Abstract
Candida albicans represents the most common commensal and opportunistic fungal pathogen colonizing humans. As a member of the normal microflora, it is present on the skin and the mucous membranes of the upper respiratory tract, gastrointestinal tract and female genital tracts. It is therefore not transmitted. It lies in wait for a change in some aspect of the host physiology that normally suppress growth and invasiveness through an enigmatic phenomenon called Phenotypic Switch System or White-Opaque Transition. This system involves reversible and heritable switching between alternative cellular phenotypes. White–opaque switching in Candida albicans was first discovered in 1987. This was initially identified in strain WO-1. Switching has been demonstrated to occur at sites of infection and to occur between recurrent episodes of infection in select cases esp. AIDS and diabetes.
Keywords: Candida albicans, opaque cells, phenotype, phenotypic switch system, white cells
INTRODUCTION
Candida albicans represents the most permeative fungal pathogen colonizing humans. C. albicans is an unicellular yeast of the Cryptococcaceae family with a single bud.[1] As a member of the normal microflora, it is present on the skin and the mucous membranes of the upper respiratory tract, gastrointestinal tract and female genital tracts. It is, therefore, not transmitted. It reproduces by asexual budding and grows rapidly at 25–37°C and within a pH range of 2–8. There are several medically important Candida species, of which C. albicans, Candida tropicalis, Candida glabrata, Candida parapsilosis and Candida krusei are the most frequently isolated. Among these, C. albicans is the far most common species present in health and disease.[2,3,4]
C. albicans exists in two forms – pseudohyphae and yeast forms – a trait known as dimorphism (grows as either yeast or hyphal cells). The yeast form is believed to be innocuous, but the hyphae form is usually associated with invasion into the host tissue. The hyphae are approximately 2 μm in diameter and showed a large population of mitochondria localized at the tip region. This organization is due to large energy required for hyphae extension.[5] This transition from a benign yeast type to highly invasive hyphae type depends on changes in the host defenses, i.e., elude the immune system by altering the surface antigens and on environmental signals that allow it to express pathogenic factors which are normally repressed including changes in the fungal response to antifungal agents.
The factors involved its pathogenicity are – mannoprotein component, i.e., the extracellular polymeric material that coats the surface of C. albicans plays a role in adherence. Second, C. albicans produces a range of proteinases and phospholipases which are particularly concentrated on the tips of the fungal hyphae that disrupts the cell membrane and results in invasion of the pathogen into host tissue. Third, to be a successful pathogen, C. albicans has developed the capacity to vary phenotype by spontaneously generating variants within infecting populations. This empirical axiomatic phenomenon is called as Phenotypic Switching System.[2] It is also known as phenotypic plasticity or bistable switching system (white to opaque) or tristable (white to gray to opaque) switching system.[6]
WHAT IS PHENOTYPIC SWITCHING: EVOLUTION OF IT'S TERMINOLOGY
Phenotypic switching is defined as “an in vitro reversible phenomenon with spontaneous emergence of colonies with altered colony morphology at rates higher than somatic mutation rates.”[7,8] This insidious strategy allows them to undergo rapid adaptation in response to environmental challenges such as individual body locations that may exhibit drastic differences in temperature and pH. The ability to grow in different morphological forms is critical for both its commensal lifestyle and its existence as a pathogen.[6,9,10]
The ‘’white-opaque’’ transition is a well-known bistable phenotypic switching system in C. albicans.[11] Recently, it has been observed that an intermediate phase between the white and opaque phenotypes does exist and proposed that the phenotypic switching system in this species may be tristable; the so-called ‘’white-gray-opaque’’ tristable phenotypic switching system in C. albicans[12] as illustrated in Figure 1. Switching can also be seen in C. glabrata[13] and Candida neoformans.[7] Switching has been demonstrated to occur at sites of infection and between recurrent episodes of infection in selected cases, especially AIDS and diabetes.[8]
Figure 1.
