Abstract
Biotin (vitamin B7), an essential micronutrient for normal cellular functions, is obtained from both dietary sources as well as gut microbiota. Absorption of biotin in both the small and large intestine is via a carrier-mediated process that involves the sodium-dependent multivitamin transporter (SMVT). Although different physiological and molecular aspects of intestinal biotin uptake have been delineated, nothing is known about the effect of LPS on the process. We addressed this issue using in vitro (human colonic epithelial NCM460 cells) and in vivo (mice) models of LPS exposure. Treating NCM460 cells with LPS was found to lead to a significant inhibition in carrier-mediated biotin uptake. Similarly, administration of LPS to mice led to a significant inhibition in biotin uptake by native colonic tissue. Although no changes in total cellular SMVT protein and mRNA levels were observed, LPS caused a decrease in the fraction of SMVT expressed at the cell surface. A role for casein kinase 2 (CK2) (whose activity was also inhibited by LPS) in mediating the endotoxin effects on biotin uptake and on membrane expression of SMVT was suggested by findings that specific inhibitors of CK2, as well as mutating the putative CK2 phosphorylation site (Thr78Ala) in the SMVT protein, led to inhibition in biotin uptake and membrane expression of SMVT. This study shows for the first time that LPS inhibits colonic biotin uptake via decreasing membrane expression of its transporter and that these effects likely involve a CK2-mediated pathway.
Keywords: biotin, LPS, transport colon
biotin (vitamin B7) is an essential micronutrient for normal human health because of its involvement in a variety of critical metabolic reactions. The vitamin acts as a cofactor for five carboxylases that are critical for fatty acid, glucose, and amino acid metabolism (36, 38, 46). Important roles for biotin in the regulation of cellular oxidative stress (35), gene expression [in which expression of over 2,000 human genes appears to be affected by biotin status (44, 55, 56)], and in normal function of the immune system (29) have also been recognized. In reference to the latter, biotin was shown to be important for activity, generation, maturation, and/or responsiveness of immune cells (5, 7, 28, 42); also deficiency of the vitamin has been shown to lead to an increase in the levels of proinflammatory cytokines like tumor necrosis factor-α, interleukin-1β, and interferon-γ (18, 30, 31). A role for biotin in the maintenance of normal intestinal integrity and homeostasis (18, 45), in influencing the colonization/invasiveness of certain entero-pathogenic bacteria (57), and in mediating the effect of probiotic bacteria on gut microbial community (54) has, in addition, been recently reported. It is not surprising, therefore, that deficiency/suboptimal levels of biotin lead to disturbances in the normal function of many systems and ultimately to clinical manifestations (38, 46, 54). Deficiency/suboptimal levels of biotin occur in a variety of conditions including inflammatory bowel disease (IBD) (1, 14), inborn errors in biotin metabolism, multiple carboxylase deficiency (12), and chronic alcoholism (10).
Humans and other mammals cannot synthesize biotin and thus must obtain the vitamin from exogenous sources via intestinal absorption. The intestine is exposed to two sources of biotin, a dietary source (absorbed in the small intestine) and a microbiota-generated source (absorbed in the large intestine) (41, 48, 49). Absorption of biotin in both the small and large intestine occurs via a carrier-mediated process that involves the sodium-dependent multivitamin transporter (SMVT) system encoded by the SLC5A6 gene. This carrier system is exclusively expressed at the apical membrane domain of polarized absorptive epithelial cells (52). Studies from our laboratory and others have delineated different cell and molecular aspects of the intestinal biotin uptake process, how the process is regulated at the transcriptional and posttranscriptional levels, and how specific external (environmental)/internal factors and conditions affect and interfere with the event (46–49). Nothing, however, is presently known about the effect of the bacterial LPS on intestinal biotin uptake process, so it was therefore examined in this study.
LPS is a powerful bacterial virulence factor in terms of proinflammatory properties and is a source of considerable clinical morbidity and mortality. This endotoxin is a major component of the outer membrane of Gram-negative bacteria that is released from bacterial cell walls by shedding or through bacterial lysis and acts as a potent activator of the inflammatory response in the gut (27). The concentration of LPS is highest in the gut lumen (especially colonic lumen because it harbors a large collection of bacteria) and increases markedly in patients with IBD and in those infected with enteric pathogens (e.g., Salmonella) (8, 22, 51). LPS generated in the gut lumen exerts its effects on the lining epithelia via interaction with Toll-like receptor-4 (TLR-4) (3, 4, 22). Although LPS has been shown to affect intestinal transport of a variety of substrates (2, 15, 37), its effect on the intestinal uptake of biotin has not been investigated. We addressed this issue in the present investigation using the human colonic epithelial NCM460 cells and mice as models. Our results showed that in vitro and in vivo exposure to LPS leads to a decrease in biotin uptake by colonic epithelial cells with no change in level of expression of total cellular SMVT protein or its mRNA. Rather the effect appears to be due to a decrease in membrane expression of the SMVT protein, likely mediated via a role for casein kinase 2 (CK2).
MATERIALS AND METHODS
Materials
3H-Biotin (specific activity 60 Ci/mmol; radiochemical purity >97.5%) was purchased from American Radiolabeled Chemicals (St. Louis, MO). All other cell culture reagents, reconstituted aqueous LPS (Escherichia coli 0111:B4) solution, and specific primers used for PCR amplifications were from Sigma Genosys (Woodlands, TX). CK2 inhibitor I (tetrabromobenzotriazole; TBB) and inhibitor III (tetrabromophenyl acrylic acid; TBCA) were purchased from EMD Millipore (Billerica, MA).
Methods
Culturing and transfection of the human epithelial cells.
Confluent monolayers of the colonic epithelial NCM460 cells (derived from a 68-yr-old male), the intestinal epithelial Caco-2 cells (derived from a 72-yr-old male), and the retinal pigment epithelial ARPE-19 cells (derived from a 19-yr-old male) were used in these investigations (ATCC, Manassas, VA). NCM460 cells were maintained in F12 medium (Ham), whereas Caco-2 and ARPE-19 cells were maintained in EMEM (GIBCO, Waltham, MA) medium, supplemented with FBS (10%) and streptomycin (10 mg/l), under standard conditions. Confluent cell monolayers (3–4 days postconfluence) were used to examine the effect of LPS on biotin uptake. Cells were serum starved overnight, then treated with 50 μg/ml LPS in the appropriate growth medium supplemented with 0.5% FBS. ARPE-19 cells were used because of their proven high transfection efficiency of wild-type and mutant hSMVT constructs (43). One day before transfection, cells were seeded to attain 75–85% confluence at the time of transfection.
Biotin uptake.
