Abstract
Menthol, the active ingredient in several topically applied analgesics, activates transient receptor potential melastatin 8 (TRPM8) receptors on sensory nerves and on the vasculature inducing a cooling sensation on the skin. Ilex paraguariensis is also a common ingredient in topical analgesics that has potential vasoactive properties and may alter the mechanisms of action of menthol. We sought to characterize the microvascular effects of topical menthol and ilex application and to determine the mechanism(s) through which these compounds may independently and combined alter cutaneous blood flow. We hypothesized that menthol would induce vasoconstriction and that ilex would not alter skin blood flow (SkBF). Three separate protocols were conducted to examine menthol and ilex-mediated changes in SkBF. In protocol 1, placebo, 4% menthol, 0.7% ilex, and combination menthol + ilex gels were applied separately to the skin and red cell flux was continuously measured utilizing laser speckle contrast imaging (LSCI). In protocol 2, seven concentrations of menthol gel (0.04%, 0.4%, 1%, 2%, 4%, 7%, 8%) were applied to the skin to model the dose-response curve. In protocol 3, placebo, menthol, ilex, and menthol + ilex gels were applied to skin under local thermal control (34°C) both with and without sensory nerve blockage (topical lidocaine 4%). Post-occlusive reactive hyperemia (PORH) and local heating (42°C) protocols were conducted to determine the relative contribution of endothelium derived hyperpolarizing factors (EDHFs)/sensory nerves and nitric oxide (NO), respectively. Red cell flux was normalized to mean arterial pressure expressed as cutaneous vascular conductance (CVC: flux•mmHg-1) in all protocols. Topical menthol application increased SkBF compared to placebo (3.41±0.33 v 1.1±0.19 CVC: p<0.001). During the dose-response, SkBF increased with increasing doses of menthol (main effect, p<0.05) with an ED50 of 1.0%. Similarly, SkBF was increased after menthol application during PORH (3.62±0.29 v 2.50±0.21 flux•mmHg-1; p<0.001), but not local heating (2.98±0.24 v 2.86±0.32 flux•mmHg-1; p=0.44). Concurrent sensory nerve inhibition attenuated menthol-mediated vasodilation at thermoneutral baseline (1.29±0.19 flux•mmHg-1; p<0.001) and during PORH (2.79±0.28 flux•mmHg-1; p<0.001), but not during local heating (3.45±0.21 flux•mmHg-1; p=0.1). Topically applied menthol, but not ilex, dose-dependently increases blood flow in the cutaneous microvasculature. This increase in blood flow is mediated, in-part by sensory nerves and EDHFs.
Keywords: Cutaneous, menthol, TRPM8, microvascular, ilex paraguariensis
Introduction
Topically applied analgesic gels are commonly used in clinical practice to relieve muscle and joint pain. The purported analgesic mechanism of action for these agents is gate control theory (Melzack, 1996). Menthol, the active ingredient in many topically applied analgesics, activates transient receptor potential melastatin 8 (TRPM8) channels which are part of a family of non-selective cation channels which also open in response to cool temperatures (8-28°C) (McKemy et al., 2002). According to gate control theory, menthol elicits an analgesic effect by activating TRMP8 channels located on sensory nerves and on C and Aδ nociceptors to reduce pain transmission (Liu et al., 2013; Premkumar and Abooj, 2013). While these topically applied agents are effective at reducing perceived pain from various musculoskeletal injuries (Airaksinen et al., 2004; Hill and Sumida, 2002) they may also have other non-specific effects on neural and vascular tissues containing TRMP8 receptors.
TRMP8 receptors are expressed in both neural (Babes et al., 2011) and vascular cells (Johnson et al., 2009; Yang et al., 2006). The therapeutic effects of topical analgesics containing menthol may be mediated in-part through its action in the skin itself. Several skin-specific techniques have been developed to examine neurovascular signaling in the cutaneous circulation including the relative contributions of sensory nerves, endothelium derived hyperpolarizing factor(s) (EDHFs) (Lorenzo and Minson, 2007) and nitric oxide (NO)-dependent (Kellogg et al., 1999) signaling pathway. Specifically, after a brief period of arterial occlusion (5 min) the following increase in skin blood flow (SkBF) is mediated through sensory nerves and EDHF -dependent mechanisms (Lorenzo and Minson, 2007). Locally heating a small area of skin causes a brief axon reflex that is in-part mediated by sensory nerves. The axon reflex is followed by a nadir and then a prolonged plateau in vasodilation that is predominantly dependent on the production of NO from endothelial NO synthase (Minson et al., 2001). Sensory nerves do not play a role in the NO-mediated plateau in SkBF (Hodges et al., 2009). Menthol is an agonist for TRPM8 channels and induces either vasodilation or vasoconstriction dependent upon the initial level of vascular tone (Johnson et al., 2009) through alterations in sensory nerve (Johnson et al., 2009), NO (Johnson et al., 2009), and RhoA/ROCK vasoconstrictor pathways (Sun et al., 2014). Work on human arterial blood flow suggests that menthol acts as a constrictor (Olive et al., 2010; Topp et al., 2013). However, the effect of menthol at clinically relevant concentrations on cutaneous neurovascular control in humans is presently unknown.
