Abstract
Study Objectives:
Narcolepsy, a chronic disorder of the central nervous system, is clinically characterized by a symptom pentad that includes excessive daytime sleepiness, cataplexy, sleep paralysis, hypnopompic/hypnagogic hallucinations, and disrupted nighttime sleep. Ideally, screening and diagnosis instruments that assist physicians in evaluating a patient for type 1 or type 2 narcolepsy would be brief, easy for patients to understand and physicians to score, and would identify or rule out the need for electrophysiological testing.
Methods:
A search of the literature was conducted to review patient-reported measures used for the assessment of narcolepsy, mainly in clinical trials, with the goal of summarizing existing scales and identifying areas that may require additional screening questions and clinical practice scales.
Results:
Of the seven scales reviewed, the Epworth Sleepiness Scale continues to be an important outcome measure to screen adults for excessive daytime sleepiness, which may be associated with narcolepsy. Several narcolepsy-specific scales have demonstrated utility, such as the Ullanlinna Narcolepsy Scale, Swiss Narcolepsy Scale, and Narcolepsy Symptom Assessment Questionnaire, but further validation is required.
Conclusions:
Although the narcolepsy-specific scales currently in use may identify type 1 narcolepsy, there are no validated questionnaires to identify type 2 narcolepsy. Thus, there remains a need for short, easily understood, and well-validated instruments that can be readily used in clinical practice to distinguish narcolepsy subtypes, as well as other hypersomnias, and for assessing symptoms of these conditions during treatment.
Citation:
Kallweit U, Schmidt M, Bassetti CL. Patient-reported measures of narcolepsy: the need for better assessment. J Clin Sleep Med. 2017;13(5):737–744.
Keywords: cataplexy, narcolepsy, narcolepsy scales, patient-reported measures
INTRODUCTION
Narcolepsy is a chronic disorder of the central nervous system (CNS) with a putative pathophysiologic mechanism of immune-mediated loss of hypocretin (orexin)-producing neurons in genetically predisposed individuals.1 It is clinically characterized by a symptom pentad that includes excessive daytime sleepiness (EDS), cataplexy, sleep paralysis, hypnopompic/hypnagogic hallucinations, and disrupted nighttime sleep.2,3 The prevalence of narcolepsy with cataplexy (narcolepsy/cataplexy) is estimated to be 0.03% to 0.05% of the general population.4–6 The onset of narcolepsy symptoms most often occurs in adolescence, although approximately one-third of patients experience initial symptoms in adulthood.7–9
The two major symptoms of narcolepsy are EDS, with attacks of irresistible sleep, and cataplexy, defined as “generally brief (< 2 minutes), sudden episodes of loss of bilateral muscle tone (us ually symmetrical) with retained consciousness”10 often triggered by strong emotions.5,11 All patients with narcolepsy have EDS, which is the initial presenting symptom in the majority of patients, and represents the most persistently debilitating aspect of this disease.12,13 Cataplexy is pathognomonic for narcolepsy type 1 (ie, it is a confirmatory characteristic of narcolepsy with cataplexy that is present in an estimated 60% to 90% of the patients with narcolepsy).13,14 Cataplexy may be highly distressing, because triggers of cataplectic attacks include laughter, excitement, or anger, common to daily activities such as socializing, sports, and recreation. The potential to trigger a cataplexy attack may lead to curtailment of these activities, as well as potentially impairing work, school, and driving.14–16 Among other narcolepsy symptoms, the reported prevalence is approximately 60% for hypnopompic or hypnagogic hallucinations, about 50% for sleep paralysis, and as many as 80% of patients report disrupted nighttime sleep.3,13,16
The medical literature consistently reports a substantial delay, often more than 10 years, between onset of narcolepsy symptoms and diagnosis, likely resulting from the lack of symptom recognition by general practitioners and clinicians.17 This lack of recognition is demonstrated by a survey of clinicians in the United States that reported, among respondents, only 9% of 300 primary care physicians and 42% of 100 sleep-medicine specialists felt either “very” or “extremely” comfortable diagnosing narcolepsy.18 Furthermore, misdiagnosis of narcolepsy is common, especially in children,19–21 due to symptoms frequently overlapping with other conditions in patients with comorbid physical and psychiatric conditions.22,23