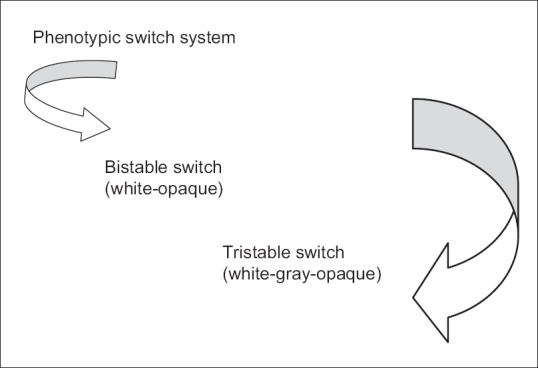
Diagrammatic illustration of phenotypic switch system with its terminology
PHENOTYPIC SWITCHING IN CANDIDA ALBICANS AND IT'S HISTORICAL BACKGROUND
C. albicans is a commensal that colonizes the human host in various niches. Three different switching systems were first described by Soll et al.[14,15] The first candida model switching strain, 3153A switching system, discovered in 1985, demonstrated that common strains of C. albicans switched reversibly between a number of variant phenotypes[16,17] that were quite heterogeneous and produced at least seven different phenotypes including “original smooth,” “star,” “irregular wrinkle,” “ring,” “mottled,” “fuzzy,” and “revertant smooth.”[8,16,18] Another system included strains that switch between colonies with and without dense myceliation.[14] Finally, the classically studied strain is white-opaque switching, first discovered in 1987 was isolated from the bloodstream of a bone marrow transplant patient at The University of Iowa Hospitals and Clinics.[19] This strain consists of two phases, one that grows as smooth white colonies called the white cells and one that is rod-like and grows as flat gray colonies called the opaque cells.
In yesteryears, most research has been performed on the white-opaque switching strain.[7] A novel morphological phenotype of C. albicans, referred to as the ‘’gray’’ phenotype was identified. This phenotype is heritable, but distinct from the previously identified white and opaque phenotypes in cellular and colony appearance, global gene expression profiles, secreted aspartyl proteinase (SAP) activities and virulence characteristics. The gray phenotype, together with the white and opaque phenotypes, forms a novel tristable and heritable switching system in C. albicans.[6]
The various distinguishing features between white and opaque cells are as follows:
White cells are round with smooth, domed colonies, whereas opaque cells are bean-shaped, flatter with translucent colonies[20,21]
The interaction of white and opaque cells with the immune system also differs, i.e., white cells secrete a chemoattractant for leukocytes, whereas opaque cells do not. In addition, white and opaque cells are phagocytosed by macrophages, suggesting that white-opaque switching may be an adaptive mechanism to favor C. albicans cells escape the host immune system[21]
Opaque cells differed from white cells in their sensitivity to neutrophils and reactive oxygen species, adhesion to human cells and plastics, secretion of proteinases and resistance to antifungals[21]
One of the most fascinating differences observed between white and opaque cells was the expression of genes involved in glucose metabolism. White cells specially expressed genes involved in fermentative metabolism, whereas opaque cells expressed genes involved in oxidative metabolism. This difference occurred under both aerobic and anaerobic conditions[21]
White and opaque cells also differ in their gene expression profiles, mating competency and virulence characteristics.[19,22] Opaque cells can mate more efficiently and are better at cutaneous infections than white cells, whereas white cells are more virulent in systemic candidiasis.[19,23] as illustrated in Figures 2 and 3.
Figure 2.
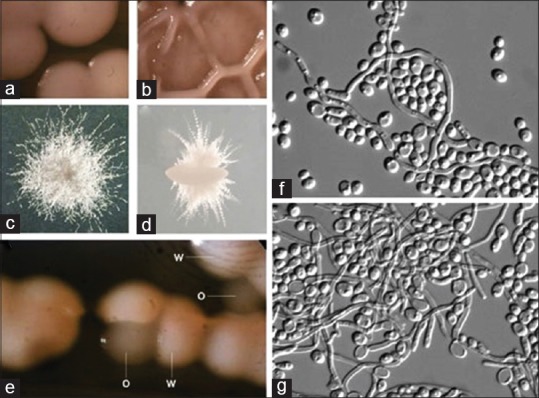
Photomicrograph of white hemispherical colony morphology referred to as the “white phase and a grey flat colony morphology, referred to as the “opaque phase as shown from (a-g). (Courtesy : Judith Berman and Peter E. Sudbery Candida albicans: A molecular revolution built on lessons from budding yeast. Nature Reviews Genetics 3, 918-932; December 2002)
Figure 3.
