Abstract
The World Health Organization ranks hypertension the leading global risk factor for disease, specifically, cardiovascular disease. Blood pressure (BP) is higher in Westernized populations consuming Na+-rich processed foods than in isolated societies consuming K+-rich natural foods. Evidence suggests that lowering dietary Na+ is particularly beneficial in hypertensive individuals who consume a high-Na+ diet. Nonetheless, numerous population studies demonstrate a relationship between higher dietary K+, estimated from urinary excretion or dietary recall, and lower BP, regardless of Na+ intake. Interventional studies with K+ supplementation suggest that it provides a direct benefit; K+ may also be a marker for other beneficial components of a “natural” diet. Recent studies in rodent models indicate mechanisms for the K+ benefit: the distal tubule Na+-Cl− cotransporter (NCC) controls Na+ delivery downstream to the collecting duct, where Na+ reabsorbed by epithelial Na+ channels drives K+ secretion and excretion through K+ channels in the same region. High dietary K+ provokes a decrease in NCC activity to drive more K+ secretion (and Na+ excretion, analogous to the actions of a thiazide diuretic) whether Na+ intake is high or low; low dietary K+ provokes an increase in NCC activity and Na+ retention, also independent of dietary Na+. Together, the findings suggest that public health efforts directed toward increasing consumption of K+-rich natural foods would reduce BP and, thus, cardiovascular and kidney disease.
Keywords: dietary potassium, dietary sodium, ENaC, hypertension, NCC
according to the World Health Organization, the leading risk factor for global disease burden is high blood pressure (BP), accounting for 7.0% of the global disability life years (DALYs) (34). In this analysis, the dietary risk factors contributing to disease burden, and relevant to hypertension, include diets low in fruits, diets high in Na+, and diets low in vegetables (4.2%, 2.5%, and 1.5% DALYs, respectively). Hypertension and these dietary factors contributed primarily to cardiovascular diseases (CVDs), such as ischemic heart disease and stroke (34). While deaths from heart disease and stroke have been halved during the past 40 yr, hypertension prevalence remains very high, and only 50% of hypertensive individuals have their BP under control (32). The thesis has been presented that the interaction of the dietary Na+ surfeit coupled to relative K+ deficiency, rather than either alone, is the critical environmental factor contributing to the hypertension in Western cultures (2, 3). These World Health Organization study findings suggest that lifestyle modification, specifically consumption of diets high in fruits and vegetables (rich sources of K+) and low in Na+, may reduce the disease burden of hypertension and, thus, morbidity and mortality of CVDs. This minireview aims to summarize the evidence for influences of dietary Na+, K+, and the Na+-to-K+ ratio on BP at the level of population studies, interventional studies, and molecular mechanisms.
Population Studies on the Influences of Dietary Sodium and Potassium
Habitual daily salt intake, which can range from 0.05 g NaCl/day in the Yanomamö population to >10 g/day in Western cultures (Table 1), correlates with the rise in BP with age across populations (38). Interestingly, populations consuming very low Na+ exhibit low BP coupled to greatly elevated components of the renin-angiotensin-aldosterone system compared with those consuming a “Western” diet (2, 45). When people move from isolated to industrialized societies, their BP and dietary Na+-to-K+ ratio rise, leading experts to postulate that the hypertension is linked to the rise in the Na+-to-K+ ratio (2, 3, 68). In a study of vegetarians living in Massachusetts, mean arterial pressures were 10–15 mmHg lower and rose less with age than in nonvegetarians (53). While vegetarians consume abundant K+ and alkali-rich fruits and vegetables, it is not clear whether these dietary constituents are simply markers for a “healthy” diet or whether a diet with a lower Na+-to-K+ ratio provides the benefit itself. Additionally, whether the putative benefit is due to lower Na+, higher K+, or the Na+-to-K+ ratio has not been clarified.
Table 1.
Urinary Na+ and K+ output levels in different populations
| Ref No. | Urine Na+, meq/24 h | Urine K+, meq/24 h | Urine Cl−, meq/24 h | Na+-to-K+ Ratio | Plasma Renin Activity, ng·ml−1·h−1 | Urine Aldosterone, µg/24 h | |
|---|---|---|---|---|---|---|---|
| Yanomamö | 44, 45 | 1 | 200 | 15 | 0.007 | 10 | 70 |
| Western diet | 2, 3 | 150 | 50 | 100 | 3 | 1–2 | 8 |
| USDA guide | 62 | 100 | 120 | 0.83 |
Urinary Na+ and K+ levels are used as surrogate biomarkers for dietary Na+ and K+ intake (which assumes input = output at steady state). Individuals in isolated communities generally consume unprocessed foods, leading to overall low-Na+–high-K+ diets; e.g., the diet of Yanomamö natives of the Amazon is very low in Na+, which provokes renin-angiotensin-aldosterone system activation (44, 45). When people move from isolated to industrialized societies and increase consumption of salted processed foods, their Na+ intake rises and K+ falls, raising the dietary Na+-to-K+ ratio above 1 and lowing levels of renin and aldosterone (2, 3). On the basis of epidemiological and interventional studies such as the DASH diet (5), the US Department of Agriculture (USDA) recommends consumption of a diet with a Na+-to-K+ ratio below 1, specifically, Na+ ≤2,300 mg/day (100 meq) and K+ 4,700 mg/day (120 meq) (62).
