Abstract
Background:
Oral submucous fibrosis (OSMF) is a chronic oral mucosal disease characterized by progressive deposition of collagen in subepithelial connective tissue and epithelial atrophy. The present study was conducted to evaluate the changes in epithelial thickness and cellular and nuclear morphometric characteristics of parabasal and spinous compartments of the epithelium in various stages of OSMF in comparison to normal epithelium.
Materials and Methods:
Sample included 30 cases of OSMF of various stages and five cases of normal buccal mucosa. Measurements for epithelial thickness and cellular morphology using morphometric analysis were performed on captured digital images using image analysis software.
Statistical Analysis:
The ANOVA analysis of epithelial thickness and correlation of epithelial thickness with cell contour and cell roundness was done using Karl Pearson's coefficient of correlation.
Results:
There was a statistically significant decrease in epithelial thickness with increase in severity/stage of OSMF. It was also observed that there is a highly statistically significant correlation (P = 0.000) between the thickness of epithelium and cell morphology in varying stages of OSMF.
Conclusion:
The morphometric parameters and the use of quantitative histological methods in determining the squamous epithelial structures thus made it possible to evaluate that there is reduction in thickness of epithelium or “atrophy” with increasing stages of OSMF which may be due to actual change in cell morphology of the individual cells of specific compartment or all compartments in the epithelium.
Keywords: Atrophy, cell morphology, morphometry, oral submucous fibrosis
INTRODUCTION
Although the evidence that oral submucous fibrosis (OSMF) predisposes to cancer is not yet absolutely conclusive, the frequency of malignant change reported in patients with OSMF ranges from 3% to 6%.[1] It is generally agreed that epithelium responds secondarily to the pathological alteration in OSMF that begins in lamina propria and which may predispose to malignant changes in epithelium.[2,3] On the evaluation of epithelial changes in different grades of OSMF, at least a few hold view that epithelium has become stretched and thinned by changes in underlying connective tissue.[3] Few authors have prompted an alternative hypothesis in favor of epithelial hypoproliferation, rather than atrophy. It has been stated that atrophic changes in the mucosa predispose to malignant changes in epithelium.[3]
Changes in epithelial cell size and shape in OSMF may have serious implications on future cell behavior, including malignant transformation. Alteration of cellular morphology and altered tissue architecture as observed from histopathology presently contributes majorly in determination and confirmation of pathological states.[4] However, visual estimation lacks reproducibility and may mislead diagnostic procedures. With the evolution of computerized image processing and analysis system for image capture, storage and analysis using specialized software and hardware, morphometric analysis has been used as an investigative tool in pathological research.
Currently, one of the greatest challenges to oral oncobiologists is to determine and identify the degree of tissue damage or stages of various precancerous states of oral tissue and to detect the exact transition of a normal tissue to precancerous state. In this current scenario with improved morphometric analysis of histopathological images may go hand in hand to provide a better diagnosis and early detection of cancer.
Thus, the present study was done to morphometrically measure and compares the changes in epithelial thickness and evaluates the shape factors, i.e., cell roundness and contour index of the epithelial cells in varying stages of OSMF in comparison to normal epithelium. The epithelial thickness was also analyzed and correlated with cell roundness and contour index in varying stages of OSMF.
MATERIALS AND METHODS
A retrospective study was conducted in the Department of Oral and Maxillofacial Pathology and Microbiology, ITS Center for Dental Studies and Research, Muradnagar, Ghaziabad (UP). The representative study sample of 30 biopsy specimens of OSMF from buccal mucosa and five of normal mucosa were recruited for morphometric analysis. The specimens were selected from the records which comprised 10 specimens of the early stage of OSMF, 10 of an intermediate stage of OSMF, 10 of the advanced stage of OSMF according to Utsunomiya et al.[5] and five of normal mucosa. Paraffin-embedded sections of 3–4 μm thickness obtained from these cases were stained routinely with hematoxylin/eosin.
For analysis of target features of normal and OSMF, high-power (×40) magnification images were captured with the help of an Olympus camera (digital E-PL3) fitted to the research microscope (Olympus BX 41, Tokyo, Japan). Photographs of five random fields of interest were obtained from the hematoxylin/eosin stained slides. Accurate calibration for (×40) magnification was done using a stage micrometer before measurements using the software.
