Abstract
Mechanochemical signal transduction occurs when mechanical forces, such as fluid shear stress, are converted into biochemical responses within the cell. The molecular mechanisms by which endothelial cells (ECs) sense/transduce shear stress into biological signals, including the nature of the mechanosensor, are still unclear. G proteins and G protein-coupled receptors (GPCRs) have been postulated independently to mediate mechanotransduction. In this study, we used in situ proximity ligation assay (PLA) to investigate the role of a specific GPCR/Gαq/11 pair in EC shear stress-induced mechanotransduction. We demonstrated that sphingosine 1-phosphate (S1P) stimulation causes a rapid dissociation at 0.5 min of Gαq/11 from its receptor S1P3, followed by an increased association within 2 min of GPCR kinase-2 (GRK2) and β-arrestin-1/2 with S1P3 in human coronary artery ECs, which are consistent with GPCR/Gαq/11 activation and receptor desensitization/internalization. The G protein activator AlF4 resulted in increased dissociation of Gαq/11 from S1P3, but no increase in association between S1P3 and either GRK2 or β-arrestin-1/2. The G protein inhibitor guanosine 5′-(β-thio) diphosphate (GDP-β-S) and the S1P3 antagonist VPC23019 both prevented S1P-induced activation. Shear stress also caused the rapid activation within 7 s of S1P3/Gαq/11. There were no increased associations between S1P3 and GRK2 or S1P3 and β-arrestin-1/2 until 5 min. GDP-β-S, but not VPC23019, prevented dissociation of Gαq/11 from S1P3 in response to shear stress. Shear stress did not induce rapid dephosphorylation of β-arrestin-1 or rapid internalization of S1P3, indicating no GPCR activation. These findings suggest that Gαq/11 participates in the sensing/transducing of shear stress independently of GPCR activation in ECs.
Keywords: endothelial cell, G protein-coupled receptors, heterotrimeric G proteins, shear stress
vascular endothelial cells (ECs) are exposed to mechanical forces from blood flow, which are sensed and transduced into intracellular biochemical responses. These signals contribute to the overall phenotype and function of normal ECs, but they can also lead to vascular pathologies (9). The identity of the primary mechanosensor, including its composition and subcellular structure, is still largely unknown. Fluid shear stress, the tangential component of hemodynamic forces, is known to activate heterotrimeric G protein subunits αq and -11 (Gαq/11) in ECs within seconds of flow onset (16, 18). Gαq/11 may also be activated independently of cytoskeletal and cytosolic components and occur in the absence of protein receptors (17), suggesting a critical role of the phospholipid bilayer. More recent evidence suggests that Gαq/11 is part of a mechanosensitive complex together with platelet endothelial cell adhesion molecule-1 (PECAM-1) and GPCRs at the EC junction (11, 30, 46). However, it has also been postulated that GPCRs can be directly stimulated by mechanical forces, including fluid shear stress and stretch (7, 48).
GPCRs are a family of membrane receptors that respond to a diverse set of extracellular physical and chemical stimuli, including light, odor, neurotransmitters, cytokines, growth factors, lipids, and hormones to mediate a wide range of biological processes. Generally, binding of agonists to their respective GPCRs leads to the activation of intracellularly associated heterotrimeric G proteins, composed of α-, β-, and γ-subunits, and the subsequent dissociation of the Gα subunit from the dimeric Gβγ subunit and GPCR. In the continuous presence of agonist, GPCRs are phosphorylated by G protein-coupled receptor kinases (GRKs). Phosphorylation is followed by the recruitment and binding of β-arrestins (typically β-arrestin-1 and -2), desensitization, and internalization, which effectively terminates G protein signaling (31).
Sphingosine 1-phosphate (S1P) is a bioactive lipid that binds to and activates a family of five GPCRs, S1P1–5, which are differentially coupled to G protein subtypes and are known to be expressed in a wide variety of tissues and cell types. Whereas S1P1 is known to couple only to Gαi and S1P4 and S1P5 to Gαi and Gα12/13, both S1P2 and S1P3 can couple to and activate Gαi, Gαq/11, and Gα12/13 (35, 37). Activation of either S1P1 or S1P3, both of which are specifically expressed by ECs, can lead to the activation of several downstream signaling pathways, including the extracellular signal-regulated kinase (ERK) pathway (43).
To investigate the specific roles of Gαq/11 and GPCRs in mechanochemical signal transduction, we compared shear stress- and ligand-induced activation of a specific GPCR/G protein pair by examining the endogenous interactions between S1P3 and Gαq/11, S1P3 and GRK2, and S1P3 and β-arrestin-1/2 in ECs upon stimulation using in situ proximity ligation assay (PLA). We found that this particular GPCR/G protein pair is mechanosensitive. By targeting Gαq/11 and S1P3 separately, we further demonstrated that shear stress-induced activation of Gαq/11 occurs independently of S1P3 activation. Our results reveal that shear stress-induced Gαq/11 activation has a molecular signature distinct from that of ligand-induced Gαq/11 activation.
MATERIALS AND METHODS
Cell culture.
Human coronary artery endothelial cells (HCAECs) from male donors were obtained from either Cell Applications (San Diego, CA) or Lonza (Walkersville, MD) and maintained in complete endothelial growth medium (EGM-2; Lonza) supplemented with 10% heat-inactivated FBS and penicillin-streptomycin. Before all experimental procedures, cells were seeded onto glass microscope slides, grown to confluence, and serum-starved overnight in ATP-free endothelial basal medium (EBM-2; Lonza) supplemented with 0.5% BSA. HCAECs within six passages were used for all experiments.
Reagents.
Sphingosine-1-phosphate (S1P) was purchased from either Tocris Bioscience (Bristol, UK) or Cayman Chemical (Ann Arbor, MI) and resuspended in PBS with 0.4% BSA. Serotonin hydrochloride (5-hydroxytryptamine, 5-HT) was purchased from Tocris Bioscience (Bristol, UK) and solubilized in water. AlF4- was prepared by mixing 30 μM AlCl3 and 10 mM NaF. Guanosine 5′-O-(2-thiodiphosphate), trilithium salt (GDP-β-S) was purchased from EMD Millipore (Billerica, MA). VPC23019 was purchased from Tocris Bioscience and resuspended in dimethyl sulfoxide (DMSO). Anti-phospho-ERK1/2 (T202/Y204) (catalog no. 9102), anti-ERK1/2, anti-phospho-β-arrestin-1 (S412) (catalog no. 2416), and anti-Akt (S473) (catalog no. 9271) antibodies were all purchased from Cell Signaling Technology (Danvers, MA). Anti-β-arrestin-1 (catalog no. 610550) and anti-Akt antibodies were purchased from BD Biosciences (San Jose, CA) and Santa Cruz Biotechnology (Dallas, TX), respectively.
