Summary
Group B streptococcus (GBS) is part of the normal genital and gastrointestinal flora in healthy humans. However, GBS is a major cause of sepsis and meningitis in newborn infants in the Western world and an important pathogen in many developing countries. The dissection of the host response to GBS may increase the general understanding of innate immunity in sepsis, since newborn infants lack a sufficient adaptive response. Inflammatory signal induction in macrophages by GBS seems largely preserved in newborn infants, as shown both in vitro and in vivo. The engagement of Toll-like receptor 2 (TLR2) by lipoproteins and a myeloid differentiation factor 88 (MyD88)-dependent pathway induced by GBS cell wall are both important in this context. TLR2 activation of microglia by GBS induces neuronal damage, which might account for the high morbidity of GBS meningitis. At the same time, TLR2 mediates activation-induced cell death (AICD), a process involved in the containment of inflammation. In newborn infants, AICD and anti-bacterial polymorphonuclear leukocyte activity appears to be compromised. Accordingly, neonatal aberrations in the pathogen-specific negative control of inflammatory signaling are likely to contribute to excessive inflammation and neurological sequelae in GBS sepsis and meningitis.
Keywords: monocytes/macrophages, bacterial infection, cytokines, Toll-like receptors, apoptosis/autophagy, cell activation
Introduction
At the beginning of extrauterine life, the newborn infant transitions from the sterile womb to an environment that is loaded with potentially harmful microorganisms. Group B streptococcus (GBS), Streptococcus agalactiae, is among the first bacterial species that the fetus encounters, as GBS colonizes the birth canal of 10−30% of all pregnant women (1). Many newborn infants have to manage a GBS challenge before mechanisms of coexistence with microorganisms are firmly established. Most newborn infants meet this challenge without any apparent irritation, although persistent mucosal and dermal colonization with GBS can be detected in about half of the exposed infants. Without intervention, up to 5% of colonized infants will eventually develop systemic invasive GBS infections, which carry significant mortality (5−10%) (2, 3). Due to its dominance in small infants, GBS is the third most frequent overall cause of bacterial meningitis in countries with highly developed infrastructure and routine immunization against Haemophilus influenzae type B (4, 5). In developing countries, the incidence of neonatal GBS shows high regional variability. In some areas of Africa, GBS is emerging as one of the most important causes of neonatal sepsis and meningitis with overall incidences as high as 1.8/1000 live births and fatality rates up to 33% (6-9).
The likelihood of exposure might explain in part why GBS is a particular threat to newborn infants. Screening for genital GBS colonization and subsequent antibiotic prophylaxis to expectant mothers, which is now routine in the US and many European countries, has significantly reduced the risk for neonatal GBS sepsis (10). However, today up to 50% of sepsis cases in newborn infants occur after the first week of life, and perinatal antibiotic prophylaxis does not influence this so-called late onset sepsis (11). Here, the neonatal skin, intestinal tract, and mucous membranes are already colonized by a variety of organisms. Therefore, the prominent role of GBS in late onset sepsis is intriguing. Beyond the first three months of life, GBS poses a significant risk to pregnant women and the elderly, particularly those with diabetes mellitus (3, 11-14). Accordingly, it is tempting to assume that newborn infants exhibit immunological alterations that allow GBS to become a primary cause of inflammation, sepsis, and death at the beginning of life. Moreover, these or functionally similar immunological idiosyncrasies appear to be unmasked in pregnancy and diabetes.
GBS is well adapted to its ecological niches, the female genital tract and the gastrointestinal tract in men and women. Adaptation to coexistence implies that selection pressure on either site of the GBS-host interface has promoted mechanisms through which host immunity can discriminate between colonization and invasion (15). Newborn and even more preterm infants are particularly dependent on the competence of their innate immune system for the control of GBS at their surface barriers: the adaptive immune system is deficient both in immunoglobulin G synthesis and the variable heavy chain gene repertoire (16), and transplacental antibodies acquired from the mother only partially compensate for this deficiency. Furthermore, GBS is capable of evading adaptive immunity through molecular mimicry of its capsular polysaccharide sialic acid substitutions with the human LewisX antigen (17).
This review aims to delineate the basic mechanisms of GBS recognition by the innate immune system. General and GBS-specific paradigms of inflammation control in sepsis are explored to improve the understanding of GBS pathogenesis.
Recognition of GBS by the innate immune system
GBS shares its basic structure with many Gram-positive bacteria such as other streptococci, staphylococci, and Listeria. Yet, GBS inhabits a specific niche on the human body surface and imposes a particular risk to defined patient populations.
At mucosal and dermal surfaces of the human body, tissue macrophages and dendritic cells act as both sentinel cells and first-line defense cells (18). During the initial contact with bacteria, several events occur in parallel. Initially, bacteria are recognized rather nonspecifically via complement receptors, e.g. β2-integrins and Fc receptors that interact with opsonizing antibodies or activated complement factors (19-21). In parallel, pathogen-associated molecular patterns (PAMPs), microbial components with structural conservation between related species and with an essential role in microbial viability, interact with so-called pattern recognition receptors on phagocytes and antigen-presenting cells. Among pattern-recognition receptors, Toll-like receptors (TLRs) are the best defined. The family of mammalian TLRs comprises 13 orthologs, 10 in humans and 12 in mice (22, 23). In the human system, specific microbial agonists for all TLRs but TLR10 have been identified. TLRs are undoubtedly essential for the recognition of many microbial substructures. However, alternative recognition systems have been discovered more recently, in particular nucleotide oligomerization domain (NOD)-like receptors (NLRs) (24). NLRs share the principal ligand recognition site with TLRs in the form of a leucine-rich domain.
Signal induction via TLRs
The most compelling evidence for TLRs as master regulators of the host response to GBS is provided by studies in mice with targeted deletions of TLR2 or the TLR adapter protein myeloid differentiation factor 88 (MyD88). In normal mice, a targeted local immune response destroys a low subcutaneous GBS inoculum without measurable systemic inflammatory activity in the blood. The same GBS dose induces 20−30% lethality in mice with deletions of either TLR2 or MyD88 (25). Accordingly, a significant bacterial load can normally be removed by host immunity in a ‘non-phlogistic’ TLR-dependent fashion. Interestingly, a contrasting effect is observed in the lethal dose 90 (LD90) model, where 90% of wildtype mice die within three days. Under these conditions, 40−50% of mice deficient In TLR2 or MyD88 are saved. As might be expected, lethality is correlated to bacterial dissemination and to cytokine levels in the blood. However, the susceptibility to GBS sepsis differs dramatically between newborn and adult mice: whereas 30 GBS organisms injected subcutaneously represent the LD50 in newborn mice, a similarly effective dose is approximately 1,000,000-fold higher in adult mice of the same strain (25). Host factors that underlie postnatal maturation and directly influence elimination of GBS from tissue are likely to significantly contribute to GBS sepsis in newborns.
The course of GBS sepsis in mice with a targeted deletion of TLR2 or MyD88 closely mimics that of wildtype mice that were subjected to an anti-tumor necrosis factor (TNF) injection before the GBS challenge. Hence TLR2, MyD88, and TNF are essential elements in the host immunity to GBS. Next to TNF, type I interferons (IFNs) and IFN-γ, interleukin-6 (IL-6), IL-12, and IL-18 contribute significantly to the course of GBS sepsis in the same neonatal mouse model (26-30).
How does GBS activate the TLR system?
GBS expresses at least two PAMPs: the first is released by GBS and engages a receptor complex consisting of TLR2, TLR6, and CD14. The second is cell bound and potently engages a MyD88-dependent pathway (21, 31). The identity of the first PAMP, the dominant TLR2 agonist both in vitro and in vivo, has just been resolved as mature lipoproteins (32). In GBS, prolipoproteins are processed by the diacyltransferase Lgt and the signal peptidase II Lsp before they are released by growing GBS through a yet to be resolved mechanism. Both enzymes, and therefore diacylation of the lipobox and cleavage of the signal peptide by the signal peptidase II (Lsp), are structural prerequisites for the ability of GBS lipoproteins to activate TLR2 both in vitro and in vivo (32). Recent data suggest that lipoproteins bind directly to TLR2 (33).
The role of lipoteichoic acid (LTA), the most widely studied TLR2 agonist in Gram-positive bacteria, is currently under debate as an inflammatory substructure (34). A large body of evidence including data generated with synthetic LTA supports a model where LTA induces cytokines in phagocytes through interaction with TLR2 (35, 36). However, several lines of genetic and biochemical evidence indicate that LTA is of minor importance in this context: LTA extracted from GBS with a targeted deletion of the acyltransferase Lgt looses most of its potency for TLR2 activation (32, 37).
In contrast to lipoproteins, the cell-wall associated PAMP from GBS has not been identified on the molecular level. Fixed GBS cells potently stimulate phagocytes to the formation of inflammatory cytokines. GBS cell wall, in the form of heat- or ethanol-fixed bacteria, very potently induces inflammatory cytokines, reactive oxygen intermediates (ROIs), and a host of signaling intermediates [nuclear factor-κB (NFκB), mitogen-associated protein kinases (MAPKs)] in strict dependence on MyD88 but independently of single TLRs (1, 2, 4, 5, 6, 9), which have been shown to mediate the response to other Gram-positive and -negative bacteria (21, 31, 38, 39). It is currently unclear which bacterial substructures of the GBS particle are actually sensed by the MyD88-dependent receptor. The GBS surface, which constitutes the obvious interface with cognate receptors expressed by phagocytes, comprises the polysaccharide capsule and the adjacent cell wall. GBS expresses two distinct polysaccharide species, a group-specific and type-specific (capsular) polysaccharide. A distinct, albeit relatively weak effect of type-specific polysaccharide has been observed, with polysaccharide from GBS serotype VIII exceeding that from GBS serotype III (40-42). However, since mutants lacking type-specific polysaccharide exhibit normal inflammatory potency, this carbohydrate can be excluded as an essential inflammatory toxin from GBS. Group-specific polysaccharide has been reported to be a relatively potent inflammatory stimulus, and the dimeric adhesion molecule CD11b/ CD18 has been implicated in its recognition (42-44). However, GBS mutants in group-specific polysaccharide are not available. The generation of highly pure polysaccharide preparations for in vitro studies is hampered by its covalent phosphodiester linkage to the peptidoglycan assembly. Hence, it remains to be clarified if polysaccharides themselves comprise inflammatory potency or whether this is accounted for by co-purified molecules. As outlined in more detail below, GBS cell wall interacts with TLR2 on mouse microglia, which results in increased formation of nitric oxide and induction of apoptosis, whereas these pathways appear not to be expressed in macrophages (45).
