Abstract
Study Objectives:
To assess effects of low-level continuous positive airway pressure (CPAP) on snoring in habitual snorers without obstructive sleep apnea (OSA).
Methods:
A multicenter prospective in-laboratory reversal crossover intervention trial was conducted between September 2013 and August 2014. Habitual snorers were included if they snored (inspiratory sound pressure level ≥ 40 dBA) for ≥ 30% all sleep breaths on a baseline sleep study (Night 1), and if significant OSA and daytime somnolence were absent. Included participants then underwent a CPAP titration study at 2, 4, or 6 cm H2O (Night 2) to examine snoring responses to step-increases in nasal pressure, a treatment night at optimal pressure (Night 3), followed by baseline night (Night 4). At each pressure, snoring intensity was measured on each breath. Snoring frequency was quantified as a percentage of sleep breaths at thresholds of 40, 45, 50, and 55 dBA. Sleep architecture and OSA severity were characterized using standard measurements.
Results:
On baseline sleep studies, participants demonstrated snoring at ≥ 40 dBA on 53 ± 3% and ≥ 45 dBA on 35 ± 4% of breaths. Snoring frequency decreased progressively as nasal pressure increased from 0 to 4 cm H2O at each threshold, and plateaued thereafter. CPAP decreased snoring frequency by 67% and 85% at 40 and 45 dBA, respectively. Intervention did not alter sleep architecture and sleep apnea decreased minimally.
Conclusions:
Low-level CPAP below the range required to treat OSA diminished nocturnal snoring, and produced uniform reduction in nightly noise production below the World Health Organization's limit of 45 dBA.
Clinical Trial Registration:
ClinicalTrials.gov, identifier: NCT01949584.
Citation:
Guzman MA, Sgambati FP, Pho H, Arias RS, Hawks EM, Wolfe EM, Ötvös T, Rosenberg R, Dakheel R, Schneider H, Kirkness JP, Smith PL, Schwartz AR. The efficacy of low-level continuous positive airway pressure for the treatment of snoring. J Clin Sleep Med. 2017;13(5):703–711.
Keywords: CPAP, noise pollution, sleep, sleep apnea, snore
INTRODUCTION
Snoring is a commonly reported problem with an estimated prevalence of approximately 40% of the adult population.1,2 It encompasses a spectrum of disorders caused by upper airway obstruction during sleep. Greater degrees of airway obstruction can compromise ventilation during sleep, leading to the development of obstructive sleep apnea (OSA) with episodic oxyhemoglobin desaturation, and repeated arousals from sleep.3 In recent years, this disorder has become an increasingly recognized source of neurocognitive, metabolic, and cardiovascular morbidity and mortality.4 In contrast, snoring can be associated with relatively mild degrees of airflow obstruction in otherwise asymptomatic individuals5 and uncertain risk of excess morbidity and mortality.6,7 In fact, the health consequences of asymptomatic snoring are disputed with some reports documenting little excess morbidity or mortality,8 yet others suggesting that snoring per se may ultimately contribute to the development of hypertension and cardiovascular disease.9–12 Snoring constitutes a recognized source of noise pollution that may disrupt the sleep of bed partners and degrade a couple's overall quality of life,13,14 particularly when noise levels exceed 45 dBA.15 Nonetheless, little consensus exists on how to treat snoring and whether it should be treated at all.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Continuous positive airway pressure (CPAP) is the gold standard for the treatment of obstructive sleep apnea. Snoring is a manifestation of partial upper airway obstruction during sleep and can be eliminated by CPAP at levels considerably lower than those used to treat obstructive sleep apnea.
Study Impact: Snoring is a potent source of noise pollution in the bedroom that can degrade the quality of sleep in bed partners. Low-level CPAP can mitigate snoring and potentially improve the sleep quality and daytime function of bed partners.
Efforts to mitigate asymptomatic snoring are hindered by a lack of suitable treatment strategies. Agents that reduce nasal airflow obstruction including nasal splints, nasal saline sprays, nasal decongestants, and anti-inflammatory medications have limited clinical efficacy in snorers.2 Alternative approaches are designed to relieve pharyngeal obstruction with a variety of devices including mandibular advancement, tongue retaining, and head and neck positioning devices, which stabilize soft tissue and bony structures around the airway. Nasal continuous positive airway pressure (CPAP) can reliably relieve snoring, although it is usually reserved for treatment of sleep apnea16 and its use at home has been plagued with a lack of adherence over the long term.17,18 Finally, surgical treatments including alar nasal valve repair, turbinectomy, nasal septoplasty, palatal battens, and uvulopalatopharyngoplasty19 have been promulgated for this purpose. Despite the wide range of available treatment options, many snorers remain untreated due to limited tolerability and/or therapeutic efficacy of therapies.
Although highly efficacious, widespread adoption of nasal CPAP for treatment of asymptomatic snorers is limited due to difficulties in tolerating the interface and the relatively high pressures that are applied.18 In theory, the pressure required to mitigate snoring should be substantially lower than that required to treat OSA,20 specifically, lower than 4 cm H2O. This concept stems from the fact that the pharynx is less collapsible in the asymptomatic snorer than in the apneic patient, and requires proportionately less pressure to splint it open during sleep.21 Reductions in the pressure requirement may ultimately make the nasal interface more tolerable, and enhance adoption in the home. Objective data documenting CPAP efficacy for snoring, however, is still lacking.
The major aim of the current study was to examine the efficacy of low-level nasal CPAP in relieving habitual snoring in asymptomatic individuals without sleep apnea. We hypothesized that progressive decreases in snoring severity would occur as CPAP is incremented stepwise over a range from 2 to 6 cm H2O, and that the application of CPAP in this range would decrease objective measurements of snoring severity over the course of an entire night.
METHODS
Study Design
A three-center prospective clinical trial was conducted to test the efficacy of low-level CPAP in habitual simple snorers with subjects serving as their own control. Participants were screened for the presence of habitual snoring and absence of significant sleep apnea. Qualifying subjects underwent a sequence of four sleep studies: (1) a baseline polysomnographic study to assess for sleep apnea and snoring severity, (2) a CPAP titration study to determine the response in snoring to increments in nasal pressure, (3) a treatment night on a fixed low level of CPAP pressure (as determined from the titration night), and (4) another baseline sleep study off CPAP to examine snoring variability over time.
All studies were conducted in sound-attenuated sleep laboratories at the Johns Hopkins Center for Interdisciplinary Sleep Research and Education in Baltimore, Maryland; Doctors Community Hospital in Lanham, Maryland; and Neurotrials Research, Inc. in Atlanta, Georgia. The study protocol was approved by Institutional Review Boards at the Johns Hopkins School of Medicine, Doctors Community Hospital, and by the Sterling Institutional Review Board. This clinical trial was registered on www.clinicaltrials.gov (#NCT01949584).