In addition to menthol, commonly used topical analgesic gels (BioFreeze®) also contain ilex paraguariensis, a plant-derived additive that contributes to the texture of BioFreeze®. Ilex has documented antioxidant properties (Boaventura et al., 2012; Gao et al., 2013b; Leonard et al., 2010) and increases NO bioavailability, while decreasing vasoconstrictors including endothelin and thromboxane B2 (Gao et al., 2013a), in both human and rat models (Muccillo Baisch et al., 1998; Schinella et al., 2005). However, the effects of topically applied ilex and possible interactions with menthol on cutaneous blood flow are unclear.
The aim of this study was to determine the separate and combined effects of menthol and ilex on SkBF. We also sought to elucidate mechanisms by which ilex and menthol may alter SkBF by utilizing non-invasive skin specific stimuli (PORH, and local heating). We hypothesized that topical menthol would induce cutaneous vasoconstriction through sensory nerve mediated mechanisms, while topical ilex would have no effect on SkBF.
Material and methods
Subjects
Experimental protocols were approved by the institutional review board of The Pennsylvania State University and conformed to the Declaration of Helsinki. Voluntary written and verbal consent were obtained from all subjects prior to participation in the study. Each protocol was performed on different groups of 10 young, healthy subjects. All subjects were apparently healthy, normally active, nonsmokers, not on prescription medication, and did not present with any chronic disease.
Instrumentation
All experiments took place in a thermoneutral laboratory with subjects in a semi-supine position. Subjects refrained from consuming alcohol for at least 12 hours before the experiment and did not consume caffeine or exercise on the day of the experiment. The subject's left arm was placed in a supinated position on a vacuum cushion to limit movement artifact during data collection.
Protocol 1
Each subject completed four trials where forearm blood flow over a 60 cm2 area of the ventral forearm was measured with a full-field laser perfusion imager (Moor instruments) that utilizes laser speckle contrast imaging (LSCI) to obtain relative measures of SkBF, expressed as flux. LSCI works through measuring fluctuations in the speckle pattern of laser light as blood flows through the skin (Briers, 2007). The full-field laser perfusion imager was set with a time constant of 1.0 seconds, display rate of 25 Hz, and exposure time of 4 milliseconds. During each trial, one of four gels was applied to the skin in a double blind randomized fashion: (1) placebo (2) menthol (4%) (3) ilex (0. 07%) and (4) menthol + ilex. The menthol + ilex gel was commercially available BioFreeze® gel. The other gels were formulated by the manufacturer, de-identified and coded. The formulations were exactly as the commercially available gel except for the removal of menthol and/or ilex. All investigators were blinded as to the identity of the gels until the completion of data collection and analysis.
The measurement area was marked on each subject and clear cellophane wrap was placed over the arm to limit evaporation. LCSI was conducted with and without cellophane wrap prior to gel application in nine experiments and was found not to alter baseline blood flow (p=0.185). Baseline blood flow was measured on the forearm for 15 minutes, after which the cellophane wrap was temporarily removed and 1ml of test gel was applied evenly over the marked area. The clear cellophane wrap was placed back into position and blood flow was measured until a minimum of 15 minutes of a steady plateau in SkBF was obtained. Subjects completed one trial for each treatment condition (placebo, menthol, ilex, menthol + ilex). Pilot work indicated that a 24 hour washout was sufficient to allow abatement of the effects of previous gel exposure, so a minimum 24 hour washout between trials was employed.
Arterial blood pressure was measured via brachial auscultation every five minutes and heart rate was continuously monitored throughout the experiment (Datex-Ohmeda Cardiocap/5). Mean arterial pressure (MAP) was calculated as diastolic blood pressure plus one third pulse pressure. All data were normalized to cutaneous vascular conductance (CVC:flux/MAP).
Data analysis
Data were analyzed, recorded, and stored offline for analysis (moorFLPI Review V3.0). Baseline values were determined as the average of a five minute periods of stable blood flow prior to gel application. Peak CVC was determined as the average of a five minute plateau in SkBF post gel application. A two-way mixed model repeated-measures ANOVA was conducted to detect gel and condition (i.e., post-gel) differences in SkBF. Alpha was set at P<0.05.
Protocol 2
After establishing 4% menthol gel as a vasodilator in protocol 1, we sought to characterize the dose-response curve between menthol concentration and changes in CVC. Seven different gels containing 0.04%, 0.4%, 1%, 2%, 4%, 7%, and 8% menthol were utilized for this experiment. These concentrations were chosen to test menthol concentrations above and below those contained in commercially available analgesic gels, with 7-8% menthol representing the highest concentrations available that would emulsify in the gel.
In a group of 10 subjects, four 15 cm2 areas for gel application were identified on the ventral forearm. Volumes of 0.25 ml of each gel were applied to the marked sites in a randomized manner. This volume of gel was chosen to match the same amount of gel/cm2 as utilized in protocol 1. Both researchers and subjects were blind to the identity of each gel. Immediately after gel application, all four sites were covered with clear cellophane wrap as in protocol 1. SkBF was measured with LSCI. Once flux at any individual site reached a plateau for five minutes the cellophane wrap was removed from that location only and any excess gel was removed. SkBF was then continuously measured until flux returned to basal values for a minimum of five minutes. Blood pressure and heart rate were monitored as in protocol 1.
