Abstract
There are intense published data in literature related to cell engulfment phenomena such as emperipolesis, entosis and cell cannibalism. All these are closely related phenomena with a very fine line of differences. Its correct identification has a significant diagnostic and prognostic value. After extensive literature search, a gap of knowledge was found in concept designing and clarity about understanding of aforementioned terminologies. The authors have attempted to review data of these closely knit terminologies and further organize its characteristic appearances, pathogenetic aspects and prognostic implications. The data published in English Language, from 1925 to 2015, were collected using keywords such as emperipolesis, entosis and cell cannibalism through scientific database systems such as MEDLINE, Science Direct, Cochrane Library and Google Scholar. Articles were selected which have focused to explain the phenomenon, presentation and pathogenesis of one or more of this phenomenon. A total of 48 articles were retrieved, thirty of which were selected. The various cell engulfment phenomena are very similar looking but operate through entirely different pathways.
Keywords: Cell cannibalism, emperipolesis, entosis, phagocytosis
INTRODUCTION
Cell engulfment is a well-known phenomenon. The interfacial energies of the host cell and the engulfed cell play an important role in cell–to-cell interactions.[1] The phenomenon of a cell harboring an another living cell is a very extraordinary finding. Study of cell-in-cell phenomena began with the desire of Lewis to understand his observation of nonphagocytotic process of cell eating a cell.[1] Some such phenomena are such as emperipolesis, cell cannibalism and entosis. Scarcity of sufficient evidence has lead different researches to propose varying concepts and hypothesize about their occurrence. These terminologies, as previously mentioned, are very closely knit but differ in the variety of the involved host cell and the internalized cells. The molecular events and pathogenesis controlling them also vary considerably. However, following cell internalization, it is very difficult to differentiate one from the other.[2] With this view in mind, the current review attempts to throw light on these related terminologies. Focus has been put on the occurrence, pathogenesis and appearance of emperipolesis, entosis and cell cannibalism.
EMPERIPOLESIS
Emperipolesis is a condition, wherein hematopoietic cells in living and intact state are seen in the cytoplasm of host cell without any damage.[3] The cells taken in frequently are neutrophils, lymphocytes and plasma cells. The host cells may be a megakaryocyte, monocyte, endothelial cell, fibroblast and a malignant cell.[3,4] In this phenomenon with both cells being viable, there is not any physiological and morphological consequences to either of them.[5,6] The term was first described by Humble et al. in 1956 as, “the active penetration of one cell by another which remains intact.”[3,4] The term comes from the Greek word, “Emperipolesis,” which means “inside round about wandering.”[4] Many physiologic and pathologic conditions exhibit this phenomenon [Table 1].[3]
Table 1.
Physiological and pathological conditions exhibiting emperipolesis

Phagocytosis differs from emperipolesis in the way that the engulfing cell here does not engulf a living cell, rather focuses on dead or dying cells and other materials floating around the extracellular space. Also that in phagocytosis, there is an active participation of the host cell cytoskeleton (actin microfilaments) to form pseudopodia or extensions to engulf the dead material. Then, engulfed material is then transported inside the cytoplasm with the help of endoplasmic reticulum and lysosomal complex. The released metabolic breakdown products of the process can then be further used for synthesis of cellular structures or released outside the cell.[2]
Emperipolesis has been detected in bone marrow aspirates, tissue cultures, cerebrospinal fluid (CSF), fine-needle aspiration cytology (FNAC) and imprint cytology. Amita et al. described a case of adult T-cell lymphoblastic lymphoma (mediastinal type) showing emperipolesis, which was detected in FNAC and imprint cytology. It has been more commonly seen in non-Hodgkin's lymphoma than in Hodgkin's lymphoma.[3]
Rosai–Dorfman disease (RDD) is a benign proliferative disorder of histiocytes.[1] Here, the involved histiocytes exhibit emperipolesis of lymphocytes, plasma cells, neutrophils and red blood cells [Figure 1].[7,8,9,10] Emperipolesis in a lymph node with mixed inflammatory infiltrate is a feature suggestive of RDD [Table 2].[10,11,12] Even CSF in patients of RDD is diagnostic of emperipolesis.[11]
Figure 1.
