Abstract
Association between circulating lipopolysaccharide (LPS) and metabolic diseases (such as type 2 diabetes and atherosclerosis) has shifted the focus from high-fat high-cholesterol containing Western-type diet (WD)-induced changes in gut microbiota per se to release of gut bacteria-derived products (e.g., LPS) into circulation due to intestinal barrier dysfunction as the possible mechanism for the chronic inflammatory state underlying the development of these diseases. We demonstrated earlier that oral supplementation with curcumin attenuates WD-induced development of type 2 diabetes and atherosclerosis. Poor bioavailability of curcumin has precluded the establishment of a causal relationship between oral supplementation and it is in vivo effects. We hypothesized that curcumin attenuates WD-induced chronic inflammation and associated metabolic diseases by modulating the function of intestinal epithelial cells (IECs) and the intestinal barrier function. The objective of the present study was to delineate the underlying mechanisms. The human IEC lines Caco-2 and HT-29 were used for these studies and modulation of direct as well as indirect effects of LPS on intracellular signaling as well as tight junctions were examined. Pretreatment with curcumin significantly attenuated LPS-induced secretion of master cytokine IL-1β from IECs and macrophages. Furthermore, curcumin also reduced IL-1β-induced activation of p38 MAPK in IECs and subsequent increase in expression of myosin light chain kinase involved in the phosphorylation of tight junction proteins and ensuing disruption of their normal arrangement. The major site of action of curcumin is, therefore, likely the IECs and the intestinal barrier, and by reducing intestinal barrier dysfunction, curcumin modulates chronic inflammatory diseases despite poor bioavailability.
Keywords: chronic inflammatory metabolic diseases, intestinal epithelial cell inflammation, tight junction proteins
in addition to Western diet (WD)-induced dyslipidemia, chronic inflammatory state is increasingly being recognized as an important contributor to the development of metabolic diseases such as type 2 diabetes and atherosclerosis. The mechanisms underlying the development of this chronic inflammation are poorly defined and whether WD feeding directly contributes to this process is not known. The observed increase in circulating LPS following WD feeding has shifted the focus from WD-induced changes in gut microbiota per se to release of gut bacteria-derived products into circulation as the possible mechanism for the chronic inflammatory state underlying the development of these diseases. In human studies, the plasma LPS concentration is positively correlated with visceral fat volume and indexes of insulin resistance such as homeostasis model assessment-insulin resistance (HOMA-IR) or HbA1c (24, 25). In a healthy intestine, the translocation of LPS from the gut lumen into the circulation is restricted by the intestinal epithelial barrier. However, disruption of this intestinal barrier function leads to an increase in systemic LPS levels. We and others have reported an increase in circulating LPS levels in response to WD feeding due to an increase in intestinal permeability (8, 12).
Intestinal barrier can be visualized as composed of four different “layers” namely, 1) intestinal alkaline phosphatase that dephosphorylates and detoxifies LPS; 2) mucin layer that limits the direct interaction between gut bacteria and the intestinal epithelial cells; 3) The tight junctions of the intestinal epithelial cell layer; and 4) the antibacterial proteins secreted by the Paneth cells into the lumen. Modulation of one or more of these layers to improve intestinal barrier function is likely to limit the generation or translocation of gut bacteria-derived LPS into the systemic circulation. Although the effects of WD in modulating the diversity of gut bacteria are well characterized (4, 26), the mechanism(s) by which it increases intestinal permeability is not known. We earlier reported a WD-induced decrease in intestinal alkaline phosphatase activity (12) indicating the disruption of this first layer of the intestinal barrier by WD that results in effectively increasing the luminal LPS concentration.
Luminal LPS not only activates the intestinal epithelial cells (IECs) but also activates intestinal macrophages that “survey” the intestinal lumen and constitute the intestinal innate immune system (6). IECs as well as macrophages secrete proinflammatory cytokines when activated by LPS, e.g., interleukin-1β (IL-1β), that can further activate these cells and perpetuate inflammatory responses as well as intracellular signaling. IL-1β-dependent activation of p38 MAP kinase and subsequent activation of myosin light chain kinase (MLCK) leading to phosphorylation of myosin light chains result in disruption of intestinal tight junctions and an increase in intestinal permeability emphasizing the importance of understanding the processes that regulate luminal LPS or LPS-induced IL-1β production as well as strategies to modulate them.
We have earlier demonstrated that oral supplementation with curcumin significantly increased the activity of intestinal alkaline phosphatase and reduced the levels of plasma LPS in WD-fed mice demonstrating its protective effects against WD-induced disruption of intestinal barrier function (12). Curcumin is reported to exert potent anti-inflammatory effects in vitro, but its poor bioavailability has raised doubts about a causal relationship between oral supplementation and the in vivo effects. Based on our data demonstrating significant improvement of intestinal barrier function by curcumin, it is hypothesized that curcumin attenuates WD-induced chronic inflammation and associated metabolic diseases by modulating the function of IECs and the objective of the present study was to delineate the underlying mechanisms. The data presented here demonstrate that curcumin attenuates activation of IECs as well as macrophages resulting in decreased secretion of IL-1β. Reduced intracellular IL-1β signaling and subsequent reduction in disruption of tight junctions represent the mechanisms underlying the beneficial effects of oral curcumin, despite its poor bioavailability.
MATERIALS AND METHODS
Two human colon carcinoma cell lines (Caco-2 and HT-29), model cell systems for IECs used in these studies, were obtained from ATCC and maintained in DMEM supplemented with 20% fetal bovine serum. Lipopolysaccharide (LPS) from Escherichia coli (055:B5) and curcumin were purchased from Sigma-Aldrich. The MAPK p38 inhibitor SB203580 was purchased from Cell Signaling. Primary antibodies were either from Cell Signaling (JNK, 9252; p-JNK, 9251; Erk1/2, 4695; p-Erk1/2, 4370; p38, 9212; p-p38, 9216; and p-MKP1 2857) or from Santa Cruz Biotechnology (ZO-1, sc-10804; claudin-1, sc-17658 and sc-22932, MKP1, and sc-370) or Invitrogen (claudin-7, 34-9100) and were used at dilutions recommended by the manufacturer. Secondary antibodies for Western blot analyses were from LICOR. Biotinylated secondary antibodies as well as NeutrAvidin-conjugated AlexaFluors were from Pierce/ThermoFisher. Ready-go-set ELISA kit for measuring human IL-1β, IL-10, and IL-6 were from Affymetrix-eBiosciences. Optimized Taqman gene expression assay kits for IL-1β (Hs00174097_m1), IL-6 (Hs00985639_m1), IL-10 (Hs00961622_m1), and MLCK (Hs00364926_m1) were obtained from Applied Biosciences (ThermoFisher), and qPCR conditions recommended by the manufacturer were used to determine the respective mRNA levels and normalized to β-actin (4326315E) serving as the housekeeping gene.