Peak SkBF was measured as the highest CVC recorded during menthol administration for each menthol dose. Total hyperemic response (THR) was determined for each menthol dose as the integrated increase in SkBF with baseline subtracted, expressed as CVC multiplied by time in seconds (CVC•s), as previously described (Wong et al., 2003). One-way ANOVAs were run to detect between gel differences. Alpha was set at P<0.05. Menthol concentrations were log transformed and data were curve modelled as a four parameter logistic equation without minimum or maximum constraints (GraphPad Prism 6). Effective dose 50% (ED50), the dose of menthol required to elicit half maximum vasodilation was determined from the curve model.
Protocol 3
Protocol 3 was conducted to investigate the mechanisms by which menthol-induced cutaneous vasodilation. Experiments for protocol 3 took place under the same conditions as protocols 1 and 2. Four local heater units (moor instruments skin heater/temperature monitor SH02) were affixed to the ventral surface of the forearm with adhesive disks. Heaters were placed to avoid large, superficial blood vessels. The same four treatments from protocol 1 were utilized in protocol 3. Volumes of 0.1 mL of each treatment gel were applied to the skin within separate local heaters in a randomized double blind manner. After gel application, local heaters were filled with distilled water which could be temperature controlled to maintain baseline skin temperature (Tsk) and heat the skin during the local heating protocol. The heaters were then covered with a transparent cap to prevent water loss. Heart rate and blood pressure were monitored in protocol 3 in the same manner as protocol 1. Figure 1 illustrates the experimental procedure for protocol 3.
Figure 1.
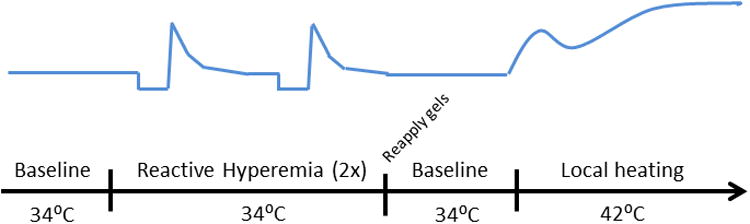
Reactive Hyperemia
A reactive hyperemia protocol was performed to assess EDHF and sensory nerve-mediated vasodilation. An automatic, pneumatic cuff was placed around the left-upper arm (Hokanson E 20 rapid cuff inflator). Upon completion of instrumentation, local heater temperatures were set to clamp local Tsk at 34.0°C. Twenty minutes of baseline data were obtained. After completion of baseline measurements, the pneumatic cuff was inflated > 50 mmHg suprasystolic pressure and remained inflated for five minutes. After five minutes, pressure was released from the cuff and the reactive hyperemic response was imaged. Flux was allowed to return to baseline values and blood flow was measured during a stable baseline for a minimum of five minutes. A second identical reactive hyperemia protocol was then performed.
Local Heating
After completion of the reactive hyperemic protocol, a local heating protocol was performed to assess NO-mediated vasodilation. Pilot studies indicated that the reactive hyperemia protocol did not affect local heating data when the two protocols were separated by a minimum of 20 minutes. Local heaters were drained of water and each gel was reapplied. Gels were applied identically to initial application and the local heaters were refilled with distilled water and again set to clamp local Tsk at 34.0°C. Baseline flux was measure for 20 minutes. Local heating of the skin was then conducted at a rate of 0.5°C every 30 seconds until water temperature reached 42.0°C. Heating was maintained until flux reached a stable plateau (30-40 minutes).
Sensory nerve blockade
Concurrent with reactive hyperemia and local heating protocols, 4% lidocaine (EMLA®) was applied to the opposite forearm and covered with a bio-occlusive patch for a minimum of one hour to block sensory nerve transduction. Sensory nerve blockade was confirmed through lack of sensation to a needle prick (Minson et al., 2001; Wong, 2013) before and after the protocol. Both the reactive hyperemia and local heating protocols were conducted after topical lidocaine application applied to determine sensory nerve contribution to menthol- and ilex- mediated changes in SkBF.
Data analysis
Data were analyzed, recorded, and stored offline for analysis (moorFLPI Review V3.0). Baseline values were determined as the average of a five minute periods of stable flux before commencement of the reactive hyperemia protocol. Peak flux was determined as the highest absolute flux value obtained during reactive hyperemia. The THR for SkBF in protocol 3 was characterized as the integration of post-occlusion flux above baseline from the time of cuff release until a return to baseline flux, this is representative of the total increase in blood flow post-occlusion. The local heating plateau was measured as the average of a period of five minute stable flux during heating to 42.0°C. All values were standardized to CVC or CVC•s in the case of THR integration. A two-way mixed model repeated-measures ANOVA was conducted to detect gel and condition (i.e., local heating) differences in blood flow. Alpha was set at P<0.05.
Results
Subject characteristics for all three protocols are illustrated in Table 1
Table 1.
| Subjects (men, women) | Age (y) | Height (m) | Weight (kg) | MAP (mmHg) | BMI (kg•m-2) | |
|---|---|---|---|---|---|---|
| Protocol 1 | 5,5 | 24 ± 3 | 1.6 ± 0.1 | 74 ± 11 | 82 ± 9 | 23.4 ± 2.7 |
| Protocol 2 | 4,6 | 24 ± 1 | 1.7 ± 0.1 | 68 ± 4 | 83 ± 2 | 22.6 ± 0.9 |
| Protocol 3 | 7,3 | 23 ± 1 | 1.8 ± 0.1 | 69 ± 3 | 82 ± 2 | 22.3 ± 0.5 |
Data from protocol 1 are shown in figure 2. Compared to pre-gel application, menthol (pre: 0.99±0.04 vs post: 3.41±0.33 flux•mmHg-1; p<0.001) and menthol + ilex (pre: 0.95±0.04 vs post: 3.50±0.34 flux•mmHg-1; p<0.001) increased CVC, whereas CVC was not affected by the application of ilex (pre: 0.98±0.06 vs post: 0.97±0.11 flux•mmHg-1; p=0.98) or placebo gels (pre: 1.03±0.05 vs post: 1.120±0.19 flux•mmHg-1; p=0.68).