To guide diagnosis, the third edition of the International Classification of Sleep Disorders classifies narcolepsy as either type 1, narcolepsy/cataplexy, or type 2, narcolepsy without cataplexy.10 These diagnostic criteria place a strong emphasis on ancillary tests, which is a controversial issue. Although both narcolepsy types require the presence of EDS for ≥ 3 months, type 1 not only requires the presence of cataplexy, but also a mean sleep latency of ≤ 8 minutes and two or more sleep-onset rapid eye movement periods (SOREMPs) on a standard Multiple Sleep Latency Test (MSLT) (one of these SOREMPs may come from a preceding nocturnal polysomnogram), and/ or a hypocretin-1 concentration in cerebrospinal fluid (CSF) of ≤ 110 pg/mL or less than one-third of mean values obtained in normal subjects as measured by immunoreactivity assay. In contrast, diagnosis of type 2 narcolepsy requires the same EDS, MSLT, and SOREMPs criteria as type 1 narcolepsy, but with an absence of cataplexy, and either no measurement of hypocretin-1 CSF, or CSF hypocretin-1 concentration > 110 pg/mL or more than one-third of mean values of normal subjects, and lack of a better explanation for the objective hyper-somnolence and/or MSLT findings.10
In the largest European multicenter study of the clinical and polysomnographic characteristics of patients with type 1 narcolepsy (n = 1,099), only 2.4% of 294 patients with available data had normal hypocretin levels, and among 927 patients with MSLT data, only 8% had a mean MSLT > 8 minutes and 8.5% had fewer than two SOREMPs.6 Prior to the advent of hypocretin testing, an earlier study (n = 306) found that the combination of a history of definitive cataplexy and two or more SOREMPs during MSLT was the best determinant of narcolepsy with cataplexy.24
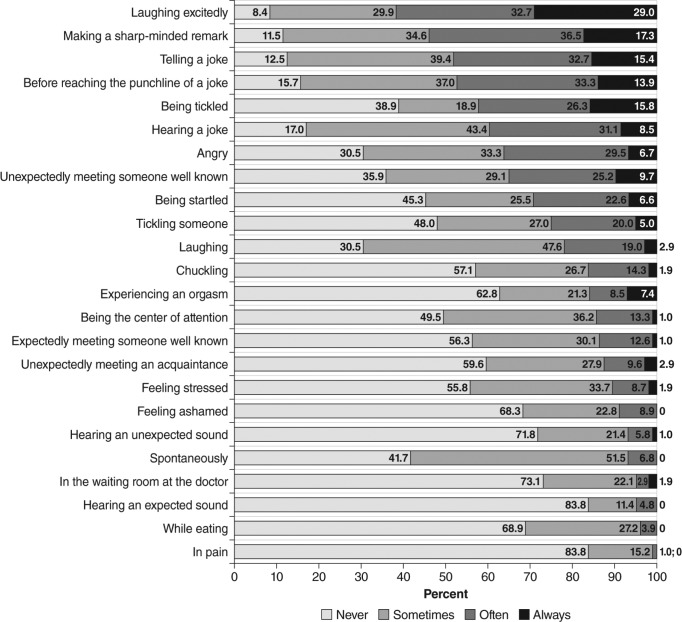
In the clinical setting, cataplexy is not frequently observed by clinicians. Although documentation of its presence relies on patient self-report, its occurrence may not necessarily be recognized by patients, parents, or caregivers. This lack of recognition may potentially lead to inaccurate or misleading reports of its occurrence and frequency,25,26 because presentation of cataplexy varies with regard to triggers (Figure 1) and manifestations (Table 1 and Table 2).15,27 Episodes range from complete attacks that involve bilateral loss of muscle tone of both upper and lower body muscles with collapse to the ground, to partial attacks that affect muscles of the arms and/or neck and face, leading to drooping of the head, jaw sagging, and garbled speech.13,15,27 Partial attacks may be subtle in presentation and more likely to be detected by the patient's partner or caregiver. Additionally, early manifestations in children may also include complex movement disorders such as facial (or generalized) hypotonia with droopy eyelids, mouth opening, and protruding tongue or gait unsteadiness, as well as facial and masticatory movements that do not meet the classic cataplexy definition.28 Frequency of attacks varies widely, from less than one per year to several per day,11,27,29 and cataplexy-like phenomena may occur in narcoleptic and non-narcoleptic patients, further complicating reporting and diagnosis.15,26,28,30
Figure 1. Most frequent cataplexy triggers reported in a questionnaire survey of 109 patients with definite cataplexy and documented hypocretin-1 deficiency.
Reprinted from Sleep Medicine, Vol 12(1), Overeem S, et al. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency, pages 12-18, Copyright 2011, with permission from Elsevier.27
Table 1.
Cataplexy characteristics reported in a questionnaire survey of 109 patients with definite cataplexy and documented low hypocretin-1 levels.
Table 2.
Cataplexy and cataplexy-like symptoms in narcolepsy patients with definite cataplexy (n = 41).

Guidelines for management of narcolepsy recommend first-line treatment with sodium oxybate (gamma hydroxy-butyrate) for both EDS and cataplexy in narcolepsy, and modafinil for EDS in narcolepsy.31,32 Both agents are approved for these indications by the United States Food and Drug Administration; the European Medicines Agency has also approved modafinil for treatment of EDS in narcolepsy, and sodium oxybate for cataplexy and EDS in adults with narcolepsy.33–35 Other pharmacologic therapies with less supporting data, which lack approval for narcolepsy but have been traditionally used, include amphetamines and amphetamine-like stimulants for EDS and antidepressants for cataplexy.31,32 Clinical studies of agents to treat EDS, cataplexy, and narcolepsy have used a variety of instruments for diagnosis and efficacy assessment, highlighting that an unmet need still exists for standardized and easily administered instruments for screening/diagnosis of narcolepsy, as well as to assess the effects of treatment over time with application for both clinical practice and clinical studies. Ideally, screening/diagnosis instruments would be brief, easy for patients to understand and for physicians to score, and would identify or rule out the need for further electrophysiological or laboratory testing. The following is a review of patient-reported measures that have been used for the assessment of narcolepsy, mainly in clinical trials, with the goal of summarizing existing scales and identifying areas where additional screening and clinical practice scales would be useful. This review, which reflects the experience and knowledge of the authors in the clinical setting, is qualitative rather than a more formal, structured, or systematic review, and therefore does not necessarily include all patient-reported measures that potentially may be used for evaluation of narcolepsy.
