Abstract
Suvorexant is a new insomnia drug, and it is generally safe and well tolerated. Here, we report a rare but potentially important adverse effect of suvorexant in a patient with Parkinson disease.
Citation:
Tabata H, Kuriyama A, Yamao F, Kitaguchi H, Shindo K. Suvorexant-induced dream enactment behavior in Parkinson disease: a case report. J Clin Sleep Med. 2017;13(5):759–760.
Keywords: nightmare, Parkinson disease, polysomnography, REM sleep behavioral disorder, suvorexant
INTRODUCTION
Suvorexant (Belsomra, Merck & Co. Inc., Whitehouse Station, New Jersey) is the first dual orexin receptor antagonist for the treatment of primary insomnia. Suvorexant improves both sleep latency and sleep maintenance in patients with insomnia. It is generally a well-tolerated and safe drug at approved doses. Therefore, it represents a novel alternative for the treatment of insomnia, especially in patients in whom traditional drugs, such as benzodiazepines, are contraindicated. However, some rare but significant adverse effects to suvorexant have been reported. The United States Food and Drug Administration (FDA) reported that suvorexant was associated with symptoms that resembled rapid eye movement (REM) sleep behavior disorder (RBD) in one patient.1 Here, we report a case of suvorexant-induced nightmares and dream enactment behavior during sleep in a patient with Parkinson disease (PD) who also had REM sleep without atonia.
REPORT OF CASE
This case reports on a 72-year-old man who had been previously diagnosed with PD (diagnosis occurred 8 years previous to this report). He had also been previously diagnosed with sleep apnea and insomnia. He was taking daily levodopa 300 mg, pergolide mesilate 750 mcg, and selegi-line hydrochloride 5 mg for PD. He was taking no selective serotonin re-uptake inhibitors. Mild bradykinesia and limb rigidity on his left side remained despite his treatment for PD. Oropharyngeal examination revealed class III modified Mallampati classification. He had been receiving continuous positive airway pressure (CPAP) therapy for three years for the management of sleep apnea. His apnea-hypopnea index (AHI) was 22.8 events/h (REM AHI = 12.7 events/h and non-REM AHI = 25.0 events/h). He had been compliant with CPAP therapy. He had never experienced abnormal behavior during sleep.
For the management of insomnia, he was taking daily rilmazafone 2 mg and etizolam 0.5 mg. He sometimes required zolpidem 10 mg to help him fall asleep, and the frequency with which he was taking zolpidem had gradually increased. Hence, we changed his therapy from rilmazafone and zolpidem to suvorexant, a newly launched drug at that time, after discussion with the patient. The dose of suvorexant prescribed was 15 mg, which was the approved dose for elderly patients.
After he began taking suvorexant, the patient started having nightmares. The patient's family also reported that he sometimes yelled out and talked during sleep. In addition to this vocalization, an abnormal behavior during sleep appeared. The patient started kicking his bedding, and grabbed and pulled his wife's hair, which led to her feeling threatened and caused her to sleep in another room. Although he repeatedly experienced nightmares and vocalization, the violent behavior against his wife during sleep occurred once. He had been on suvorexant for seven weeks when he reported this event. After he stopped taking suvorexant, such vocalization and abnormal behavior promptly disappeared.
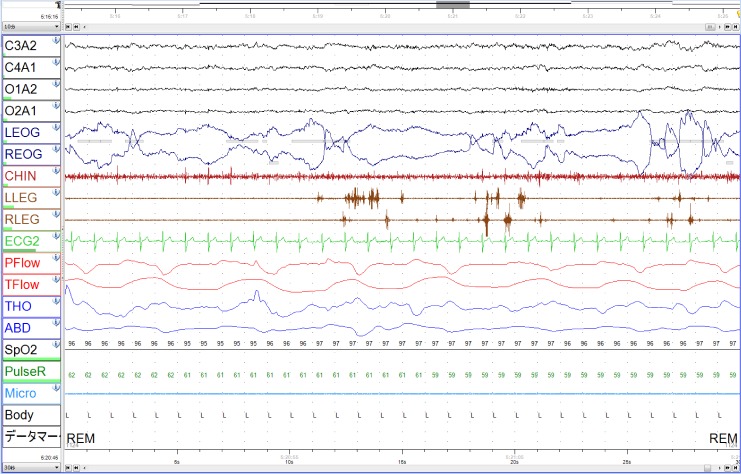
After these episodes were reported, we reviewed data from the patient's polysomnography (PSG) that was performed 3 years prior and led to the patient's diagnosis of sleep apnea. A 30-second epoch of the patient's PSG data during REM sleep is shown in Figure 1. The electromyogram showed activity of the leg muscles in more than 50% of successive 3-second miniepochs (right leg trace on electromyography and left leg trace on electromyography, Figure 1). In another 30-second epoch, excessive phasic muscle activities of the chin were recorded during REM sleep (data not shown). The REM sleep without atonia was observed in 13.8% of REM sleep (%REM = 17.5% of total sleep time). However, a video monitor recording did not show any abnormal behavior throughout the patient's sleep.