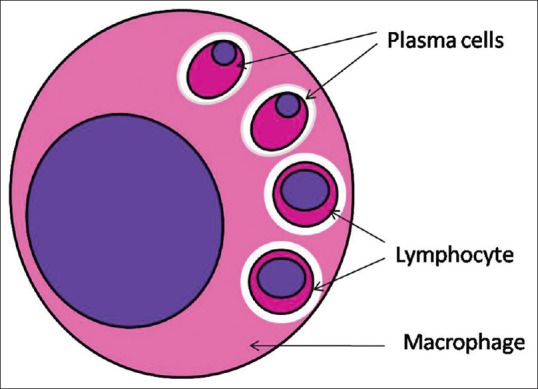
Emperipolesis of different variety of cells (red blood cells and plasma cells) in a vacuolated space by a megakaryocyte
Table 2.
Immunoreactivity of histiocytes diagnostic for emperipolesis

In neutrophilic emperipolesis seen associated with fibrosis, the neutrophils interact with megakaryocyte through abnormal P-selectin localization on the demarcation membrane system (DMS).[12] In bone marrow, megakaryocytic emperipolesis has been reported to increase in incidences of granulocytic or erythropoietic hyperplasia. The emperipoletic activity of erythroblasts in liver has been found to increase in periods of high level of hepatic erythropoietic activity and relatively anemic fetal state.[13]
HISTOPATHOLOGIC CHARACTERISTICS
In emperipolesis, the engulfed cell is enclosed in a membrane-bound vacuole in the cytoplasm of host cell. This gives a rim of clear halo around the engulfed cell [Figures 1 and 2]. Sometimes, vacuolations are also seen in the cytoplasm.[3] On occasion, internalized cells appear to continue living within the host cell for brief periods of time following the engulfment, sometimes even dividing within the vacuole, in which they are housed. Some internalized cells manage to escape from the host cell and survive to continue life as an individual cell. As opposite to phagocytosis, the engulfed cell here is not destroyed even after entering the cytoplasm of the host cell.[3]
Figure 2.
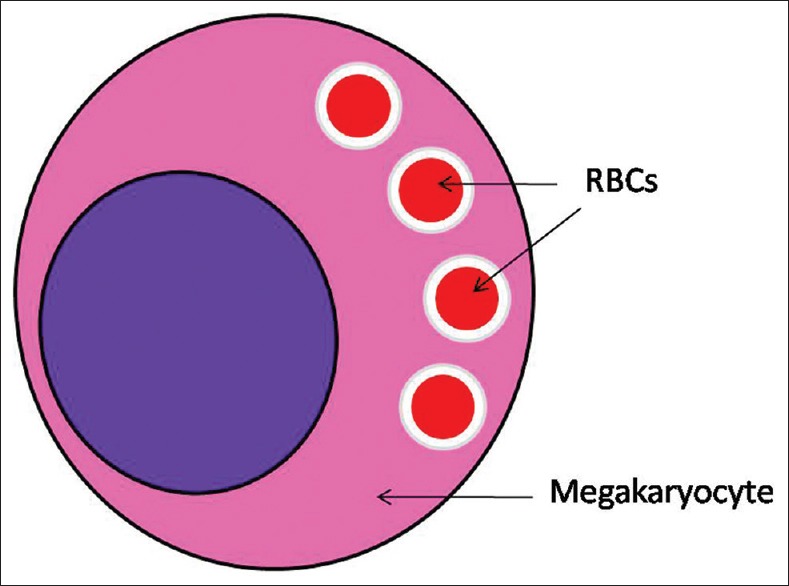
Emperipolesis of red blood cells in a vacuolated space by a megakaryocyte
PATHOGENESIS
Since the term “emperipolesis” was coined, several attempts have been made to understand how a cell inhabits the other, and the biological relevance of this co-habitation with their final respective fates. Emperipolesis is now understood as a mechanism to improve cell survival and help prevent apoptosis of cells within the host cell or control cells that could be cytotoxic to the host cells.[13]
Occurrence of emperipolesis in lymphomas is correlated with the role of cytokines released by lymphoma cells. Some investigators opine that the phenomenon is a result of active adherence of lymphocytes to tumor cells or macrophages with a subsequent inclusion in vacuoles inside the cytoplasm of this cells.[8,14]
Wang and Li attempted to propose the mechanism behind emperipolesis. They described this as a mechanism of the natural killer cell to mediate tumor cell death. For this, the membranes of both the target cell and the natural killer cell should be fluid enough to make this heterogeneous interaction successful enough, for its engagement with natural killer cells. They demonstrated at ultrastructural level the disintegration of host tumor cells following emperipolesis of natural killer through a lysosome-mediated degradation pathway. However, unexpectedly, few years later, they also discovered that some natural killer cells were disintegrated by tumor cells through a lysosome-mediated mechanism, similar to what was observed in entosis. The authors suggested emperipolesis is a pathway to mediate natural killer cell-mediated tumor cell death. Target cell membrane fluidity is essential for this interaction. This process requires free calcium, adhesion molecules and actin-based cytoskeleton.[15] Occasionally, mitotic activity was also observed in the invaded natural killer cells inside the host tumor cells after emperipolesis, similar to those observed in Overholtzer's report, suggesting an attempt to cell division within the host cell.[15,16]