SCALES FOR SCREENING AND DIAGNOSIS
Epworth Sleepiness Scale
Developed in 1991 by Johns,36 the Epworth Sleepiness Scale (ESS) is the most widely used standardized tool for patient-reported measurement of EDS, regardless of etiology. The ESS consists of eight questions related to specific activities of daily life, with each rated as to how likely the patient is to “doze off or fall asleep” on a four-point scale from 0 (“would never doze”) to 3 (“high chance of dozing”). The total score range is 0–24; a cutoff score higher than 10 has been reported to have a sensitivity for EDS of 93.5% and a specificity of 100%, which is greater than the corresponding values for the MSLT (94.5% and 73.3%, respectively, at < 8 minute cutoff) and maintenance of wakefulness test (MWT) (20-minute MWT; 84.3% and 98.4%, respectively, at < 12 minute cutoff).37 In patients with narcolepsy/cataplexy, mean ESS scores of 17 to 20 have been consistently reported, and these scores are significantly greater than those of healthy controls and patients with other hypersomnias.6,15,38,39
A systematic literature review found that among 35 studies that evaluated the psychometric properties of the ESS in adults, the study quality ranged from excellent to poor, with the majority rated as fair.40 Most studies were of patients with obstructive sleep apnea (OSA) and/or insomnia, but several included narcolepsy. Although internal consistency was good (Cronbach alpha 0.73–0.86), pooled correlations with other constructs ranged from moderate for the MWT (rho = −0.43) to weak for the MSLT (rho = −0.27) and OSA-related variables (rho 0.11–0.23); evidence for test-retest reliability was also lacking.40
A major gap in the psychometric data for the ESS with respect to narcolepsy, given that most patients experience onset of symptoms during childhood or adolescence, is a paucity of validation studies in pediatric populations. Although modified versions of the ESS for use in children have been reported,41–43 psychometric studies demonstrating their validity for use as an indicator of EDS in pediatric populations are still lacking.
The Ullanlinna Narcolepsy Scale
The Ullanlinna Narcolepsy Scale (UNS) was developed by Hublin et al.44 to find a simple method for identifying symptoms typical of the narcoleptic syndrome and distinguish them from symptoms of other conditions that mimic narcolepsy. The UNS is an 11-item questionnaire that assesses EDS and cataplexy, with 7 items measuring sleep latency and propensity to fall asleep in various situations, and 4 items addressing typical manifestations of cataplexy occurring “when laughing, becoming glad or angry in an exciting situation.” Responses are scored from “Never” (0) to “Daily” or “Almost daily/Several times daily” (4), with a total score range of 0 to 44 and higher scores indicating worse outcomes.
The initial validation study of the UNS was conducted in patients with narcolepsy and cataplexy and 7 comparison groups of patients with other conditions that could be associated with EDS (including OSA, multiple sclerosis, and depression), but did not necessarily have EDS. In the narcolepsy/cataplexy patient group, the mean UNS score of 27.3 was higher than in the other disease comparison groups (Figure 2). Using 14 as a cutoff score, which was the lowest value among the narcolepsy patients, the UNS demonstrated a sensitivity of 100% and specificity of 98.8% in the 435 patients studied.44
Figure 2. Box plot graphics of the Ullanlinna Narcolepsy Scale score distributions in patients with narcolepsy (mean 27.3; 95% confidence interval 24.4–33.1; range 14–41) and 7 comparison groups.
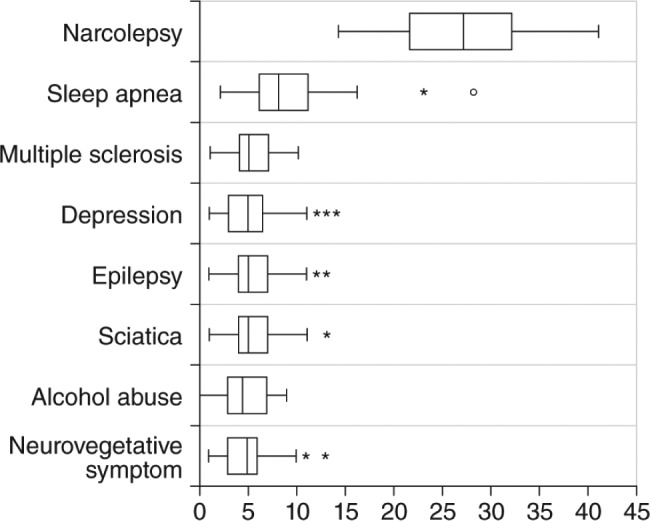
The box represents the interquartile range (IQR) and the median is marked by the central vertical line. The horizontal line segments indicate values falling within 1.5 IQRs from the box. Asterisks indicate outside values and open circles indicate far outside values (> 3 IQRs from box). Reprinted from Journal of Sleep Research, Vol 3(1), Hublin C, et al. The Ullanlinna Narcolepsy Scale: validation of a measure of symptoms in the narcoleptic syndrome, pages 52-59, Copyright 1994, with permission of John Wiley & Sons, Inc.44
Although a validation study of a Chinese version of the UNS reported that the mean (standard deviation [SD]) score of 18.6 (4.7) in narcolepsy patients was substantially lower than the score of 27.3 reported by Hublin et al.,44 it still significantly differentiated narcolepsy patients from the comparison groups (P < .001).45 Additionally, the Chinese version also reported high sensitivity (94.1%) and specificity (93.5%).
In contrast, a study by Sturzenegger and Bassetti15 reported a mean UNS score in patients with narcolepsy that was similar to the original validation study but reported a low specificity (56%), despite a high sensitivity (98%) using a cutoff value higher than 14. However, the low specificity may have been related to the inclusion in the study of patients with non-narcoleptic hypersomnia. Although the UNS is the most widely used narcolepsy screening instrument, its inconsistent specificity and length of 11 items suggest it may be less than optimal for the clinical setting.