Experimental Measurements and Materials
Polysomnography
Participants were assessed using standard polysomnography as recommended by American Academy of Sleep Medicine guidelines.22 Electroencephalograms, electrooculograms, submental and pretibial electromyograms, modified V5 electrocardiogram, body position, pulse oximetry, and respiratory inductance plethysmography signals were digitized to assess sleep stage, respiratory effort, respiratory movements, and leg movements. On control nights 1 and 4, airflow was also monitored with a nasal pressure cannula and oronasal thermistor to detect apneas and hypopneas (see next paragraphs). On CPAP nights 2 and 3, nasal pressure was monitored in the breathing circuit and airflow was monitored with a lightweight, low-resistance pneumotachograph23 affixed to a nasal interface and placed in line with the CPAP breathing circuit. Additionally, video and audio were recorded. All signals were recorded continuously using the REMLogic 1.3 data acquisition systems (Natus Medical Inc.; Broomfield, Colorado, United States) for each overnight sleep study, and transmitted to the Scoring Center at the Johns Hopkins Center for Interdisciplinary Sleep Research and Education for further evaluation.
Epworth Sleepiness Scale
This survey asked participants to report the likelihood of falling asleep (on a four-point Likert scale) in eight commonly encountered wake time situations, and was utilized to characterize participants' subjective report of daytime somnolence. A value of greater than 10 was taken as evidence for daytime hypersomnolence.24
Measurement of Snoring
Snoring frequency and intensity were captured with a high-accuracy class 2 digital sound pressure level meter with an accuracy ± 1.4 dB (DT-8851, Ruby Electronics, Saratoga, California, United States) in compliance with IEC 61672-1 standards. Device settings were A-frequency weighted and fast-time weighted (125 ms) to accurately capture sound pressure levels for each breath within the acoustic range of human hearing.25 The system was calibrated using an industrial sound level calibrator (SC-05, Reed, Inc., Wilmington, North Carolina, United States) with an accuracy ± 0.5 dB in compliance with IEC 942. A DC analog sound level output of 10 mV/ dB was digitized by the REMLogic data acquisition system and sound pressure measurements (dBA) were recorded continuously throughout each sleep study and synchronized with the airflow signal. Pink noise was applied for 10-second intervals to calibrate the sound (snoring) signal.26–29 Each sleep study was conducted in closed sound-attenuated laboratory bedrooms where background ambient noise levels were ≤ 35 dBA. The sound pressure level meter was affixed 65 cm above the surface of the bed during each study night to approximate the distance between the head of the bed partner and snorer.
Low-Level CPAP Device
A specialized device (Cloud9, inSleep Technologies, Weston, Florida, United States) was used in this study to deliver CPAP at low-levels ranging from 2 to 6 cm of H2O via a custom nasal interface. The device consisted of a small airflow generator, which incorporated a controlled mechanism for maintaining a specified low level of nasal CPAP by monitoring pressure in the nasal interface. Flow in the nasal circuit varied dynamically over a range from 0 to 80 L/min to stabilize pressure in the nasal interface throughout the respiratory cycle. The flow generator prevented rebreathing exhaled air at low nasal pressures by providing a continuous biased flow through the breathing circuit out of a fixed leak in the nasal interface. The effect of low-level CPAP on end-tidal CO2 (ETCO2) was examined in several subjects (n = 4) by comparing ETCO2 before and after 10 minutes exposure to 2 cm H2O. In each individual, ETCO2 was measured for 5 to 15 consecutive breaths in each condition. In mixed-model linear regression analysis, we demonstrated no significant difference in ETCO2 between baseline and CPAP conditions. Specifically, we found that ETCO2 was 4.38 ± 0.12 and 4.33 ± 0.12%, respectively. A customized nasal “butterfly” interface was designed to cradle the nares, and connect to the flow generator via tubing that branched over the cheeks toward the crown of the head (Figure 1). During normal operations, the breathing circuit, butterfly interface, and flow generator emitted background noise well below 40 dBA.
Figure 1. Image of customized butterfly nasal interface with custom noise baffling pathway therein.

The interface connects in series with the breathing circuit and flow generator. The breathing circuit wraps around both cheeks toward the crown of the head before connecting to the continuous positive airway pressure hose and flow generator.
Subjects
Self-reported habitual snorers greater than 18 years were recruited from participating clinics and the surrounding communities through brochures, flyers, newspaper advertisements, and social media. A total of 611 participants were screened with an initial telephone interview at a centralized screening site (Figure 2). Candidates were excluded if they reported a history of OSA, unstable cardiovascular disease, chronic obstructive pulmonary disease, asthma, stroke, uncontrolled hypertension (blood pressure > 170/100), nocturnal choking or gasping and/or witnessed apnea, current pregnancy or suspected pregnancy, or current participation in another research study (n = 447). Informed consent was obtained from the remaining 164 subjects, who were subsequently evaluated with a standardized history and physical examination, Epworth Sleepiness Scale (ESS) score, and a baseline sleep study (Night 1). Participants were included if snoring was present on this sleep study at levels ≥ 40 dBA for ≥ 30% all breaths during sleep. They were excluded if they had a body mass index > 35 kg/m2 on examination, a prior diagnosis of sleep apnea, or evidence of sleep apnea syndrome, as defined by either a respiratory disturbance index (RDI) ≥ 15 events/h or an RDI ≥ 5 events/h and ESS score > 10. Twenty-six participants met all screening criteria and were enrolled in the treatment trial. A single participant withdrew and another exhibited persistent elevations in background noise > 40 dBA due to excessive mouth leak. Twenty-four subjects (males = 12; females = 12; age: 46 ± 13 years; body mass index: 29 ± 5 kg/m2; RDI: 9 ± 3 events/h) participated in the CPAP intervention trial.
Figure 2. Study flow diagram describing screen failures and final study sample.
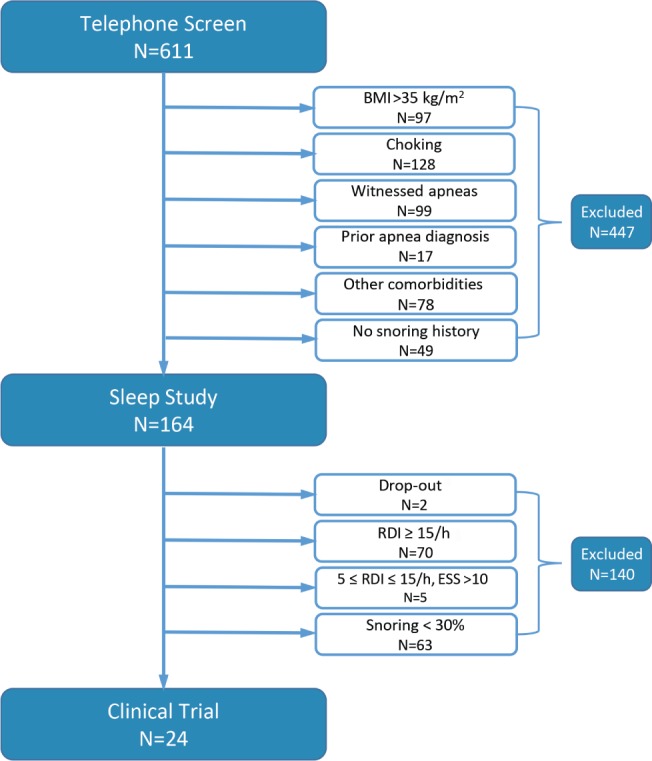
A two stage screening process was implemented by means of telephone survey and baseline sleep study. For details on “Drop-out,” see Subjects section in text. BMI = body mass index, ESS = Epworth Sleepiness Scale, RDI = respiratory disturbance index.
