Abstract
Peroxisomes are indispensable organelles for lipid metabolism in humans, and their biogenesis has been assumed to be under regulation by peroxisome proliferator-activated receptors (PPARs). However, recent studies in hepatocytes suggest that the mitochondrial proliferator PGC-1α (peroxisome proliferator-activated receptor gamma coactivator-1α) also acts as an upstream transcriptional regulator for enhancing peroxisomal abundance and associated activity. It is unknown whether the regulatory mechanism(s) for enhancing peroxisomal function is through the same node as mitochondrial biogenesis in human skeletal muscle (HSkM) and whether fatty acid oxidation (FAO) is affected. Primary myotubes from vastus lateralis biopsies from lean donors (BMI = 24.0 ± 0.6 kg/m2; n = 6) were exposed to adenovirus encoding human PGC-1α or GFP control. Peroxisomal biogenesis proteins (peroxins) and genes (PEXs) responsible for proliferation and functions were assessed by Western blotting and real-time qRT-PCR, respectively. [1-14C]palmitic acid and [1-14C]lignoceric acid (exclusive peroxisomal-specific substrate) were used to assess mitochondrial oxidation of peroxisomal-derived metabolites. After overexpression of PGC-1α, 1) peroxisomal membrane protein 70 kDa (PMP70), PEX19, and mitochondrial citrate synthetase protein content were significantly elevated (P < 0.05), 2) PGC-1α, PMP70, key PEXs, and peroxisomal β-oxidation mRNA expression levels were significantly upregulated (P < 0.05), and 3) a concomitant increase in lignoceric acid oxidation by both peroxisomal and mitochondrial activity was observed (P < 0.05). These novel findings demonstrate that, in addition to the proliferative effect on mitochondria, PGC-1α can induce peroxisomal activity and accompanying elevations in long-chain and very-long-chain fatty acid oxidation by a peroxisomal-mitochondrial functional cooperation, as observed in HSkM cells.
Keywords: β-oxidation, obesity, peroxisome proliferator-activated receptors, lignoceric acid oxidation, human skeletal muscle cells
because of its relative mass (~40% of total body weight in men), skeletal muscle is the major organ responsible for lipid oxidation (14, 27). Consequently, impairments in skeletal muscle lipid oxidation, due in part to reported reductions in mitochondrial capacity or function, have been implicated in the development of cellular lipotoxicity and oxidative stress and are associated with insulin resistance observed in the obese and diabetic conditions (10, 21, 27, 38, 57).
The peroxisome is a dynamic cellular organelle discovered in 1954 by J. Rhodin (named as microbodies) (31, 43) and later characterized by de Duve and Baudhuin (18). Since this initial identification and characterization, they have been found in most human tissues, especially in heart, liver, and kidney. Peroxisomes are indispensable for chemical reactions involving lipid catabolism in mammals (34, 55); for example: 1) α-oxidation of branched-chain fatty acids and 2) β-oxidation (partial chain shortening to 6 carbons) of long-chain fatty acids (LCFA). Peroxisomes are also the exclusive organelle for oxidation of very-long-chain fatty acids (VLCFA; >22 carbons). Importantly, the latter cannot be metabolized directly by mitochondria. Instead, VLCFA (for example, C24:0 lignoceric acid) first must be degraded by peroxisomes to medium- or short-chain fatty acids and exported as acyl-carnitine metabolites for subsequent complete oxidation within mitochondria. Despite the proportion of VLCFAs occupying a relatively small portion of the dietary fatty acid pool in the Western diet (for example, lignoceric acid: ~0.02% of total fat) (6), they are present in commonly consumed foods including vegetable oils, fatty fish and meats, plant cover and cuticle, fruit peel and seeds, and grains and nuts (41). VLCFAs are present in varying lipid species pools, including sphingomyelin, gangliosides, glycerophosphatides, and cholesterol esters. Pathologically, certain peroxisomal disorders such as X-linked adrenoleukodystrophy are associated with an accumulation of VLCFAs, especially hexacosanoic acid (C26:0) and tetracosanoic acid/lignoceric acid (C24:0), which could be derived from both dietary and endogenous origin (7). The most severely affected tissues are myelin in the central nervous system, the adrenal cortex, and the leydig cells in the testes. Furthermore, peroxisomal β-oxidation of diet-rich LCFAs, such as palmitate and oleate, could be a potential contributor for attenuating lipotoxicity in multiple tissues, including skeletal muscle.
Peroxisomal biogenesis and associated functions require a group of concerted movement of critical proteins called peroxins, which are expressed by specific PEX genes (16). The expression of PEX genes and associated peroxins facilitate bioactions of peroxisomal membrane elongation followed by subsequent fission events by shared fission machinery with mitochondria; for example, DLP1/Drp1, Mff, and Fis1, which are also utilized for mitochondria biogenesis in mammals (46). Overall, peroxisomes function in multiple roles such as providing potential support to mitochondrial oxidation under conditions of intramyocellular lipid accumulation.
Peroxisomes are intimately associated with mitochondria in many other metabolic aspects of the cellular environment (48). In addition to sharing the fission machinery (15), peroxisomes have functional similarities with mitochondria in terms of reactive oxygen species degradation (40). Despite the identification of these functions attributed to the peroxisome, our understanding concerning how the organelle metabolically cooperates with mitochondria in terms of lipid metabolism is less well known in mammals. Furthermore, what is understood is based predominantly from animal studies and in liver. The study of peroxisomes in skeletal muscle is rarely reported, most especially in humans (39, 57). Recent findings from our group, however, demonstrate that in skeletal muscle from rodents, an enhancement of peroxisomal function occurs under a condition of excess intramyocellular lipid (39).
Peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α) is a powerful transcriptional coactivator that modulates a wide spectrum of physiological, energy homeostatic responses at the transcriptional level in different mammalian tissues. For example, PGC-1α cooperates with numerous transcription factors, such as estrogen-related receptor-α and nuclear respiratory factor-1 or -2 (NRF-1 and -2), to promote enhanced expression of downstream genes encoding mitochondrial biogenesis and oxidative respiratory functions (23, 36, 58). PGC-1α also plays a crucial role in regulating the cellular redox environment by upregulating antioxidant genes and derived function (51) and interacts with peroxisome proliferator-activated receptors (PPARs) to increase fatty acid oxidation (FAO) (37). Aforementioned transcriptional regulation pathways are inducible by several metabolic cues, such as low environmental temperatures, fasting, oxidative stress, and physical exercise (4).
The notion that PGC-1α overexpression promotes peroxisomal biogenesis/function and fatty acid oxidation (FAO) gene expression has been demonstrated previously in mouse brown adipose cells and hepatic tissues (5) but has never been considered in human skeletal muscle (HSkM). Historically, this has been due in part to a limitation of profiling techniques defining peroxisomal structure in HSkM using traditional cytochemical staining techniques developed thus far (54), However, Wanders et al. (56) have indicated that skeletal muscle cells derived from human tissue are an acceptable model in vitro for the study of peroxisomal function or their activities.
The conventional view of peroxisomal biogenesis in mammals has assumed that it is under the transcriptional regulation by PPARs, which are responsible for hepatocyte peroxisomal gene expression in mammals. PPARα induces several lipid metabolism activities in mice (20). However, recent studies in hepatic cells from PPARα-knockout (KO) mice have demonstrated that PGC-1α can induce PEX gene expression and peroxin abundance in a PPARα-independent fashion (5). It is unknown whether the regulatory mechanism for peroxisomal biogenesis and its associated activity is through the same node as mitochondrial biogenesis in HSkM and whether FAO is affected. That is, are there functional interactions between mitochondria and peroxisomes in terms of FAO in HSkM similar to what occurs in liver, as described above (47)? In this regard, PGC-1α might be considered an ideal candidate for coordinating both mitochondrial and peroxisomal biogenesis (or its activity) and for enhancing peroxisomal contributions toward mitochondrial function in terms of FAO.
