Abstract
Skeletal muscles have a fundamental role in locomotion and whole body metabolism, with muscle mass and quality being linked to improved health and even lifespan. Optimizing nutrition in combination with exercise is considered an established, effective ergogenic practice for athletic performance. Importantly, exercise and nutritional approaches also remain arguably the most effective countermeasure for muscle dysfunction associated with aging and numerous clinical conditions, e.g., cancer cachexia, COPD, and organ failure, via engendering favorable adaptations such as increased muscle mass and oxidative capacity. Therefore, it is important to consider the effects of established and novel effectors of muscle mass, function, and metabolism in relation to nutrition and exercise. To address this gap, in this review, we detail existing evidence surrounding the efficacy of a nonexhaustive list of macronutrient, micronutrient, and “nutraceutical” compounds alone and in combination with exercise in relation to skeletal muscle mass, metabolism (protein and fuel), and exercise performance (i.e., strength and endurance capacity). It has long been established that macronutrients have specific roles and impact upon protein metabolism and exercise performance, (i.e., protein positively influences muscle mass and protein metabolism), whereas carbohydrate and fat intakes can influence fuel metabolism and exercise performance. Regarding novel nutraceuticals, we show that the following ones in particular may have effects in relation to 1) muscle mass/protein metabolism: leucine, hydroxyl β-methylbutyrate, creatine, vitamin-D, ursolic acid, and phosphatidic acid; and 2) exercise performance: (i.e., strength or endurance capacity): hydroxyl β-methylbutyrate, carnitine, creatine, nitrates, and β-alanine.
Keywords: nutrients, metabolism, exercise, skeletal muscle, nutraceuticals
skeletal muscle represents the largest organ in the body, comprising ~40% of whole body mass (123). The functions of skeletal muscle extend beyond the widely recognized role of locomotion, serving as the body’s largest tissue for glucose storage and utilization (101, 121) and a primary site of lipid metabolism (104). Muscle also stores ~40% of total body amino acids (AA), which can act as a source of fuel and an AA substrate for other tissues in times of illness or fasting via release of glucogenic, ketogenic AA (264). Changes in muscle mass are regulated by dynamic turnover of the muscle protein pool (~1–1.5%/day), with skeletal muscle mass remaining constant when muscle protein synthesis (MPS) and muscle protein breakdown (MPB) are in balance (8). During situations of muscle growth [e.g., resistance exercise training (RET) combined with AA substrate], net MPS exceeds MPB (8). Conversely, net MPB is greater than MPS in conditions of muscle loss (e.g., bed rest, cachexia, and sarcopenia (75)); in humans, such wasting conditions are typically due predominantly to reduced MPS under fasted and/or fed conditions (191)). In addition to the regulation of muscle and function being clinically relevant, optimal strategies to promote growth, maintenance of muscle mass, and exercise performance (i.e., strength and endurance capacity) are of great interest to performance scientists. Therefore, a major area of interest surrounds the role of macronutrients, micronutrients, and nutraceuticals that influence muscle metabolism and function.
The consumption of nutritional supplements with “ergogenic” claims occurs in many populations, including athletes (186), the elderly (24), chronic disease sufferers (78), and sedentary (201) adults, often without sound empirical evidence. As such, there is a need to review the continually growing area of nutrients/nutraceuticals and associated mechanisms on aspects of skeletal muscle health to formulate evidence-based recommendations. Indeed, previous reviews have summarized the effects of multiple nutrient/nutraceutical compounds on aspects of skeletal muscle metabolism and exercise performance (53, 171). Often such reviews target a specific population (e.g., athletes), end point (i.e., aerobic performance), or dosing regime (e.g., timing and amount). As such, the present review adopts a more wide-ranging scope, including data irrespective of age, training status, or other independent variables, to highlight universal skeletal muscle effects of each nutritional compound.
Herein, we detail existing evidence for a nonexhaustive list of established and emerging nutrients in relation to some or all of the following end points: 1) muscle mass, 2) metabolism (protein and fuel), and 3) exercise performance (i.e., strength and endurance capacity). Since nutrition and exercise are the two key modifiable lifestyle factors for maintaining muscle health, this review will critique available literature examining the muscular responses to nutrient supplementation alone, nutrient supplementation plus acute exercise, and chronic nutrient supplementation combined with chronic exercise training (i.e., >1 bout of exercise). We shall include responses to both resistance exercise (RE)/RET and endurance exercise (EE)/endurance exercise training (EET) since exercise mode may differentially influence muscular responses to nutrition. Finally, because of the emerging nature of some nutrients, where mechanisms have not been well defined in humans, data from other models (e.g., cell/rodents) have been drawn upon where necessary. Therefore, this review should be of interest to scientists, clinicians, and athletes aiming to optimize muscle mass and function in clinical and athletic populations. Out of the scope of this review are a selection of established nutrients with purported effects on muscle (e.g., caffeine and green tea) due to the large volume of existing review literature available. Furthermore, some emerging nutrients (e.g., tomatidine and minerals) have been omitted from this review due to the paucity of existing literature. Therefore, we direct readers to the following publications for further reading regarding nutrients not discussed herein (53), in particular, caffeine (96), green tea (114), tomatidine (69), and minerals (209). Since we have not performed a systematic analysis, we apologize to those whose work we have not alluded to. Finally, in reading this review, we urge readers to refer to Supplemental Table S1 (all Supplemental Material for this article is available online at the AJP-Endocrinology and Metabolism website) as a resource, which illustrates source data relating to the impact(s) upon skeletal muscle mass, metabolism, and performance.
Definitions of Macro/Micronutrients and “Nutraceuticals”
From the outset, it is important that we define what is meant when we refer to macronutrients, micronutrients, and nutraceuticals, since the classification can be misinterpreted due to obscure classification boundaries. Proteins, fats, and carbohydrate (CHO) are required by the body in large amounts (i.e., g·kg−1·day−1), and therefore they are termed macronutrients (139). Micronutrients are defined as vitamins and trace elements (minerals) (212, 213) that are essential to our diet, albeit in small amounts (i.e., mg·kg−1·day−1), to maintain normal physiological and metabolic function. “Nutraceuticals” is an emerging term within the scientific literature that has not been well defined. A recent review defined a nutraceutical as a nutrient compound “with added extra health benefits” (i.e., in addition to the basic nutritional value contained in foods) (210). For the purpose of this review, we define a nutraceutical as “a compound that alone or in tandem with exercise impacts major physiological end point(s)” e.g., effectors of whole body metabolism, skeletal muscle mass, and/or whole body/muscle function.
Established Macronutrients and Exercise
Providing a mixed macronutrient feed containing protein, CHO, and fat stimulates MPS (200). The absolute stimulation of MPS is highly dependent on the AA content, with the provision of AA alone being sufficient to maximally stimulate MPS (15); this effect is entirely attributable to the essential AA (EAA) (218). Of the EAA, the branched-chain AA (BCAA) provide the most potent anabolic stimulation (9), particularly leucine (9, 256). This stimulation of MPS by AA is highly dose dependent and saturable, with maximal stimulation provided by between 20 and 40 g of high-quality protein (166, 167, 230, 263) or 10–20 g of EAA (58). Furthermore, this MPS stimulation is finite, where following an initial lag period of ~30 min during intravenous infusion (or ~45–60 min following oral ingestion to allow for the digestion, absorption, and transport of AA into the systemic circulation) the rate of MPS is increased approximately two- to threefold, reaching a maximum by 1.5–3 h. Subsequently, rates of MPS return to baseline (~2–3 h postingestion) despite continued plasma and muscle AA availability and elevated anabolic signaling (7). Thereafter, muscle remains refractory to further stimulation for an as yet undefined period, a phenomenon coined “muscle full” (7). This ~2- to 3-h period of MPS stimulation can be extended, depending on the type and dose of AA and macronutrient coingestion in combination with resistance exercise (RE) (51). The timing of protein ingestion in close proximity with the performance of acute RE, which when performed alone stimulates MPS for ~48 h (190), is thought to be important. This is because there is an enhanced sensitivity of the muscle to the anabolic properties of AA for ≥24 h postexercise (36), synergistically impacting MPS. However, protein ingestion before (236), during (14), and 1 or 3 h (199) after RE have all elicited similar postexercise increases in MPS.
The mechanisms underlying the anabolic effects to nutrition involve both the stimulation of MPS (200) and suppression of MPB (255); however, it is generally accepted that increases in MPS are the primary driver (8). Following transportation into the muscle cell, leucine in particular stimulates mammalian target of rapamycin complex 1 (mTORC1) (9), which is considered a key regulator of cell growth. mTORC1 activation leads to the phosphorylation of the downstream translation eukaryotic initiation factor 4E-binding protein (4E-BP1) and 70-kDa ribosomal protein S6 kinase 1 (p70S6K1) (see Fig. 1), stimulating the binding of eukaryotic initiation factors 4A (eIF4A) and 4E (eIF4E) to 4G (eIF4G) to form the 4F (eIF4F) complex (135). The eIF4F complex promotes the assembly of the 48S preinitiation complex via mediating the binding of mRNA to the 43S preinitiation complex, thereby promoting MPS (135). Currently the AA sensor coupling intracellular AA signaling to mTORC1 remains to be fully defined, although Rag GTPases (207), leucyl-tRNA synthetase (105), and sestrin2 (265) are all proposed candidates. This has led to intense interest in the development of novel leucine-enriched supplementation regimes to aid maintenance of muscle mass (44, 249). Unlike dietary protein, neither fats nor CHO lead to a direct stimulation of MPS (91, 95, 138); nonetheless, they can influence the bioavailability of AA when provided as part of a mixed meal, slowing plasma AA appearance and increasing AA retention (84) without blunting muscle anabolism (95). Finally, CHO (as well as AA; see Refs. 172 and 173) are insulin secretagogues, positively impacting net muscle anabolism via inhibition of MPB (255) (rather than stimulation of MPS; see Refs. 102 and 255).
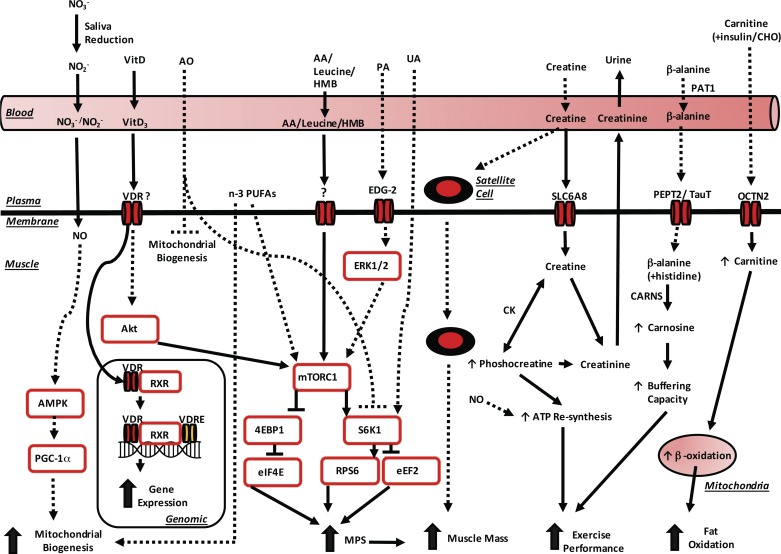
Fig. 1.
Proposed metabolism and mechanisms of action for nutrients/nutraceuticals. Solid arrows, activation; Solid verticle line perpendicular to solid horizontal line, inhibition; dashed arrows, purported activation; dashed vertical line perpendicular to dashed horizontal line, purported suppression; question mark, unknown. n-3 PUFA, n-3 polyunsaturated fatty acids; RXR, retinoid X receptor; 4E-BP1, eukaryotic initiation factor 4E-binding protein-1; AA, amino acids; AMPK, 5′-AMP-activated protein kinase; AO, antioxidants; CARNS, carnosine synthase; CHO, carbohydrate; CK, creatine kinase; EDG-2, endothelial differentiation gene; eEF2, eukaryotic elongation factor 2; eIF4E, eukaryotic initiation factor 4E; HMB, β-hydroxy-β-methylbutyrate; MPS, muscle protein synthesis; mTORC1, mammalian target of rapamycin complex 1; , nitrate; , nitrite; NO, nitric oxide; OCTN2, organic cation transporter 2; PA, phosphatidic acid; PAT1, proton-coupled amino acid transporter 1; PEPT2, peptide transporter 2; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; RPS6, ribosomal protein S6; SLC6AS, solute carrier family 6, member 8; TauT, taurine transporter; UA, ursolic acid; VDR, vitamin D receptor; VDRE, vitamin D response elements; VitD, vitamin D; VitD3, active vitamin D.
Exercise combined with feeding extends the stimulation of MPS (59), thereby delaying the “muscle full” set-point (8). It is the cumulative stimulation of muscle protein turnover with repeated bouts of exercise and feeding that drives exercise-induced skeletal muscle remodeling and hypertrophy (29). The impact of macronutrient supplements on exercise adaptation is multifarious. It is established that CHO intake helps to spare muscle and liver glycogen stores while also leading to a more rapid recovery of these stores postexercise (47, 162). The benefits of chronic protein supplementation alongside exercise are more inconsistent, with a number of studies showing positive (120, 134, 259) or negligible findings (149, 198, 242). However, a recent meta-analysis suggested that, overall, protein supplementation does lead to an augmentation of muscle mass and strength gains during chronic RET (49). To conclude, it is now well established that macronutrients play key roles in promoting muscle mass maintenance/ growth and functional adaptations. Future work should focus on identifying the underlying cellular mechanisms and associated refractory period of “muscle full.”
Emerging Nutraceuticals and Exercise
Leucine metabolites.
Leucine as a BCAA can be metabolized within muscle, engendering the possibility that its metabolites harbor anabolic effects. For instance, the keto-acid derivative of leucine metabolism, α-ketoisocaproate (α-KIC), was shown to stimulate MPS when provided by infusion; however, this effect could simply be due to KIC being reversibly transaminated to leucine (74). However, there is good evidence of anabolic activities of the more distal leucine metabolite β-hydroxy-β-methylbutyrate (HMB) produced via cytosolic α-KIC dioxygenase (174). Ingestion of ~3 g of HMB in humans elicited comparable increases in MPS to 3 g of leucine while also suppressing MPB independently of insulin (256). Similarly to leucine, the stimulation of MPS by HMB is attributable to enhanced mTORC1 signaling (256). To understand the insulin-independent suppression of MPB associated with HMB, numerous molecular targets associated with different proteolytic pathways [beclin 1, calpain 1, muscle RING finger 1 (MuRF1), muscle atrophy F-box, and cathepsin L] have been investigated, although no detectable changes in the protein abundance or posttranslational modifications were observed (256). Although it has been shown previously that there is a disparity between protein breakdown and the abundance in proteolytic proteins (102), it should be noted that only small amounts of HMB (~5%) are generated from normal leucine metabolism (239a), meaning that to obtain 3 g of HMB (a commonly supplemented amount) one would have to consume 60 g of leucine (260). Thus, when supplementing with physiological doses of leucine, it is unlikely that HMB is the main anabolic constituent, hence the practical use of HMB as a stand-alone nutritional supplement.
Indeed, longer-term studies have found that HMB preserved muscle mass during periods of disuse (65), whereas year-long supplementation of HMB (plus arginine and lysine) in the elderly led to improved preservation of lean body mass, possibly due to an augmentation in muscle protein turnover (10), although since HMB was administered as part of a nutritional cocktail it is impossible to delineate whether HMB was solely responsible for the effects on lean body mass. However, recent meta-analysis of 287 elderly participants (147 HMB-supplemented and 140 controls) found that HMB supplementation led to greater gains in muscle mass compared with controls, indicating that HMB is an effective ergogenic aid, at least in the elderly population, for preventing the loss of lean body mass (268). These anabolic properties of HMB have also been suggested to facilitate favorable RET adaptations. For example, supplementation of HMB (3 g/day) with RET for between 4 and 7 wk led to heightened increases in muscle strength (181), lean body mass (261), and fat free mass (174) compared with RET alone. However, not all studies have reported positive effects; for instance, RET for 1 mo combined with between 3 and 6 g/day of HMB did not change parameters of body composition in RE-trained males (140). In this latter case, HMB was provided in its calcium form (CaHMB) (140), which compared with the free acid form (FA-HMB) may have lower bioavailability and, therefore, might not enhance anabolism to the same extent (although this premise remains to be tested) (82).