Regarding dietary Na+, Mente and colleagues recently reported, in a pooled study of >100,000 people from 40 countries, that the association of urinary Na+ excretion (a surrogate marker for Na+ intake) with cardiovascular events was actually U-shaped (39, 40). In 50% of participants, Na+ excretion in this meta-analysis was 4–6 g/day. When stratified into hypertensive and nonhypertensive populations (n ≥ 60,000 each), high Na+ excretion (6–12 g/day) was associated with increased risk of cardiovascular events and death in the hypertensive population alone, whereas there was no significant association within the normotensive population, with Na+ excretion between 4 and 12 g/day (39). Mente and colleagues recommended that people with hypertension and high-Na+ diets should be specifically targeted for lowering dietary Na+. The recommended salt intake of 2.3 g/day (28) is about half the average amount consumed in this study. In hypertensive and nonhypertensive groups, Na+ excretion in this recommended range was associated with significantly elevated risk of cardiovascular events and death (39). This unexpected finding stimulated commentaries that suggested the following caveats: 1) numbers were low in the low-Na+ analysis—only 10% of the population excreted <3 g/day and only 4% excreted <2.4 g/day, the current recommended maximum; 2) results were based on a single morning urine; 3) Na+ excretion may have been low because some individuals were ill; and 4) there was no consideration of K+ excretion (12). Related to the last point, a previous study of the same population did extract the relationship between K+ excretion, Na+ excretion, and BP (40): the highest BPs were observed in the group with Na+ >5 g/day and K+<1.9 g/day (Na+-to-K+ ratio >2.5) and the lowest BPs in the group with Na+ <3 g/day and K+ >2.5 g/day (Na+-to-K+ ratio ~1), indicating that the Na+-to-K+ ratio was an important predictor. Before we leave the topic of dietary Na+, it is worth discussing the salt-reduction program undertaken in the United Kingdom (24). Between 2003 and 2011, there was a 15% reduction in salt intake in the general population (from 9.5 to 8.0 g/day, estimated from 24-h urine Na+). During this time period, BP decreased 3 mmHg and stroke deaths and ischemic heart disease decreased 40% after adjustment for multiple factors, including fruit and vegetable intake and body mass index, both of which increased.
Earlier studies are worth noting. The Third National Health and Nutrition Examination Survey Linked Mortality File (69), conducted between 1988 and 2006 in 12,000 people, used 24-h dietary recalls and reported that higher dietary Na+-to-K+ ratio increased the risk of CVD and all-cause mortality. Interestingly, the ratio was a stronger predictor than dietary Na+ or K+ alone. For the more recent Prevention of Renal and Vascular Endstage Disease prospective study of >5,000 nonmedicated normotensive individuals, 24-h urine (a more rigorous assessment of intakes than dietary recall) was collected twice at 3- to 4-yr intervals and participants were followed for 7.6 yr, during which time 20% developed hypertension. The authors calculated that “six percent of all incident hypertension cases seemed to be attributable to suboptimal dietary potassium” and concluded that strategies to increase K+ intake to the recommended 90 mmol/day (3.5 g/day) have the potential to reduce the incidence of hypertension (30). Three additional studies in smaller populations are also worth noting. Hedayati and colleagues (26) used robust linear regression to examine the relationship between urinary Na+-to-K+ ratio and BP in 3,303 African Americans and non-African Americans of both sexes. They discovered that higher BPs correlated with higher urine Na+-to-K+ ratio (1.6-mmHg rise in systolic pressure for each 3-unit increase in urine Na+-to-K+ ratio); the effect was more pronounced in men than women and in African Americans. Rodrigues and colleagues (51) investigated the effect of K+ on the contribution of dietary Na+ to BP in a population of 1,661 Brazilians with mean urinary Na+-to-K+ ratio of 5. No apparent benefit of dietary K+ was evident in participants excreting <6 g NaCl/day. In contrast, an inverse association of BP with K+ intake was evident in participants consuming >6 g NaCl/day, and those in the highest quartile of K+ excretion exhibited no hypertension, supporting the idea that K+ blunts the influence of high Na+ intake on BP. There have been very few studies of the development of hypertension in children, yet a prospective study in adolescent girls warrants discussion. Buendia et al. (7) assessed Na+ and K+ intake by dietary recall and BP in 2,185 girls age 9–10 yr followed up yearly for 10 yr. The study did not detect an effect of Na+ intake on BP with age [range 2.5 g/day (low) to > 4 g/day (high)]. On the other hand, K+ intake [range <1.8 g/day (low) to >2.4 g/day (high)] was inversely correlated with BP. The study concluded that adolescents should eat K+-rich foods to blunt the developmental rise in BP.
In further support of a K+-rich diet, in a study of >500,000 people in China between 30 and 79 yr old, without CVD or antihypertensive treatment at baseline, participants were queried regarding frequency of fresh fruit consumption, a rich source of dietary K+ (14). BP (systolic reduced 4 mmHg), blood glucose (reduced 9.0 mg/dl), and risks of major CVDs such as ischemic heart disease and stroke (adjusted hazard ratios of 0.60 for cardiovascular deaths) were significantly lower in participants who reported daily or frequent fruit consumption than in those who ate fruit rarely to never. Based on studies like these, Drewnowski and colleagues (13) analyzed the monetary cost of diets based on K+, Na+, and Na+-to-K+ ratios and concluded that K+-rich diets were associated with higher costs, while higher-Na+ diets were not. These investigators recommended that lowering costs of certain K+-rich foods could be an effective mechanism to lower the dietary Na+-to-K+ ratio and recommended identifying low-cost high-K+ foods that should be promoted (e.g., dried beans, potatoes, coffee, skim milk, and bananas).
A number of studies also indicate that higher dietary K+ slows the decline in renal function during chronic diseases. In a study of Japanese type 2 diabetic patients followed for 11 yr, Araki et al. (6) reported that higher K+ excretion was associated with a slower decline in renal function and CVD progression and suggested that future studies should evaluate the effects of increasing dietary K+ intake in this burgeoning population. A 2014 study from the ONTARGET and TRANSCEND investigators (58) evaluated 29,000 participants with established vascular disease or diabetes at high cardiovascular risk for a mean of 4.5 yr and evaluated renal outcomes, including glomerular filtration rate decline, proteinuria, and hyperkalemia, as a function of fasting morning urine Na+ and K+ (as surrogates for intake). They observed no association between Na+ and any renal outcome over a range of intakes from 3 to 6 g/day. In contrast, there was a strong linear association between higher K+ intake and reduced renal outcomes over a range of intakes from 1.7 to 2.7 g/day. The authors state that their findings do not support recommendations to lower Na+ intake to <3 g/day, and they suggest that high-K+ diets may benefit the kidney; they recommend against prematurely restricting dietary K+, especially in patients with normal plasma K+ concentration ([K+]). A 2016 study addressing the effects of dietary Na+ and K+ on the progression of patients from chronic kidney disease to end-stage renal disease or death concluded that progression is reduced when chronic kidney disease patients consumed the lowest quartiles of Na+ and K+, suggesting that as the kidney fails and medications that affect Na+ and K+ transport are added, the renal capacity to regulate K+ homeostasis is compromised and K+ is no longer beneficial (25).