MyD88 is one of five Toll/interleukin-1 receptor (TIR) containing TLR adapter proteins. The others are TRIF (TIR-containing adapter inducing IFN-β), TRAM (TRIF-related adapter molecule), MyD88-adapter-like/TIR adapter protein (MAL/TIRAP), and SARM (sterile α and HEAT-Armadillo motifs). Interactions between TIR domains in the TLR adapter are believed to occur via conserved peptides, so-called boxes (46, 47). MyD88 is the only known adapter that mediates the cytokine response to GBS cell wall (S. Kenzel and P. Henneke, unpublished observation), although the role of SARM has not been tested. The composition of the TLR adapter has important implications for downstream signaling events, e.g. the resulting cytokine profile. However, the interplay between the adapters remains poorly understood. For example, it is unknown why TLR4 requires four adapters for inflammatory cytokine formation, whereas MyD88 alone is sufficient for TLR9 and GBS (48-50).
In addition to TLRs, the type I IL-1 receptor (IL-1R1) interacts with MyD88. However, IL-1R1 does not mediate the MyD88-dependent cytokine response to GBS. This conclusion is based on the following findings: (i) IL-1β is a very weak inducer of TNF; (ii) IL-1R1-deficient macrophages respond normally to GBS; and (iii) inhibition of IL-1- and IL-18-maturation through inhibition of caspase-1 does not affect GBS-induced TNF formation (P. Henneke, unpublished observations). Downstream of MyD88, GBS cell wall activates inflammatory cytokines with particular dependence on the MAPKs ERK1/2 (extracellular signal-regulated kinases 1 and 2), JNK (c-Jun N-terminal kinase), and p38 (51). Despite these similarities, JNK and p38 are clearly distinct in the engagement of downstream events. JNK but not p38 directly induces NFκB activation. In contrast, p38 but not JNK is essential for directly antimicrobial properties such as phagocytosis and oxygen radical formation. In accordance with these observations, treatment with the JNK inhibitor SP600125 both decreased TNF serum concentrations and improved survival in neonatal mice with GBS sepsis without interfering with bacterial clearance (52, 53).
Another putative PAMP from GBS is β-hemolysin. Although the gene encoding GBS βhemolytic activity has been identified as cylE, the encoded protein has not been purified to homogeneity (54). A number of studies found that expression of β-hemolysin is associated with typically receptor-mediated events such as cytokine formation and apoptosis (55, 56). However, a cognate receptor for GBS β-hemolysin has not been identified.
Signal induction via NODs and other intracellular receptors
Macromolecular peptidoglycan was the first putative PAMP from Gram-positive bacteria that was recognized by TLR2 (57, 58). However, the association with other cell-wall-embedded putative TLR2 ligands, such as lipoproteins and LTA, constitutes an obstacle to purification to homogeneity and makes firm conclusions on the agonistic activity of peptidoglycan for TLR2 difficult. Since careful removal of contaminating lipids from peptidoglycan preparations abrogated the TLR2 agonistic effects, most investigators no longer accept peptidoglycan as a TLR2 agonist (59, 60). In contrast, it is now widely acknowledged that peptidoglycan from a number of Gram-positive species in the form of the minimal fragments muramyl dipeptides (MDP, MurNAc-L-Ala-D-IsoGln) is a PAMP for the intracellular receptor NOD2 (61, 62). NOD2 belongs to the family of NLRs, which comprises 20 members according to bioinformatics data from the human genome. NLRs are cytosolic receptors that interact with bacteria that have escaped the phagolysosome or with bacterial substructures leaking from this compartment. Interaction is believed to occur through the leucine-rich repeat (LRR) domain, which NLRs share with TLRs (63). With respect to streptococci, MDPs from Streptococcus pneumoniae appear to be recognized by NOD2; however, no data on GBS are available in this context (64).
At least one further signaling pathway for Gram-positive bacteria beyond TLRs and NODs exists, and this involves the inflammasome. The inflammasome is a cytosolic protein complex, which induces processing of IL-1β and IL-18 via caspase-1. The inflammasome components ASC and cryopyrin (or NALP3 or CIAS1) are essential for IL-1β production by live S. aureus and Listeria monocytogenes (65). Furthermore, Listeria monocytogenes as a model cytosolic pathogen has been shown to induce a so-called ‘late response cluster’, which contains mainly MyD88-independent IFN genes (β and γ), IL-12, RANTES (regulated upon activation, normal T-cell expressed, and presumably secreted), and MCP-1 (monocyte chemotactic protein-1). Expression of the cholesterol-binding toxin listeriolysin is essential for the activation of the inflammasome and the late response cluster. Notably, listeriolysin constitutes a classical escape mechanism from endosomes, since it is activated by decreasing pH in this cellular compartment. The most attractive model for activation of these intracellular pathways is that components of Listeria (and S.aureus), such as DNA, engage intracellular sensors (66, 67). Alternatively, activation of the inflammasome or the late cluster might be mediated through perturbation of intracellular K+ levels (68). Data on the inflammasome and the cellular response to streptococci are not available. However, engagement of an intracellular recognition system by the usually extracellular GBS seems conceivable, since GBS was found free in the cytoplasm of Hep-2 epithelial cells (69) and persists in macrophages for up to 48 h (70, 71).
Signal induction viaβ2-integrins
In addition to TLRs and intracellular receptors, β2-integrins, in particular CD11b/CD18, have been suggested as GBS signaling receptors (42, 72). Constitutive and inducible CD11b expression levels are lower on polymorphonuclear lymphocytes (PMLs) and monocytes from very preterm but not term infants as compared to adults (73). Some investigators found that fibroblasts gain inflammatory signaling properties to GBS upon heterologous expression of CD11b/CD18 (74, 75), although not confirmed by other studies (21). In addition, whole blood from mice with a targeted deletion of CD11b responded with lower cytokine concentrations to GBS as compared to wildtype control samples (76). In contrast, macrophages from the same CD11b-deficient mice were found to respond normally to GBS, as indicated by the potent formation of cytokines, oxygen radicals, and a series of signaling intermediates (21). Accordingly, CD11b/CD18 is not an essential signaling receptor for GBS on macrophages but appears to affect cellular activation in a mixed leukocyte environment. Studies with CD11b−/− cells are potentially influenced by interaction with extracellular matrix proteins, such as fibronectin, which has been shown to bind to GBS and to contribute to the formation of TNF and reactive oxygen intermediates (ROIs) (77-79).
β2-integrins have been shown to regulate lipid biosynthesis, specifically phosphatidylinositol 4,5-bisphosphate (PIP2), via the guanosine triphosphatase ARF6 and the downstream phosphatidylinositol 4-phosphate 5-kinase (PI5K) (80, 81). In full agreement with the model of PIP2 as the partner for MAL/TIRAP with respect to localization at the plasma membrane, Kagan et al. (82) demonstrated that Mal/TIRAP trafficking to PIP2-rich membrane ruffles is largely dependent on CD11b, the only β2-integrin expressed on macrophages. In the same study, CD11b-deficient macrophages exhibited a similar defect in lipopolysaccharide (LPS)-induced IL-6 formation as compared to macrophages deficient in MAL/TIRAP. These data might explain in part how CD11b/CD18 contributes to inflammatory signaling in response to GBS under some circumstances.
Neonatal GBS sepsis: a highly inflammatory disease
Since newborn and particularly preterm infants are susceptible to invasive bacterial infections, they can be regarded as suffering from (transient) immunodeficiency. Most but not all investigators find neonatal monocytes to respond with a diminished in vitro cytokine response to purified bacterial substructures (83-88). Levy et al. (89) reported a decreased TNF response of cord blood mononuclear cells to agonists of TLR2, TLR4, and TLR7 as compared to cells from healthy adults. Accordingly, a weak inflammatory cytokine response is conventionally regarded as a parameter of immunodeficiency in newborn infants. However, the decreased cytokine response to TLR agonists appears to be selective for TNF, whereas IL-6 production is preserved. Alterations in the expression of TLRs, MyD88, and CD14 and in adenosine sensing by monocytes might account in part for the reduced TNF response by newborn cells (73, 83, 86, 90-92). These data are potentially important for disease progression, especially at early stages of infection when a robust immune response to spurious bacterial components may limit bacterial dissemination. However, a global deficiency in cytokine formation of newborn cells is not backed up by other studies, which examined the response to whole GBS. Under these circumstances, neonatal monocytes mount an inflammatory cytokine response, which is at least as potent as that from adult cells (76, 78, 85, 93). Furthermore, very high cytokine concentrations such as IL-1β and IL-8 are found in septic newborn infants, in part with even higher concentration in very immature infants (88, 94-96). Surface expression of TLR2 of both PML and monocytes increases substantially in newborn infants during the course of sepsis. Hence, data generated with cells from healthy infants might not reflect the response during sepsis (97). In accordance with the concept of overwhelming inflammation, all clinical sequels of a cytokine storm, such as coagulation disorders, cardio-respiratory depression, and multi-organ failure, can be observed in septic newborn infants. Finally, anti-inflammatory cytokines (IL-10, IL-1ra), anti-bacterial mediators (type I and II IFNs), or antibodies directed against inflammatory cytokines (TNF, IL-6, IL-12) protect neonatal mice and piglets from GBS sepsis (26-30, 98). Accordingly, the following sections explore whether neonatal immunity lacks regulation of the inflammatory response in the form of signal termination rather than signal induction.
Signal termination by PMLs: not good enough in newborn infants?
It seems beyond doubt that PMLs exert anti-inflammatory action through removal of inflammatory microbial structures via phagocytosis, microbial killing, and intracellular degradation (99). The elimination of invading GBS by PMLs requires a series of tightly controlled processes, such as chemotaxis, recognition of GBS, and subsequent chemokine formation, phagocytosis, and killing of the internalized GBS (100).
Several lines of evidence indicate that the global killing machinery is deficient in neonatal phagocytes. In a primate model of neonatal GBS pneumonia, GBS may reach a density as high as 109 to 1011 CFU/g in lung tissue (101). As outlined above, the LD90 in neonatal mice is 1,000,000-fold lower than in adult mice in the same model (25). Importantly, PMLs from newborn infants exhibit a specific deficiency in GBS killing, whereas they are competent in the killing of E. coli and S. pyogenes (102).