In the in vitro LPS exposure studies, confluent monolayers of NCM460 (as well as Caco-2 and ARPE-19 cells) were used, and biotin uptake was examined as described by us previously (16). In brief, cells were incubated (5 min; initial rate) in Krebs-Ringer (KR) buffer (133.00 mM NaCl, 4.93 mM KCl, 1.23 mM MgSO4, 0.85 mM CaCl2, 5.00 mM glucose, 5.00 mM glutamine, 10.00 mM HEPES, and 10.00 mM MES, pH 7.4) at 37°C in presence of 3H-biotin (6.4 nM). At the end of incubation, buffer was aspirated, and cells were washed twice with ice-cold KR buffer, lysed with 1 N NaOH (followed by neutralization with 10 N HCl) then counted for radioactivity in a liquid scintillation counter (Beckman Coulter, Brea, CA). Biotin uptake by the carrier-mediated process was determined by subtracting uptake of a physiological concentration of 3H-biotin in the presence of 1 mM unlabeled biotin from total uptake (i.e., from uptake in the absence of unlabeled biotin).
For in vivo studies, we used 12-wk-old male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME). The animal protocol was approved by the Long Beach VAMC Animal Care and Use Committee. Full-thickness proximal colonic sheets, prepared as described previously (17, 53), were used to examine the effect of in vivo exposure to LPS on colonic biotin uptake physiology and molecular biology parameters. In these experiments, colonic tissue was removed immediately after killing the animals, and equal pieces (1 cm) of colonic sheets were incubated (5 min) in KR (pH 7.4) buffer in the presence of 3H-biotin (64 nM), washed, and then processed for 3H content.
CK2 activity assay.
NCM460 cells were homogenized in cell lysis buffer (Cell Signaling Technology, Danvers, MA). The phospho-transferase activity of CK2 was measured using a CK2 assay kit (Millipore, Temecula, CA) following manufacturer’s recommendations. Briefly, cell lysates, substrate peptide, protein kinase inhibitor cocktail, and [γ-32P]-ATP from American Radiolabeled Chemicals in the assay dilution buffer were incubated for 10 min at 30°C. The reaction was stopped by 40% trichloroacetic acid. The phosphorylated substrate was then separated on BA-85 Protan paper, washed with ortho-phosphoric acid, and quantified in a scintillation counter.
Site-directed mutagenesis.
To introduce a mutation in the hSMVT protein, we used full-length hSMVT construct cloned in pcDNA 3.1(−) vector (Invitrogen, Carlsbad, CA) generated previously (19). Quick Change II site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) was used for mutating putative CK2 phosphorylation residues using specific mutant primers (Table 1). The hSMVT-pcDNA 3.1(−) construct was used as a template, and PCR was performed using specific mutant primers following the manufacturer's instruction. The mutated clones were verified by sequencing from the isolated plasmid DNA (Laragen, Culver City, CA).
Table 1.
Combination of primers used for PCR
| Forward and Reverse Primers (5′–3′) | |
|---|---|
| hSMVT-RT | TGTCTACCTTCTCCATCATGGA; TAGAGCCCAATGGCAAGAGA |
| hβ-Actin-RT | CATCCTGCGTCTGGACCT; TAATGTCACGCACGATTTCC |
| mSMVT-RT | GGATCTGTGGGACTGTGA; CACATCTGTCCAGATGACA |
| mβ-Actin-RT | ATCCTCTTCCTCCCTGGA; TTCATGGATGCCACAGGA |
| Thr78Ala | CTGTCCCTGCTGGCCGCCTTCCAGTCAGCCGTG; CACGGCTGACTGGAAGGCGGCCAGCAGGGACAG |
| Ser128Ala | CGCCTGCATCTCACCGCTGCCTATGAGTACCTG; CAGGTACTCATAGGCAGCGGTGAGATGCAGGCG |
| Ser242Ala | CAGCACGGCCGCATCGCTGGGTTTGAGCTGGAT; ATCCAGCTCAAACCCAGCGATGCGGCCGTGCTG |
| Thr366Ala | AGCGGCTCTCTCAGCGCTATATCCTCTGCTTTT; AAAAGCAGAGGATATAGCGCTGAGAGAGCCGCT |
| Thr627Ala | CAGGGGAGCAGCTCCGCCTGCATCCTCCAGGAG; CTCCTGGAGGATGCAGGCGGAGCTGCTCCCCTG |
SMVT-RT and β-actin-RT represent the primer sequences used for quantitative real-time PCR for SMVT and β-actin, respectively. The bold-underlined nucleotides represent the mutation in respective primers used for site-directed mutagenesis in hSMVT. “h” and “m” prefixes denote human and mouse, respectively.
Real-time PCR analysis.
Total RNA was isolated from cells and from colonic tissue of mice treated with LPS and their controls using TRIzol reagent as described by the manufacturer (Life Technologies, Carlsbad, CA) and then treated with DNase to remove DNA contaminations. The RNA pool was reverse transcribed to cDNA using i-script reverse transcriptase kit (Bio-Rad, Hercules, CA) and then used for quantitative real-time PCR using specific primers (Table 1) in a CFX96 real-time i-cycler (Bio-Rad). After initial denaturation at 95°C for 5 min, the amplification program was repeated 45 times (95°C with a 30-s hold, 55°C with 15-s hold followed by 72°C with 30-s hold for extension and fluorescence measurement). Melt-curve analysis was performed immediately after the amplification protocol under the following conditions: 1-min denaturation at 95°C, 1-min annealing at 55°C, 80 cycles of 0.5°C increments (10 s each) beginning at 55°C. The relative expression of different mRNAs was normalized relative to β-actin as an internal control and quantified by 2-ΔΔCt method (34).
Western blot analysis.
For Western blotting, cultured cells and mouse colonic tissues were homogenized in RIPA buffer (Sigma) in the presence of a complete protease inhibitor cocktail (Roche, Nutley, NJ). The soluble fraction was isolated by centrifugation at 8,000 g for 10 min, and the protein content was measured using DC protein assay kit (Bio-Rad). An equal amount of protein (60 μg) was loaded onto a 10% mini gel (Invitrogen) and transferred to a polyvinylidene difluoride (PVDF) membrane for Western blot analysis. The membrane was blocked overnight and then probed with specific anti-SMVT polyclonal antibodies (1:200 dilutions; Santa Cruz Biotechnology, Santa Cruz, CA); specificity of the antibodies has been previously validated in our laboratory using protein samples from the intestine of the conditional (intestinal-specific) SMVT knockout mice (18). Anti β-actin monoclonal antibody (in 1:2,000 dilutions; Thermo Fisher, Waltham, MA) was also included in the probing buffer along with anti-SMVT polyclonal antibodies for 2 h at room temperature followed by corresponding secondary antibodies (LI-COR Biosciences, Lincoln, NE) in 1:25,000 dilutions for 1 h at room temperature described previously (18). Relative expression of the protein was quantified by normalizing the intensities with respect to corresponding β-actin using Odyssey application software (version 3.0) in an Odyssey Infrared imaging system (LI-COR).
Cell surface biotinylation assay.