Morphometric parameters
Measurement of epithelial thickness
Images of normal mucosa and OSMF were morphometrically measured (in μm) at 3 points from epithelial surface to epithelial connective tissue interface using Magnus-Pro image analysis software in 5 different fields. The mean of the 3 measurements in 5 fields were taken as the thickness of the epithelium as shown in Figure 1.
Figure 1.
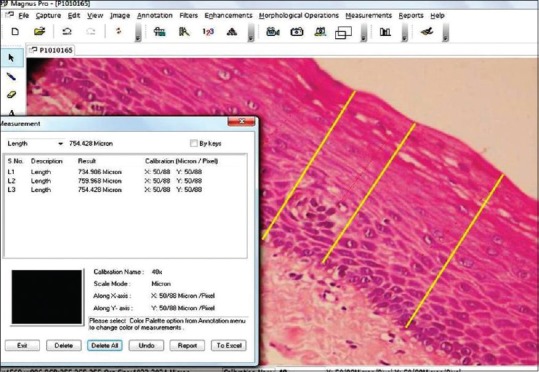
The mean of the 3 measurements in 5 fields were taken as the thickness of the epithelium
Measurement of cell roundness and contour index
Five random cells with clear outline from each of the five fields were traced in spinous cell layer of normal mucosa and OSMF and thereby cell area and cell perimeter was calculated by the Magnus Pro image analysis software as shown in Figure 2. Using a commercially available SPSS statistical software program the data were analyzed.[4]
Figure 2.
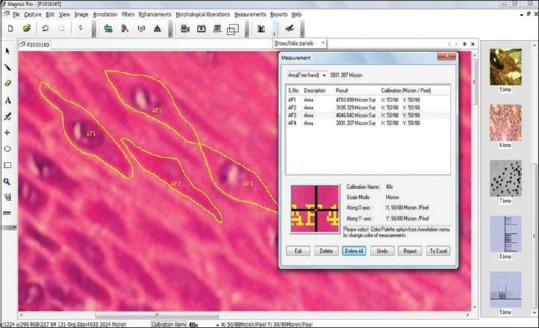
Five random cells with clear outline from each of the five fields were traced in spinous cell layer of normal mucosa and oral submucous fibrosis and thereby cell area and cell perimeter was calculated by the Magnus-Pro image analysis software
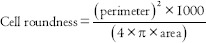
With 1.0 = Perfect circle and
>1.0 = Oblong and noncircular objects.
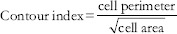
With 3.54 = Contour from a perfect circle.
>3.54 = Increase in indentation or convolutions of the outline.
STATISTICAL ANALYSIS AND RESULTS
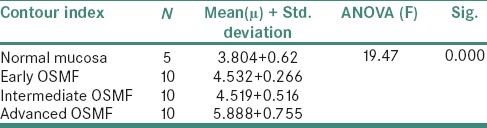
The inferential statistics of the epithelial thickness in various stages of OSMF and normal mucosa recorded are shown in Table 1. The ANOVA analysis of epithelial thickness was found to be highly significant. The inferential statistics of cell roundness and contour index in various stages of OSMF and normal mucosa recorded are shown in Tables 2 and 3. The analysis of cell roundness and contour index was found to be highly significant.
Table 1.
Inferential statistics of the epithelial thickness in various stages of oral submucous fibrosis and normal mucosa
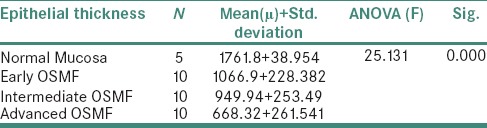
Table 2.
Inferential statistics of cell roundness in various stages of oral submucous fibrosis and normal mucosa

Table 3.
Inferential statistics of cell contour index in various stages of oral submucous fibrosis and normal mucosa
Statistical analysis for correlation of epithelial thickness with cell contour and cell roundness was done using Karl Pearson's coefficient of correlation. The analysis shows that the correlation between cell contour and thickness of epithelium is highly significant. Similarly, the coefficient of correlation between cell roundness and thickness of epithelium is highly significant.