PMLs from newborn infants were found to be impaired in several aspects related to migration, such as formation of lamellipodia, rolling adhesion to activated endothelium, transmigration to the subendothelial tissue, and absolute speed of directed migration (103-108). These phenotypic alterations correspond well to several findings on the molecular level, such as a decreased expression of the adhesion molecule L-selectin, particularly in preterm infants (109, 110), and a deficiency in the ability to rapidly polymerize monomeric G-actin to microtubules, which results in a qualitatively deficient bipolar cell shape (111, 112). Importantly, PML chemotaxis is only transiently impaired and reaches ‘normal’ values at about 10 days, which corresponds to the exquisite susceptibility for GBS infections in the first week of life (113). GBS has been shown to both activate the formation of chemokines (IL-8, leukotriene B4) and chemotactic complement factors (C3b, C5a) (114, 115) and evade chemotaxis via its conserved surface protein C5a peptidase (116). Data from animal models suggest that phagocytosis of GBS by neonatal PMLs is reduced. Alveolar macrophages from newborn rats phagocytose GBS less efficiently than cells from adult rats (117). Phagocytosis of GBS is dependent on CD11b/CD18 and opsonization with complement and surfactant proteins A and D, all of which are low in newborns, especially preterm infants. In contrast, specific anti-serum appears to be dispensable for phagocytosis (21, 77, 118-120). Accordingly, C3-deficient mice are more susceptible to GBS than wildtype controls (121). Interaction of the C3 receptor CD11b/CD18 with capsular polysaccharide from GBS has been observed and suggested to occur via the lectin-binding site of the receptor (118, 122). Phagocytes from term infants express similar levels of CD11b/CD18 as cells from adults, whereas expression is decreased on PMLs and monocytes from preterm infants. The implications of these expression levels are currently unclear (73, 123-125). In preterm infants, Fc receptor-mediated phagocytosis can be assumed to be further decreased, since both specific antibodies and expression of the Fc receptor III are low, whereas both parameters should not limit phagocytosis in term infants (113).
Subsequent to phagocytosis, phagosomes fuse with endosomal lysosomes to phagolysosomes, where GBS is killed by both oxygen-dependent and oxygen-independent enzymatic and nonenzymatic mechanisms. In piglet and mouse models of invasive GBS infections, killing of GBS occurs largely through ROIs (126). In humans, ROIs seem less important, since GBS is not a common pathogen in patients with defects in the NADPH (nicotinamide adenine dinucleotide phosphate) oxidase system (chronic granulomatous disease) (15). GBS-induced ROI formation was found to be normal in term infants but significantly decreased in preterm infants (127).
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is critical for ROI-induced GBS clearance from the mouse lung. The exaggerated cytokine response in GMCSF-deficient mice, which do not clear GBS efficiently, provides support for the hypothesis of inflammatory signal termination via GBS clearance (128). With respect to ROI-independent killing mechanisms, elastase formation is reduced in preterm infants but not in term infants (73). Background lysozyme concentrations are decreased in resting neonatal PMLs but can be induced to adult levels. Isolated deficiencies in the non-oxidative, nonenzymatic machinery have been detected, e.g. lactoferrin and bactericidal/permeability-increasing protein are decreased in both resting and activated PMLs from newborn infants (129, 130). In contrast, cathelicidin, which together with β-defensin-2 exerts synergistic antimicrobial activity against GBS, is increased in neonatal mouse skin (131). The complexity and redundancy of the intracellular, anti-bacterial killing apparatus makes firm conclusions on the molecular basis of the diminished anti-bacterial response in newborn phagocytes impossible at this stage (132, 133). Furthermore, it remains to be established how far the outlined deficiencies in antimicrobial properties affect signal termination in neonatal GBS sepsis.
An ongoing controversy exists concerning the capacity of PMLs to propagate or dampen inflammation through the formation of cytokines. Uncertainties in this regard are largely due to difficulties in completely removing contaminating peripheral blood mononuclear cells (PBMCs), which are a much better source of inflammatory cytokines. Accordingly, numerous investigators have demonstrated that purified TLR agonists and whole bacteria induce the formation of TNF and IL1β in PMLs (134). However, another report with a specific emphasis on PML purity found a very restricted repertoire of anti-inflammatory cytokines and chemokines [IL-1ra, IL-8, macrophage inflammatory protein-1α (MIP-1α), MIP-1β, growth-related oncogene-α, and M-CSF]. Co-culture of PMLs with PBMCs showed significantly reduced TNF-α levels as compared with PBMCs alone (135).
Another PML-specific mechanism of inflammation resolution is the class switch in arachidonic acid-derived eicosanoids from the inflammatory prostaglandins and leukotrienes to the anti-inflammatory lipotoxins (136). A predominantly anti-inflammatory response pattern of PMLs upon bacterial challenge remains an attractive hypothesis, despite the outlined uncertainties. Unfortunately, no specific information on the regulatory programs of PMLs in newborn infants is available.
Specific TLR2-dependent signal termination
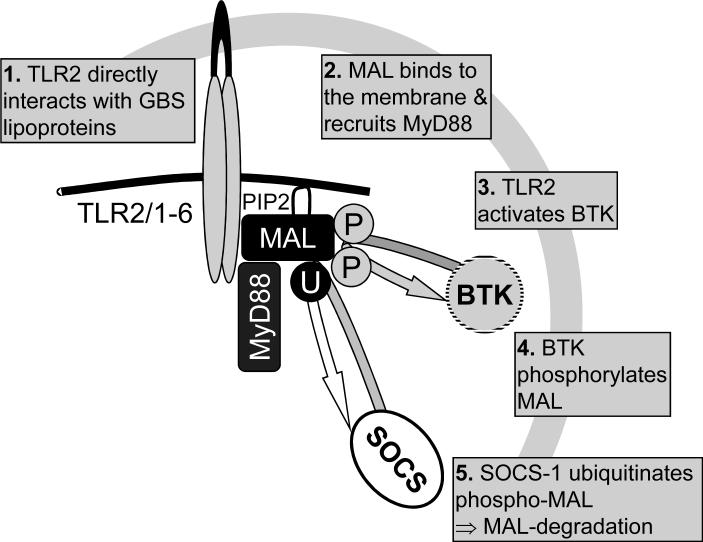
GBS activates TLR2 through lipoproteins, and this interaction is important for the outcome of GBS sepsis in vivo (32). TLR2 interacts with either TLR1 or TLR6 and two intracellular adapter proteins, MyD88 and MAL/TIRAP. The interaction between these four molecules is a striking example of tightly regulated signal induction and termination. The initial event is the direct interaction of acylated lipoproteins with the binding groove of the TLR2/1 or TLR2/6 heteromer. Direct interaction has recently been demonstrated for triacylated lipoproteins and the binding groove of TLR2/1 via co-crystallization of the interaction partners (33). A similar direct interaction can be assumed to occur between diacylated lipoproteins from GBS and TLR2/6, although not proven. Subsequently, MAL/TIRAP is recruited to PIP2 in the plasma membrane through its N-terminal PIP2-binding domain (82). Current evidence suggests that MAL/TIRAP serves as a bridging molecule between TLR2 and MyD88, which triggers a downstream signaling cascade. This culminates in the transcription of many inflammatory genes. Alternatively, TLR2 activation induces Bruton's tyrosine kinase, which in turn phosphorylates tyrosine residues of MAL/TIRAP at positions 86 and 187 (137). Tyrosine phosphorylation appears to be a prerequisite for NFκB p65 serine phosphorylation (138). At the same time, activation of TLR2 activates the suppressor of cytokine signaling-1 (SOCS-1), which recognizes a proline-glutamine-serine-threonine-rich (PEST) domain in MAL/TIRAP and targets the latter to 26S proteasome-dependent degradation through polyubiquitination of MAL/ TIRAP (139) (Fig.1).
Fig. 1. Negative regulation of TLR2-dependent signaling.
Bacterial lipoproteins directly interact with the TLR2/1 or TLR2/6 dimer. Then MAL/TIRAP is recruited to PIP2 in the plasma membrane through its PIP2-binding domain. MAL/TIRAP links the TLR2-dimer to MyD88, which activates a complex downstream signaling cascade. A parallel event upon TLR2 activation is the tyrosine phosphorylation (aa 86 and 187) of MAL/TIRAP by Bruton's tyrosine kinase, which leads to NFκB p65 serine phosphorylation. TLR2 signaling is terminated through a negative feedback loop which comprises SOCS-1. SOCS-1 recognizes the MAL/TIRAP via its PEST domain, polyubiquitinates MAL/TIRAP, and targets it to 26S proteasome-dependent degradation.
Several further negative regulators of the TLR2-dependent signaling pathway have been revealed. In view of the important role of TLR2 in GBS, these mechanisms can be assumed to modulate the response to GBS, although they have not been specifically evaluated in this context. Soluble TLR2, which inhibits TLR2-dependent signaling, occurs in human plasma and breast milk (140). Phosphatidylinositol 3-kinase (PI3K), which is induced by GBS lipoproteins, has the potential to inhibit TLR2-induced IL-12 in dendritic cells through a pathway that involves p38 (32, 141, 142). Tollip, a TLR adapter without a TIR domain, was reported to inhibit TLR2 through autophosphorylation of IL1 receptor-associated kinase-1 (IRAK-1) (143). However, more recent data from the same investigators, which made use of a TOLLIP−/− mouse, did not confirm any inhibitory role for TOLLIP in TLR2 or TLR4 signaling (144). IRAK-1-binding protein has recently been reported to negatively regulate transcriptional activation by the presumed TLR2 agonist LTA (145). The ubiquitin-editing enzyme A20 is essential for the termination of TLR-induced IκB-kinase-β activity via de-ubiquitination of TNF receptor-associated factor-6 (TRAF-6) (146). However, A20 is not a specific inhibitor for TLRs, since it inhibits other pathways, e.g. the NLR pathway (147). ST2L, the soluble variant of the IL-33 receptor ST2, disrupts TLR2 signaling via sequestration of MAL/TIRAP and MyD88 (148, 149). The TNF-related apoptosis-inducing ligand receptor (TRAIL-R) stabilizes IκB-α and therefore inhibits TLR2-dependent transcriptional activation (150). Whether MyD88s, a splice variant with inhibitory potential for TLR4 and the IL-1R1, inhibits TLR2 function has not been tested but seems highly likely given the central role of MyD88 in TLR2 signaling (151). Among the outlined molecular mechanism of signal termination, some have been shown to contribute to a cellular program denominated ‘endotoxin tolerance’. This term was originally coined to describe the inability of mononuclear cells to respond to a second endotoxin challenge (typically 12 − 24 h after the first) with TNF formation. Endotoxin tolerance is not a global downmodulation of cellular activity. Rather, it is a program that specifically targets certain inflammatory cytokines (IL-1β and TNF) but does not inhibit certain anti-inflammatory cytokines such as IL-1ra (152) or anti-bacterial properties of mononuclear cells. It appears that gene-specific chromatin modifications are associated with component-specific regulation of inflammatory genes (153).