The effect of LPS, CK2 inhibitors, and mutating the putative CK2 phosphorylation sites in the SMVT protein on expression of the carrier protein at the cell membrane of NCM460 and ARPE-19 cells was examined by means of biotinylation assay using a cell surface biotinylation kit (Pierce Biotechnology, Rockford, IL), as previously described (6, 21, 33, 39). Briefly, cells were maintained under serum-starved condition overnight and treated with LPS (50 μg/ml; 72 h) in suitable medium supplemented with 0.5% FBS. For in vitro CK2 inhibition studies, cells were treated with CK2 inhibitors in serum-free medium (30 μM, 1 h). Cell surface biotinylation was performed by treating cells with sulfo-NHS-SS-biotin (4°C; 30 min; Pierce Biotechnology) followed by isolation of surface proteins by incubating the cell lysate with streptavidin-agarose beads following the manufacturer’s protocol. Protein assays were conducted after biotinylation and respective protein samples were loaded onto an SDS-polyacrylamide premade gel (10%; Invitrogen). Following electrophoresis, the proteins were electro-blotted onto a PVDF immunoblot membrane (Bio-Rad) overnight, washed (3 times) with PBS-Tween-20 for 5 min, and blocked with Odyssey blocking buffer (LI-COR). The SMVT protein bands were detected using the specific polyclonal anti-SMVT antibodies (18).
Statistical analysis.
Uptake data presented in the study are means ± SE of multiple separate experimental determinations. Uptake was expressed as a percentage relative to simultaneously performed controls or femtomoles per milligram protein per 5 min. Statistical significance was determined using Student’s t-test or one-way ANOVA with statistical significance set at >0.05.
RESULTS
Effect of LPS on Biotin Uptake by Colonic Epithelial Cells
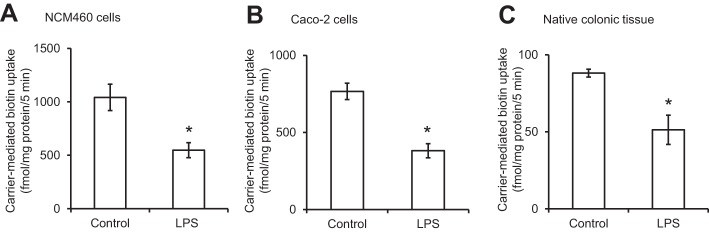
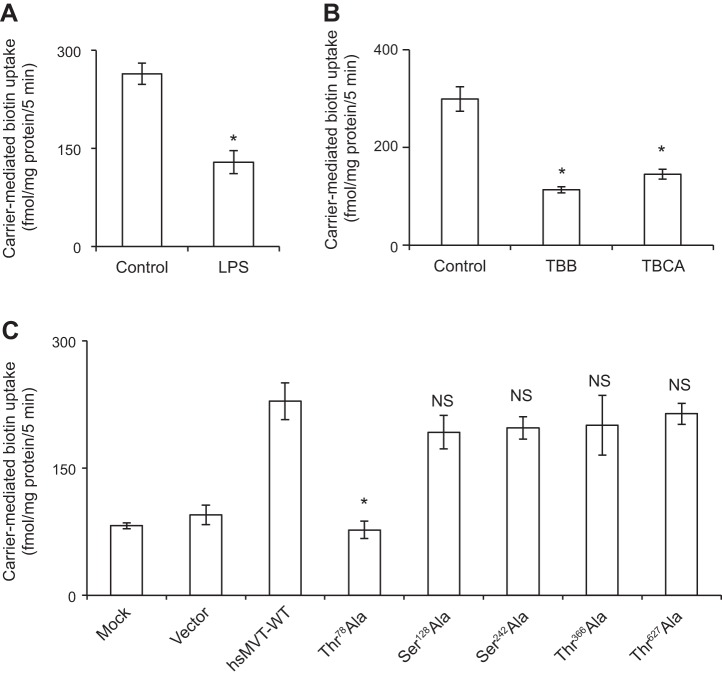
In this study, we examined the effect of treating (for 72 h) cultured human colonic epithelial NCM460 monolayers with the LPS (50 μg/ml, a concentration that is relevant to the endotoxin level that can be reached in colonic lumen) (24, 32, 50) on the initial rate (5 min) of carrier-mediated 3H-biotin (6.4 nM) uptake. The results showed a significant (P < 0.01) inhibition in the vitamin uptake by LPS-treated cells compared with uptake by simultaneously examined untreated controls (Fig. 1A). Similar results were obtained in studies utilizing the human intestinal epithelial Caco-2 cells, where again LPS (50 μg/ml; 72 h) caused a significant (P < 0.01) inhibition in carrier-mediated biotin uptake (Fig. 1B).
Fig. 1.
Effect of LPS on biotin uptake by human colonic epithelial NCM460 cells (A), human intestinal epithelial Caco-2 cells (B), and mouse native colonic tissue (C). For the in vitro LPS studies, confluent cells were serum starved overnight and then treated with LPS (50 μg/ml; 72 h). For the in vivo LPS study, mice were injected (5 mg/kg body wt ip) and then used 72 h later for the study. Biotin uptake was measured as described in Methods. Data are means ± SE of at least 3 independent experiments (*P < 0.01).
To establish physiological relevance of the above in vitro LPS exposure study, we also examined the effect of in vivo exposure of mice to LPS on biotin uptake by native colonic tissue. For this, adult mice were given a single dose of LPS (5 mg/kg body wt, i.p.) as described before (22), followed by examination (72 h later) of carrier-mediated biotin uptake by native colonic sheets as described by us before (17, 53). The results showed that in vivo exposure to LPS again leads to a significant (P < 0.01) inhibition in carrier-mediated biotin uptake by native colonic tissue (Fig. 1C).
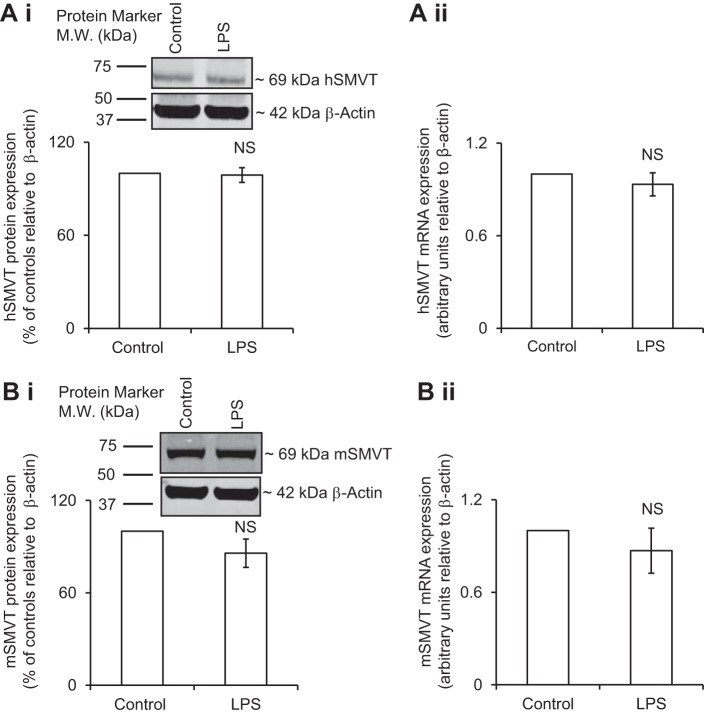
Effect of In Vitro and In Vivo Exposure to LPS on Colonic Level of Expression of SMVT Protein and mRNA
Recent investigations from our laboratory have established that the SMVT is the only transport system responsible for biotin uptake in the gut (18). Thus we examined whether the above-described inhibition in carrier-mediated biotin uptake by in vitro exposure of the colonic epithelial NCM460 cells to LPS is associated with changes in expression of SMVT protein and mRNA levels. Total cellular SMVT protein level was determined by means of Western blotting using whole cell homogenate and specific anti-SMVT polyclonal antibodies, whereas level of the SMVT mRNA was determined by real-time PCR (see Methods). The results showed no significant changes in the level of expression of total cellular hSMVT protein (Fig. 2Ai) or mRNA (Fig. 2Aii) in cells treated with LPS compared with untreated controls. Similarly, in vivo treatment of mice with LPS did not affect the level of expression of total cellular mSMVT protein or mRNA in the colonic mucosa compared with their levels in the colonic mucosa of the untreated controls (Fig. 2B, i and ii). These results suggest that the effect of LPS on colonic biotin uptake is not mediated via changes in the rate of transcription of the SLC5A6 gene or in total cellular level of the SMVT protein.