In the bone marrow, erythroblastic emperipolesis is believed to be due to binding of the erythroblasts to the ED2 antigen or to the 30 kDa heparin-binding protein of the macrophages.[13] No such obvious cell-to-cell binding and/or communication has been noted in the occurrence of emperipolesis of erythroblasts by Kupffer cells in fetal liver.[13]
Emperipolesis of neutrophils in marrow fibrosis has been proposed to be a result of detection of P-selectin on the DMS of neutrophils. This is believed to be due to the release of neutrophil enzymes, in close proximity to the atypical granules within the cytoplasm of megakaryocyte, causing the granules themselves to release their content including growth factors such as transforming growth factor in the microenvironment through the DMS.[12] Abnormal P-selectin localization on the DMS triggers therefore might mechanism of pathologic neutrophil emperipolesis within the MK, which may cause marrow fibrosis and also extramedullary hemopoiesis in these mutants.
ENTOSIS
Overholtzer et al. observed a homogeneous cell-in-cell phenomenon similar to emperipolesis and gave the term “entosis” to it (from the Greek word “entos” for “inside” or “into”).[16] Here, a cell engulfs a cell of same type. While cells that are internalized by this mechanism are initially viable, with the majority of them eventually undergoing a nonapoptotic form of cell death that requires autophagy proteins.[17] This was proposed as a process of cell-in-cell invasion, which plays a physiological role in the elimination of cells detached from a surface.[18]
PATHOGENESIS
Entotic cell engulfment is induced by detachment of a cell from the extracellular matrices and further potentiated by imbalances in actomyosin contraction between the neighboring cells. Entosis is driven by imbalances in actomyosin contraction between the neighboring cells.[17] This similar cell–to-cell engulfment is mediated by adherens junction molecules such as E-cadherins, Rho GTPase and Rho-kinase (ROCK)-mediated actomyosin contraction within engulfing cells.[17] There is absence of integrin signaling and force-driven invasion of one cell into another cell. Cell engulfment requires actin, myosin II, Rho and ROCK activity of the invading cell and myosin-based contractile force from the recipient cell. Entosis also has been found to be leading to lysosomal cell death [Figure 3].[19]
Figure 3.
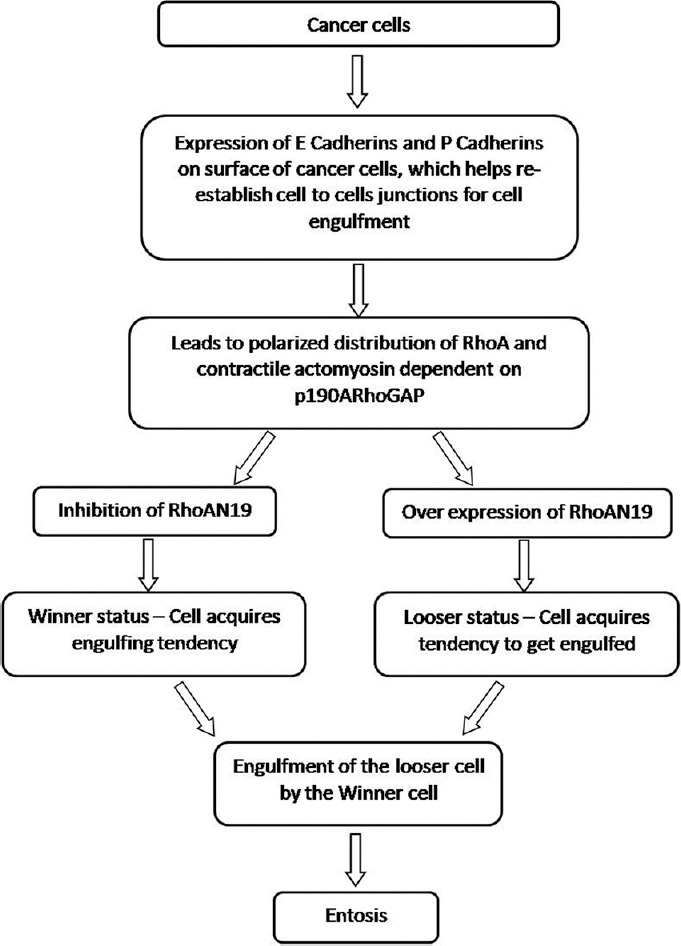
Pathogenesis of entosis
In this event, some invading cells try to escape through a transcytosis-like movement, whereas a small percentage of invading cells undergo cell division within the host cells.[6] This suggests that emperipolesis and entosis primarily differ in the mechanism though the outcomes of both processes may vary from existence of the engulfed cell, to cell division, to escape through transcytosis.