Another ergogenic effect of HMB is the purported ability to attenuate exercise-induced muscle damage. For example, oral HMB supplementation (3 g/day for 6 wk) in EE athletes attenuated the increase in creatine phosphokinase and lactate dehydrogenase (plasma markers of exercise-induced muscle damage) after a 20-km time trial run compared with placebo (136). This protective effect of HMB may be due to HMB being a precursor of de novo cholesterol synthesis (175), which is critical for cell membrane (sarcolemmal) maintenance. Thus, HMB may maintain muscle membrane integrity during bouts of damaging exercise.
Furthermore, HMB has been shown to be efficacious for improving EE performance. For example, Vukovich and Dreifort (246) reported that HMB in combination with EE prolonged the time to reach the onset of blood lactate accumulation and V̇o2peak, albeit via an unknown mechanism. Others have investigated markers of endurance performance following high-intensity interval training (HIIT) with or without HMB supplementation. To exemplify, following 5 wk of HIIT-based running in combination with 3 g/day ca-HMB, V̇o2max improved more compared with placebo (144). These authors speculated that the performance benefits were attributable to the preservation of the cell membrane; however, membrane stability was not measured in the study, and thus no mechanistic conclusions can be drawn. Furthermore, HMB in untrained participants potentiated the effects of HIIT on physical working capacity at the onset of neuromuscular fatigue compared with HIIT training alone (163).
In summary, the literature supports a role for HMB supplementation in promoting 1) muscle mass, demonstrated by the preservation or increase in muscle mass when combined with RET; 2) muscle metabolism, since HMB stimulates MPS and inhibits MPB; and 3) aerobic and strength performance. However, data reporting negligible effects of HMB do exist (140, 214); prior exercise training history and/or being accustomed to an exercise stimulus may determine the effectiveness of the intervention. This is supported by evidence that HMB supplementation combined with RET in trained individuals had no effect on muscle strength or lean body mass vs. placebo (214). Further research is warranted that rigorously investigates 1) the mechanisms regulating the insulin-independent suppression of MPB associated with HMB supplementation, 2) the effects of novel and accustomed exercise in combination with HMB on endurance performance, and 3) the effects of EET and HMB on muscle mass.
Creatine.
Creatine (Cr) is an endogenously formed metabolite synthesized from arginine, glycine, and methionine (20). Found almost exclusively in skeletal muscle, Cr levels can be increased via endogenous synthesis in the liver and pancreas or exogenously from foodstuff, particularly meat and fish (43, 99). Following oral consumption of Cr, Cr is absorbed into the systemic circulation and is taken up by skeletal muscle via the sarcolemal Na+/Cl−-dependent transporter soluble carrier family 6 member 8 (126). Intramuscular Cr can then be phosphorylated to phosphocreatine in a reversible reaction facilitated by the enzyme creatine kinase. During high energy demands, the phosphate of phosphocreatine plus free ADP is used for ATP synthesis (126). Another fate of intramuscular Cr is the conversion to the end product creatinine, which due to its muscle exclusivity correlates with muscle mass (110). Creatinine diffuses out of the muscle cell and is removed from the body via urine (126). Oral Cr administration (20–30 g/day for ≥2 days) increases total muscle Cr stores by >20%, of which 20–30% is stored in the form of phosphocreatine (PCr) (107). The greatest Cr loading effects are seen in those with the lowest basal Cr pool levels, i.e., vegetarians (99); thus basal muscle Cr levels are an important determinant of Cr uptake (43, 107). The ergogenic effects of Cr are facilitated by elevated resting PCr, which sustains PCr-mediated ATP resynthesis during intense anaerobic exercise (42) primarily in fatigue-susceptible type II fibers (43), thus improving acute high-intensity performance. Increased basal muscle PCr levels also expedite the replenishment of PCr stores during recovery from intense exercise, leading to improved performance over repeated bouts of sprint exercise (43, 99). For example, 20 g/day of Cr for 5 days led to sustained isokinetic torque compared with placebo during repeated bouts of maximal voluntary contractions (100). Similar results have been obtained when different exercise modes such as cycling are employed (18, 70). In contrast, some studies have shown no effect of Cr supplementation on exercise performance (55, 170, 219, 234). For example, despite increased total muscle Cr following 5 days of 30-g Cr (and 30-g dextrose) supplementation, there were no improvements in sprint exercise performance (219). A lack of ergogenic effect may be attributable to the small total muscle Cr levels of ~12 mmol/kg dry wt (219), where previous reports show that a total Cr of >20 mmol/kg dry mass results in ergogenic benefit (42). Factors affecting the extent to which muscle Cr stores increase are not well known, although preexisting muscle Cr, exercise (107), and CHO ingestion (98) may be potential factors. Also with regard to performance, Cr supplementation improves the rate of functional recovery following exercise (54), which might be mediated by Cr promoting gene expression, thereby aiding MPS during the recovery periods (54, 258), ultimately increasing the deposition of newer functional proteins for improved functional recovery. Indeed, Cr supplementation will also increase muscle PCr, which might increase local rephosphorylation from ADP to ATP (54), thus providing more energy for contraction. As such, performance during successive bouts is maximized (i.e., can work at higher training loads), which in turn may contribute to the gains in strength observed when combined with RET (31, 63, 66).
In addition to energetic impacts, evidence supports a role for chronic Cr supplementation, typically provided as a loading dose (i.e., ~5 days of 20–30 g) followed by maintenance doses (~5 g) (32), for increasing muscle mass (25, 31, 245). For example, 12 wk of RET plus Cr (25 g/day for the 1st wk, followed by a maintenance dose of 5 g/day for the rest of the training duration) resulted in significantly greater fat-free mass, strength, and fiber cross-sectional area gains compared with placebo (245). Similarly, 14 wk of whole body RET (3 times/week) combined with Cr (5 g/day plus 2 g of dextrose) led to significantly greater gains in fat-free mass (31). Furthermore, a recent meta-analysis concluded that Cr supplementation combined with RET elicited further increases in fat-free mass compared with RET alone (albeit in older adults) (66). This meta-analysis reported a weighted mean difference of 1.33 kg for RET combined with Cr (66) compared with 0.69 kg for RET with protein (49), demonstrating the potent ergogenic effect of Cr on fat-free mass. The mechanisms regulating the effects of Cr on muscle mass remain to be fully elucidated, although it is known that acute provision of Cr does not directly stimulate MPS either with (152) or without RE (153). However, Cr did augment the satellite cell (SC) response following RE (178), which may contribute to hypertrophic gains since increased SC content is observed following chronic RET (241). Although the contribution of SC to hypertrophy is still debated (158), theoretically, the nucleus content in hypertrophying muscle fibers becomes diluted such that additional nuclei are required for continued growth. As such, SCs fuse and donate nuclei to the preexisting muscle fibers, thereby increasing the transcriptional capacity of the muscle cell and thus the potential for growth (30). Additionally, augmented PCr availability and ATP resynthesis during intense exercise likely permits greater work output. Greater work may be a factor that stimulates greater muscle gene expression, thereby promoting muscle mass accretion observed with Cr supplementation (32, 204, 257). It is possible that changes in fat-free mass may be in part attributable to the osmotic potential of elevated intracellular Cr, leading to myocellular water retention (204, 273). This potential increase in cell volume from Cr-induced fluid retention may then act as an anabolic signal, activating intracellular signaling cascades that maintain cellular function (204). For example, the attachment complex protein focal adhesion kinase, which is critical for osmosensing and hypertrophic signaling (56), is upregulated following Cr supplementation (204).
To summarize, Cr supplementation is capable of increasing total muscle Cr stores, which improves performance via maintaining PCr-mediated ATP resynthesis, although not all studies have shown improved exercise performance. Beyond performance, chronic Cr supplementation combined with RET is capable of stimulating muscle mass accretion. Although acute effects of Cr supplementation on MPS are not shown, potentiating RET capacity and enhanced recovery likely mediates increased muscle mass. Further studies are needed to firmly establish factors that determine the variability of Cr storage in muscle, since this could have implications for optimizing the dosing regime of Cr.
Carnitine.
Carnitine is synthesized endogenously from AA precursors and can also be obtained exogenously from the diet, particularly red meat, with the majority of whole body carnitine (95%) being stored in skeletal muscles (26). Carnitine has well-documented roles in regulating the translocation of long-chain fatty acids into the mitochondrial matrix for subsequent β-oxidation (223). This process is regulated via the mitochondrial enzyme carnitine palmitoyltransferase (CPT) 1 catalyzing the esterification of carnitine with long-chain acyl-CoA (223). The long-chain acylcarnitine is transported across the mitochondrial membrane into the mitochondrial matrix concurrently with the exchange of free carnitine from the mitochondrial matrix (94). Inside the mitochondrial matrix, acylcarnitine is transesterified to long-chain acyl-CoA and free carnitine via CPT2 (223). Subsequently, the long-chain acyl-CoA is able to undergo β-oxidation. Readers are directed toward the review by Stephens et al. (223) for a more comprehensive overview regarding the role of carnitine in fatty acid translocation.
Therefore, increasing muscle carnitine content could hypothetically enhance fat oxidation while sparing glycogen, therein posing an attractive ergogenic strategy for delaying fatigue during prolonged aerobic exercise and aiding body weight control by promoting fat oxidation. However, a number of studies have failed to increase muscle carnitine via intravenous infusion despite increasing plasma carnitine availability (225). Similarly, oral consumption of carnitine acutely (220) and chronically (247) failed to increase muscle carnitine levels. It is likely that the poor bioavailability of oral carnitine and rapid urinary clearance (106) explain, at least partly, why carnitine supplementation alone does not increase muscle carnitine stores (225). Consequently, several strategies have been tested to stimulate muscle carnitine accretion; concurrent hyperinsulinemia and hypercarnitinemia increased human muscle carnitine content by ~15% (225), and carnitine plus CHO supplementation promoted muscle carnitine accretion (211). Mechanisms by which insulin can facilitate increased muscle carnitine are purported to be due to insulin increasing Na+-dependent active transport of carnitine into the muscle via organic cation transporter (OCTN2) (225). Similarly, Na+-dependent uptake of AA (274) and Cr (97) by skeletal muscle is increased by insulin, thereby supporting the proposed mechanisms of carnitine uptake (225). However, CHO in addition to protein blunts the stimulation of muscle carnitine uptake (211). This was previously suggested to be related to AA inhibiting carnitine intestinal absorption (233); however, since the combination of CHO and protein led to greater plasma and urinary carnitine vs. CHO alone, this suggests otherwise (211). The precise mechanisms underlying the blunting effect of protein on carnitine uptake into skeletal muscle remain to be fully identified.
By increasing muscle carnitine content, human fuel metabolism can be manipulated. For example, acute increases in resting skeletal muscle carnitine content led to an inhibited glycolytic flux (denoted by reduced lactate) and CHO oxidation (demonstrated via reduced pyruvate dehydrogenase complex activity) concurrent with increased muscle glycogen and long-chain acyl-CoA accumulation (224). Therefore, these studies support the notion that carnitine can enhance fat oxidation while sparing glycogen. A subsequent study by the same group found a 30% increase in muscle carnitine content following dietary carnitine (1.36 g) and CHO (80 g) twice a day for 6 mo and an ~55% reduction in glycogen use during low-intensity exercise (30 min cycling at 50% V̇o2max) compared with controls (250). Additionally, following 3 mo of supplementation, carnitine and CHO feeding prevented the 2-kg increase in body mass, which was seen in the control group (250). The authors speculate that the lack of increase in body mass in the carnitine group may be due to carnitine-induced increases in long-chain fatty acid oxidation (250).
Subsequent studies have supported the role of carnitine combined with CHO for the prevention of fat gain, which was associated with increased fat oxidation during low-intensity exercise (227). Conversely, increased CHO but not fat oxidation during steady-state exercise has been reported following 2 wk of carnitine supplementation (3 g/day carnitine and tartrate combined with CHO meals) (1), and 1 mo of carnitine intake (3 g/day carnitine and tartrate) had no effect on substrate oxidation during steady-state exercise (27). These findings conflict with those reported at rest and differ from hypotheses suggesting that limited carnitine availability may limit fat oxidation during exercise (224). Interestingly, in the study by Broad et al. (27), there was no mention of daily carnitine supplementation being coingested with supplemental CHO, which is critical for increasing muscle carnitine stores (226). Therefore, the protocol might have been suboptimal for increasing muscle carnitine stores, which was not measured within the study, and thus may explain the negligible effect of carnitine on substrate utilization.
Thus, insulin-stimulated carnitine uptake is capable of increasing muscle carnitine stores which promotes fat oxidation, spares muscle glycogen, and thereby improves endurance performance. Further work is required to fully elucidate the mechanisms regulating the blunting of carnitine uptake when combined with CHO and protein.
n-3 polyunsaturated fatty acids.
n-3 Polyunsaturated fatty acids (n-3 PUFA) contain a double bond at the third carbon atom from the end of the carbon chain. Abundantly found in walnuts and oily fish, there are three types of n-3 PUFA: 1) α-linoleic acid (ALA), 2) eicosapentaenoic acid (EPA), and 3) docosahexaenoic acid (DHA). n-3 PUFA serve well-established roles as critical components of cell membranes and as substrates for lipid signaling (37). Early evidence demonstrated a role for n-3 PUFA in muscle anabolism when n-3 PUFA-enriched feed provided to growing steers increased the phosphorylation of anabolic signaling and the nonoxidative whole body disposal of AA, which was representative of increased whole body protein synthesis (85). Additionally, fish oil containing 18% EPA attenuated the loss of skeletal muscle following 30% burn in guinea pigs, which may be mediated by EPA reducing inflammatory related prostanoids (4). Hence, there is interest in the application of n-3 PUFA as a nutritional supplement in humans. It has been suggested that fish oil supplementation in humans may increase muscle n-3 PUFA content (160), have anti-inflammatory properties (128) via reduced leukotriene B4 formation (an inducer of inflammation) (79), and attenuate the loss of muscle mass in disease states, possibly via reductions in proinflammatory cytokines (203). Furthermore, n-3 PUFA might potentiate anabolic responses to nutrition in skeletal muscle. In support of this, 8 wk of n-3 PUFA supplementation (1.86 g of EPA + 1.5 g DHA/day) was shown to augment hyperaminoacidemia/hyperinsulinemia-induced increases in mixed MPS compared with corn oil controls in young, middle-aged, and older adults (215, 216). Indeed, enhanced phosphorylation of mTORC1 and the downstream target p70S6K1 were observed in young, middle-aged, and older adults (215, 216). However, MPS increases were observed in the context of hyperaminoacidemia and hyperinsulinemia, which may not be physiologically obtainable. Moreover, supplementation of n-3 PUFA for 3 (151) and 6 mo (217) led to increases in muscle mass and function in older adults. A recent study in C2C12 skeletal muscle cells found a 25% increase in MPS following EPA that was not observed following DHA (131), suggesting that EPA may be the more anabolic constituent of n-3 PUFA. Interestingly, both EPA and DHA stimulated p70S6K1, and thus EPA might stimulate MPS via a p70S6K1-independent mechanism (131).
Despite being less well defined, these positive effects of n-3 PUFA on muscle appear to be recapitulated when combined with exercise (202). Supplementation during 3 mo of RET promoted increases in muscle strength in older women (202), suggesting that n-3 PUFA could have a positive role on muscle protein metabolism by enhancing the anabolic response to RE (90). Despite recent contrasting findings that chronic fish oil supplementation failed to increase muscle anabolism in younger people under rested and exercise-trained conditions (161), the lack of pre- and postintervention measurements confound interpretation of these results. Additionally, positive findings regarding the efficacy of n-3 PUFA supplementation have been observed largely in older adults. Because aging associates with blunted anabolic responses to AA and exercise, the muscular benefits of n-3 PUFA may be more pronounced in those in whom anabolic responses are already suboptimal.