Study Protocol
History and Physical Examination
Snoring subjects underwent history and physical examination to confirm health and all other inclusion criteria. Medical history was taken as well as documentation of current medications. Each subject also completed the ESS.
Baseline Sleep Study (Night 1 and 4)
Subjects underwent a full standard overnight polysomnography to rule out apnea (Night 1) and assess snoring severity. Subjects were encouraged to sleep supine on all study nights. All sleep studies commenced at approximately 11:30 PM (lights out), and terminated at 6:00 AM (lights on) the following morning.
Titration Night Sleep Study (Night 2)
Subjects underwent full overnight polysomnography and snoring monitoring while using the Cloud9 device. The nasal pressure was titrated upward in a stepwise fashion through the night from 2 to 6 cm H2O to assess snoring responses to varying levels of CPAP.
Treatment Night Sleep Study (Night 3)
Subjects underwent full standard overnight polysomnography to test CPAP efficacy in mitigating snoring while exposing subjects to a fixed level of nasal pressure for the entire night. The pressure was selected to minimize snoring and inspiratory flow limitation based on Night 2 responses.
Data Analysis
Polysomnography Scoring
Full polysomnography was acquired digitally, as previously mentioned, to assess for the presence or absence of OSA. Sleep stages N1, N2, N3, and rapid eye movement (REM) were visually scored in 30-second sequential epochs using standard American Academy of Sleep Medicine criteria.22 Sleep architecture was summarized by calculating the total sleep time (TST), sleep efficiency, and percentages of TST spent in each sleep stage on control and treatment nights. A successful study was defined by more than 4 hours of sleep.
Respiratory events and arousals were also scored according to standard criteria. An obstructive apnea was defined as ≥ 90% drop in airflow for ≥ 10 seconds in the presence of continued respiratory effort. Hypopnea was defined as ≥ 30% drop in airflow for ≥ 10 seconds in association with ≥ 4% oxygen desaturation and/or arousal from sleep. The RDI was determined by calculating the number of apneas and hypopneas per hour of TST.
Snoring Analysis
Custom software was deployed to assess the frequency and intensity of snoring on each breath during non-rapid eye movement (NREM) and REM sleep. Our algorithm automatically detected each inspiration from the airflow signal, and measured the peak inspiratory amplitude from the sound level envelope. Each recording was then visually scanned for sound artifacts that did not coincide with inspiration. These artifacts were tagged and omitted from the array of sound amplitudes associated with each breath. All snores with peak decibel values exceeding a minimum threshold of ≥ 40 dB were tabulated because this threshold sound level has been recognized to be associated with nocturnal noise pollution and sleep disruption.15,30 The frequency of snoring was calculated as a percentage of all breaths during sleep. Snoring sound intensity was further categorized by determining the percentage of breaths associated with peak decibel levels greater than or equal to threshold levels of 40, 45, 50, and 55 dBA during sleep.
Statistical Analysis
Statistical analyses were structured to examine responses in sleep architecture, respiratory parameters, and snoring severity (the frequency of snoring at specific intensity levels) to low-level CPAP. Initial exploratory analyses were conducted to determine whether outcome variables were normally distributed and to transform non-normally distributed variables accordingly. Mixed-model linear regression was applied to determine the effect of CPAP treatment on outcome variables, while accounting for random differences among participants using [SAS/STAT] software, Version [9.3] (SAS Institute Inc., Cary, North Carolina, United States). When significant main effects were identified (P < .05), post hoc least squares means testing was utilized to compare differences in outcome variables among CPAP levels or treatment nights.
RESULTS
Subject Characteristics
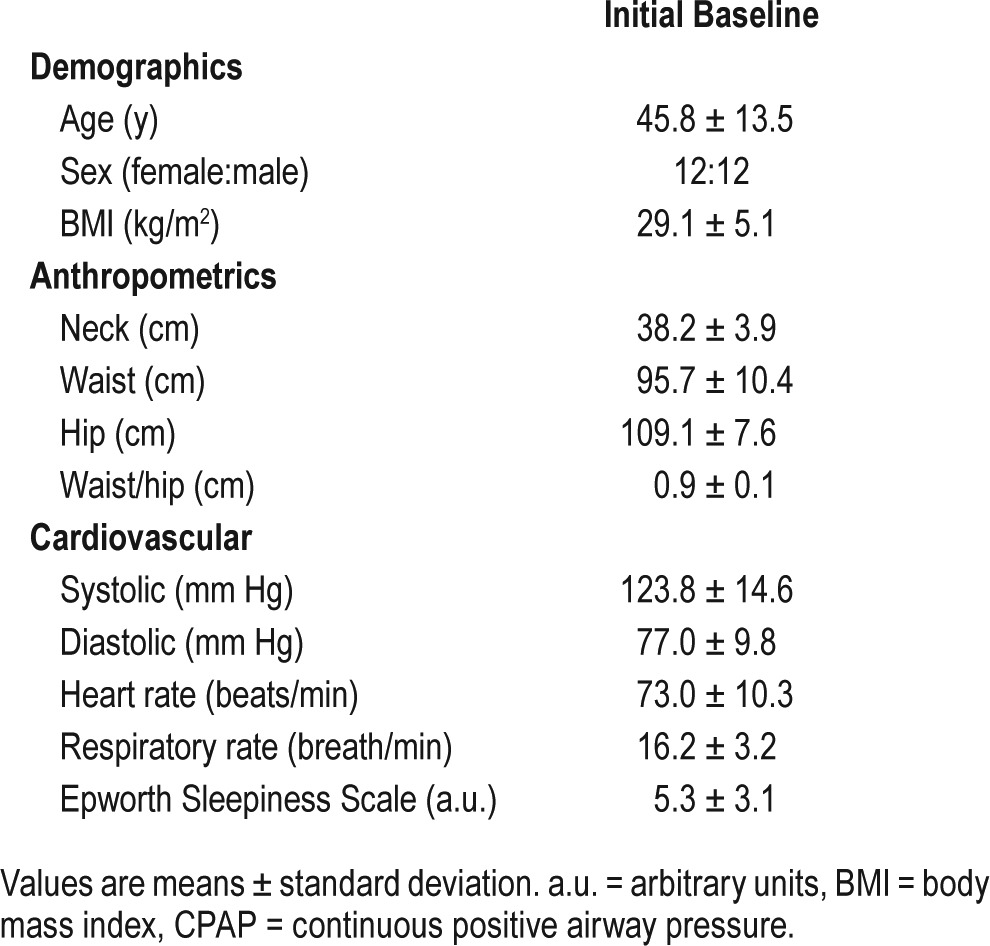
Participants' demographic and anthropometric characteristics as well as ESS scores are presented in Table 1. The study population included an equal number of men (n = 12) and women (n = 12) with an average age of 45.8 ± 13.5 years (mean ± standard deviation). In general, our subjects were mild to moderately obese. Their ESS score was 5.3 ± 3.1, indicating no significant daytime sleepiness. Subject characteristics did not differ significantly among the study sites (not shown).