The equivalent of in vitro endotoxin tolerance in septic patients is called immunoparalysis, which is believed to contribute substantially to the morbidity and mortality of sepsis (154, 155). In recent years, it has been shown that this phenomenon is not confined to signaling events associated with the endotoxin receptor TLR4 but also occurs after stimulation of TLR2 (156). Furthermore, it is found in many cell types, e.g. PMLs and epithelial cells. Various mechanisms on all levels of the signaling cascade have been reported to contribute to tolerance development, and a comprehensive review of these findings is far beyond the scope of this review. The summary of some recent findings with potential implication for GBS sepsis is important in this context. A substantial body of evidence suggests that endotoxin tolerance is mediated on the level of cytokine gene transcription, such as the specific induction of anti-inflammatory genes and the formation of inhibitory NFκB p50 homodimers (157, 158). In addition, mice deficient in the IRAK-M, which is an inactive kinase restricted to monocytes and macrophages, exhibit reduced endotoxin tolerance, and IRAK-M expression is increased in a human model of endotoxin tolerance (159, 160). Medvedev et al. (161) recently reported that tolerance manifests even further upstream in the signaling cascade on the level of the TLR complex itself. Tyrosine phosphorylation of both TLR4 and the adapter Mal/TIRAP, which regulates the formation of the signalosome, is downmodulated in endotoxin tolerance (161). Similar effects can be assumed to exist with TLR2, since it comprises two putative phosphotyrosines at positions 617 and 761 (142). In 2007, Kramer et al. (162) firmly established that endotoxin tolerance can be induced in PBMCs from neonatal mammals: intraamniotic LPS injection resulted in LPS-hyporesponsiveness of neonatal PBMCs and lung cells (162). A decreased response to LPS in PMLs from septic newborn infants was documented subsequently (163). Furthermore, intestinal epithelial cells from fetal mice acquire endotoxin tolerance immediately after birth by exposure to exogenous LPS. Notably, acquisition of endotoxin tolerance was related to the mode of delivery and occurred only in vaginally born mice but not in those delivered by Caesarean section (164).
Signal termination via apoptosis
GBS has been reported to induce apoptosis in neurons both in vivo and in vitro (165-168) and, important in the context of this review, in peripheral monocytes, macrophages, and the resident macrophage-like microglia of the central nervous system (45, 169-176). It has been a long-standing dispute whether bacteria-induced apoptosis of immune cells is advantageous or harmful to the host. It seems reasonable to assume that apoptosis is an advantageous process per se in host immunity, since it developed through evolutionary forces despite its energy-consuming nature. It leads to the non-inflammatory clearance of internalized bacteria and therefore limits inflammation. In contrast, apoptosis of phagocytes may be detrimental to the host, as it potentially weakens the first-line immune defense and might be manipulated by certain pathogens during immune evasion (155). Furthermore, apoptosis is deleterious in tissues with low self-regenerative potential like neurons (177) .
With respect to causative toxins in GBS, β-hemolysin has been shown to promote apoptosis in monocytes and macrophages. An increase of intracellular calcium, activation of protein kinase C, calpain, and apoptosis-inducing factor are essential mediators of this effect (169, 171). However, β-hemolysin is not the only apoptotic component of GBS, since GBS deficient in this toxin potently induce apoptosis in immune cells (173). Accordingly, Ulett et al. (173) proposed that β-hemolysin expression and apoptosis induction are associated rather than causally linked, since both are influenced independently by glucose levels in the culture medium.
TLR2 and MyD88 are critical components of a receptor complex that mediates GBS-induced apoptosis in immune cells in a β-hemolysin-independent fashion (45). In view of the recently published finding that lipoproteins are the dominant TLR2-activating toxins from GBS, they can be assumed to mediate apoptosis as well (32). However, TLR2 activation alone appears not to be sufficient for apoptosis induction in microglia, since GBS supernatant, which is rich in lipoproteins, does not induce apoptosis. These findings are in line with the finding that endotoxin but not TLR2 agonists from various species induces microglia apoptosis (178). It seems safe to assume that GBS induces a second signal beyond TLR2 activation that is required for the induction of apoptosis. Importantly, TLR2-dependent recognition of GBS cell wall by microglia differs substantially from the largely TLR2-independent recognition by macrophages.
On the molecular level, GBS induces both the intrinsic and the extrinsic apoptosis pathways dependent on the host cell type. In mouse macrophages, GBS induces the intrinsic pathway, which comprises activation of caspase-9 and caspase-3 but not caspase-8 (176). In microglia, GBS induces caspase-8, the classical caspase of the extrinsic pathway (45). However, this form of apoptosis does not involve the common effector caspase-3. This is yet another example for differences between macrophages and microglia in GBS-induced signal activation. Apoptosis induction in microglia by GBS is reminiscent of apoptosis induction by pure TLR2 agonists in epithelial cells, which activates components of the canonical extrinsic pathway (179-182). In summary, TLR2 and MyD88 are part of a death receptor complex for GBS in microglia.
Interaction of GBS with macrophages and microglia induces the release of various soluble factors, which have the potential to induce apoptosis. TNF plays a major role in apoptosis of rat hippocampal neurons in GBS meningitis (166). Furthermore, soluble TRAIL and IFN-α have been associated with GBS-induced cell death (165, 166, 168, 175, 183). In a rat model of GBS meningitis, ROIs contributed to both necrotic injury and apoptotic cell death, whereas nitric oxide was protective (165). This observation is in contrast to in vitro data, where both are apoptotic effector molecules upon GBS stimulation (165, 168). Discrepancies between in vitro and in vivo effects of nitric oxide are probably due to its role in the regulation of cerebral blood flow (184). Importantly, apoptosis in microglia mediated by TLR2 does not involve soluble mediators like nitric oxide, whereas the latter is instrumental for neuronal apoptosis (45, 168).
Interaction of GBS with TLR2 on microglia both induces the release of inflammatory mediators like nitric oxide and terminates inflammation through the extrinsic apoptotic pathway. This phenomenon reflects the concept of activation-induced cell death (AICD). The linkage of immune cell activation, which is tailored to prevent microbial spread, and signal termination through one receptor seems particularly important in the context of neuronal tissue, which is not capable of self-regeneration. Beyond microglia, AICD has been reported for many immune cell types, such as T and B lymphocytes, PMLs, and macrophages (185-190). Signal termination is completed with the phagocytosis of apoptotic bodies, cell debris-containing vesicles. Several studies found that this process elicits an anti-inflammatory program in macrophages, which suppresses the formation of TNF and induces secretion of anti-inflammatory cytokines like transforming growth factor-β1 and platelet-activating factor (191, 192). In contrast, apoptotic cell death has been linked to inflammation, for example through the activation of caspase-1 and its cleavage product IL-1β (193-195). Apoptosis of immune cells profoundly influences the cytokine milieu (136), and pathogen-specific effects might bias bystander immune cells towards termination of inflammation.
Downstream of GBS-TLR interaction, MAPKs might play an important role, as has been shown for the inflammatory response (51, 52). Exhaustive information on the specific role of MAPKs in GBS-induced death of macrophages or microglia is not available. However, GBS-induced macrophage apoptosis was associated with persistent activation of JNK and p38. In contrast, the MAPK ERK was inhibited (170). In macrophages stimulated with LPS, p38 mediates apoptotic cell death, which is associated with the release of IL-1β (194). It is tempting to speculate that MAPKs constitute another level of control, where signal activating and terminating events interdigitate, hence where AICD manifests.
Few data on pathogen-induced signal termination via apoptosis in newborn infants are available. In support of the proposed model of reduced signal termination in neonatal sepsis, Gille et al. (196) recently reported on a substantially reduced rate of apoptosis in neonatal monocytes upon infection with Escherichia coli. Data on a low apoptosis rate in monocytes infected with respiratory syncytial virus are in accordance with these data (197). Furthermore, deletion of MyD88 in mice impairs leukocyte apoptosis but increases lethality in a cecal ligation sepsis model. The contrasting effects of MyD88 deficiency on apoptosis and sepsis-related outcome in mice might be in part applicable to the neonatal situation, since MyD88 expression is reduced in monocytes from newborn infants (86, 198). Apoptosis of neonatal PMLs has been more intensively studied and was found to be impaired. Low expression of apoptotic proteins such as Fas receptor and caspase-3 are associated with reduced apoptosis (103). Furthermore, labor reduces PML apoptosis, probably due to alterations in eicosanoid expression such as the apoptotic lipoxins (199). Unfortunately, GBS-specific information on AICD in newborn infants is not available.
Conclusions
The sudden and heavy exposure to GBS during birth constitutes an obvious challenge to the local immunity of skin, mucous membranes, and lung in newborn infants. This challenge can only be met by rapid and potent activation of inflammation, to destroy invading GBS, and termination of inflammation, to prevent systemic inflammation. The neonatal immune response to GBS appears to be tilted towards enhanced inflammation, since many mechanisms of stimulus removal and signal termination are deficient at the beginning of life. It remains to be established whether a genetic predisposition for a failure in inflammation control puts patients at increased risk of GBS sepsis. Finally, adjunctive therapeutic interventions in neonatal sepsis have to be carefully evaluated with respect to potentially counterproductive effects on GBS clearance and signal termination.
Acknowledgments
This work was supported in part by Deutsche Forschungsgemeinschaft (He 3127/ 2-3 and 3-1) and the National Institutes of Health (R01AI052455).