Although the combination of EE and n-3 PUFA has not been investigated in the context of muscle mass and protein metabolism, there is sound evidence to suggest that n-3 PUFA supplementation may alter fuel metabolism by improving metabolic flexibility, i.e., the ability to switch between using fat or CHO as a fuel source. For example, 6 g/day of fish oil for 3 wk led to a 35% increase in fat oxidation following a glucose or fructose bolus (61). In the context of exercise, 3 wk of fish oil supplementation (6 g/day) led to a nonsignificant trend for greater fat oxidation during an acute bout of cycling (90 min at 60% O2 output), a possible compensatory response for the lower CHO oxidation (62). Further studies have found significantly greater fat oxidation during EE in humans following 3 wk of fish oil supplementation (119). Although each of these studies lacked comprehensive investigation into the mechanisms regulating changes in metabolic flexibility, n-3 PUFA have been shown to mediate the upregulation of genes regulating mitochondrial biogenesis, such as peroxisome proliferator-activated receptor-α and -γ and the transcription factor nuclear respiratory factor 1 in mice (146), offering a potential explanation for these findings. Additionally, rats fed a low-fat diet supplemented with DHA had higher oxygen consumption and apparent Km for ADP in permeabilized muscle fibers compared with placebo, which was indicative of improved mitochondrial function (103). Thus, effects on mitochondrial biogenesis and function may underpin the synergistic effects of n-3 PUFA and EE-associated metabolic adaptation.
Collectively, n-3 PUFA supplementation beneficially affects muscle protein metabolism, which may contribute to chronic gains in muscle mass, and also shows promise for impacting metabolic flexibility. Further human research that investigates the effects of EPA and DHA individually on aspects of skeletal muscle health is warranted to establish which is the main anabolic constituent.
Nitrates.
Nutrients that contain dietary inorganic nitrates (e.g., beetroot and lettuce) or related precursors (e.g., arginine) can increase nitric oxide (NO) availability, which is capable of modulating muscle-related processes, including contraction, glucose homeostasis, blood flow (127), and satellite cell activation (5, 35). Following oral ingestion of dietary nitrate-rich foods, nitrate () is reduced to nitrite () via nitrate reductases within the mouth (68). Subsequently, is converted into NO and additional reactive nitrogen species in the acidic environment of the stomach (2). Oral - increases plasma and levels, indicating that nitrates are bioavailable. With regard to muscle protein turnover, these compounds are thought to promote anabolism via improving blood flow (through increased NO production), thus enhancing nutrient delivery to the muscle, providing more substrates for MPS. However, it has been shown on several occasions that enhanced muscle blood flow does not augment anabolic responses in young or older males (164, 187–189). Nonetheless, dietary arginine (the principle substrate for endothelial nitric oxide synthase for endogenous production of NO) supplementation did increase the weight of the soleus and EDL muscle in obese rats (125). However, in humans, Luiking et al. (154) and Tang et al. (232) found that oral arginine (10 g), of which ~70% is bioavailable following ingestion, had no effect on muscle blood flow or MPS when provided alone or in combination with AA or acute RE. In contrast, vasodilatory effects of arginine have been shown when administered by intravenous (iv) infusion at higher doses (30 g) (23). By comparison, the peak in plasma arginine was considerably lower following 10 g of oral arginine (~225 µmol/l) (232) vs. 30 g iv infused arginine (~6,223 µmol/) (23), and thus the dose of arginine used by Luiking et al. (154) and Tang et al. (232) may not have been sufficient to increase plasma arginine to an amount that elicits effects on vasodilation. In fact, these authors projected that, on the premise of 70% bioavailability, a total of ~43 g of oral arginine would have been required to reach similar plasma levels reported following iv infusion (232). An alternative may be to utilize the arginine precursor citrulline (156), which bypasses splanchnic extraction (267). Supplementation of citrulline in rodents was shown to stimulate MPS (179) via the mTORC1 pathway (193). However, similar effects have not been observed in humans, since there was no additional impact of citrulline (10 g), when coingested with whey, on MPS or blood flow with or without acute RE vs. whey combined with nonessential AA (52). Finally, flavanols such as in cocoa (39, 109) also promote vasodilation via NO pathways (80, 132). It was reported recently that despite an acute dose of cocoa flavanols (350 mg) increasing macro- and microvascular blood flow, this was not associated with enhanced muscle anabolic responses to nutrition (188), suggesting that in healthy individuals nutrient delivery is not rate limiting for muscle anabolism (189).
In contrast to muscle mass and strength-related studies, a plethora of research has investigated the effects of nitrates and EE on whole body metabolism and endurance performance. An early study by Larsen et al. (148) reported that sodium nitrate supplementation reduced the O2 cost of submaximal cycling exercise, whereas similar results have been reported following nitrate-rich beetroot juice supplementation (11), which is indicative of improved aerobic metabolism or mechanical efficiency (147). In addition to metabolic improvements, nitrate supplementation provided in the form of 500 ml of beetroot juice improved 4- and 16.1-km cycling time trial performance in trained cyclists (145). These improvements are likely attributable to an enhanced rate of PCr recovery (239) increasing the rate of ATP synthesis, although this mechanism remains speculative at present. Emerging evidence from cell culture studies suggests that nitrate supplementation enhances mitochondrial biogenesis and oxidative metabolism via increased 5′-adenosine monophosphate-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor-γ coactivator-1α gene expression (240), although in vivo data is lacking. Although others have also reported nitrate-mediated improvements in EE performance (169, 269), several authors have shown no improvements (6, 48, 254). For example, consuming 140 ml of beetroot juice 2.5 h before a 1-h cycling time trial did not improve time trial performance in trained cyclists compared with placebo (48). These discrepant findings may be explained by methodological differences such as the dose of nitrates (since the increase in plasma and is somewhat dose dependent; see Ref. 270), control of nitrate intake, the source of nitrates provided, and the training status of the participants. For example, since numerous studies demonstrate nitrate supplementation to have no beneficial effect on performance in well-trained participants (6, 48, 254), it is likely that fitness status influences the ergogenic potential of nitrate supplementation (127). Indeed, higher plasma levels of were present in trained vs. untrained participants pre- and post-acute exercise (195). This may be explained by higher nitric oxide synthase activity (159) and/or higher plasma nitrate values (195) in trained participants.
Thus it is established that nitrates reduce the O2 cost of aerobic exercise. Further in vivo work is required to understand whether oral doses of arginine larger than those already tested can enhance vasodilation and effect protein metabolism across different ages. Furthermore, precise mechanisms regulating the nitrate-induced beneficial effect on O2 cost remain to be delineated in vivo.
β-Alanine and carnosine.
β-alanine (BA) is a β-AA produced endogenously in the liver and found primarily in meat (238). BA is the rate-limiting precursor for the synthesis of carnosine, which is a dipeptide of BA and histidine that improves the muscle buffering capacity (222). BA supplementation has generated interest as an ergogenic aid since early studies found BA supplementation to be capable of increasing muscle carnosine stores by ~40–65%, demonstrating good bioavailability, a consistent and reproducible finding (16, 108, 222), although the extent to which carnosine content increases may be dependent on the dosing protocol (108). Other factors have been shown to cause muscle carnosine variability, including sex, age, dietary BA intake, vegetarianism (76), and fiber type distribution, since carnosine content is double in type II compared with type I fibers (106a). The regulation of muscle carnosine stores from dietary/supplemental sources is still under investigation (222). Oral BA may be transported across the gut via the H+-coupled PAT1 AA transporter (235), which increases plasma availability of BA for muscle carnosine synthesis. Transport of BA into skeletal muscle has been shown to be regulated via both peptide transporter 2 (67) and the taurine transporter (237), although this remains to be confirmed in humans. Once within the muscle cell, BA and sarcoplasmic histidine synthesize carnosine via carnosine synthase (222).
Increased muscle carnosine stores may increase RE work capacity via regulation of the muscle-buffering capacity during RE, and therefore, interest in the potential of BA supplementation for promoting RET adaptations has been gained (133). However, 10 wk of RET combined with 6.4 g/day BA did not enhance body mass or strength changes in 26 males despite increased muscle carnosine (133).
During high-intensity exercise, the buildup of H+ ions reduces the intramuscular pH, leading to fatigue that is likely due to acidosis-induced reductions in ATP generation (205). Increased muscle carnosine via BA supplementation is capable of reducing intramuscular acidity during high-intensity exercise, therefore enhancing exercise performance (57, 112, 229). For example, 4 and 10 wk of BA supplementation increased cycling capacity (total work done) in untrained males when cycling at 110% of maximum power (112), which is hypothesized to be due to improved intracellular buffering. In sprint-trained athletes, 4–5 wk of BA supplementation (4.8 g/day) led to increased knee torque but did not enhance sprint performance (64). Importantly, this study found increased muscle carnosine stores (+47%), demonstrating that it is possible to increase muscle carnosine even in trained athletes (64). Women supplemented with BA for 28 days delayed the onset of neuromuscular fatigue (denoted by improved ventilatory threshold, physical working capacity, and time to exhaustion), which was likely the result of improved intracellular buffering capacity (228).
BA supplementation is associated with paresthesia (i.e., flushing) following acute doses of ≥800 mg (60, 108). This side effect is deemed to be dose dependent and likely related to BA plasma kinetics (108). Compared with pure BA, slow-releasing BA capsules eliminate all paresthesia side effects, which is most likely explained by the attenuated BA plasma concentration and delayed time to peak (60), and thus offer a suitable alternative supplement option.
Therefore, BA supplementation may be implemented to increase muscle carnosine stores, which in turn enhances acute EE performance, which is mediated likely via an enhanced intracellular buffering capacity. However, the effects of BA combined with RET need to be studied further in vivo.
Micronutrients: Vitamins and Exercise
Vitamins are essential for many metabolic processes; however consuming vastly more or less than recommended can likely result in toxicity or deficiency, respectively (212), which can be detrimental for muscle health. For example, vitamin D (VitD) deficiency has been linked to muscle wasting (86), and as such, vitamins have been implicated in regulating muscle mass, metabolism, and performance, as discussed below.
Vitamin D.
VitD is a steroid hormone, the deficiency of which in humans throughout the world is reaching epidemic levels mostly because of reduced sun exposure (116). VitD deficiency is prevalent in many debilitating conditions, including osteoporosis and rickets (116, 117), and is associated with reduced muscle mass and strength (244). For example, rodent models have demonstrated that VitD deficiency induced muscle loss, a consequence of increased MPB and reduced MPS compared with controls (17). The VitD receptor (VDR) is present in many tissues, including muscle (89), which has led to increasing interest in the effects of VitD on muscle metabolism. Although conflicting reports exist regarding the presence of the VDR (192, 251), these discrepancies are most likely due to the use of nonvalidated antibodies, lack of controls, or differences in antibody specificity (89).
Following sun exposure or consumption of VitD-rich dietary sources/supplements, circulating VitD bound to VitD-binding protein increases and transports to the liver, where hydroxylation (via 25-hydroxylase) generates 25-hydroxyvitamin D. A second hydroxylation in the kidney (via 1α-hydroxylase) produces the biologically active form of VitD [1,25(OH)2D] (87). Mechanisms underpinning the effects of VitD on muscle metabolism are not fully understood but are believed to be in part related to the regulation of gene expression via the VDR or secondary messenger protein signaling (194). The binding of 1,25(OH)2D to the VDR causes conformational changes, allowing VDR to heterodimerize with the retinoid X receptor. This complex then binds to VitD response elements on the DNA, promoting gene transcription (45, 87). 1,25(OH)2D may also have nongenomic effects on intramuscular signaling by binding to a cell surface receptor (40), which in turn activates intracellular signaling pathways such as the Akt and mitogen-activated protein kinases (MAPK) pathway (33). For example, VitD treatment increased myotube size, downregulated myostatin (88), upregulated Akt (33), and sensitized the Akt/mTORC1 pathway and MPS responses to leucine and insulin (206) in muscle cell cultures. Thus, there is growing in vitro evidence for an anabolic role of VitD in skeletal muscle. In humans, supplementation of VitD has been proposed to increase muscle strength (13), function (83, 252), fiber area (46, 208, 221), and lean body mass (72) and reduce falls (83, 130), although a recent meta-analysis found no overall effects of VitD supplementation on muscle mass (13). Of importance, benefits of VitD supplements are observed particularly in the elderly or in those who are VitD deficient (13), which may be a potential explanation for some of the discrepant findings within the literature.
Since VitD supplementation has been suggested to promote muscle mass and function, concurrent VitD supplementation with RET may be expected to potentiate exercise-induced adaptations. Indeed, 4 mo of VitD3 supplementation (1,920 IU/day + 800 mg/day calcium) in combination with lower body RET for 3 mo led to a greater reduction in myostatin mRNA expression, a negative regulator of muscle mass, and a greater change in the percentage of type IIa muscle fibers in young males (3). However, these changes did not translate into greater muscle strength or hypertrophy above RET alone (3). Elderly adults undertaking RET combined with VitD improved muscle quality (strength/cross-sectional area) more so than young males, thus demonstrating that elderly individuals may benefit more from VitD supplementation (3). VitD-insufficient (according to VitD previously reported ranges; see Ref. 118) overweight and obese adults did not augment gains in lean body mass compared with placebo following 3 mo of RET and 4,000 IU/day VitD3 (41). This may be due to the fact that VitD is deposited in body fat, reducing bioavailability (266) and requiring greater levels of VitD supplementation to promote muscle anabolism in this population. Similarly, others reported no change in body composition after 9 mo supplementation of 400 IU/day and RET twice/wk in overweight males and females (34). Since no change in body composition was seen in the training only group either, these findings may result from low training adherence (~53%) (34).
Therefore, although there is some evidence to suggest an emerging role for the supplementation of VitD for the promotion of muscle mass and protein metabolism, more high-quality in vivo work is required. For example, investigations into the direct effect of VitD on MPS in humans are needed, as are more acute and chronic EE studies to understand the potential synergistic effects of VitD supplementation and exercise on muscle health. These studies need to be well controlled, accounting for basal VitD status, and should determine true VitD bioavailability.
Vitamins C and E (i.e., “antioxidants”).
High levels of free radicals (an atom with a single, unpaired electron) and reactive oxygen species (ROS) can disrupt protein homeostasis (196). This is likely due to ROS promoting catabolism via increases in the ubiquitin-conjugating activity (150) and diminishing anabolism via attenuation of MPS and signaling proteins (182), with evidence for these mechanisms arising from cell culture studies. Therefore, it is thought that consuming dietary antioxidants [i.e., vitamins C (VitC) and E (VitE)] that are capable of donating an electron to neutralize free radicals (168) may reduce ROS, thus minimizing disruption of protein homeostasis. For instance, a positive relationship was observed between VitC intake and appendicular lean body mass (209), which may be related to the fact that muscle is a major storage site for VitC (253).
However, physiological levels of ROS such as that produced during exercise (248) promote gene expression (e.g., manganese superoxide dismutase) (185) and cell signaling (e.g., c-Jun NH2-terminal kinases and MAPKs) (92, 185) in healthy skeletal muscle. Thus, it may be hypothesized that provision of antioxidants combined with RET could hamper exercise-induced adaptations. Human studies assessing the interactions of RET and antioxidant supplementation have produced varied results, with support for positive (22, 143), negative (19, 184), and negligible (21, 184) effects of antioxidants. For example, greater gains in fat-free mass were observed following 6 mo of RET combined with VitC (1,000 mg/day) and VitE (600 mg/day) compared with RET alone, which was postulated to be a result of antioxidants increasing protein synthesis, although this was not measured (22, 143). However, 3-mo supplementation of daily VitC (1,000 mg) and VitE (235 mg) alongside whole body RET led to blunted gains in total lean body mass and muscle thickness (19). Ten weeks of whole body RET combined with 1,000 mg of VitC and 235 mg of VitE daily found negligible effects on acute MPS and muscle mass; however, the phosphorylation of anabolic signaling proteins was blunted compared with placebo (184). Supporting the lack of ability to potentiate exercise-induced adaptations, RET and antioxidants increased fat-free mass but no more than RET alone (21). This may be a result of the low participant numbers or due to the fact that the participants were not vitamin deficient, and therefore, it may be that additional vitamin intake provides little or no added benefits. The absorption of antioxidants, particularly VitC, may also be limited (21), further reducing the antioxidant-induced anabolic potential. Another factor that may explain the efficacy of antioxidant supplements is the age of the participants, since the elderly have an altered redox status (184), which could impact the efficacy of the antioxidants.