Table 1.
CPAP treatment trial characteristics.
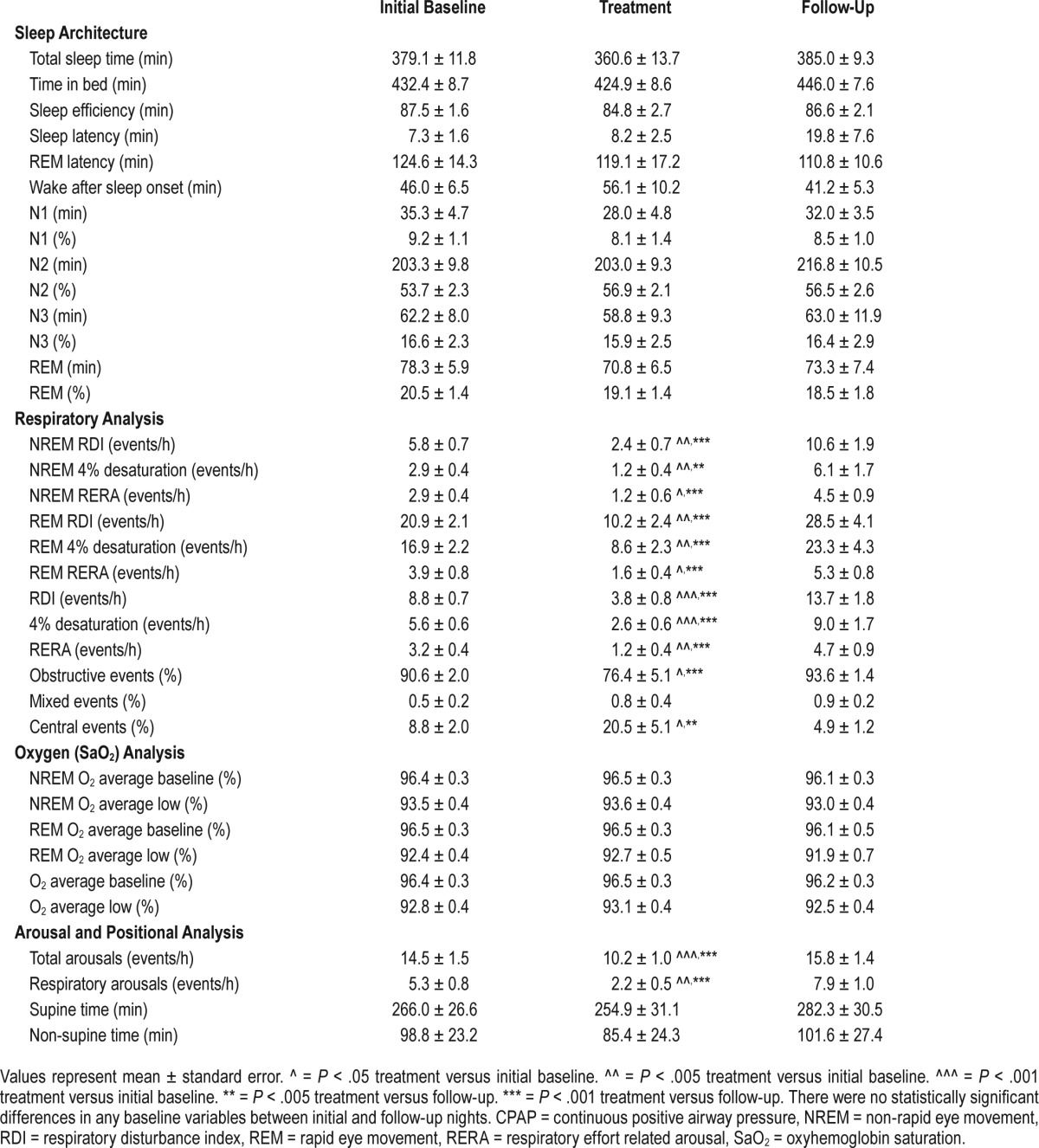
Our subjects had relatively mild OSA, which was greater in REM than NREM sleep (Table 2, initial baseline and follow-up nights). The four sleep studies spanned a time period of 64.1 ± 1.7 days, with a mean of 21.4 ± 0.6 days between each visit. Among the 96 overnight studies performed, 8 were repeated due to technical failures, whereas none needed to be repeated due to CPAP intolerance or insufficient sleep. Subjects were encouraged to sleep supine through the night and spent most of their sleep in the supine position (Table 2). No statistically significant differences were observed in the amount of time spent in supine versus non-supine positions, or in the amount and distribution of sleep stages among the study nights. Moreover, sleep architecture, sleep apnea severity, and oxygenation and arousal indices did not change significantly between baseline and follow-up Nights 1 and 4 (Table 2).
Table 2.
Sleep study results for CPAP treatment trial.
Titration Response (Night 2)
Snoring responses to the application of low-level CPAP are illustrated for one subject during NREM sleep (Figure 3). At atmospheric pressure, loud continuous snoring was observed to nearly 50 dBA (left panel). Peak snoring intensity decreased to approximately 45 dBA at 2 cm H2O (middle panel), and was completely abolished at 4 cm H2O (right panel, below 40 dBA, background ambient noise). Audible snoring was associated with the development of inspiratory airflow limitation, as characterized by an early plateau in inspiratory airflow while inspiratory effort continued to increase.31 At each level of nasal pressure, oxyhemoglobin saturation, nasal airflow, and thoracic and abdominal efforts remained stable. A detailed view of airflow signals is illustrated at each level of nasal pressure (Figure 3, bottom panel), and demonstrate inspiratory flow limitation at 0 and 2 cm H2O, which was abolished at 4 cm H2O. Cardinal features of inspiratory flow limitation (1) plateauing of mid-inspiratory airflow with prolongation of the inspiratory duty cycle, (2) a variable degree of negative effort dependence, (3) a prolongation of the inspiratory duty cycle (compared to non-flow-limited breaths), (4) high frequency fluctuations in flow (from snoring) in mid- to late inspiration, and (5) a dissociation between maximal inspiratory airflow and peak inspiratory effort over a series of adjacent breaths were present at low pressures of 0 and 2 cm H2O, and eliminated at 4 cm H2O.