Previous literature supports the stimulation of entosis by oncogene Kras, as one class of tumor suppressors. This involves the regulation of epithelial cadherins E and P.[19] This oncogenic transformation in a cell leads to its capability to engulf the other cells [Figure 4]. This leads to physical elimination of the “loser” cells, which usually succumb to nonapoptotic cell death when the phagosome envelopes the engulfed cell. This phagosome has membrane surface LC3 which then fuses with lysosomes. Entosis exhibits β-catenin localization patterns that are indicative of a cell junction-mediated mechanism of engulfment.[19] The prognostic implications of entosis are varying in various studies. High number of cancer-initiating cells (CICs) in high-grade, aggressive breast cancers has been found to be indicative of poor prognosis. In contrast, high levels of CICs correlated with a lower incidence of metastases in pancreatic adenocarcinomas.[19]
Figure 4.
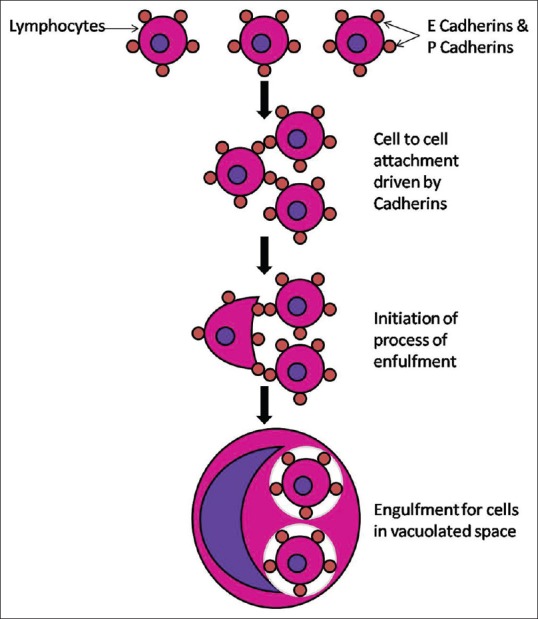
Schematic presentation of phenomenon of entosis
Sun et al. proposed that the mechanic deformability of the engulfing cell makes its function easier. As tumors are more mechanically heterogeneous than normal tissues, the tendency for tumor progression is further increased with an elevated mechanically deformable tendency of the cancer cells. This increases motility, entotic activity and the metastatic potential of the tumor.[20] Transfection enforced expression of active KrasV12 further increases this mechanical tendency to deformation. The effect of KrasV12 relies on Rac1.[20] Knockdown of Rac1 suppresses engulfment tendency (so-called “winner” phenotype status) conferred by KrasV12. On the other hand, expression of active Rac1 induces a “loser” status [Figure 3]. Entosis carried out by “winner” cells constitutes a competitive advantage to the aggressive tumor cells by giving the capability to the “winner cells” to retrieve amino acids and other building blocks from the engulfed cell or increasing their genomic instability subsequent to mitotic aberrations.[21]
CELLULAR CANNIBALISM
A much related phenomenon is cellular cannibalism, which is the ability of a cell to engulf another living cell of its own type or another. Leyden used the term, “birds” eye cells for cells exhibiting this phenomenon.[22] Other terms used in the literature to describe this phenomenon are “cellular phagocytosis,” “cell phagocytosis,” “cell–in-cell appearance,” “cell-in-cell pattern,” “one cell delicately wrapped around the next,” “tumor cell within a tumor cell,” “phagocytosis of tumor cell by tumor cell” and “tumor cell embraced by another tumor cell.”[23]
Self-cannibalism (macroautophagy) has been described as a process of cell repair, wherein there is a molecule and organelle recycling of the engulfed cell, allowing it to survive. In contrast to this term, xeno-cannibalism signifies a process where the engulfed cell is completely digested to death.[22]
The cannibalistic cell can engulf similar or dissimilar cell types. Most of the times an inactive lying cell becomes a favorite target of the cannibalistic cell. “Homotypic cell engulfment” is a term used when similar cell types have been engulfed, and heterotypic cell engulfment, wherein dissimilar cell is engulfed [Table 3].[22] Homotypic live cell engulfment (among cells of the same type) mostly occurs in cancers, probably reflecting major alterations in cellular physiology, which are associated with oncogenesis and tumor progression. Heterotypic live cell engulfment usually involves the ingestion of leukocytes by nonleukocytes (such as epithelial cells or fibroblasts).[19]
Table 3.