The purpose of these studies was to test the hypothesis that PGC-1α acts as a biological “driver” for both the enhancement of peroxisomal activity and mitochondrial biogenesis in HSkM, leading to interorganelle metabolic interactions that enhance FAO by mitochondria. Establishing a role for PGC-1α in peroxisomal function would represent a new frontier in HSkM fatty acid metabolism that could be exploited to develop as yet unknown targets for the treatment of skeletal muscle insulin resistance, obesity, Type 2 diabetes, and related metabolic diseases. Herein, we report the novel finding of a role for PGC-1α in the simultaneous induction of both peroxisomal activity and mitochondrial biogenesis and the concomitant functional interactions between the organelles with respect to FAO.
MATERIALS AND METHODS
Human subjects and muscle biopsies.
Six female subjects were recruited from the local area of Greenville, NC. Inclusion criteria for the studies were 1) nondiabetic (HOMA-IR <3.6) premenopausal; 2) sedentary; 3) lean (BMI ≤26 kg/m2); and 4) young female subjects (<30 yr of age) who reported no recent history of substantial weight loss or gain (body weight within ±2 kg for the previous 12 mo), enrollment in nutritional intervention protocols, hypertension, and metabolic and/or musculoskeletal diseases. HSkM biopsies were performed using a modification of the percutaneous needle biopsy technique as reported (24). All procedures were approved by the East Carolina University Institutional Review Board.
Materials.
[1-14C]palmitate was purchased from PerkinElmer (Boston, MA), and [1-14C]lignoceric acid was obtained from American Radiolabeled Chemicals (St. Louis, MO). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified as follows: Dulbecco’s phosphate-buffered saline (DPBS), fetal bovine serum (FBS), heat-inactivated horse serum (HS), penicillin-streptomycin, 0.05% trypsin EDTA, Hanks’ balanced salt solution, and Dulbecco’s modified Eagle’s medium (DMEM; 5 mM glucose) were purchased from Life Technologies (Grand Island, NY). Collagen-coated tissue culture plates and dishes were obtained from Fisher Scientific (Pittsburgh, PA).
Human skeletal muscle cell culture and adenovirus infection strategies.
Satellite cells were isolated from 50 to 80 mg of fresh muscle tissues from human vastus lateralis and cultured into myoblasts, as described previously (8). Briefly, tissues from biopsies were transferred to ice-cold DMEM, and all fat and connective tissues were removed by sterilized dissecting blades and enzymatic digestion. For the current studies, myoblasts, all at passage four, were subcultured into collagen-coated, 10-cm cell culture dishes or six-well plates in growth media containing DMEM supplemented with 10% FBS, SkGM SingleQuot Kit (5 ml of BSA, 5 ml of fetuin, 0.5 ml of human epidermal growth factor, and 0.5 ml of dexamethasone; Lonza, Walkersville, MD), 100 U/ml penicillin, and 100 μg/ml streptomycin. Upon obtaining 80–90% confluency during cell growth, cells were switched to differentiation media made from DMEM containing 2% of HS, 0.3% of bovine serum albumin, 0.05% of fetuin, 100 U/ml of penicillin, and 100 μg/ml of streptomycin. On day 4 post-differentiation, premature myotubes were exposed to differentiation medium containing adenovirus encoding for human PGC-1α coexpressing GFP (Ad-PGC-1α) or adenovirus containing GFP alone (Ad-GFP). We measured cesium chloride gradient-purified Ad-PGC-1α titer as 7.5 × 106 plaque-forming units (pfu: an indicator of active virus)/μl using an end point dilution assay and presented adenovirus dose as pfu/ml media throughout this study. On day 7 post-differentiation, human primary myotubes were harvested for subsequent experiments. Adenoviruses expressing PGC-1α or GFP constructs were described previously (45, 52).
Cell homogenization for total protein.
HSkM cells (HSkMC) were used to probe for key peroxisomal proteins (peroxins; PEX19 and PMP70 mandatory for peroxisomal biogenesis) via standard Western blotting procedures. Briefly, whole cell lysates were prepared using homogenization buffer containing 50 mM HEPES, 10 mM EDTA, 100 mM NaF, 50 mM sodium pyrophosphate, pH = 7.4, 5 μl/ml protease inhibitor cocktail (cat. no. P8340), and 10 μl/ml phosphatase inhibitors (cat. no. P5726 and P0044) from Sigma-Aldrich (St. Louis, MO). Cells were harvested by adding 1% Triton X-100 and transferred to 1.7-ml microcentrifuge tubes. Lysates were prepared by sonication twice for 5 s each and placed on an orbital shaker at 4°C for 2 h. Supernatants were collected after centrifugation at 12,000 g for 15 min. Total protein content was determined by the Pierce BCA (bicinchoninic acid) protein assay (Thermo Scientific, Rockford, IL).
Western blotting assessment.
Western blotting assessments were performed using standard PAGE-SDS procedures. Briefly, 20 μg of protein from cell lysates suspended in sample buffer containing Lammeli buffer (Bio-Rad 161-0710) and β-mercaptoethanol (9:1 ratio) were boiled at 95°C for 5 min, and then protein was equally loaded (verified by BCA protein determination) into wells of precast polyacrylamide 10% Tris·HCl gels (Bio-Rad). Electrophoresis occurred at 100 V for 30 min and then at 150 V for 1 h. Afterward, proteins were transferred at 95 V for 1.5 h onto a PVDF membrane (Millipore, Billerica, MA) prewetted with methanol, and rinsed briefly with transfer buffer. After membranes were dried for 1.5 h, they were incubated in blocking buffer (Li-Cor 927-40000) for 1 h and then incubated at 4°C overnight in the blocking buffer with the antibodies anti-PGC-1α (ab106814), anti-citrate synthetase (ab96600), anti-mtTFA (ab47517), anti-PEX19 (ab139684), and anti-PMP70 (ab85550) purchased from Abcam (Cambridge, MA). After washing with TBST (0.1% Tween 20) for three times, membranes were incubated with the appropriate secondary antibodies accordingly at room temperature for 1 h: goat anti-mouse 680LT (926-68020), goat anti-mouse 800CW (926-32210), donkey anti-rabbit 680LT (926-68023), goat anti-rabbit 800CW (no. 926-32211), and donkey anti-goat 800CW (926-32214) purchased from Li-Cor (Lincoln, NE). After additional washes in TBST, detection, quantification, and imaging were performed using an Odyssey infrared imaging system (Li-Cor).
RNA extraction and mRNA expression quantification.
PGC-1α, peroxisomal β-oxidation genes (ACOX1, EHHADH/PBFE, and ACAA1/PTHIO), peroxisomal biogenesis factors (PEXs), the peroxisomal biogenesis-related gene (DNM1L), lipid droplet metabolism-associated genes (DGAT1, ATGL, and PLIN5), and the ether phospholipid synthesis gene (GNPAT) were quantified from cell lysates. Total RNA was isolated from cultured human primary myotubes after Ad-PGC-1α or Ad-GFP infection for 72 h with the RNeasy plus mini kit (Qiagen, Germantown, MD) according to the manufacturer’s instructions. Determination of RNA concentrations was performed using NanoDrop 1000 spectrophotometric analysis (Thermo Scientific, Waltham, MA). RNA concentrations of samples were diluted to 100 ng/μl with nuclease-free H2O. One microgram of RNA from each sample was used to perform reverse transcription reactions with a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA) using a PTC-200 PCR Thermal Cycler (MJ Research, Waltham, MA). Real-time qRT-PCR was performed with the ViiA 7 Real-Time PCR System (Applied Biosystems, Carlsbad, CA), using the TaqMan gene expression assay [PGC-1α (Hs01016719_m1), ABCD1 (Hs00163610_m1), ABCD3/PMP70 (Hs01082796_m1), PEX19 (Hs00267867_m1), PEX3 (Hs00920532_m1), PEX11A (Hs00610156_m1), PEX11B (Hs00187237_m1), PEX13 (Hs00159996_m1), ACOX1 (Hs01074241_m1), EHHADH/PBFE (Hs00157347_m1), ACAA1/PTHIO (Hs01576070_m1), CrAT (Hs00912963_m1), CrOT (Hs00221733_m1), DNM1L/DLP1 (Hs01552605_m1), DGAT1 (Hs01017541_m1), ATGL (Hs00386101_m1), PLIN5 (Hs00965990_m1), and GNPAT/DHAPAT (Hs_00204517_m1) and 18S (Hs99999901_s1)], following standard protocols with thermal cycling conditions: 50°C for 2 min and 95°C for 10 min (hold stage) and 40 cycles of 95°C for 15 s followed by 60°C for 1 min. mRNA expression was determined using the comparative CT method (ΔΔCT) with an endogenous control (18S ribosomal RNA) and converted to a linear function by using a base 2 antilog transformation ().