Interventional Studies on the Influence of Dietary Sodium and Potassium
While studies show an inverse association of BP with K+ intake, it remains possible that K+ intake is an indicator, rather than the actual mediator, of the benefit. Interventional studies have been conducted to address whether K+ intake is an actual mediator of the cardiovascular benefit. Twenty-five years ago, in a small study of hypertensive individuals with controlled BP, Siani and colleagues examined the impact of simple nutritional advice aimed at increasing K+ intake from natural foods (56). In those given dietary advice (n = 26), K+ intake, estimated from urinary K+ excretion, increased to 50-70 meq/day, but urine K+ did not change in the controls (n = 21); urine Na+ remained unchanged in both groups. After 1 yr, 81% of the patients with the elevated dietary K+ reduced their medications by 50% compared with 29% of the patients not consuming more K+. A meta-analysis of 33 studies in which dietary K+ supplementation was the only intervention variable (with 2,609 participants) concluded that K+ supplementation significantly reduced systolic and diastolic BP by 3.11 and 1.97 mmHg, respectively; the effects were larger in the studies in which participants consumed more Na+ (68).
Not specifically a K+ supplementation study, the highly regarded Dietary Approaches to Stop Hypertension (DASH) studies investigated the impact of consuming a diet rich in vegetables, fruits, and low-fat dairy products (including whole grains, poultry, fish, and nuts) and reduced in fat, red meat, sweets, and sugar-containing beverages. The DASH diet, administered to participants consuming low, intermediate, or high levels of Na+ for 30 days, lowered BP at all Na+ levels. The greatest impact of the DASH diet was observed in the group consuming the highest levels of Na+ (5, 54). The effects of fruits and vegetables per se, studied in parallel, were shown to lower BP but were not as effective as the DASH diet. The DASH diet has been shown to be effective in hypertensive individuals, the elderly, and African Americans (4). Recently, recognizing that poor dietary habits are leading drivers of CVD and that lifestyle modifications are the first recommendation in hypertension, Gay et al. carried out a meta-analysis comparing the efficacy of a number of different dietary interventions on BP: the DASH diet, a low-Na+–high-K+ diet, a low-Na+–low-calorie diet, a low-Na+ diet, and the Mediterranean diet (20). The DASH diet had the largest effect, reducing systolic and diastolic BPs by 7.6 and 4.22 mmHg, respectively. In comparison, the low-Na+–high-K+ diets decreased systolic and diastolic BPs by about half as much as the DASH diet, and the other diets had even smaller effects. The impact of the DASH diet score (calculated on the basis of reported intake of fruits and vegetables, low-fat dairy, whole grains, legumes and nuts, red meat, and soda) on subsequent kidney disease was examined prospectively over 23 yr in a cohort of 15,000 participants in the Atherosclerosis Risk in Communities study (49). More than 3,000 participants developed kidney disease. While the study did not include measures of albuminuria (an indicator of renal disease), the results did indicate that participants in the highest DASH score quartile were 16% less likely to develop kidney disease than those in the lowest DASH score quartile; interestingly, the dietary components that appeared most beneficial were nuts, legumes, and low-fat dairy. Overall, a DASH-style diet, which has a lower Na+-to-K+ ratio, appears beneficial for lowering BP and slowing progression of kidney disease, but the exact component(s) that provide the benefit are not clear.
One of the most deliberate interventional studies to examine the effect of dietary K+ on cardiovascular health was conducted a decade ago by Chang et al. (9) in a population of elderly Taiwanese male veterans with normal renal function living in retirement communities. K+-enriched salt (49% NaCl-49% KCl) was provided to two kitchens (n = 635) and regular salt to three kitchens (n = 916); condiments such as soy sauce and monosodium glutamate were not restricted. Participants were studied over 31 mo. Intakes of Na+ and K+ were estimated on the basis of urinary excretion normalized to urine creatinine (UK/UCr, UNa/UCr) in ~25% of the participants: UNa/UCr decreased 10%, and UK/UCr increased 70%. At 30 mo, a 40% relative risk reduction in CVD mortality was evident, indicating that a minor adjustment in dietary salt intake, raising K+ 70% and lowering Na+ 10%, can have a major impact on CVD mortality. On the basis of the results of this study, a 2014 study in 282 hypertensive Tibetans living at high altitude asked whether a 65% NaCl-25% KCl-10% MgSO4 salt substitute could lower BP (71). After 3 mo, systolic and diastolic BPs (after adjustment for baseline and other variables) decreased ~8 and 3.5 mmHg, respectively, lending support to this simple approach to reduce BP in remote populations.
A review of 52 studies conducted between 1990 and 2013 by Aaron and Sanders (1) concurs with the findings of the population and interventional studies discussed above. The authors do not provide a recommendation for a lower limit for Na+ or an upper limit for K+ consumption, but they do conclude that raising dietary K+ blunts the effects of high dietary Na+, especially in overweight and aging adults. They recommend a general strategy of modest Na+ restriction with increasing K+ intake to control BP, prevent kidney disease and stroke, and reduce CVD mortality.
Molecular Mechanisms That Facilitate the Cardiovascular Benefits of Raising Dietary Potassium
Why does elevating dietary K+ reduce the incidence of hypertension and CVD, even in the presence of normal-to-high dietary Na+? The answer appears to lie in homeostasis hierarchies: the body does a balancing act to maintain circulating Na+ mass and volume, K+, and osmolality, among other factors. K+ has a high homeostatic priority because of its critical importance in determining membrane potential and, thus, nerve and muscle function. Na+ and volume homeostasis appear to take a backseat to K+ homeostasis, because K+ homeostasis is maintained by the concerted regulation of Na+, as well as K+, transporters and channels in the renal distal nephron, the subject of numerous recent reviews (15, 16, 23, 47). In this aldosterone-sensitive distal nephron (Fig. 1), Na+ is reabsorbed along the distal convoluted tubule (DCT) via Na+-Cl− cotransporters (NCC), influenced by NCC distribution in the plasma membrane vs. subapical vesicles and, by activating phosphorylation, “NCCp” (46, 55). Na+ reabsorption by NCC determines the fractional delivery of Na+ downstream to the epithelial Na+ channels (ENaC), which reabsorb Na+ when activated by proteolytic cleavage in the plasma membrane (31). Na+ reabsorbed by ENaC provides an electrochemical driving force for K+ secretion via the renal outer medullary K+ channel, as well as flow-sensitive K+ channels (27, 67). Thus K+ secretion and excretion are dependent on the state of Na+ transporter (NCC and ENaC) activation, which is also key to Na+ and effective circulating volume (ECV) homeostasis. Recent studies investigating the molecular mechanisms responsible for excreting K+ shed light on this relationship (48, 50, 59). Whether K+ is provided by gavage, with a meal, or infused into a rodent, [K+] in the extracellular fluid provides a signal to rapidly dephosphorylate NCC, which reduces Na+ reabsorption in the DCT, provoking a downstream shift in Na+ reabsorption to ENaC, where it drives K+ secretion and excretion (Fig. 2B) (50, 59). Since ENaC activation cannot completely compensate for the inhibition of higher-capacity NCC, the K+-rich meal provokes acute increases in urinary Na+, as well as K+ (50, 59, 64). As summarized in Table 2, columns III and IV, the natriuretic consequences of K+ adaptation can contribute to reducing ECV and BP, likely contributing to the association between high-K+ diets and lower BP in the population studies discussed above (15, 47) and in individuals consuming the DASH diet (5). Conversely, a low-K+ diet (or factors that increase urinary K+ loss) provokes an increase in NCC abundance and phosphorylation, especially evident with high-salt diet consumption, in order to reduce Na+ delivery to ENaC and reduce urinary K+; the NCC activation during Na+-replete–K+-deficient feeding in rodents is associated with a significant antinatriuresis and a rise in BP (61, 66). These findings are reminiscent of the hypertension evident in industrialized populations consuming high-Na+–low-K+ Western diets and suggest that BP rises because the body places a higher priority on K+ than Na+ and volume homeostasis (Table 2, columns I and II).