Detrimental and negligible interactions have also been reported following EE and antioxidant supplementation (183, 272). For example, daily VitC (1,000 mg) and VitE (235 mg) during an 11-wk EE training program consisting of steady state and HIIT in humans led to blunted increases in mitochondrial protein content, which was indicative of blunted mitochondrial biogenesis, although no differences were observed in V̇o2max compared with placebo (183). Similarly, VitC hampered running time to exhaustion in rats, perhaps as a result of impaired mitochondrial biogenesis (93). Others have reported no alterations in EE-induced adaptations (measured as maximal O2 consumption, power output, and workload at lactate threshold) following antioxidant supplementation (272). Differences in the antioxidant dosing regimens might explain some divergent findings between studies (183). Thus, although VitC and VitE are vital for maintaining health, the benefits of supplementation are debatable and likely to depend on the age group and deficiency status. The poor bioavailability described in several studies may further impact any benefits of supplementation (21).
Currently, it is difficult to conclude whether antioxidant supplementation is beneficial or detrimental for muscle mass, protein metabolism, and performance/adaptation. Close et al. (53) highlighted that confusion and misguided conclusions are often drawn due to inappropriate methodological techniques. For example, the lipid peroxidation markers thiobarbituric acid reactive substances can be the result of non-redox-related sources and are thus no longer recommended for use as oxidative stress markers (81), yet they are often published in the context of antioxidant supplementation (111, 155, 157). It is believed that diets rich in fruits and vegetables as opposed to large supplemental doses of antioxidants are preferable since no investigations to date support attenuations in adaptations to training in response to fruits and vegetables, which have naturally occurring antioxidants (53).
Emerging Nutraceuticals
Ursolic acid.
Despite the paucity of research at present, other novel nutraceuticals have gained recent attention for their potential to promote muscle mass, protein metabolism, and/or exercise adaptations. For example, the naturally occurring phytochemical ursolic acid (UA) found in apple peel has drawn attention ever since UA-supplemented mice gained 7% muscle weight (142), suggesting that UA may be capable of promoting muscle hypertrophy (71, 124, 141, 142). UA-induced hypertrophic effects are proposed to be due to the attenuation of the atrophy-related genes MuRF1 and atrogin-1 and the upregulation in IGF gene expression (142). Contrary to this, UA incubations in cell cultures were reported to inhibit leucine-stimulated mTORC1 signaling by inhibiting mTORC1 localization to the lysosome (180), a key step in AA-induced anabolic signaling (207). Research is warranted to detail the effects of UA on muscle metabolism in humans.
With regard to exercise interactions, UA injection following RE in rats stimulated p70S6K1 at 1 h and was maintained 6 h later, which began the descent to baseline in the exercise-only group, reflecting prolonged mTORC1 activity and thus anabolic potential when RE was combined with UA (177). Despite an unclear mechanism, these authors speculated that IGF-I may contribute to the UA-induced p70S6K1 activation, and previous work supports this hypothesis (142). Contrary, data in humans (not in the context of UA) show no change in IGF-I but increased anabolic signaling after acute RE (28). In RE-trained males, RET six times/wk (at 60–80% of 1-RM) for 2 mo combined with 450 mg/day UA improved leg strength but had no effect on lean body mass, although RET alone also had no effects on lean body mass (12). This may be due the fact that the participants had >3 yr RET experience, and hypertrophic responses predominate in the early stages of RET (29). To the authors’ knowledge, no evidence exists regarding UA supplementation combined with EE. An important issue to consider is the low and variable bioavailability of UA following oral ingestion, which is likely due to its lack of solubility in aqueous solutions (113). This could markedly impact its potential as a nutraceutical. However, recent efforts have been made to improve the bioavailability of UA and other triterpenoids by, for instance, using nanoliposomes to aid solubility (271). The varied and low bioavailability of UA in humans is demonstrated by the lack of UA content in some participants following a 1-g oral dose, and in those that did display UA content, it was observed only ≤12 h postconsumption (113). Additional findings show that oral UA ingestion (3 g) led to increased plasma UA 2 and 6 h postexercise (50). As such, the true bioavailability of UA in response to time and dose should be investigated further.
Phosphatidic acid.
Phosphatidic acid (PA) is a diacyl-glycerophospholipid found endogenously in mammalian cell membranes that can be obtained exogenously from raw cabbage (231). Both endogenous and exogenous PA are believed to positively influence muscle protein metabolism, whereby endogenous PA can be increased by RE and binds directly to mTORC1, influencing MPS. Exogenous PA indirectly stimulates mTORC1 activation (77, 165) via extracellular-signal regulated protein kinase-dependent (262) and phosphatidylinositol-3-kinase-independent (176) mechanisms and may also attenuate MPB via attenuation of atrophy-related genes (210). Exogenous PA in cultured muscle cells also prevented atrophy in the presence of the atrophy-inducing substances tumor necrosis factor-α (TNFα) and dexamethasone (122). Recently, acute PA supplementation in rodents tended to increase MPS in the fasted state; however, PA blunted the whey protein-induced rise in MPS (165). Possibly the addition of PA to whey alters the pathways of mTORC1 activation, thus shifting peak MPS (165); research is needed to understand the signaling responses of PA alone vs. PA plus whey. In a human case study, orally ingested PA metabolized into lysophosphatidic acid (LPA) and glycerophosphate increased plasma PA and LPA 30 min postingestion (of 1.5 g of PA), which plateaued at 1–3 h and remained elevated above baseline at 7 h (197). Thus, it seems PA is bioavailable in humans, although beyond 7 h postingestion the bioavailability is unknown, and further studies with a larger cohort are needed to determine the true bioavailability of PA. PA supplementation (750 mg daily) combined with 2 mo of supervised whole body RET in RE-trained males found increased lean body mass and cross-sectional area compared with the placebo group (129). Conversely, others have shown nonsignificant increases (+2.6%) in lean body mass despite utilizing a similar RET and supplementation program (115). The differential findings between these studies may be due to the fact that training was unsupervised in the later study. To our knowledge, no data assessing the interactions of PA plus EE currently exist.
Combined Nutraceuticals
Although not the focus of this review, it is worth speculating that combining nutraceuticals may provide multiple benefits to skeletal muscle health or potentiate skeletal muscle health benefits in response to exercise. Consequently, some studies have investigated the potential of combined nutritional “cocktails.” For example, a supplement containing PA, HMB, and VitD in combination with 2 mo of RET led to greater gains in lean body mass and strength compared with the placebo group, providing support that the combined supplement possessed anabolic properties (73). The combination of VitD, leucine, and whey twice daily in tandem with RET three times/wk for 13 wk prevented the loss of appendicular muscle mass during intentional weight loss in obese males and females (243). The caveat with implementing combined nutritional supplementation is that it is difficult to attribute changes in the end point to the responsible individual or combination of nutrients unless rigorous study designs are implemented with adequate control groups.
Conclusion and Future Directions
Although it is extremely unlikely that a single nutraceutical will prove to be a “magic bullet,” it is clear that certain nutraceuticals, under certain conditions, do indeed possess ergogenic potential. Of the nutrients discussed herein, strong evidence exists for leucine, HMB, and Cr for muscle mass, leucine and HMB for protein metabolism, carnitine for fuel metabolism and leucine, and HMB, carnitine, Cr, nitrates, and β-alanine for athletic (strength or endurance) performance. Further empirical in vivo evidence is required to firmly establish the currently emerging roles of VitD, UA, and PA for promoting muscle mass and n-3 PUFA, UA, and PA for muscle protein metabolism. This review highlights 1) the need for better-controlled, longer-duration human studies that investigate the role of individual nutrients on muscle mass, protein/fuel metabolism, and indices of exercise performance/adaptation, 2) the lack of in vivo “mechanistic” studies, and 3) the need to determine the bioavailability of emerging nutrients. As mentioned in the introduction to this review, please refer to Supplemental Table S1 for a summary outlining the outcomes of individual studies relating to nutraceutical supplementation(s).
GRANTS
C. S. Deane is a Ph.D student funded by Bournemouth University. D. J. Wilkinson is a postdoctoral research fellow funded through the MRC-ARUK Centre for Musculoskeletal Aging Research. The Medical Research Council-Arthritis Research UK (MRC-ARUK) Centre for Musculoskeletal Aging Research was funded by grants from the MRC (grant no. MR/K00414X/1) and ARUK (grant no.19891) awarded to the Universities of Nottingham and Birmingham.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.SD prepared figures; C.S.D., D.J.W., and B.E.P. drafted manuscript; C.S.D., D.J.W., B.E.P., K.S., T.E., and P.J.A. edited and revised manuscript; C.S.D., D.J.W., B.E.P., K.S., T.E., and P.J.A. approved final version of manuscript.
Supplementary Material
References
- 1.Abramowicz WN, Galloway SDR. Effects of acute versus chronic L-carnitine L-tartrate supplementation on metabolic responses to steady state exercise in males and females. Int J Sport Nutr Exerc Metab 15: 386–400, 2005. doi: 10.1123/ijsnem.15.4.386. [DOI] [PubMed] [Google Scholar]
- 2.Affourtit C, Bailey SJ, Jones AM, Smallwood MJ, Winyard PG. On the mechanism by which dietary nitrate improves human skeletal muscle function. Front Physiol 6: 211, 2015. doi: 10.3389/fphys.2015.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agergaard J, Trøstrup J, Uth J, Iversen JV, Boesen A, Andersen JL, Schjerling P, Langberg H. Does vitamin-D intake during resistance training improve the skeletal muscle hypertrophic and strength response in young and elderly men? - a randomized controlled trial. Nutr Metab (Lond) 12: 32, 2015. doi: 10.1186/s12986-015-0029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander JW, Saito H, Trocki O, Ogle CK. The importance of lipid type in the diet after burn injury. Ann Surg 204: 1–8, 1986. doi: 10.1097/00000658-198607000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson JE. A role for nitric oxide in muscle repair: nitric oxide-mediated activation of muscle satellite cells. Mol Biol Cell 11: 1859–1874, 2000. doi: 10.1091/mbc.11.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold JT, Oliver SJ, Lewis-Jones TM, Wylie LJ, Macdonald JH. Beetroot juice does not enhance altitude running performance in well-trained athletes. Appl Physiol Nutr Metab 40: 590–595, 2015. doi: 10.1139/apnm-2014-0470. [DOI] [PubMed] [Google Scholar]
- 7.Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92: 1080–1088, 2010. doi: 10.3945/ajcn.2010.29819. [DOI] [PubMed] [Google Scholar]
- 8.Atherton PJ, Smith K. Muscle protein synthesis in response to nutrition and exercise. J Physiol 590: 1049–1057, 2012. doi: 10.1113/jphysiol.2011.225003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 38: 1533–1539, 2010. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- 10.Baier S, Johannsen D, Abumrad N, Rathmacher JA, Nissen S, Flakoll P. Year-long changes in protein metabolism in elderly men and women supplemented with a nutrition cocktail of beta-hydroxy-beta-methylbutyrate (HMB), L-arginine, and L-lysine. JPEN J Parenter Enteral Nutr 33: 71–82, 2009. doi: 10.1177/0148607108322403. [DOI] [PubMed] [Google Scholar]
- 11.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol (1985) 107: 1144–1155, 2009. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 12.Bang HS, Seo DY, Chung YM, Oh KM, Park JJ, Arturo F, Jeong SH, Kim N, Han J. Ursolic Acid-induced elevation of serum irisin augments muscle strength during resistance training in men. Korean J Physiol Pharmacol 18: 441–446, 2014. doi: 10.4196/kjpp.2014.18.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, Petermans J, Reginster J-Y, Bruyère O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 99: 4336–4345, 2014. doi: 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- 14.Beelen M, Koopman R, Gijsen AP, Vandereyt H, Kies AK, Kuipers H, Saris WH, van Loon LJ. Protein coingestion stimulates muscle protein synthesis during resistance-type exercise. Am J Physiol Endocrinol Metab 295: E70–E77, 2008. doi: 10.1152/ajpendo.00774.2007. [DOI] [PubMed] [Google Scholar]
- 15.Bennet WM, Connacher AA, Scrimgeour CM, Smith K, Rennie MJ. Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: studies of incorporation of [1-13C]leucine. Clin Sci (Lond) 76: 447–454, 1989. doi: 10.1042/cs0760447. [DOI] [PubMed] [Google Scholar]
- 16.Bex T, Chung W, Baguet A, Stegen S, Stautemas J, Achten E, Derave W. Muscle carnosine loading by beta-alanine supplementation is more pronounced in trained vs. untrained muscles. J Appl Physiol (1985) 116: 204–209, 2014. doi: 10.1152/japplphysiol.01033.2013. [DOI] [PubMed] [Google Scholar]
- 17.Bhat M, Kalam R, Qadri SS, Madabushi S, Ismail A. Vitamin D deficiency-induced muscle wasting occurs through the ubiquitin proteasome pathway and is partially corrected by calcium in male rats. Endocrinology 154: 4018–4029, 2013. doi: 10.1210/en.2013-1369. [DOI] [PubMed] [Google Scholar]
- 18.Birch R, Noble D, Greenhaff PL. The influence of dietary creatine supplementation on performance during repeated bouts of maximal isokinetic cycling in man. Eur J Appl Physiol Occup Physiol 69: 268–276, 1994. doi: 10.1007/BF01094800. [DOI] [PubMed] [Google Scholar]
- 19.Bjørnsen T, Salvesen S, Berntsen S, Hetlelid KJ, Stea TH, Lohne-Seiler H, Rohde G, Haraldstad K, Raastad T, Køpp U, Haugeberg G, Mansoor MA, Bastani NE, Blomhoff R, Stølevik SB, Seynnes OR, Paulsen G. Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scand J Med Sci Sports 26: 755–763, 2016. doi: 10.1111/sms.12506. [DOI] [PubMed] [Google Scholar]
- 20.Bloch K, Schoenheimer R. Biological precursors of creatine. J Biol Chem 138: 167–194, 1940. [Google Scholar]
- 21.Bobeuf F, Labonte M, Dionne IJ, Khalil A. Combined effect of antioxidant supplementation and resistance training on oxidative stress markers, muscle and body composition in an elderly population. J Nutr Health Aging 15: 883–889, 2011. doi: 10.1007/s12603-011-0097-2. [DOI] [PubMed] [Google Scholar]