Quantification of very-long-chain and long-chain FAO rates.
Human primary myotube homogenates were prepared according to established validated methods in our laboratory (27, 39). Briefly, on day 7 post-differentiation, primary myotubes were harvested by trypsinization and then counted using a Vi-CELL cell viability analyzer (Beckman Coulter, Atlanta, GA). Primary myotubes (9,000,000 cells) were quickly and thoroughly suspended in 600 μl of SET buffer containing 250 mM sucrose, 1 mM EDTA, 10 mM Tris·HCl, and 2 mM ATP, pH = 7.4, and then transferred to a 3-ml glass mortar and homogenized on ice with a Teflon pestle for 10 passes over 30 s at 1,200 rpm. Cell homogenates were kept on ice until oxidation experiments were performed. The experiments employed [1-14C]palmitate and [1-14C]lignoceric acid (unoxidizable by mitochondria unless peroxisomes are present) to evaluate the functionalities of both mitochondria and peroxisomes. Final concentrations of 200 μM palmitate or 25 μM lignoceric acid were diluted in 2× reaction buffer containing the following final concentrations: 100 mM sucrose, 10 mM Tris·HCl, 10 mM KPO4, 100 mM KCl, 1 mM MgCl2·6H2O, 1 mM l-carnitine, 0.1 mM malate, 2 mM ATP, 0.05 mM coenzyme A, and 1 mM dithiothreitol, pH = 7.4. Incubation times for palmitate and lignoceric acid oxidation were 2 and 4 h, respectively. Quantification of complete (as 14CO2) and incomplete [as acid-soluble metabolites (ASM) from both mitochondrial and peroxisomal β-oxidation] FAO from cell homogenates was based on previously published and validated methods (39) using liquid scintillation analysis (PerkinElmer, Waltham, MA) (39). Data are expressed as picomole (lignoceric acid) or nanomole (palmitic acid) of substrate oxidized per milligram protein per hour.
Fluorescent imaging.
Before the aforementioned assays were performed, the human primary myotubes were stained with 2-(4-amidinophenyl)-1H -indole-6-carboxamidine (DAPI) for visualization of nuclei and imaged at 20× under an EVOS FL autoimaging system (Fisher Scientific, Atlanta, GA).
Triacylglycerol assay.
To confirm differences in intramyocellular TAG content within cell lysates and between groups, we employed the method established and validated in our laboratory. We utilized a commercial triglyceride assay (cat. no. TR0100; Sigma) to measure glycerol as the indirect measure of total triglycerides (IMTG) in the skeletal muscle cell lysates according to the manufacturer’s instructions. Unknown samples were quantified for IMTG against glycerol standard curves generated. All data were expressed as microgram per milligram of protein.
Statistical analysis.
Data analyses were performed using SPSS Statistics for Windows version 20.0 (IBM, Armonk, NY). Comparisons of group means between Ad-PGC-1α and Ad-GFP were assessed using Student’s t-test. All data collected from the studies are presented as means ± SE, and the probability for acceptance of statistical differences was set a priori at an α-level of P ≤ 0.05.
RESULTS
Participant characteristics.
Table 1 depicts demographic profiles for the six sedentary lean subjects. Subjects were 27.7 ± 1.9 yr old. The average BMI for the subjects was 24.0 ± 0.6 kg/m2. HOMA-IR index was 1.6 ± 0.2, which was considered insulin sensitive (<3.6) for these individuals as defined by Stern et al. (50).
Table 1.
Subject profiles
| Characteristics | Value |
|---|---|
| Age, yr | 27.7 ± 1.9 |
| Height, cm | 166.6 ± 4.2 |
| Weight, kg | 66.4 ± 2.7 |
| BMI, kg/m2 | 24.0 ± 0.6 |
| HOMA-IR | 1.6 ± 0.5 |
Values are means ± SE (n = 6). BMI, body mass index; HOMA-IR, homeostasis model assessment for insulin resistance (50). Subject profiles from female subjects.
Dose and time-course responses of Ad-PGC-1α overexpression.
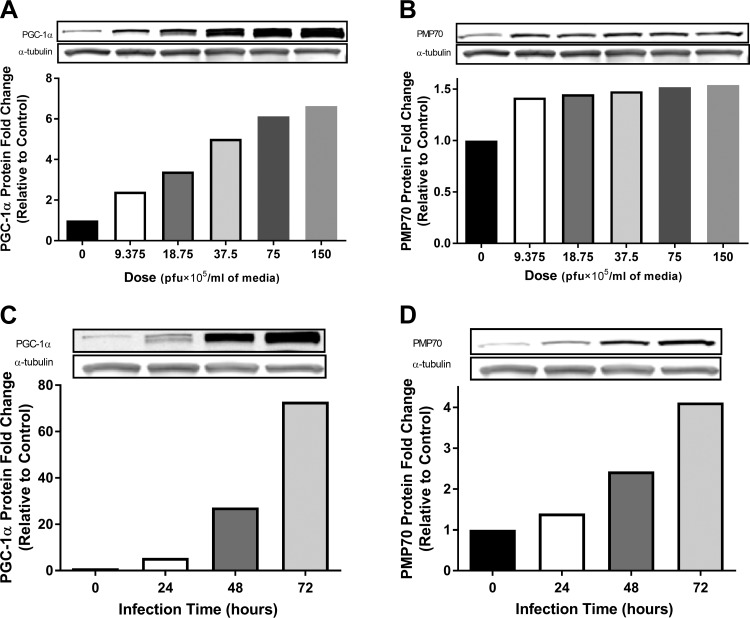
To establish infection strategies, pooled HSKMCs from lean sedentary donors (n = 3) were exposed to the adenovirus with different doses or time exposures during differentiation. PGC-1α and PMP70 (peroxisomal-specific LCFA transporter) protein contents were quantified by Western blotting. Following the 48-h adenoinfection with varying doses, Fig. 1A demonstrates that PGC-1α protein increased in a dose-dependent manner, which was associated with increases in the peroxisome-specific membrane marker PMP70 protein that peaked at the dose of 18.75 × 105 pfu/ml media compared with the 0 pfu/ml media[nonvirus control (NVC), diffrentiation media without any virus; Fig. 1B). It was also observed that protein contents of PGC-1α and PMP70 increased in a time-dependent fashion with an equal dose of 37.5 × 105 pfu/ml media (Fig. 1, C and D). According to these findings, the subsequent experiments adopted a dose of the 18.75 × 105 pfu/ml media due to this level being sufficient to increase the PGC-1α protein 3.4-fold (vs. 0 pfu/ml media). This response is similar to the physiologically relevant extent of reported protein levels induced by aerobic exercise training in humans (44). For the time frame employed for PGC-1α overexpression, 72-h postinfection was used as the end point for harvesting and assaying the samples. The rationale for this approach was based on data obtained from Western blotting analyses of PMP70 protein content, which suggested that peroxisomal biogenesis/activity was elevated fourfold over that of 0 pfu/ml media control samples at 72 h.
Fig. 1.
Overexpression of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) increases peroxisomal membrane protein 70 kDa (PMP70) in a time-dependent fashion in human primary myotubes. Human skeletal muscle cells (HSkMC) from vastus lateralis muscle biopsies were exposed to varying doses [plaque-forming units (pfu)/ml media] or time (h) of adenovirus packed with human PGC-1α on days 4 and 5 post-differentiation and harvested and assayed on day 7 for PMP70 and PGC-1α proteins by Western blotting. Results are shown as Western blots and fold change vs. control (0 pfu/ml media) (pooled cells from lean donors, BMI = 23.3 ± 1.6; n = 3). A and C: dose and time-course responses of PGC-1α protein contents. B and D: dose and time-course responses of PMP70 protein. The data supported our hypothesis that PGC-1α drives the peroxisomal marker in human primary myotubes. Pfu: an indicator of active virus.