Figure 2.
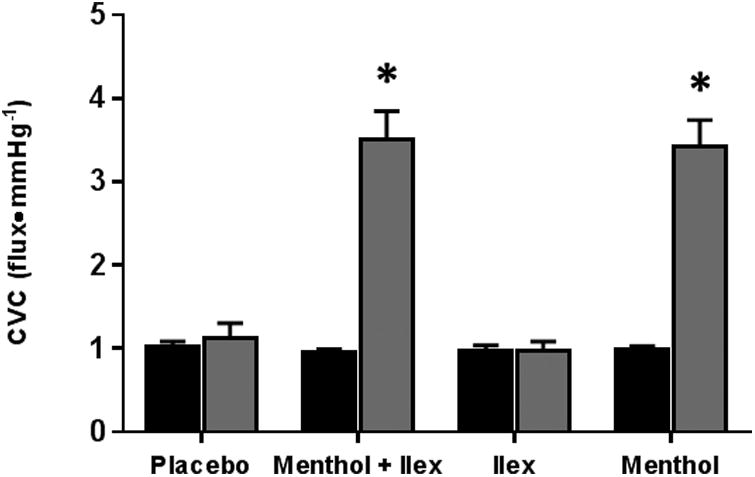
The menthol dose-response data are shown in Figure 3. Peak CVC and area under the curve both increased with increasing doses of menthol. Through pharmacological curve modelling, the ED50 was determined to be 1.0% menthol.
Figure 3.
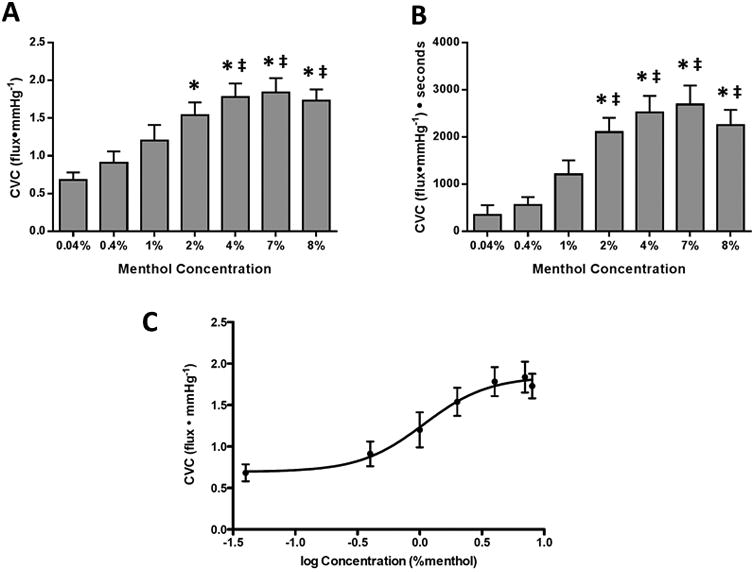
Figure 4 shows CVC at thermoneutral (34.0°C) baseline taken before the first reactive hyperemia for all sites both with and without sensory nerve blockade. With intact sensory nerves, baseline CVC at the menthol (menthol: 2.28±0.28, placebo: 0.72±0.1 flux•mmHg-1; p<0.001) and menthol + ilex (menthol + ilex: 2.20±0.21 flux•mmHg-1; p<0.001) sites was increased compared to placebo. Sites treated with ilex were not different from placebo (ilex: 0.69±0.8 flux•mmHg-1; p=0.85).
Figure 4.
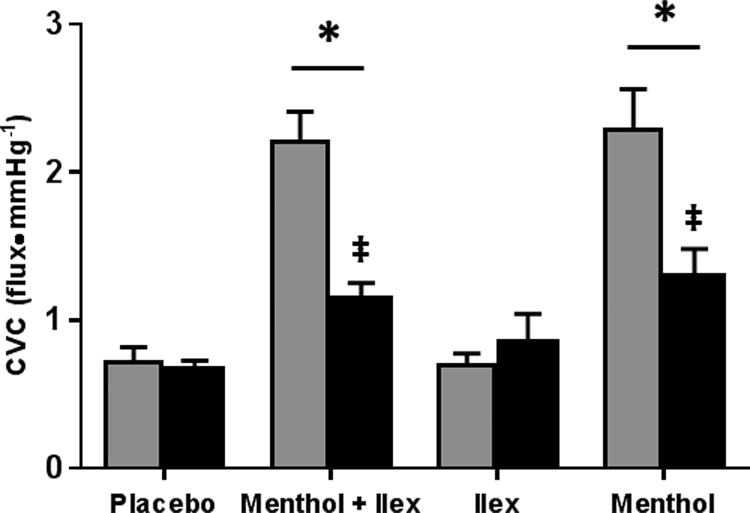
At baseline, sensory nerve blockade partially attenuated CVC at the menthol (1.29±0.19 flux•mmHg-1; p<0.001) and menthol + ilex sites (1.14± flux•mmHg-1; p<0.001). However, CVC at both menthol containing sites remained elevated compared to placebo site (placebo with blockade: 0.67±0.06 flux•mmHg-1; p<0.001).