DISCUSSION
In modern literature, Schwartz first described the condition as “atrophia idiopathica (atropica) mucosae oris” among five east African women of Indian origin.[6] This was followed by the first description of this condition in India by Lal as “diffuse OSMF”[7] and by Joshi as “submucous fibrosis.”[8] The condition has also been described by Su[9] as idiopathic scleroderma of the mouth, by Rao[10] as idiopathic palatal fibrosis and by Behl (1962) as sclerosing stomatitis.
Pindborg and Sirsat defined OSMF as “an insidious, chronic disease affecting any part of the oral cavity and sometimes the pharynx. Although occasionally preceded by and/or associated with the vesicle formation, it is always associated with juxta-epithelial inflammatory reaction followed by a fibroelastic change of the lamina propria, with epithelial atrophy leading to stiffness of the oral mucosa and causing trismus and inability to eat.”[11]
OSMF has generally been considered a precancerous condition which can be defined as a “generalized state associated with a significantly increased risk for oral cancer.”[12]
The hypothesis that submucous fibrosis is a precancerous condition was observed by Pindborg et al. when he evaluated the malignant potential of OSMF based on observation of 89 patients with the disease in Ernakulam District, Kerala, India.[2] Considering malignant transformation and coexistence together, oral cancer was observed in 13% of patients and leukoplakia was found in 26% of the patients with OSMF. Out of 42 OSMF patients biopsied, 12% showed squamous cell carcinoma, 26% epithelial dysplasia and 76% atrophic epithelium.
It is generally agreed that the pathological alteration in OSMF begins with fibrosis of lamina propria and epithelium responds only secondarily to it in the form of atrophy that may predispose to malignant changes in epithelium.[2,3] Here, the term epithelial atrophy is used to describe the thinning of epithelium. However, the nature of the thinning or atrophy has not been addressed so far. The literature available on histopathology of OSMF deals mainly with changes in connective tissue and very sparsely on the nature of epithelial changes.
In this study, morphometric analysis and correlation between epithelial thickness with cell roundness and contour index was done in varying stages of OSMF compared to normal epithelium.
According to various hypotheses by earlier investigators, epithelium changes are described alternatively as normal, atrophic, thinned out or hyperplastic in different grades of OSMF. Pindborg et al. reported a study of 23 cases of OSMF, 91% showed an atrophic epithelium with cell layers as low as 3–4 cell rows in some cases.[13] Shear and Lemmer reported that epithelial thickness varied in different parts of the section, and from case to case. In their study of 6 cases, number of cell layers was 7 at narrowest and 20 at its widest.[14] Wahi et al. studied changes in epithelium of OSMF in three different clinical groups and showed that more than 90% of cases in all the three clinical groups reflected hyperplastic epithelium.[15] Pindborg et al. studied 53 biopsies of OSMF and noted that 71.7% had atrophic epithelium, 26.4% normal and 1.9% had hyperplastic epithelium when compared to normal epithelium.[16] Mani and Singh in 38 patients study noted that 42.1% normal epithelium, 34.2% atrophic epithelium and 23.7% had hyperplastic epithelium.[17] Canniff et al. in 44 cases study found that 87% of cases had atrophic epithelium of which 27% had flattened epidermal-dermal junction. There was no evidence of epithelial hyperplasia.[18]
However, the present study provided a statistically valid technique to achieve accurate clues of epithelium changes in different grades of OSMF compared to normal epithelium by the use of quantitative histological methods which has not been addressed so far morphometrically.
It was observed that the decrease in epithelial thickness with the increase in severity/stage of OSMF which was supported by morphometric measurements was statistically significant (P = 0.000). Furthermore, the reason behind the thinning of epithelium may be due to the reduced cell division in the progenitor compartment (hypoproliferation) or it can be due to accelerated tissue turnover time or due to an actual change in cell morphology of the individual cells of specific compartment or all compartments. In support to the previous studies, the hypotheses of hypoproliferation and increased tissue turnover time had been discarded as being the reason behind the thinning of epithelium.
Hence, the present morphometric study was undertaken to see the actual changes occurring in the cellular dimensions in the epithelial cells of OSMF in comparison to normal oral epithelium. In the assessment of cellular morphology, to overcome the unreliability in the subjective examination of cell shape and contour index, these features were analyzed with a more objective approach employing the value of computer-based image analysis techniques.