PGC-1α overexpression induces peroxisomal function/peroxisomal β-oxidation-related gene expression in human primary myotubes.
To test whether PGC-1α is involved in elevating peroxisomal activity in HSkM, a gain-of-function approach was used. Real-time qRT-PCR was performed to determine the levels of peroxisomal proliferator gene (PEX) mRNA between human primary myotubes infected with Ad-GFP or Ad-PGC-1α. Results demonstrated that PGC-1α mRNA increased ∼21-fold compared with Ad-GFP (P < 0.005; Fig. 2A). As we hypothesized, this elevation in PCG-1α mRNA was associated with upregulation of numerous peroxisomal-related genes. Peroxisomal PMP70 and PEX19 mRNA expression increased 4.2- and 1.4-fold, respectively, 72 h-post adenovirus infection (P < 0.005; Fig. 2C), whereas other PEXs, PEX3, and PEX13 (P < 0.005) also increased. Furthermore, there was a significant increase in the expression of ACOX1 (acyl-CoA oxidase 1), the rate-limiting enzyme responsible for the initial step of peroxisomal β-oxidation (P < 0.05), as well as PBFE (P < 0.001), PTHIO (P < 0.001), and DNM1L required for mitochondrial fission (P < 0.001). However, some peroxisomal biogenesis-associated genes were unchanged (e.g., PEX 11A and PEX 11B), and others were significantly reduced, e.g., CrOT (P < 0.001) and ABCD1 (P < 0.05). Concomitant with our mRNA studies, fluorescent images from human primary myotubes infected with Ad-GFP alone or Ad-PGC-1α (which also encodes for GFP) were visualized by fluorescent microscopy (Fig. 2B). Green fluorescent signals detected by microscopy provided visual evidence that our methodology reached a sufficient level of primary myotube infection (nearly 100% of infection efficiency observed in both groups).
Fig. 2.
PGC-1α drives peroxisomal gene expression in human primary myotubes. HSkMCs from muscle biopsies were exposed to adenovirus packed with human PGC-1α (Ad-PGC-1α) or GFP (Ad-GFP) on days 4 and 5 post-differentiation and harvested on day 7 postdifferentiation and measured for peroxisomal biogenesis gene expression by qRT-PCR. A: PGC-1α mRNA expression (ΔΔCT value: −4.34). B: fluorescent images from fluorescent microscopy showed infection efficiency after 72-h infection with either Ad-PGC-1α (coexpressed GFP) or Ad-GFP in human primary myotubes (magnification ×20). C: peroxisomal biogenesis factor (PEX) mRNA expression. ABCD1, ATP-binding cassette subfamily D member 1 responsible for transporting very long-chain fatty acids (VLCFA) across the peroxisomal membrane (ΔΔCT value: 0.61). PMP70, peroxisomal membrane protein 70 kDa, a marker of peroxisomal biogenesis (ΔΔCT value: −1.97); PEX3, PEX11A, PEX11B, PEX13, and PEX19, peroxisomal biogenesis genes initiated to peroxisomal proliferation (ΔΔCT value: −3.20, −0.10, 0.30, −0.60, and −0.37); ACOX1, peroxisomal acyl-coenzyme A oxidase 1 (ΔΔCT value: −0.47); PBFE, peroxisomal bifunctional enzyme (ΔΔCT value: −1.73); PTHIO, peroxisomal 3-ketoacyl-CoA thiolase for the peroxisomal β-oxidation (ΔΔCT value: −2.61); CrAT, carnitine O-acetyltransferase for converting acetyl-CoA to acetyl-carnitine (ΔΔCT value: −3.45); CrOT, carnitine octanoyltransferase for converting acyl-CoA to acyl-carnitine (ΔΔCT value: 1.25); DNM1L, dynamin 1-like protein involved in fission of both peroxisomal and mitochondrial biogenesis (ΔΔCT value: −1.15). mRNA expression was determined using the comparative CT method (ΔΔCT) with an endogenous control (18S ribosomal RNA) for normalization and then compared with Ad-GFP. Results are shown as means ± SE (n = 6). *P < 0.05, **P < 0.005, and ***P < 0.001 vs. Ad-GFP groups by paired 2-tailed t-test. DAPI, 2-(4-amidinophenyl)-1H -indole-6-carboxamidine; GFP, green fluorescent protein. [(2R,3R,4S,5S,6R)-3-fluoro-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl] dihydrogen phosphate.
Both mitochondrial biogenesis and peroxisomal activity-associated protein content are significantly elevated in primary myotubes following overexpression of PGC-1α.
Western blotting was performed to probe peroxins and several important markers for mitochondrial biogenesis in HSkMC in response to induction by PGC-1α. Findings indicted that following 72 h-post infection, PMP70 and PEX19 protein content was significantly elevated 3- (P < 0.05) and 1.4-fold (P < 0.05), respectively, relative to the Ad-GFP group and no virus control (NVC) in primary myotubes (Fig. 3). Likewise, we examined the effects of overexpression of PGC-1α on mitochondrial biogenesis. As shown in Fig. 3, citrate synthetase (CS) was significantly elevated nearly fivefold compared with the Ad-GFP groups (P < 0.05). Likewise, the mitochondrial transcription factor A (mtTFA), a critical molecule necessary for mitochondrial DNA transcription, was increased >2.2-fold following overexpression of PGC-1α (P < 0.05).
Fig. 3.
PGC-1α induces both mitochondrial biogenesis marker and peroxisomal biogenesis proteins in human primary myotubes. Western blotting data of whole cell lysates from human primary myotubes incubated with differentiation media without any virus [nonvirus control (NVC; 0 pfu/ml media): 0 pfu/ml media] or infected with GFP or human PGC-1α adenovirus for 72 h. All values were normalized to α-tubulin and then compared with NVC. Results are shown as means ± SE (n = 6). *P < 0.05 vs. Ad-GFP groups by paired 2-tailed t-test. mtTFA, mitochondrial transcription factor A; CS, citrate synthetase; PMP70, peroxisomal membrane protein 70 kDa; PEX19, peroxisomal biogenesis protein initiated to peroxisomal proliferation.
Peroxisomal-mitochondrial oxidative function is significantly enhanced in human primary myotubes following PGC-1α overexpression.
To determine the functional implications to which PGC-1α-induced peroxisomal activity and mitochondrial biogenesis would confer on VLCFA and LCFA oxidation, we performed assays with radiolabeled palmitic acid (C16:0) and lignoceric acid (C24:0), respectively, using HSkMC homogenates after adenoinfection. Complete oxidation of palmitic acid was significantly elevated in the Ad-PGC-1α group vs. the Ad-GFP (P < 0.05). Likewise, acid-soluble metabolites (ASM) representing incomplete oxidation products from both peroxisomes and mitochondria following the Ad-PGC-1α overexpression were significantly increased (Fig. 4A). Furthermore, the ratio of CO2 to ASM (complete/incomplete oxidation) has become recognized as an index of FAO efficiency (13). In the Ad-PGC-1α group, the oxidation efficiency with palmitate incubation increased significantly compared with the Ad-GFP group (P < 0.05; Fig. 4B).
Fig. 4.
PGC-1α overexpression increases both palmitic acid and lignoceric acid oxidation in human primary myotubes. Fatty acid oxidation (FAO) with radiolabeled substrates was performed by using primary myotubes homogenates. A: [1-14C]palmitate oxidation and acid-soluble metabolites (ASM). B: palmitate oxidation efficiency was expressed by an index of complete oxidation/acid-soluble metabolites (CO2/ASM); an increase in the index of CO2/ASM in the Ad-PGC-1α group indicates that oxidation efficiency is elevated post-PGC-1α overexpression. C: [1-14C]lignoceric acid (C24:0). VLCFA, which is necessarily oxidized first by peroxisomes, was used to assess mitochondrial oxidation, which utilized peroxisomal-derived fatty acid metabolites to boost mitochondrial/peroxisomal fatty acid disposal. D: lignoceric acid oxidation efficiency. E: indirect measure of total triglyceride (IMTG) content by measurement of triacylglycerol (TAG) content in cell lysates. F: lipid droplet metabolism and ether phospholipid synthesis gene expression by real-time qRT-PCR. mRNA expression was determined using the comparative CT method (ΔΔCT) with an endogenous control (18S ribosomal RNA) for normalization and then compared with the Ad-GFP group. Results are shown as means ± SE (n = 6). *P < 0.05, **P < 0.005, and ***P < 0.001 vs. Ad-GFP groups by paired, 2-tailed t-test. DGAT1, diacylglycerol acyltransferase 1 responsible for TAG synthesis (ΔΔCT value: −2.48); ATGL, adipose triglyceride lipase (ΔΔCT value: −1.54); PLIN5, perilipin 5 involved in lipid droplet mobilization (ΔΔCT value: −4.04); GNPAT/DHAPAT, glyceronephosphate acyltransferase-dihydroxyacetone phosphate acyltransferase involved in ether phospholipid synthesis (ΔΔCT value: −0.46) vs. Ad-GFP groups by paired 2-tailed t-test.