Secretion of IL-1β.
IECs, PMA-differentiated human THP1 macrophages (29), or thioglycolate-elicited mouse peritoneal macrophages (isolated using Institutional Animal Care and Use Committee approved protocol and as described before; Ref. 28) were pretreated with curcumin (5 μM) for 48 h and then primed with LPS (1 μg/ml) overnight. Inflammasome was activated (second signal) either by nigericin (20 μM, 20 min) or ALUM (200 μg/ml). Conditioned medium was collected after 6 h and secreted IL-1β was measured by ELISA. To assess the effects on the expression of cytokines (IL-1β, IL-6, and IL-10), total RNA was extracted from the cells following exposure to LPS and mRNA levels determined by real-time PCR and normalized to β-actin using Taqman assays.
Intracellular IL-1β signaling.
IECs were pretreated with curcumin (5 μM) for 48 h and then activated by incubation with IL-1β (10 ng/ml, 10 min). Where indicated, cells were treated for 1 h with SB203580 (10 μM), before the addition of IL-1β. Total protein lysates were prepared and the level of p38, JNK, or Erk1/2 phosphorylation was assessed by Western blotting. The membranes were stripped and probed for total p38, JNK, or Erk1/2 protein for normalization. For measurement of MLCK expression, total RNA was prepared from the cells and MLCK mRNA levels determined by real-time RT-PCR and normalized to β-actin using optimized Taqman assays.
Immunocytochemistry.
IECs were plated in four-well chamber slides and treated with LPS (1 μg/ml) or IL-1β (10 ng/ml) with or without pretreatment with curcumin (5 μM). Cells were fixed in 4% buffered formalin for 1 h, permeabilized with Triton X-100 (0.1% in cold PBS for 3 min on ice), blocked in 1% BSA for 1 h and then incubated overnight with primary antibody (ZO-1, sc-17658 or claudin-1, sc-17658 or sc-22932 or claudin-7, 34–9100) at dilutions recommended by the manufacturer. Specific staining was detected using biotinylated secondary antibody and NeutrAvidin-conjugated AlexaFluors. Actin filaments were stained using phalloidin and images were acquired using Carl Zeiss inverted fluorescence microscope fitted with a digital camera.
Translocation of mannitol.
Effect of curcumin on translocation of [3H]mannitol was determined as described earlier (12). In brief, Caco-2 cells were plated on polycarbonate Transwells at 1 × 105 cells/cm2 and cultured for 21 days. Before the start of the experiment the transepithelial electrical resistance (TEER) was measured and cultures displaying a TEER between 300 and 400 Ω/cm2 were considered suitable for the study. Cells were pretreated with 5 µM curcumin (in DMSO) for 24 h, either from the apical or from the basolateral side before being stimulated with IL-1β (10 ng/ml) from the apical side only. After a wash in HBBS, 0.5 ml [3H]mannitol (0.5 mCi/ml HBSS) was added to the apical side. Medium (50 µl, in duplicate) was collected from the basolateral side at 2 and 4 h and associated radioactivity determined.
RESULTS
Curcumin attenuates LPS-induced secretion of IL-1β from IECs.
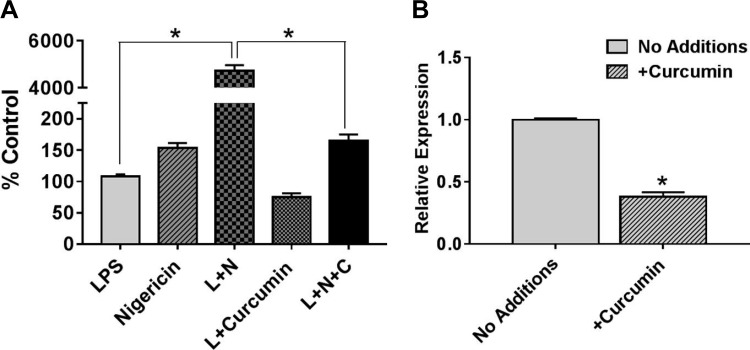
In vivo, IECs as well as intestinal macrophages are continually exposed to gut bacteria-derived LPS and secrete proinflammatory cytokine IL-1β that in turn can potentiate proinflammatory signaling in these cells. IEC inflammation is central to increasing intestinal permeability and we have earlier demonstrated a twofold increase in the secretion of intestinal alkaline phosphatase (IAP) by oral supplementation with curcumin (12); IAP dephosphorylates/detoxifies LPS thereby reducing its proinflammatory effects on IECs. In the present study, we examined the effect of curcumin on LPS-induced secretion of IL-1β from IECs. As shown in Fig. 1, pretreatment of Caco-2 cells with curcumin significantly reduced LPS-induced secretion of IL-1β (Fig. 1A) as well as expression of IL-1β mRNA expression (Fig. 1B); nigericin was used as the second signal to activate inflammasome and processing of pro-IL-1β required for its secretion (10).
Fig. 1.
Curcumin attenuates IL-1β production from IECs. A: confluent Caco-2 monolayers were pretreated with curcumin (C; 5 μM) for 48 h and then primed with LPS (L; 1 μg/ml) overnight. Inflammasome was activated (second signal) by nigericin (N; 20 μM, 20 min). Conditioned medium was collected after 6 h and secreted IL-1β was measured by ELISA. Data (means ± SD, n = 3) are presented as percent control where control represents cells treated with LPS alone. B: total RNA was extracted from confluent monolayers of Caco-2 cells with or without treatment with curcumin (5 μM) for 48 h and IL-1β expression was determined by real-time RT-PCR. Data (means ± SD; n = 3) are presented as IL-1β expression relative to β-actin. *P < 0.05.