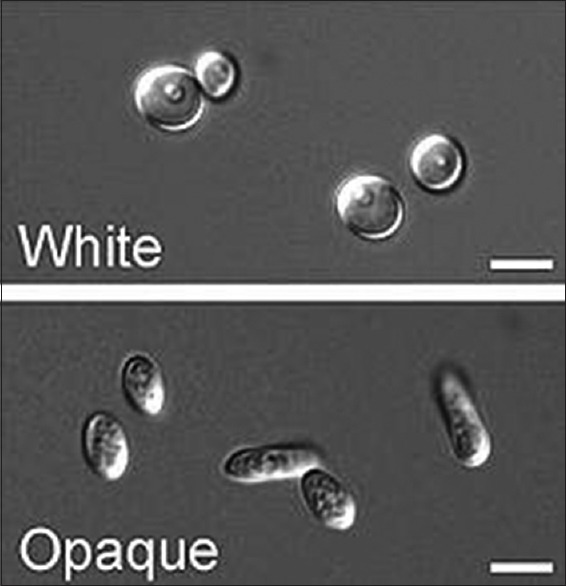
Photomicrograph of white cells phase and opaque cells. (Courtesy: Judith Berman and Peter E. Sudbery Candida albicans: A molecular revolution built on lessons from budding yeast. Nature Reviews Genetics 3, 918-932; December 2002)
MOLECULAR PATHOGENESIS OF SWITCHING SYSTEM
Antigenic variation, a common adaptive strategy of microbial pathogens, is mechanisms used by these pathogens to stochastically change their cell surface composition. Strains of C. albicans, the most important fungal pathogen of humans, are able to spontaneously and reversibly switch phenotypes that affects the size and shape of cells, their ability to form hyphae, their surface properties of adhesion and permeability, membrane composition, range of secretory products, sensitivity to neutrophils and oxidants, antigenicity and drug susceptibility. These traits are transcriptionally controlled by expression of various genes such as SAP genes SAP1 and SAP3,[24,25,26,27,28] a glucose-lipid-regulated protein gene WH11,[29,30] membrane protein gene OP4,[26] two component regulator gene CaNIK1,[31] transcription factor gene EFG1[32] and a transporter gene CDR3.[33] Studies using high-density oligonucleotide microarray analysis to profile global gene expression ratified that these genes were associated with wide range of functional identification that inferred metabolic preferences and concluded that switching extends beyond antigenic variation to include metabolic pathways, which itself may reflect inherent differences in the efficiency with which each switch phenotype is able to exploit available nutrients and adapt to ambient conditions. For example, opaque cells expressed a transcriptional profile consistent with oxidative metabolism and white cells expressed a fermentative one. Furthermore, the virulence of W- and O-cells depends on the site of infection; W-cells were more virulent in disseminated infections and O-cells were more virulent in cutaneous ones, which is consistent with the availability of glucose in the bloodstream and interstitial spaces, but its absence in the skin.[34] This bias was obtained regardless of carbon source, suggesting a connection between phenotypic switching and metabolic flexibility, where metabolic specialization of switch phenotypes enhances selection in relation to the nutrients available at different anatomical sites.
HOST PATHOGEN INTERACTION
C. albicans is a communistic microorganism, in which phenotypic switching play an important role in the biology of C. albicans and in the interactions of this fungus with its host. In response to various environmental signals, C. albicans changes its growth mode from the budding yeast form to filamentous growth, which facilitates tissue invasion.[35] The various strains of C. albicans as described previously are homozygous at the mating type locus (MTL) and can spontaneously and reversibly switch from the normal yeast morphology (white) to an elongated cell type (opaque), which is the mating-competent form of the fungus. This influences the ability of C. albicans to colonize and proliferate in specific host niches and its susceptibility to host defense mechanisms. It is currently not understood why C. albicans has integrated a morphological switch into its life cycle to undergo sexual development. C. albicans cells that have become homozygous for the MTL first have to switch from the normal yeast morphology to the opaque cell form to become mating competent.[1,36] This again is influenced by various environmental signals,[21,37] and once these signals are dominated, the switch is turn on and causes superficial infections of skin, mucosa as well as life-treating disseminated infections, especially in immunocompromised individuals.