Fig. 1.
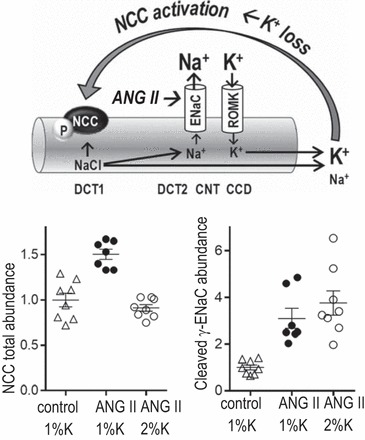
Angiotensin II (ANG II) infusion (400 ng·kg−1·min−1) in rats fed 1% K+ diet activates the epithelial Na+ channel (ENaC), evident as increased abundance of the cleaved γ-subunit, and increases distal convoluted tubule (DCT1 and DCT2) Na+-Cl− cotransporter (NCC) abundance (ANG II, 1% K) compared with control 1% K+ diet-fed rats (65). Rats fed 1% K+ also presented with higher urinary K+ and lower plasma K+ concentration after an overnight fast (not shown), leading to the hypothesis that the increased NCC abundance was secondary to K+ depletion, a response that decreases Na+ delivery to ENaC, which drives K+ secretion via K+ channels such as the renal outer medullary K+ (ROMK) channel. This hypothesis (top) was supported by data from the study of Vieras et al. (65) of rats fed a diet in which K+ was doubled during ANG II infusion (ANG II, 2%K) to prevent K+ depletion (bottom): NCC was unchanged, and γENaC cleavage was elevated. CNT, connecting tubule, CCD, cortical collecting duct.
Fig. 2.
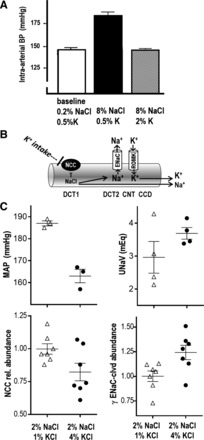
Raising dietary K+ counteracts hypertension in spontaneously hypertensive rats (SHR). A: at baseline, with dietary electrolytes at 0.2% NaCl and 0.5% K+, blood pressure (BP) is 148 mmHg. Raising dietary NaCl to 8% increases BP to 182 mmHg if K+ is kept at 0.5%, but BP does not increase if K+ is also raised to 2% (146 mmHg). [Redrawn from results of Ganguli and Tobian (18).] B: dietary K+ intake, under conditions of normokalemia, inhibits Na+ reabsorption via DCT NCC, which increases Na+ delivery downstream to ENaC, where Na+ reabsorption drives increased K+ secretion. Net result is increased excretion of Na+ and K+. C: summary of results from a small study of SHR fed 2% NaCl + 1% KCl or 2% NaCl + 4% KCl from 6 to 13 wk of age (37). Tail cuff BP [mean arterial pressure (MAP), mean of weekly measurements from weeks 11–14 after 3 wk of adaptation] was 24 mmHg lower in SHR fed 4% KCl; overnight urine Na+ (UNaV) was marginally increased by 25%; NCC total abundance, measured by immunoblotting, was decreased ~15%, and ENaC activation, estimated from abundance of the cleaved (clvd) γ-subunit, was increased ~20%, supporting the model linking increased K+ secretion to decreased Na+ reabsorption.
Table 2.
Predicted outcomes of consuming diets with different Na+-to-K+ ratios
|
I Processed Food Western Diet (high Na+-low K+) |
II Reduced-Na+ Western Diet (low Na+-low K+) |
III K+-Enriched Western Diet (high Na+-high K+) |
IV Recommended DASH Diet (low Na+-high K+) |
|---|---|---|---|
| ↓ Renin, ANG II | ↑ Renin, ANG II | ↑ Aldo | ↑ Renin, ANG II, Aldo |
| ↑ NCC activity | ↑ NCC activity | ↓NCC activity | ↓ NCC activity |
| ↓ ENaC activity | ↓ ENaC activity | ↑ ENaC activity | ↑ ENaC activity |
| K+ retention | K+ retention | K+ excretion | K+ excretion |
| Na+ retention | Na+ retention | Na+ excretion | Na+ excretion |
| ↑ Blood pressure | ← → Blood pressure | ↓ Blood pressure | ↓ Blood pressure |
Raising dietary K+ provokes a decrease in NCC activity, independent of dietary Na+, which provokes an increase in NCC activity, independent of dietary Na+. ANG II, angiotensin II; NCC, Na+-Cl− cotransporter; ENaC, epithelial Na+ channel; Aldo, aldosterone.