Shear stress.
Glass microscope slides with HCAECs were mounted on a conventional parallel-plate flow chamber (14) and subjected to a steady fluid shear stress of 14 dyn/cm2 by perfusion with CO2-equilibrated EBM-2 containing 0.5% BSA using a PHD 2000 syringe pump (Harvard Apparatus, Holliston, MA). Cells on slides that were mounted but not subjected to shear stress served as “Sham” controls.
In situ proximity ligation assay.
HCAEC monolayers on glass slides were immediately quenched in cold methanol-acetone (−20°C), rehydrated in ice-cold PBS, and then treated according to the manufacturer’s protocol (Olink Biosciences, Uppsala, Sweden). This method of fixing cells with cold methanol is routinely used to quench metabolic activity on a subsecond time scale for quantitative metabolomics (10). Cells were probed using primary antibodies that have been previously shown to be specific for S1P3, Gαq/11, and 5-hydroxytryptamine receptor 2A (5-HT2A) (1, 27, 29). Primary antibodies used were a custom-made rabbit anti-Gαq/11 (clone no. 47; Epitomics), goat anti-S1P3/EDG-3 (V-20) (catalog no. sc-16076; Santa Cruz Biotechnology), mouse anti-SR-2A/5-HT2A (A-4) (catalog no. sc-166775; Santa Cruz Biotechnology), rabbit anti-GRK2 (catalog no. 3982; Cell Signaling Technology), and rabbit anti-pan arrestin (catalog no. ab2914; Abcam, Cambridge, MA). The two fluorescence-labeled PLA probes used were Duolink In Situ PLA Probe Anti-Rabbit PLUS (catalog no. DUO92002) and Duolink In Situ PLA Probe Anti-Goat MINUS (catalog no. DUO92006), purchased from Sigma-Aldrich (Carlsbad, CA). When the two target proteins are bound with both primary and secondary antibodies, the oligonucleotide probes are hybridized to each other and ligated to form a closed circle. Polymerase-driven rolling circle amplification (RCA) generates a product to which the oligonucleotide probes hybridize, thereby forming a visible PLA signal. All primary antibody pairs and PLA probes were tested at different concentrations to ensure that the density of PLA signals were in the linear range for detection of effects and did not reach a saturation point (data not shown). As a negative control to assess background signal for each experiment, one of the primary antibodies for each pair was omitted (13) and showed very few PLA signal (Table 1). Single-sliced images were acquired on a LSM5 PASCAL confocal fluorescence microscope (Carl Zeiss, Germany) equipped with a Plan Apochromatic 63/1.4 numerical aperture oil immersion objective and both the PLA signal (i.e., single dots or pixels) and cell nuclei were quantified using a custom ImageJ image analysis macro. A minimum of ten fields of acquisition were acquired for each of at least three individual experiments.
Table 1.
PLA signal of HCAECs under basal conditions
| Antibodies | Average Number of Fluorescent Dots per Microscope Field | Average Number of Nuclei per Microscope Field |
|---|---|---|
| S1P3 only | 35.8 ± 2.9 | 17.2 ± 1.3 |
| Gαq/11 only | 21.2 ± 2.7 | 19.1 ± 0.6 |
| GRK2 only | 17.7 ± 1.8 | 19.7 ± 1.0 |
| β-Arrestins only | 25.2 ± 2.2 | 18.8 ± 0.9 |
| S1P3/Gαq/11 pair | 401.9 ± 18.8 | 22.2 ± 0.9 |
| S1P3/GRK2 pair | 87.8 ± 4.5 | 15.4 ± 0.6 |
| S1P3/β-arrestins pair | 83.8 ± 3.2 | 19.4 ± 0.9 |
Values are from a representative experiment and depicted as means ± SE. HCAECs, human coronary artery endothelial cells; S1P3, sphingosine-1-phosphate receptor 3; GRK2, G protein-coupled receptor kinase-2.
Immunofluorescence receptor localization assay.
HCAEC monolayers on glass slides were incubated with 2.5 μM S1P or subjected to shear stress for 0, 5, and 10 min. Cells were then fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.3% Triton X-100 in PBS for 10 min, and blocked in 3% BSA in PBS for 1 h. Cells were incubated with goat anti-S1P3/EDG-3 (V-20) (catalog no. sc-16076; Santa Cruz Biotechnology) diluted 1:100 in blocking buffer followed by incubation with a 1:200 dilution of Alexa Fluor 488 donkey anti-goat IgG (Molecular Probes, Eugene, OR).
Western blot analysis.
Proteins were separated on NuPAGE 4–12% Bis-Tris gels (Thermo Fisher Scientific) in MOPS SDS running buffer (Thermo Fisher Scientific) and transferred to PVDF membranes (Immobilon-P; Millipore, Temecula, CA). Membranes were blocked for 1 h with 3% BSA in Tris-buffered saline (TBS) and then incubated with a primary antibody for 2 h or overnight in 3% BSA-TBST (TBS with 0.1% Tween 20) at 4°C. After washing and incubating with horseradish peroxidase-conjugated secondary antibodies for 1 h was completed, the membranes were incubated with chemiluminescence substrate (SuperSignal West Pico or West Femto; Thermo Scientific, Rockford, IL). Images were acquired using a C-DiGit Blot Scanner (LI-COR Biosciences, Lincoln, NE).
Statistical analyses.
All experimental data are expressed as means ± SE from at least three independent experiments. Single comparisons between groups were performed using Student’s t-test, whereas multiple group comparisons were analyzed using one-way ANOVA with Bonferroni post hoc tests. P values of <0.05 were considered statistically significant.
RESULTS
S1P3/Gαq/11 activation is induced by both S1P and shear stress.