As we all know in humans, the two important cells that act as the first line of defense in innate immunity are the neutrophils and the dendritic cells. Earlier work by eminent researchers has shown that opaque cells do not readily undergo filamentation under most conditions that promote hyphal formation in white cells, which may result in a reduced ability to invade tissues.[38] Geiger et al.[39] in his study showed that opaque cells do not produce a chemoattractant for neutrophils that is secreted by white cells pointed to the possibility that opaque cells can also avoid detection by these important first-line host defense cells. Kolotila and Diamond[40] hypothesized that opaque cells are in fact more susceptible than white cells to killing by neutrophils and also stimulate the production of reactive oxygen species, which is one mechanism by which these phagocytes destroy invading pathogens, more strongly than do white cells.
Soloviev et al.,[41] in 2007, said that there was another attractive candidate, the pH-regulated antigen Pra1, which is predominantly expressed in a highly glycosylated form on the surface of C. albicans hyphae, but not yeast cells and bound by the αmβ2 receptor on PMNs. Pra1 also acts as a chemoattractant for PMNs, and deletion of PRA1 decreased PMN migration and PMN adhesion to and killing of C. albicans. In 2011, he also reported that dendritic cells can recognize C. albicans by an αmβ2 independent mechanism, which would explain our finding that dendritic cells phagocytosed opaque cells under conditions, in which they were not attacked by neutrophils. Dwivedi et al.,[42,43] in 2011, concluded that the hyphae express many other cell surface proteins that are not found on yeast cells. One of these, Hyr1, confers even increased resistance to killing by neutrophils and other phagocytes. Hence, by ratification of various studies, this conflicting bias remains to be established to know which hypha-specific antigens and corresponding receptors on neutrophils are responsible for the selective phagocytosis of germinating C. albicans.
CONCLUSION
Understanding the regulatory mechanisms of phenotypic switching system will provide insights into several fundamental questions such as how pathogens adapt to the host and survive and propagate under diverse niches. This treatise review explores phenotypic switching in the most common pathogenic fungi, C. albicans and describes the various traits involved, molecular pathogenesis of this switching system and its role in immune cells.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Soll DR, Lockhart SR, Zhao R. Relationship between switching and mating in Candida albicans. Eukaryot Cell. 2003;2:390–7. doi: 10.1128/EC.2.3.390-397.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh A, Verma R, Murari A, Agrawal A. Oral candidiasis: An overview. J Oral Maxillofac Pathol. 2014;18(Suppl 1):S81–5. doi: 10.4103/0973-029X.141325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasanna KR. Oral candidiasis – A review. Scholarly J Med. 2012;2:6–30. [Google Scholar]
- 4.Dangi YS, Soni MS, Namdeo KP. Oral candidiasis: A review. Int J Pharm Pharm Sci. 2010;2:36–41. [Google Scholar]
- 5.Lehmann PF. Fungal structure and morphology. Med Mycol. 1998;4:57–8. [Google Scholar]
- 6.Tao L, Du H, Guan G, Dai Y, Nobile CJ, Liang W, et al. Discovery of a “white-gray-opaque” tristable phenotypic switching system in Candida albicans: Roles of non-genetic diversity in host adaptation. PLoS Biol. 2014;12:e1001830. doi: 10.1371/journal.pbio.1001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain N, Hasan F, Fries BC. Phenotypic switching in fungi. Curr Fungal Infect Rep. 2008;2:180–8. doi: 10.1007/s12281-008-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soll DR. High-frequency switching in Candida albicans. Clin Microbiol Rev. 1992;5:183–203. doi: 10.1128/cmr.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiteway M, Bachewich C. Morphogenesis in Candida albicans. Annu Rev Microbiol. 