References
- 1.Natarajan G, Johnson YR, Zhang F, Chen KM, Worsham MJ. Real-time polymerase chain reaction for the rapid detection of group B streptococcal colonization in neonates. Pediatrics. 2006;118:14–22. doi: 10.1542/peds.2005-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baltimore RS, Huie SM, Meek JI, Schuchat A, O'Brien KL. Early-onset neonatal sepsis in the era of group B streptococcal prevention. Pediatrics. 2001;108:1094–1098. doi: 10.1542/peds.108.5.1094. [DOI] [PubMed] [Google Scholar]
- 3.Heath PT, et al. Group B streptococcal disease in UK and Irish infants younger than 90 days. Lancet. 2004;363:292–294. doi: 10.1016/S0140-6736(03)15389-5. [DOI] [PubMed] [Google Scholar]
- 4.Hyde TB, Hilger TM, Reingold A, Farley MM, O'Brien KL, Schuchat A. Trends in incidence and antimicrobial resistance of early-onset sepsis: population-based surveillance in San Francisco and Atlanta. Pediatrics. 2002;110:690–695. doi: 10.1542/peds.110.4.690. [DOI] [PubMed] [Google Scholar]
- 5.Schuchat A, et al. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337:970–976. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 6.Laving AM, Musoke RN, Wasunna AO, Revathi G. Neonatal bacterial meningitis at the newborn unit of Kenyatta National Hospital. East African Med J. 2003;80:456–462. doi: 10.4314/eamj.v80i9.8742. [DOI] [PubMed] [Google Scholar]
- 7.Osrin D, Vergnano S, Costello A. Serious bacterial infections in newborn infants in developing countries. Curr Opin Infect Dis. 2004;17:217–224. doi: 10.1097/00001432-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 8.English M, et al. Causes and outcome of young infant admissions to a Kenyan district hospital. Arch Dis Child. 2003;88:438–443. doi: 10.1136/adc.88.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray KJ, Bennett SL, French N, Phiri AJ, Graham SM. Invasive group B streptococcal infection in infants, Malawi. Emerg Infect Dis. 2007;13:223–229. doi: 10.3201/eid1302.060680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrag SJ, Stoll BJ. Early-onset neonatal sepsis in the era of widespread intrapartum chemoprophylaxis. Pediatr Infect Dis J. 2006;25:939–940. doi: 10.1097/01.inf.0000239267.42561.06. [DOI] [PubMed] [Google Scholar]
- 11.Fluegge K, Supper S, Siedler A, Berner R. Serotype distribution of invasive group B streptococcal isolates in infants: results from a nationwide active laboratory surveillance study over 2 years in Germany. Clin Infect Dis. 2005;40:760–763. doi: 10.1086/427942. [DOI] [PubMed] [Google Scholar]
- 12.Blancas D, Santin M, Olmo M, Alcaide F, Carratala J, Gudiol F. Group B streptococcal disease in nonpregnant adults: incidence, clinical characteristics, and outcome. Eur J Clin Microbiol Infect Dis. 2004;23:168–173. doi: 10.1007/s10096-003-1098-9. [DOI] [PubMed] [Google Scholar]
- 13.Zaleznik DF, et al. Invasive disease due to group B Streptococcus in pregnant women and neonates from diverse population groups. Clin Infect Dis. 2000;30:276–281. doi: 10.1086/313665. [DOI] [PubMed] [Google Scholar]
- 14.Edwards MS, Rench MA, Palazzi DL, Baker CJ. Group B streptococcal colonization and serotype-specific immunity in healthy elderly persons. Clin Infect Dis. 2005;40:352–357. doi: 10.1086/426820. [DOI] [PubMed] [Google Scholar]
- 15.Henneke P, Berner R. Interaction of neonatal phagocytes with group B streptococcus: recognition and response. Infect Immun. 2006;74:3085–3095. doi: 10.1128/IAI.01551-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer K, et al. Diversification of Ig heavy chain genes in human preterm neonates prematurely exposed to environmental antigens. J Immunol. 2002;169:1349–1356. doi: 10.4049/jimmunol.169.3.1349. [DOI] [PubMed] [Google Scholar]
- 17.Carlin AF, Lewis AL, Varki A, Nizet V. Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. J Bacteriol. 2007;189:1231–1237. doi: 10.1128/JB.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 19.Sobota A, Strzelecka-Kiliszek A, Gladkowska E, Yoshida K, Mrozinska K, Kwiatkowska K. Binding of IgG-opsonized particles to Fc gamma R is an active stage of phagocytosis that involves receptor clustering and phosphorylation. J Immunol. 2005;175:4450–4457. doi: 10.4049/jimmunol.175.7.4450. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Guerrero C, et al. Suppressors of cytokine signaling regulate Fc receptor signaling and cell activation during immune renal injury. J Immunol. 2004;172:6969–6977. doi: 10.4049/jimmunol.172.11.6969. [DOI] [PubMed] [Google Scholar]
- 21.Henneke P, et al. Cellular activation, phagocytosis, and bactericidal activity against group B streptococcus involve parallel myeloid differentiation factor 88-dependent and independent signaling pathways. J Immunol. 2002;169:3970–3977. doi: 10.4049/jimmunol.169.7.3970. [DOI] [PubMed] [Google Scholar]
- 22.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 24.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Mancuso G, et al. Dual role of TLR2 and myeloid differentiation factor 88 in a mouse model of invasive group B streptococcal disease. J Immunol. 2004;172:6324–6329. doi: 10.4049/jimmunol.172.10.6324. [DOI] [PubMed] [Google Scholar]
- 26.Mancuso G, Cusumano V, Genovese F, Gambuzza M, Beninati C, Teti G. Role of interleukin 12 in experimental neonatal sepsis caused by group B streptococci. Infect Immun. 1997;65:3731–3735. doi: 10.1128/iai.65.9.3731-3735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancuso G, et al. Type I IFN signaling is crucial for host resistance against different species of pathogenic bacteria. J Immunol. 2007;178:3126–3133. doi: 10.4049/jimmunol.178.5.3126. [DOI] [PubMed] [Google Scholar]
- 28.Mancuso G, et al. Beneficial effects of interleukin-6 in neonatal mouse models of group B streptococcal disease. Infect Immun. 1994;62:4997–5002. doi: 10.1128/iai.62.11.4997-5002.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cusumano V, et al. Interleukin-18 is an essential element in host resistance to experimental group B streptococcal disease in neonates. Infect Immun. 2004;72:295–300. doi: 10.1128/IAI.72.1.295-300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cusumano V, Genovese F, Mancuso G, Carbone M, Fera MT, Teti G. Interleukin-10 protects neonatal mice from lethal group B streptococcal infection. Infect Immun. 1996;64:2850–2852. doi: 10.1128/iai.64.7.2850-2852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henneke P, et al. Novel engagement of CD14 and multiple toll-like receptors by group B streptococci. J Immunol. 2001;167:7069–7076. doi: 10.4049/jimmunol.167.12.7069. [DOI] [PubMed] [Google Scholar]
- 32.Henneke P, et al. Lipoproteins are critical TLR2-activating toxins in a model of group B streptococcal sepsis. J Immunol. 2008;180:6149–6158. doi: 10.4049/jimmunol.180.9.6149. [DOI] [PubMed] [Google Scholar]
- 33.Jin MS, et al. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 34.von Aulock S, Hartung T, Hermann C. Comment on ”Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus”. J Immunol. 2007;178:2610. doi: 10.4049/jimmunol.178.5.2610. author reply 2610−2611.
- 35.Morath S, Geyer A, Hartung T. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J Exp Med. 2001;193:393–397. doi: 10.1084/jem.193.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morath S, Stadelmaier A, Geyer A, Schmidt RR, Hartung T. Synthetic lipoteichoic acid from Staphylococcus aureus is a potent stimulus of cytokine release. J Exp Med. 2002;195:1635–1640. doi: 10.1084/jem.20020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hashimoto M, et al. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J Immunol. 2006;177:3162–3169. doi: 10.4049/jimmunol.177.5.3162. [DOI] [PubMed] [Google Scholar]
- 38.Draper DW, Bethea HN, He YW. Toll-like receptor 2-dependent and -independent activation of macrophages by group B streptococci. Immunol Lett. 2006;102:202–214. doi: 10.1016/j.imlet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Flo TH, et al. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J Immunol. 2000;164:2064–2069. doi: 10.4049/jimmunol.164.4.2064. [DOI] [PubMed] [Google Scholar]
- 40.Vallejo JG, Baker CJ, Edwards MS. Roles of the bacterial cell wall and capsule in induction of tumor necrosis factor alpha by type III group B streptococci. Infect Immun. 1996;64:5042–5046. doi: 10.1128/iai.64.12.5042-5046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mikamo H, Johri AK, Paoletti LC, Madoff LC, Onderdonk AB. Adherence to, invasion by, and cytokine production in response to serotype VIII group B Streptococci. Infect Immun. 2004;72:4716–4722. doi: 10.1128/IAI.72.8.4716-4722.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuzzola M, et al. Human monocyte receptors involved in tumor necrosis factor responses to group B streptococcal products. Infect Immun. 2000;68:994–998. doi: 10.1128/iai.68.2.994-998.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mancuso G, Tomasello F, von Hunolstein C, Orefici G, Teti G. Induction of tumor necrosis factor alpha by the group- and type-specific polysaccharides from type III group B streptococci. Infect Immun. 1994;62:2748–2753. doi: 10.1128/iai.62.7.2748-2753.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Hunolstein C, et al. Soluble antigens from group B streptococci induce cytokine production in human blood cultures. Infect Immun. 1997;65:4017–4021. doi: 10.1128/iai.65.10.4017-4021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lehnardt S, et al. TLR2 and caspase-8 are essential for group B Streptococcus-induced apoptosis in microglia. J Immunol. 2007;179:6134–6143. doi: 10.4049/jimmunol.179.9.6134. [DOI] [PubMed] [Google Scholar]
- 46.Medzhitov R, Janeway C., Jr. Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 47.Xu Y, et al. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature. 2000;408:111–115. doi: 10.1038/35040600. [DOI] [PubMed] [Google Scholar]
- 48.Medzhitov R, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 49.Wesche H, Gao X, Li X, Kirschning CJ, Stark GR, Cao Z. IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J Biol Chem. 1999;274:19403–19410. doi: 10.1074/jbc.274.27.19403. [DOI] [PubMed] [Google Scholar]