The issue of the homeostatic hierarchy of Na+ vs. K+ in the distal nephron was directly addressed in a 2011 study in rats by Frindt et al. (17), who investigated the physiological consequences of Na+ depletion with and without accompanying K+ depletion. The results showed that ENaC activity was increased, as expected, during Na+ depletion with normokalemia and that ENaC activity was unexpectedly reduced during Na+ depletion plus low K+ intake, presumably to reduce the driving force for K+ secretion. In the low Na+-low K+ intake set, the abundance of DCT NCC doubled. This coordinated pattern of NCC and ENaC regulation in response to low K+ intake supports the notion that K+ homeostasis takes the higher priority and that other pathways are called in to manage Na+ and volume (19). Additionally, the results of this low Na+-low K+ feeding experiment provide an explanation for why lowering dietary Na+ may not lower BP in patients with low dietary K+ intake and why simply raising dietary K+ may lower BP (Table 2, column II vs. column III).
Recently, progress has been made in understanding the renal sensing of K+ status. Interestingly, a key sensor is located in the DCT (upstream from the location of K+ secretion), where regulation of NCC activation controls Na+ delivery to ENaC and the drive to secrete K+. Specifically, low plasma [K+] activates a heteromer of K+ channels located in the basolateral membrane of the DCT cells (Kir4.1/5.1, the K+ sensor), which hyperpolarizes the membrane potential and lowers cell Cl− concentration, which relieves the inhibition of the WNK-SPAK kinase cascade, leading to NCC phosphorylation (NCCp) and activation, which reduces Na+ delivery to ENaC downstream (60, 61).
How does the renin-angiotensin system interface with K+ homeostasis in the control of plasma [K+] vs. ECV and BP? The stimulus to increase NCC during low Na+ intake has been attributed to a direct effect of angiotensin II (ANG II) stimulation in many studies (52). In response to acute ANG II stimulation, we observed increased NCC trafficking to the plasma membrane (55) but not acute changes in NCC activating phosphorylation (33). In contrast, there is agreement across laboratories that chronic ANG II infusion is associated with increases in both NCC abundance and activating phosphorylation (8, 43, 63). Relevant to NCC phosphorylation, ANG II also directly stimulates ENaC activity acutely (35) and chronically (21, 36), thus increasing the driving force for K+ secretion and excretion. We postulated that K+ depletion during chronic ANG II infusion was the primary stimulus driving increased NCCp, rather than ANG II per se. Our recent results support this hypothesis (65): ANG II (vs. control)-infused male rats presented with ENaC activation (assessed as cleaved γENaC) along with the predicted urinary K+ loss, lower plasma [K+], and increased NCCp (Fig. 1). A single K+-rich meal did not reduce NCCp or increase K+ excretion, suggesting a K+-depleted state in which the ingested K+ is directed to replenish intracellular stores. However, doubling dietary K+ intake during the chronic ANG II infusion normalized plasma [K+] and prevented the increase in NCCp (Fig. 1), in support of the conclusion that the rise in NCCp during ANG II infusion is due to the K+ loss, which stimulates the DCT K+ sensors, depolarizes the membrane, decreases cell Cl−, and activates NCCp. Incidentally, the dietary K+ supplementation did not blunt the ANG II hypertension, likely because of the multiple antinatriuretic effects of ANG II (e.g., ENaC activation). Nonetheless, coupled to the evidence that a low-K+–Na+-replete diet can raise BP (66), these findings suggest that recommendation of a DASH-style diet to prevent K+ depletion and reduce distal tubule NCC-mediated Na+ reabsorption should be considered.
The observation that raising dietary K+ can lower BP (even in the face of high Na+ intake) is not new. In 1990, Ganguli and Tobian (18) demonstrated that the level of dietary K+ determined the degree of salt-sensitive hypertension in spontaneously hypertensive rats (SHR). With a ratio of 0.25% NaCl to 0.5% K+, baseline BP was 146 mmHg in this hypertensive rat strain. Raising dietary NaCl to 8% increased BP 36 mmHg when K+ was kept at 0.5%, but 8% NaCl did not raise BP when dietary K+ was also increased to 2% (Fig. 2A). Mortality in these stroke-prone SHR after 14 wk on diets was 90% in the 8% NaCl-0.5% K+ group and only 5% in the 8% NaCl-2% K+ group. Remarkably, decreasing NaCl from 8% to 0.24% in 2% K+-fed SHR lowered BP <10 mmHg. Thus, as verified in the DASH diet studies in humans, K+ lowers BP more in populations consuming high-Na+ diets (5).
We conducted a limited follow-up study, also in SHR fed elevated NaCl and low or elevated KCl. SHR were fed 2% NaCl (as in a Western diet) with 1% KCl [equivalent to 0.5% K+ in the study of Ganguli and Tobian (18)] or 4% KCl between 6 and 14 wk of age (Fig. 2C) (37). BP was blunted by raising KCl, confirming the findings of Ganguli and Tobian (18); additionally, overnight urinary Na+ was elevated in the 4% KCl-fed group, confirming the natriuretic effect of elevating K+ intake (Fig. 2B). At the molecular level, NCC levels were lower and activated ENaC levels (assessed as cleaved γENaC) were higher in the 4% KCl-fed group, consistent with adaptations to increase K+ excretion (changes were rather small, as Na+ transporters are suppressed by the high Na+ intake). Together, the results summarized in Fig. 2 provide molecular mechanisms that may account for how raising dietary K+ lowers BP in the SHR. Basically, the results reinforce the conclusion that consuming a high-K+ diet is similar to taking a NCC-inhibiting thiazide diuretic, without the side effects (15).
Muscle also plays an important role in K+ adaptation and homeostasis. Muscle serves as the main intracellular K+ reservoir and buffer pool, containing >90% of the body’s K+. Muscle actively takes up K+ during dietary K+ excess, mediated by Na+-K+-ATPase activation, and releases K+ during K+ deficiency or prolonged fasting; both adaptations are aimed at maintaining extracellular [K+] in the normal range (11, 70). Additionally, exercise improves extrarenal K+ adaptation, as it optimizes K+ fluxes across muscles not only by increasing mass but also by increasing Na+-K+-ATPase activity (10, 42). Thus muscle works in concert with the kidneys to maintain and optimize K+ homeostasis (37a).