Based on subcellular fractionation studies in rat liver, lignoceric acid oxidation is exclusively oxidized first by peroxisomes (49). Although peroxisomes are incapable of fully oxidizing VLCFA, their acyl-carnitine or acetyl-carnitine metabolites are exported to the mitochondria, where they can undergo completion of β-oxidation, yielding 14CO2 as the byproduct. Thus measurement of 14CO2 can be used as a readout of mitochondrial complete FAO using peroxisomal-derived products (no 14CO2 can be derived from peroxisomal FAO, as it lacks a TCA cycle). In this regard, functional interactions between the two organelles can be established. In the present studies, a significant increase in complete oxidation rates (mitochondrial 14CO2 production) of lignoceric acid was observed in the Ad-PGC-1α group vs. Ad-GFP (P < 0.05) This was accompanied by elevations in the ASM production from lignoceric acid between the two groups (P < 0.05; Fig. 4C). However, we found no statistical difference between the groups with respect to oxidization efficiency of lignoceric acid, as evidenced by evaluation of the CO2-to-ASM ratio (P = 0.08; Fig. 4D).
PGC-1α overexpression induces lipid droplet metabolism and peroxisomal ether lipid synthesis gene expression in human primary myocytes.
To assess the intramyocellular lipid pool, we performed a triacylglycerol (TAG) assay to biochemically measure TAG content using HSkMC lysates since TAG is the major form of lipid stored in skeletal muscle. Results demonstrated that TAG content was elevated in the Ad-PGC-1α group vs. Ad-GFP by 72.4% (P = 0.07; Fig. 4E). Lipid droplet metabolism-associated gene expression following PGC-1α overexpression was also assessed by real-time qRT-PCR. Significant elevations of DGAT1, ATGL, and PLIN5 were found in the Ad-PGC-1α group vs. Ad-GFP (P < 0.005). Furthermore, one peroxisomal ether phospholipid synthesis gene, GNPAT, was significantly elevated (P < 0.001; Fig. 4F).
DISCUSSION
Employing gain-of-function approaches, these studies demonstrate that the master gene transcription coactivator PGC-1α induces both peroxisomal activity and mitochondrial biogenesis in HSkMC. In addition, we describe the novel finding that the functional outcome of a PGC-1α-driven increase in peroxisomal activity and mitochondrial biogenesis is the elevation of FAO by mitochondria. To the best of our knowledge, this represents the first evidence to demonstrate functional interactions between peroxisomes and mitochondria in HSkMC and that the functions of both organelles are induced under the same nexus of control by PGC-1α.
Bagattin et al. (5) utilized an approach similar to the current study employing adenoinfection of brown adipose tissue, bone osteosarcoma U2OS cells, and hepatic-derived FAO cells to overexpress PGC-1α over a 72-h time period. Results were congruent with our gene expression findings in that PGC-1α induced peroxisomal PEX3, PEX13, PEX19, PMP70, ACOX1, peroxisomal bifunctional enzyme (PBFE), and peroxisomal 3-ketoacyl-CoA thiolase (PTHIO) gene expression as well as the fission-related gene DNM1L. Together, the current findings and evidence from other cell lines (5) support the contention that PGC-1α can act as a cotransducer for peroxisomal activity as well as mitochondrial biogenesis in multiple tissues, which now includes HSkMC by the present report.
Interestingly, PGC-1α did not upregulate the expression of all PEX genes studied. Since our paper demonstrated that PGC-1α overexpression resulted in increased peroxisomal activity, the finding that some of the peroxisomal genes were either unchanged (PEX11A and PEX11B) or reduced (CrOT and ABCD1) suggests that not all PEX genes are required for induction of peroxisomal activity in skeletal muscle. Assuming that mRNA reflects protein expression, only a core set of peroxisomal genes may be required to elicit increased peroxisomal-related fatty acid metabolism. Clearly, our understanding of the specific genes that influence peroxisomal activity driven by PGC-1α in skeletal muscle is still in its infancy and requires additional investigations, notably in humans.
It is important to note, however, that PGC-1α binds to and activates other transcription factors such as PPARs to implement its regulatory influences on organelle content and function. This is based on the established understanding that the coactivator PGC-1α does not bind directly to DNA itself. However, the conventional wisdom about peroxisome proliferation and peroxisomal β-oxidation by nuclear receptor PPARs was based largely on rodent studies of hepatic and other nonskeletal muscle tissues (17, 25). For instance, PPARα is regarded as a key regulator for hepatic peroxisomal biogenesis (5), whereas in adipocytes PPARγ is believed to play a pivotal role in initiating peroxisomal gene expression, leading to peroxisomal biogenesis in this tissue (34). In this regard, it is intriguing that in the recent report by Bagattin et al. (5), another transcriptional regulatory mechanism accounting for peroxisomal biogenesis by PGC-1α that may act via an as yet unidentified target of PGC-1α was suggested. In the study by Bagattin et al. (5) utilizing a PPARα liver-specific KO mouse, peroxisomal biogenesis gene expression was noted by PGC-1α despite the absence of PPARα, the presumed signal transducer for peroxisomal biogenesis in liver (5). Findings such as these suggest a rather naïve understanding of the cellular control mechanisms leading to peroxisomal biogenesis/function, most especially in skeletal muscle.
Therefore, further scientific pursuit would serve to expand the current understanding of the transcriptional control for peroxisomal biogenesis and function(s) in a variety of tissue types, especially skeletal muscle. As such, in addition to specific PPAR isoform involvement, these studies may unmask yet unknown coregulators for the organelle’s proliferation and function. For example, BAF60a (SMARCD1) was identified as a molecular target within a PGC-1α transcriptional network for both mitochondrial and peroxisomal β-oxidation gene expression in rodent hepatocytes using genome-wide screening analysis (33). PGC-1α has been suggested to mediate recruitment of BAF60a to PPARα-binding sites, leading to transcriptional activation of peroxisomal and mitochondrial fat oxidation genes (33). Given the report by Bagattin et al. (5) above, other PPAR isoforms may also be a targets for cofactors such as BAF60a to induce peroxisomal/mitochondrial biogenesis in HSkM.
Another noteworthy outcome of the current studies is the novel finding that both peroxisomal (elevated PMP70 and PEX19) and mitochondrial (CS and mtTFA) biogenesis/function was positively influenced by PGC-1α overexpression in HSkM. This is likely to have multiple physiological implications. For example, what has been lacking in past studies until the current report was a demonstration of the functional significance of the simultaneous increase in the biogenesis/function of both organelles, which was demonstrated in the present study as measurements of FAO. In this regard, it can be hypothesized that an increased activity of peroxisomes would lead to elevations in acyl- and acetyl-carnitine export products from the peroxisome that in animal models has been demonstrated to enhance complete oxidation of peroxisomal chain/shorten metabolites by the mitochondria (39).