Types of cell cannibalism
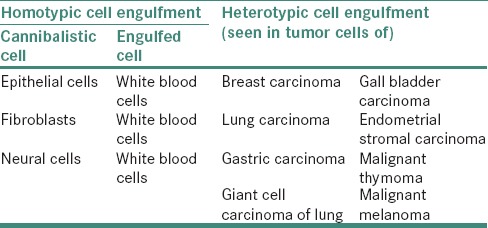
OCCURRENCE
The earliest report of a cell–in-cell phenomenon in tumors was reported by Steinhans in 1891. The engulfed cell still remains alive when internalized, but the process implies its death.[18] The process of cannibalism has been reported in breast carcinoma, giant cell carcinoma of lungs, gallbladder carcinoma, endometrial stromal carcinoma, malignant thymoma, malignant lymphoma, etc., In the literature, cancer cannibalism has been used to differentiate between benign tumors from malignant, but few studies recently have reported its occurrence in giant cell tumor of tendon sheath and central and peripheral giant cell granuloma (PGCG) of the oral cavity.[18]
In cannibalism, the tumor cells are seen engulfing cells such as other tumor cells, neutrophils and erythrocytes. Sachin et al. have noted and studied the phenomenon of neutrophil-tumor cell cannibalism (NTCC) in 500 cases of oral squamous cell carcinoma (OSCC). They found NTCC in 1.4% of cases of OSCC. The internalized neutrophil showed different stages of degeneration. They concluded that NTCC can predict the biological behavior and could be a useful prognostic marker for OSCC. The authors have proposed NTCC as an important histopathological prognostic indicator in OSCC.[24]
NTCC has also been reported in carcinoma arising from gallbladder (anaplastic carcinoma), small intestine (adenocarcinoma), pancreatic adenocarcinoma, infiltrating duct carcinoma of breast, squamous cell carcinoma of larynx and pleomorphic giant cell carcinoma of lung, gallbladder, pancreas and intestine. This neutrophilic engulfment helps in immune evasion and tumor survival.[25,26,27,28,29]
HISTOPATHOLOGIC CHARACTERISTICS
In cellular cannibalism, a cell gets entrapped within another cell and is present in a vacuolated space. This pushes the nucleus to the periphery. This mechanism has been proposed to create a favorable stay house for the tumor cells within the host immune cells, thereby escaping destruction [Figure 5].[22]
Figure 5.