2007;61:529–53. doi: 10.1146/annurev.micro.61.080706.093341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pande K, Chen C, Noble SM. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat Genet. 2013;45:1088–91. doi: 10.1038/ng.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slutsky B, Staebell M, Anderson J, Risen L, Pfaller M, Soll DR. “White-opaque transition”: A second high-frequency switching system in Candida albicans. J Bacteriol. 1987;169:189–97. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie J, Du H, Guan G, Tong Y, Kourkoumpetis TK, Zhang L, et al. N-acetylglucosamine induces white-to-opaque switching and mating in Candida tropicalis, providing new insights into adaptation and fungal sexual evolution. Eukaryot Cell. 2012;11:773–82. doi: 10.1128/EC.00047-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lachke SA, Joly S, Daniels K, Soll DR. Phenotypic switching and filamentation in Candida glabrata. Microbiology. 2002;148(Pt 9):2661–74. doi: 10.1099/00221287-148-9-2661. [DOI] [PubMed] [Google Scholar]
- 14.Soll DR, Langtimm CJ, McDowell J, Hicks J, Galask R. High-frequency switching in Candida strains isolated from vaginitis patients. J Clin Microbiol. 1987;25:1611–22. doi: 10.1128/jcm.25.9.1611-1622.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soll DR. Molecular biology of Candida pathogenesis. In: Kirsch DR, Kelly R, Kurtz MB, editors. The Genetics of Candida. Boca Raton, FL: CRC Press; 1990. pp. 147–76. [Google Scholar]
- 16.Slutsky B, Buffo J, Soll DR. High-frequency switching of colony morphology in Candida albicans. Science. 1985;230:666–9. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- 17.Pomés R, Gil C, Nombela C. Genetic analysis of Candida albicans morphological mutants. J Gen Microbiol. 1985;131:2107–13. doi: 10.1099/00221287-131-8-2107. [DOI] [PubMed] [Google Scholar]
- 18.Ramsey H, Morrow B, Soll DR. An increase in switching frequency correlates with an increase in recombination of the ribosomal chromosomes of Candida albicans strain 3153A. Microbiology. 1994;140(Pt 7):1525–31. doi: 10.1099/13500872-140-7-1525. [DOI] [PubMed] [Google Scholar]
- 19.Soll DR. Why does Candida albicans switch? FEMS Yeast Res. 2009;9:973–89. doi: 10.1111/j.1567-1364.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JM, Soll DR. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J Bacteriol. 1987;169:5579–88. doi: 10.1128/jb.169.12.5579-5588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alby K, Bennett RJ. Stress-induced phenotypic switching in Candida albicans. Mol Biol Cell. 2009;20:3178–91. doi: 10.1091/mbc.E09-01-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller MG, Johnson AD. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell. 2002;110:293–302. doi: 10.1016/s0092-8674(02)00837-1. [DOI] [PubMed] [Google Scholar]
- 23.Bennett RJ, Johnson AD. Mating in Candida albicans and the search for a sexual cycle. Annu Rev Microbiol. 2005;59:233–55. doi: 10.1146/annurev.micro.59.030804.121310. [DOI] [PubMed] [Google Scholar]
- 24.Hube B, Monod M, Schofield DA, Brown AJ, Gow NA. Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol Microbiol. 1994;14:87–99. doi: 10.1111/j.1365-2958.1994.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 25.Morrow B, Srikantha T, Soll DR. Transcription of the gene for a pepsinogen, PEP1, is regulated by white-opaque switching in Candida albicans. Mol Cell Biol. 1992;12:2997–3005. doi: 10.1128/mcb.12.7.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrow B, Srikantha T, Anderson J, Soll DR. Coordinate regulation of two opaque-phase-specific genes during white-opaque switching in Candida albicans. Infect Immun. 1993;61:1823–8. doi: 10.1128/iai.61.5.1823-1828.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrow B, Ramsey H, Soll DR. Regulation of phase-specific genes in the more general switching system of Candida albicans strain 3153A. J Med Vet Mycol. 1994;32:287–94. doi: 10.1080/02681219480000361. [DOI] [PubMed] [Google Scholar]