Fig. 2.
Effect of LPS on the level of expression of total cellular sodium-dependent multivitamin transporter (SMVT) protein and mRNA in human colonic epithelial NCM460 cells (A) and mouse native colonic tissue (B). NCM460 cells were pretreated with LPS (50 μg/ml; 72 h) followed by determination of the level of expression of hSMVT protein (A, i) and mRNA (A, ii). Total cellular mSMVT protein (B, i) and mRNA level (B, ii) were determined in colonic tissue of LPS-treated mice (5 mg/kg body wt ip; 72 h). In both Western blot analyses, identical amounts of protein (60 μg) were loaded onto gels. Data were normalized relative to β-actin for mRNA and protein (see Methods). Data are means ± SE of at least 3 independent experiments. NS, not significant.
LPS Inhibition of Colonic Biotin Uptake Is Mediated Via Reduction in Cell Surface Expression of the hSMVT Protein
With the above-described findings suggesting that LPS-mediated inhibition in colonic biotin uptake is not exerted at the level of transcription of the SLC5A6 gene or via changes in level of total cellular SMVT protein, we tested the possibility that the effect is mediated via changes in the fraction of the SMVT protein that is expressed at the cell surface. This was done by means of biotinylation assay followed by Western blotting. The results indeed showed a significant (P < 0.01) reduction in the level of expression of the hSMVT protein at the cell surface in LPS-treated NCM460 cells compared with untreated control cells (Fig. 3). These findings suggest that LPS interferes with the level of expression of SMVT at the cell surface of the colonic epithelial cells.
Fig. 3.
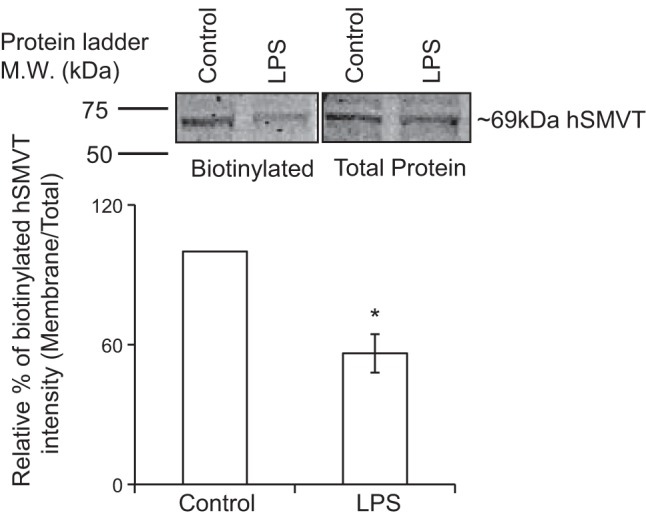
Effect of LPS on expression of the hSMVT protein at the surface of NCM460 cells. Biotinylation assay was performed on LPS-treated (50 µg/ml; 72 h) and untreated cells. Inset: representative images from biotinylation assay. In the Western blot analysis, identical amounts of protein (60 μg) were loaded onto gels. Data are means ± SE of at least 3 independent experiments (*P < 0.01).
Role for CK2 in Mediating the LPS Effect on Colonic Biotin Uptake and on Membrane of Expression of the hSMVT Protein
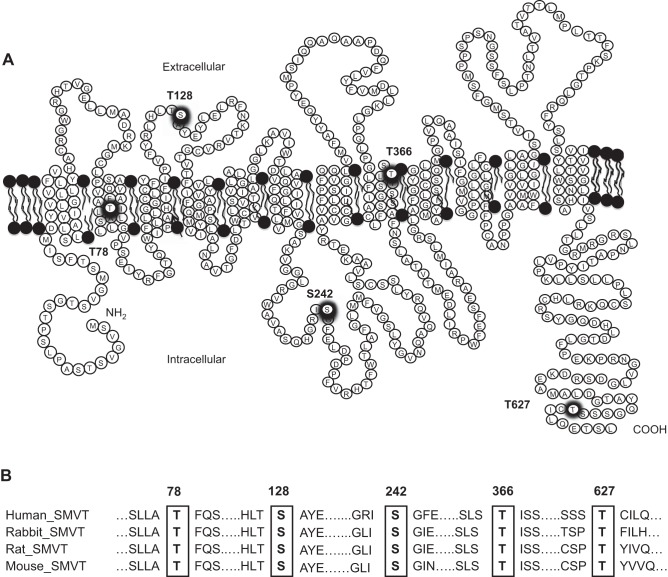
Recent studies have reported a role for CK2 (11, 13) and protein kinase C (PKC) (23, 40) in the process of cell surface expression of membrane transporters in a variety of cellular systems. Thus we considered possible involvement of these pathways in mediating the LPS effect on membrane expression of the hSMVT protein (and on biotin uptake). We focused on the potential role of CK2 because our previous investigations have shown that PKC does not affect the expression of hSMVT at the cell surface (20) and that the sequence of the SMVT protein appears to have a number of putative CK2 phosphorylation sites (Thr78, Ser128, Ser242, Thr366, and Thr627; Fig. 4), which are conserved among species.
Fig. 4.
Schematic diagram showing the putative CK2 phosphorylation site in the hSMVT protein. A: the hSMVT protein is predicted [by NetPhos 3.1 Server (9)] to have five putative CK2 phosphorylation sites (thick circles) that are conserved in different species. B: conserved amino acid residues (in rectangles) in different species were identified by multiple sequence alignment using “PRALINE” software (www.ibi.vu.nl/programs/pralinewww/).