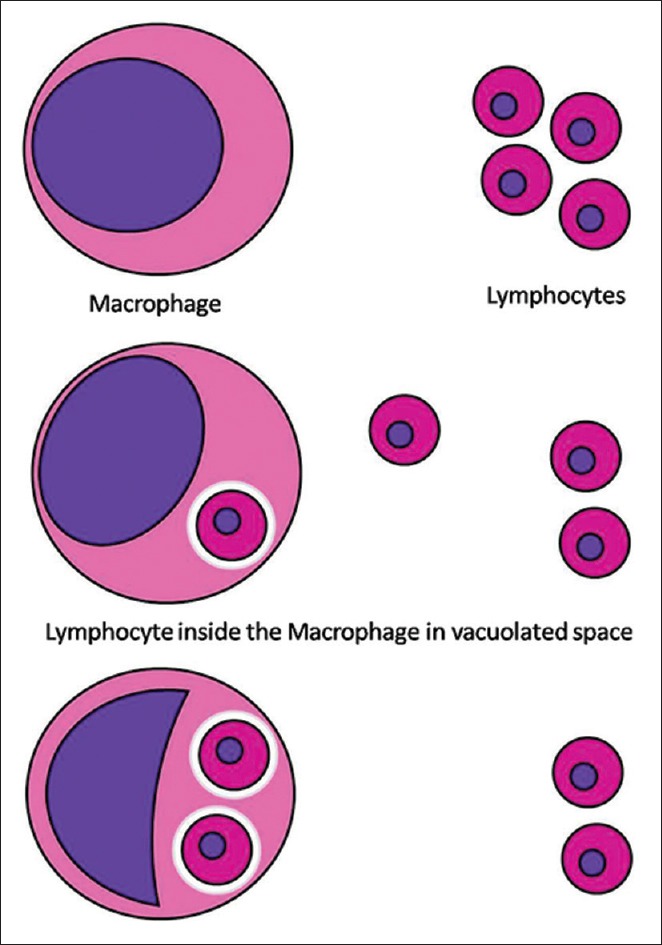
Schematic presentation of cellular cannibalism
PATHOGENESIS OF CELL CANNIBALISM
The mechanism of cannibalism has been attributed to the metabolic alterations in the malignant tumor cells, which encourages cannibalization of these cells within other similar tumor cells, in adverse conditions of hypoxia, decreased nutritional state and acidic states [Figure 6]. This leads to the selection of certain resistant cell phenotypes over others in caustic environment. These malignant cells are highly virulent and cannibalize other malignant cells and help them survive and progress in such difficult conditions. However, this pathogenesis cannot be considered for the giant cells as seen in PGCG and central giant cell granuloma, as they possess an inherent property of engulfment.[18]
Figure 6.
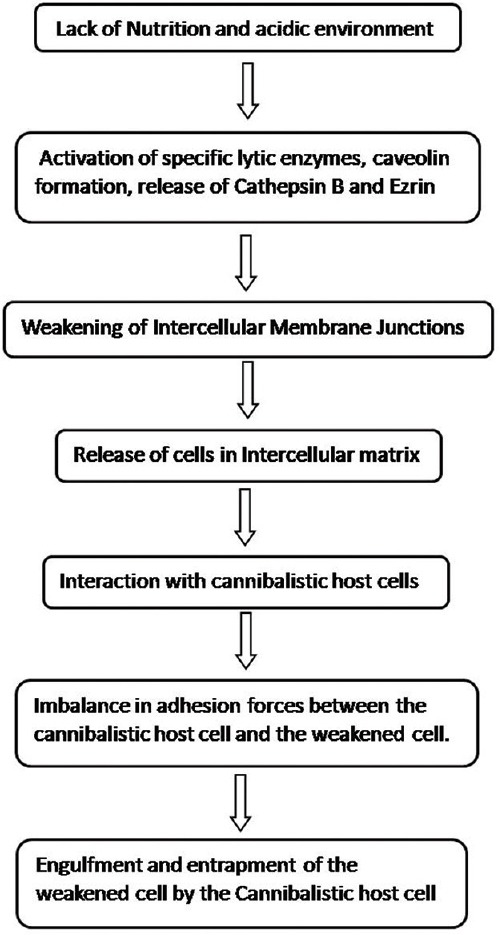
Pathogenesis of cell cannibalism
Krajcovic et al. suggested that cannibalistic cell engulfment leads to polyploidy, as internalized cells disrupt the cytokinesis of the engulfing cell. This behavior can promote tumor progression by inducing aneuploidy. Cannibalism-mediated chromosomal instability and aneuploidy may be one of the reasons for aggressive behavior of any cancer.[18]
At times, a malignant cell which has engulfed another malignant cell might, in turn, get engulfed by a third malignant cell. The process is referred to as complex cannibalism [Figure 7]. Cannibalistic giant cells as well as stromal cells express histiocytic markers. The internalized cells do not express bcl2, suggesting that this internalization induces apoptotic cell death.[22]
Figure 7.