Conclusions and Implications
Medical communities, First Lady Michelle Obama, and common sense tell us to eat more fruits and vegetables and to get regular exercise to optimize our health. Along with exercise, consuming a surfeit of dietary K+ is a good strategy, since our physiology evolved and was optimized to deal with high-K+–low-Na+ intake, often referred to a Paleolithic diet (29). How can a society implement these straightforward lifestyle adjustment recommendations? A commentary by Silver and Farley (57) on a study of Na+ and K+ intake and mortality from NHANES III (69) notes that more than three-fourths of the NaCl in the US diet is added during food processing and, thus, is difficult to control or assess. They suggest the following feasible and concrete recommendations: 1) promote efforts to reduce Na+ artificially added to food during processing to reduce mortality, as implemented in England and discussed above (24), 2) promote public policies to increase dietary intake of K+ from plant-based sources, 3) make fruits and vegetables available at lower cost and more accessible, as discussed by Drewnowski et al. (13), 4) develop food-processing technology that retains natural composition of cations, and 5) require manufacturers to display K+ content on nutrition facts panels. Recent advice from the Rogosin Roundtable to medical doctors, nurses, and nutritionists who treat renal patients suggests an “ABCD” approach (41): Access to affordable, fresh foods; Back to basics; Cooking: You can do it!; and Deliver information a patient can understand. The specifics included making nutrition health a priority and giving people the tools to make healthier food choices; encouraging the best of technology-based tools for nutrition management; increasing access to fresh foods by activating and working with community leaders to bring fresh food to the patients, as has been implemented by Goraya et al. (22); encouraging patients and their families to enjoy cooking healthy meals together; and promoting policy changes to increase dietitian-to-patient ratios. All these feasible suggestions bring with them the promise of reducing the incidence of hypertension and CVD, as well as their enormous costs. The challenge now is implementation.
GRANTS
This work is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-083785 and American Heart Association Western States Affiliate Grant-in-Aid 15GRNT23160003 to A. A. McDonough.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A.M., L.C.V., and D.L.R. conceived and designed the research; A.A.M., L.C.V., C.A.G., and D.L.R. analyzed the data; A.A.M., L.C.V., and C.A.G. prepared the figures; A.A.M. drafted the manuscript; A.A.M., L.C.V., and D.L.R. edited and revised the manuscript; A.A.M., L.C.V., C.A.G., and D.L.R. approved the final version of the manuscript.
REFERENCES
- 1.Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc 88: 987–995, 2013. doi: 10.1016/j.mayocp.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adrogué HJ, Madias NE. The impact of sodium and potassium on hypertension risk. Semin Nephrol 34: 257–272, 2014. doi: 10.1016/j.semnephrol.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Adrogué HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med 356: 1966–1978, 2007. doi: 10.1056/NEJMra064486. [DOI] [PubMed] [Google Scholar]
- 4.Appel LJ. Lifestyle modification as a means to prevent and treat high blood pressure. J Am Soc Nephrol 14, Suppl 2: S99–S102, 2003. doi: 10.1097/01.ASN.0000070141.69483.5A. [DOI] [PubMed] [Google Scholar]
- 5.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM; American Heart Association . Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 47: 296–308, 2006. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- 6.Araki S, Haneda M, Koya D, Kondo K, Tanaka S, Arima H, Kume S, Nakazawa J, Chin-Kanasaki M, Ugi S, Kawai H, Araki H, Uzu T, Maegawa H. Urinary potassium excretion and renal and cardiovascular complications in patients with type 2 diabetes and normal renal function. Clin J Am Soc Nephrol 10: 2152–2158, 2015. doi: 10.2215/CJN.00980115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buendia JR, Bradlee ML, Daniels SR, Singer MR, Moore LL. Longitudinal effects of dietary sodium and potassium on blood pressure in adolescent girls. JAMA Pediatr 169: 560–568, 2015. doi: 10.1001/jamapediatrics.2015.0411. [DOI] [PubMed] [Google Scholar]
- 8.Castañeda-Bueno M, Cervantes-Pérez LG, Vázquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G. Activation of the renal Na+:Cl− cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA 109: 7929–7934, 2012. doi: 10.1073/pnas.1200947109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang HY, Hu YW, Yue CS, Wen YW, Yeh WT, Hsu LS, Tsai SY, Pan WH. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr 83: 1289–1296, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Clausen T. Excitation of skeletal muscle is a self-limiting process, due to run-down of Na+, K+ gradients, recoverable by stimulation of the Na+, K+ pumps. Physiol Rep 3: e12373, 2015. doi: 10.14814/phy2.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev 83: 1269–1324, 2003. doi: 10.1152/physrev.00011.2003. [DOI] [PubMed] [Google Scholar]
- 12.Cogswell ME, Mugavero K, Bowman BA, Frieden TR. Dietary sodium and cardiovascular disease risk—measurement matters. N Engl J Med 375: 580–586, 2016. doi: 10.1056/NEJMsb1607161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drewnowski A, Rehm CD, Maillot M, Monsivais P. The relation of potassium and sodium intakes to diet cost among US adults. J Hum Hypertens 29: 14–21, 2015. doi: 10.1038/jhh.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du H, Li L, Bennett D, Guo Y, Key TJ, Bian Z, Sherliker P, Gao H, Chen Y, Yang L, Chen J, Wang S, Du R, Su H, Collins R, Peto R, Chen Z; China Kadoorie Biobank Study . Fresh fruit consumption and major cardiovascular disease in China. N Engl J Med 374: 1332–1343, 2016. doi: 10.1056/NEJMoa1501451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellison DH, Terker AS. Why your mother was right: how potassium intake reduces blood pressure. Trans Am Clin Climatol Assoc 126: 46–55, 2015. [PMC free article] [PubMed] [Google Scholar]