Figure 5 shows the peak CVC response during PORH. Similar to thermoneutral baseline, peak CVC was increased in the menthol (menthol: 3.62±0.29, placebo: 2.50±0.21 flux•mmHg-1; p<0.001) and menthol + ilex (menthol + ilex: 3.54±0.25 flux•mmHg-1; p<0.001) sites compared to placebo. Peak CVC at the ilex site was not different from placebo (ilex: 2.41±0.08 flux•mmHg-1; p=0.52).
Figure 5.
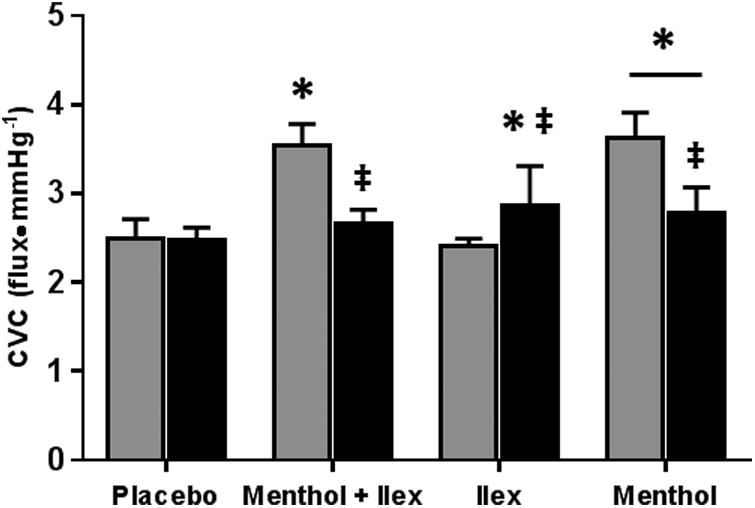
Sensory nerve blockade did not alter the peak CVC at the placebo site (with blockade: 2.47±0.15 flux•mmHg-1; p=0.82). Sensory nerve blockade reduced peak CVC at the menthol (with blockade: 2.79±0.28 flux•mmHg-1; p<0.001) and menthol + ilex (with blockade: 2.67±0.15 flux•mmHg-1; p<0.001) sites. With blockade, peak CVC at the menthol site was greater than placebo (p=0.02), but peak CVC at the menthol + ilex site was not (p=0.15). Peak CVC was augmented at the ilex site with sensory nerve blockade (with blockade: 2.87±0.44 flux•mmHg-1; p<0.001) so that is was greater than placebo (p=0.004)
Figure 6 shows the THR, the integrated increase in cutaneous blood flow above baseline, with and without sensory nerve inhibition. THR at the menthol (menthol 622.7±79.9 v placebo: 191.6±39.8 CVC•s; p<0.003) and menthol +ilex sites (menthol + ilex: 722.0±169.0 CVC•s; p<0.001) were increased compared to placebo. Ilex treatment alone (154.1±25.1 CVC•s; p=0.79) was no different from placebo.
Figure 6.
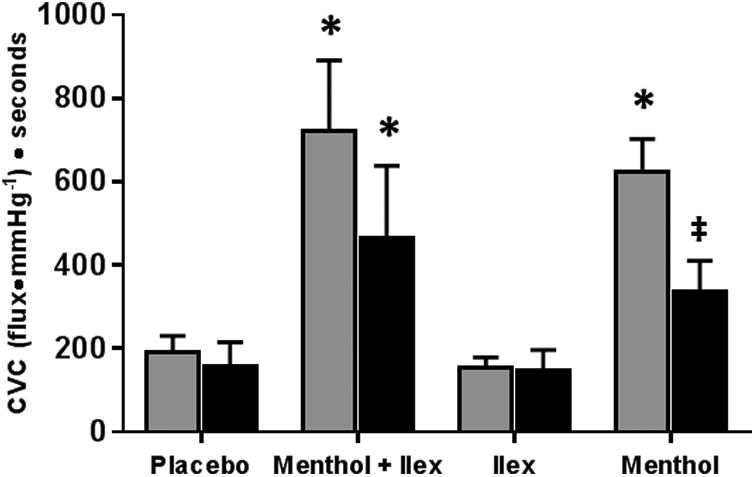
Sensory nerve blockade reduced the THR at the menthol site (with blockade: 335.5±75.2 CVC•s; p=0.045) so that it was no longer different from placebo (with blockade: 158.6±56.6 CVC•s; p=0.21). Sensory nerve blockade did not attenuate THR in the menthol + ilex site (with blockade: 464.0±175.0 CVC•s; p=0.07) and THR remained elevated compared to the placebo site (p=0.03). THR at neither the ilex (with blockade 146.2±51.0 CVC•s, p=0.95) nor placebo (p=0.82) site was altered with sensory nerve inhibition.