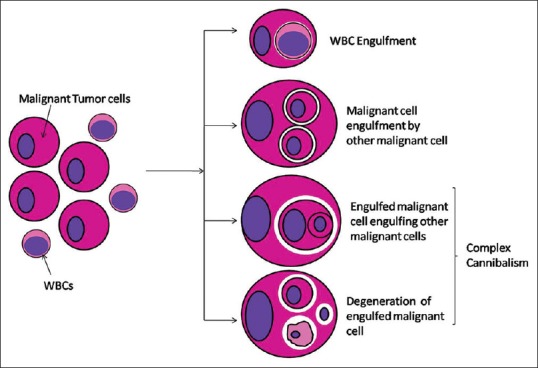
Schematic presentation of malignant tumor cell cannibalism and complex cannibalism
The hidden state holds great possibility of the escape of tumor cells from host immune system (immune escape). The number of cannibalistic cells directly correlates with the poor prognosis of the tumor. Cannibalistic cells are thus well related to aggressive behavior, anaplasia, invasiveness and metastatic potential of the malignancy.[22,23] This opens a new arena of research to definitely target such cells in tumor immunotherapy. Studying the degree and extent of cannibalistic cells stands as a vital prognostic indicator to know the aggressive biological behavior of a tumor. Studies related to mechanism of cell division of invading cell, molecular delineation of these events through nanoscale imaging and the involved protein-protein interactions is the need of the hour.
CONCLUSIONS
The process of emperipolesis, entosis and cannibalism is a very similar-appearing phenomenon but primarily differs in the patterns of operation and mechanisms involved. They provide a fascinating platform to study cell–in-cell interaction. As entosis and cannibalism are common findings in many tumors, further studies in relation to use in cancer diagnosis and prognosis would help us solve the hidden mystery behind the immunosurveillance exhibited by cancer. As these are easily identifiable morphological features under light microscopy, tumor aggressiveness can be routinely assessed, avoiding the use of expensive molecular techniques. In this review, an attempt was made to understand the pathways involved in these processes to distinctly characterize them.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Stuart AE. Phagocytic engulfment and cell adhesiveness as cellular surface phenomena. J Clin Pathol. 1977;30:592. [Google Scholar]
- 2.Jef A. It's a Cell-Eat-Cell World. In The Scientist. Exploring life Inspiring Innovation. [Last updated on 2011 Aug 01; Last assessed on 2015 Dec 25]. Available from: http://www.the-scientist.com/?articles.view/articleNo/30956/title/It-s-a-Cell-Eat-Cell-World/
- 3.Amita K, Vijay Shankar S, Abhishekh MG, Geethalakshmi U. Emperipolesis in a case of adult T cell lymphoblastic lymphoma (mediastinal type) – Detected at FNAC and imprint cytology. Online J Health Allied Sci. 2011;10:11. [Google Scholar]
- 4.Raja H, Subramanyam SG, Govindaraj S, Babu MK. A rare cause of massive lymphadenopathy. Indian J Surg Oncol. 2011;2:212–4. doi: 10.1007/s13193-011-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sable MN, Sehgal K, Gadage VS, Subramanian PG, Gujral S. Megakaryocytic emperipolesis: A histological finding in myelodysplastic syndrome. Indian J Pathol Microbiol. 2009;52:599–600. doi: 10.4103/0377-4929.56153. [DOI] [PubMed] [Google Scholar]
- 6.Janssen CE, Rose CD, Naranjo A, Meunier BB, Cimaz R, Harjacek M, et al. Emperipolesis and cell death in NOD2-related Blau syndrome and Crohn's disease. Pediatr Rheumatol Online J. 2011;9(Suppl 1):P293. [Google Scholar]
- 7.Lewis WH. The engulfment of living blood cells by others of the same type. Anat Rec. 1925;31:43–9. [Google Scholar]
- 8.Humble JG, Jayne WH, Pulvertaft RJ. Biological interaction between lymphocytes and other cells. Br J Haematol. 1956;2:283–94. doi: 10.1111/j.1365-2141.1956.tb06700.x. [DOI] [PubMed] [Google Scholar]
- 9.Vemuganti GK, Naik MN, Honavar SG. Rosaidorfman disease of the orbit. J Hematol Oncol. 2008;1:7. doi: 10.1186/1756-8722-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bi Y, Huo Z, Meng Y, Wu H, Yan J, Zhou Y, et al. Extranodal Rosai-Dorfman disease involving the right atrium in a 60-year-old male. Diagn Pathol. 2014;9:115. doi: 10.1186/1746-1596-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraeft SK, Honig M, Krishnamurthy S. Emperipolesis in the cerebrospinal fluid from a patient with Rosai-Dorfman disease. Diagn Cytopathol. 2008;36:67–8. doi: 10.1002/dc.20742. [DOI] [PubMed] [Google Scholar]