- 22.Bobeuf F, Labonté M, Khalil A, Dionne IJ. Effects of resistance training combined with antioxidant supplementation on fat-free mass and insulin sensitivity in healthy elderly subjects. Diabetes Res Clin Pract 87: e1–e3, 2010. doi: 10.1016/j.diabres.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Bode-Böger SM, Böger RH, Galland A, Tsikas D, Frölich JC. L-arginine-induced vasodilation in healthy humans: pharmacokinetic-pharmacodynamic relationship. Br J Clin Pharmacol 46: 489–497, 1998. doi: 10.1046/j.1365-2125.1998.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosaeus I, Rothenberg E. Nutrition and physical activity for the prevention and treatment of age-related sarcopenia. Proc Nutr Soc 75: 174–180, 2016. doi: 10.1017/S002966511500422X. [DOI] [PubMed] [Google Scholar]
- 25.Branch JD. Effect of creatine supplementation on body composition and performance: a meta-analysis. Int J Sport Nutr Exerc Metab 13: 198–226, 2003. doi: 10.1123/ijsnem.13.2.198. [DOI] [PubMed] [Google Scholar]
- 26.Brass EP. Pharmacokinetic considerations for the therapeutic use of carnitine in hemodialysis patients. Clin Ther 17: 176–185, 1995. doi: 10.1016/0149-2918(95)80017-4. [DOI] [PubMed] [Google Scholar]
- 27.Broad EM, Maughan RJ, Galloway SDR. Effects of four weeks L-carnitine L-tartrate ingestion on substrate utilization during prolonged exercise. Int J Sport Nutr Exerc Metab 15: 665–679, 2005. doi: 10.1123/ijsnem.15.6.665. [DOI] [PubMed] [Google Scholar]
- 28.Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Phillips BE, Szewczyk NJ, Greenhaff PL, Smith K, Atherton PJ. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J Physiol 594: 7399–7417, 2016. doi: 10.1113/JP272857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Szewczyk NJ, Greenhaff PL, Smith K, Atherton PJ. Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide-derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. FASEB J 29: 4485–4496, 2015. doi: 10.1096/fj.15-273755. [DOI] [PubMed] [Google Scholar]
- 30.Brook MS, Wilkinson DJ, Phillips BE, Perez-Schindler J, Philp A, Smith K, Atherton PJ. Skeletal muscle homeostasis and plasticity in youth and ageing: impact of nutrition and exercise. Acta Physiol (Oxf) 216: 15–41, 2016. doi: 10.1111/apha.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brose A, Parise G, Tarnopolsky MA. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J Gerontol A Biol Sci Med Sci 58: 11–19, 2003. doi: 10.1093/gerona/58.1.B11. [DOI] [PubMed] [Google Scholar]
- 32.Buford TW, Kreider RB, Stout JR, Greenwood M, Campbell B, Spano M, Ziegenfuss T, Lopez H, Landis J, Antonio J. International Society of Sports Nutrition position stand: creatine supplementation and exercise. J Int Soc Sports Nutr 4: 6, 2007. doi: 10.1186/1550-2783-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buitrago CG, Arango NS, Boland RL. 1α,25(OH)2D3-dependent modulation of Akt in proliferating and differentiating C2C12 skeletal muscle cells. J Cell Biochem 113: 1170–1181, 2012. doi: 10.1002/jcb.23444. [DOI] [PubMed] [Google Scholar]
- 34.Bunout D, Barrera G, Leiva L, Gattas V, de la Maza MP, Avendaño M, Hirsch S. Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Exp Gerontol 41: 746–752, 2006. doi: 10.1016/j.exger.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Buono R, Vantaggiato C, Pisa V, Azzoni E, Bassi MT, Brunelli S, Sciorati C, Clementi E. Nitric oxide sustains long-term skeletal muscle regeneration by regulating fate of satellite cells via signaling pathways requiring Vangl2 and cyclic GMP. Stem Cells 30: 197–209, 2012. doi: 10.1002/stem.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burd NA, West DWD, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Phillips SM. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr 141: 568–573, 2011. doi: 10.3945/jn.110.135038. [DOI] [PubMed] [Google Scholar]
- 37.Burdge GC, Calder PC. Introduction to fatty acids and lipids. World Rev Nutr Diet 112: 1–16, 2015. doi: 10.1159/000365423. [DOI] [PubMed] [Google Scholar]
- 39.Campia U, Panza JA. Flavanol-rich cocoa a promising new dietary intervention to reduce cardiovascular risk in type 2 diabetes? J Am Coll Cardiol 51: 2150–2152, 2008. doi: 10.1016/j.jacc.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 40.Capiati D, Benassati S, Boland RL. 1,25(OH)2-vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. J Cell Biochem 86: 128–135, 2002. doi: 10.1002/jcb.10191. [DOI] [PubMed] [Google Scholar]
- 41.Carrillo AE, Flynn MG, Pinkston C, Markofski MM, Jiang Y, Donkin SS, Teegarden D. Impact of vitamin D supplementation during a resistance training intervention on body composition, muscle function, and glucose tolerance in overweight and obese adults. Clin Nutr 32: 375–381, 2013. doi: 10.1016/j.clnu.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casey A, Constantin-Teodosiu D, Howell S, Hultman E, Greenhaff PL. Creatine ingestion favorably affects performance and muscle metabolism during maximal exercise in humans. Am J Physiol Endocrinol Metab 271: E31–E37, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Casey A, Greenhaff PL. Does dietary creatine supplementation play a role in skeletal muscle metabolism and performance? Am J Clin Nutr 72, Suppl: 607S–617S, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Casperson SL, Sheffield-Moore M, Hewlings SJ, Paddon-Jones D. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin Nutr 31: 512–519, 2012. doi: 10.1016/j.clnu.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ceglia L. Vitamin D and its role in skeletal muscle. Curr Opin Clin Nutr Metab Care 12: 628–633, 2009. doi: 10.1097/MCO.0b013e328331c707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ceglia L, Niramitmahapanya S, da Silva Morais M, Rivas DA, Harris SS, Bischoff-Ferrari H, Fielding RA, Dawson-Hughes B. A randomized study on the effect of vitamin D3 supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab 98: E1927–E1935, 2013. doi: 10.1210/jc.2013-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cermak NM, van Loon LJ. The use of carbohydrates during exercise as an ergogenic aid. Sports Med 43: 1139–1155, 2013. doi: 10.1007/s40279-013-0079-0. [DOI] [PubMed] [Google Scholar]
- 48.Cermak NM, Res P, Stinkens R, Lundberg JO, Gibala MJ, van Loon LJ. No improvement in endurance performance after a single dose of beetroot juice. Int J Sport Nutr Exerc Metab 22: 470–478, 2012. doi: 10.1123/ijsnem.22.6.470. [DOI] [PubMed] [Google Scholar]
- 49.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJC. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 96: 1454–1464, 2012. doi: 10.3945/ajcn.112.037556. [DOI] [PubMed] [Google Scholar]
- 50.Church DD, Schwarz NA, Spillane MB, McKinley-Barnard SK, Andre TL, Ramirez AJ, Willoughby DS. l-Leucine Increases Skeletal Muscle IGF-1 but Does Not Differentially Increase Akt/mTORC1 Signaling and Serum IGF-1 Compared to Ursolic Acid in Response to Resistance Exercise in Resistance-Trained Men. J Am Coll Nutr 35: 627–638, 2016. doi: 10.1080/07315724.2015.1132019. [DOI] [PubMed] [Google Scholar]
- 51.Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK, Phillips SM. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr 99: 276–286, 2014. doi: 10.3945/ajcn.113.068775. [DOI] [PubMed] [Google Scholar]
- 52.Churchward-Venne TA, Cotie LM, MacDonald MJ, Mitchell CJ, Prior T, Baker SK, Phillips SM. Citrulline does not enhance blood flow, microvascular circulation, or myofibrillar protein synthesis in elderly men at rest or following exercise. Am J Physiol Endocrinol Metab 307: E71–E83, 2014. doi: 10.1152/ajpendo.00096.2014. [DOI] [PubMed] [Google Scholar]
- 53.Close GL, Hamilton DL, Philp A, Burke LM, Morton JP. New strategies in sport nutrition to increase exercise performance. Free Radic Biol Med 98: 144–158, 2016. doi: 10.1016/j.freeradbiomed.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 54.Cooke MB, Rybalka E, Williams AD, Cribb PJ, Hayes A. Creatine supplementation enhances muscle force recovery after eccentrically-induced muscle damage in healthy individuals. J Int Soc Sports Nutr 6: 13, 2009. doi: 10.1186/1550-2783-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooke WH, Grandjean PW, Barnes WS. Effect of oral creatine supplementation on power output and fatigue during bicycle ergometry. J Appl Physiol (1985) 78: 670–673, 1995. [DOI] [PubMed] [Google Scholar]
- 56.Crossland H, Kazi AA, Lang CH, Timmons JA, Pierre P, Wilkinson DJ, Smith K, Szewczyk NJ, Atherton PJ. Focal adhesion kinase is required for IGF-I-mediated growth of skeletal muscle cells via a TSC2/mTOR/S6K1-associated pathway. Am J Physiol Endocrinol Metab 305: E183–E193, 2013. doi: 10.1152/ajpendo.00541.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Culbertson JY, Kreider RB, Greenwood M, Cooke M. Effects of beta-alanine on muscle carnosine and exercise performance: a review of the current literature. Nutrients 2: 75–98, 2010. doi: 10.3390/nu2010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19: 422–424, 2005. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 59.Cuthbertson DJ, Babraj J, Smith K, Wilkes E, Fedele MJ, Esser K, Rennie M. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab 290: E731–E738, 2006. doi: 10.1152/ajpendo.00415.2005. [DOI] [PubMed] [Google Scholar]
- 60.Décombaz J, Beaumont M, Vuichoud J, Bouisset F, Stellingwerff T. Effect of slow-release β-alanine tablets on absorption kinetics and paresthesia. Amino Acids 43: 67–76, 2012. doi: 10.1007/s00726-011-1169-7. [DOI] [PubMed] [Google Scholar]
- 61.Delarue J, Couet C, Cohen R, Bréchot JF, Antoine JM, Lamisse F. Effects of fish oil on metabolic responses to oral fructose and glucose loads in healthy humans. Am J Physiol Endocrinol Metab 270: E353–E362, 1996. [DOI] [PubMed] [Google Scholar]
- 62.Delarue J, Labarthe F, Cohen R. Fish-oil supplementation reduces stimulation of plasma glucose fluxes during exercise in untrained males. Br J Nutr 90: 777–786, 2003. doi: 10.1079/BJN2003964. [DOI] [PubMed] [Google Scholar]
- 63.Dempsey RL, Mazzone MF, Meurer LN. Does oral creatine supplementation improve strength? A meta-analysis. J Fam Pract 51: 945–951, 2002. [PubMed] [Google Scholar]
- 64.Derave W, Ozdemir MS, Harris RC, Pottier A, Reyngoudt H, Koppo K, Wise JA, Achten E. beta-Alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters. J Appl Physiol (1985) 103: 1736–1743, 2007. doi: 10.1152/japplphysiol.00397.2007. [DOI] [PubMed] [Google Scholar]
- 65.Deutz NE, Pereira SL, Hays NP, Oliver JS, Edens NK, Evans CM, Wolfe RR. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr 32: 704–712, 2013. doi: 10.1016/j.clnu.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 66.Devries MC, Phillips SM. Creatine supplementation during resistance training in older adults-a meta-analysis. Med Sci Sports Exerc 46: 1194–1203, 2014. doi: 10.1249/MSS.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 67.Dieck ST, Heuer H, Ehrchen J, Otto C, Bauer K. The peptide transporter PepT2 is expressed in rat brain and mediates the accumulation of the fluorescent dipeptide derivative β-Ala-Lys-Nε-AMCA in astrocytes. Glia 25: 10–20, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 68.Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith L, Golden M, Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med 1: 546–551, 1995. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 69.Dyle MC, Ebert SM, Cook DP, Kunkel SD, Fox DK, Bongers KS, Bullard SA, Dierdorff JM, Adams CM. Systems-based discovery of tomatidine as a natural small molecule inhibitor of skeletal muscle atrophy. J Biol Chem 289: 14913–14924, 2014. doi: 10.1074/jbc.M114.556241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Earnest CP, Snell PG, Rodriguez R, Almada AL, Mitchell TL. The effect of creatine monohydrate ingestion on anaerobic power indices, muscular strength and body composition. Acta Physiol Scand 153: 207–209, 1995. doi: 10.1111/j.1748-1716.1995.tb09854.x. [DOI] [PubMed] [Google Scholar]
- 71.Ebert SM, Dyle MC, Bullard SA, Dierdorff JM, Murry DJ, Fox DK, Bongers KS, Lira VA, Meyerholz DK, Talley JJ, Adams CM. Identification and small molecule inhibition of an ATF4-dependent pathway to age-related skeletal muscle weakness and atrophy. J Biol Chem 290: 25497–25511, 2015. doi: 10.1074/jbc.M115.681445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El-Hajj Fuleihan G, Nabulsi M, Tamim H, Maalouf J, Salamoun M, Khalife H, Choucair M, Arabi A, Vieth R. Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab 91: 405–412, 2006. doi: 10.1210/jc.2005-1436. [DOI] [PubMed] [Google Scholar]
- 73.Escalante G, Alencar M, Haddock B, Harvey P. The effects of phosphatidic acid supplementation on strength, body composition, muscular endurance, power, agility, and vertical jump in resistance trained men. J Int Soc Sports Nutr 13: 24, 2016. doi: 10.1186/s12970-016-0135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Escobar J, Frank JW, Suryawan A, Nguyen HV, Van Horn CG, Hutson SM, Davis TA. Leucine and alpha-ketoisocaproic acid, but not norleucine, stimulate skeletal muscle protein synthesis in neonatal pigs. J Nutr 140: 1418–1424, 2010. doi: 10.3945/jn.110.123042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr 91: 1123S–1127S, 2010. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- 76.Everaert I, Mooyaart A, Baguet A, Zutinic A, Baelde H, Achten E, Taes Y, De Heer E, Derave W. Vegetarianism, female gender and increasing age, but not CNDP1 genotype, are associated with reduced muscle carnosine levels in humans. Amino Acids 40: 1221–1229, 2011. doi: 10.1007/s00726-010-0749-2. [DOI] [PubMed] [Google Scholar]
- 77.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 294: 1942–1945, 2001. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 78.Fetterman JW Jr, Zdanowicz MM. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am J Health Syst Pharm 66: 1169–1179, 2009. doi: 10.2146/ajhp080411. [DOI] [PubMed] [Google Scholar]
- 79.Fischer R, Konkel A, Mehling H, Blossey K, Gapelyuk A, Wessel N, von Schacky C, Dechend R, Muller DN, Rothe M, Luft FC, Weylandt K, Schunck W-H. Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J Lipid Res 55: 1150–1164, 2014. doi: 10.1194/jlr.M047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fisher NDL, Hughes M, Gerhard-Herman M, Hollenberg NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens 21: 2281–2286, 2003. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 81.Forman HJ, Augusto O, Brigelius-Flohe R, Dennery PA, Kalyanaraman B, Ischiropoulos H, Mann GE, Radi R, Roberts LJ II, Vina J, Davies KJA. Even free radicals should follow some rules: a guide to free radical research terminology and methodology. Free Radic Biol Med 78: 233–235, 2015. doi: 10.1016/j.freeradbiomed.2014.10.504. [DOI] [PubMed] [Google Scholar]
- 82.Fuller JC, Sharp RL, Angus HF, Khoo PY, Rathmacher JA. Comparison of availability and plasma clearance rates of β-hydroxy-β-methylbutyrate delivery in the free acid and calcium salt forms. Br J Nutr 114: 1403–1409, 2015. doi: 10.1017/S0007114515003050. [DOI] [PubMed] [Google Scholar]
- 83.Gallagher JC. The effects of calcitriol on falls and fractures and physical performance tests. J Steroid Biochem Mol Biol 89-90: 497–501, 2004. doi: 10.1016/j.jsbmb.2004.03.059. [DOI] [PubMed] [Google Scholar]
- 84.Gaudichon C, Mahé S, Benamouzig R, Luengo C, Fouillet H, Daré S, Van Oycke M, Ferrière F, Rautureau J, Tomé D. Net postprandial utilization of [15N]-labeled milk protein nitrogen is influenced by diet composition in humans. J Nutr 129: 890–895, 1999. [DOI] [PubMed] [Google Scholar]