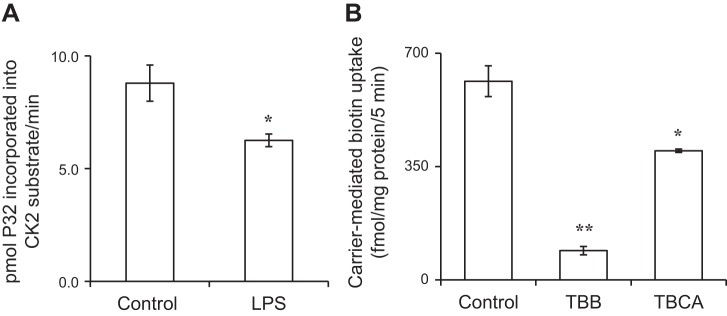
First we examined whether treatment of the colonic epithelial NCM460 cells with LPS affects the activity of CK2 (see Methods). The results showed that such a treatment indeed leads to a significant (P < 0.05) inhibition in CK2 activity (Fig. 5A). We then examined the effect of pretreating (for 1 h) the colonic epithelial NCM460 cells with the CK2-specific inhibitors TBB and TBCA (both at 30 μM) (58) on carrier-mediated biotin (6.4 nM) uptake. The results showed a significant (P < 0.01 for TBB and P < 0.05 TBCA compared with untreated control) inhibition in biotin uptake by both of the CK2 inhibitors tested (Fig. 5B). In a related study, we also tested whether exposing the LPS-treated NCM460 cells with TBB could further augment the inhibitory effect caused by LPS alone on biotin uptake. The result indeed showed that this is to be the case, with uptake of 1048.36 ± 19.99, 671.20 ± 86.02, and 330.09 ± 46.69 fmol/mg protein/5 min ± SE for control, LPS-treated cells, and LPS plus TBB-treated cells, respectively.
Fig. 5.
A: effect of LPS on activity of CK2 in NCM460 cells. Cells were treated with LPS (50 µg/ml; 4 h). Activity of CK2 was determined on cell lysate as described in Methods. B: effect of specific inhibitors of CK2 on biotin uptake by NCM460 cells. Cells were treated with the CK2 inhibitors (TBB and TBCA, 30 µM) for 1 h, followed by determination of biotin uptake (see Methods). Data are means ± SE of at least 3 independent experiments (*P < 0.05, **P < 0.01).
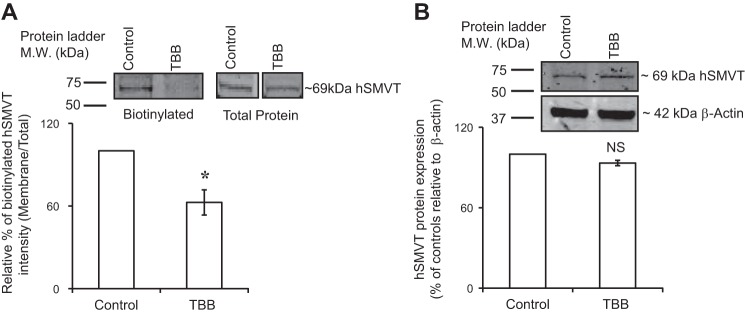
In another investigation, we examined whether the inhibition in biotin uptake caused by TBB is mediated via a decrease in the level of expression of the SMVT protein at the cell surface. We used the biotinylation assay described in Methods for this purpose. The results showed a significant (P < 0.01) decrease in the level of expression of the hSMVT protein at the cell membrane (Fig. 6A), with no change in its level in the total cell homogenate (Fig. 6B).
Fig. 6.
Effect of the CK2 inhibitor TBB on expression of the hSMVT protein at the surface of NCM460 cells. Cells (maintained in serum-free medium) were treated with the TBB (30 µM; 1 h), followed by biotinylation assay (see Methods). A: surface expression of hSMVT was quantified using anti-hSMVT polyclonal antibodies and was normalized relative to total cellular hSMVT level. B: total cellular hSMVT was quantified and then normalized relative to β-actin. Inset: representative images from biotinylation assay. In both Western blot analyses, identical amounts of protein (60 μg) were loaded onto gels. Data are means ± SE of at least 3 independent experiments (*P < 0.01).
To further investigate the potential involvement of CK2 in the inhibitory effect of LPS on biotin uptake by colonic epithelial NCM460 cells and on cell surface expression of the hSMVT protein, we investigated the effect of mutating (to alanine) the putative CK2 phosphorylation sites in the hSMVT protein (Thr78Ala, Ser128Ala, Ser242Ala, Thr366Ala, and Thr627Ala) on carrier-mediated biotin uptake and on membrane expression of SMVT. The individual mutation was introduced into the hSMVT protein by means of site-directed mutagenesis followed by expression of the different mutant into ARPE-19 cells. We chose the latter cell type for this study because of their excellent transfection efficiency (unlike the poor transfection efficiency of NCM460 cells) and because the previous findings have established their suitability in studies of SMVT function and regulation (20, 25, 26). In these investigations, we first showed that treatment of the ARPE-19 cells with LPS or with the CK2 inhibitors TBB and TBCA also leads to inhibition (P < 0.01 for all) in biotin uptake (Fig. 7, A and B, respectively). We then examined the ability of the different hSMVT mutants (Thr78Ala, Ser128Ala, Ser242Ala, Thr366Ala, and Thr627Ala; Fig. 6) to transport biotin following their expression in ARPE-19 cells. The results showed a significant (P < 0.01; when compared with the hSMVT-WT construct) inhibition in carrier-mediated biotin (6.4 nM) uptake by cells expressing the Thr78Ala mutant but not those expressing the wild-type hSMVT or mutants Ser128Ala, Ser242Ala, Thr366Ala, and Thr627Ala (Fig. 7C).
Fig. 7.
Effect of LPS (A) and CK2 inhibitors (B) on biotin uptake by the human retinal pigment epithelial ARPE-19 cells. Postconfluency cells were incubated with LPS (50 μg/ml, 72 h) or with the CK2 inhibitors TBB and TBCA (30 µM; 1 h) followed by examination of biotin uptake (see Methods). C: effect of mutating the putative CK2 phosphorylation sites in the hSMVT protein on biotin uptake. Equal amounts of the wild-type and the mutated hSMVT constructs (3 µg/well, in 12-well culture plates) were transfected into ARPE-19 cells. Biotin uptake was then determined 48 h after transfection. Data are means ± SE from at least 3 independent experiments (*P < 0.01).
To determine whether the decrease in the ability of the Thr78Ala hSMVT mutant to transport biotin is due to a decrease in level of expression of the protein at the cell surface, we again resorted to biotinylation assay. In this study, we transfected the ARPE-19 cells with the mutant Thr78Ala or with wild-type hSMVT followed by biotinylation assay. The results showed a significant (P < 0.05) reduction in the level of expression of the Thr78Ala mutant at the cell surface compared with level of expression of the wild-type hSMVT protein (Fig. 8).
Fig. 8.