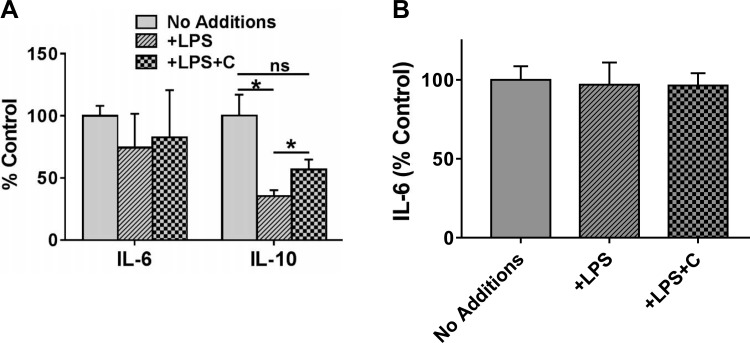
To determine whether exposure to LPS affects the expression of other cytokines, mRNA levels of IL-6 and IL-10 were monitored. While no significant change in the expression of IL-6 was observed, exposure to LPS significantly reduced the expression of IL-10 (Fig. 2A). Consistent with its anti-inflammatory role, pretreatment with curcumin attenuated the LPS-induced decrease in IL-10 expression (Fig. 2A). Secretion of IL-6 into the culture medium was, similarly, not affected by LPS exposure (Fig. 2B); IL-10 could not be detected in the culture medium by the commercially available ELISA kits with lowest detection limits in the picogram range indicating low levels of IL-10 secretion by IECs.
Fig. 2.
Effects of curcumin on expression of IL-6 and IL-10. A: confluent Caco-2 monolayers were pretreated with curcumin (C; 5 μM) for 48 h and then primed with LPS (L; 1 μg/ml) overnight. Total RNA was extracted and IL-6 as well as IL-10 expression was determined by real-time RT-PCR. Data (relative expression, normalized to β-actin) are presented (means ± SD, n = 3). *P < 0.05; ns, nonsignificant difference. B: confluent Caco-2 monolayers were pretreated with curcumin (C; 5 μM) for 48 h and then primed with LPS (L; 1 μg/ml) overnight. Conditioned medium was collected after 6 h and secreted IL-6 and IL-10 were measured by ELISA. Data (means ± SD; n = 3) are presented as percent control where control represents untreated cells (No additions).
Curcumin reduces IL-1β secretion from macrophages.
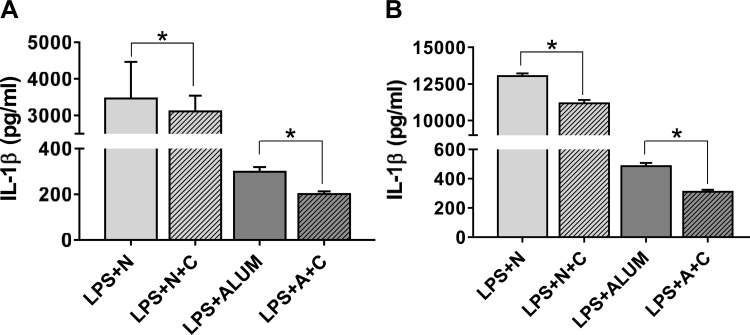
In addition to IECs, intestinal macrophages are also exposed to gut bacteria-derived LPS and activated macrophages contribute to the perpetuation of inflammatory processes by secreting IL-1β. The direct effects of curcumin on secretion of IL-1β from human THP1 as well as mouse peritoneal macrophages were examined in response to stimulation with LPS; nigericin or ALUM were used as the second signals (10). While stimulation of LPS-primed cells with nigericin led to significantly higher secretion of IL-1β compared with LPS + ALUM, curcumin significantly attenuated IL-1β secretion under both conditions in human THP1 macrophages (Fig. 3A) and mouse peritoneal macrophages (Fig. 3B). These data demonstrate that in addition to reducing the potency of luminal LPS by increasing IAP activity in vivo, curcumin effectively reduces the perpetuation of LPS-induced IEC inflammation by reducing IL-1β production from IECs as well as macrophages.
Fig. 3.
Curcumin attenuates LPS-induced IL-1β production from macrophages: PMA-differentiated human THP1 macrophages (A) or thioglycolate-elicited mouse peritoneal macrophages (B) were pretreated with curcumin (5 μM) for 48 h and then primed with LPS (50 ng/ml) overnight. Inflammasome was activated (second signal) either by nigericin (N; 20 μM, 20 min) or by ALUM (A; 200 μg/ml) Conditioned medium was collected after 6 h and secreted IL-1β measured by ELISA. Data (means ± SD, n = 6) are presented as IL-1β (pg/ml). *P < 0.05.
Curcumin reduces IL-1β-induced activation of p38 MAPK.