The cell shape was measured which assesses the deviation from circularity. By the relations, a circle has a value of one and an ellipse, or an irregular structure is <1. Another shape measuring variable is the Contour Index, which measures the regularity of the particle surface. A value of 3.54 is obtained for a circle by the formula presented above. Higher values are obtained with the increase in the indentations or convolutions of the outline.[4]
The present study demonstrated that there is a highly significant correlation between the thickness of epithelium and cell morphology that is, cell roundness and contour index in varying stages of OSMF compared to normal epithelium which has not been correlated so far.
Shabana et al. studied the size and shape of the cells in the basal cell layer of the oral epithelium in four groups of white lesions (traumatic keratosis, lichen planus, leukoplakia and a “risk group”) in addition to two control groups (normal mucosa and squamous cell carcinoma) from oral mucosa by using an interactive image analysis system (IBAS-1). The results showed a progressive increase in the dimensions (area, perimeter and maximum diameter) of the cells from normal mucosa through traumatic keratosis, lichen planus, leukoplakia and the “risk group” to carcinoma, with considerable differences. A substantial increase in the dimensions of the cell was found in the “risk group.” They concluded that the shape factors and contour index seemed to be less helpful in the identification of the “risk group” and the size of the basal cell and its nucleus can be of diagnostic value for lesions with a high risk of malignant transformation.[4] Gao using IBAS-II studied morphometry of the cells in the spinous cells of oral epithelium in five groups, normal mucosa, OSMF, leukoplakia, epithelial dysplasia and squamous cell carcinoma. The results showed a progressive reduction in the dimensions (area, perimeter, all kinds of diameter) of the cells from normal mucosa, through OSMF, leukoplakia, dysplasia to carcinoma. According to the author, shape factors and contour index seemed to be less helpful in identification. It indicated that the decrease of cell area of the spinous cell could reflect a malignant progress and might be an objective feature to distinguish carcinoma from other lesions. The result also suggested that the pathological grade of OSMF is between normal mucosa and mild epithelial dysplasia.[19]
Gao et al. studied the morphometry of spinous cell in 16 specimens of OSMF using IBAS-II. Nineteen parameters of the size and shape were chosen and compared with normal mucosa, leukoplakia, dysplasia and carcinoma. The results indicated that the cell dimensions (area, perimeter, all kinds of diameter) in OSMF were between normal mucosa and dysplasia as well as carcinoma. This showed a progressive decrease (P < 0.01) and thus the decrease of cell area reflected a malignant progress.[20]
In agreement with, the present study also indicated a reduction in cell dimensions (area, perimeter) in OSMF with the increase in the severity of OSMF as compared to normal mucosa. However, the study also reflected that there was a significant increase in cell roundness with cells more oblong and flattened out in contrast to the polygonal shape of the cell with the decrease in thickness of epithelium. The study also reflected that the cells showed higher contour index with increased cellular outline indentations with the increasing severity of OSMF. With this, the dimensional variables of the cell seemed to be important for distinguishing the risk group.
These results have thus shown that the use of quantitative histological methods in determining the squamous epithelial structures made it possible to evaluate that there is thinning of epithelium in increasing stages of OSMF which may be due to an actual change in cell morphology of the individual cells of specific compartment or all compartments.