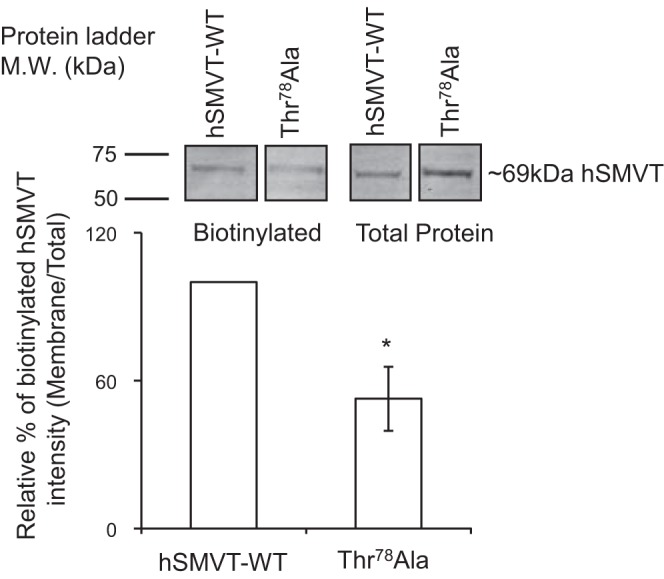
Effect of mutating Thr78 of the hSMVT protein on its level of expression at the surface of ARPE-19 cells. Cells were transiently transfected with equal amounts of wild-type or mutated hSMVT (Thr78Ala). Biotinylation assay was performed (48 h after transfection), and surface expression of the SMVT protein was quantified by means of Western blotting (see Methods). Identical amounts of protein (60 μg) were loaded onto gels. Data are means ± SE of at least 3 independent experiments (*P < 0.01).
DISCUSSION
Emerging evidence has pointed to the involvement of biotin in critical metabolic activities and physiological functions beyond its classical role as a cofactor for enzymes like carboxylases. This includes an expanded role for this micronutrient in normal immune function and response (5, 7, 18, 28, 29, 45), in the regulation of cellular oxidative stress (35), in the maintenance of normal gut mucosal physiology and homeostasis (18, 45), in influencing the colonization/invasiveness of certain entero-pathogenic bacteria (57), and in influencing gut microbial community (54). Thus studies that aim at furthering our understanding of the factors that affect/interfere with normal body homeostasis of biotin are of clear physiological and nutritional importance.
The intestinal tract plays a critical role in regulating and determining body level of biotin, as humans (mammals) cannot synthesize the vitamin endogenously and thus must obtain the essential micronutrient from exogenous sources. The intestine encounters two sources of biotin, a dietary source (absorbed in the small intestine) and a microbiota-generated source (absorbed in the large intestine). Studies from our laboratory and others over the past quarter century have delineated different aspects of the carrier-mediated biotin uptake process and how specific external/internal conditions and factors affect the event. It is well established now that the intestinal biotin absorption process is mediated exclusively by the SMVT system, which is exclusively expressed at the apical membrane domain of absorptive epithelia (52). Thus any interference with the function/expression of this uptake system would have the potential to affect the normal intestinal absorption process of the vitamin.
In the present investigation, our aim was to examine the effect of LPS on the intestinal biotin uptake process. We focused on the effect of this bacterial endotoxin on colonic biotin uptake because the large intestinal lumen contains high levels of LPS (24, 32, 50) as it harbors the largest level of bacteria in the intestinal tract. We employed in vitro (cultured colonic epithelial NCM460 cells) and in vivo (mice) LPS exposure approaches in our investigations. Results of both approaches showed that exposure to LPS leads to a significant inhibition in colonic carrier-mediated biotin uptake. This inhibition was not mediated via changes in the level of expression of total cellular SMVT protein or in the level of its mRNA; rather it appeared to be mediated via a decrease in the fraction of the hSMVT protein that is expressed at the surface of colonic epithelial cells as indicated by the results of biotinylation assay.
It has been reported that both the CK2 and the PKC-mediated pathways play a role in regulating membrane expression of different proteins in a variety of cell types. Thus we considered the possible role of these two pathways in mediating the effect of LPS on membrane expression of the hSMVT protein and on biotin uptake. We focused on the role of the CK2-mediated pathway in this study because our previous investigations have shown no role for PKC in membrane expression of the hSMVT system (20) and because the hSMVT polypeptide appears to have a number of potential CK2 phosphorylation sites. Our investigations showed that LPS inhibits the activity of CK2 in colonic epithelial NCM460 cells. Also inhibiting CK2 with the use of specific pharmacological inhibitors led to significant inhibition in both biotin uptake and in the level of expression of the hSMVT protein at the cell membrane without affecting the level of expression of the transporter in total cell homogenate. These findings suggest a role for CK2 in mediating the inhibitory effect of LPS on biotin uptake and on membrane expression of the hSMVT protein.
To further confirm the possible role of CK2 in regulating biotin uptake and in expression of the hSMVT protein at the cell surface, we examined the effect of mutating (individually) the putative CK2 phosphorylation sites in the SMVT polypeptide on biotin uptake. The results showed that mutating the Thr78 site, but not the other putative sites (Ser128, Ser242, Thr366, and Thr627), led to a significant inhibition in biotin uptake. This inhibition was again found to be associated with a decrease in the level of expression of the hSMVT protein at the cell surface with no change in total cellular level for the hSMVT protein.
In summary, results of these investigations show that colonic biotin uptake is sensitive to the inhibitory effect of the bacterial endotoxin LPS and that such an effect is mediated via a decrease in cell surface expression of the hSMVT protein. Furthermore, the latter appears to be exerted via involvement of a CK2-mediated pathway.
GRANTS
This study was supported by the Department of Veterans Affairs and National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grants DK58057 and DK56057.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.L. performed experiments; R.L. and H.M.S. analyzed data; R.L. and H.M.S. interpreted results of experiments; R.L. and H.M.S. prepared figures; R.L. and H.M.S. drafted manuscript; R.L. and H.M.S. edited and revised manuscript; R.L. and H.M.S. approved final version of manuscript.