Stanford Center for Narcolepsy Sleep Inventory
The Stanford Sleep Inventory (SSI) is a 146-item questionnaire, divided into 9 sections, that attempts to comprehensively capture the demographic and clinical characteristics of patients with narcolepsy. Sections 1 and 2 report demographic information; section 3 comprises ESS questions; section 4 addresses various sleep disturbances; section 5 includes 51 items on cataplexy; sections 6, 7, and 8 address hypnagogic hallucinations, sleep paralysis, and automatic behavior, respectively; and section 9 invites the patient to add any further remarks or information.26 Because the length of the questionnaire precludes its use in daily clinical practice, the cataplexy section was evaluated for use as a self-administered questionnaire to screen for definite cataplexy.26 Although the cataplexy section was found to be a valid measure, the number of questions, 51, makes it unwieldy for regular use in the clinical setting. However, factor analysis and stepwise logistic regression analysis further identified “telling a joke,” “laughter,” and “anger” as the three strongest predictors of cataplexy, and these factors were confirmed using receiver operating characteristic curve analysis. Based on these results, a prospective decision-tree algorithm was devised to classify patients with clear-cut cataplexy,26 but this algorithm is not proven and hence is not widely accepted nor frequently used in clinical practice.
Cataplexy Emotional Trigger Questionnaire
The data generated by the validation study of the cataplexy section of the SSI were used as the basis for a short screening questionnaire, the Cataplexy Emotional Trigger Questionnaire (CETQ; Figure 3).46 In addition to the SSI data that focus on the types of triggers as the strongest predictors of cataplexy, the CETQ also drew from another study that administered the SSI to 55 patients with narcolepsy/cataplexy and 47 comparators with OSA and found that 2 physical manifestations of a cataplexy attack, slurred speech and difficulty hearing, were also significant predictors.46,47
Figure 3. Cataplexy Emotional Trigger Questionnaire.
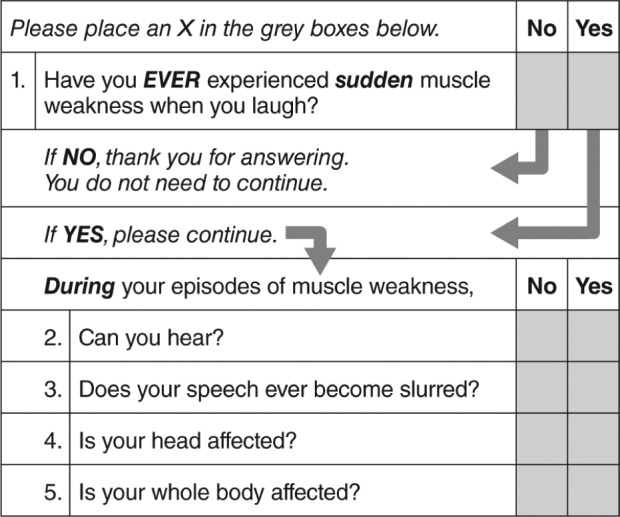
Reprinted from Journal of Clinical Sleep Medicine, Vol 3(1), Moore WR, et al. Cataplexy Emotional Trigger Questionnaire (CETQ) – a brief patient screen to identify cataplexy in patients with narcolepsy, pages 37-40, Copyright 2007, with permission of the American Academy of Sleep Medicine.46
A pilot validation study of the CETQ demonstrated that Question 1 (“Have you ever experienced sudden muscle weakness when you laugh?”) provided the highest sensitivity (94%; 95% confidence interval, 86% to 98%) and specificity (99%; 95% confidence interval, 93% to 100%), and that addition of the other questions did not result in improved discrimination. The authors concluded that these results demonstrated that a short and simple screening instrument using either only Question 1 or the full CETQ, depending on the circumstances, can improve recognition of cataplexy in a sleep center setting, and differentiate cataplexy-like symptoms in disorders other than narcolepsy from definite cataplexy.46 However, despite these promising results, the CETQ has not been widely used.
Swiss Narcolepsy Scale
The Swiss Narcolepsy Scale (SNS) is a 5-question narcolepsy screening instrument developed by Sturzenegger et al.,15,48 with 3 items addressing EDS and 2 for cataplexy, scored with negative and positive values (Figure 4). Although brief, the questions aim at multiple aspects of sleep-wake habits and symptoms to provide a potentially more extensive clinical assessment. The initial validation study, which compared the SNS with the UNS in 57 patients with narcolepsy/cataplexy, 56 with non-narcoleptic hypersomnia, and 40 healthy controls, suggested that an SNS score less than zero had similar sensitivity (96%) and higher specificity (98%) for narcolepsy than the UNS value higher than 14, which had 98% and 56% sensitivity and specificity, respectively.15
Figure 4. The Swiss Narcolepsy Scale.