- 16.Ellison DH, Terker AS, Gamba G. Potassium and its discontents: new insight, new treatments. J Am Soc Nephrol 27: 981–989, 2016. doi: 10.1681/ASN.2015070751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frindt G, Ergonul Z, Palmer LG. Surface expression of epithelial Na channel protein in rat kidney. J Gen Physiol 131: 617–627, 2008. doi: 10.1085/jgp.200809989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganguli M, Tobian L. Dietary K determines NaCl sensitivity in NaCl-induced rises of blood pressure in spontaneously hypertensive rats. Am J Hypertens 3: 482–484, 1990. doi: 10.1093/ajh/3.6.482. [DOI] [PubMed] [Google Scholar]
- 19.Garty H. Complex challenges—what will the collecting duct do when both Na+ and K+ have to be conserved. Am J Physiol Renal Physiol 301: F12–F13, 2011. doi: 10.1152/ajprenal.00190.2011. [DOI] [PubMed] [Google Scholar]
- 20.Gay HC, Rao SG, Vaccarino V, Ali MK. Effects of different dietary interventions on blood pressure: systematic review and meta-analysis of randomized controlled trials. Hypertension 67: 733–739, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06853. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, Seth DM, Fuchs S, Eladari D, Picard N, Bachmann S, Delpire E, Peti-Peterdi J, Navar LG, Bernstein KE, McDonough AA. The absence of intrarenal ACE protects against hypertension. J Clin Invest 123: 2011–2023, 2013. doi: 10.1172/JCI65460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goraya N, Simoni J, Jo C, Wesson DE. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int 81: 86–93, 2012. doi: 10.1038/ki.2011.313. [DOI] [PubMed] [Google Scholar]
- 23.Gumz ML, Rabinowitz L, Wingo CS. An integrated view of potassium homeostasis. N Engl J Med 373: 60–72, 2015. doi: 10.1056/NEJMra1313341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He FJ, Pombo-Rodrigues S, Macgregor GA. Salt reduction in England from 2003 to 2011: its relationship to blood pressure, stroke and ischaemic heart disease mortality. BMJ Open 4: e004549, 2014. doi: 10.1136/bmjopen-2013-004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He J, Mills KT, Appel LJ, Yang W, Chen J, Lee BT, Rosas SE, Porter A, Makos G, Weir MR, Hamm LL, Kusek JW; Chronic Renal Insufficiency Cohort Study Investigators . Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol 27: 1202–1212, 2016. doi: 10.1681/ASN.2015010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedayati SS, Minhajuddin AT, Ijaz A, Moe OW, Elsayed EF, Reilly RF, Huang CL. Association of urinary sodium/potassium ratio with blood pressure: sex and racial differences. Clin J Am Soc Nephrol 7: 315–322, 2012. doi: 10.2215/CJN.02060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holtzclaw JD, Grimm PR, Sansom SC. Role of BK channels in hypertension and potassium secretion. Curr Opin Nephrol Hypertens 20: 512–517, 2011. doi: 10.1097/MNH.0b013e3283488889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Institute of Medicine Dietary Reference Intakes: The Essential Guide to Nutrient Requirements (Online). Washington, DC: National Academies Press, 2006. https://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 29.Kamel KS, Schreiber M, Halperin ML. Integration of the response to a dietary potassium load: a paleolithic perspective. Nephrol Dial Transplant 29: 982–989, 2014. doi: 10.1093/ndt/gft499. [DOI] [PubMed] [Google Scholar]
- 30.Kieneker LM, Gansevoort RT, Mukamal KJ, de Boer RA, Navis G, Bakker SJ, Joosten MM. Urinary potassium excretion and risk of developing hypertension: the prevention of renal and vascular end-stage disease study. Hypertension 64: 769–776, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03750. [DOI] [PubMed] [Google Scholar]
- 31.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotchen TA. Historical trends and milestones in hypertension research: a model of the process of translational research. Hypertension 58: 522–538, 2011. doi: 10.1161/HYPERTENSIONAHA.111.177766. [DOI] [PubMed] [Google Scholar]
- 33.Lee DH, Maunsbach AB, Riquier-Brison AD, Nguyen MT, Fenton RA, Bachmann S, Yu AS, McDonough AA. Effects of ACE inhibition and ANG II stimulation on renal Na-Cl cotransporter distribution, phosphorylation, and membrane complex properties. Am J Physiol Cell Physiol 304: C147–C163, 2013. doi: 10.1152/ajpcell.00287.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2260, 2012. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, Pochynyuk O. Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J Biol Chem 287: 660–671, 2012. doi: 10.1074/jbc.M111.298919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mamenko M, Zaika O, Prieto MC, Jensen VB, Doris PA, Navar LG, Pochynyuk O. Chronic angiotensin II infusion drives extensive aldosterone-independent epithelial Na+ channel activation. Hypertension 62: 1111–1122, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonough AA, Nguyen MT, Lee D, Riquier-Brison A. Beneficial effects of 4% KCl-1% NaCl and 4% KCl-2% NaCl vs. 1% KCl-2% NaCl diets in SHR rats (Abstract). J Am Soc Nephrol 21: 744A, 2010. [Google Scholar]
- 37a.McDonough AA, Youn JH. Potassium homeostasis: the knowns, the unknowns, and the health benefits. Physiology (Bethesda) 32: 100–111, 2017. doi: 10.1152/physiol.00022.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85: 679–715, 2005. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 39.Mente A, O’Donnell M, Rangarajan S, Dagenais G, Lear S, McQueen M, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Li W, Lu Y, Yi S, Rensheng L, Iqbal R, Mony P, Yusuf R, Yusoff K, Szuba A, Oguz A, Rosengren A, Bahonar A, Yusufali A, Schutte AE, Chifamba J, Mann JF, Anand SS, Teo K, Yusuf S; PURE, EPIDREAM and ONTARGET/TRANSCEND Investigators . Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet 388: 465–475, 2016. doi: 10.1016/S0140-6736(16)30467-6. [DOI] [PubMed] [Google Scholar]
- 40.Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, Morrison H, Li W, Wang X, Di C, Mony P, Devanath A, Rosengren A, Oguz A, Zatonska K, Yusufali AH, Lopez-Jaramillo P, Avezum A, Ismail N, Lanas F, Puoane T, Diaz R, Kelishadi R, Iqbal R, Yusuf R, Chifamba J, Khatib R, Teo K, Yusuf S; PURE Investigators . Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med 371: 601–611, 2014. doi: 10.1056/NEJMoa1311989. [DOI] [PubMed] [Google Scholar]
- 41.Moe SM, Crews DC, Gutiérrez OM, Hoyt-Hudson P, Lew SQ, Shanaman B, Smith BH, Weiner DE, Wesson D. Food as medicine: no more renal “diet”. ASN Kidney News Online 8: 16–17, 2016. [Google Scholar]