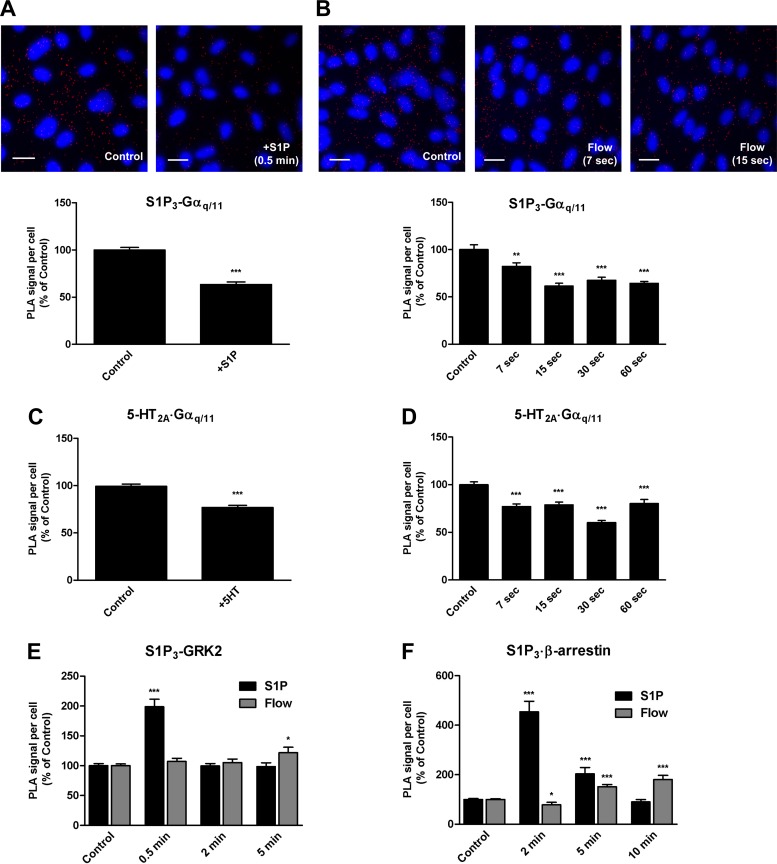
To gain insight into the mechanism(s) by which shear stress induces heterotrimeric G protein activation in endothelial cells, we examined the endogenous interactions between the specific GPCR/G protein pair, S1P3/Gαq/11, using in situ proximity ligation assay (PLA). We detected the presence of S1P3 in close proximity to Gαq/11 in HCAECs under basal conditions as indicated by the presence of red fluorescent dots, which we refer to as PLA signal and is indicative of the relative number of complexes (Fig. 1A). In cells treated with S1P (2.5 μM) for 0.5 min, we observed a significant decrease (37%) in PLA signal detected by the S1P3/Gαq/11 antibody pair compared with that in the untreated control condition, which suggests rapid dissociation of Gαq/11 from S1P3 and therefore rapid activation of the complex.
Fig. 1.
Ligand- and shear stress-induced activation of the sphingosine-1-phosphate (S1P3)-Gαq/11 complex. In situ proximity ligation assay (PLA) was performed using antibodies directed against S1P3 and Gαq/11 (A and B), serotonin (5-HT)2A and Gαq/11 (C and D), S1P3 and G protein-coupled receptor kinases (GRK2; E), and S1P3 and β-arrestin-1/2 (F) on human coronary artery endothelial cells (HCAECs) treated with vehicle alone (Control), S1P; 2 μM for 30 s) (A, E, and F), or 5-HT (100 nM for 30 s) (C) and on HCAECs that were either unstimulated (Sham) or subjected to a step change in shear stress (Flow) for the indicated times (B, D, E, and F). Representative confocal images in a single z-plane of cells probed with the S1P3/Gαq/11 antibody pair are shown (A and B). Scale bar, 20 μm. Each bar graph shows quantification of at least 3 independent experiments as PLA signal (red dots) per cell (blue nuclei) relative to the control condition, with error bars indicating SE; n = 6 for C, n = 5 for A, B, E (Flow), and F (Flow), n = 3 for D, E (S1P), and F (S1P). *P < 0.05; **P < 0.01; ***P < 0.001.
To determine whether shear stress can activate the S1P3/Gαq/11 complex, we examined the association between S1P3 and Gαq/11 in HCAECs by PLA. Interestingly, shear stress induced an 18% decrease in PLA signal as early as 7 s after stimulation (Fig. 1B). The PLA signal is decreased even further at 15 s (39%) and remains decreased through 30 s (32%) and up to 60 s (37%).
To verify the general applicability of the in situ PLA technique to monitor the activation of GPCR/G proteins, we examined the endogenous interactions between the serotonin receptor, 5-HT2A, and Gαq/11 in response to both its natural ligand, serotonin (Fig. 1C), and shear stress (Fig. 1D). HCAECs stimulated with 5-HT (100 nM) for 30 s showed a decrease in the number of 5-HT2A·Gαq/11 complexes (23%). Similarly, the number of complexes was decreased with the rapid onset of flow at 7 s (23%) and reaching a maximum decrease at 30 s (40%) compared with the sham control condition.
S1P, but not shear stress, induces association of GRK2 and β-arrestin1/2 with S1P3.
We next examined the association of S1P3 with GRK2 in response to S1P and shear stress stimulation to determine the recruitment of GPCR kinases to activated GPCRs. Stimulation of HCAECs with S1P for 0.5 min resulted in a marked increase (99%) in PLA signal compared with untreated controls (Fig. 1E). However, this relative change in signal was transient as the number of fluorescent dots decreased to baseline levels at 2 and 5 min. In contrast, in cells subjected to shear stress, there was no increase in association between S1P3 and GRK2 at 0.5 and 2 min and only a modest increase at 5 min (22%).
Since β-arrestins bind to and negatively regulate almost all activated GPCRs, we examined the association of S1P3 with β-arrestin-1/2 to determine the recruitment of arrestins to activated GPCRs. An increase was observed in the number of dots detected by the S1P3/ β-arrestin-1/2 antibody pair with a peak (4.5-fold) at 2 min upon stimulation with S1P (Fig. 1F). At 10 min, the number of dots detected was slightly below the baseline level, perhaps reflecting internalization of the receptor. In contrast, shear stress did not increase the PLA signal detected by proximity of S1P3 and β-arrestin-1/2 at 2 min, but there was a steady increase that began at 5 min after exposure to shear stress and continued through 10 min (51 and 81%, respectively).
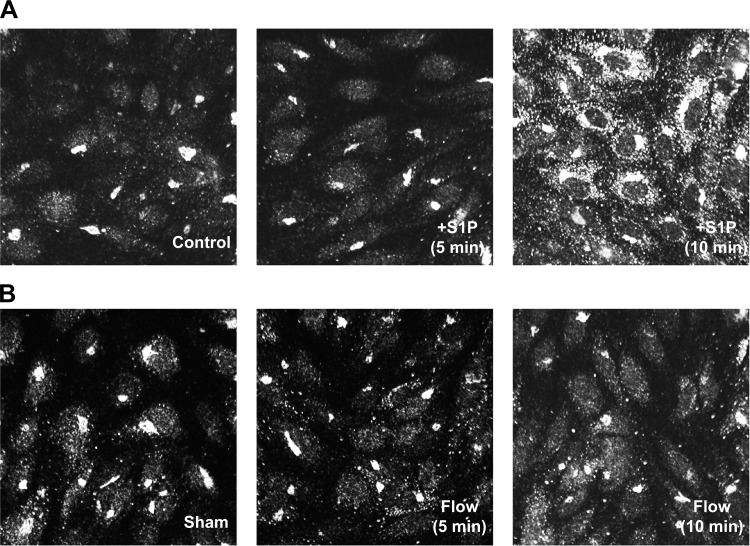
S1P, but not shear stress, induces S1P3 internalization.