Figure 3. Recording example illustrating snoring response to low-level CPAP in one habitual snorer during NREM sleep.
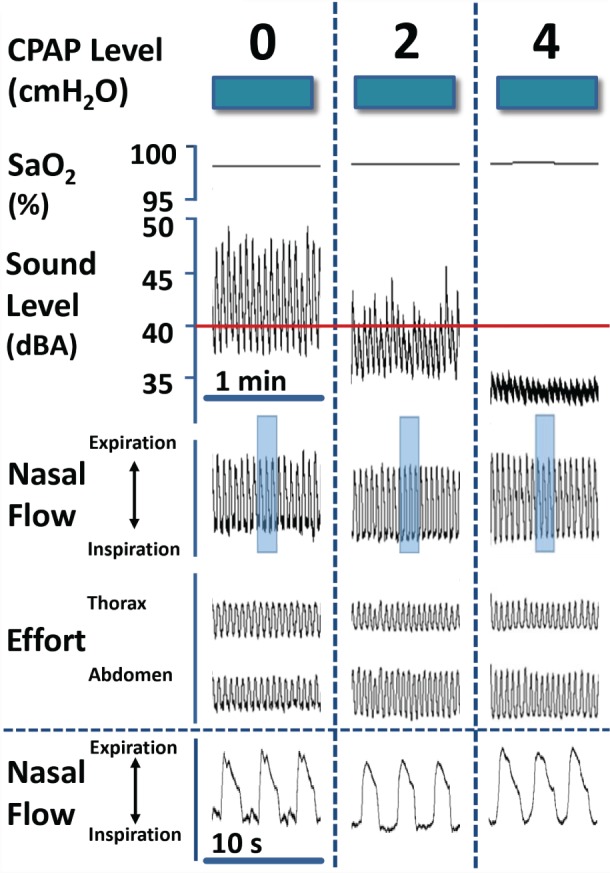
Snoring amplitude decreased progressively at 0, 2 and 4 cm H2O. Snoring was associated with the development of IFL, which was abolished at 4 cm H2O. In bottom panel, airflow signals are illustrated in greater detail from shaded regions of compressed signals above at each CPAP level. IFL was present at 0 and 2 cm H2O, as indicated by mid-inspiratory flattening of the airflow signal and an increase in the inspiratory duty cycle, but was abolished at 4 cm H2O. CPAP = continuous positive airway pressure, IFL = inspiratory airflow limitation, NREM = non-rapid eye movement, SaO2 = oxyhemoglobin saturation.
Titration responses to low-level CPAP are represented in Figure 4 for the entire group. Progressive increases in CPAP from 0 to 4 cm H2O resulted in graded decreases in the frequency of snoring (P < .005), which plateaued with further increases in nasal pressure from 4 to 6 cm H2O (P = n.s.). Increases in CPAP treatment to 2 and 4 cm H2O led to an approximately 49% and 67% reduction in the percentage of all snoring breaths (≥ 40 dBA), respectively, compared to baseline levels at atmospheric pressure. Loud snoring (≥ 45 dBA) also decreased markedly, to approximately 59 and 83% of baseline with the application of 2 and 4 cm H2O (P < .01), respectively. In contrast, snoring did not decrease further with increases in CPAP from 4 to 6 cm H2O (P = n.s.).
Figure 4. Snoring frequency (as a percentage of all breaths during sleep) versus CPAP level during the titration Night 2 for the entire group at threshold levels of 40 and 45 dBA.
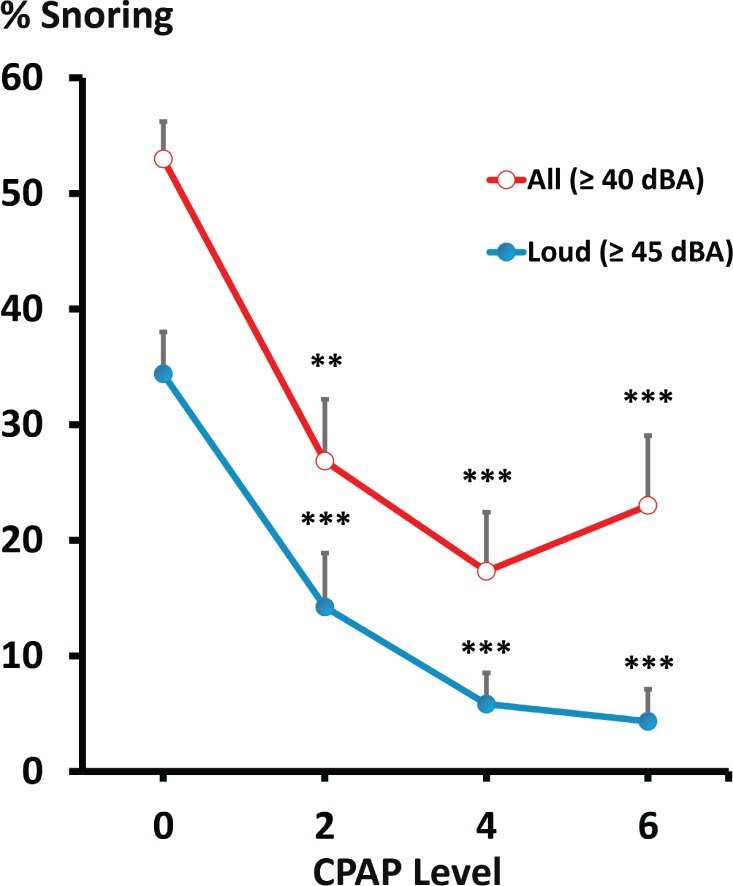
** = P < .005 treatment versus initial baseline. *** = P < .001 treatment versus initial baseline. CPAP = continuous positive airway pressure.