- 85.Gingras A-A, White PJ, Chouinard PY, Julien P, Davis TA, Dombrowski L, Couture Y, Dubreuil P, Myre A, Bergeron K, Marette A, Thivierge MC. Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-mTOR-S6K1 pathway and insulin sensitivity. J Physiol 579: 269–284, 2007. doi: 10.1113/jphysiol.2006.121079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Girgis CM. Vitamin D and muscle function in the elderly: the elixir of youth? Curr Opin Clin Nutr Metab Care 17: 546–550, 2014. doi: 10.1097/MCO.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 87.Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev 34: 33–83, 2013. doi: 10.1210/er.2012-1012. [DOI] [PubMed] [Google Scholar]
- 88.Girgis CM, Clifton-Bligh RJ, Mokbel N, Cheng K, Gunton JE. Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology 155: 347–357, 2014. doi: 10.1210/en.2013-1205. [DOI] [PubMed] [Google Scholar]
- 89.Girgis CM, Mokbel N, Cha KM, Houweling PJ, Abboud M, Fraser DR, Mason RS, Clifton-Bligh RJ, Gunton JE. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 155: 3227–3237, 2014. doi: 10.1210/en.2014-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Di Girolamo FG, Situlin R, Mazzucco S, Valentini R, Toigo G, Biolo G. Omega-3 fatty acids and protein metabolism: enhancement of anabolic interventions for sarcopenia. Curr Opin Clin Nutr Metab Care 17: 145–150, 2014. doi: 10.1097/MCO.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 91.Glynn EL, Fry CS, Timmerman KL, Drummond MJ, Volpi E, Rasmussen BB. Addition of carbohydrate or alanine to an essential amino acid mixture does not enhance human skeletal muscle protein anabolism. J Nutr 143: 307–314, 2013. doi: 10.3945/jn.112.168203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gomez-Cabrera MC, Borrás C, Pallardó FV, Sastre J, Ji LL, Viña J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol 567: 113–120, 2005. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Viña J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87: 142–149, 2008. [DOI] [PubMed] [Google Scholar]
- 94.Gonzalez JT, Stevenson EJ. New perspectives on nutritional interventions to augment lipid utilisation during exercise. Br J Nutr 107: 339–349, 2012. doi: 10.1017/S0007114511006684. [DOI] [PubMed] [Google Scholar]
- 95.Gorissen SHM, Burd NA, Hamer HM, Gijsen AP, Groen BB, van Loon LJ. Carbohydrate coingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. J Clin Endocrinol Metab 99: 2250–2258, 2014. doi: 10.1210/jc.2013-3970. [DOI] [PubMed] [Google Scholar]
- 96.Graham TE, Battram DS, Dela F, El-Sohemy A, Thong FS. Does caffeine alter muscle carbohydrate and fat metabolism during exercise? Appl Physiol Nutr Metab 33: 1311–1318, 2008. doi: 10.1139/H08-129. [DOI] [PubMed] [Google Scholar]
- 97.Green AL, Hultman E, Macdonald IA, Sewell DA, Greenhaff PL. Carbohydrate ingestion augments skeletal muscle creatine accumulation during creatine supplementation in humans. Am J Physiol Endocrinol Metab 271: E821–E826, 1996. [DOI] [PubMed] [Google Scholar]
- 98.Green AL, Simpson EJ, Littlewood JJ, Macdonald IA, Greenhaff PL. Carbohydrate ingestion augments creatine retention during creatine feeding in humans. Acta Physiol Scand 158: 195–202, 1996. doi: 10.1046/j.1365-201X.1996.528300000.x. [DOI] [PubMed] [Google Scholar]
- 99.Greenhaff PL, Bodin K, Soderlund K, Hultman E. Effect of oral creatine supplementation on skeletal muscle phosphocreatine resynthesis. Am J Physiol Endocrinol Metab 266: E725–E730, 1994. [DOI] [PubMed] [Google Scholar]
- 100.Greenhaff PL, Casey A, Short AH, Harris R, Soderlund K, Hultman E. Influence of oral creatine supplementation of muscle torque during repeated bouts of maximal voluntary exercise in man. Clin Sci (Lond) 84: 565–571, 1993. doi: 10.1042/cs0840565. [DOI] [PubMed] [Google Scholar]
- 101.Greenhaff PL, Hultman E, Harris RC. Carbohydrate Metabolism. In: Principles of Exercise Biochemistry. Basel, Switzerland: Karger, 2003, p. 108–151. doi: 10.1159/000074367 [DOI] [Google Scholar]
- 102.Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab 295: E595–E604, 2008. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Le Guen M, Chaté V, Hininger-Favier I, Laillet B, Morio B, Pieroni G, Schlattner U, Pison C, Dubouchaud H. A 9-wk docosahexaenoic acid-enriched supplementation improves endurance exercise capacity and skeletal muscle mitochondrial function in adult rats. Am J Physiol Endocrinol Metab 310: E213–E224, 2016. doi: 10.1152/ajpendo.00468.2014. [DOI] [PubMed] [Google Scholar]
- 104.Guo Z, Burguera B, Jensen MD. Kinetics of intramuscular triglyceride fatty acids in exercising humans. J Appl Physiol (1985) 89: 2057–2064, 2000. [DOI] [PubMed] [Google Scholar]
- 105.Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 149: 410–424, 2012. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 106.Harper P, Elwin CE, Cederblad G. Pharmacokinetics of intravenous and oral bolus doses of L-carnitine in healthy subjects. Eur J Clin Pharmacol 35: 555–562, 1988. doi: 10.1007/BF00558253. [DOI] [PubMed] [Google Scholar]
- 106a.Harris RC, Dunnett M, Greenhaff PL. Carnosine and taurine contents in individual fibres of human vastus lateralis muscle. J Sports Sci 16: 639–643, 1998. doi: 10.1080/026404198366443. [DOI] [Google Scholar]
- 107.Harris RC, Söderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci (Lond) 83: 367–374, 1992. doi: 10.1042/cs0830367. [DOI] [PubMed] [Google Scholar]
- 108.Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, Fallowfield JL, Hill CA, Sale C, Wise JA. The absorption of orally supplied β-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 30: 279–289, 2006. doi: 10.1007/s00726-006-0299-9. [DOI] [PubMed] [Google Scholar]
- 109.Heiss C, Kleinbongard P, Dejam A, Perré S, Schroeter H, Sies H, Kelm M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol 46: 1276–1283, 2005. doi: 10.1016/j.jacc.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 110.Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr 37: 478–494, 1983. [DOI] [PubMed] [Google Scholar]
- 111.Higashida K, Kim SH, Higuchi M, Holloszy JO, Han DH. Normal adaptations to exercise despite protection against oxidative stress. Am J Physiol Endocrinol Metab 301: E779–E784, 2011. doi: 10.1152/ajpendo.00655.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA. Influence of β-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 32: 225–233, 2007. doi: 10.1007/s00726-006-0364-4. [DOI] [PubMed] [Google Scholar]
- 113.Hirsh S, Huber L, Zhang P, Stein R, Joyal S. A single ascending dose, initial clinical pharmacokinetic and safety study of ursolic acid in healthy adult volunteers (Abstract). FASEB J 28: 1044.6, 2014.24253251 [Google Scholar]
- 114.Hodgson AB, Randell RK, Jeukendrup AE. The effect of green tea extract on fat oxidation at rest and during exercise: evidence of efficacy and proposed mechanisms. Adv Nutr 4: 129–140, 2013. doi: 10.3945/an.112.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoffman JR, Stout JR, Williams DR, Wells AJ, Fragala MS, Mangine GT, Gonzalez AM, Emerson NS, McCormack WP, Scanlon TC, Purpura M, Jäger R. Efficacy of phosphatidic acid ingestion on lean body mass, muscle thickness and strength gains in resistance-trained men. J Int Soc Sports Nutr 9: 47, 2012. doi: 10.1186/1550-2783-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest 116: 2062–2072, 2006. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 81: 353–373, 2006. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 118.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96: 1911–1930, 2011. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 119.Huffman DM, Michaelson JL, Thomas TR, Derek M, Huffman JL, Michaelson TR. Chronic supplementation with fish oil increases fat oxidation during exercise in young men. J Exerc Physiol 7: 48–57, 2004. [Google Scholar]
- 120.Hulmi JJ, Kovanen V, Selänne H, Kraemer WJ, Häkkinen K, Mero AA. Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids 37: 297–308, 2009. doi: 10.1007/s00726-008-0150-6. [DOI] [PubMed] [Google Scholar]
- 121.Ivy JL, Katz AL, Cutler CL, Sherman WM, Coyle EF. Muscle glycogen synthesis after exercise: effect of time of carbohydrate ingestion. J Appl Physiol (1985) 64: 1480–1485, 1988. [DOI] [PubMed] [Google Scholar]
- 122.Jaafar R, De Larichaudy J, Chanon S, Euthine V, Durand C, Naro F, Bertolino P, Vidal H, Lefai E, Némoz G. Phospholipase D regulates the size of skeletal muscle cells through the activation of mTOR signaling. Cell Commun Signal 11: 55, 2013. doi: 10.1186/1478-811X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985) 89: 81–88, 2000. [DOI] [PubMed] [Google Scholar]
- 124.Jeong JW, Shim JJ, Choi ID, Kim SH, Ra J, Ku HK, Lee DE, Kim TY, Jeung W, Lee J-H, Lee KW, Huh CS, Sim JH, Ahn YT. Apple Pomace Extract Improves Endurance in Exercise Performance by Increasing Strength and Weight of Skeletal Muscle. J Med Food 18: 1380–1386, 2015. doi: 10.1089/jmf.2014.3401. [DOI] [PubMed] [Google Scholar]
- 125.Jobgen W, Meininger CJ, Jobgen SC, Li P, Lee MJ, Smith SB, Spencer TE, Fried SK, Wu G. Dietary L-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr 139: 230–237, 2009. doi: 10.3945/jn.108.096362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Joncquel-Chevalier Curt M, Voicu PM, Fontaine M, Dessein AF, Porchet N, Mention-Mulliez K, Dobbelaere D, Soto-Ares G, Cheillan D, Vamecq J. Creatine biosynthesis and transport in health and disease. Biochimie 119: 146–165, 2015. doi: 10.1016/j.biochi.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 127.Jones AM. Dietary nitrate supplementation and exercise performance. Sports Med 44, Suppl 1: S35–S45, 2014. doi: 10.1007/s40279-014-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jouris KB, McDaniel JL, Weiss EP. The effect of omega-3 fatty acid supplementation on the inflammatory response to eccentric strength exercise. J Sports Sci Med 10: 432–438, 2011. [PMC free article] [PubMed] [Google Scholar]
- 129.Joy JM, Gundermann DM, Lowery RP, Jäger R, McCleary SA, Purpura M, Roberts MD, Wilson SM, Hornberger TA, Wilson JM. Phosphatidic acid enhances mTOR signaling and resistance exercise induced hypertrophy. Nutr Metab (Lond) 11: 29, 2014. doi: 10.1186/1743-7075-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kalyani RR, Stein B, Valiyil R, Manno R, Maynard JW, Crews DC. Vitamin D treatment for the prevention of falls in older adults: systematic review and meta-analysis. J Am Geriatr Soc 58: 1299–1310, 2010. doi: 10.1111/j.1532-5415.2010.02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kamolrat T, Gray SR. The effect of eicosapentaenoic and docosahexaenoic acid on protein synthesis and breakdown in murine C2C12 myotubes. Biochem Biophys Res Commun 432: 593–598, 2013. doi: 10.1016/j.bbrc.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 132.Karim M, McCormick K, Kappagoda CT. Effects of cocoa extracts on endothelium-dependent relaxation. J Nutr 130, Suppl: 2105S–2108S, 2000. [DOI] [PubMed] [Google Scholar]
- 133.Kendrick IP, Harris RC, Kim HJ, Kim CK, Dang VH, Lam TQ, Bui TT, Smith M, Wise JA. The effects of 10 weeks of resistance training combined with beta-alanine supplementation on whole body strength, force production, muscular endurance and body composition. Amino Acids 34: 547–554, 2008. doi: 10.1007/s00726-007-0008-3. [DOI] [PubMed] [Google Scholar]
- 134.Kerksick CM, Rasmussen CJ, Lancaster SL, Magu B, Smith P, Melton C, Greenwood M, Almada AL, Earnest CP, Kreider RB. The effects of protein and amino acid supplementation on performance and training adaptations during ten weeks of resistance training. J Strength Cond Res 20: 643–653, 2006. doi: 10.1519/R-17695.1. [DOI] [PubMed] [Google Scholar]
- 135.Kimball SR. Integration of signals generated by nutrients, hormones, and exercise in skeletal muscle. Am J Clin Nutr 99: 237S–242S, 2014. doi: 10.3945/ajcn.113.068387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Knitter AE, Panton L, Rathmacher JA, Petersen A, Sharp R. Effects of beta-hydroxy-beta-methylbutyrate on muscle damage after a prolonged run. J Appl Physiol (1985) 89: 1340–1344, 2000. [DOI] [PubMed] [Google Scholar]
- 138.Koopman R, Beelen M, Stellingwerff T, Pennings B, Saris WH, Kies AK, Kuipers H, van Loon LJ. Coingestion of carbohydrate with protein does not further augment postexercise muscle protein synthesis. Am J Physiol Endocrinol Metab 293: E833–E842, 2007. doi: 10.1152/ajpendo.00135.2007. [DOI] [PubMed] [Google Scholar]
- 139.Kraemer WJ, Fleck SJ, Deschenes MR. Exercise Physiology: Integrating Theory and Application. Philadelphia, PA: Lippincott Williams & Wilkins, 2011. [Google Scholar]
- 140.Kreider RB, Ferreira M, Wilson M, Almada AL. Effects of calcium β-hydroxy-β-methylbutyrate (HMB) supplementation during resistance-training on markers of catabolism, body composition and strength. Int J Sports Med 20: 503–509, 1999. doi: 10.1055/s-1999-8835. [DOI] [PubMed] [Google Scholar]
- 141.Kunkel SD, Elmore CJ, Bongers KS, Ebert SM, Fox DK, Dyle MC, Bullard SA, Adams CM. Ursolic acid increases skeletal muscle and brown fat and decreases diet-induced obesity, glucose intolerance and fatty liver disease. PLoS One 7: e39332, 2012. doi: 10.1371/journal.pone.0039332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kunkel SD, Suneja M, Ebert SM, Bongers KS, Fox DK, Malmberg SE, Alipour F, Shields RK, Adams CM. mRNA expression signatures of human skeletal muscle atrophy identify a natural compound that increases muscle mass. Cell Metab 13: 627–638, 2011. doi: 10.1016/j.cmet.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Labonté M, Dionne IJ, Bouchard DR, Sénéchal M, Tessier D, Khalil A, Labonté M, Bobeuf F, Tessier D, Khalil A, Dionne IJ. Effects of antioxidant supplements combined with resistance exercise on gains in fat-free mass in healthy elderly subjects: a pilot study. J Am Geriatr Soc 56: 1766–1768, 2008. doi: 10.1111/j.1532-5415.2008.01810.x. [DOI] [PubMed] [Google Scholar]
- 144.Lamboley CRH, Royer D, Dionne IJ. Effects of beta-hydroxy-beta-methylbutyrate on aerobic-performance components and body composition in college students. Int J Sport Nutr Exerc Metab 17: 56–69, 2007. doi: 10.1123/ijsnem.17.1.56. [DOI] [PubMed] [Google Scholar]
- 145.Lansley KE, Winyard PG, Bailey SJ, Vanhatalo A, Wilkerson DP, Blackwell JR, Gilchrist M, Benjamin N, Jones AM. Acute dietary nitrate supplementation improves cycling time trial performance. Med Sci Sports Exerc 43: 1125–1131, 2011. doi: 10.1249/MSS.0b013e31821597b4. [DOI] [PubMed] [Google Scholar]
- 146.Lanza IR, Blachnio-Zabielska A, Johnson ML, Schimke JM, Jakaitis DR, Lebrasseur NK, Jensen MD, Sreekumaran Nair K, Zabielski P. Influence of fish oil on skeletal muscle mitochondrial energetics and lipid metabolites during high-fat diet. Am J Physiol Endocrinol Metab 304: E1391–E1403, 2013. doi: 10.1152/ajpendo.00584.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab 13: 149–159, 2011. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 148.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 191: 59–66, 2007. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 149.Lemon PW, Tarnopolsky MA, MacDougall JD, Atkinson SA. Protein requirements and muscle mass/strength changes during intensive training in novice bodybuilders. J Appl Physiol (1985) 73: 767–775, 1992. [DOI] [PubMed] [Google Scholar]