Since most canonical GPCRs undergo rapid β-arrestin-dependent internalization upon ligand activation, we investigated whether S1P3 is internalized in response to either S1P stimulation or shear stress in HCAECs by using immunofluorescence staining. In vehicle-stimulated cells, S1P3 had a perinuclear Golgi-like staining in addition to a diffuse cell surface localization (Fig. 2A). Stimulation of cells with S1P for 5 min did not appear to change this S1P3 localization pattern. At 10 min, however, S1P3 immunostaining became more punctate and localized in the cytoplasm. Shear stress, on the other hand, did not induce cytoplasmic localization of S1P3 at any of the time points that were evaluated (Fig. 2B). This finding suggests that shear stress, in contrast to S1P, does not induce S1P3 internalization.
Fig. 2.
Ligand- and shear-induced internalization of S1P3. HCAECs were treated with S1P at 2.5 μM (A) or exposed to flow at 14 dyn/cm2 (B) for indicated time points. Cells were then fixed, permeabilized, blocked, and immunostained with an anti-S1P3/EDG-3 primary antibody followed by an Alexa fluor 488-conjugated secondary antibody. Representative images from 3 independent experiments are shown.
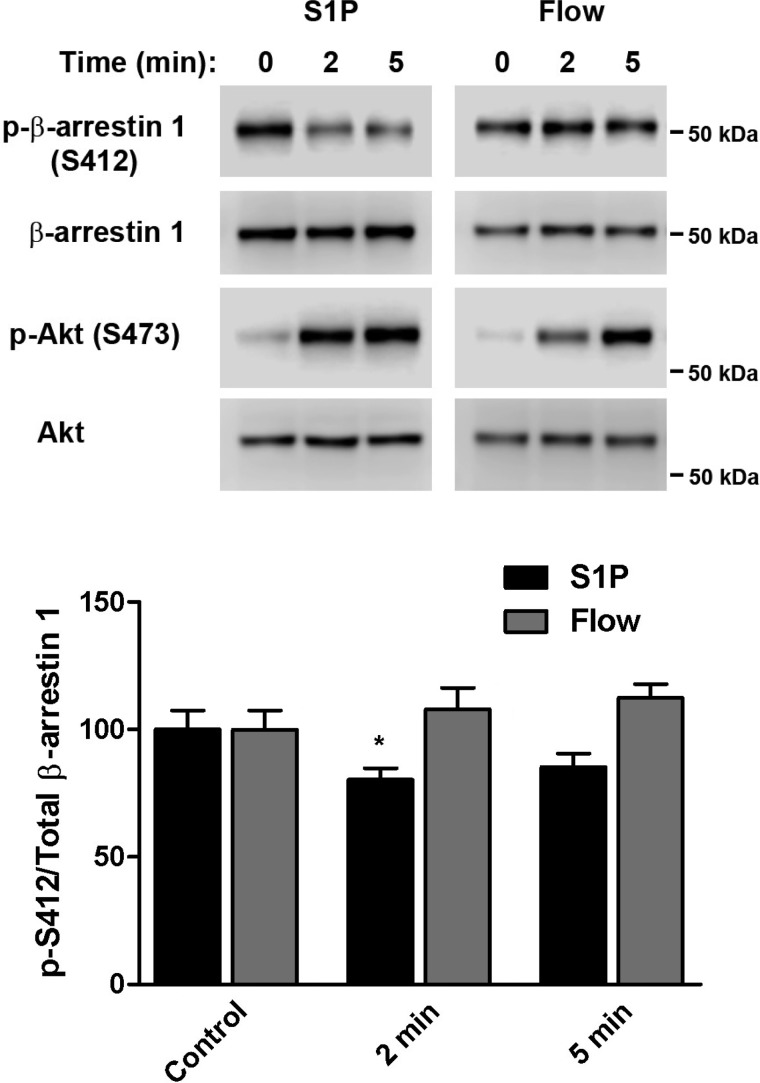
β-Arrestin-1 is rapidly dephosphorylated in response to S1P but not shear stress.
Cytosolic β-arrestin-1 is known to be constitutively phosphorylated on serine 412 (S412) and is recruited to and rapidly dephosphorylated at the plasma membrane upon agonist stimulation, a process that is required for clathrin-mediated receptor internalization (24). We therefore measured the phosphorylation/dephosphorylation status of β-arrestin-1 in HCAECs that were stimulated with either S1P or shear stress. Western blot analyses of lysates prepared from S1P-activated cells showed rapid dephosphorylation, as reflected by a decrease in phosphorylation of S412 at both 2 and 5 min poststimulation (20 and 15%, respectively) (Fig. 3). In contrast, flow did not induce any β-arrestin-1 dephosphorylation at the same time points. As a positive control, immunoblotting for phosphorylated Akt, which is a known downstream event of both signaling pathways (12, 20), confirmed that cells were indeed activated by each stimulus.
Fig. 3.
Ligand- and shear stress-induced dephosphorylation of β-arrestin-1. HCAECs were treated with S1P (2.5 μM) or exposed to flow (14 dyn/cm2) for indicated time points. Immunoblotting was performed on cell lysates using antibodies against phosphorylated β-arrestin-1 (S412) and total β-arrestin-1 to assess the level of phosphorylation/dephosphorylation. As a positive control for cell stimulation, lysates were also probed for phosphorylated Akt (S473) and total Akt. Representative blots from 5 independent experiments are shown. Bar graph represents the quantification as a ratio of phospho-S412 over total β-arrestin-1, with the mean value of the control condition arbitrarily set to 100% and error bars indicating SE; n = 5. *P < 0.05.