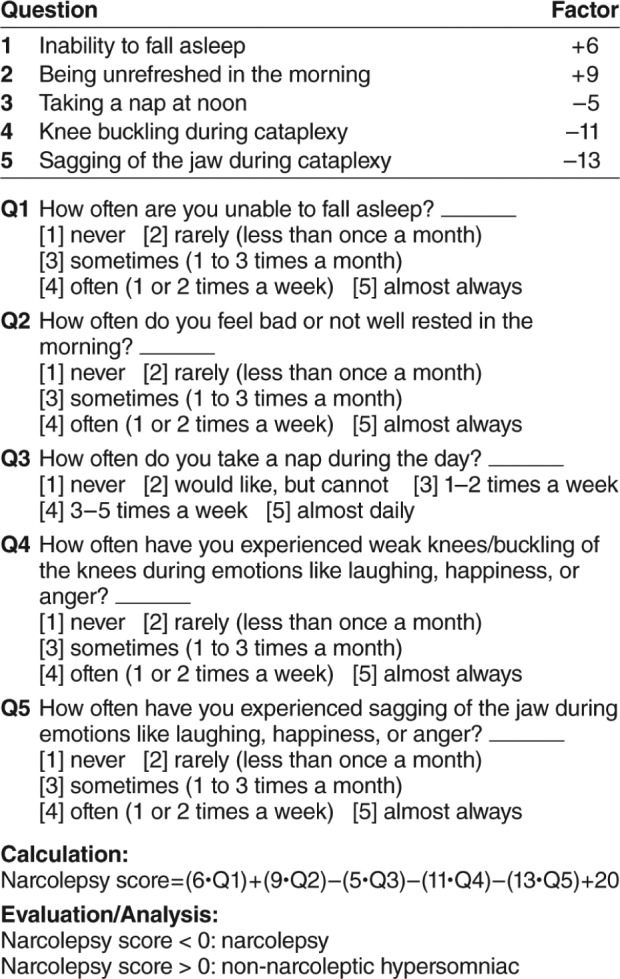
Adapted from Journal of Sleep Research, Vol 13(4), Sturzenegger C, Bassetti CL. The clinical spectrum of narcolepsy with cataplexy: a reappraisal, pages 395-406, Copyright 2004, with permission of John Wiley & Sons, Inc.15
A larger follow-up study evaluated SNS, UNS, and ESS in 33 patients with narcolepsy/cataplexy (26 with CSF hypo-cretin-1 assessment) and 142 patients with EDS due to a wide variety of other sleep disorders.49 This study confirmed the validity of the SNS for identifying narcolepsy, both overall, as well as in the subgroup with low CSF hypocretin, and demonstrated superior specificity relative to the other measures. An additional study again compared these three measures for prediction of narcolepsy in 80 patients with narcolepsy/cataplexy and 111 patients with EDS due to various non-narcoleptic sleep disorders.48 This study reported sensitivity and specificity for the SNS (score less than zero) of 89% and 88%, whereas the UNS (score of 14 or higher) had corresponding values of 100% and 62%, respectively. The ESS (score of 14 or higher) had a sensitivity of 91% and specificity of 54%. Among patients with low hypocretin-1, sensitivity was slightly higher for the SNS, although values for the UNS and ESS were similar to those observed in the overall population. Hence, this latter study supported previous findings suggesting the superiority of the SNS, especially for those with low CSF hypocretin.
ASSESSING EFFECTS OF TREATMENT
Associated with the need for useful screening instruments for narcolepsy is the need for measures than can demonstrate sensitivity to change (improvement or worsening) in order to evaluate patient response to treatment. Ideally, such measures could be mapped to the minimal clinically important difference (MCID), which identifies the minimum change that is perceived by the patient as being of clinical importance, and is a key metric both for patient care and clinical trials of therapies.50
Studies evaluating narcolepsy therapies have used a variety of efficacy measures that include the MWT, MSLT, ESS, actigraphy, and Global Impression of Change from the clinician and patient perspectives.51 Polysomnographic data have also been used to measure changes in sleep architecture, such as increased duration in stage 3 and 4 sleep, whereas cataplexy has largely been measured by change in number of weekly attacks.51 However, because most of these measures are not narcolepsy specific, and some are objective measures requiring in-clinic testing, they do not help patients capture and record specific aspects of their experience with narcolepsy. It should also be noted that the MCID has not been determined for any of the measures that have been used to evaluate treatment effects in patients with narcolepsy.
Narcolepsy Symptom Status Questionnaire
The Narcolepsy Symptom Status Questionnaire (NSSQ) was introduced by Mitler et al.52 in 1982 in an attempt to better evaluate and quantify treatment efficacy. This measure is in the form of a questionnaire that uses a 7-point scale to quantify the severity of 5 common narcolepsy symptoms (sleepiness, sleep attacks, cataplexy, sleep paralysis, and hypnagogic hallucinations) during 12 daily situations and mood states (working, driving, sitting, watching television, exercising, lying in bed, excitement, boredom, anger, sadness, and tension). Values for each symptom range from 12 (“No symptom”) to 84 (“Severe”). The initial study using this instrument, conducted in 10 patients with narcolepsy and 8 healthy volunteers, found that following treatment (unspecified), the change in total NSSQ score was consistent with improvements in MWT and MSLT. However, a subsequent study found that the NSSQ did not consistently correlate with other outcomes, such as the MWT.53 However, no formal validation studies of the NSSQ have been reported in the literature. Additionally, because of the number of items in the questionnaire, the NSSQ may result in an administrative burden that is unlikely to be acceptable in daily clinical practice.
