The primary cilium was first so named by Sergei Sorokin in 1968.[1] Since the discovery of primary cilium in 1898 by Zimmerman, three major hypotheses for their function have been put forth. The first is that primary cilia are vestigial organelles inherited from an ancestor whose cells had motile cilia and now are of no purpose in multicellular organisms. The second is that they are involved in the control of cell cycle. And the third is that primary cilia are sensory organelles. There has been virtually no experimental evidence in support of atavistic hypothesis. Increasing evidence suggests that primary cilia are the key coordinators of signaling pathways during development and in tissue homeostasis and, when defective, are a major cause of human diseases and developmental disorders (now commonly referred to as ciliopathies).[1,2]
Most mammalian cells possess a solitary, nonmotile cilium known as primary cilium which projects from the apical surface of polarized and differentiated cells to the internal lumen of the tissues. Like the mitochondria, Golgi apparatus and endoplasmic reticulum, cilia function as specialized cellular organelles. All cilia are formed during interphase of the cell cycle from an ancestral basal body or elder centriole of the centrosome. They consist of an axoneme of nine doublet microtubules (MTs) that extends from a basal body, which is derived from the older [mother] centriole of the centrosome, surrounded by the ciliary membrane (a specialized domain extension of the cell membrane).[3] The MT pattern of the ciliary axoneme is conventionally abbreviated by referring to the numbers of peripheral doublets and single central MTs as 9 + 2, 9 + 0, etc. In contrast to those of motile 9 + 2 cilia, axonemes of nonmotile primary cilia lack key elements involved in ciliary motility, including the central pair of MTs and the proteins that surround them, mostly if not all radial spokes and, importantly, outer and inner dynein arms that power MT sliding to produce motility [Figure 1].[2,3]
Figure 1.
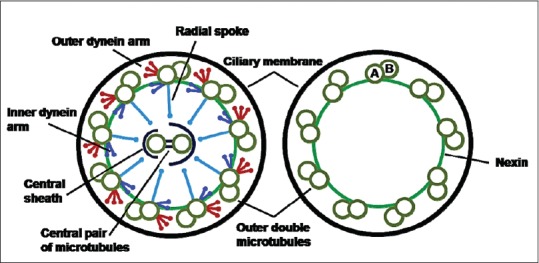
Schematic illustration of motile 9 + 2 cilia and primary 9 + 0 cilia. Motile cilia have inner and outer dynein arms, radial spokes, nexin links and a central microtubule pair surrounded by the central sheath. Primary cilia only have the nine outer microtubule doublets with the nexin links to stabilize the structure. The microtubule doublets are composed of A and B subfibers
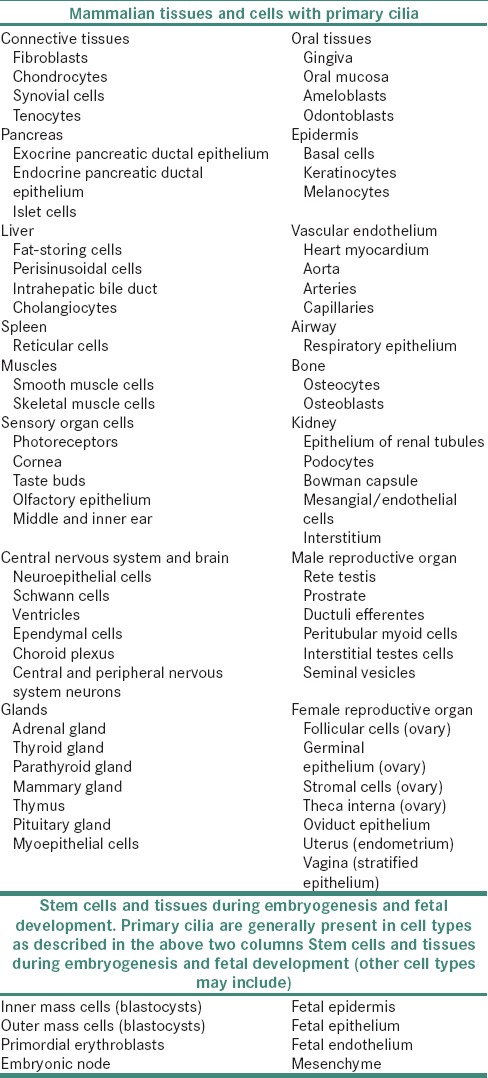
Single 9 + 0 primary cilia are found on a large number of cells in the mammalian body, including stem, epithelial, endothelial, connective tissue and muscle cells as well as neurons [Table 1].[1,3,4]
Table 1.
Expression of primary cilia in various mammalian tissues and cells
Both primary and motile cilia are assembled and maintained through a highly conserved process called intraflagellar transport (IFT). Functions of cilia that are not related to motility are thought to involve sensing of environmental cues. Because cilia protrude from the cell surface, they might act as antennae that receive signals from the periphery. The remote information may be converted into signaling cascades that are initiated within the ciliary compartment and then transduced to the cell body. Consistently, the ciliary membrane (which is continuous with the plasma membrane) contains various cilia-specific receptors, ion channels and signaling molecules. The signaling pathways coordinated by primary cilia are quite diverse and depend on the cell type [Table 2].[1]
Table 2.
Types of Ciliary signaling pathways

A single primary cilium can be set up for several different kinds of signaling and can respond, for example, to mechanical strain as well as to several morphogens, hormones or growth factors. Different receptors or channels can be present in the same cilium at the same time or at different times.[1,3]
Signaling through the primary cilium is of paramount importance during development and probably remains so in stem cell populations in various tissues. In the adult, primary cilia might still function in fibroblast cell cycle control and/or cell migration during tissue regeneration and wound healing. Most other differentiated, nondividing cells of the adult body, including neurons and kidney cells, possess primary cilia.
