. These studies suggested that reduced nuclear size and increased cytoplasm size are useful early indicators of malignant transformation.[14]
The N:C ratio of spinous cells was significantly higher in our study denoting increased NA compared to the control group, and the observation is similar to Smitha et al.[1]
One important finding noted in our study is that there is no statistically significant difference in morphometry of cells in OSCC cases showing various grades of OSCC [Table 2]. The cellular and nuclear dimensions in all included grades of OSCC were larger than of controls group. Very few reported cases show grade-wise difference in the morphometric studies before. Most of the studies on the contrary have pooled samples from a specific type of SCC cases. It is also seen in our study that there is no statistically significant difference found in cases compared with gender and of different age groups.
Many of the studies in the literature have focused on the nuclear changes in the development of malignancy. Similar results were observed in our study, only CP and CA showed less deviation.
To summarize, the objectives of this morphometric analyses were to study the (a) size and (b) N:C ratio of the spinous cells and nuclei in normal mucosa and OSCC using image analysis. The results have revealed the following:
The morphometric parameter size was useful to differentiate spinous cells in normal mucosa and OSCC. The N:C ratio parameter was also useful to differentiate between normal mucosa and OSCC.
In summary, this study supports and extends the view that morphometric evaluation of epithelial cells can serve as a useful diagnostic adjunct for early detection of oral cancer. An increase in the nuclear size and N:C ratio is characteristics of malignant epithelial cells. Evaluation of a greater number of cases is essential to establish the cutoff values of these parameters (i.e., sensitivity and specificity) that can be used as definitive indicators.
CONCLUSIONS
The increase in cellular and nuclear size in OSCC has shown it to be more prone for malignant change, and therefore, their measurements may provide an objective means for the assessment of epithelial dysplasia and to predict their malignant potential. Techniques of image analysis offer an opportunity to quantify the nuclear and cell changes associated with malignancy and provide an objective basis for grading dysplasia and tumors. Application of computer and image analysis software for accurate assessment of other dysplastic features should be of great help in the proper evaluation of dysplasia and in determining its malignant potential. Overall, these results have shown that the quantitative histomorphometric techniques can detect features that may be overlooked by routine histological examination.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Smitha T, Sharada P, Girish H. Morphometry of the basal cell layer of oral leukoplakia and oral squamous cell carcinoma using computer-aided image analysis. J Oral Maxillofac Pathol. 2011;15:26–33. doi: 10.4103/0973-029X.80034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markopoulos AK. Current aspects on oral squamous cell carcinoma. Open Dent J. 2012;6:126–30. doi: 10.2174/1874210601206010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Natrajan S, Mahajan S, Boaz K, George T. Morphometric analysis of nuclear features and volume-corrected mitotic index in the prognosis of oral squamous cell carcinoma. Oral Sci Int. 2009;6:85–94. [Google Scholar]
- 4.Neha U, Shubhangi K, Alka D, Kumar MR, Rohit M. A study of morphometrical differences between normal mucosa, dysplasia, squamous cell carcinoma and pseudoepitheliomatous hyperplasia of the oral mucosa. IOSR J Pharm Biol Sci. 2013;5:66–70. [Google Scholar]
- 5.Nandini DB, Subramanyam RV. Nuclear features in oral squamous cell carcinoma: A computer-assisted microscopic study. J Oral Maxillofac Pathol. 2011;15:177–81. doi: 10.4103/0973-029X.84488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raju Ragavendra T, Rammanohar M, Sowmya K. Morphometric computer-assisted image analysis of oral epithelial cells in normal epithelium and leukoplakia. J Oral Pathol Med. 2010;39:149–54. doi: 10.1111/j.1600-0714.2009.00860.x. [DOI] [PubMed] [Google Scholar]
- 7.Swaminathan U, Joshua E, Rao UK, Ranganathan K. Expression of p53 and Cyclin D1 in oral squamous cell carcinoma and normal mucosa: An immunohistochemical study. J Oral Maxillofac Pathol. 2012;16:172–7. doi: 10.4103/0973-029X.98451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khandelwal S, Solomon MC. Cytomorphological analysis of keratinocytes in oral smears from tobacco users and oral squamous cell carcinoma lesions – A histochemical approach. Int J Oral Sci. 2010;2:45–52. doi: 10.4248/IJOS10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frost JK. Pathologic processes affecting cells from inflammation to cancer. In: Bibbo M, editor. Comprehensive Cytopathology. 2nd ed. Philadelphia: W.B Saunder's Company; 1997. p. 845. [Google Scholar]
- 10.Evenson JW, Mc Donald DG. Hamster tongue carcinogenesis I. Characteristics of theexperimental model. J Oral Pathol Med. 1981;10:322–41. doi: 10.1111/j.1600-0714.1981.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 11.Shabana AH, el-Labban NG, Lee KW, Kramer IR. Morphometric analysis of suprabasal cells in oral white lesions. J Clin Pathol. 1989;42:264–70. doi: 10.1136/jcp.42.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shabana AH, el-Labban NG, Lee KW. Morphometric analysis of basal cell layer in oral premalignant white lesions and squamous cell carcinoma. J Clin Pathol. 1987;40:454–8. doi: 10.1136/jcp.40.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowpe JG. Quantitative exfoliative cytology of normal and abnormal oral mucosal squames: Preliminary communication. J R Soc Med. 1984;77:928–31. doi: 10.1177/014107688407701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramaesh T, Mendis BR, Ratnatunga N, Thattil RO. Cytomorphometric analysis of squames obtained from normal oral mucosa and lesions of oral leukoplakia and squamous cell carcinoma. J Oral Pathol Med. 1998;27:83–6. doi: 10.1111/j.1600-0714.1998.tb02099.x. [DOI] [PubMed] [Google Scholar]