Figure 1. A 30-second epoch of PSG during REM sleep.
Muscle activity in both legs is seen in the LLEG and RLEG traces. LEOG and REOG traces showed evidence of rapid eye movement. LEOG = left electro-oculogram, LLEG = left leg trace on electromyography, PSG = polysomnography, REOG = right electro-oculogram, RLEG = right leg trace on electromyography.
DISCUSSION
A feature of suvorexant is its smaller effect on sleep neuro-physiology compared with other drugs used to treat insomnia. Previous PSG studies showed that 10–20 mg doses of suvorexant caused minimal changes in REM sleep duration.2 In addition, animal studies showed that suvorexant increases REM sleep.3 The long-term effects of suvorexant are unclear, although a recent review raised the concern that the nonsuppressive or promoting effect on REM sleep might cause RBD in some patients receiving suvorexant.4 A recent meta-analysis showed that suvorexant increased the incidence of abnormal dreams. Compared to a placebo, the relative risk for suvorexant was 2.08.5 Thus, suvorexant might exacerbate the adverse effects related to REM sleep.
Gagnon et al. reported that 58% of patients with PD had REM sleep without atonia, and 42% did not show behavioral manifestations of RBD,6 which suggests that subclinical RBD is not a rare complication in PD patients. Here, we reported on a patient with PD in whom use of 15 mg suvorexant induced both nightmares and abnormal behavior during sleep, although he did not exhibit such dream enactment behavior when not taking suvorexant. Importantly, previous PSG data showed a frequent increase in activity of the chin and leg muscles during sleep, suggesting the presence of subclinical RBD. To our knowledge, this is the first report of suvorexant-induced dream enactment behavior in a patient with PD. RBD may be an important factor to consider when selecting hypnotic drugs for patients with PD. Further study is necessary to discuss the tolerability and safety of suvorexant for the treatment of insomnia in patients with PD.
DISCLOSURE STATEMENT
Institution at which the work was performed: Kurashiki Central Hospital. This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Farkas R for the United States Food and Drug Administration. Suvorexant safety and efficacy. [Slides for the May 22, 2013 Meeting of the peripheral and central nervous system drugs advisory committee] [Accessed January 31, 2017]. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PeripheralandCentralNervousSystemDrugsAdvisoryCommittee/UCM354215.pdf.
- 2.Sun H, Kennedy WP, Wilbraham D, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013;36(2):259–267. doi: 10.5665/sleep.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox CD, Breslin MJ, Whitman DB, et al. Discovery of the dual orexin receptor antagonist methanone (MK-4305) for the treatment of insomnia. J Med Chem. 2010;53(14):5320–5332. doi: 10.1021/jm100541c. [DOI] [PubMed] [Google Scholar]
- 4.Sutton EL. Profile of suvorexant in the management of insomnia. Drug Des Devel Ther. 2015;9:6035–6042. doi: 10.2147/DDDT.S73224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuriyama A, Tabata H. Suvorexant for the treatment of primary insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2016 Oct 28;35:1–7. doi: 10.1016/j.smrv.2016.09.004. doi: 10.1016/j.smrv.2016.09.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Gagnon JF, Bedard MA, Gantini ML, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson's disease. Neurology. 2002;59(4):585–589. doi: 10.1212/wnl.59.4.585. [DOI] [PubMed] [Google Scholar]