The primary cilia play critical roles associated with the epithelium–mesenchyme interaction in various tissues, including the tooth germ. Accumulating evidence shows that many growth factors/receptors and cross-talk through these factors between adjacent tissues precisely regulate tooth development and morphological and functional differentiation of ameloblasts.[5,6,7] Primary cilia are known to exert a specific negative regulatory effect on sonic hedgehog activity that functions to repress tooth formation and thus determine tooth number.[5] Cilia are aligned parallel to the dentin walls, with the top part oriented toward the pulp core, crucial for both dentin formation and tooth pain transmission. Analysis of the literature suggests putative role of cilia in sensing the microenvironment, probably related to dentin secretion. Thus, this organelle could represent a critical link between signals that influence cell fate (terminal differentiation of odontoblasts) and signals that influence cell movement toward the pulp matrix and consequently dentin architecture during the life of the tooth.[7,8] IFT proteins of primary cilia are essential in the development of bone and cartilage, as well as the differentiation and mechanotransduction of mesenchymal stem cells, osteoblasts, osteocytes and chondrocytes.
The primary cilia in these tissues might be necessary for the maintenance of differentiated state and suppression of cyst formation or oncogenesis.[9] In many tissues, aberrant activation or absence of ciliary signaling is correlated with uncontrolled cell division and cancer. Primary cilia are known to be associated with pathogenesis of keratocystic odontogenic tumor and dentigenous cyst.
A ciliopathy is classified as a disorder that results from aberrant form or function of primary cilia. As a class of diseases, ciliopathies have an extraordinarily broad range of clinical manifestations. The spectrum of phenotypes has been attributed to the purported ubiquitous nature of primary cilia. Ciliopathies with craniofacial defects include Bardet–Biedl syndrome, oral-facial-digital syndrome type 1, Meckel syndrome or Meckel–Gruber syndrome, Joubert syndrome, cranioectodermal dysplasia, frontonasal dysplasia and Ellis-van Creveld syndrome.[10,11]
Normal structure and function of primary cilia are required for a cell to transduce molecular signals from the environment. Several lines of evidence show that primary cilia have significant implications on both normal and abnormal maxillofacial development through the signaling molecules during development. Furthermore, the relationship between primary cilia and these signaling molecules raises the possibility that a number of craniofacial dysmorphologies may arise as a consequence of abnormal signaling secondary to ciliary dysfunction.
In the near future, it is likely that a number of craniofacial disorders, in which a genetic cause is not presently known, will be shown to involve impaired ciliogenesis or ciliary function. Recent advances in the understanding of how primary cilia contribute to craniofacial development have introduced a new class of genetic candidates for craniofacial syndromes of unknown etiology. The continued engineering of transgenic, ciliopathic animal models will allow for a deeper comprehension of how each tissue involved in the development of the craniofacial complex utilizes primary cilia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Dr. S. S. Vanaki and Dr. R. S. Puranik, Department of Oral and Maxillofacial Pathology and Microbiology, P M N M Dental College & Hospital, Bagalkot, Karnataka.
REFERENCES
- 1.Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010;123(Pt 4):499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohazama A, Haycraft CJ, Seppala M, Blackburn J, Ghafoor S, Cobourne M, et al. Primary cilia regulate Shh activity in the control of molar tooth number. Development. 2009;136:897–903. doi: 10.1242/dev.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abou Alaiwi WA, Lo ST, Nauli SM. Primary cilia: Highly sophisticated biological sensors. Sensors. 2009;9:7003–20. doi: 10.3390/s90907003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson CT, Castillo AB, Brugmann SA, Helms JA, Jacobs CR, Stearns T. Primary cilia: Cellular sensors for the skeleton. Anat Rec. 2008;291:1074–8. doi: 10.1002/ar.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hisamoto M, Goto M, Muto M, Nio-Kobayashi J, Iwanaga T, Yokoyama A. Developmental changes in primary cilia in the mouse tooth germ and oral cavity. Biomed Res. 2016;37:207–14. doi: 10.2220/biomedres.37.207. [DOI] [PubMed] [Google Scholar]
- 6.Liu B, Chen S, Cheng D, Jing W, Helms JA. Primary cilia integrate hedgehog and Wnt signaling during tooth development. J Dent Res. 2014;93:475–82. doi: 10.1177/0022034514528211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thivichon-Prince B, Couble ML, Giamarchi A, Delmas P, Franco B, Romio L, et al. Primary cilia of odontoblasts: Possible role in molar morphogenesis. J Dent Res. 2009;88:910–5. doi: 10.1177/0022034509345822. [DOI] [PubMed] [Google Scholar]
- 8.Magloire H, Couble ML, Romeas A, Bleicher F. Odontoblast primary cilia: Facts and hypotheses. Cell Biol Int. 2004;28:93–9. doi: 10.1016/j.cellbi.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Michaud EJ, Yoder BK. The primary cilium in cell signaling and cancer. Cancer Res. 2006;66:6463–7. doi: 10.1158/0008-5472.CAN-06-0462. [DOI] [PubMed] [Google Scholar]
- 10.Brugmann SA, Cordero DR, Helms JA. Craniofacial ciliopathies: A new classification for craniofacial disorders. Am J Med Genet A. 2010;152A:2995–3006. doi: 10.1002/ajmg.a.33727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fliegauf M, Benzing T, Omran H. When cilia go bad: Cilia defects and ciliopathies. Mol Cell Biol. 2007;8:880–93. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]