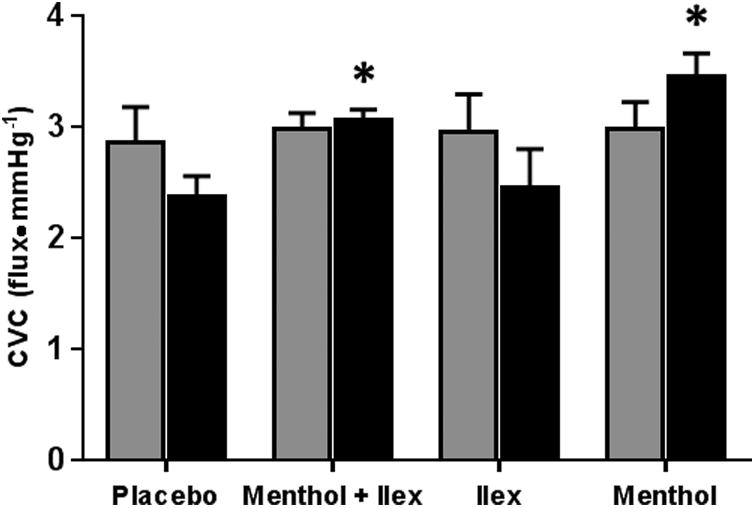
Figure 7 shows the NO-dependent plateau in CVC during local heating of the skin. CVC was not different from placebo at the menthol (menthol: 2.98±0.24 flux•mmHg-1, placebo: 2.86±0.32; p=0.44), ilex (2.96±0.34 flux•mmHg-1; p=0.49) or menthol + ilex (2.98±0.15 flux•mmHg-1; p=0.46) sites. Blockade of sensory nerves during local heating did not significantly alter CVC at the menthol (with blockade: 3.45±0.21 flux•mmHg-1; p=0.13), menthol + ilex (with blockade: 3.06±0.09 flux•mmHg-1; p=0.78), ilex (2.45±0.35 flux•mmHg-1; p=0.053) or placebo (with blockade: 2.37±0.18 flux•mmHg-1; p=0.23) sites. However, with sensory nerve inhibition, CVC at both the menthol (p<0.001) and menthol + ilex (p=0.03) sites was significantly greater than at the placebo site.
Figure 7.
Discussion
The main findings from this study were that 1) topical menthol application dose-dependently increases cutaneous blood flow in thermoneutral conditions (Tsk=34.0°C) but ilex had no effect, 2) the PORH response was augmented after topical application of menthol but there was no effect of menthol on the local heating response. Collectively these data suggest that menthol acts as a vasodilator when topically applied to the skin. These data also suggest that menthol increases skin blood flow through EDHF- and sensory-nerve dependent mechanisms with little or no relative contribution from NO.
Menthol elicits a cold sensation when topically applied to the skin and is clinically used to mimic the pain relieving properties of cryotherapy. Traditional cryotherapy is typically accompanied by a decrease in blood flow to the treated area (Ho et al., 1994; Thorsson et al., 1985). In contrast to studies examining arterial blood flow with ultrasonography measurements (Olive et al., 2010; Topp et al., 2013; Topp et al., 2011), we and others (Johnson et al., 2009) have found that menthol containing gels increase cutaneous blood flow, not resembling cryotherapy despite the cold sensation. It is possible that these differences are due to differences in the macro- and microvasculature, and the depth of penetration of the gels, and possible influences of evaporation of the gels on the skin surface. The present data were collected using relatively large amounts of gel per surface area and with the use of plastic covering to prevent evaporation. Other studies inn conduit arteries have used a smaller volume of gel per surface area and allowed for free evaporation. We observed an increase in cutaneous blood flow, but we did not simultaneously measure arterial blood flow to examine macrovascular function in response to topical menthol and ilex application.
We found that menthol containing gels produced a robust increase in cutaneous blood flow, while ilex application alone (without menthol) did not alter SkBF. Menthol has been shown to induce vasodilation in various vascular beds and animal models through NO, RhoA/Rho kinase, and EDHF mechanisms (Cheang et al., 2013; Johnson et al., 2009; Sun et al., 2014). Our initial observation of menthol-induced cutaneous vasodilation lead us to further characterize the SkBF response to topical menthol (protocol 2) and to further investigate the mechanisms involved in menthol-mediated vasodilation (protocol 3).
In protocol 2, using doses ranging from 0.04% to 8% menthol, a range that includes the menthol concentration found in most commercially available menthol gels, menthol increased SkBF dose-dependently. While menthol did have a dose-dependent effect, using greater than 4% menthol did not further increase SkBF compared to 4% menthol, the concentration found in over-the-counter menthol formulations (BioFreeze®, Wonder Freeze®, Bengay Cold Therapy Gel® [5%]).
Our next protocol was designed to elucidate the potential mechanism(s) through which menthol elicits cutaneous vasodilation. At thermoneutral baseline (34°C) and during reactive hyperemia, the menthol and menthol + ilex gels significantly increased SkBF compared to placebo. The increase in SkBF during PORH was predominantly mediated by either sensory nerves or EDHFs, a group of putative vasodilators that hyperpolarize vascular smooth muscle through activation of calcium- and ATP-activated potassium channels (Edwards et al., 2010). While uncovering the exact pathway through which menthol interacts with EDHFs was outside the scope of these experiments, the robust increase in the THR observed with menthol application suggests that menthol augmented one or more EDHFs to increase cutaneous blood flow.