- 42.Murphy KT, Nielsen OB, Clausen T. Analysis of exercise-induced Na+-K+ exchange in rat skeletal muscle in vivo. Exp Physiol 93: 1249–1262, 2008. doi: 10.1113/expphysiol.2008.042457. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen MT, Lee DH, Delpire E, McDonough AA. Differential regulation of Na+ transporters along nephron during ANG II-dependent hypertension: distal stimulation counteracted by proximal inhibition. Am J Physiol Renal Physiol 305: F510–F519, 2013. doi: 10.1152/ajprenal.00183.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nowaczynski W, Oliver WJ, Neel JV. Serum aldosterone and protein-binding variables in Yanomama Indians: a no-salt culture as compared to partially acculturated Guaymi Indians. Clin Physiol Biochem 3: 289–306, 1985. [PubMed] [Google Scholar]
- 45.Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation 52: 146–151, 1975. doi: 10.1161/01.CIR.52.1.146. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA. Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int 78: 160–169, 2010. doi: 10.1038/ki.2010.130. [DOI] [PubMed] [Google Scholar]
- 47.Penton D, Czogalla J, Loffing J. Dietary potassium and the renal control of salt balance and blood pressure. Pflügers Arch 467: 513–530, 2015. doi: 10.1007/s00424-014-1673-1. [DOI] [PubMed] [Google Scholar]
- 48.Penton D, Czogalla J, Wengi A, Himmerkus N, Loffing-Cueni D, Carrel M, Rajaram RD, Staub O, Bleich M, Schweda F, Loffing J. Extracellular K+ rapidly controls NCC phosphorylation in native DCT by Cl−-dependent and -independent mechanisms. J Physiol 594: 6319–6331, 2016. doi: 10.1113/JP272504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rebholz CM, Crews DC, Grams ME, Steffen LM, Levey AS, Miller ER 3rd, Appel LJ, Coresh J. DASH (Dietary Approaches to Stop Hypertension) diet and risk of subsequent kidney disease. Am J Kidney Dis 68: 853–861, 2016. doi: 10.1053/j.ajkd.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA. Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 306: F1059–F1068, 2014. doi: 10.1152/ajprenal.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodrigues SL, Baldo MP, Machado RC, Forechi L, Molina MC, Mill JG. High potassium intake blunts the effect of elevated sodium intake on blood pressure levels. J Am Soc Hypertens 8: 232–238, 2014. doi: 10.1016/j.jash.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Rojas-Vega L, Gamba G. Regulation of the renal NaCl cotransporter by hormones. Am J Physiol Renal Physiol 310: F10–F14, 2016. doi: 10.1152/ajprenal.00354.2015 [DOI] [PubMed] [Google Scholar]
- 53.Sacks FM, Kass EH. Low blood pressure in vegetarians: effects of specific foods and nutrients. Am J Clin Nutr 48, Suppl: 795–800, 1988. [DOI] [PubMed] [Google Scholar]
- 54.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons-Morton DG, Karanja N, Lin PH, Aickin M, Most-Windhauser MM, Moore TJ, Proschan MA, Cutler JA; DASH-Sodium Collaborative Research Group . Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 344: 3–10, 2001. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 55.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. ANG II provokes acute trafficking of distal tubule Na+-Cl− cotransporter to apical membrane. Am J Physiol Renal Physiol 293: F662–F669, 2007. doi: 10.1152/ajprenal.00064.2007. [DOI] [PubMed] [Google Scholar]
- 56.Siani A, Strazzullo P, Giacco A, Pacioni D, Celentano E, Mancini M. Increasing the dietary potassium intake reduces the need for antihypertensive medication. Ann Intern Med 115: 753–759, 1991. doi: 10.7326/0003-4819-115-10-753. [DOI] [PubMed] [Google Scholar]
- 57.Silver LD, Farley TA. Sodium and potassium intake: mortality effects and policy implications: comment on “Sodium and potassium intake and mortality among US adults”. Arch Intern Med 171: 1191–1192, 2011. doi: 10.1001/archinternmed.2011.271. [DOI] [PubMed] [Google Scholar]
- 58.Smyth A, Dunkler D, Gao P, Teo KK, Yusuf S, O’Donnell MJ, Mann JF, Clase CM; ONTARGET and TRANSCEND Investigators . The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int 86: 1205–1212, 2014. doi: 10.1038/ki.2014.214. [DOI] [PubMed] [Google Scholar]
- 59.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 60.Su XT, Wang WH. The expression, regulation, and function of Kir4.1 (Kcnj10) in the mammalian kidney. Am J Physiol Renal Physiol 311: F12–F15, 2016. doi: 10.1152/ajprenal.00112.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.US Department of Health and Human Services and Agriculture. US Department of Agriculture 2015–2020 Dietary Guidelines for Americans. Washington, DC: US Dept. of Agriculture, 2015. [Google Scholar]
- 63.van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zietse R, Hoorn EJ. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int 79: 66–76, 2011. doi: 10.1038/ki.2010.290. [DOI] [PubMed] [Google Scholar]
- 64.van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJ. K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl− cotransporter. Am J Physiol Renal Physiol 305: F1177–F1188, 2013. doi: 10.1152/ajprenal.00201.2013. [DOI] [PubMed] [Google Scholar]
- 65.Veiras LC, Han J, Ralph DL, McDonough AA. Potassium supplementation prevents sodium chloride cotransporter stimulation during angiotensin II hypertension. Hypertension 68: 904–912, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vitzthum H, Seniuk A, Schulte LH, Müller ML, Hetz H, Ehmke H. Functional coupling of renal K+ and Na+ handling causes high blood pressure in Na+ replete mice. J Physiol 592: 1139–1157, 2014. doi: 10.1113/jphysiol.2013.266924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Welling PA. Regulation of renal potassium secretion: molecular mechanisms. Semin Nephrol 33: 215–228, 2013. doi: 10.1016/j.semnephrol.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 68.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA 277: 1624–1632, 1997. doi: 10.1001/jama.1997.03540440058033. [DOI] [PubMed] [Google Scholar]
- 69.Yang Q, Liu T, Kuklina EV, Flanders WD, Hong Y, Gillespie C, Chang MH, Gwinn M, Dowling N, Khoury MJ, Hu FB. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 171: 1183–1191, 2011. doi: 10.1001/archinternmed.2011.257. [DOI] [PubMed] [Google Scholar]
- 70.Youn JH, McDonough AA. Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol 71: 381–401, 2009. doi: 10.1146/annurev.physiol.010908.163241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao X, Yin X, Li X, Yan LL, Lam CT, Li S, He F, Xie W, Sang B, Luobu G, Ke L, Wu Y. Using a low-sodium, high-potassium salt substitute to reduce blood pressure among Tibetans with high blood pressure: a patient-blinded randomized controlled trial. PLoS One 9: e110131, 2014. doi: 10.1371/journal.pone.0110131. [DOI] [PMC free article] [PubMed] [Google Scholar]