- 50.Arbibe L, et al. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 51.Mancuso G, et al. Mitogen-activated protein kinases and NF-kappa B are involved in TNF-alpha responses to group B streptococci. J Immunol. 2002;169:1401–1409. doi: 10.4049/jimmunol.169.3.1401. [DOI] [PubMed] [Google Scholar]
- 52.Kenzel S, Henneke P. The innate immune system and its relevance to neonatal sepsis. Current opinion in infectious diseases. 2006;19:264–270. doi: 10.1097/01.qco.0000224821.27482.bd. [DOI] [PubMed] [Google Scholar]
- 53.Vallejo JG, Knuefermann P, Mann DL, Sivasubramanian N. Group B Streptococcus induces TNF-alpha gene expression and activation of the transcription factors NF-kappa B and activator protein-1 in human cord blood monocytes. J Immunol. 2000;165:419–425. doi: 10.4049/jimmunol.165.1.419. [DOI] [PubMed] [Google Scholar]
- 54.Pritzlaff CA, Chang JC, Kuo SP, Tamura GS, Rubens CE, Nizet V. Genetic basis for the beta-haemolytic/cytolytic activity of group B Streptococcus. Mol Microbiol. 2001;39:236–247. doi: 10.1046/j.1365-2958.2001.02211.x. [DOI] [PubMed] [Google Scholar]
- 55.Doran KS, Liu GY, Nizet V. Group B streptococcal beta-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J Clin Invest. 2003;112:736–744. doi: 10.1172/JCI17335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nizet V. Streptococcal beta-hemolysins: genetics and role in disease pathogenesis. Trends Microbiol. 2002;10:575–580. doi: 10.1016/s0966-842x(02)02473-3. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 58.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 59.Dziarski R, Gupta D. Staphylococcus aureus peptidoglycan is a toll-like receptor 2 activator: a reevaluation. Infect Immun. 2005;73:5212–5216. doi: 10.1128/IAI.73.8.5212-5216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Travassos LH, et al. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep. 2004;5:1000–1006. doi: 10.1038/sj.embor.7400248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Girardin SE, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 62.Girardin SE, et al. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2:736–742. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 64.Opitz B, et al. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem. 2004;279:36426–36432. doi: 10.1074/jbc.M403861200. [DOI] [PubMed] [Google Scholar]
- 65.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 66.Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, Pamer EG. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003;19:891–901. doi: 10.1016/s1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
- 67.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Leber JH, Crimmins GT, Raghavan S, Meyer-Morse NP, Cox JS, Portnoy DA. Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS pathogens. 2008;4:e6. doi: 10.1371/journal.ppat.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valenti-Weigand P, Benkel P, Rohde M, Chhatwal GS. Entry and intracellular survival of group B streptococci in J774 macrophages. Infect Immun. 1996;64:2467–2473. doi: 10.1128/iai.64.7.2467-2473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cornacchione P, et al. Group B streptococci persist inside macrophages. Immunology. 1998;93:86–95. doi: 10.1046/j.1365-2567.1998.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marodi L, Kaposzta R, Nemes E. Survival of group B streptococcus type III in mononuclear phagocytes: differential regulation of bacterial killing in cord macrophages by human recombinant gamma interferon and granulocyte-macrophage colony- stimulating factor. Infect Immun. 2000;68:2167–2170. doi: 10.1128/iai.68.4.2167-2170.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goodrum KJ, Dierksheide J, Yoder BJ. Tumor necrosis factor alpha acts as an autocrine second signal with gamma interferon to induce nitric oxide in group B streptococcus- treated macrophages. Infect Immun. 1995;63:3715–3717. doi: 10.1128/iai.63.9.3715-3717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Henneke P, Osmers I, Bauer K, Lamping N, Versmold HT, Schumann RR. Impaired CD14-dependent and independent response of polymorphonuclear leukocytes in preterm infants. J Perinat Med. 2003;31:176–183. doi: 10.1515/JPM.2003.024. [DOI] [PubMed] [Google Scholar]
- 74.Cuzzola M, et al. Beta 2 integrins are involved in cytokine responses to whole Gram-positive bacteria. J Immunol. 2000;164:5871–5876. doi: 10.4049/jimmunol.164.11.5871. [DOI] [PubMed] [Google Scholar]
- 75.Medvedev AE, et al. Involvement of CD14 and complement receptors CR3 and CR4 in nuclear factor-kappaB activation and TNF production induced by lipopolysaccharide and group B streptococcal cell walls. J Immunol. 1998;160:4535–4542. [PubMed] [Google Scholar]
- 76.Levy O, et al. Critical role of the complement system in group B streptococcus-induced tumor necrosis factor alpha release. Infect Immun. 2003;71:6344–6353. doi: 10.1128/IAI.71.11.6344-6353.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang KD, Bathras JM, Shigeoka AO, James J, Pincus SH, Hill HR. Mechanisms of bacterial opsonization by immune globulin intravenous: correlation of complement consumption with opsonic activity and protective efficacy. J Infect Dis. 1989;159:701–707. doi: 10.1093/infdis/159.4.701. [DOI] [PubMed] [Google Scholar]
- 78.Peat EB, Augustine NH, Drummond WK, Bohnsack JF, Hill HR. Effects of fibronectin and group B streptococci on tumour necrosis factor-alpha production by human culture-derived macrophages. Immunology. 1995;84:440–445. [PMC free article] [PubMed] [Google Scholar]
- 79.Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. The leukocyte integrins. J Biol Chem. 2000;275:23409–23412. doi: 10.1074/jbc.R000004200. [DOI] [PubMed] [Google Scholar]
- 80.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 81.Honda A, et al. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- 82.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 83.Chelvarajan RL, et al. Defective macrophage function in neonates and its impact on unresponsiveness of neonates to polysaccharide antigens. J Leukoc Biol. 2004;75:982–994. doi: 10.1189/jlb.0403179. [DOI] [PubMed] [Google Scholar]
- 84.Peters AM, Bertram P, Gahr M, Speer CP. Reduced secretion of interleukin-1 and tumor necrosis factor-alpha by neonatal monocytes. Biol Neonate. 1993;63:157–162. doi: 10.1159/000243926. [DOI] [PubMed] [Google Scholar]
- 85.Williams PA, Bohnsack JF, Augustine NH, Drummond WK, Rubens CE, Hill HR. Production of tumor necrosis factor by human cells in vitro and in vivo, induced by group B streptococci. J Pediatr. 1993;123:292–300. doi: 10.1016/s0022-3476(05)81706-8. [DOI] [PubMed] [Google Scholar]
- 86.Yan SR, Qing G, Byers DM, Stadnyk AW, Al-Hertani W, Bortolussi R. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect Immun. 2004;72:1223–1229. doi: 10.1128/IAI.72.3.1223-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yerkovich ST, Wikstrom ME, Suriyaarachchi D, Prescott SL, Upham JW, Holt PG. Postnatal development of monocyte cytokine responses to bacterial lipopolysaccharide. Pediatr Res. 2007;62:547–552. doi: 10.1203/PDR.0b013e3181568105. [DOI] [PubMed] [Google Scholar]
- 88.Rogers BB, Alexander JM, Head J, McIntire D, Leveno KJ. Umbilical vein interleukin-6 levels correlate with the severity of placental inflammation and gestational age. Human Pathol. 2002;33:335–340. doi: 10.1053/hupa.2002.32214. [DOI] [PubMed] [Google Scholar]
- 89.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173:4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 90.Forster-Waldl E, et al. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr Res. 2005;58:121–124. doi: 10.1203/01.PDR.0000163397.53466.0F. [DOI] [PubMed] [Google Scholar]
- 91.Angelone DF, et al. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr Res. 2006;60:205–209. doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 92.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berner R, Welter P, Brandis M. Cytokine expression of cord and adult blood mononuclear cells in response to Streptococcus agalactiae. Pediatr Res. 2002;51:304–309. doi: 10.1203/00006450-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 94.Berner R, et al. Plasma levels and gene expression of granulocyte colony-stimulating factor, tumor necrosis factor-alpha, interleukin (IL)-1beta, IL-6, IL- 8, and soluble intercellular adhesion molecule-1 in neonatal early onset sepsis. Pediatr Res. 1998;44:469–477. doi: 10.1203/00006450-199810000-00002. [DOI] [PubMed] [Google Scholar]
- 95.Krueger M, Nauck MS, Sang S, Hentschel R, Wieland H, Berner R. Cord blood levels of interleukin-6 and interleukin-8 for the immediate diagnosis of early-onset infection in premature infants. Biol Neonate. 2001;80:118–123. doi: 10.1159/000047130. [DOI] [PubMed] [Google Scholar]
- 96.Dollner H, Vatten L, Linnebo I, Zanussi GF, Laerdal A, Austgulen R. Inflammatory mediators in umbilical plasma from neonates who develop early-onset sepsis. Biol Neonate. 2001;80:41–47. doi: 10.1159/000047118. [DOI] [PubMed] [Google Scholar]
- 97.Viemann D, Dubbel G, Schleifenbaum S, Harms E, Sorg C, Roth J. Expression of toll-like receptors in neonatal sepsis. Pediatr Res. 2005;58:654–659. doi: 10.1203/01.PDR.0000180544.02537.FD. [DOI] [PubMed] [Google Scholar]
- 98.Vallette JD, Jr., et al. Effect of an interleukin-1 receptor antagonist on the hemodynamic manifestations of group B streptococcal sepsis. Pediatr Res. 1995;38:704–708. doi: 10.1203/00006450-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 99.Aderem A. Phagocytosis and the inflammatory response. J Infect Dis. 2003;187(Suppl):S340–345. doi: 10.1086/374747. [DOI] [PubMed] [Google Scholar]
- 100.Henneke P, Golenbock DT. Phagocytosis, innate immunity, and host-pathogen specificity. J Exp Med. 2004;199:1–4. doi: 10.1084/jem.20031256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rubens CE, Raff HV, Jackson JC, Chi EY, Bielitzki JT, Hillier SL. Pathophysiology and histopathology of group B streptococcal sepsis in Macaca nemestrina primates induced after intraamniotic inoculation: evidence for bacterial cellular invasion. J Infect Dis. 1991;164:320–330. doi: 10.1093/infdis/164.2.320. [DOI] [PubMed] [Google Scholar]
- 102.Marodi L, Leijh PC, van Furth R. Characteristics and functional capacities of human cord blood granulocytes and monocytes. Pediatr Res. 1984;18:1127–1131. doi: 10.1203/00006450-198411000-00014. [DOI] [PubMed] [Google Scholar]