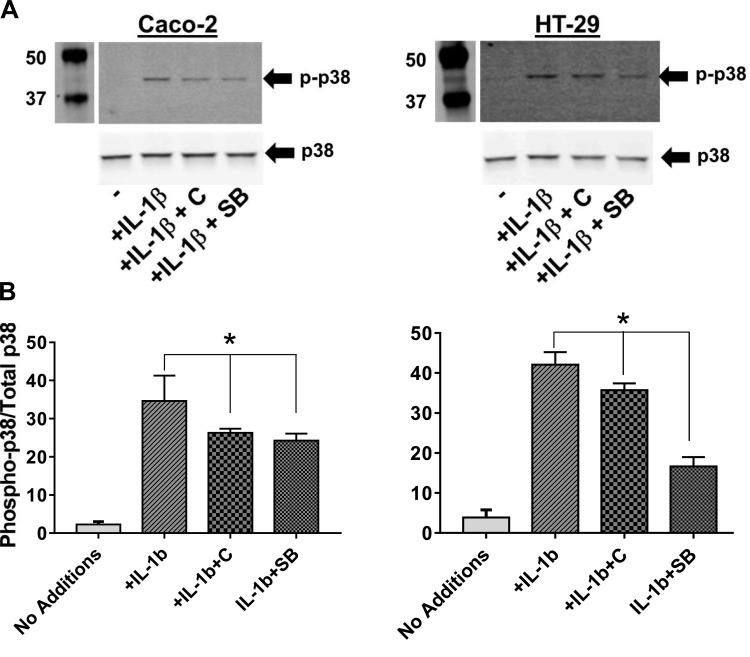
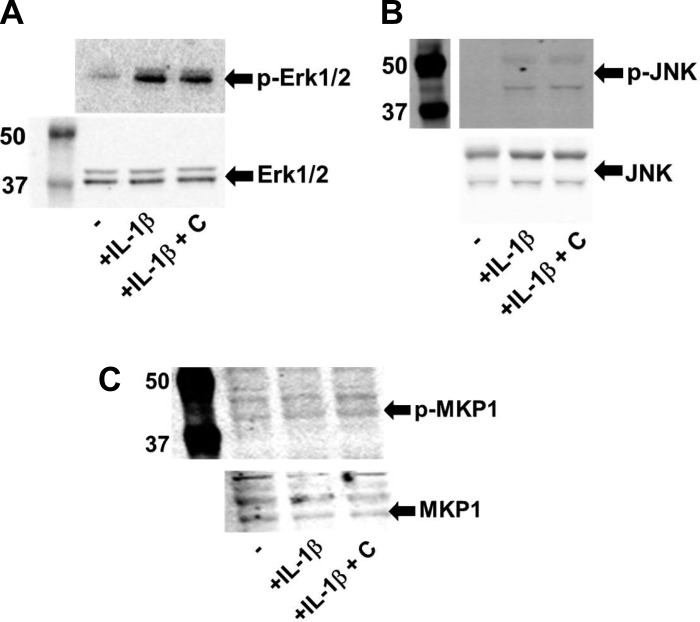
Proinflammatory signaling in IECs is mediated by activation of MAPK and exposure to IL-1β has been shown to increase the activation of p38 MAPK (2). To examine whether in addition to reducing LPS-induced IL-1β production from IECs (Fig. 1) or intestinal macrophages as shown (Fig. 3), curcumin also modulates inflammatory signaling in IECs by affecting activation of p38 MAPK, IECs namely Caco-2 or HT-29 cells were exposed to IL-1β with or without pretreatment with curcumin. As shown in Fig. 4, exposure to IL-1β increased phosphorylation/activation of p38 MAPK. Pretreatment with curcumin significantly attenuated this effect and effect of curcumin was comparable to the bona fide p38 MAPK inhibitor SB203580 demonstrating curcumin-dependent reduction in intracellular IL-1β signaling. The levels of intracellular phosphorylated p38 MAPK are regulated by a balance between IL-1β signaling and MKP-1-mediated dephosphorylation. Pretreatment of IECs with curcumin did not affect the expression/phosphorylation of MKP-1 (Fig. 5) indicating that the observed effects of curcumin on attenuating phospho-p38 levels may not involve MKP-1-mediated dephosphorylation. Curcumin did not affect the activation/phosphorylation of other MAPKs, namely Erk1/2 and JNK (Fig. 5).
Fig. 4.
Curcumin reduces IL-1β-induced activation of p38 MAPK. IECs (Caco-2 or HT-29) were pretreated with curcumin (C, 5 μM) for 48 h and then activated by incubation with IL-1β (10 ng/ml, 10 min). Where indicated, cells were treated for 1 h with SB203580 (SB; 10 μM), before the addition of IL-1β. Total protein lysates were prepared and the level of p38 phosphorylation was assessed by Western blotting. A: representative Western blots; B: densitometric quantification. Data (means ± SD; n = 3) are expressed as the ratio of phospho-p38 and total p38. *P < 0.05.
Fig. 5.
Curcumin does not affect IL-1β-induced activation of Erk1/2 or JNK. IECs (Caco-2 or HT-29) were pretreated with curcumin (C, 5 μM) for 48 h and then activated by incubation with IL-1β (10 ng/ml, 10 min). Total protein lysates were prepared and the level of phosphorylated as well as total Erk1/2 (A), JNK (B), and MKP-1 (C) was assessed by Western blotting.
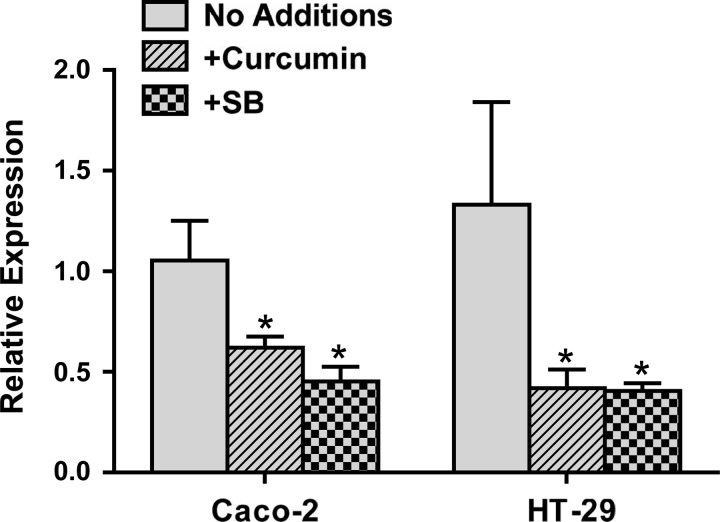
Curcumin reduces MLCK expression in IECs.
Myosin light chain phosphorylation mediated by MLCK is critical to the pathophysiological regulation of intestinal barrier function (9). Activation of p38 MAPK leads to increased expression of MLCK and likely represents the mechanism underlying the p38 MAPK-dependent disruption of intestinal barrier function (3). In addition to decreasing p38 phosphorylation, whether pretreatment with curcumin also affects MLCK expression was examined in IECs. MLCK expression was significantly reduced with curcumin pretreatment (Fig. 6). More importantly, curcumin-mediated decrease in MLCK expression was similar to that seen with the p38 MAPK inhibitor SB203580. Taken together with our earlier demonstration that curcumin increases IAP activity in vivo and the data shown in Fig. 1, these data demonstrate that curcumin can effectively attenuate intestinal inflammation by acting at multiple levels, namely, detoxification of LPS, attenuation of IL-1β production, decrease in IL-1β-induced proinflammatory signaling in IECs, and reduced expression of MLCK.
Fig. 6.
Curcumin attenuates expression of MLCK in IECs. Total RNA was extracted from confluent monolayers of Caco-2 or HT-29 cells with or without treatment with curcumin (5 μM) for 48 h and MLCK expression was determined by real-time RT-PCR. Data (means ± SD; n = 3) are presented as MLCK expression relative to β-actin. *P < 0.05.