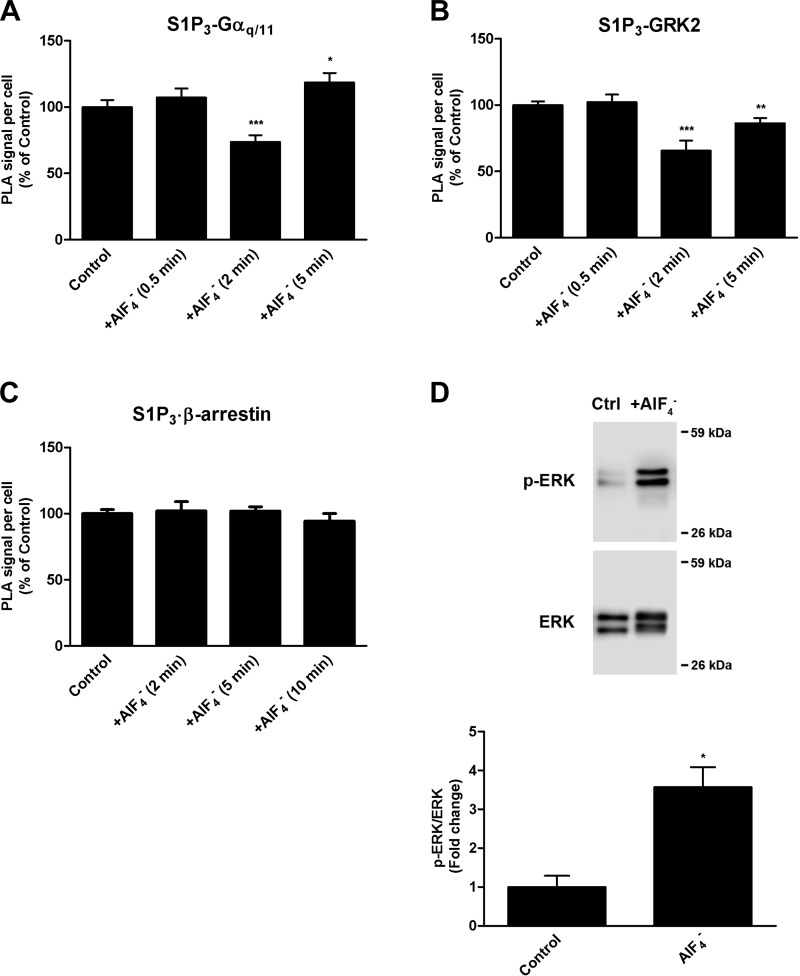
These differences in the molecular association patterns, receptor internalization, and β-arrestin-1 phosphorylation/dephosphorylation status between the S1P-induced and shear stress-induced conditions led us to hypothesize that shear stress may cause Gαq/11 activation independently of GPCR activation. We therefore sought to determine the patterns if Gαq/11 was directly activated independently of S1P3 activation. To this end, we examined cells treated with the G protein activator AlF4− in the absence of S1P stimulation. Our results showed that Gαq/11 is transiently dissociated from S1P3 at 2 min (Fig. 4A). In contrast to S1P-induced cells, in which S1P3 was directly activated by ligand binding, there was no observable increase in association of S1P3 with either GRK2 (Fig. 4B) or β-arrestin-1/2 (Fig. 4C) in AlF4--treated ECs. In fact, GRK2 appeared to be dissociated from S1P3 at 2 min, as suggested by the decrease in PLA signal. To verify that AlF4− was used at an active concentration, we examined ERK1/2 activation, a pathway known to be mediated by Gαq/11. Western blot analysis showed that AlF4- is a potent activator of ERK1/2 activation, as indicated by its marked increase in phosphorylation at 5 min (Fig. 4D).
Fig. 4.
AlF4 (30 μM AlCl3 + 10 mM NaF)-induced activation of Gαq/11. HCAECs were treated with vehicle or AlF4- for indicated time points. In situ PLA was performed using antibodies against S1P3 and Gαq/11 (A), S1P3 and GRK2 (B), and S1P3 and β-arrestin-1/2 (C). Each bar graph shows quantification of 3 independent experiments as PLA signal per cell relative to the vehicle-treated control condition, with error bars indicating SE; n = 3. D: immunoblotting for phospho-ERK1/2 was performed on lysates prepared from cells stimulated with AlF4- for 5 min. A representative blot from 3 independent experiments is shown. Bar graph represents the quantification as a ratio of phospho-ERK1/2 over total ERK1/2 levels, with the mean value of the control condition set to 1 and error bars indicating SE; n = 3. *P < 0.05; **P < 0.01; ***P < 0.001.
GDP-β-S prevents both S1P- and shear stress-induced Gαq/11 activation.
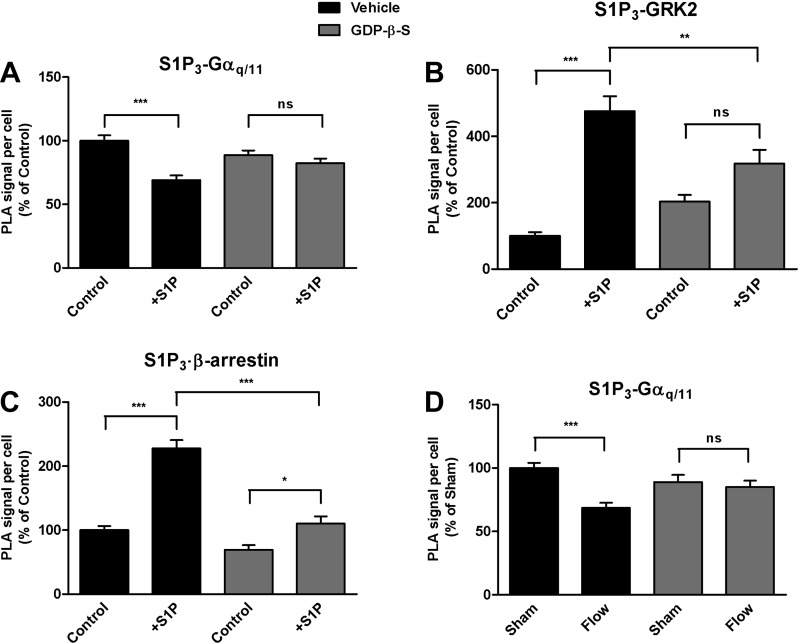
We next investigated whether the observed S1P-induced dissociation of the S1P3/Gαq/11 complex represents Gαq/11 activation, S1P3 activation, or both. For the first set of studies, cells were treated with GDP-β-S, a metabolically stable analog of GDP that binds to Gα proteins and inhibits binding and activation by GTP. GDP-β-S (300 μM, 4 h), but not vehicle, prevented the decrease in association between S1P3 and Gαq/11 in S1P-induced cells at 30 s (Fig. 5A). GDP-β-S also abrogated S1P-induced association of GRK2 with S1P3 at 30 s (Fig. 5B). Interestingly, GDP-β-S did not completely block the S1P-induced increase in association of β-arrestin-1/2 with S1P3, but caused a significant decrease in their S1P-induced association relative to vehicle control at 2 min (Fig. 5C). These findings suggest that the dissociation of Gαq/11 from S1P3 in response to S1P treatment is indicative of its activation.
Fig. 5.