CPAP Treatment Trial (Night 3 Versus Nights 1 and 4)
Subjects were treated with low-level CPAP at 4.5 ± 1.5 cm H2O on Night 3, at a nasal pressure of 2 (n = 2), 3 (n = 1), 4 (n = 8) and 6 (n = 11) cm H2O. In Table 2, sleep study parameters are described for Night 3 and for the initial and follow-up control Nights 1 and 4. Comparing the baseline nights, we found no differences in sleep architecture, sleep-disordered breathing severity, or oxygenation. Compared to initial and follow-up nights off CPAP, CPAP decreased RDI, 4% desaturation indices, and respiratory effort-related arousals during NREM, REM, and all sleep time (P < .005) without changing oxygenation parameters. It also decreased the inspiratory duty cycle32 and shifted the proportion of sleep-disordered breathing events from obstructive to central episodes, consistent with relief of upper airway obstruction during the CPAP treatment night.33
Snoring Intensity and Frequency
Snoring frequency is illustrated at specific thresholds of snoring intensity in Figure 5. At snoring intensity thresholds of 40, 45, 50, and 55 dBA, snoring prevalence did not differ significantly between the initial and follow-up baseline sleep study nights. In contrast, the application of fixed low-level CPAP decreased snoring frequency markedly at each level of snoring intensity (P < .01).
Figure 5. Snoring frequency during the low-level CPAP treatment (Night 3) and untreated baseline nights before and after treatment (Nights 1 and 4) for the entire group at threshold levels of 40, 45, 50, and 55 dBA.
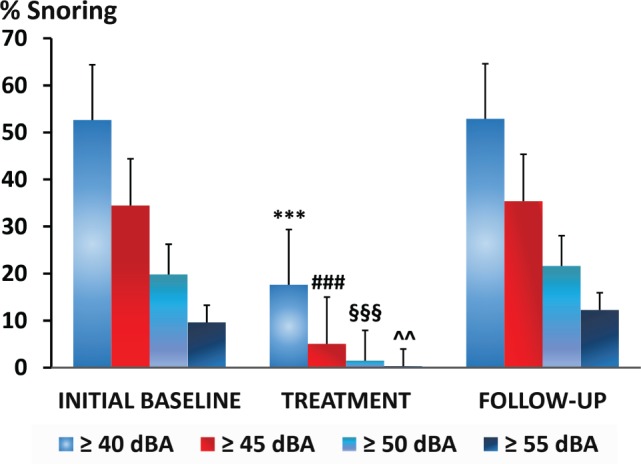
*** = P < .001 treatment versus initial baseline and follow-up. ### = P < .001 treatment versus initial baseline and follow-up. §§§ = P < .001 treatment versus initial baseline and follow-up. ^^ = P < .01 treatment versus initial baseline and follow-up. CPAP = continuous positive airway pressure.
DISCUSSION
This study demonstrates that low-level CPAP during sleep is highly efficacious in mitigating snoring in habitual snorers without significant sleep apnea. On CPAP titration nights, we found that CPAP decreased snoring frequency markedly at all levels of snoring intensity. Progressive decreases in snoring severity were observed when CPAP was increased stepwise from 0 to 4 cm H2O. Snoring frequency decreased from greater than 50% to less than 20% of all breaths during sleep (≥ 40 dBA) and loud snoring (≥ 45 dBA) was almost completely eliminated. We also demonstrated that the effects of CPAP plateaued with further increases in CPAP from 4 to 6 cm H2O, suggesting that snoring had indeed fallen to background levels above the 4 cm H2O level. During low-level CPAP application for an entire night, we confirmed marked reductions in snoring frequency at each level of snoring intensity. We also detected modest decreases in background sleep apneic activity without substantial reductions in sleep quality or quantity, suggesting that low-level CPAP was well tolerated without instituting any prior acclimatization measures. Our study indicates that low-level CPAP markedly reduces objective measures of snoring severity. The findings suggest that low-level CPAP can eliminate noise pollution from snoring in the bedroom, and potentially improve sleep quality of both bed partners and snorers.
The amount of pressure required to eliminate snoring is largely determined by the collapsibility of the pharynx (Pcrit).21,33 Prior studies have demonstrated that quantitative differences in pharyngeal collapsibility distinguish those with varying degrees of upper airway obstruction, as determined by measuring the nasal pressure level below which the upper airway occludes during sleep (also termed pharyngeal critical pressure). The critical pressure is higher in habitual snorers than normal non-snoring individuals, leading to pharyngeal collapse whenever negative inspiratory pressures fall below a critical pressure of approximately 5 cm H2O.33 Under these circumstances, airflow plateaus at a maximal level while the pressure gradient across the pharynx widens during inspiration. This gradient can be dissipated by repeated closure and reopening of the airway, which produces low-pitched audible oscillations in airflow or snoring sounds.34–38 The airway only partially collapses in the habitual snorer, and requires considerably less nasal pressure to restore full patency than that of an apneic patient, whose airway completely obstructs (when its critical pressure becomes positive) during sleep. Our findings confirm those of previous studies, which have demonstrated that low-level CPAP is sufficient to alleviate snoring and partial upper airway obstruction (inspiratory airflow limitation) during sleep. It extends these findings with objective measurements demonstrating graded reductions in snoring severity with the application of low-level CPAP from 0 to 4 cm H2O.
Both social and medical considerations govern treatment decisions in habitual snorers. It is well recognized that snoring can impair sleep quality, personal relationships, and quality of life in bed partners.13,39,40 These consequences can be attributed to concomitant sleep apnea, which was found in many of the snorers screened in our study (Figure 2). In fact, the subjects included in our final study sample generally had mild yet asymptomatic sleep apnea (see final study sample, Table 1). We found that low-level CPAP decreased apnea severity and associated movement arousals (see Table 2), which could further improve the quality of sleep and life in both snorers and bed partners.14,19,41 Even snorers without frank apnea demonstrate pronounced inspiratory flow limitation (Figure 3), a recognized physiologic correlate of snoring with an extraordinarily high prevalence in the general population.32,42 In these subjects, nocturnal respiratory loads from partial upper airway obstruction have been linked to functional somatic disorders including headaches, fibromyalgia, irritable bowel syndrome, temporomandibular joint pain, insomnia and generalized somatic distress,43–46 all of which may ultimately be amenable to treatment with CPAP.45 The current study provides evidence that treating habitual snorers with low-level CPAP produces robust reductions in snoring and the severity of upper airway obstruction during sleep, both of which could generate substantial improvements in quality of life and sleep in snorers and their bed partners.
Several limitations should be considered in interpreting our findings. First, our trial was conducted in a relatively small, select group of habitual snorers, leaving open the question about the overall generalizability of our findings. Nonetheless, our subjects were well characterized with objective measures of snoring and sleep apnea severity, and therapeutic responses were demonstrated across three study sites. In fact, we selected snorers with ≥ 30% of breaths at ≥ 40 dBA, who represented the most severe snorers in our patient sample. Second, we acknowledge that our findings provide evidence for efficacy of low-level CPAP in the laboratory setting rather than effectiveness in the home. Nevertheless, our study design controlled for any time-dependent variability in snoring severity by tightly bracketing assessments with baseline snoring measurements before and after treatment nights. Rigorous laboratory methods were utilized to measure snoring severity, which did not differ between control nights, and therapeutic efficacy was demonstrated on both titration and full-night treatment trials. Third, we recognize that some of the habitual snorers included in our trial had mild sleep apnea. We nonetheless restricted our sampling frame by excluding those with daytime hypersomnolence and possible sleep apnea syndrome in order to determine the efficacy of low-level CPAP for purposes of noise abatement rather than sleep apnea therapy.