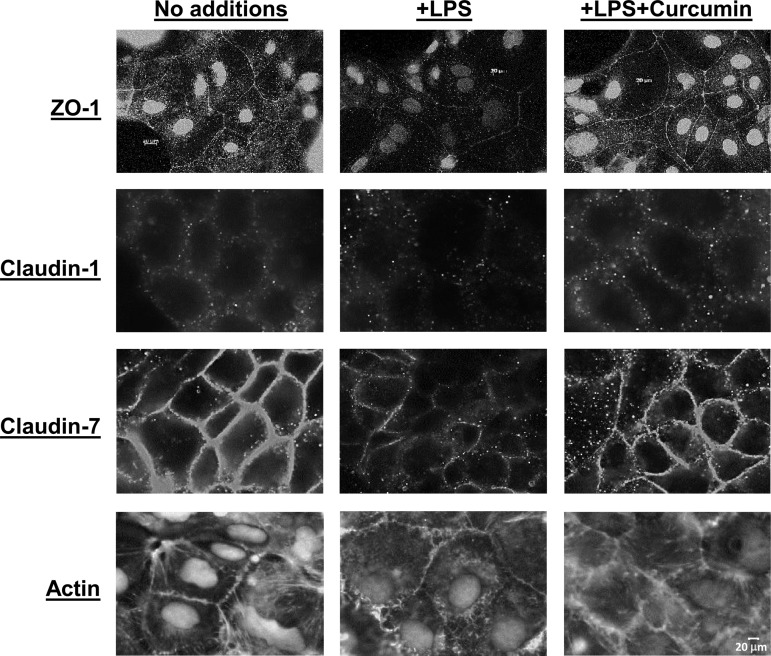
Curcumin prevents disruption of the organization of tight junction proteins.
In addition to appropriate expression, proper organization of the tight junction proteins (e.g., zona occludens, ZO-1, and claudins) is critical for the maintenance of an intact intestinal permeability barrier. The proposed model includes binding of ZO-1, 2, and 3 with membrane associated claudin-1 and anchoring of this complex by intracellular actin filaments (27). We examined the effects of curcumin on LPS-mediated disruption of the organization of tight junction proteins in Caco-2 cells. As expected, exposure to LPS alters the organization of ZO-1, claudin-1, and claudin-7 on the cell surface (Fig. 7). It should be pointed out that in Caco-2 cells ZO-1 is also present in the nucleus in addition to being associated with plasma membrane (13). Under normal conditions (no treatment), actin filaments are present in a lattice-like formation within the cells and facilitate the anchoring of occludin-ZO-1 complexes. Exposure to LPS alters this actin filament arrangement as seen in Fig. 7, bottom. Pretreatment with curcumin, prevents this LPS-induced disorganization of ZO-1, claudin-1, claudin-7, as well as actin filaments. Similar effects were also noted in the other IEC cell line, HT-29 (data not shown). These data suggest that the observed improvement in intestinal permeability as well as permeability of Caco-2 cell monolayers by curcumin (12) is plausibly facilitated by curcumin-mediated maintenance of tight junction protein organization.
Fig. 7.
Curcumin prevents LPS-induced disruption of the organization of tight junction proteins. Confluent monolayers of Caco-2 cells were pretreated with curcumin (5 μM) for 48 h and then exposed to LPS overnight. After fixation, distribution of ZO-1, claudin-1, claudin-7, and actin was assessed by immunocytochemistry as described under materials and methods. Images were acquired under identical exposures and representative images are presented. Scale bar, 20 μm.
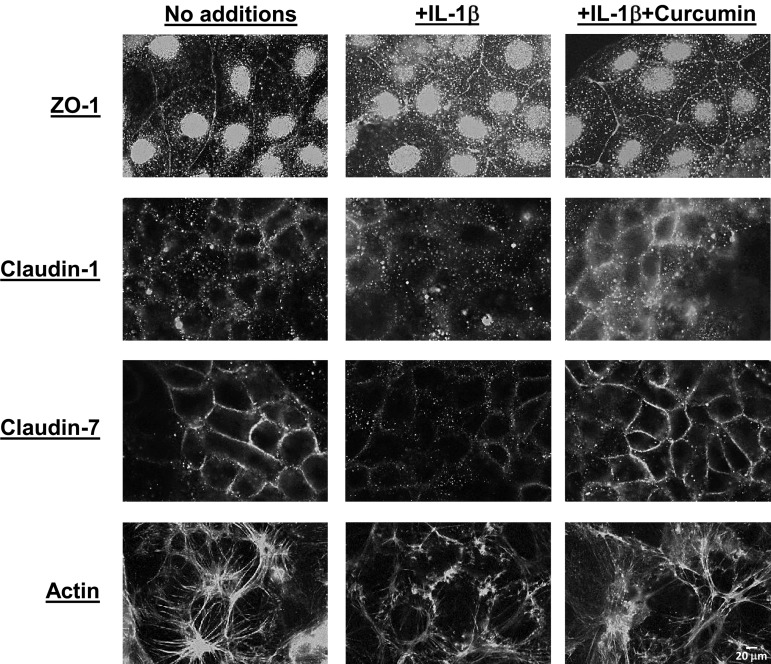
As shown above, curcumin also attenuates LPS-induced IL-1β secretion as well as the downstream proinflammatory signaling. The effects of curcumin pretreatment on IL-1β-induced changes in organization of tight junction proteins were also examined. Similar to the effects seen with LPS, exposure to IL-1β altered the surface expression of ZO-1, claudin-1, and claudin-7 as well as the intracellular organization of actin filaments (Fig. 8). Pretreatment with curcumin reduced IL-1β-induced disruption of the organization of tight junction proteins and actin filaments. Taken together, these data demonstrate that curcumin protects against the direct effects of LPS on the organization of tight junction proteins as well as similar indirect effects via attenuation of IL-1β secretion.
Fig. 8.
Curcumin prevents IL-1β-induced disruption of the organization of tight junction proteins. Confluent monolayers of Caco-2 cells were pretreated with curcumin (5 μM) for 48 h and then exposed to IL-1β (10 ng/ml) overnight. After fixation, distribution of ZO-1, claudin-1, claudin-7, and actin was assessed by immunocytochemistry as described under materials and methods. Images were acquired under identical exposures and representative images are presented. Scale bar, 20 μm.