Effects of G protein inhibition on ligand- and shear stress-induced Gαq/11 activation. HCAECs were pretreated with vehicle or guanosine 5′-(β-thio)diphosphate (GDP-β-S; 300 μM, 4 h), an inhibitor of G protein activation, before stimulation with S1P (2 μM). In situ PLA was performed using antibodies directed against the pairs S1P3 and Gαq/11 (A) and S1P3 and GRK2 (B) at 0.5 min, and the pair S1P3 and β-arrestin-1/2 (C) at 2 min. Each bar graph shows quantification of at least 3 independent experiments as PLA signal per cell relative to the vehicle-treated control condition, with error bars indicating SE; n = 6 for A, n = 4 for B, n = 3 for C. D: HCAECs were pretreated with vehicle or with GDP-β-S (300 μM, 4 h) before exposure to shear stress (Flow; 15 s). In situ PLA was performed with antibodies against S1P3 and Gαq/11. Bar graph shows quantification of 3 independent experiments as PLA signal per cell relative to the vehicle-treated control condition with error bars indicating SE; n = 3. *P < 0.05; **P < 0.01; ***P < 0.001.
To determine whether activation of the S1P3/Gαq/11 complex by shear stress is due to direct activation of Gαq/11 or is mediated specifically through S1P3, we sought to target each molecule separately. GDP-β-S blocked the shear stress-induced rapid dissociation of S1P3/Gαq/11 at 15 s (Fig. 5D), while vehicle alone did not.
VPC23019 blocks Gαq/11 activation by S1P but not by shear stress.
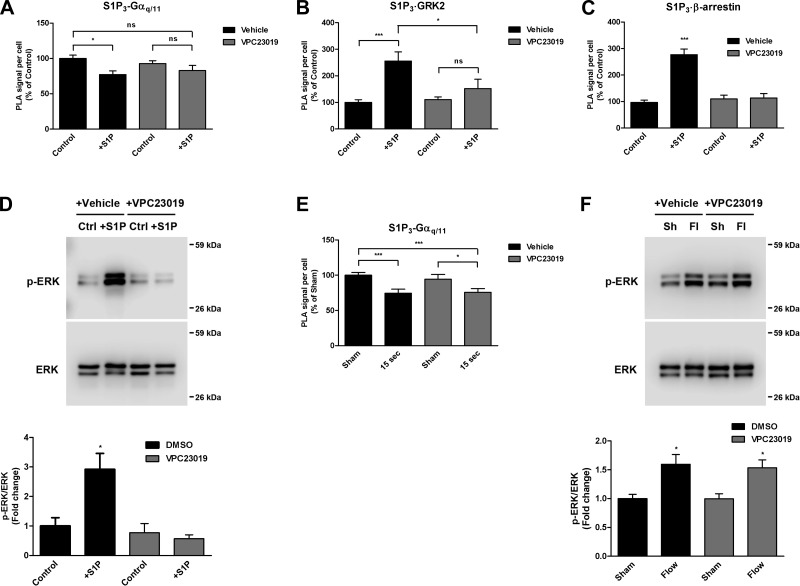
To determine whether the dissociation that we observed between S1P3 and Gαq/11 within 0.5 min of HCAEC stimulation with S1P is also indicative of S1P3 activation, we performed PLA experiments using cells that were pretreated for 2 min with either vehicle or the S1P3-selective antagonist VPC23019 at 10 μM (Fig. 6A). In vehicle control-treated cells induced with S1P for 0.5 min, we observed a significant decrease in PLA signal detected by the S1P3/Gαq/11 antibody pair. VPC23019 prevented this decrease in PLA signal, as indicated by the lack of a significant change in the number of S1P3/Gαq/11 complexes detected at 0.5 min.
Fig. 6.
Effects of S1P3 inhibition on ligand- and shear stress-induced Gαq/11 activation. A and C: HCAECs were pretreated with vehicle or the S1P receptor antagonist VPC23019 (10 μM), for 2 min before stimulation with S1P (2.5 μM). In situ PLA was performed using antibodies directed against S1P3 and Gαq/11 on cells stimulated for 0.5 min (A), S1P3 and GRK2 on cells stimulated for 0.5 min (B), and S1P3 and β-arrestin-1/2 on cells stimulated for 2 min (C). Each bar graph shows quantification of 3 independent experiments as PLA signal per cell relative to the vehicle-treated control condition with error bars indicating SE; n = 3. D: immunoblotting for phospho-ERK1/2 was performed on cell lysates at 5 min poststimulation with S1P in the absence or presence of VPC23019 pretreatment. A representative blot from 3 independent experiments is shown. Bar graph represents the quantification as a ratio of phospho-ERK1/2 over total ERK1/2 levels, with the mean value of the control condition set to 1 and error bars indicating SE. E: HCAECs were pretreated with vehicle or VPC23019 (10 μM, 2 min) before flow. In situ PLA was performed using antibodies against S1P3 and Gαq/11. Bar graph shows quantification of 3 independent experiments as PLA signal per cell relative to the vehicle-treated control condition, with error bars indicating SE; n = 3. F: immunoblotting for phospho-ERK1/2 was performed on lysates from cells subjected to flow for 5 min in the absence or presence of VPC23019 pretreatment. A representative blot from 4 independent experiments is shown. Bar graph represents the quantification as a ratio of phospho-ERK1/2 over total ERK1/2 levels, with the mean value of the control condition set to 1 and error bars indicating SE; n = 4. *P < 0.05; ***P < 0.001.
The effects of VPC23019 on the S1P-induced associations of S1P3 with GRK2 and β-arrestin-1/2 were also investigated. The dramatic increase in association of S1P3 with GRK2 by S1P at 0.5 min in the presence of vehicle alone was blocked by the pretreatment with VPC23019 (Fig. 6B). Likewise, there was an increase in the number of S1P3-β-arrestin-1/2 complexes in vehicle-treated cells after 2 min of S1P stimulation, but no apparent increase in S1P3-β-arrestin-1/2 complexes with VPC23019 (Fig. 6C).
Western blot analysis was also performed to confirm that VPC23019 under the selected conditions inhibits downstream signaling induced by S1P stimulation (Fig. 6D). As expected, we observed a significant increase in ERK1/2 phosphorylation (2.9-fold increase) with vehicle control at 5 min poststimulation with S1P, which was completely abrogated by VPC23019.
For cells subjected to shear stress in the presence of VPC23019, there was still a significant decrease in the number of S1P3/Gαq/11 complexes (Fig. 6E), just as there had been in the absence of the S1P3 inhibitor, indicating that S1P3 inhibition does not have an effect on shear stress-induced S1P3/Gαq/11 activation. Additionally, shear stress-induced ERK1/2 phosphorylation, which is known to be mediated by Gαq/11 activation (15), was unchanged in the presence of VPC23019 at 5 min (Fig. 6F). It should be noted here that ERK1/2 phosphorylation was examined at 5 min as opposed to 10 min poststimulation to avoid autocrine effects due to shear stress-induced S1P secretion (40). Collectively, these data demonstrate that shear stress-induced activation of the S1P3/Gαq/11 complex is not dependent on activation of S1P3.