- 150.Li YP, Chen Y, Li AS, Reid MB. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am J Physiol Cell Physiol 285: C806–C812, 2003. doi: 10.1152/ajpcell.00129.2003. [DOI] [PubMed] [Google Scholar]
- 151.Logan SL, Spriet LL. Omega-3 Fatty Acid Supplementation for 12 Weeks Increases Resting and Exercise Metabolic Rate in Healthy Community-Dwelling Older Females. PLoS One 10: e0144828, 2015. doi: 10.1371/journal.pone.0144828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Louis M, Poortmans JR, Francaux M, Berré J, Boisseau N, Brassine E, Cuthbertson DJR, Smith K, Babraj JA, Waddell T, Rennie MJ. No effect of creatine supplementation on human myofibrillar and sarcoplasmic protein synthesis after resistance exercise. Am J Physiol Endocrinol Metab 285: E1089–E1094, 2003. doi: 10.1152/ajpendo.00195.2003. [DOI] [PubMed] [Google Scholar]
- 153.Louis M, Poortmans JR, Francaux M, Hultman E, Berre J, Boisseau N, Young VR, Smith K, Meier-Augenstein W, Babraj JA, Waddell T, Rennie MJ. Creatine supplementation has no effect on human muscle protein turnover at rest in the postabsorptive or fed states. Am J Physiol Endocrinol Metab 284: E764–E770, 2003. doi: 10.1152/ajpendo.00338.2002. [DOI] [PubMed] [Google Scholar]
- 154.Luiking YC, Ten Have GA, Wolfe RR, Deutz NE. Arginine de novo and nitric oxide production in disease states. Am J Physiol Endocrinol Metab 303: E1177–E1189, 2012. doi: 10.1152/ajpendo.00284.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Makanae Y, Kawada S, Sasaki K, Nakazato K, Ishii N. Vitamin C administration attenuates overload-induced skeletal muscle hypertrophy in rats. Acta Physiol (Oxf) 208: 57–65, 2013. doi: 10.1111/apha.12042. [DOI] [PubMed] [Google Scholar]
- 156.Mandel H, Levy N, Izkovitch S, Korman SH. Elevated plasma citrulline and arginine due to consumption of Citrullus vulgaris (watermelon). J Inherit Metab Dis 28: 467–472, 2005. doi: 10.1007/s10545-005-0467-1. [DOI] [PubMed] [Google Scholar]
- 157.Marzani B, Balage M, Vénien A, Astruc T, Papet I, Dardevet D, Mosoni L. Antioxidant supplementation restores defective leucine stimulation of protein synthesis in skeletal muscle from old rats. J Nutr 138: 2205–2211, 2008. doi: 10.3945/jn.108.094029. [DOI] [PubMed] [Google Scholar]
- 158.McCarthy JJ, Esser KA. Counterpoint: Satellite cell addition is not obligatory for skeletal muscle hypertrophy. J Appl Physiol (1985) 103: 1100–1102, 2007. doi: 10.1152/japplphysiol.00101.2007a. [DOI] [PubMed] [Google Scholar]
- 159.McConell GK, Bradley SJ, Stephens TJ, Canny BJ, Kingwell BA, Lee-Young RS. Skeletal muscle nNOS mu protein content is increased by exercise training in humans. Am J Physiol Regul Integr Comp Physiol 293: R821–R828, 2007. doi: 10.1152/ajpregu.00796.2006. [DOI] [PubMed] [Google Scholar]
- 160.McGlory C, Galloway SDR, Hamilton DL, McClintock C, Breen L, Dick JR, Bell JG, Tipton KD. Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins Leukot Essent Fatty Acids 90: 199–206, 2014. doi: 10.1016/j.plefa.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 161.McGlory C, Wardle SL, Macnaughton LS, Witard OC, Scott F, Dick J, Bell JG, Phillips SM, Galloway SD, Hamilton DL, Tipton KD. Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. Physiol Rep 4: e12715, 2016. doi: 10.14814/phy2.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McLellan TM, Pasiakos SM, Lieberman HR. Effects of protein in combination with carbohydrate supplements on acute or repeat endurance exercise performance: a systematic review. Sports Med 44: 535–550, 2014. doi: 10.1007/s40279-013-0133-y. [DOI] [PubMed] [Google Scholar]
- 163.Miramonti AA, Stout JR, Fukuda DH, Robinson EH IV, Wang R, La Monica MB, Hoffman JR. The effects of four weeks of high intensity interval training and β-hydroxy-β-methylbutyric free acid supplementation on the onset of neuromuscular fatigue. J Strength Cond Res 30: 626–634, 2016. doi: 10.1519/JSC.0000000000001140. [DOI] [PubMed] [Google Scholar]
- 164.Mitchell WK, Phillips BE, Wilkinson DJ, Williams JP, Rankin D, Lund JN, Smith K, Atherton PJ. Supplementing essential amino acids with the nitric oxide precursor, l-arginine, enhances skeletal muscle perfusion without impacting anabolism in older men. Clin Nutr S0261-5614: 31271–31277, 2016. doi: 10.1016/j.clnu.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 165.Mobley CB, Hornberger TA, Fox CD, Healy JC, Ferguson BS, Lowery RP, McNally RM, Lockwood CM, Stout JR, Kavazis AN, Wilson JM, Roberts MD. Effects of oral phosphatidic acid feeding with or without whey protein on muscle protein synthesis and anabolic signaling in rodent skeletal muscle. J Int Soc Sports Nutr 12: 32, 2015. doi: 10.1186/s12970-015-0094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 70: 57–62, 2015. doi: 10.1093/gerona/glu103. [DOI] [PubMed] [Google Scholar]
- 167.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89: 161–168, 2009. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- 168.Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 35: 411–429, 2007. doi: 10.1002/mus.20743. [DOI] [PubMed] [Google Scholar]
- 169.Muggeridge DJ, Howe CC, Spendiff O, Pedlar C, James PE, Easton C. A single dose of beetroot juice enhances cycling performance in simulated altitude. Med Sci Sports Exerc 46: 143–150, 2014. doi: 10.1249/MSS.0b013e3182a1dc51. [DOI] [PubMed] [Google Scholar]
- 170.Mujika I, Chatard JC, Lacoste L, Barale F, Geyssant A. Creatine supplementation does not improve sprint performance in competitive swimmers. Med Sci Sports Exerc 28: 1435–1441, 1996. doi: 10.1097/00005768-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 171.Naderi A, de Oliveira EP, Ziegenfuss TN, Willems ME. Timing, Optimal Dose and Intake Duration of Dietary Supplements with Evidence-Based Use in Sports Nutrition. J Exerc Nutrition Biochem 20: 1–12, 2016. doi: 10.20463/jenb.2016.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Newsholme P, Bender K, Kiely A, Brennan L. Amino acid metabolism, insulin secretion and diabetes. Biochem Soc Trans 35: 1180–1186, 2007. doi: 10.1042/BST0351180. [DOI] [PubMed] [Google Scholar]
- 173.Newsholme P, Krause M. Nutritional regulation of insulin secretion: implications for diabetes. Clin Biochem Rev 33: 35–47, 2012. [PMC free article] [PubMed] [Google Scholar]
- 174.Nissen S, Sharp R, Ray M, Rathmacher JA, Rice D, Fuller JC Jr, Connelly AS, Abumrad N. Effect of leucine metabolite β-hydroxy-β-methylbutyrate on muscle metabolism during resistance-exercise training. J Appl Physiol (1985) 81: 2095–2104, 1996. [DOI] [PubMed] [Google Scholar]
- 175.Nissen SL, Abumrad NN. Nutritional role of the leucine metabolite β-hydroxy β-methylbutyrate (HMB). J Nutr Biochem 8: 300–311, 1997. doi: 10.1016/S0955-2863(97)00048-X. [DOI] [Google Scholar]
- 176.O’Neil TK, Duffy LR, Frey JW, Hornberger TA. The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. J Physiol 587: 3691–3701, 2009. doi: 10.1113/jphysiol.2009.173609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ogasawara R, Sato K, Higashida K, Nakazato K, Fujita S. Ursolic acid stimulates mTORC1 signaling after resistance exercise in rat skeletal muscle. Am J Physiol Endocrinol Metab 305: E760–E765, 2013. doi: 10.1152/ajpendo.00302.2013. [DOI] [PubMed] [Google Scholar]
- 178.Olsen S, Aagaard P, Kadi F, Tufekovic G, Verney J, Olesen JL, Suetta C, Kjaer M. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol 573: 525–534, 2006. doi: 10.1113/jphysiol.2006.107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Osowska S, Duchemann T, Walrand S, Paillard A, Boirie Y, Cynober L, Moinard C. Citrulline modulates muscle protein metabolism in old malnourished rats. Am J Physiol Endocrinol Metab 291: E582–E586, 2006. doi: 10.1152/ajpendo.00398.2005. [DOI] [PubMed] [Google Scholar]
- 180.Ou X, Liu M, Luo H, Dong LQ, Liu F. Ursolic acid inhibits leucine-stimulated mTORC1 signaling by suppressing mTOR localization to lysosome. PLoS One 9: e95393, 2014. doi: 10.1371/journal.pone.0095393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Panton LB, Rathmacher JA, Baier S, Nissen S. Nutritional supplementation of the leucine metabolite β-hydroxy-β-methylbutyrate (hmb) during resistance training. Nutrition 16: 734–739, 2000. doi: 10.1016/S0899-9007(00)00376-2. [DOI] [PubMed] [Google Scholar]
- 182.Patel J, McLeod LE, Vries RG, Flynn A, Wang X, Proud CG. Cellular stresses profoundly inhibit protein synthesis and modulate the states of phosphorylation of multiple translation factors. Eur J Biochem 269: 3076–3085, 2002. doi: 10.1046/j.1432-1033.2002.02992.x. [DOI] [PubMed] [Google Scholar]
- 183.Paulsen G, Cumming KT, Holden G, Hallén J, Rønnestad BR, Sveen O, Skaug A, Paur I, Bastani NE, Østgaard HN, Buer C, Midttun M, Freuchen F, Wiig H, Ulseth ET, Garthe I, Blomhoff R, Benestad HB, Raastad T. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double-blind, randomised, controlled trial. J Physiol 592: 1887–1901, 2014. doi: 10.1113/jphysiol.2013.267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Paulsen G, Hamarsland H, Cumming KT, Johansen RE, Hulmi JJ, Børsheim E, Wiig H, Garthe I, Raastad T. Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J Physiol 592: 5391–5408, 2014. doi: 10.1113/jphysiol.2014.279950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Petersen AC, McKenna MJ, Medved I, Murphy KT, Brown MJ, Della Gatta P, Cameron-Smith D. Infusion with the antioxidant N-acetylcysteine attenuates early adaptive responses to exercise in human skeletal muscle. Acta Physiol (Oxf) 204: 382–392, 2012. doi: 10.1111/j.1748-1716.2011.02344.x. [DOI] [PubMed] [Google Scholar]
- 186.Petróczi A, Naughton DP, Pearce G, Bailey R, Bloodworth A, McNamee M. Nutritional supplement use by elite young UK athletes: fallacies of advice regarding efficacy. J Int Soc Sports Nutr 5: 22, 2008. doi: 10.1186/1550-2783-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Phillips BE, Atherton PJ, Varadhan K, Limb MC, Wilkinson DJ, Sjøberg KA, Smith K, Williams JP. The effects of resistance exercise training on macro- and micro-circulatory responses to feeding and skeletal muscle protein anabolism in older men. J Physiol 593: 2721–2734, 2015. doi: 10.1113/JP270343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Phillips BE, Atherton PJ, Varadhan K, Limb MC, Williams JP, Smith K. Acute cocoa flavanol supplementation improves muscle macro- and microvascular but not anabolic responses to amino acids in older men. Appl Physiol Nutr Metab 41: 548–556, 2016. doi: 10.1139/apnm-2015-0543. [DOI] [PubMed] [Google Scholar]
- 189.Phillips BE, Atherton PJ, Varadhan K, Wilkinson DJ, Limb M, Selby AL, Rennie MJ, Smith K, Williams JP. Pharmacological enhancement of leg and muscle microvascular blood flow does not augment anabolic responses in skeletal muscle of young men under fed conditions. Am J Physiol Endocrinol Metab 306: E168–E176, 2014. doi: 10.1152/ajpendo.00440.2013. [DOI] [PubMed] [Google Scholar]
- 190.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997. [DOI] [PubMed] [Google Scholar]
- 191.Phillips SM, McGlory C. CrossTalk proposal: The dominant mechanism causing disuse muscle atrophy is decreased protein synthesis. J Physiol 592: 5341–5343, 2014. doi: 10.1113/jphysiol.2014.273615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pike JW. Expression of the vitamin D receptor in skeletal muscle: are we there yet? Endocrinology 155: 3214–3218, 2014. doi: 10.1210/en.2014-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Le Plénier S, Walrand S, Noirt R, Cynober L, Moinard C. Effects of leucine and citrulline versus non-essential amino acids on muscle protein synthesis in fasted rat: a common activation pathway? Amino Acids 43: 1171–1178, 2012. doi: 10.1007/s00726-011-1172-z. [DOI] [PubMed] [Google Scholar]
- 194.Pojednic RM, Ceglia L. The emerging biomolecular role of vitamin D in skeletal muscle. Exerc Sport Sci Rev 42: 76–81, 2014. doi: 10.1249/JES.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Poveda JJ, Riestra A, Salas E, Cagigas ML, López-Somoza C, Amado JA, Berrazueta JR. Contribution of nitric oxide to exercise-induced changes in healthy volunteers: effects of acute exercise and long-term physical training. Eur J Clin Invest 27: 967–971, 1997. doi: 10.1046/j.1365-2362.1997.2220763.x. [DOI] [PubMed] [Google Scholar]
- 196.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276, 2008. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Purpura M, Jäger R, Joy JM, Lowery RP, Moore JD, Wilson JM. Effect of oral administration of soy-derived phosphatidic acid on concentrations of phosphatidic acid and lyso-phosphatidic acid molecular species in human plasma. J Int Soc Sports Nutr 10, Suppl 1: P22, 2013. doi: 10.1186/1550-2783-10-S1-P22. [DOI] [Google Scholar]
- 198.Rankin JW, Goldman LP, Puglisi MJ, Nickols-Richardson SM, Earthman CP, Gwazdauskas FC. Effect of post-exercise supplement consumption on adaptations to resistance training. J Am Coll Nutr 23: 322–330, 2004. doi: 10.1080/07315724.2004.10719375. [DOI] [PubMed] [Google Scholar]
- 199.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol (1985) 88: 386–392, 2000. [DOI] [PubMed] [Google Scholar]
- 200.Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 63: 519–523, 1982. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- 201.Robinson MM, Turner SM, Hellerstein MK, Hamilton KL, Miller BF. Long-term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. FASEB J 25: 3240–3249, 2011. doi: 10.1096/fj.11-186437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rodacki CL, Rodacki AL, Pereira G, Naliwaiko K, Coelho I, Pequito D, Fernandes LC. Fish-oil supplementation enhances the effects of strength training in elderly women. Am J Clin Nutr 95: 428–436, 2012. doi: 10.3945/ajcn.111.021915. [DOI] [PubMed] [Google Scholar]
- 203.Ryan AM, Reynolds JV, Healy L, Byrne M, Moore J, Brannelly N, McHugh A, McCormack D, Flood P. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg 249: 355–363, 2009. doi: 10.1097/SLA.0b013e31819a4789. [DOI] [PubMed] [Google Scholar]
- 204.Safdar A, Yardley NJ, Snow R, Melov S, Tarnopolsky MA. Global and targeted gene expression and protein content in skeletal muscle of young men following short-term creatine monohydrate supplementation. Physiol Genomics 32: 219–228, 2008. doi: 10.1152/physiolgenomics.00157.2007. [DOI] [PubMed] [Google Scholar]
- 205.Sahlin K, Ren JM. Relationship of contraction capacity to metabolic changes during recovery from a fatiguing contraction. J Appl Physiol (1985) 67: 648–654, 1989. [DOI] [PubMed] [Google Scholar]
- 206.Salles J, Chanet A, Giraudet C, Patrac V, Pierre P, Jourdan M, Luiking YC, Verlaan S, Migné C, Boirie Y, Walrand S. 1,25(OH)2-vitamin D3 enhances the stimulating effect of leucine and insulin on protein synthesis rate through Akt/PKB and mTOR mediated pathways in murine C2C12 skeletal myotubes. Mol Nutr Food Res 57: 2137–2146, 2013. doi: 10.1002/mnfr.201300074. [DOI] [PubMed] [Google Scholar]
- 207.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290–303, 2010. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis 20: 187–192, 2005. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- 209.Scott D, Blizzard L, Fell J, Giles G, Jones G. Associations between dietary nutrient intake and muscle mass and strength in community-dwelling older adults: the Tasmanian Older Adult Cohort Study. J Am Geriatr Soc 58: 2129–2134, 2010. doi: 10.1111/j.1532-5415.2010.03147.x. [DOI] [PubMed] [Google Scholar]