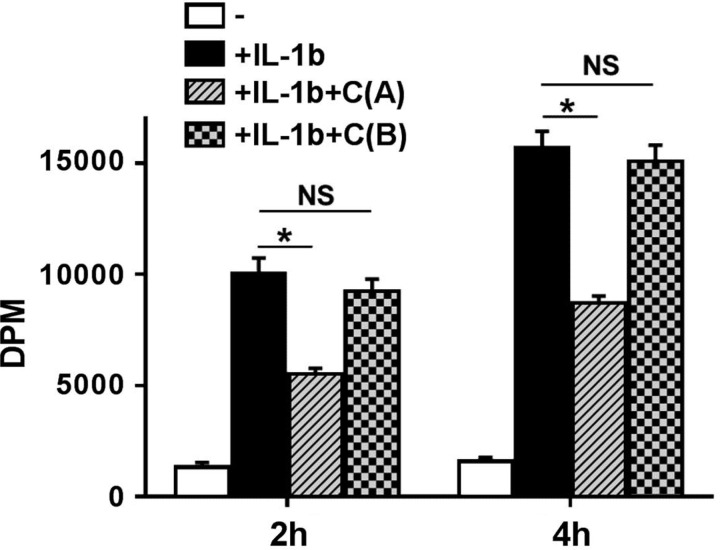
Curcumin attenuates IL-1β-induced paracellular transport.
Since paracellular transport will be the final physiological effect of intracellular IL-1β signaling and the downstream effects on expression/organization of tight junction proteins, the effects of curcumin on paracellular transport of [3H]mannitol across polarized Caco-2 cell monolayers was monitored. In accordance with the effects of curcumin in reducing IL-1β mediated signaling and disruption of tight junction protein organization, treatment of monolayers by curcumin from the apical side significantly attenuated IL-1β-induced paracellular transport of [3H]mannitol (Fig. 9). These data are consistent with the observed curcumin-mediated protection of intestinal barrier function resulting in reduced translocation of luminal LPS to systemic circulation in WD-fed mice (11). In contrast, no significant effect was noted when cells were pretreated with curcumin from the basolateral side.
Fig. 9.
Apical treatment with curcumin attenuates IL-1β-induced paracellular transport. Fully differentiated Caco-2 cell monolayers in Transwell inserts were pretreated overnight with curcumin (10 μM) either from the apical side [+IL-1b+C(A)] or basolateral side [+IL-1b+C(B)] followed by exposure to IL-1β (10 ng/ml). Following a wash with HBBS, [3H]mannitol was then added to the apical side and its transport (expressed as dpm, means ± SE; n = 6) to the bottom chamber was monitored at indicated time points. *P < 0.05; NS, not significant.
DISCUSSION
Chronic inflammation underlies the development of WD-induced metabolic diseases including diabetes or atherosclerosis and evidence is increasingly accumulating that translocation of gut bacteria-derived endotoxin LPS underlies the perpetuation of this chronic inflammatory state (7). Furthermore, continuous infusion of low dose LPS recapitulates the disease phenotype associated with the WD-induced chronic inflammatory state and interactions between LPS-activated macrophages and metabolic cells such as adipocytes are thought to underlie the observed effects (19). The translocation of luminal gut bacteria-derived LPS to systemic circulation is restricted by an intact intestinal barrier and translocation of LPS can only occur when this barrier is disrupted. The data presented herein demonstrate that LPS initiates proinflammatory signaling in IECs leading to perpetuation of inflammation and resulting in the disruption of intestinal tight junctions. More importantly curcumin attenuates this inflammatory pathway by its action at multiple steps.
The mechanisms underlying the potent beneficial effects of curcumin including attenuation of inflammation have largely been evaluated by in vitro cell culture-based studies. However, orally administered curcumin is very poorly absorbed; with a detection limit of 1 μg/ml, no curcumin was detected following oral supplementation with 12 g of curcumin (16) hindering the establishment of a causal relationship between oral curcumin and the observed in vivo anti-inflammatory or anticancer effects. Earlier studies from our laboratory demonstrated the potent effects of oral supplementation with curcumin on the development of WD-induced glucose intolerance and atherosclerosis (12). Furthermore, curcumin supplementation attenuated WD-induced increase in plasma LPS levels by not only increasing the activity of intestinal alkaline phosphatase that detoxifies LPS in the gut lumen but also by improving intestinal barrier function. The data presented herein establishes the intracellular mechanisms by which curcumin improves the intestinal barrier. It is noteworthy that basolateral exposure to curcumin did not affect IL-1β-induced changes in paracellular transport of mannitol consistent with the differences in the physiological responses by exposure from apical and basolateral surface of IECs (1, 14). Since curcumin is very poorly absorbed resulting in limited basolateral exposure, these data further suggest that apical or luminal exposure to curcumin might underlie its observed effects on improvement of intestinal barrier function by oral administration (12).
While direct contact between luminal bacteria and IECs is restricted by the presence of a firmly attached mucin layer, bacteria-derived LPS not only can activate IECs but also the surveying intestinal macrophages leading to the production of proinflammatory mediators such as IL-1β. Mucosal balance between IL-1β and IL-1Ra regulates inflammatory bowel diseases in humans (5) and animals (18). Activity of SHIP that reduces expression of IL-1β is reduced in Crohn’s disease patients (20) underscoring the importance of tight regulation of intestinal IL-1β production and also identifying interventions to attenuate IL-1β production associated with these inflammatory diseases. Curcumin treatment significantly reduces the expression and secretion of IL-1β by IECs (Fig. 1) and increases the expression of anti-inflammatory IL-10 (Fig. 2). It is noteworthy that oral curcumin is currently being considered as a therapeutic agent for inflammatory bowel disease (23).