Therefore, an important additional purpose of the present study was to determine the functional significance of a PGC-1α-driven increase in peroxisomal function on lipid catabolism. It is known from previous studies in HSkMC that overexpression of PGC-1α leads to increased long-chain FAO potential with marked and simultaneous elevations in mitochondrial oxidative capacity (e.g., increased protein content of COX-IV and mtTFA, which stimulates mitochondrial biogenesis) (13). Congruent with these past reports, the current findings demonstrate that radiolabeled palmitic acid oxidization was significantly elevated following PGC-1α overexpression (Fig. 4A). Notably, we extended the findings by Consitt et al. (13) and evaluated the potential changes in oxidation efficiency following PGC-1α overexpression (oxidation efficiency can be indirectly assessed using an index of ASM/CO2; higher values for the ratio signify reduced mitochondrial oxidative efficiency). Our data suggest an increase in oxidation efficiency (CO2/ASM) following Ad-PGC-1α exposure in human primary myotubes, which is presumably due in part to elevations in peroxisomal activity. The observation of elevated mitochondrial oxidation efficiency was also congruent to findings by Koves et al. (28). Evidence from their study in rat L6 skeletal myotubes suggested that mitochondrial function and fatty acid oxidation efficiency increase (a reduction of ASM/CO2) with the elevation in PGC-1α protein content, but the explanation was in contrast to the present studies (Fig. 4B). That is, they provided an alternative explanation for their finding, which was due to enhancement of PGC-1α-mediated coupling events between the mitochondrial β-oxidation system and the tricarboxylic acid cycle flux. Again, the current studies serve to contribute additional mechanisms by which PGC-1α may act to maintain cellular lipid homeostasis.
For the sake of further discussion, we acknowledge differences in lipid oxidization capacities by particular skeletal muscle fiber phenotypes. For example, slow-twitch (type I) fibers possess greater oxidative enzyme activities vs. type II fibers (19). To illustrate, a correlational study in HSkM demonstrated that the expression of PGC-1α mRNA levels was positively correlated with type I muscle fibers (30). In addition, the evidence demonstrating lower type I muscle fibers in obese vs. in lean subjects (53) suggests that PGC-1α-induced peroxisomal function may play a role in contributing to an elevated oxidative capacity of type I fiber phenotypes in obesity. However, a difference in peroxisomal activity among varying fiber types is unknown in HSkM. These investigations merit further investigation to discern whether PGC-1α may influence peroxisomal biology in skeletal muscle based on fiber type expression as noted in the obese vs. lean humans (53).
A further strength of current experimental design was the employment of [1-14C]lignoceric acid (C24:0). Thus, the present investigation also provides the first direct evidence that PGC-1α can increase VLCFA oxidation in HSkMC (primary myotubes; Fig. 4C). This VLCFA is activated by a very long-chain acyl-CoA synthetase and then is catabolized by peroxisomal β-oxidation by several enzymes (e.g., ACOX1, PBFE, and PTHIO) (42). The end products of peroxisomal partial β-oxidation are converted to acetyl-carnitine or acyl-carnitine metabolites such as C2-acetyl-carnitine and C8 octanoyl-carnitine [by the enzymes: carnitine acetyltransferase (CrAT) and carnitine octanoyltransferase (CrOT); Fig. 5] upon export from the peroxisomal matrix, as described above (9). Evidence from the present study suggests that the peroxisomal-derived acyl- and acetyl-carnitine metabolites were channeled to mitochondria for subsequent complete oxidation. Recent reports also imply that the varying chain length acylcarnitine moieties (C2 and C8) bypass the long-chain regulatory pathway (carnitine palmitoyl transferase I located on the outer membrane of mitochondria), possibly by a reputed mitochondrial carnitine acylcarnitine transporter (Fig. 5) (26, 55). Thereafter, varying chain length acylcarnitine substrates can be converted back to acyl- or acetyl-CoA derivatives by mitochondrial CPTII for subsequent β-oxidation and complete oxidation in the tricarboxylate cycle with CO2 as the byproduct. Importantly, elevations in 14CO2 production were noted in the present report following PGC-1α infection, suggesting that the peroxisomal chain/shorten acyl- and acetyl-carnitines export products were incorporated into the mitochondrial matrix to undergo complete oxidation. These results indicate a capacity for peroxisomes to cooperate with mitochondria for lipid disposal in HSkM.
Fig. 5.
A schematic model of PGC-1α-induced peroxisomal activity and mitochondrial biogenesis and subsequent functional cooperation for enhanced FAO in human skeletal muscle. PGC-1α overexpression enhanced nuclear transcription of peroxisomal proliferator genes and subsequent peroxisomal activity and mitochondrial biogenesis. Elevated peroxisomal function/activity leads to elevated peroxisomal β-oxidation. Increased export of peroxisomal acyl- and acetyl-carnitine products is reconverted by mitochondrial CPT II and undergoes β-oxidation, thereby increasing lipid disposal under conditions of mitochondrial metabolites overload (low energy demand). Further mechanistic investigations are needed to define potential downstream targets of PGC-1α or involved transcriptional control networks such as specific PPAR isoforms and/or other unidentified transcriptional cofactors (TF?) involved in both peroxisomal activity, and mitochondrial biogenesis in HSkM represents fruitful areas for future scientific exploration. LCFA, long-chain fatty acid; VLCFA, very-long-chain fatty acid; CrOT, carnitine octanoyltransferase; CrAT, carnitine O-acetyltransferase.
Although the VLCFAs are substantially less abundant as LCFAs [for instance palmitate (C16:0) and oleate (C18:1)] in the typical Western diet (6) or in the circulating lipid pool, some ceramide species can be derived from VLCFAs [e.g., lignoceric acid (C24:0)]. Ceramides have been observed to be significantly elevated in HSkM from obese individuals vs. sedentary counterparts (1, 2). Many laboratories have also demonstrated a link between insulin resistance and ceramide species in skeletal muscle (11). In this regard, this may imply a functional importance for peroxisomal β-oxidation in catabolizing VLCFAs, which may be relevant to insulin resistance and obesity in HSkM. Because the function of peroxisomes are as of yet poorly understood in skeletal muscle, additional investigations may be insightful toward defining additional functions for peroxisomes in the metabolism of bioactive lipids in this tissue, notably in HSkM.
Accordingly, the novel finding in this report may represent a new paradigm in HSkM lipid metabolism and perhaps provide a new target to consider for the treatment of metabolic diseases such as obesity and Type 2 diabetes. Both of these diseases are characterized by lipid overload that imposes on a dysregulated mitochondrial oxidative capacity, leading to accumulation of bioactive long-chain fatty acids (e.g., acyl-CoA and ceramide lipid species), which have been identified as causal factors in the development of insulin resistance in skeletal muscle (3, 35). In this regard, stimulation of peroxisomal activity and the subsequent enhancement of functional interactions with mitochondria could serve as a compensatory adaptation to raise the “metabolic ceiling” for lipid oxidation that would otherwise lead to lipid accumulation, lipotoxicity, and associated metabolic disease, as noted by many investigators (21, 27, 35, 38).
As a final note, because peroxisomes are also involved with lipid synthesis, we assayed the derived primary myocytes for total lipid synthesis as measured by triacylglycerol content assays as well as genes associated with lipid synthesis. Results from the assessment of TAG content revealed nearly statistically significant differences between the Ad-GFP and PGC-1α overexpression cells (P = 0.07). More so, given the observed 72% increase in IMTG estimates in the infected myotubes, the result is consistent with a previous study by Koves et al. (29) that indicated that overexpression of PGC-1α in mice and in human primary myotubes can induce upregulation of key lipid synthesis-associated gene expression, including diglyceride acyltransferase 1 (DGAT1), and some lipid droplet mobilization genes, for example, adipose triglyceride lipase (ATGL) and perilipin family protein 5 (PLIN5) and concomitant lipid droplets/IMTG increments. Therefore, we performed additional qRT-PCR for several genes associated with lipid synthesis. Likewise, elevations in gene expression for IMTG metabolism were observed (Fig. 4F). Also, an additional peroxisomal gene for ether lipid synthesis (GNPAT) was significantly elevated in this study. Despite these intriguing findings, further studies seem warranted with regard to the influences of peroxisomal induction on the synthesis of specific lipid species as they relate to basic cellular function and perhaps metabolic diseases associated with changes in the lipid profiles of HSkM. This would necessitate enhanced lipidomic profiling investigations beyond the scope of the present investigations, but it is certainly warranted in the future.