- 12.Centurione L, Di Baldassarre A, Zingariello M, Bosco D, Gatta V, Rana RA, et al. Increased and pathologic emperipolesis of neutrophils within megakaryocytes associated with marrow fibrosis in GATA-1low mice. Blood. 2004;104:3573–80. doi: 10.1182/blood-2004-01-0193. [DOI] [PubMed] [Google Scholar]
- 13.Lee WB, Erm SK, Kim KY, Becker RP. Emperipolesis of erythroblasts within Kupffer cells during hepatic hemopoiesis in human fetus. Anat Rec. 1999;256:158–64. doi: 10.1002/(SICI)1097-0185(19991001)256:2<158::AID-AR6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Poppema S. Sternberg-Reed cells with intracytoplasmic lymphocytes. Phagocytosis or emperipolesis? Virchows Arch A Pathol Anat Histol. 1978;380:355–9. doi: 10.1007/BF00431321. [DOI] [PubMed] [Google Scholar]
- 15.Xia P, Wang S, Guo Z, Yao X. Emperipolesis, entosis and beyond: Dance with fate. Cell Res. 2008;18:705–7. doi: 10.1038/cr.2008.64. [DOI] [PubMed] [Google Scholar]
- 16.Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–79. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 17.McCall K, Klein C. Methods in Molecular Biology. 1st ed. New York, N.J: Humana Press: Springer Science Business Media, LLC; 2013. [Google Scholar]
- 18.Sarode SC, Sarode GS. Cellular cannibalism in central and peripheral giant cell granuloma of the oral cavity can predict biological behavior of the lesion. J Oral Pathol Med. 2014;43:459–63. doi: 10.1111/jop.12119. [DOI] [PubMed] [Google Scholar]
- 19.Kroemer G, Perfettini JL. Entosis, a key player in cancer cell competition. Cell Res. 2014;24:1280–1. doi: 10.1038/cr.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Q, Luo T, Ren Y, Florey O, Shirasawa S, Sasazuki T, et al. Competition between human cells by entosis. Cell Res. 2014;24:1299–310. doi: 10.1038/cr.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krajcovic M, Krishna S, Akkari L, Joyce JA, Overholtzer M. mTOR regulates phagosome and entotic vacuole fission. Mol Biol Cell. 2013;24:3736–45. doi: 10.1091/mbc.E13-07-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kale A. Cellular cannibalism. J Oral Maxillofac Pathol. 2015;19:7–9. doi: 10.4103/0973-029X.157191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarode GS, Sarode SC, Karmarkar S. Complex cannibalism: An unusual finding in oral squamous cell carcinoma. Oral Oncol. 2012;48:e4–6. doi: 10.1016/j.oraloncology.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Sarode SC, Sarode GS. Neutrophil-tumor cell cannibalism in oral squamous cell carcinoma. J Oral Pathol Med. 2014;43:454–8. doi: 10.1111/jop.12157. [DOI] [PubMed] [Google Scholar]
- 25.Bak M, Teglbjaerg PS. Pleomorphic (giant cell) carcinoma of the intestine. An immunohistochemical and electron microscopic study. Cancer. 1989;64:2557–64. doi: 10.1002/1097-0142(19891215)64:12<2557::aid-cncr2820641225>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Caruso RA, Famulari C, Giuffré G, Mazzeo G. Pleomorphic carcinoma of the gallbladder: Report of a case. Tumori. 1991;77:523–6. doi: 10.1177/030089169107700615. [DOI] [PubMed] [Google Scholar]
- 27.Fishback NF, Travis WD, Moran CA, Guinee DG, Jr, McCarthy WF, Koss MN. Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer. 1994;73:2936–45. doi: 10.1002/1097-0142(19940615)73:12<2936::aid-cncr2820731210>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 28.Guo KJ, Yamaguchi K, Enjoji M. Undifferentiated carcinoma of the gallbladder. A clinicopathologic, histochemical, and immunohistochemical study of 21 patients with a poor prognosis. Cancer. 1988;61:1872–9. doi: 10.1002/1097-0142(19880501)61:9<1872::aid-cncr2820610925>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 29.Gupta RK, Wakefield SJ. Needle aspiration cytology, immunocytochemistry, and electron microscopic study of unusual pancreatic carcinoma with pleomorphic giant cells. Diagn Cytopathol. 1992;8:522–7. doi: 10.1002/dc.2840080513. [DOI] [PubMed] [Google Scholar]