While defining the causal relationship between curcumin exposure of IECs and inflammatory bowel diseases is straightforward and is not affected by the issue of poor bioavailability, the beneficial systemic effects are difficult to define. Considerable efforts are currently being directed to increasing the bioavailability of curcumin with the underlying rationale of increasing the effective concentration of curcumin at the site of action (2, 11, 17, 21). By modulating the inflammatory signaling in the intestine, both at the level of IECs as well as intestinal macrophages, curcumin modulates the translocation of gut bacteria-derived LPS into systemic circulation by attenuating the disruption of the intestinal barrier function. Intracellular IL-1β signaling resulting in p38 MAPK activation, increased MLCK expression and final alteration in the expression/organization of tight junction proteins is established as the pathway underlying inflammation-induced disruption of intestinal barrier function. The data presented herein demonstrate that exposure to curcumin attenuated this pathway at multiple steps providing direct evidence for curcumin mediated protection of inflammation-induced disruption of intestinal barrier function. Although not monitored in the present study, curcumin has also been shown to inhibit IL-1β signaling by blocking IRAK recruitment in murine thymoma cells (15). In contrast to the observed lack of effects on MKP-1 in the current study, Song et al. (22) reported activation of MKP-1 by intragastric administration of curcumin in a rat enteritis model.
Collectively, acting on multiple steps of the pathway leading from luminal LPS to changes in the expression and/or organization of tight junction proteins (Fig. 10), curcumin is expected to not only reduce local inflammation in the gut, but by altering intestinal barrier function it will also reduce systemic inflammation triggered by the release of LPS into circulation. These studies, therefore, provide evidence that despite poor absorption and low bioavailability, oral curcumin likely mediates its anti-inflammatory (and inflammation-dependent downstream effects) by its local action in the gut.
Fig. 10.
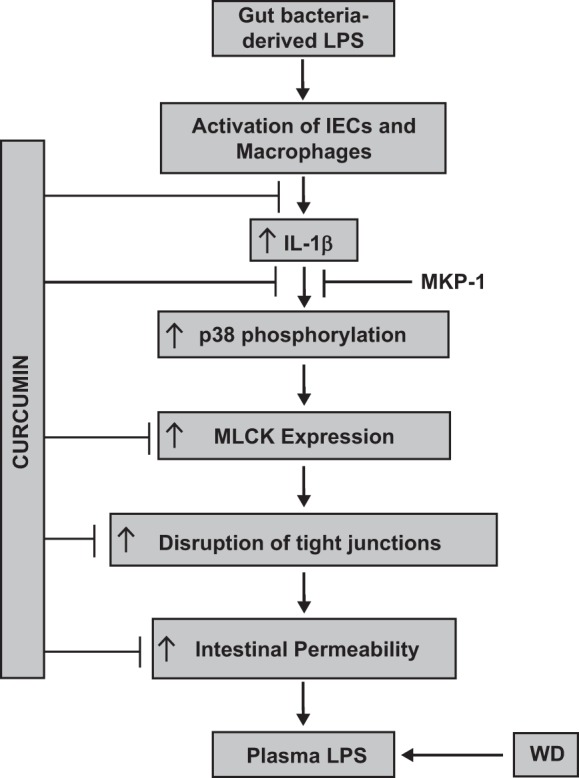
Proposed sites of action of curcumin in attenuating disruption of intestinal epithelial barrier function. LPS is continually being produced in the intestinal lumen by the gut bacteria and an intact intestinal epithelial barrier restricts the translocation of this luminal LPS to systemic circulation. Luminal LPS not only activates IECs but also stimulates intestinal macrophages to produce IL-1β that can in turn activate these cells and initiate proinflammatory signaling by increasing p38 MAPK phosphorylation and an increase in MLCK expression. The final result is disruption of intestinal tight junctions, resulting in an increase in intestinal permeability. These processes are, therefore, tightly regulated under normal physiological conditions. High-fat-, high-cholesterol-containing Western-type diet (WD) increases intestinal permeability, leading to increase in plasma LPS levels. The data presented herein identify the multiple steps that are modulated by curcumin and provide the mechanism by which oral supplementation with curcumin decreases systemic inflammation.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-097346 and Innovative Basic Science Award 1–16-IBS-105 from the American Diabetes Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.W., S.S.G., and S.G. conception and design of the research; J.W. and S.G. performed experiments; J.W. and S.G. analyzed data; J.W., S.S.G., and S.G. interpreted results of experiments; J.W., S.S.G., and S.G. approved final version of manuscript; S.S.G. and S.G. edited and revised manuscript; S.G. prepared figures; S.G. drafted manuscript.
ACKNOWLEDGMENTS
We thank Paul J. Yannie for immunofluorescence imaging.
REFERENCES
- 1.Alhamoruni A, Lee AC, Wright KL, Larvin M, O’Sullivan SE. Pharmacological effects of cannabinoids on the Caco-2 cell culture model of intestinal permeability. J Pharmacol Exp Ther 335: 92–102, 2010. doi: 10.1124/jpet.110.168237. [DOI] [PubMed] [Google Scholar]
- 2.Allam AN, Komeil IA, Fouda MA, Abdallah OY. Preparation, characterization and in vivo evaluation of curcumin self-nano phospholipid dispersion as an approach to enhance oral bioavailability. Int J Pharm 489: 117–123, 2015. doi: 10.1016/j.ijpharm.2015.04.067. [DOI] [PubMed] [Google Scholar]
- 3.Al-Sadi R, Guo S, Ye D, Dokladny K, Alhmoud T, Ereifej L, Said HM, Ma TY. Mechanism of IL-1β modulation of intestinal epithelial barrier involves p38 kinase and activating transcription factor-2 activation. J Immunol 190: 6596–6606, 2013. doi: 10.4049/jimmunol.1201876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amato KR, Yeoman CJ, Cerda G, Schmitt CA, Cramer JD, Miller ME, Gomez A, Turner TR, Wilson BA, Stumpf RM, Nelson KE, White BA, Knight R, Leigh SR. Variable responses of human and non-human primate gut microbiomes to a Western diet. Microbiome 3: 53, 2015. doi: 10.1186/s40168-015-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev 13: 323–340, 2002. doi: 10.1016/S1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 6.Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev 260: 102–117, 2014. doi: 10.1111/imr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutagy NE, McMillan RP, Frisard MI, Hulver MW. Metabolic endotoxemia with obesity: is it real and is it relevant? Biochimie 124: 11–20, 2016. doi: 10.1016/j.biochi.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57: 1470–1481, 2008. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Wang P, Su Q, Wang S, Wang F. Myosin light chain kinase mediates intestinal barrier disruption following burn injury. PLoS One 7: e34946, 2012. doi: 10.1371/journal.pone.0034946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cullen SP, Kearney CJ, Clancy DM, Martin SJ. Diverse activators of the NLRP3 inflammasome promote IL-1β secretion by triggering necrosis. Cell Reports 11: 1535–1548, 2015. doi: 10.1016/j.celrep.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Douglass BJ, Clouatre DL. Beyond yellow curry: assessing commercial curcumin absorption technologies. J Am Coll Nutr 34: 347–358, 2015. doi: 10.1080/07315724.2014.950392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh SS, Bie J, Wang J, Ghosh S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR−/− mice–role of intestinal permeability and macrophage activation. PLoS One 9: e108577, 2014. doi: 10.1371/journal.pone.0108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottardi CJ, Arpin M, Fanning AS, Louvard D. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell-cell contacts. Proc Natl Acad Sci USA 93: 10779–10784, 1996. doi: 10.1073/pnas.93.20.10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grenier E, Mailhot G, Dion D, Ravid Z, Spahis S, Bendayan M, Levy E. Role of the apical and basolateral domains of the enterocyte in the regulation of cholesterol transport by a high glucose concentration. Biochem Cell Biol 91: 476–486, 2013. doi: 10.1139/bcb-2013-0053. [DOI] [PubMed] [Google Scholar]
- 15.Jurrmann N, Brigelius-Flohé R, Böl GF. Curcumin blocks interleukin-1 (IL-1) signaling by inhibiting the recruitment of the IL-1 receptor-associated kinase IRAK in murine thymoma EL-4 cells. J Nutr 135: 1859–1864, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Klickovic U, Doberer D, Gouya G, Aschauer S, Weisshaar S, Storka A, Bilban M, Wolzt M. Human pharmacokinetics of high dose oral curcumin and its effect on heme oxygenase-1 expression in healthy male subjects. BioMed Res Int 2014: 458592, 2014. doi: 10.1155/2014/458592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latimer B, Ekshyyan O, Nathan N, Moore-Medlin T, Rong X, Ma X, Khandelwal A, Christy HT, Abreo F, McClure G, Vanchiere JA, Caldito G, Dugas T, McMartin K, Lian T, Mehta V, Nathan CA. Enhanced systemic bioavailability of curcumin through transmucosal administration of a novel microgranular formulation. Anticancer Res 35: 6411–6418, 2015. [PubMed] [Google Scholar]
- 18.Maeda S, Ohno K, Nakamura K, Uchida K, Nakashima K, Fukushima K, Tsukamoto A, Goto-Koshino Y, Fujino Y, Tsujimoto H. Mucosal imbalance of interleukin-1β and interleukin-1 receptor antagonist in canine inflammatory bowel disease. Vet J 194: 66–70, 2012. doi: 10.1016/j.tvjl.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 19.Nakarai H, Yamashita A, Nagayasu S, Iwashita M, Kumamoto S, Ohyama H, Hata M, Soga Y, Kushiyama A, Asano T, Abiko Y, Nishimura F. Adipocyte-macrophage interaction may mediate LPS-induced low-grade inflammation: potential link with metabolic complications. Innate Immun 18: 164–170, 2012. doi: 10.1177/1753425910393370. [DOI] [PubMed] [Google Scholar]
- 20.Ngoh EN, Weisser SB, Lo Y, Kozicky LK, Jen R, Brugger HK, Menzies SC, McLarren KW, Nackiewicz D, van Rooijen N, Jacobson K, Ehses JA, Turvey SE, Sly LM. Activity of SHIP, which prevents expression of interleukin 1β, is reduced in patients with crohn’s disease. Gastroenterology 150: 465–476, 2016. doi: 10.1053/j.gastro.2015.09.049. [DOI] [PubMed] [Google Scholar]
- 21.Patil S, Choudhary B, Rathore A, Roy K, Mahadik K. Enhanced oral bioavailability and anticancer activity of novel curcumin loaded mixed micelles in human lung cancer cells. Phytomedicine 22: 1103–1111, 2015. doi: 10.1016/j.phymed.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Song WB, Wang YY, Meng FS, Zhang QH, Zeng JY, Xiao LP, Yu XP, Peng DD, Su L, Xiao B, Zhang ZS. Curcumin protects intestinal mucosal barrier function of rat enteritis via activation of MKP-1 and attenuation of p38 and NF-κB activation. PLoS One 5: e12969, 2010. doi: 10.1371/journal.pone.0012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sreedhar R, Arumugam S, Thandavarayan RA, Karuppagounder V, Watanabe K. Curcumin as a therapeutic agent in the chemoprevention of inflammatory bowel disease. Drug Discov Today 21: 843–849, 2016. doi: 10.1016/j.drudis.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Moreira AP, Alves RD, Teixeira TF, Macedo VS, de Oliveira LL, Costa NM, Bressan J, do Carmo Gouveia Peluzio M, Mattes R, de Cássia Gonçalves Alfenas R. Higher plasma lipopolysaccharide concentrations are associated with less favorable phenotype in overweight/obese men. Eur J Nutr 54: 1363–1370, 2015. doi: 10.1007/s00394-014-0817-6. [DOI] [PubMed] [Google Scholar]
- 25.Trøseid M, Nestvold TK, Rudi K, Thoresen H, Nielsen EW, Lappegård KT. Plasma lipopolysaccharide is closely associated with glycemic control and abdominal obesity: evidence from bariatric surgery. Diabetes Care 36: 3627–3632, 2013. doi: 10.2337/dc13-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 1: 6ra14, 2009. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J Biol Chem 274: 35179–35185, 1999. doi: 10.1074/jbc.274.49.35179. [DOI] [PubMed] [Google Scholar]
- 28.Zhao B, Song J, Chow WN, St Clair RW, Rudel LL, Ghosh S. Macrophage-specific transgenic expression of cholesteryl ester hydrolase significantly reduces atherosclerosis and lesion necrosis in Ldlr mice. J Clin Invest 117: 2983–2992, 2007. doi: 10.1172/JCI30485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao B, Song J, St Clair RW, Ghosh S. Stable overexpression of human macrophage cholesteryl ester hydrolase results in enhanced free cholesterol efflux from human THP1 macrophages. Am J Physiol Cell Physiol 292: C405–C412, 2007. doi: 10.1152/ajpcell.00306.2006. [DOI] [PubMed] [Google Scholar]