- 103.Hanna N, et al. Mechanisms underlying reduced apoptosis in neonatal neutrophils. Pediatr Res. 2005;57:56–62. doi: 10.1203/01.PDR.0000147568.14392.F0. [DOI] [PubMed] [Google Scholar]
- 104.Anderson DC, Hughes BJ, Smith CW. Abnormal mobility of neonatal polymorphonuclear leukocytes. Relationship to impaired redistribution of surface adhesion sites by chemotactic factor or colchicine. J Clin Invest. 1981;68:863–874. doi: 10.1172/JCI110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carr R, Pumford D, Davies JM. Neutrophil chemotaxis and adhesion in preterm babies. Archives of disease in childhood. 1992;67:813–817. doi: 10.1136/adc.67.7_spec_no.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klein RB, Fischer TJ, Gard SE, Biberstein M, Rich KC, Stiehm ER. Decreased mononuclear and polymorphonuclear chemotaxis in human newborns, infants, and young children. Pediatrics. 1977;60:467–472. [PubMed] [Google Scholar]
- 107.Pahwa SG, Pahwa R, Grimes E, Smithwick E. Cellular and humoral components of monocyte and neutrophil chemotaxis in cord blood. Pediatr Res. 1977;11:677–680. doi: 10.1203/00006450-197705000-00010. [DOI] [PubMed] [Google Scholar]
- 108.Wolach B, Sonnenschein D, Gavrieli R, Chomsky O, Pomeranz A, Yuli I. Neonatal neutrophil inflammatory responses: parallel studies of light scattering, cell polarization, chemotaxis, superoxide release, and bactericidal activity. Am J Hematol. 1998;58:8–15. doi: 10.1002/(sici)1096-8652(199805)58:1<8::aid-ajh2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 109.Anderson DC, Abbassi O, Kishimoto TK, Koenig JM, McIntire LV, Smith CW. Diminished lectin-, epidermal growth factor-, complement binding domain-cell adhesion molecule-1 on neonatal neutrophils underlies their impaired CD18-independent adhesion to endothelial cells in vitro. J Immunol. 1991;146:3372–3379. [PubMed] [Google Scholar]
- 110.Mariscalco MM, Tcharmtchi MH, Smith CW. P-Selectin support of neonatal neutrophil adherence under flow: contribution of L-selectin, LFA-1, and ligand(s) for P-selectin. Blood. 1998;91:4776–4785. [PubMed] [Google Scholar]
- 111.Harris MC, Shalit M, Southwick FS. Diminished actin polymerization by neutrophils from newborn infants. Pediatr Res. 1993;33:27–31. doi: 10.1203/00006450-199301000-00006. [DOI] [PubMed] [Google Scholar]
- 112.Sacchi F, Augustine NH, Coello MM, Morris EZ, Hill HR. Abnormality in actin polymerization associated with defective chemotaxis in neutrophils from neonates. Int Arch Allergy Appl Immunol. 1987;84:32–39. doi: 10.1159/000234395. [DOI] [PubMed] [Google Scholar]
- 113.Carr R. Neutrophil production and function in newborn infants. Br J Haematol. 2000;110:18–28. doi: 10.1046/j.1365-2141.2000.01992.x. [DOI] [PubMed] [Google Scholar]
- 114.Rowen JL, Smith CW, Edwards MS. Group B streptococci elicit leukotriene B4 and interleukin-8 from human monocytes: neonates exhibit a diminished response. J Infect Dis. 1995;172:420–426. doi: 10.1093/infdis/172.2.420. [DOI] [PubMed] [Google Scholar]
- 115.Shigeoka AO, Gobel RJ, Janatova J, Hill HR. Neutrophil mobilization induced by complement fragments during experimental group B streptococcal (GBS) infection. Am J Pathol. 1988;133:623–629. [PMC free article] [PubMed] [Google Scholar]
- 116.Takahashi S, Nagano Y, Nagano N, Hayashi O, Taguchi F, Okuwaki Y. Role of C5a-ase in group B streptococcal resistance to opsonophagocytic killing. Infect Immun. 1995;63:4764–4769. doi: 10.1128/iai.63.12.4764-4769.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martin TR, Ruzinski JT, Rubens CE, Chi EY, Wilson CB. The effect of type-specific polysaccharide capsule on the clearance of group B streptococci from the lungs of infant and adult rats. J Infect Dis. 1992;165:306–314. doi: 10.1093/infdis/165.2.306. [DOI] [PubMed] [Google Scholar]
- 118.Albanyan EA, Vallejo JG, Smith CW, Edwards MS. Nonopsonic binding of type III Group B Streptococci to human neutrophils induces interleukin-8 release mediated by the p38 mitogen-activated protein kinase pathway. Infect Immun. 2000;68:2053–2060. doi: 10.1128/iai.68.4.2053-2060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shappell SB, Toman C, Anderson DC, Taylor AA, Entman ML, Smith CW. Mac-1 (CD11b/CD18) mediates adherence-dependent hydrogen peroxide production by human and canine neutrophils. J Immunol. 1990;144:2702–2711. [PubMed] [Google Scholar]
- 120.Drossou V, et al. Concentrations of main serum opsonins in early infancy. Arch Dis Child Fetal Neonatal Ed. 1995;72:F172–175. doi: 10.1136/fn.72.3.f172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wessels MR, Butko P, Ma M, Warren HB, Lage AL, Carroll MC. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci USA. 1995;92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Edwards MS, Wessels MR, Baker CJ. Capsular polysaccharide regulates neutrophil complement receptor interactions with type III group B streptococci. Infect Immun. 1993;61:2866–2871. doi: 10.1128/iai.61.7.2866-2871.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abughali N, Berger M, Tosi MF. Deficient total cell content of CR3 (CD11b) in neonatal neutrophils. Blood. 1994;83:1086–1092. [PubMed] [Google Scholar]
- 124.McEvoy LT, Zakem-Cloud H, Tosi MF. Total cell content of CR3 (CD11b/CD18) and LFA-1 (CD11a/CD18) in neonatal neutrophils: relationship to gestational age. Blood. 1996;87:3929–3933. [PubMed] [Google Scholar]
- 125.Nupponen I, et al. Neutrophil activation in preterm infants who have respiratory distress syndrome. Pediatrics. 2002;110:36–41. doi: 10.1542/peds.110.1.36. [DOI] [PubMed] [Google Scholar]
- 126.Bowdy BD, Marple SL, Pauly TH, Coonrod JD, Gillespie MN. Oxygen radical-dependent bacterial killing and pulmonary hypertension in piglets infected with group B streptococci. Am Rev Respir Dis. 1990;141:648–653. doi: 10.1164/ajrccm/141.3.648. [DOI] [PubMed] [Google Scholar]
- 127.Kallman J, Schollin J, Schalen C, Erlandsson A, Kihlstrom E. Impaired phagocytosis and opsonisation towards group B streptococci in preterm neonates. Arch Dis Child Fetal Neonatal Ed. 1998;78:F46–50. doi: 10.1136/fn.78.1.f46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.LeVine AM, Reed JA, Kurak KE, Cianciolo E, Whitsett JA. GM-CSF-deficient mice are susceptible to pulmonary group B streptococcal infection. J Clin Invest. 1999;103:563–569. doi: 10.1172/JCI5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gahr M, Schulze M, Scheffczyk D, Speer CP, Peters JH. Diminished release of lactoferrin from polymorphonuclear leukocytes of human neonates. Acta Haematologica. 1987;77:90–94. doi: 10.1159/000205965. [DOI] [PubMed] [Google Scholar]
- 130.Levy O, et al. Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein. Pediatrics. 1999;104:1327–1333. doi: 10.1542/peds.104.6.1327. [DOI] [PubMed] [Google Scholar]
- 131.Dorschner RA, Lin KH, Murakami M, Gallo RL. Neonatal skin in mice and humans expresses increased levels of antimicrobial peptides: innate immunity during development of the adaptive response. Pediatr Res. 2003;53:566–572. doi: 10.1203/01.PDR.0000057205.64451.B7. [DOI] [PubMed] [Google Scholar]
- 132.Reeves EP, et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 133.Levy O. Antimicrobial proteins and peptides: anti-infective molecules of mammalian leukocytes. J Leukoc Biol. 2004;76:909–925. doi: 10.1189/jlb.0604320. [DOI] [PubMed] [Google Scholar]
- 134.Greten FR, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schroder AK, et al. Polymorphonuclear leucocytes selectively produce anti-inflammatory interleukin-1 receptor antagonist and chemokines, but fail to produce pro-inflammatory mediators. Immunology. 2006;119:317–327. doi: 10.1111/j.1365-2567.2006.02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 137.Gray P, Dunne A, Brikos C, Jefferies CA, Doyle SL, O'Neill LA. MyD88 adapter-like (Mal) is phosphorylated by Bruton's tyrosine kinase during TLR2 and TLR4 signal transduction. J Biol Chem. 2006;281:10489–10495. doi: 10.1074/jbc.M508892200. [DOI] [PubMed] [Google Scholar]
- 138.Jefferies CA, et al. Bruton's tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor kappaB activation by Toll-like receptor 4. J Biol Chem. 2003;278:26258–26264. doi: 10.1074/jbc.M301484200. [DOI] [PubMed] [Google Scholar]
- 139.Mansell A, et al. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol. 2006;7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 140.LeBouder E, et al. Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J Immunol. 2003;171:6680–6689. doi: 10.4049/jimmunol.171.12.6680. [DOI] [PubMed] [Google Scholar]
- 141.Fukao T, et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 142.Henneke P, et al. Role of lipoteichoic acid in the phagocyte response to group B streptococcus. J Immunol. 2005;174:6449–6455. doi: 10.4049/jimmunol.174.10.6449. [DOI] [PubMed] [Google Scholar]
- 143.Burns K, et al. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat Cell Biol. 2000;2:346–351. doi: 10.1038/35014038. [DOI] [PubMed] [Google Scholar]
- 144.Didierlaurent A, et al. Tollip regulates proinflammatory responses to interleukin-1 and lipopolysaccharide. Mol Cell Biol. 2006;26:735–742. doi: 10.1128/MCB.26.3.735-742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Conner JR, Smirnova II, Poltorak A. Forward genetic analysis of Toll-like receptor responses in wild-derived mice reveals a novel antiinflammatory role for IRAK1BP1. J Exp Med. 2008;205:305–314. doi: 10.1084/jem.20071499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boone DL, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 147.Hitotsumatsu O, et al. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity. 2008;28:381–390. doi: 10.1016/j.immuni.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brint EK, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–379. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 149.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 150.Diehl GE, et al. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004;21:877–889. doi: 10.1016/j.immuni.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 151.Janssens S, Burns K, Tschopp J, Beyaert R. Regulation of interleukin-1- and lipopolysaccharide-induced NF-kappaB activation by alternative splicing of MyD88. Curr Biol. 2002;12:467–471. doi: 10.1016/s0960-9822(02)00712-1. [DOI] [PubMed] [Google Scholar]