REFERENCES
- 1.Abad-Lacruz A, Fernandez-Bañares F, Cabre E, Gil A, Esteve M, Gonzalez-Huix F, Xiol X, Gassull MA. The effect of total enteral tube feeding on the vitamin status of malnourished patients with inflammatory bowel disease. Int J Vitam Nutr Res 58: 428–435, 1988. [PubMed] [Google Scholar]
- 2.Abad B, Mesonero JE, Salvador MT, García-Herrera J, Rodríguez-Yoldi MJ. Cellular mechanism underlying LPS-induced inhibition of in vitro L-leucine transport across rabbit jejunum. J Endotoxin Res 8: 127–133, 2002. doi: 10.1177/09680519020080020601. [DOI] [PubMed] [Google Scholar]
- 3.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: How bacterial recognition shapes intestinal function. Nat Rev Immunol 10: 131–144, 2010. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 4.Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol 174: 4453–4460, 2005. doi: 10.4049/jimmunol.174.8.4453. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal S, Agrawal A, Said HM. Biotin deficiency enhances the inflammatory response of human dendritic cells. Am J Physiol Cell Physiol 311: C386–C391, 2016. doi: 10.1152/ajpcell.00141.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akhter S, Cavet ME, Tse CM, Donowitz M. C-terminal domains of Na(+)/H(+) exchanger isoform 3 are involved in the basal and serum-stimulated membrane trafficking of the exchanger. Biochemistry 39: 1990–2000, 2000. doi: 10.1021/bi991739s. [DOI] [PubMed] [Google Scholar]
- 7.Báez-Saldaña A, Díaz G, Espinoza B, Ortega E. Biotin deficiency induces changes in subpopulations of spleen lymphocytes in mice. Am J Clin Nutr 67: 431–437, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Bao S, Beagley KW, France MP, Shen J, Husband AJ. Interferon-gamma plays a critical role in intestinal immunity against Salmonella typhimurium infection. Immunology 99: 464–472, 2000. doi: 10.1046/j.1365-2567.2000.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4: 1633–1649, 2004. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]
- 10.Bonjour JP. Vitamins and alcoholism. V. Riboflavin, VI. Niacin, VII. Pantothenic acid, and VIII. Biotin. Int J Vitam Nutr Res 50: 425–440, 1980. [PubMed] [Google Scholar]
- 11.Chan T, Cheung FSG, Zheng J, Lu X, Zhu L, Grewal T, Murray M, Zhou F. Casein kinase 2 is a novel regulator of the human organic anion transporting polypeptide 1A2 (OATP1A2) trafficking. Mol Pharm 13: 144–154, 2016. doi: 10.1021/acs.molpharmaceut.5b00576. [DOI] [PubMed] [Google Scholar]
- 12.Cowan MJ, Wara DW, Packman S, Ammann AJ, Yoshino M, Sweetman L, Nyhan W. Multiple biotin-dependent carboxylase deficiencies associated with defects in T-cell and B-cell immunity. Lancet 2: 115–118, 1979. doi: 10.1016/S0140-6736(79)90002-3. [DOI] [PubMed] [Google Scholar]
- 13.de Groot REA, Rappel SB, Lorenowicz MJ, Korswagen HC. Protein kinase CK2 is required for Wntless internalization and Wnt secretion. Cell Signal 26: 2601–2605, 2014. doi: 10.1016/j.cellsig.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Banares F, Abad-Lacruz A, Xiol X, Gine JJ, Dolz C, Cabre E, Esteve M, Gonzalez-Huix F, Gassull MA. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol 84: 744–748, 1989. [PubMed] [Google Scholar]
- 15.García-Herrera J, Abad B, Rodríguez-Yoldi MJ. Effect of lipopolysaccharide on D-fructose transport across rabbit jejunum. Inflamm Res 52: 177–184, 2003. doi: 10.1007/s000110300069. [DOI] [PubMed] [Google Scholar]
- 16.Ghosal A, Chatterjee NS, Chou T, Said HM. Enterotoxigenic Escherichia coli infection and intestinal thiamin uptake: Studies with intestinal epithelial Caco-2 monolayers. Am J Physiol Cell Physiol 305: C1185–C1191, 2013. doi: 10.1152/ajpcell.00276.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosal A, Jellbauer S, Kapadia R, Raffatellu M, Said HM. Salmonella infection inhibits intestinal biotin transport: Cellular and molecular mechanisms. Am J Physiol Gastrointest Liver Physiol 309: G123–G131, 2015. doi: 10.1152/ajpgi.00112.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosal A, Lambrecht N, Subramanya SB, Kapadia R, Said HM. Conditional knockout of the Slc5a6 gene in mouse intestine impairs biotin absorption. Am J Physiol Gastrointest Liver Physiol 304: G64–G71, 2013. doi: 10.1152/ajpgi.00379.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosal A, Said HM. Structure-function activity of the human sodium-dependent multivitamin transporter: Role of His115 and His254. Am J Physiol Cell Physiol 300: C97–C104, 2011. doi: 10.1152/ajpcell.00398.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosal A, Subramanian VS, Said HM. Role of the putative N-glycosylation and PKC-phosphorylation sites of the human sodium-dependent multivitamin transporter (hSMVT) in function and regulation. Biochim Biophys Acta 1808: 2073–2080, 2011. doi: 10.1016/j.bbamem.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest 117: 428–437, 2007. doi: 10.1172/JCI29625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol 182: 375–387, 2013. doi: 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong M, Hong W, Ni C, Huang J, Zhou C. Protein kinase C affects the internalization and recycling of organic anion transporting polypeptide 1B1. Biochim Biophys Acta 1848: 2022–2030, 2015. doi: 10.1016/j.bbamem.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Im E, Riegler FM, Pothoulakis C, Rhee SH. Elevated lipopolysaccharide in the colon evokes intestinal inflammation, aggravated in immune modulator-impaired mice. Am J Physiol Gastrointest Liver Physiol 303: G490–G497, 2012. doi: 10.1152/ajpgi.00120.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janoria KG, Boddu SH, Wang Z, Paturi DK, Samanta S, Pal D, Mitra AK. Vitreal pharmacokinetics of biotinylated ganciclovir: Role of sodium-dependent multivitamin transporter expressed on retina. J Ocul Pharmacol Ther 25: 39–49, 2009. doi: 10.1089/jop.2008.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jwala J, Vadlapatla RK, Vadlapudi AD, Boddu SH, Pal D, Mitra AK. Differential expression of folate receptor-alpha, sodium-dependent multivitamin transporter, and amino acid transporter (B (0, +)) in human retinoblastoma (Y-79) and retinal pigment epithelial (ARPE-19) cell lines. J Ocul Pharmacol Ther 28: 237–244, 2012. doi: 10.1089/jop.2011.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest 100: 6–10, 1997. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kung JT, Mackenzie CG, Talmage DW. The requirement for biotin and fatty acids in the cytotoxic T-cell response. Cell Immunol 48: 100–110, 1979. doi: 10.1016/0008-8749(79)90103-5. [DOI] [PubMed] [Google Scholar]
- 29.Kuroishi T. Regulation of immunological and inflammatory functions by biotin. Can J Physiol Pharmacol 93: 1091–1096, 2015. doi: 10.1139/cjpp-2014-0460. [DOI] [PubMed] [Google Scholar]
- 30.Kuroishi T, Endo Y, Muramoto K, Sugawara S. Biotin deficiency up-regulates TNF-alpha production in murine macrophages. J Leukoc Biol 83: 912–920, 2008. doi: 10.1189/jlb.0607428. [DOI] [PubMed] [Google Scholar]