CONCLUSION
Thus, we conclude that deviation in the shape of epithelial cells (increase in cell roundness and contour index) in OSMF compared to normal epithelium seems to play a promising morphometric feature in causing thinning of epithelium, which has previously received no attention. Finally, we believe that the use of quantitative analysis offers a valuable and objective criterion, thus improving the differentiation of this condition from lesions carrying a greater risk of malignant change.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rajendran R. Oral submucous fibrosis: Etiology, pathogenesis, and future research. Bull World Health Organ. 1994;72:985–96. [PMC free article] [PubMed] [Google Scholar]
- 2.Pindborg JJ, Murti PR, Bhonsle RB, Gupta PC, Daftary DK, Mehta FS. Oral submucous fibrosis as a precancerous condition. Scand J Dent Res. 1984;92:224–9. doi: 10.1111/j.1600-0722.1984.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 3.Rajendran R, Vijayakumar T, Vasudevan DM. An alternative pathogenetic pathway for oral submucous fibrosis (OSMF) Med Hypotheses. 1989;30:35–7. doi: 10.1016/0306-9877(89)90122-9. [DOI] [PubMed] [Google Scholar]
- 4.Shabana AH, El-Labban NG, Lee KW. Morphometric analysis of basal cell layer in oral premalignant white lesions and squamous cell carcinoma. J Clin Pathol. 1987;40:454–8. doi: 10.1136/jcp.40.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Utsunomiya H, Tilakaratne WM, Oshiro K, Maruyama S, Suzuki M, Ida-Yonemochi H, et al. Extracellular matrix remodeling in oral submucous fibrosis: Its stage-specific modes revealed by immunohistochemistry and in situ hybridization. J Oral Pathol Med. 2005;34:498–507. doi: 10.1111/j.1600-0714.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz J. Demonstrated at the th International Dental Congress. London: 1952. Atrophia Idiopathica (Tropica) Mucosa Oris. [Google Scholar]
- 7.Lal D. Diffuse oral submucous fibrosis. J All India Dent Assoc. 1953;26:14–5. [Google Scholar]
- 8.Joshi SG. Submucous fibrosis of the palate and pillars. Indian J Otolaryngol. 1953;4:1–4. [Google Scholar]
- 9.Su JP. Idiopathic scleroderma of the mouth; report of three cases. AMA Arch Otolaryngol. 1954;59:330–2. doi: 10.1001/archotol.1954.00710050342011. [DOI] [PubMed] [Google Scholar]
- 10.Rao AB. Idiopathic palatal fibrosis. Br J Surg. 1962;50:23–5. doi: 10.1002/bjs.18005021907. [DOI] [PubMed] [Google Scholar]
- 11.Pindborg JJ, Sirsat SM. Oral submucous fibrosis. Oral Surg Oral Med Oral Pathol. 1966;22:764–79. doi: 10.1016/0030-4220(66)90367-7. [DOI] [PubMed] [Google Scholar]
- 12.Mehta FS, Hamner JE., 3rd . Tobacco Related Oral Mucosal Lesions and Conditions in India. Bombay: Basic Dental Research Unit; 1993. [Google Scholar]
- 13.Pindborg JJ, Chawla TN, Srivastava AN, Gupta D. Epithelial changes in oral submucous fibrosis. Acta Odontol Scand. 1965;23:277–86. doi: 10.3109/00016356509007516. [DOI] [PubMed] [Google Scholar]
- 14.Shear M, Lemmer J. Histological features of oral submucous fibrosis. Dent Pract. 1967;18:49–53. [PubMed] [Google Scholar]
- 15.Wahi PN, Luthra UK, Kapur VL. Submucous fibrosis of the oral cavity. Histomorphological studies. Br J Cancer. 1966;20:676–87. doi: 10.1038/bjc.1966.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pindborg JJ, Mehta FS, Daftary DK. Occurrence of epithelial atypia in 51 Indian villagers with oral submucous fibrosis. Br J Cancer. 1970;24:253–7. doi: 10.1038/bjc.1970.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mani NJ, Singh B. Studies on oral submucous fibrosis. III. Epithelial changes. Oral Surg. 1976;41:203–14. doi: 10.1016/0030-4220(76)90232-2. [DOI] [PubMed] [Google Scholar]
- 18.Canniff JP, Harvey W, Harris M. Oral submucous fibrosis: Its pathogenesis and management. Br Dent J. 1986;160:429–34. doi: 10.1038/sj.bdj.4805876. [DOI] [PubMed] [Google Scholar]
- 19.Gao S. Cell morphometric analysis in oral submucous fibrosis, leukoplakia and squamous cell carcinoma. Zhonghua Kou Qiang Yi Xue Za Zhi. 1992;27:145–7. 189. [PubMed] [Google Scholar]
- 20.Gao S, Liu S, Shen Z, Peng L. Morphometric analysis of spinous cell in oral submucous fibrosis. Comparison with normal mucosa, leukoplakia and squamous cell carcinoma. Chin Med J (Engl) 1995;108:351–4. [PubMed] [Google Scholar]