- 152.Learn CA, Mizel SB, McCall CE. mRNA and protein stability regulate the differential expression of pro- and anti-inflammatory genes in endotoxin-tolerant THP-1 cells. J Biol Chem. 2000;275:12185–12193. doi: 10.1074/jbc.275.16.12185. [DOI] [PubMed] [Google Scholar]
- 153.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 154.Munford RS, Pugin J. Normal Responses to Injury Prevent Systemic Inflammation and Can Be Immunosuppressive. Am J Respir Crit Care Med. 2001;163:316–321. doi: 10.1164/ajrccm.163.2.2007102. [DOI] [PubMed] [Google Scholar]
- 155.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 156.Medvedev AE, et al. Induction of tolerance to lipopolysaccharide and mycobacterial components in Chinese hamster ovary/CD14 cells is not affected by overexpression of Toll-like receptors 2 or 4. J Immunol. 2001;167:2257–2267. doi: 10.4049/jimmunol.167.4.2257. [DOI] [PubMed] [Google Scholar]
- 157.Bohuslav J, et al. Regulation of an essential innate immune response by the p50 subunit of NF-kappaB. J Clin Invest. 1998;102:1645–1652. doi: 10.1172/JCI3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ziegler-Heitbrock HW, et al. Tolerance to lipopolysaccharide involves mobilization of nuclear factor kappa B with predominance of p50 homodimers. J Biol Chem. 1994;269:17001–17004. [PubMed] [Google Scholar]
- 159.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr., Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 160.van 't Veer C, et al. Induction of IRAK-M is associated with lipopolysaccharide tolerance in a human endotoxemia model. J Immunol. 2007;179:7110–7120. doi: 10.4049/jimmunol.179.10.7110. [DOI] [PubMed] [Google Scholar]
- 161.Medvedev AE, et al. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J Biol Chem. 2007;282:16042–16053. doi: 10.1074/jbc.M606781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kallapur SG, et al. Pulmonary and systemic endotoxin tolerance in preterm fetal sheep exposed to chorioamnionitis. J Immunol. 2007;179:8491–8499. doi: 10.4049/jimmunol.179.12.8491. [DOI] [PubMed] [Google Scholar]
- 163.Molloy EJ, O'Neill AJ, Grantham-Sloan JJ, Webb DW, Watson RW. Maternal and neonatal lipopolysaccharide and Fas responses are altered by antenatal risk factors for sepsis. Clin Exp Immunol. 2008;151:244–250. doi: 10.1111/j.1365-2249.2007.03540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Leib SL, Kim YS, Chow LL, Sheldon RA, Tauber MG. Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J Clin Invest. 1996;98:2632–2639. doi: 10.1172/JCI119084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bogdan I, Leib SL, Bergeron M, Chow L, Tauber MG. Tumor necrosis factor-alpha contributes to apoptosis in hippocampal neurons during experimental group B streptococcal meningitis. J Infect Dis. 1997;176:693–697. doi: 10.1086/514092. [DOI] [PubMed] [Google Scholar]
- 167.Bifrare YD, Kummer J, Joss P, Tauber MG, Leib SL. Brain-derived neurotrophic factor protects against multiple forms of brain injury in bacterial meningitis. J Infect Dis. 2005;191:40–45. doi: 10.1086/426399. [DOI] [PubMed] [Google Scholar]
- 168.Lehnardt S, et al. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol. 2006;177:583–592. doi: 10.4049/jimmunol.177.1.583. [DOI] [PubMed] [Google Scholar]
- 169.Fettucciari K, et al. Group B Streptococcus induces apoptosis in macrophages. J Immunol. 2000;165:3923–3933. doi: 10.4049/jimmunol.165.7.3923. [DOI] [PubMed] [Google Scholar]
- 170.Fettucciari K, Fetriconi I, Bartoli A, Rossi R, Marconi P. Involvement of mitogen-activated protein kinases in Group B Streptococcus-induced macrophage apoptosis. Pharmacol Res. 2003;47:355–362. doi: 10.1016/s1043-6618(03)00004-5. [DOI] [PubMed] [Google Scholar]
- 171.Fettucciari K, et al. Group B Streptococcus induces macrophage apoptosis by calpain activation. J Immunol. 2006;176:7542–7556. doi: 10.4049/jimmunol.176.12.7542. [DOI] [PubMed] [Google Scholar]
- 172.Buratta S, Fettucciari K, Mambrini R, Fetriconi I, Marconi P, Mozzi R. Group B streptococcus (GBS) modifies macrophage phosphatidylserine metabolism during induction of apoptosis. FEBS Lett. 2002;520:68–72. doi: 10.1016/s0014-5793(02)02769-2. [DOI] [PubMed] [Google Scholar]
- 173.Ulett GC, Bohnsack JF, Armstrong J, Adderson EE. Beta-hemolysin-independent induction of apoptosis of macrophages infected with serotype III group B streptococcus. J Infect Dis. 2003;188:1049–1053. doi: 10.1086/378202. [DOI] [PubMed] [Google Scholar]
- 174.Liu GY, et al. Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc Natl Acad Sci USA. 2004;101:14491–14496. doi: 10.1073/pnas.0406143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ulett GC, Adderson EE. Nitric oxide is a key determinant of group B streptococcus-induced murine macrophage apoptosis. J Infect Dis. 2005;191:1761–1770. doi: 10.1086/429693. [DOI] [PubMed] [Google Scholar]
- 176.Ulett GC, Maclean KH, Nekkalapu S, Cleveland JL, Adderson EE. Mechanisms of group B streptococcal-induced apoptosis of murine macrophages. J Immunol. 2005;175:2555–2562. doi: 10.4049/jimmunol.175.4.2555. [DOI] [PubMed] [Google Scholar]
- 177.van der Flier M, Geelen SP, Kimpen JL, Hoepelman IM, Tuomanen EI. Reprogramming the host response in bacterial meningitis: how best to improve outcome? Clin Microbiol Rev. 2003;16:415–429. doi: 10.1128/CMR.16.3.415-429.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jung DY, et al. TLR4, but not TLR2, signals autoregulatory apoptosis of cultured microglia: a critical role of IFN-beta as a decision maker. J Immunol. 2005;174:6467–6476. doi: 10.4049/jimmunol.174.10.6467. [DOI] [PubMed] [Google Scholar]
- 179.Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J. 2000;19:3325–3336. doi: 10.1093/emboj/19.13.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lopez M, Sly LM, Luu Y, Young D, Cooper H, Reiner NE. The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2. J Immunol. 2003;170:2409–2416. doi: 10.4049/jimmunol.170.5.2409. [DOI] [PubMed] [Google Scholar]
- 181.Into T, et al. Stimulation of human Toll-like receptor (TLR) 2 and TLR6 with membrane lipoproteins of Mycoplasma fermentans induces apoptotic cell death after NF-kappa B activation. Cell Microbiol. 2004;6:187–199. doi: 10.1046/j.1462-5822.2003.00356.x. [DOI] [PubMed] [Google Scholar]
- 182.Salaun B, Romero P, Lebecque S. Toll-like receptors’ two-edged sword: when immunity meets apoptosis. Eur J Immunol. 2007;37:3311–3318. doi: 10.1002/eji.200737744. [DOI] [PubMed] [Google Scholar]
- 183.Halaas O, et al. Monocytes stimulated with group B streptococci or interferons release tumour necrosis factor-related apoptosis-inducing ligand. Scand J Immunol. 2004;60:74–81. doi: 10.1111/j.0300-9475.2004.01448.x. [DOI] [PubMed] [Google Scholar]
- 184.Leib SL, Kim YS, Black SM, Tureen JH, Tauber MG. Inducible nitric oxide synthase and the effect of aminoguanidine in experimental neonatal meningitis. J Infect Dis. 1998;177:692–700. doi: 10.1086/514226. [DOI] [PubMed] [Google Scholar]
- 185.Arnold R, Brenner D, Becker M, Frey CR, Krammer PH. How T lymphocytes switch between life and death. Eur J Immunol. 2006;36:1654–1658. doi: 10.1002/eji.200636197. [DOI] [PubMed] [Google Scholar]
- 186.Donjerkovic D, Scott DW. Activation-induced cell death in B lymphocytes. Cell Res. 2000;10:179–192. doi: 10.1038/sj.cr.7290047. [DOI] [PubMed] [Google Scholar]
- 187.Adler B, Adler H, Jungi TW, Peterhans E. Interferon-alpha primes macrophages for lipopolysaccharide-induced apoptosis. Biochem Biophys Res Commun. 1995;215:921–927. doi: 10.1006/bbrc.1995.2552. [DOI] [PubMed] [Google Scholar]
- 188.Kim SO, Ono K, Tobias PS, Han J. Orphan nuclear receptor Nur77 is involved in caspase-independent macrophage cell death. J Exp Med. 2003;197:1441–1452. doi: 10.1084/jem.20021842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mayo L, Stein R. Characterization of LPS and interferon-gamma triggered activation-induced cell death in N9 and primary microglial cells: induction of the mitochondrial gateway by nitric oxide. Cell Death Differ. 2007;14:183–186. doi: 10.1038/sj.cdd.4401989. [DOI] [PubMed] [Google Scholar]
- 190.Liu B, Wang K, Gao HM, Mandavilli B, Wang JY, Hong JS. Molecular consequences of activated microglia in the brain: overactivation induces apoptosis. J Neurochem. 2001;77:182–189. doi: 10.1046/j.1471-4159.2001.t01-1-00216.x. [DOI] [PubMed] [Google Scholar]
- 191.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Albert ML. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat Rev. 2004;4:223–231. doi: 10.1038/nri11308. [DOI] [PubMed] [Google Scholar]
- 193.Philpott DJ, Edgeworth JD, Sansonetti PJ. The pathogenesis of Shigella flexneri infection: lessons from in vitro and in vivo studies. Philos Trans R Soc Lond. 2000;355:575–586. doi: 10.1098/rstb.2000.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fukui M, Imamura R, Umemura M, Kawabe T, Suda T. Pathogen-associated molecular patterns sensitize macrophages to Fas ligand-induced apoptosis and IL-1 beta release. J Immunol. 2003;171:1868–1874. doi: 10.4049/jimmunol.171.4.1868. [DOI] [PubMed] [Google Scholar]
- 195.Saleh M, Green DR. Caspase-1 inflammasomes: choosing between death and taxis. Cell Death Differ. 2007;14:1559–1560. doi: 10.1038/sj.cdd.4402203. [DOI] [PubMed] [Google Scholar]
- 196.Gille C, et al. Diminished phagocytosis-induced cell death (PICD) in neonatal monocytes upon infection with Escherichia coli. Pediatr Res. 2008;63:33–38. doi: 10.1203/PDR.0b013e31815b8e9f. [DOI] [PubMed] [Google Scholar]
- 197.Krilov LR, McCloskey TW, Harkness SH, Pontrelli L, Pahwa S. Alterations in apoptosis of cord and adult peripheral blood mononuclear cells induced by in vitro infection with respiratory syncytial virus. J Infect Dis. 2000;181:349–353. doi: 10.1086/315203. [DOI] [PubMed] [Google Scholar]
- 198.Peck-Palmer OM, Unsinger J, Chang KC, Davis CG, McDunn JE, Hotchkiss RS. Deletion of MyD88 markedly attenuates sepsis-induced T and B lymphocyte apoptosis but worsens survival. J Leukoc Biol. 2008;83:1009–1018. doi: 10.1189/jlb.0807528. [DOI] [PubMed] [Google Scholar]
- 199.Weinberger B, et al. Influence of labor on neonatal neutrophil apoptosis, and inflammatory activity. Pediatr Res. 2007;61:572–577. doi: 10.1203/pdr.0b013e318045be38. [DOI] [PubMed] [Google Scholar]