A limitation to the current studies revolves around organelle contributions toward elevations in palmitate oxidation following PGC-1α overexpression. In this regard, our findings could be explained by elevations in the peroxisomal β-oxidation system, but they also could occur due to concomitant mitochondrial biogenesis induced by PGC-1α overexpression. Therefore, future studies are required to discern the relative contributions of each organelle toward long-chain FAO and perhaps the potential for peroxisomal influences on lipid synthesis induced by PGC-1α overexpression. Additional future directions also include employing PGC-1α underexpression in models of increased lipid accumulation, as is evident in skeletal muscle obtained from obese individuals (12, 22, 32), which could provide further insights with respect to the necessity of elevations in peroxisomal function via PGC-1α regulation.
In conclusion, the study of peroxisomal biology in skeletal muscle remains in its infancy. The present studies provide the first direct evidence supporting the hypothesis that PGC-1α-driven metabolic transcription can initiate both mitochondrial biogenesis and peroxisomal activity in HSkM. The resultant outcome is an increase in peroxisomal FAO, which can be effectively coupled to the mitochondrial β-oxidation system, leading to an enhanced complete oxidation of intramyocellular lipids in HSkM. Finally, further mechanistic investigations are needed to define potential downstream targets of PGC-1α or involved transcriptional control networks such as specific PPAR isoforms and/or other unidentified transcription factors (denoted as TF? in Fig. 5) involved in both peroxisomal activity and mitochondrial biogenesis in HSkM. Since PGC-1α does not induce the expression of all PEX genes, and since PGC-1α must bind transcription factors to directly affect gene expression, our findings suggest that different PEX genes are regulated by different transcription factors. Additional areas of investigation also include the potential effects of enhanced peroxisomal activity on changes in the pool of specific lipid species such as ceramides and phospholipids.
Understanding the mechanistic aspects of a peroxisomal/mitochondrial cooperation in FAO may lead to a new paradigm in HSkM lipid biology. The potential for pharmacological exploitation of an expanded scientific realization regarding peroxisomal biology may be productive in terms of developing novel strategies to combat lipid accumulation in HSkM, as observed in metabolic diseases such as obesity and Type 2 diabetes.
GRANTS
This work was supported in part by a seed grant from the Diabetes Obesity Institute, East Carolina University (R. N.Cortright), NIH-DK-056112 (J. A. Houmard), and NIH-1R15-HL-113854-01A1 (R. C. Hickner).
AUTHOR CONTRIBUTIONS
T-Y.H., D.Z., and R.C.H. performed experiments; T-Y.H. and R.N.C. analyzed data; T-Y.H., D.Z., J.A.H., J.J.B., and R.N.C. interpreted results of experiments; T-Y.H. prepared figures; T-Y.H. drafted manuscript; T-Y.H., J.J.B., R.C.H., and R.N.C. edited and revised manuscript; T-Y.H., J.A.H., J.J.B., R.C.H., and R.N.C. approved final version of manuscript; R.N.C. conceived and designed research.
ACKNOWLEDGMENTS
We are grateful to Dr. Kai Zou and Dr. Sanghee Park for assistance with myocytes isolations and Thomas Green for assistance with fluorescent imaging.
REFERENCES
- 1.Adams JM II, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53: 25–31, 2004. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Amati F, Dubé JJ, Alvarez-Carnero E, Edreira MM, Chomentowski P, Coen PM, Switzer GE, Bickel PE, Stefanovic-Racic M, Toledo FGS, Goodpaster BH. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes 60: 2588–2597, 2011. doi: 10.2337/db10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW III, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin S, St-Pierre J. PGC1α and mitochondrial metabolism—emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci 125: 4963–4971, 2012. doi: 10.1242/jcs.113662. [DOI] [PubMed] [Google Scholar]
- 5.Bagattin A, Hugendubler L, Mueller E. Transcriptional coactivator PGC-1alpha promotes peroxisomal remodeling and biogenesis. Proc Natl Acad Sci USA 107: 20376–20381, 2010. doi: 10.1073/pnas.1009176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baylin A, Kabagambe EK, Siles X, Campos H. Adipose tissue biomarkers of fatty acid intake. Am J Clin Nutr 76: 750–757, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Berger J, Gärtner J. X-linked adrenoleukodystrophy: clinical, biochemical and pathogenetic aspects. Biochim Biophys Acta 1763: 1721–1732, 2006. doi: 10.1016/j.bbamcr.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Berggren JR, Tanner CJ, Houmard JA. Primary cell cultures in the study of human muscle metabolism. Exerc Sport Sci Rev 35: 56–61, 2007. doi: 10.1249/JES.0b013e31803eae63. [DOI] [PubMed] [Google Scholar]
- 9.Le Borgne F, Ben Mohamed A, Logerot M, Garnier E, Demarquoy J. Changes in carnitine octanoyltransferase activity induce alteration in fatty acid metabolism. Biochem Biophys Res Commun 409: 699–704, 2011. doi: 10.1016/j.bbrc.2011.05.068. [DOI] [PubMed] [Google Scholar]
- 10.Boyle KE, Zheng D, Anderson EJ, Neufer PD, Houmard JA. Mitochondrial lipid oxidation is impaired in cultured myotubes from obese humans. Int J Obes 36: 1025–1031, 2012. doi: 10.1038/ijo.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab 15: 585–594, 2012. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Coen PM, Dubé JJ, Amati F, Stefanovic-Racic M, Ferrell RE, Toledo FGS, Goodpaster BH. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes 59: 80–88, 2010. doi: 10.2337/db09-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Consitt LA, Bell JA, Koves TR, Muoio DM, Hulver MW, Haynie KR, Dohm GL, Houmard JA. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha overexpression increases lipid oxidation in myocytes from extremely obese individuals. Diabetes 59: 1407–1415, 2010. doi: 10.2337/db09-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dagenais GR, Tancredi RG, Zierler KL. Free fatty acid oxidation by forearm muscle at rest, and evidence for an intramuscular lipid pool in the human forearm. J Clin Invest 58: 421–431, 1976. doi: 10.1172/JCI108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delille HK, Alves R, Schrader M. Biogenesis of peroxisomes and mitochondria: linked by division. Histochem Cell Biol 131: 441–446, 2009. doi: 10.1007/s00418-009-0561-9. [DOI] [PubMed] [Google Scholar]
- 16.Distel B, Erdmann R, Gould SJ, Blobel G, Crane DI, Cregg JM, Dodt G, Fujiki Y, Goodman JM, Just WW, Kiel JA, Kunau WH, Lazarow PB, Mannaerts GP, Moser HW, Osumi T, Rachubinski RA, Roscher A, Subramani S, Tabak HF, Tsukamoto T, Valle D, van der Klei I, van Veldhoven PP, Veenhuis M. A unified nomenclature for peroxisome biogenesis factors. J Cell Biol 135: 1–3, 1996. doi: 10.1083/jcb.135.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell 68: 879–887, 1992. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 18.De Duve C, Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev 46: 323–357, 1966. [DOI] [PubMed] [Google Scholar]
- 19.Essén B, Jansson E, Henriksson J, Taylor AW, Saltin B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol Scand 95: 153–165, 1975. doi: 10.1111/j.1748-1716.1975.tb10038.x. [DOI] [PubMed] [Google Scholar]
- 20.Fiamoncini J, Lima TM, Hirabara SM, Ecker J, Gorjão R, Romanatto T, ELolimy A, Worsch S, Laumen H, Bader B, Daniel H, Curi R. Medium-chain dicarboxylic acylcarnitines as markers of n-3 PUFA-induced peroxisomal oxidation of fatty acids. Mol Nutr Food Res 59: 1573–1583, 2015. doi: 10.1002/mnfr.201400743. [DOI] [PubMed] [Google Scholar]
- 21.Gaster M, Rustan AC, Aas V, Beck-Nielsen H. Reduced lipid oxidation in skeletal muscle from type 2 diabetic subjects may be of genetic origin: evidence from cultured myotubes. Diabetes 53: 542–548, 2004. doi: 10.2337/diabetes.53.3.542. [DOI] [PubMed] [Google Scholar]