DISCUSSION
GPCRs are activated by a wide array of external stimuli, which can be either chemical or physical in nature. Here, we show that the specific GPCR/G protein pair S1P3/Gαq/11 is mechanosensitive and is activated within 7 s of the onset of fluid shear stress. Although mechanical stimulation, in the form of either membrane stretch or fluid shear stress, has been previously shown to activate a number of different GPCRs in different cell types (38), the majority of the studies either examined events that were distal from receptor activation (i.e., Ca2+ measurements and ERK phosphorylation), or utilized approaches [i.e., fluorescence resonance energy transfer (FRET) and bioluminescence resonance energy transfer (BRET)], which required the transfection and overexpression of constructs. Furthermore, these expression constructs contain fluorescence proteins on the third intracellular loop and COOH terminus of GPCRs, which are known to decrease ligand-binding affinity and coupling to G proteins (41). Our study presents a novel approach to investigate a more proximal event in the activation of endogenous GPCRs and measure it in the context of their native cellular and subcellular environment, without the need for overexpression.
In situ PLA has recently been utilized to demonstrate GPCR heterodimerization without the need for heterologous expression (5) and has even been used as a stand-alone method in detecting and quantifying dimerization between transcription factors (2) as well as interactions between receptors and their effector molecules (39). This technique has also been used to confirm junctional associations between Gαq/11 and PECAM-1 in quiescent endothelial cells and to detect dissociations between these two proteins in response to a sudden temporal change in shear stress (11). The association between Gαq/11 and PECAM-1 under basal conditions and their dissociation from one another in response to shear stress had previously been shown independently using coimmunoprecipitation (co-IP) (30). It should be noted that endogenous levels of PECAM-1 in ECs are typically high, which eliminates the need for its heterologous expression. There have also been several other studies that have shown interactions between two proteins by in situ PLA and verified by co-IP or vice versa (4, 33, 34, 36, 45). In the majority of the aforementioned studies, co-IP was performed using lysates from cells that were transfected with constructs to overexpress their proteins of interest. In situations in which the endogenous levels of two proteins of interest are relatively low, the likelihood of detecting an interaction by conventional co-IP is also predictably low and therefore other alternative methods, such as in situ PLA, must be utilized. The solubility of a target protein, especially a seven-transmembrane receptor such as S1P3, in low-stringency conditions that would favor protein-protein interactions, may also be low. We attribute the lack of detection of an interaction between endogenous S1P3 and Gαq/11 in ECs by co-IP (data not shown) in our studies to these two aforementioned reasons. The strengths of in situ PLA are that it allows for detection of associations between endogenous proteins without the need for expression constructs, the resolution is on the order of individual interactions between two proteins, and it is highly sensitive due to rolling circle amplification (8). Through the use of in situ PLA, we demonstrated that S1P stimulation and exposure of endothelial cells to shear stress both cause rapid dissociation of Gαq/11 from S1P3, which suggests the activation and release of Gαq/11 from the GPCR/G protein complex in both instances.
In the case of S1P stimulation, there was also a rapid and transient increase in the association of S1P3, first with GRK2, and then with β-arrestin-1/2. These events were then followed by a return to their respective baseline complex levels. Together, these findings are consistent with the recruitment of GRK2, which functions to phosphorylate ligand-bound and activated GPCRs, and the subsequent binding of β-arrestin-1/2, which either serves to initiate GPCR inactivation through the canonical pathway of desensitization and internalization or to mediate G protein-independent signaling (42). S1P stimulation indeed caused a change in S1P3 localization from the cell surface to the cytoplasm after 10 min, which is consistent with two previous studies, one that showed that S1P induces internalization of S1P3 within 45 min in human umbilical vein endothelial cells and another that demonstrates epitope-tagged S1P3 labeling to be punctate and accumulate in the cytoplasm in cells treated with S1P for 15 min (19, 22). This result strongly suggests that the β-arrestin-1/2 recruitment observed by in situ PLA at 2 min may be initiating S1P3 internalization in response to S1P stimulation.
In addition to demonstrating for the first time that shear stress rapidly induces the dissociation of Gαq/11 from a specific GPCR, we also provide evidence that it was not a result of direct activation of the GPCR but rather direct activation of Gαq/11, independent of GPCR activation. The timing of endogenous Gαq/11 activation in the present study is consistent with previous work showing Gαq/11 to be rapidly activated within 1 s of flow onset (18). Gαq/11 mediates both shear stress-induced Ras activation (16) and intracellular Ca2+ release (27) within 5–20 s, which implies that Gαq/11 activation must occur before these events. Indeed, detection of shear-induced Gαq/11 activation by in situ PLA places it right at or before these two relatively early signal transduction events. There is evidence that GPCRs can be directly activated by shear stress. For example, it has been shown that the activities of the Gαq/11-coupled GPCRs, bradykinin receptor B2 (B2R) and parathyroid hormone type 1 receptor are increased by shear stress (7, 47). However, in these particular studies, each GPCR was overexpressed in cells as a FRET sensor, and activation was inferred from conformational changes. Additionally, responses to shear stress were detected only after long times (e.g., 80 s) (7), which are far longer than it takes for shear stress to activate Gαq/11.
Direct activation of Gαq/11 with the G protein activator AlF4- induced Gαq/11 dissociation from S1P3. However, GRK2 and β-arrestin-1/2 were not recruited to S1P3, as AlF4- activates G proteins independently of GPCR activation. Unexpectedly, a decreased association of GRK2 with S1P3 was observed, which coincided with the dissociation of Gαq/11 from S1P3. This is likely due to the higher binding affinity that activated Gαq/11 has for GRK2 (6). Activated Gαq/11 may function to sequester GRK2, preventing it from triggering desensitization/internalization of S1P3 in the absence of its ligand.
Although S1P ligand stimulation and shear stress both appear to rapidly activate the S1P3/Gαq/11 complex in endothelial cells with similar temporal kinetics (within 0.5 min), our collective data support the notion that shear stress activates the complex through a mechanism that is distinct from the classical model of ligand-induced GPCR signaling. First, VPC23019, a selective S1P3 antagonist, dramatically inhibited S1P-induced S1P3/Gαq/11 dissociation, S1P3/GRK2 and S1P3/β-arrestin-1/2 associations, and ERK1/2 phosphorylation but had no effect on the shear stress-induced dissociation of Gαq/11 from S1P3. This indicates that shear stress activates S1P3-associated Gαq/11 without activation of S1P3. Second, S1P stimulation, but not shear stress exposure, induces a change in S1P3 localization from the cell surface to the cytoplasm, indicating that shear stress does not initiate S1P3 internalization upon EC activation. Finally, β-arrestin-1 is not dephosphorylated at S412 in response to shear stress as it is upon stimulation with S1P. Dephosphorylation of β-arrestin-1 occurs upon activation of a GPCR, as has been shown specifically for the follicle-stimulating hormone (FSH) receptor in Sertoli cells upon FSH stimulation (26). This suggests that shear stress does not activate GPCRs which utilize β-arrestin-1 for clathrin-mediated receptor internalization. Since β-arrestin-2 can also be dephosphorylated upon GPCR activation (23), one cannot exclude the possibility that shear stress activates GPCRs that utilize β-arrestin-2 for this purpose.