Because of the role of sensory nerves in the PORH response we also pretreated the skin with lidocaine to inhibit sensory nerves. Sensory nerve inhibition partially attenuated the menthol-mediated rise in cutaneous blood flow during thermoneutral baseline and reactive hyperemia, suggesting that menthol interacts with sensory nerves, possibly sensitizing the nerves to induce greater vasodilation. Surprisingly, sensory nerve inhibition did not attenuate blood flow at either the placebo or ilex sites during reactive hyperemia. This is in contrast to the findings by Lorenzo & Minson, who showed that sensory never inhibition with topically applied anesthetics attenuated SkBF during reactive hyperemia (Lorenzo and Minson, 2007). The reason for these disparate findings is not readily apparent as we used similar protocols for both sensory nerve inhibition and reactive hyperemia as Lorenzo & Minson. However, because menthol increased cutaneous blood flow during EDHF-dependent reactive hyperemia, and was partially inhibited by sensory nerve blockade these data suggest that menthol elicits increases in cutaneous blood flow through a combination of EDHFs and sensory nerves.
NO-dependent vasodilation was also assessed through local heating of the skin to 42.0°C (Holowatz et al., 2010). Neither menthol nor menthol + ilex changed SkBF in response to local heating, suggesting that menthol-mediated increase in SkBF is NO-independent. Others have demonstrated that NO plays a significant role in menthol-mediated cutaneous vasodilation at thermoneutral temperatures (Johnson et al., 2009) using different methodologies. In the present study we did not pharmacologically inhibit NO synthesis and thus only have indirect measures of relative NO-dependent vasodilation. It is possible that we did not observe a direct NO contribution to menthol-mediated vasodilation due to a ceiling effect, where local heating to 42.0°C elicited near maximal cutaneous vasodilation.
In this study we found that topical application of ilex had no effect on the majority of our skin blood flow measures. The only effect of ilex was an increase in peak CVC during sensory nerve inhibition in protocol 3. Ilex augments vasodilation when applied directly to isolated vessels through NO-dependent mechanisms (Muccillo Baisch et al., 1998; Schinella et al., 2005). Ilex has robust antioxidant properties, and likely increases NO synthase activity by quenching reactive oxygen species (Schinella et al., 2005). Despite the potential vasodilatory mechanisms, in this study we observed little effect of ilex on cutaneous blood flow. This could be due to the low concentrations of ilex in the gel, possible change in the properties of ilex in commercial preparation, and/or an inability of ilex to reach the vasculature when applied topically. Our data clearly show that, under basal conditions, topically applied ilex at the concentration found in topical analgesics, such as BioFreeze®, does not alter cutaneous microvascular blood flow.
The ability of topically applied menthol to attenuate pain is well established (Airaksinen et al., 2004; Hill and Sumida, 2002; Sundstrup et al., 2014). These data do not refute using topical menthol gels as analgesics for muscle and joint pain. However, our findings suggest that topical menthol gels may have clinical uses other than pain relief. Through increasing SkBF, topical menthol application can be utilized in clinical conditions were increasing blood flow over a localized region would be beneficial. For example elevating SkBF in the hands or feet may also increase distal sensation and help those with peripheral neuropathy.
Conclusions
In summary, we found that menthol applied topically, with or without ilex paraguariensis, induced cutaneous vasodilation in a dose-dependent manner, with an ED50 at approximately a 1% menthol concentration. Furthermore, menthol increased SkBF during thermoneutral baseline and EDHF/sensory nerve-dependent reactive hyperemia. Sensory nerve inhibition partially attenuated the menthol-mediated rise in SkBF. Taken together, these data suggest that menthol induces cutaneous vasodilation by acting through EDHFs and sensory nerves. Collectively, our findings support menthol as a vasodilator in the human cutaneous microvasculature.
Acknowledgments
The authors would like to acknowledge the help of Christian Conlon, Jessica Kutz, Kelly McGuinness, James Spinuzza, Kieran Nichols, and Sean Shank in collecting data for this project. We would also like to thank all study volunteers for their time and participation.