- 22.Gray RE, Tanner CJ, Pories WJ, MacDonald KG, Houmard JA. Effect of weight loss on muscle lipid content in morbidly obese subjects. Am J Physiol Endocrinol Metab 284: E726–E732, 2003. doi: 10.1152/ajpendo.00371.2002. [DOI] [PubMed] [Google Scholar]
- 23.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27: 728–735, 2006. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 24.Houmard JA, Hortobágyi T, Neufer PD, Johns RA, Fraser DD, Israel RG, Dohm GL. Training cessation does not alter GLUT-4 protein levels in human skeletal muscle. J Appl Physiol (1985) 74: 776–781, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347: 645–650, 1990. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 26.Jakobs BS, Wanders RJ. Fatty acid beta-oxidation in peroxisomes and mitochondria: the first, unequivocal evidence for the involvement of carnitine in shuttling propionyl-CoA from peroxisomes to mitochondria. Biochem Biophys Res Commun 213: 1035–1041, 1995. doi: 10.1006/bbrc.1995.2232. [DOI] [PubMed] [Google Scholar]
- 27.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 279: E1039–E1044, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 280: 33588–33598, 2005. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 29.Koves TR, Sparks LM, Kovalik JP, Mosedale M, Arumugam R, DeBalsi KL, Everingham K, Thorne L, Phielix E, Meex RC, Kien CL, Hesselink MK, Schrauwen P, Muoio DM. PPARγ coactivator-1α contributes to exercise-induced regulation of intramuscular lipid droplet programming in mice and humans. J Lipid Res 54: 522–534, 2013. doi: 10.1194/jlr.P028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krämer DK, Ahlsén M, Norrbom J, Jansson E, Hjeltnes N, Gustafsson T, Krook A. Human skeletal muscle fibre type variations correlate with PPARα, PPARδ and PGC-1α mRNA. Acta Physiol (Oxf) 188: 207–216, 2006. doi: 10.1111/j.1748-1716.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 31.Latruffe N, Vamecq J. Evolutionary aspects of peroxisomes as cell organelles, and of genes encoding peroxisomal proteins. Biol Cell 92: 389–395, 2000. doi: 10.1016/S0248-4900(00)01083-2. [DOI] [PubMed] [Google Scholar]
- 32.Laurens C, Moro C. Intramyocellular fat storage in metabolic diseases. Horm Mol Biol Clin Investig 26: 43–52, 2016. [DOI] [PubMed] [Google Scholar]
- 33.Li S, Liu C, Li N, Hao T, Han T, Hill DE, Vidal M, Lin JD. Genome-wide coactivation analysis of PGC-1alpha identifies BAF60a as a regulator of hepatic lipid metabolism. Cell Metab 8: 105–117, 2008. doi: 10.1016/j.cmet.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lodhi IJ, Semenkovich CF. Peroxisomes: a nexus for lipid metabolism and cellular signaling. Cell Metab 19: 380–392, 2014. doi: 10.1016/j.cmet.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 307: 384–387, 2005. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 36.Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, Willy PJ, Schulman IG, Heyman RA, Lander ES, Spiegelman BM. Errα and Gabpa/b specify PGC-1α-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci USA 101: 6570–6575, 2004. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muoio DM, Koves TR. Skeletal muscle adaptation to fatty acid depends on coordinated actions of the PPARs and PGC1 alpha: implications for metabolic disease. Appl Physiol Nutr Metab 32: 874–883, 2007. doi: 10.1139/H07-083. [DOI] [PubMed] [Google Scholar]
- 38.Muoio DM, Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab 15: 595–605, 2012. doi: 10.1016/j.cmet.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noland RC, Woodlief TL, Whitfield BR, Manning SM, Evans JR, Dudek RW, Lust RM, Cortright RN. Peroxisomal-mitochondrial oxidation in a rodent model of obesity-associated insulin resistance. Am J Physiol Endocrinol Metab 293: E986–E1001, 2007. doi: 10.1152/ajpendo.00399.2006. [DOI] [PubMed] [Google Scholar]
- 40.Nordgren M, Fransen M. Peroxisomal metabolism and oxidative stress. Biochimie 98: 56–62, 2014. doi: 10.1016/j.biochi.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 41.Prieto Tenreiro A, Penacho Lázaro MÁ, Andrés Celda R, Fernández Fernández M, González Mateo C, Díez Hernández A. Dietary treatment for X-linked adrenoleukodystrophy: is “Lorenzo’s oil” useful? Endocrinol Nutr 60: 37–39, 2013. doi: 10.1016/j.endonu.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Reubsaet FA, Brückwilder ML, Veerkamp JH, Trijbels JM, Hashimoto T, Monnens LA. Immunochemical analysis of the peroxisomal beta-oxidation enzymes in rat and human heart and skeletal muscle and in skeletal muscle of Zellweger patients. Biochem Med Metab Biol 45: 197–203, 1991. doi: 10.1016/0885-4505(91)90021-C. [DOI] [PubMed] [Google Scholar]
- 43.Rhodin J. Correlation of Ultrastructural Organization and Function in Normal and Experimentally Changed Proximal Convoluted Tubule Cells of the Mouse Kidney. Stockholm, Sweden: Karoliniska Institute, Akitbolagat Godvil, 1954. [Google Scholar]
- 44.Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino J-P, Dériaz O. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 52: 2874–2881, 2003. doi: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- 45.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004. doi: 10.1016/S0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schrader M, Bonekamp NA, Islinger M. Fission and proliferation of peroxisomes. Biochim Biophys Acta 1822: 1343–1357, 2012. doi: 10.1016/j.bbadis.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Schrader M, Costello J, Godinho LF, Islinger M. Peroxisome-mitochondria interplay and disease. J Inherit Metab Dis 38: 681–702, 2015. doi: 10.1007/s10545-015-9819-7. [DOI] [PubMed] [Google Scholar]
- 48.Schrader M, Yoon Y. Mitochondria and peroxisomes: are the ‘big brother’ and the ‘little sister’ closer than assumed? BioEssays 29: 1105–1114, 2007. doi: 10.1002/bies.20659. [DOI] [PubMed] [Google Scholar]
- 49.Singh I, Moser AE, Goldfischer S, Moser HW. Lignoceric acid is oxidized in the peroxisome: implications for the Zellweger cerebro-hepato-renal syndrome and adrenoleukodystrophy. Proc Natl Acad Sci USA 81: 4203–4207, 1984. doi: 10.1073/pnas.81.13.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes 54: 333–339, 2005. doi: 10.2337/diabetes.54.2.333. [DOI] [PubMed] [Google Scholar]
- 51.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127: 397–408, 2006. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 52.St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM. Bioenergetic analysis of peroxisome proliferator-activated peceptor γ coactivators 1α and 1β (PGC-1α and PGC-1β) in muscle mells. J Biol Chem 278: 26597–26603, 2003. doi: 10.1074/jbc.M301850200. [DOI] [PubMed] [Google Scholar]
- 53.Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PRG, Swanson MS, Houmard JA. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab 282: E1191–E1196, 2002. doi: 10.1152/ajpendo.00416.2001. [DOI] [PubMed] [Google Scholar]
- 54.Wakayama Y. Peroxisomes in regenerating human skeletal myofibers. Acta Anat (Basel) 136: 121–124, 1989. doi: 10.1159/000146809. [DOI] [PubMed] [Google Scholar]
- 55.Wanders RJ, Ferdinandusse S, Brites P, Kemp S. Peroxisomes, lipid metabolism and lipotoxicity. Biochim Biophys Acta 1801: 272–280, 2010. doi: 10.1016/j.bbalip.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Wanders RJ, Barth PG, van Roermund CW, Ofman R, Wolterman R, Schutgens RB, Tager JM, van den Bosch H, Bolhuis PA. Peroxisomes and peroxisomal functions in muscle: studies with muscle cells from controls and a patient with the cerebro-hepato-renal (Zellweger) syndrome. Exp Cell Res 170: 147–152, 1987. doi: 10.1016/0014-4827(87)90123-6. [DOI] [PubMed] [Google Scholar]
- 57.Wicks SE, Vandanmagsar B, Haynie KR, Fuller SE, Warfel JD, Stephens JM, Wang M, Han X, Zhang J, Noland RC, Mynatt RL. Impaired mitochondrial fat oxidation induces adaptive remodeling of muscle metabolism. Proc Natl Acad Sci USA 112: E3300–E3309, 2015. doi: 10.1073/pnas.1418560112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]