- 210.Shad BJ, Smeuninx B, Atherton PJ, Breen L. The mechanistic and ergogenic effects of phosphatidic acid in skeletal muscle. Appl Physiol Nutr Metab 40: 1233–1241, 2015. doi: 10.1139/apnm-2015-0350. [DOI] [PubMed] [Google Scholar]
- 211.Shannon CE, Nixon AV, Greenhaff PL, Stephens FB. Protein ingestion acutely inhibits insulin-stimulated muscle carnitine uptake in healthy young men. Am J Clin Nutr 103: 276–282, 2016. doi: 10.3945/ajcn.115.119826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Muita JW. Micronutrients in health and disease. East Afr Med J 78: 449–450, 2001. doi: 10.4314/eamj.v78i9.8971. [DOI] [PubMed] [Google Scholar]
- 213.Shenkin A. The key role of micronutrients. Clin Nutr 25: 1–13, 2006. doi: 10.1016/j.clnu.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 214.Slater G, Jenkins D, Logan P, Lee H, Vukovich M, Rathmacher JA, Hahn AG. Beta-hydroxy-beta-methylbutyrate (HMB) supplementation does not affect changes in strength or body composition during resistance training in trained men. Int J Sport Nutr Exerc Metab 11: 384–396, 2001. doi: 10.1123/ijsnem.11.3.384. [DOI] [PubMed] [Google Scholar]
- 215.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr 93: 402–412, 2011. doi: 10.3945/ajcn.110.005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin Sci (Lond) 121: 267–278, 2011. doi: 10.1042/CS20100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Smith GI, Julliand S, Reeds DN, Sinacore DR, Klein S, Mittendorfer B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr 102: 115–122, 2015. doi: 10.3945/ajcn.114.105833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol Endocrinol Metab 275: E73–E78, 1998. [DOI] [PubMed] [Google Scholar]
- 219.Snow RJ, McKenna MJ, Selig SE, Kemp J, Stathis CG, Zhao S. Effect of creatine supplementation on sprint exercise performance and muscle metabolism. J Appl Physiol (1985) 84: 1667–1673, 1998. [DOI] [PubMed] [Google Scholar]
- 220.Soop M, Björkman O, Cederblad G, Hagenfeldt L, Wahren J. Influence of carnitine supplementation on muscle substrate and carnitine metabolism during exercise. J Appl Physiol (1985) 64: 2394–2399, 1988. [DOI] [PubMed] [Google Scholar]
- 221.Sørensen OH, Lund B, Saltin B, Lund B, Andersen RB, Hjorth L, Melsen F, Mosekilde L. Myopathy in bone loss of ageing: improvement by treatment with 1 alpha-hydroxycholecalciferol and calcium. Clin Sci (Lond) 56: 157–161, 1979. doi: 10.1042/cs0560157. [DOI] [PubMed] [Google Scholar]
- 222.Stellingwerff T, Decombaz J, Harris RC, Boesch C. Optimizing human in vivo dosing and delivery of β-alanine supplements for muscle carnosine synthesis. Amino Acids 43: 57–65, 2012. doi: 10.1007/s00726-012-1245-7. [DOI] [PubMed] [Google Scholar]
- 223.Stephens FB, Constantin-Teodosiu D, Greenhaff PL. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol 581: 431–444, 2007. doi: 10.1113/jphysiol.2006.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. An acute increase in skeletal muscle carnitine content alters fuel metabolism in resting human skeletal muscle. J Clin Endocrinol Metab 91: 5013–5018, 2006. doi: 10.1210/jc.2006-1584. [DOI] [PubMed] [Google Scholar]
- 225.Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. Insulin stimulates L-carnitine accumulation in human skeletal muscle. FASEB J 20: 377–379, 2006. doi: 10.1096/fj.05-4985fje. [DOI] [PubMed] [Google Scholar]
- 226.Stephens FB, Evans CE, Constantin-Teodosiu D, Greenhaff PL. Carbohydrate ingestion augments L-carnitine retention in humans. J Appl Physiol (1985) 102: 1065–1070, 2007. doi: 10.1152/japplphysiol.01011.2006. [DOI] [PubMed] [Google Scholar]
- 227.Stephens FB, Wall BT, Marimuthu K, Shannon CE, Constantin-Teodosiu D, Macdonald IA, Greenhaff PL. Skeletal muscle carnitine loading increases energy expenditure, modulates fuel metabolism gene networks and prevents body fat accumulation in humans. J Physiol 591: 4655–4666, 2013. doi: 10.1113/jphysiol.2013.255364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Stout JR, Cramer JT, Zoeller RF, Torok D, Costa P, Hoffman JR, Harris RC, O’Kroy J. Effects of beta-alanine supplementation on the onset of neuromuscular fatigue and ventilatory threshold in women. Amino Acids 32: 381–386, 2007. doi: 10.1007/s00726-006-0474-z. [DOI] [PubMed] [Google Scholar]
- 229.Suzuki Y, Ito O, Mukai N, Takahashi H, Takamatsu K. High level of skeletal muscle carnosine contributes to the latter half of exercise performance during 30-s maximal cycle ergometer sprinting. Jpn J Physiol 52: 199–205, 2002. doi: 10.2170/jjphysiol.52.199. [DOI] [PubMed] [Google Scholar]
- 230.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc 109: 1582–1586, 2009. doi: 10.1016/j.jada.2009.06.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tanaka T, Kassai A, Ohmoto M, Morito K, Kashiwada Y, Takaishi Y, Urikura M, Morishige J, Satouchi K, Tokumura A. Quantification of phosphatidic acid in foodstuffs using a thin-layer-chromatography-imaging technique. J Agric Food Chem 60: 4156–4161, 2012. doi: 10.1021/jf300147y. [DOI] [PubMed] [Google Scholar]
- 232.Tang JE, Lysecki PJ, Manolakos JJ, MacDonald MJ, Tarnopolsky MA, Phillips SM. Bolus arginine supplementation affects neither muscle blood flow nor muscle protein synthesis in young men at rest or after resistance exercise. J Nutr 141: 195–200, 2011. doi: 10.3945/jn.110.130138. [DOI] [PubMed] [Google Scholar]
- 233.Taylor PM. Absorbing competition for carnitine. J Physiol 532: 283, 2001. doi: 10.1111/j.1469-7793.2001.0283f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Thompson CH, Kemp GJ, Sanderson AL, Dixon RM, Styles P, Taylor DJ, Radda GK. Effect of creatine on aerobic and anaerobic metabolism in skeletal muscle in swimmers. Br J Sports Med 30: 222–225, 1996. doi: 10.1136/bjsm.30.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Thwaites DT, Anderson CMH. H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp Physiol 92: 603–619, 2007. doi: 10.1113/expphysiol.2005.029959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tipton KD, Elliott TA, Cree MG, Aarsland AA, Sanford AP, Wolfe RR. Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am J Physiol Endocrinol Metab 292: E71–E76, 2007. doi: 10.1152/ajpendo.00166.2006. [DOI] [PubMed] [Google Scholar]
- 237.Tomi M, Tajima A, Tachikawa M, Hosoya K. Function of taurine transporter (Slc6a6/TauT) as a GABA transporting protein and its relevance to GABA transport in rat retinal capillary endothelial cells. Biochim Biophys Acta 1778: 2138–2142, 2008. doi: 10.1016/j.bbamem.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 238.Trexler ET, Smith-Ryan AE, Stout JR, Hoffman JR, Wilborn CD, Sale C, Kreider RB, Jäger R, Earnest CP, Bannock L, Campbell B, Kalman D, Ziegenfuss TN, Antonio J. International society of sports nutrition position stand: Beta-Alanine. J Int Soc Sports Nutr 12: 30, 2015. doi: 10.1186/s12970-015-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Vanhatalo A, Fulford J, Bailey SJ, Blackwell JR, Winyard PG, Jones AM. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J Physiol 589: 5517–5528, 2011. doi: 10.1113/jphysiol.2011.216341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239a.Van Koevering M, Nissen S. Oxidation of leucine and α-ketoisocaproate to β-hydroxy-β-methylbutyrate in vivo. Am J Physiol Endocrinol Metab 262: E27–E31, 1992. [DOI] [PubMed] [Google Scholar]
- 240.Vaughan RA, Gannon NP, Carriker CR. Nitrate-containing beetroot enhances myocyte metabolism and mitochondrial content. J Tradit Complement Med 6: 17–22, 2015. doi: 10.1016/j.jtcme.2014.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, van Loon LJ. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci 64: 332–339, 2009. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH, Wodzig WK, Dendale P, van Loon LJ. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr 89: 608–616, 2009. doi: 10.3945/ajcn.2008.26626. [DOI] [PubMed] [Google Scholar]
- 243.Verreijen AM, de Wilde J, Engberink MF, Swinkels S, Verlaan S, Weijs PJ. A High Whey Protein, Leucine Enriched Supplement Preserves Muscle Mass During Intentional Weight Loss in Obese Older Adults: a Double Blind Randomized Controlled Trial. Clin Nutr 32: S3, 2013. doi: 10.1016/S0261-5614(13)60009-6. [DOI] [PubMed] [Google Scholar]
- 244.Visser M, Deeg DJH, Lips P; Longitudinal Aging Study Amsterdam . Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab 88: 5766–5772, 2003. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 245.Volek JS, Duncan ND, Mazzetti SA, Staron RS, Putukian M, Gómez AL, Pearson DR, Fink WJ, Kraemer WJ. Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Med Sci Sports Exerc 31: 1147–1156, 1999. doi: 10.1097/00005768-199908000-00011. [DOI] [PubMed] [Google Scholar]
- 246.Vukovich MD, Dreifort GD. Effect of beta-hydroxy beta-methylbutyrate on the onset of blood lactate accumulation and V(O)(2) peak in endurance-trained cyclists. J Strength Cond Res 15: 491–497, 2001. [PubMed] [Google Scholar]
- 247.Wächter S, Vogt M, Kreis R, Boesch C, Bigler P, Hoppeler H, Krähenbühl S. Long-term administration of L-carnitine to humans: effect on skeletal muscle carnitine content and physical performance. Clin Chim Acta 318: 51–61, 2002. doi: 10.1016/S0009-8981(01)00804-X. [DOI] [PubMed] [Google Scholar]
- 248.Wadley GD. A role for reactive oxygen species in the regulation of skeletal muscle hypertrophy. Acta Physiol (Oxf) 208: 9–10, 2013. doi: 10.1111/apha.12078. [DOI] [PubMed] [Google Scholar]
- 249.Wall BT, Hamer HM, de Lange A, Kiskini A, Groen BBL, Senden JMG, Gijsen AP, Verdijk LB, van Loon LJC. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin Nutr 32: 412–419, 2013. doi: 10.1016/j.clnu.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 250.Wall BT, Stephens FB, Constantin-Teodosiu D, Marimuthu K, Macdonald IA, Greenhaff PL. Chronic oral ingestion of L-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans. J Physiol 589: 963–973, 2011. doi: 10.1113/jphysiol.2010.201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wang Y, DeLuca HF. Is the vitamin d receptor found in muscle? Endocrinology 152: 354–363, 2011. doi: 10.1210/en.2010-1109. [DOI] [PubMed] [Google Scholar]
- 252.Ward KA, Das G, Roberts SA, Berry JL, Adams JE, Rawer R, Mughal MZ. A randomized, controlled trial of vitamin D supplementation upon musculoskeletal health in postmenarchal females. J Clin Endocrinol Metab 95: 4643–4651, 2010. doi: 10.1210/jc.2009-2725. [DOI] [PubMed] [Google Scholar]
- 253.Welch AA. Nutritional influences on age-related skeletal muscle loss. Proc Nutr Soc 73: 16–33, 2014. doi: 10.1017/S0029665113003698. [DOI] [PubMed] [Google Scholar]
- 254.Wilkerson DP, Hayward GM, Bailey SJ, Vanhatalo A, Blackwell JR, Jones AM. Influence of acute dietary nitrate supplementation on 50 mile time trial performance in well-trained cyclists. Eur J Appl Physiol 112: 4127–4134, 2012. doi: 10.1007/s00421-012-2397-6. [DOI] [PubMed] [Google Scholar]
- 255.Wilkes EA, Selby AL, Atherton PJ, Patel R, Rankin D, Smith K, Rennie MJ. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am J Clin Nutr 90: 1343–1350, 2009. doi: 10.3945/ajcn.2009.27543. [DOI] [PubMed] [Google Scholar]
- 256.Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, Etheridge T, Rathmacher JA, Smith K, Szewczyk NJ, Atherton PJ. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J Physiol 591: 2911–2923, 2013. doi: 10.1113/jphysiol.2013.253203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Willoughby DS, Rosene J. Effects of oral creatine and resistance training on myosin heavy chain expression. Med Sci Sports Exerc 33: 1674–1681, 2001. doi: 10.1097/00005768-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 258.Willoughby DS, Rosene JM. Effects of oral creatine and resistance training on myogenic regulatory factor expression. Med Sci Sports Exerc 35: 923–929, 2003. doi: 10.1249/01.MSS.0000069746.05241.F0. [DOI] [PubMed] [Google Scholar]
- 259.Willoughby DS, Stout JR, Wilborn CD. Effects of resistance training and protein plus amino acid supplementation on muscle anabolism, mass, and strength. Amino Acids 32: 467–477, 2007. doi: 10.1007/s00726-006-0398-7. [DOI] [PubMed] [Google Scholar]
- 260.Wilson GJ, Wilson JM, Manninen AH. Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: A review. Nutr Metab (Lond) 5: 1–17, 2008. doi: 10.1186/1743-7075-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wilson JM, Lowery RP, Joy JM, Andersen JC, Wilson SM, Stout JR, Duncan N, Fuller JC, Baier SM, Naimo MA, Rathmacher J. The effects of 12 weeks of beta-hydroxy-beta-methylbutyrate free acid supplementation on muscle mass, strength, and power in resistance-trained individuals: a randomized, double-blind, placebo-controlled study. Eur J Appl Physiol 114: 1217–1227, 2014. doi: 10.1007/s00421-014-2854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Winter JN, Fox TE, Kester M, Jefferson LS, Kimball SR. Phosphatidic acid mediates activation of mTORC1 through the ERK signaling pathway. Am J Physiol Cell Physiol 299: C335–C344, 2010. doi: 10.1152/ajpcell.00039.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr 99: 86–95, 2014. doi: 10.3945/ajcn.112.055517. [DOI] [PubMed] [Google Scholar]
- 264.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 84: 475–482, 2006. [DOI] [PubMed] [Google Scholar]
- 265.Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351: 43–48, 2016. doi: 10.1126/science.aab2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72: 690–693, 2000. [DOI] [PubMed] [Google Scholar]
- 267.Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J, Carey Satterfield M, Smith SB, Spencer TE, Yin Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37: 153–168, 2009. doi: 10.1007/s00726-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wu H, Xia Y, Jiang J, Du H, Guo X, Liu X, Li C, Huang G, Niu K. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr 61: 168–175, 2015. doi: 10.1016/j.archger.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 269.Wylie LJ, Mohr M, Krustrup P, Jackman SR, Ermιdis G, Kelly J, Black MI, Bailey SJ, Vanhatalo A, Jones AM. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur J Appl Physiol 113: 1673–1684, 2013. doi: 10.1007/s00421-013-2589-8. [DOI] [PubMed] [Google Scholar]
- 270.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol (1985) 115: 325–336, 2013. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]
- 271.Xia E-Q, Wang B-W, Xu X-R, Zhu L, Song Y, Li HB. Microwave-assisted extraction of oleanolic acid and ursolic acid from Ligustrum lucidum Ait. Int J Mol Sci 12: 5319–5329, 2011. doi: 10.3390/ijms12085319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yfanti C, Akerström T, Nielsen S, Nielsen AR, Mounier R, Mortensen OH, Lykkesfeldt J, Rose AJ, Fischer CP, Pedersen BK. Antioxidant supplementation does not alter endurance training adaptation. Med Sci Sports Exerc 42: 1388–1395, 2010. doi: 10.1249/MSS.0b013e3181cd76be. [DOI] [PubMed] [Google Scholar]
- 273.Ziegenfuss T, Lowery LM, Lemon PW. Acute fluid volume changes in men during three days of creatine supplementation. J Exerc Physiol 1: 1–9, 1998. [Google Scholar]
- 274.Zorzano A, Fandos C, Palacín M. Role of plasma membrane transporters in muscle metabolism. Biochem J 349: 667–688, 2000. doi: 10.1042/bj3490667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.