Narcolepsy Symptom Assessment Questionnaire
The Narcolepsy Symptom Assessment Questionnaire (NSAQ) consists of 26 items that evaluate the main symptoms of narcolepsy, including sleep disturbances and ability to concentrate. Individual symptoms of cataplexy, EDS, hypnagogic hallucinations, sleep paralysis, and disrupted nighttime sleep are rated as “Increased,” “Decreased,” or “Remains the same,” and items evaluating overall narcolepsy status, overall sleep quality, and concentration are rated using a five-point Likert-type scale of “Much improved” to “Much worse.”54 The measure addressing the ability to concentrate is unusual for narcolepsy assessments. However, this outcome may represent an under-recognized but important problem in narcolepsy patients, because it reflects a frequent complaint of “brain fog” by patients with narcolepsy.55
Although the NSAQ has yet to be validated, it has been used in two trials of sodium oxybate for treatment of narcolepsy symptoms in order to provide a patient-reported perspective that complemented objective outcome measures.54,56
OTHER CONSIDER ATIONS
Although a number of screening, diagnostic, and treatment-monitoring measures have been developed and evaluated for narcolepsy, it is notable how little attention has been given to addressing their efficacy and utility for use in the pediatric population. Parents/caregivers or other proxy responders may have been allowed to answer for children unable to understand the instruments reviewed, but this factor introduces a number of variables and uncertainty that could affect accuracy of reporting. Thus, measures are needed that not only can be administered to pediatric patients but have also been validated for use by proxies. Moreover, the question still remains of whether the language describing cataplexy, such as “muscle weakness,” and other symptoms, including restless legs syndrome, hallucinations, and fatigue, has been sufficiently refined so that a consistency of data and outcomes may eventually be obtained through commonly used measures across responder populations. Measures used in clinical practice might also vary from those used in academic research settings with respect to comprehensiveness, detail, recall periods, and other factors. Perhaps pertinent to this goal is the development and validation of parallel measures for use in these two settings, with the differences reflecting language and detail appropriate for each.
CONCLUSIONS
There remains a paucity of validated, patient-reported measures specific for narcolepsy and its symptoms. The ESS continues to be an important outcomes measure, and several narcolepsy-specific scales have demonstrated utility, such as the UNS, SNS, and NSAQ, but further validation is required. Although these narcolepsy-specific scales may identify narcolepsy with cataplexy, or type 1 narcolepsy, there are currently no validated questionnaires to identify narcolepsy without cataplexy, or type 2 narcolepsy (see Table S1 in the supplemental material). Thus, there remains a need for short, easily understood, and validated instruments that can be readily used in clinical practice to distinguish narcolepsy symptoms from those of other hypersomnias and for assessing these symptoms during treatment. Ideally, scales demonstrating sensitivity to change must also not only incorporate the patient's perspective and provide an estimate of the MCID, but also enable assessment of frequency versus severity of cataplexy. The importance of cataplexy as a symptom further suggests that a measure analogous to the ESS needs to be developed for this key symptom. Although it can also be suggested that the SNS may be an appropriate measure for screening and NSAQ for assessing treatment effects, further validation of these measures is required.
DISCLOSURE STATEMENT
This research was funded by Jazz Pharmaceuticals. Dr. Kallweit has consulted for UCB Pharma, Germany, Bioprojet Pharma, France, and Heinen + Löwenstein Medizintechnik, Germany, and received a senior investigator grant from the European Narcolepsy Network. Dr. Schmidt has consulted for XenoPort, served on the speakers bureau for XenoPort and UCB, and contributed to a physician educational training video for Narcolepsy Link. Dr. Bassetti has received consultancy fees from Jazz Pharmaceuticals, Inc., Servier, UCB Pharma, and Zambon, and has received industry-funded research support from Swiss National Science Foundation (SNF), ResMed, Respironics, Vifor Pharma, UCB Pharma, Schweizerische Herzstiftung, Tropos Stiftung, and Parkinson Schweiz.
ACKNOWLEDGMENTS
This review was funded by Jazz Pharmaceuticals. Under the direction of the authors, Larry Deblinger, employee of The Curry Rockefeller Group, LLC (CRG), provided medical writing assistance for this publication. Editorial assistance in formatting, proofreading, copy editing, and fact checking was also provided by CRG. Jazz Pharmaceuticals provided funding to CRG for support in writing and editing of this manuscript.
ABBREVIATIONS
- CETQ
Cataplexy Emotional Trigger Questionnaire
- CNS
central nervous system
- CSF
cerebrospinal fluid
- EDS
excessive daytime sleepiness
- ESS
Epworth Sleepiness Scale
- MCID
minimal clinically important difference
- MSLT
multiple sleep latency test
- MWT
maintenance of wakefulness test
- NSAQ
Narcolepsy Symptom Assessment Questionnaire
- NSSQ
Narcolepsy Symptom Status Questionnaire
- OSA
obstructive sleep apnea
- SD
standard deviation
- SNS
Swiss Narcolepsy Scale
- SOREMPs
sleep-onset rapid eye movement periods
- SSI
Stanford Sleep Inventory
- UNS
Ullanlinna Narcolepsy Scale
REFERENCES
- 1.Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol. 2015;14(3):318–328. doi: 10.1016/S1474-4422(14)70218-2. [DOI] [PubMed] [Google Scholar]
- 2.Nishino S, Mignot E. Narcolepsy and cataplexy. Handb Clin Neurol. 2011;99:783–814. doi: 10.1016/B978-0-444-52007-4.00007-2. [DOI] [PubMed] [Google Scholar]
- 3.Roth T, Dauvilliers Y, Mignot E, et al. Disrupted nighttime sleep in narcolepsy. J Clin Sleep Med. 2013;9(9):955–965. doi: 10.5664/jcsm.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longstreth WT, Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007;30(1):13–26. doi: 10.1093/sleep/30.1.13. [DOI] [PubMed] [Google Scholar]