Our findings suggest that different pressure ranges are required to treat habitual snoring and sleep apnea. Habitual snoring can be largely abolished by low-level CPAP,47 whereas sleep apnea generally requires pressures well above 4 cm H2O.48–51 The findings also imply that although low-level CPAP can largely eliminate snoring in otherwise asymptomatic patients, it is not likely to mask the presence of significant OSA, for which higher pressure is usually required to fully treat this disorder. Conversely, the persistence of snoring at low pressure levels would suggest the likelihood of concomitant sleep apnea, which would warrant further evaluation and treatment. Targeting each population may require suitable technology to optimize patient comfort during low-level CPAP application. Tolerability likely will be enhanced if a constant pressure is maintained at the nasal interface by varying flow dynamically throughout the respiratory cycle,52 and if CO2 accumulation in the breathing circuit is prevented by ensuring sufficient biased flow when delivering CPAP in the low-pressure range. Nonetheless, further studies are required to demonstrate tolerability, acceptance, and effectiveness of low-level CPAP in habitual snorers and their bed partners.
DISCLOSURE STATEMENT
Funding and material support for conduct of the research study was provided by inSleep Technologies, LLC. Mr. Sgambati, Ms. Guzman, and Dr. Kirkness served as consultants in support of the CRO (Maxis LLC) for site training, site qualification and data coordination. As such, they report personal fees from inSleep Technologies, LLC, during the conduct of the study. Dr. Dakheel reports personal fees from inSleep Technologies, LLC during the conduct of the study and after completion of the trial. Drs. Schwartz, Smith, and Schneider report they have received consulting fees from the study sponsor, and Ms. Guzman became an employee of the sponsor after completion of the trial. All consulting activity with Johns Hopkins personnel has been reviewed and approved by the Johns Hopkins University in accordance with its conflicts of interest policies.
ACKNOWLEDGMENTS
This study was sponsored by inSleep LLC with technical support from the Johns Hopkins Center for Interdisciplinary Sleep Research and Education (CISRE) and database support from Research Electronic Data Capture (REDCap, http://projectredcap.org).
ABBREVIATIONS
- a.u.
arbitrary units
- BMI
body mass index
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- ETCO2
end-tidal CO2
- NREM
non-rapid eye movement
- OSA
obstructive sleep apnea
- RDI
respiratory disturbance index
- REM
rapid eye movement
- RERA
respiratory effort related arousal
- SaO2
oxyhemoglobin saturation
- TST
total sleep time
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Hoffstein V. Snoring. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th ed. St. Louis, MO: Elsevier Saunders; 2011. pp. 1172–1182. [Google Scholar]
- 3.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44(6):931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 5.Chin CH, Kirkness JP, Patil SP, et al. Compensatory responses to upper airway obstruction in obese apneic men and women. J Appl Physiol. 2012;112(3):403–410. doi: 10.1152/japplphysiol.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Pack A, Maislin G, Lee SK, Kim SH, Shin C. Prospective observation on the association of snoring with subclinical changes in carotid atherosclerosis over four years. Sleep Med. 2014;15(7):769–775. doi: 10.1016/j.sleep.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endeshaw Y, Rice TB, Schwartz AV, et al. Snoring, daytime sleepiness, and incident cardiovascular disease in the health, aging, and body composition study. Sleep. 2013;36(11):1737–1745. doi: 10.5665/sleep.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Snoring is not associated with all-cause mortality, incident cardiovascular disease, or stroke in the Busselton Health Study. Sleep. 2012;35(9):1235–1240. doi: 10.5665/sleep.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang T, Lu J, Wang W, et al. Sleep duration and snoring associate with hypertension and glycaemic control in patients with diabetes. Diabet Med. 2015;32(8):1001–1007. doi: 10.1111/dme.12809. [DOI] [PubMed] [Google Scholar]
- 10.Lindberg E, Janson C, Gislason T, Svardsudd K, Hetta J, Boman G. Snoring and hypertension: a 10 year follow-up. Eur Respir J. 1998;11(4):884–889. doi: 10.1183/09031936.98.11040884. [DOI] [PubMed] [Google Scholar]
- 11.Cho JG, Witting PK, Verma M, et al. Tissue vibration induces carotid artery endothelial dysfunction: a mechanism linking snoring and carotid atherosclerosis? Sleep. 2011;34(6):751–757. doi: 10.5665/SLEEP.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeb R, Judge P, Peterson E, Lin JC, Yaremchuk K. Snoring and carotid artery intima-media thickness. Laryngoscope. 2014;124(6):1486–1491. doi: 10.1002/lary.24527. [DOI] [PubMed] [Google Scholar]
- 13.Beninati W, Harris CD, Herold DL, Shepard JW. The effect of snoring and obstructive sleep apnea on the sleep quality of bed partners. Mayo Clin Proc. 1999;74(4):955–958. doi: 10.4065/74.10.955. [DOI] [PubMed] [Google Scholar]
- 14.McArdle N, Kingshott R, Engleman HM, Mackay TW, Douglas NJ. Partners of patients with sleep apnoea/hypopnoea syndrome: effect of CPAP treatment on sleep quality and quality of life. Thorax. 2001;56(7):513–518. doi: 10.1136/thorax.56.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Night Noise Guidelines for Europe. World Health Organization Europe website. [Accessed February 20, 2017]. http://www.euro.who.int/en/health-topics/environment-and-health/noise/publications/2009/night-noise-guidelines-for-europe. Published 2009.