- 28.White TC, Miyasaki SH, Agabian N. Three distinct secreted aspartyl proteinases in Candida albicans. J Bacteriol. 1993;175:6126–33. doi: 10.1128/jb.175.19.6126-6133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kvaal CA, Srikantha T, Soll DR. Misexpression of the white-phase-specific gene WH11 in the opaque phase of Candida albicans affects switching and virulence. Infect Immun. 1997;65:4468–75. doi: 10.1128/iai.65.11.4468-4475.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srikantha T, Soll DR. A white-specific gene in the white-opaque switching system of Candida albicans. Gene. 1993;131:53–60. doi: 10.1016/0378-1119(93)90668-s. [DOI] [PubMed] [Google Scholar]
- 31.Srikantha T, Tsai L, Daniels K, Enger L, Highley K, Soll DR. The two-component hybrid kinase regulator CaNIK1 of Candida albicans. Microbiology. 1998;144(Pt 10):2715–29. doi: 10.1099/00221287-144-10-2715. [DOI] [PubMed] [Google Scholar]
- 32.Sonneborn A, Tebarth B, Ernst JF. Control of white-opaque phenotypic switching in Candida albicans by the Efg1p morphogenetic regulator. Infect Immun. 1999;67:4655–60. doi: 10.1128/iai.67.9.4655-4660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srikantha T, Tsai LK, Daniels K, Soll DR. EFG1 null mutants of Candida albicans switch but cannot express the complete phenotype of white-phase budding cells. J Bacteriol. 2000;182:1580–91. doi: 10.1128/jb.182.6.1580-1591.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kvaal C, Lachke SA, Srikantha T, Daniels K, McCoy J, Soll DR. Misexpression of the opaque-phase-specific gene PEP1 (SAP1) in the white phase of Candida albicans confers increased virulence in a mouse model of cutaneous infection. Infect Immun. 1999;67:6652–62. doi: 10.1128/iai.67.12.6652-6662.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumamoto CA, Vinces MD. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell Microbiol. 2005;7:1546–54. doi: 10.1111/j.1462-5822.2005.00616.x. [DOI] [PubMed] [Google Scholar]
- 36.Johnson A. The biology of mating in Candida albicans. Nat Rev Microbiol. 2003;1:106–16. doi: 10.1038/nrmicro752. [DOI] [PubMed] [Google Scholar]
- 37.Huang G, Srikantha T, Sahni N, Yi S, Soll DR. CO(2) regulates white-to-opaque switching in Candida albicans. Curr Biol. 2009;19:330–4. doi: 10.1016/j.cub.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson J, Cundiff L, Schnars B, Gao MX, Mackenzie I, Soll DR. Hypha formation in the white-opaque transition of Candida albicans. Infect Immun. 1989;57:458–67. doi: 10.1128/iai.57.2.458-467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geiger J, Wessels D, Lockhart SR, Soll DR. Release of a potent polymorphonuclear leukocyte chemoattractant is regulated by white-opaque switching in Candida albicans. Infect Immun. 2004;72:667–77. doi: 10.1128/IAI.72.2.667-677.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolotila MP, Diamond RD. Effects of neutrophils and in vitro oxidants on survival and phenotypic switching of Candida albicans WO-1. Infect Immun. 1990;58:1174–9. doi: 10.1128/iai.58.5.1174-1179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soloviev DA, Fonzi WA, Sentandreu R, Pluskota E, Forsyth CB, Yadav S, et al. Identification of pH-regulated antigen 1 released from Candida albicans as the major ligand for leukocyte integrin alphaMbeta2. J Immunol. 2007;178:2038–46. doi: 10.4049/jimmunol.178.4.2038. [DOI] [PubMed] [Google Scholar]
- 42.Dwivedi P, Thompson A, Xie Z, Kashleva H, Ganguly S, Mitchell AP, et al. Role of Bcr1-activated genes Hwp1 and Hyr1 in Candida albicans oral mucosal biofilms and neutrophil evasion. PLoS One. 2011;6:e16218. doi: 10.1371/journal.pone.0016218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo G, Ibrahim AS, Spellberg B, Nobile CJ, Mitchell AP, Fu Y. Candida albicans Hyr1p confers resistance to neutrophil killing and is a potential vaccine target. J Infect Dis. 2010;201:1718–28. doi: 10.1086/652407. [DOI] [PMC free article] [PubMed] [Google Scholar]