- 5.Thorpy MJ. Cataplexy associated with narcolepsy: epidemiology, pathophysiology and management. CNS Drugs. 2006;20(1):43–50. doi: 10.2165/00023210-200620010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Luca G, Haba-Rubio J, Dauvilliers Y, et al. Clinical, polysomnographic and genome-wide association analyses of narcolepsy with cataplexy: a European Narcolepsy Network study. J Sleep Res. 2013;22(5):482–495. doi: 10.1111/jsr.12044. [DOI] [PubMed] [Google Scholar]
- 7.Okun ML, Lin L, Pelin Z, Hong S, Mignot E. Clinical aspects of narcolepsycataplexy across ethnic groups. Sleep. 2002;25(1):27–35. doi: 10.1093/sleep/25.1.27. [DOI] [PubMed] [Google Scholar]
- 8.Ohayon MM, Ferini-Strambi L, Plazzi G, Smirne S, Castronovo V. How age influences the expression of narcolepsy. J Psychosom Res. 2005;59(6):399–405. doi: 10.1016/j.jpsychores.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 9.Dauvilliers Y, Montplaisir J, Molinari N, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology. 2001;57(11):2029–2033. doi: 10.1212/wnl.57.11.2029. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 11.Dauvillers Y, Billiard M, Montplaisir J. Clinical aspects and pathophysiology of narcolepsy. Clin Neurophysiol. 2003;114(11):2000–2017. doi: 10.1016/s1388-2457(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 12.Ohayon MM, Ferini-Strambi L, Plazzi G, Smirne S, Castronovo V. Frequency of narcolepsy symptoms and other sleep disorders in narcoleptic patients and their first-degree relatives. J Sleep Res. 2005;14(4):437–445. doi: 10.1111/j.1365-2869.2005.00476.x. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed I, Thorpy M. Clinical features, diagnosis and treatment of narcolepsy. Clin Chest Med. 2010;31(2):371–381. doi: 10.1016/j.ccm.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Bassetti C, Aldrich MS. Narcolepsy. Neurol Clin. 1996;14(3):545–571. doi: 10.1016/s0733-8619(05)70273-5. [DOI] [PubMed] [Google Scholar]
- 15.Sturzenegger C, Bassetti CL. The clinical spectrum of narcolepsy with cataplexy: a reappraisal. J Sleep Res. 2004;13(4):395–406. doi: 10.1111/j.1365-2869.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- 16.Nishino S. Clinical and neurobiological aspects of narcolepsy. Sleep Med. 2007;8(4):373–399. doi: 10.1016/j.sleep.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med. 2014;15(5):502–507. doi: 10.1016/j.sleep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg R, Kim AY. The AWAKEN Survey: knowledge of narcolepsy among physicians and the general population. Postgrad Med. 2014;126(1):78–86. doi: 10.3810/pgm.2014.01.2727. [DOI] [PubMed] [Google Scholar]
- 19.Kauta SR, Marcus CL. Cases of pediatric narcolepsy after misdiagnoses. Pediatr Neurol. 2012;47(5):362–365. doi: 10.1016/j.pediatrneurol.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Macleod S, Ferrie C, Zuberi SM. Symptoms of narcolepsy in children misinterpreted as epilepsy. Epileptic Disord. 2005;7(1):13–17. [PubMed] [Google Scholar]
- 21.Kryger MH, Walid R, Manfreda J. Diagnoses received by narcolepsy patients in the year prior to diagnosis by a sleep specialist. Sleep. 2002;25(1):36–41. doi: 10.1093/sleep/25.1.36. [DOI] [PubMed] [Google Scholar]
- 22.Ohayon MM. Narcolepsy is complicated by high medical and psychiatric comorbidities: a comparison with the general population. Sleep Med. 2013;14(6):488–492. doi: 10.1016/j.sleep.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Jennum P, Ibsen R, Knudsen S, Kjellberg J. Comorbidity and mortality of narcolepsy: a controlled retro- and prospective national study. Sleep. 2013;36(6):835–840. doi: 10.5665/sleep.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moscovitch A, Partinen M, Guilleminault C. The positive diagnosis of narcolepsy and narcolepsy's borderland. Neurology. 1993;43(1):55–60. doi: 10.1212/wnl.43.1_part_1.55. [DOI] [PubMed] [Google Scholar]
- 25.Morrison I, Buskova J, Nevsimalova S, Douglas NJ, Riha RL. Diagnosing narcolepsy with cataplexy on history alone: challenging the International Classification of Sleep Disorders (ICSD-2) criteria. Eur J Neurol. 2011;18(7):1017–1020. doi: 10.1111/j.1468-1331.2010.03223.x. [DOI] [PubMed] [Google Scholar]
- 26.Anic-Labat S, Guilleminault C, Kraemer HC, Meehan J, Arrigoni J, Mignot E. Validation of a cataplexy questionnaire in 983 sleep-disorders patients. Sleep. 1999;22(1):77–87. [PubMed] [Google Scholar]
- 27.Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med. 2011;12(1):12–18. doi: 10.1016/j.sleep.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Plazzi G, Pizza F, Palaia V, et al. Complex movement disorders at disease onset in childhood narcolepsy with cataplexy. Brain. 2011;134(Pt 12):3477–3489. doi: 10.1093/brain/awr244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luca G, Vienne J, Vaucher A, Jimenez S, Tafti M. Central and peripheral metabolic changes induced by gamma-hydroxybutyrate. Sleep. 2015;38(2):305–313. doi: 10.5665/sleep.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plazzi G, Ferri R, Antelmi E, et al. Restless legs syndrome is frequent in narcolepsy with cataplexy patients. Sleep. 2010;33(5):689–694. doi: 10.1093/sleep/33.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Billiard M, Bassetti C, Dauvilliers Y, et al. EFNS guidelines on management of narcolepsy. Eur J Neurol. 2006;13(10):1035–1048. doi: 10.1111/j.1468-1331.2006.01473.x. [DOI] [PubMed] [Google Scholar]
- 32.Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705–1711. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palo Alto, CA: Jazz Pharmaceuticals, Inc; 2015. Apr, Xyrem (sodium oxybate) oral solution [US prescribing information] https://www.xyremrems.com/documents/XYREM_Prescribing_Information.pdf. [Google Scholar]
- 34.North Wales, PA: Cephalon, Inc., a wholly owned subsidiary of Teva Pharmaceutical Industries Ltd.; 2015. Feb, Provigil (modafinil) tablets [US prescribing information] http://www.provigil.com/PDFs/prescribing_info.pdf. [Google Scholar]
- 35.Modafinil: summary of product characteristics and package leaflet. European Medicines Agency Web site. [Accessed January 6, 2017]. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Modafinil_31/WC500096080.pdf.