Abbreviations
- TRPM8
transient receptor potential melastatin 8
- NO
nitric oxide
- SkBF
skin blood flow
- EDHF
endothelium derived hyperpolarizing factor
- PORH
post occlusive reactive hyperemia
- LSCI
laser speckle contrast imaging
- MAP
mean arterial pressure
- CVC
cutaneous vascular conductance
- THR
total hyperemic response
- ED50
effective dilation 50%
References
- Airaksinen OV, et al. Cold gel reduced pain and disability in minor soft-tissue injury. Journal of Bone and Joint Surgery-American. 2004;86A:1101–1101. doi: 10.2106/00004623-200405000-00040. [DOI] [PubMed] [Google Scholar]
- Babes A, et al. TRPM8, a sensor for mild cooling in mammalian sensory nerve endings. Curr Pharm Biotechnol. 2011;12:78–88. doi: 10.2174/138920111793937835. [DOI] [PubMed] [Google Scholar]
- Boaventura BC, et al. Association of mate tea (Ilex paraguariensis) intake and dietary intervention and effects on oxidative stress biomarkers of dyslipidemic subjects. Nutrition. 2012;28:657–64. doi: 10.1016/j.nut.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Briers JD. Laser speckle contrast imaging for measuring blood flow. Optica Applicata. 2007;37:139–152. [Google Scholar]
- Cheang WS, et al. Menthol relaxes rat aortae, mesenteric and coronary arteries by inhibiting calcium influx. Eur J Pharmacol. 2013;702:79–84. doi: 10.1016/j.ejphar.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Edwards G, et al. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010;459:863–79. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- Gao H, et al. Effects of Yerba Mate tea (Ilex paraguariensis) on vascular endothelial function and liver lipoprotein receptor gene expression in hyperlipidemic rats. Fitoterapia. 2013a;84:264–72. doi: 10.1016/j.fitote.2012.12.024. [DOI] [PubMed] [Google Scholar]
- Gao H, et al. Beneficial effects of Yerba Mate tea (Ilex paraguariensis) on hyperlipidemia in high-fat-fed hamsters. Exp Gerontol. 2013b;48:572–8. doi: 10.1016/j.exger.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Hill JM, Sumida KD. Acute effect of 2 topical counterirritant creams on pain induced by delayed-onset muscle soreness. J Sport Rehabil. 2002;11:202–208. [Google Scholar]
- Ho SS, et al. The effects of ice on blood flow and bone metabolism in knees. Am J Sports Med. 1994;22:537–40. doi: 10.1177/036354659402200417. [DOI] [PubMed] [Google Scholar]
- Hodges GJ, et al. The involvement of heating rate and vasoconstrictor nerves in the cutaneous vasodilator response to skin warming. Am J Physiol Heart Circ Physiol. 2009;296:H51–6. doi: 10.1152/ajpheart.00919.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, et al. Aging and the control of human skin blood flow. Front Biosci (Landmark Ed) 2010;15:718–39. doi: 10.2741/3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CD, et al. Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2009;296:H1868–77. doi: 10.1152/ajpheart.01112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DL, Jr, et al. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol (1985) 1999;86:1185–90. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- Leonard SS, et al. Analysis of free-radical scavenging of Yerba Mate (Ilex paraguriensis) using electron spin resonance and radical-induced DNA damage. J Food Sci. 2010;75:C14–20. doi: 10.1111/j.1750-3841.2009.01394.x. [DOI] [PubMed] [Google Scholar]
- Liu BY, et al. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain. 2013;154:2169–2177. doi: 10.1016/j.pain.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. J Physiol. 2007;585:295–303. doi: 10.1113/jphysiol.2007.143867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, et al. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–8. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Melzack R. Gate Control Theory - On the evolution of pain concepts. Pain Forum. 1996;5:128–138. [Google Scholar]
- Minson CT, et al. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol (1985) 2001;91:1619–26. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- Muccillo Baisch AL, et al. Endothelium-dependent vasorelaxing activity of aqueous extracts of Ilex paraguariensis on mesenteric arterial bed of rats. J Ethnopharmacol. 1998;60:133–9. doi: 10.1016/s0378-8741(97)00140-2. [DOI] [PubMed] [Google Scholar]
- Olive JL, et al. Vascular conductance is reduced after menthol or cold application. Clin J Sport Med. 2010;20:372–6. doi: 10.1227/NEU.0b013e3181e57bca. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Abooj M. TRP channels and analgesia. Life Sci. 2013;92:415–24. doi: 10.1016/j.lfs.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinella G, et al. Cardioprotective effects of Ilex paraguariensis extract: evidence for a nitric oxide-dependent mechanism. Clin Nutr. 2005;24:360–6. doi: 10.1016/j.clnu.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Sun J, et al. Activation of cold-sensing transient receptor potential melastatin subtype 8 antagonizes vasoconstriction and hypertension through attenuating RhoA/Rho kinase pathway. Hypertension. 2014;63:1354–63. doi: 10.1161/HYPERTENSIONAHA.113.02573. [DOI] [PubMed] [Google Scholar]
- Sundstrup E, et al. Acute effect of topical menthol on chronic pain in slaughterhouse workers with carpal tunnel syndrome: triple-blind, randomized placebo-controlled trial. Rehabil Res Pract. 2014;2014:310913. doi: 10.1155/2014/310913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsson O, et al. The effect of local cold application on intramuscular blood flow at rest and after running. Med Sci Sports Exerc. 1985;17:710–3. doi: 10.1249/00005768-198512000-00016. [DOI] [PubMed] [Google Scholar]
- Topp R, et al. Topical menthol, ice, peripheral blood flow, and perceived discomfort. J Athl Train. 2013;48:220–5. doi: 10.4085/1062-6050-48.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp R, et al. Comparison of the effects of ice and 3.5% menthol gel on blood flow and muscle strength of the lower arm. J Sport Rehabil. 2011;20:355–66. doi: 10.1123/jsr.20.3.355. [DOI] [PubMed] [Google Scholar]
- Wong BJ. Sensory nerves and nitric oxide contribute to reflex cutaneous vasodilation in humans. Am J Physiol Regul Integr Comp Physiol. 2013;304:R651–6. doi: 10.1152/ajpregu.00464.2012. [DOI] [PubMed] [Google Scholar]
- Wong BJ, et al. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol (1985) 2003;95:504–10. doi: 10.1152/japplphysiol.00254.2003. [DOI] [PubMed] [Google Scholar]
- Yang XR, et al. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1267–76. doi: 10.1152/ajplung.00515.2005. [DOI] [PubMed] [Google Scholar]