- 16.Monasterio C, Vidal S, Duran J, et al. Effectiveness of continuous positive airway pressure in mild sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;164(6):939–943. doi: 10.1164/ajrccm.164.6.2008010. [DOI] [PubMed] [Google Scholar]
- 17.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20(4):278–283. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 18.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 19.Miljeteig H, Mateika S, Haight JS, Cole P, Hoffstein V. Subjective and objective assessment of uvulopalatopharyngoplasty for treatment of snoring and obstructive sleep apnea. Am J Respir Crit Care Med. 1994;150(5 Pt 1):1286–1290. doi: 10.1164/ajrccm.150.5.7952554. [DOI] [PubMed] [Google Scholar]
- 20.Henke KG, Dempsey JA, Kowitz JM, Skatrud JB. Effects of sleep-induced increases in upper airway resistance on ventilation. J Appl Physiol. 1990;69(2):617–624. doi: 10.1152/jappl.1990.69.2.617. [DOI] [PubMed] [Google Scholar]
- 21.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143(6):1300–1303. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 22.Berry RB, Brooks R, Gamaldo CE, et al. for the American Academy of Sleep Medicine. The AASM Manual for Scoring Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2013. Version 2.0.2. [Google Scholar]
- 23.Kirkness JP, Verma M, McGinley BM, et al. Pitot-tube flowmeter for quantification of airflow during sleep. Physiol Meas. 2011;32(2):223–237. doi: 10.1088/0967-3334/32/2/006. [DOI] [PubMed] [Google Scholar]
- 24.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 25.Bauer B, Torik E. Researches in loudness measurement. IEEE Transactions on Audio and Electroacoustics. 1966;14(3):141–151. [Google Scholar]
- 26.Keshner MS. 1/f noise. Proceedings of the IEEE. 1982;70(3):212–218. [Google Scholar]
- 27.Rohrmeier C, Herzog M, Haubner F, Kuehnel TS. The annoyance of snoring and psychoacoustic parameters: a step towards an objective measurement. Eur Arch Otorhinolaryngol. 2012;269(5):1537–1543. doi: 10.1007/s00405-011-1878-2. [DOI] [PubMed] [Google Scholar]
- 28.Caffier PP, Berl JC, Muggli A, et al. Snoring noise pollution--the need for objective quantification of annoyance, regulatory guidelines and mandatory therapy for snoring. Physiol Meas. 2007;28(1):25–40. doi: 10.1088/0967-3334/28/1/003. [DOI] [PubMed] [Google Scholar]
- 29.Liu ZS, Luo XY, Lee HP, Lu C. Snoring source identification and snoring noise prediction. J Biomech. 2007;40(4):861–870. doi: 10.1016/j.jbiomech.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Hume KI, Brink M, Basner M. Effects of environmental noise on sleep. Noise Health. 2012;14(61):297–302. doi: 10.4103/1463-1741.104897. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz AR, Smith PL, Wise RA, Bankman I, Permutt S. Effect of positive nasal pressure on upper airway pressure-flow relationships. J Appl Physiol. 1989;66(4):1626–1634. doi: 10.1152/jappl.1989.66.4.1626. [DOI] [PubMed] [Google Scholar]
- 32.Schneider H, Krishnan V, Pichard LE, Patil SP, Smith PL, Schwartz AR. Inspiratory duty cycle responses to flow limitation predict nocturnal hypoventilation. Eur Respir J. 2009;33(5):1068–1076. doi: 10.1183/09031936.00063008. [DOI] [PubMed] [Google Scholar]
- 33.Gold AR, Schwartz AR. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest. 1996;110(4):1077–1088. doi: 10.1378/chest.110.4.1077. [DOI] [PubMed] [Google Scholar]
- 34.Beck R, Odeh M, Oliven A, Gavriely N. The acoustic properties of snores. Eur Respir J. 1995;8(12):2120–2128. doi: 10.1183/09031936.95.08122120. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Padilla JR, Slawinski E, Difrancesco LM, Feige RR, Remmers JE, Whitelaw WA. Characteristics of the snoring noise in patients with and without occlusive sleep apnea. Am Rev Respir Dis. 1993;147(3):635–644. doi: 10.1164/ajrccm/147.3.635. [DOI] [PubMed] [Google Scholar]
- 36.Gavriely N, Jensen O. Theory and measurements of snores. J Appl Physiol. 1993;74(6):2828–2837. doi: 10.1152/jappl.1993.74.6.2828. [DOI] [PubMed] [Google Scholar]
- 37.Grotberg JB, Davis SH. Fluid-dynamic flapping of a collapsible channel: sound generation and flow limitation. J Biomech. 1980;13(3):219–230. doi: 10.1016/0021-9290(80)90365-6. [DOI] [PubMed] [Google Scholar]
- 38.Pevernagie D, Aarts RM, De MM. The acoustics of snoring. Sleep Med Rev. 2010;14(2):131–144. doi: 10.1016/j.smrv.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Virkkula P, Bachour A, Hytonen M, Malmberg H, Salmi T, Maasilta P. Patient-and bed partner-reported symptoms, smoking, and nasal resistance in sleep-disordered breathing. Chest. 2005;128(4):2176–2182. doi: 10.1378/chest.128.4.2176. [DOI] [PubMed] [Google Scholar]
- 40.Ulfberg J, Carter N, Talback M, Edling C. Adverse health effects among women living with heavy snorers. Health Care Women Int. 2000;21(2):81–90. doi: 10.1080/073993300245311. [DOI] [PubMed] [Google Scholar]
- 41.Kiely JL, McNicholas WT. Bed partners' assessment of nasal continuous positive airway pressure therapy in obstructive sleep apnea. Chest. 1997;111(5):1261–1265. doi: 10.1378/chest.111.5.1261. [DOI] [PubMed] [Google Scholar]
- 42.Palombini LO, Tufik S, Rapoport DM, et al. Inspiratory flow limitation in a normal population of adults in Sao Paulo, Brazil. Sleep. 2013;36(11):1663–1668. doi: 10.5665/sleep.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gold AR, Dipalo F, Gold MS, Broderick J. Inspiratory airflow dynamics during sleep in women with fibromyalgia. Sleep. 2004;27(3):459–466. doi: 10.1093/sleep/27.3.459. [DOI] [PubMed] [Google Scholar]
- 44.Gold AR, Broderick JE, Amin MM, Gold MS. Inspiratory airflow dynamics during sleep in irritable bowel syndrome: a pilot study. Sleep Breath. 2009;13(4):397–407. doi: 10.1007/s11325-009-0262-6. [DOI] [PubMed] [Google Scholar]
- 45.Gold AR. Functional somatic syndromes, anxiety disorders and the upper airway: a matter of paradigms. Sleep Med Rev. 2011;15(6):389–401. doi: 10.1016/j.smrv.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Broderick JE, Gold MS, Amin MM, Gold AR. The association of somatic arousal with the symptoms of upper airway resistance syndrome. Sleep Med. 2014;15(4):436–443. doi: 10.1016/j.sleep.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 47.Mitler MM, Dawson A, Henriksen SJ, Sobers M, Bloom FE. Bedtime ethanol increases resistance of upper airways and produces sleep apneas in asymptomatic snorers. Alcohol Clin Exp Res. 1988;12(6):801–805. doi: 10.1111/j.1530-0277.1988.tb01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164(4):608–613. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- 49.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353(9170):2100–2105. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 50.Barbe F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med. 2001;134(11):1015–1023. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 51.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171. [PMC free article] [PubMed] [Google Scholar]
- 52.Farré R, Peslin R, Montserrat JM, Rotger M, Navajas D. Flow-dependent positive airway pressure to maintain airway patency in sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1855–1863. doi: 10.1164/ajrccm.157.6.9710056. [DOI] [PubMed] [Google Scholar]