- 36.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 37.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9(1):5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 38.Parkes JD, Chen SY, Clift SJ, Dahlitz MJ, Dunn G. The clinical diagnosis of the narcoleptic syndrome. J Sleep Res. 1998;7(1):41–52. doi: 10.1046/j.1365-2869.1998.00093.x. [DOI] [PubMed] [Google Scholar]
- 39.Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck narcolepsy cohort. J Clin Sleep Med. 2013;9(8):805–812. doi: 10.5664/jcsm.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kendzerska TB, Smith PM, Brignardello-Petersen R, Leung RS, Tomlinson GA. Evaluation of the measurement properties of the Epworth sleepiness scale: a systematic review. Sleep Med Rev. 2014;18(4):321–331. doi: 10.1016/j.smrv.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Chan EY, Ng DK, Chan CH, et al. Modified Epworth Sleepiness Scale in Chinese children with obstructive sleep apnea: a retrospective study. Sleep Breath. 2009;13(1):59–63. doi: 10.1007/s11325-008-0205-7. [DOI] [PubMed] [Google Scholar]
- 42.Lecendreux M, Gamble H, Sanchez-Garrido L, Giordanella J-P, Konofal E. An evaluation study of the Epworth sleepiness scale adapted for children and adolescents: a tool for case finding of pediatric excessive daytime sleepiness (EDS) [abstract] Eur Psychiatry. 2011;26(Suppl 1):1559. [Google Scholar]
- 43.Wang YG, Benmedjahed K, Lambert J, et al. Developing outcome measures for assessing narcolepsy with cataplexy in children and adolescents [abstract] Sleep. 2015;38(Abstract Suppl):A388–A389. [Google Scholar]
- 44.Hublin C, Kaprio J, Partinen M, Koskenvuo M, Heikkilä K. The Ullanlinna Narcolepsy Scale: validation of a measure of symptoms in the narcoleptic syndrome. J Sleep Res. 1994;3(1):52–59. doi: 10.1111/j.1365-2869.1994.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 45.Wing YK, Li RH, Ho CK, Fong SY, Chow LY, Leung T. A validity study of Ullanlinna Narcolepsy Scale in Hong Kong Chinese. J Psychosom Res. 2000;49(5):355–361. doi: 10.1016/s0022-3999(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 46.Moore WR, Silber MH, Decker PA, et al. Cataplexy Emotional Trigger Questionnaire (CETQ)--a brief patient screen to identify cataplexy in patients with narcolepsy. J Clin Sleep Med. 2007;3(1):37–40. [PubMed] [Google Scholar]
- 47.Krahn LE, Lymp JF, Moore WR, et al. Characterizing the emotions that trigger cataplexy. J Neuropsychiatry Clin Neurosci. 2005;17(1):45–50. doi: 10.1176/jnp.17.1.45. [DOI] [PubMed] [Google Scholar]
- 48.Sturzenegger C, Baumann CR, Kallweit U, Lammers GJ, Bassetti C. Swiss Narcolepsy Scale: a valid tool for the identification of hypocretin-1 deficient patients with narcolepsy [abstract] J Sleep Res. 2014;23(Suppl 1):297. [Google Scholar]
- 49.Sturzenegger C, Baumann CR, Kallweit U, Lammers GJ, Bassetti C. Swiss narcolepsy scale: a valid tool for the identification of hypocretin-1 deficient patients with narcolepsy [abstract] J Sleep Res. 2006;15(Suppl 1):74. [Google Scholar]
- 50.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 51.Poryazova R, Tartarotti S, Khatami R, et al. Sodium oxybate in narcolepsy with cataplexy: Zurich sleep center experience. Eur Neurol. 2011;65(3):175–182. doi: 10.1159/000324549. [DOI] [PubMed] [Google Scholar]
- 52.Mitler MM, Gujavarty KS, Browman CP. Maintenance of wakefulness test: a polysomnographic technique for evaluation treatment efficacy in patients with excessive somnolence. Electroencephalogr Clin Neurophysiol. 1982;53(6):658–661. doi: 10.1016/0013-4694(82)90142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep. 2001;24(4):385–391. doi: 10.1093/sleep/24.4.385. [DOI] [PubMed] [Google Scholar]
- 54.Mamelak M, Swick T, Emsellem H, Montplaisir J, Lai C, Black J. A 12-week open-label, multicenter study evaluating the safety and patient-reported efficacy of sodium oxybate in patients with narcolepsy and cataplexy. Sleep Med. 2015;16(1):52–58. doi: 10.1016/j.sleep.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 55.The Voice of the Patient Report: Narcolepsy. United States Food and Drug Administration Web site. [Accessed January 4, 2017]. http://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM402907.pdf. Published June 2014.
- 56.Mamelak M, Black J, Montplaisir J, Ristanovic R. A pilot study on the effects of sodium oxybate on sleep architecture and daytime alertness in narcolepsy. Sleep. 2004;27(7):1327–1334. doi: 10.1093/sleep/27.7.1327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.