- 31.Kuroishi T, Kinbara M, Sato N, Tanaka Y, Nagai Y, Iwakura Y, Endo Y, Sugawara S. Biotin status affects nickel allergy via regulation of interleukin-1beta production in mice. J Nutr 139: 1031–1036, 2009. doi: 10.3945/jn.108.097543. [DOI] [PubMed] [Google Scholar]
- 32.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 179: 4808–4820, 2007. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Singla A, Ao M, Gill RK, Venkatasubramanian J, Rao MC, Alrefai WA, Dudeja PK. Calcitonin receptor-mediated CFTR activation in human intestinal epithelial cells. J Cell Mol Med 15: 2697–2705, 2011. doi: 10.1111/j.1582-4934.2011.01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Madsen CT, Sylvestersen KB, Young C, Larsen SC, Poulsen JW, Andersen MA, Palmqvist EA, Hey-Mogensen M, Jensen PB, Treebak JT, Lisby M, Nielsen ML. Biotin starvation causes mitochondrial protein hyperacetylation and partial rescue by the SIRT3-like deacetylase Hst4p. Nat Commun 6: 7726, 2015. doi: 10.1038/ncomms8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMahon RJ. Biotin in metabolism and molecular biology. Annu Rev Nutr 22: 221–239, 2002. doi: 10.1146/annurev.nutr.22.121101.112819. [DOI] [PubMed] [Google Scholar]
- 37.Mendoza C, Matheus N, Iceta R, Mesonero JE, Alcalde AI. Lipopolysaccharide induces alteration of serotonin transporter in human intestinal epithelial cells. Innate Immun 15: 243–250, 2009. doi: 10.1177/1753425909104781. [DOI] [PubMed] [Google Scholar]
- 38.Mock D. Biotin. In: Handbook of Vitamins. Zempleni J, McCormic DB, Suttei JW, editors. New York, NY: CRC, 2006, p. 361–377. [Google Scholar]
- 39.Morimoto S, Nishimura N, Terai T, Manabe S, Yamamoto Y, Shinahara W, Miyake H, Tashiro S, Shimada M, Sasaki T. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface. J Biol Chem 280: 2220–2228, 2005. doi: 10.1074/jbc.M406906200. [DOI] [PubMed] [Google Scholar]
- 40.Moritz AE, Rastedt DE, Stanislowski DJ, Shetty M, Smith MA, Vaughan RA, Foster JD. Reciprocal phosphorylation and palmitoylation control dopamine transporter kinetics. J Biol Chem 290: 29095–29105, 2015. doi: 10.1074/jbc.M115.667055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Keefe SJ, Ou J, Aufreiter S, O’Connor D, Sharma S, Sepulveda J, Fukuwatari T, Shibata K, Mawhinney T. Products of the colonic microbiota mediate the effects of diet on colon cancer risk. J Nutr 139: 2044–2048, 2009. doi: 10.3945/jn.109.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okabe N, Urabe K, Fujita K, Yamamoto T, Yao T, Doi S. Biotin effects in Crohn’s disease. Dig Dis Sci 33: 1495–1496, 1988. doi: 10.1007/BF01537009. [DOI] [PubMed] [Google Scholar]
- 43.Prasad PD, Wang H, Huang W, Fei YJ, Leibach FH, Devoe LD, Ganapathy V. Molecular and functional characterization of the intestinal Na+-dependent multivitamin transporter. Arch Biochem Biophys 366: 95–106, 1999. doi: 10.1006/abbi.1999.1213. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Melendez R, Zempleni J. Regulation of gene expression by biotin (review). J Nutr Biochem 14: 680–690, 2003. doi: 10.1016/j.jnutbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Sabui S, Bohl JA, Kapadia R, Cogburn K, Ghosal A, Lambrecht NW, Said HM. Role of the sodium-dependent multivitamin transporter (SMVT) in the maintenance of intestinal mucosal integrity. Am J Physiol Gastrointest Liver Physiol 311: G561–G570, 2016. doi: 10.1152/ajpgi.00240.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Said HM. Biotin: Biochemical, physiological and clinical aspects. Subcell Biochem 56: 1–19, 2012. doi: 10.1007/978-94-007-2199-9_1. [DOI] [PubMed] [Google Scholar]
- 47.Said HM. Recent advances in transport of water-soluble vitamins in organs of the digestive system: A focus on the colon and the pancreas. Am J Physiol Gastrointest Liver Physiol 305: G601–G610, 2013. doi: 10.1152/ajpgi.00231.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Said HM, Nexo E. Intestinal absorption of water-soluble vitamins. In: Physiology of the Gastrointestinal Tract, 5th ed. Johnson LR, Merchand JL, Said HM, Wood JD, editors. San Diego, CA: Elsevier, 2012, p. 1711–1756. doi: 10.1016/B978-0-12-382026-6.00064-6. [DOI] [Google Scholar]
- 49.Said HM, Trebble T. Intestinal digestion and absorption of micronutrients. In: Slesenger and Fordtran Gastrointestinal and Liver Disease, 10th ed Feldman M, Friedman LS, Brandt LJ, editors. New York, NY: Elsevier, 2015, p. 1765–1788. [Google Scholar]
- 50.Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle T Jr, Branca M, Russo A, Gribar SC, Ma C, Hackam DJ. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology 138: 185–196, 2010. doi: 10.1053/j.gastro.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strid MA, Dalby T, Mølbak K, Krogfelt KA. Kinetics of the human antibody response against Salmonella enterica serovars Enteritidis and Typhimurium determined by lipopolysaccharide enzyme-linked immunosorbent assay. Clin Vaccine Immunol 14: 741–747, 2007. doi: 10.1128/CVI.00192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Subramanian VS, Marchant JS, Boulware MJ, Ma TY, Said HM. Membrane targeting and intracellular trafficking of the human sodium-dependent multivitamin transporter in polarized epithelial cells. Am J Physiol Cell Physiol 296: C663–C671, 2009. doi: 10.1152/ajpcell.00396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Subramanya SB, Subramanian VS, Said HM. Chronic alcohol consumption and intestinal thiamin absorption: Effects on physiological and molecular parameters of the uptake process. Am J Physiol Gastrointest Liver Physiol 299: G23–G31, 2010. doi: 10.1152/ajpgi.00132.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sugahara H, Odamaki T, Fukuda S, Kato T, Xiao JZ, Abe F, Kikuchi J, Ohno H. Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community. Sci Rep 5: 13548, 2015. doi: 10.1038/srep13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Velázquez-Arellano A. From an inborn error patient to a search for regulatory meaning: A biotin conducted voyage. Mol Genet Metab 87: 194–197, 2006. doi: 10.1016/j.ymgme.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 56.Wiedmann S, Rodriguez-Melendez R, Ortega-Cuellar D, Zempleni J. Clusters of biotin-responsive genes in human peripheral blood mononuclear cells. J Nutr Biochem 15: 433–439, 2004. doi: 10.1016/j.jnutbio.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Yang B, Feng L, Wang F, Wang L. Enterohemorrhagic Escherichia coli senses low biotin status in the large intestine for colonization and infection. Nat Commun 6: 6592, 2015. doi: 10.1038/ncomms7592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zien P, Duncan JS, Skierski J, Bretner M, Litchfield DW, Shugar D. Tetrabromobenzotriazole (TBBt) and tetrabromobenzimidazole (TBBz) as selective inhibitors of protein kinase CK2: Evaluation of their effects on cells and different molecular forms of human CK2. Biochim Biophys Acta 1754: 271–280, 2005. doi: 10.1016/j.bbapap.2005.07.039. [DOI] [PubMed] [Google Scholar]