GRK2 and β-arrestin-1/2 showed increased associations with S1P3 with shear stress stimulation, but these associations were significantly delayed compared with those for ligand-induced stimulation, and there was no rapid return to baseline levels. This delayed phenomenon may in fact be due to autocrine signaling, as it has been previously shown that ECs can synthesize and secrete S1P in response to shear stress (40). Thus it is likely that the increased and more sustained associations of S1P3 with GRK2 and β-arrestin-1/2 that we observed at the later time points (5 and 10 min) in response to shear stress are due to autocrine S1P-induced activation of S1P3.
While our data demonstrate that shear stress does not directly activate S1P3, one cannot exclude the possibility that another unidentified Gαq/11-coupled GPCR, which is heterodimerized with S1P3, is directly activated. Recent reports describe cross-signaling in GPCR heteromers whereby ligand activation of one GPCR constituent leads to the G protein activation of another GPCR (28). The S1P1 receptor, which can also be induced by S1P binding, has previously been shown to be essential for both acute and chronic shear stress signaling in ECs (21). However, the “acute” signaling events referred to in that study, i.e., ERK, Akt, and eNOS activation, were all examined 10 min after flow onset, which suggests that the delayed GRK2 and β-arrestin-1/2 that we observed in the present study, are mediated by autocrine signaling rather than direct activation of S1P1.
It has been previously reported that cyclic mechanical stretch induces the activation of the GPCR angiotensin II type I receptor (AT1R), which requires β-arrestins and GRKs but is both ligand and G protein activation independent (32). While this may appear to contradict our findings, the differences may be attributed to the fact that cyclic stretch and shear stress are distinct mechanical forces with opposing biophysical mechanisms of mechanotransduction (44). In addition, each study was performed using a different cell system, with the present study looking specifically at endogenous activity in human coronary artery ECs as opposed to cells stably expressing the GPCR of interest.
Despite our findings here that shear stress induces activation of Gαq/11 independently of S1P3 activation, S1P3 may still play an important role in endothelial mechanosensing in response to temporal changes in shear stress. However, it is unlikely that S1P3 is the only GPCR involved in mechanotransduction. In fact, there are an increasing number of reports that claim the importance of GPCRs in shear stress-induced signaling, including B2R (7, 46), S1P1 (21), formyl peptide receptor (25), and AT1R (3). We speculate that a mechanosensitive complex that contains heterooligomerized GPCRs, which function as a scaffold and reservoir for G proteins, is localized at cell-cell junctions with PECAM-1 through interactions with HSPGs (11, 30, 46). By not functioning as receptors per se, these GPCRs need not be desensitized and recycled back to the cell surface by a β-arrestin-dependent mechanism in response to shear stress. The absence of any changes in S1P3 localization in response to shear stress implies that shear stress does not trigger S1P3 activation, desensitization, and/or internalization.
Our data not only demonstrate that shear stress causes the rapid dissociation of Gαq/11 from S1P3, but that it does so without the rapid association of GRK2 and β-arrestin-1/2, without the internalization of S1P3, without the global dephosphorylation of β-arrestin-1, and despite the presence of a S1P3 antagonist. Together, these findings indicate that shear stress can activate Gαq/11 and its downstream signaling in a manner that is distinct from its activation via agonist stimulation. We also show that in situ PLA can be a powerful tool for the general detection of endogenous GPCR/G protein activation, as supported by our findings on two different GPCR/G protein pairs, which may have future applications for the screening of lead compounds for the identification of novel agonists and/or antagonists targeting GPCR/G protein activation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute MERIT Award R37 HL040696.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.G.d.P., B.M., and J.A.F. conceived and designed research; N.G.d.P. and B.M. performed experiments; N.G.d.P. and B.M. analyzed data; N.G.d.P., B.M., and J.A.F. interpreted results of experiments; N.G.d.P. prepared figures; N.G.d.P. drafted manuscript; N.G.d.P. and J.A.F. edited and revised manuscript; N.G.d.P., B.M., and J.A.F. approved final version of manuscript.
REFERENCES
- 1.Aira Z, Buesa I, Gallego M, García del Caño G, Mendiable N, Mingo J, Rada D, Bilbao J, Zimmermann M, Azkue JJ. Time-dependent cross talk between spinal serotonin 5-HT2A receptor and mGluR1 subserves spinal hyperexcitability and neuropathic pain after nerve injury. J Neurosci 32: 13568–13581, 2012. doi: 10.1523/JNEUROSCI.1364-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baan B, Pardali E, ten Dijke P, van Dam H. In situ proximity ligation detection of c-Jun/AP-1 dimers reveals increased levels of c-Jun/Fra1 complexes in aggressive breast cancer cell lines in vitro and in vivo. Mol Cell Proteomics 9: 1982–1990, 2010. doi: 10.1074/mcp.M110.000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barauna VG, Magalhaes FC, Campos LC, Reis RI, Kunapuli SP, Costa-Neto CM, Miyakawa AA, Krieger JE. Shear stress-induced Ang II AT1 receptor activation: G-protein dependent and independent mechanisms. Biochem Biophys Res Commun 434: 647–652, 2013. doi: 10.1016/j.bbrc.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Borroto-Escuela DO, Craenenbroeck K, Romero-Fernandez W, Guidolin D, Woods AS, Rivera A, Haegeman G, Agnati LF, Tarakanov AO, Fuxe K. Dopamine D2 and D4 receptor heteromerization and its allosteric receptor-receptor interactions. Biochem Biophys Res Commun 404: 928–934, 2011. doi: 10.1016/j.bbrc.2010.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borroto-Escuela DO, Romero-Fernandez W, Garriga P, Ciruela F, Narvaez M, Tarakanov AO, Palkovits M, Agnati LF, Fuxe K. G protein-coupled receptor heterodimerization in the brain. Methods Enzymol 521: 281–294, 2013. doi: 10.1016/B978-0-12-391862-8.00015-6. [DOI] [PubMed] [Google Scholar]
- 6.Carman CV, Parent JL, Day PW, Pronin AN, Sternweis PM, Wedegaertner PB, Gilman AG, Benovic JL, Kozasa T. Selective regulation of Galpha(q/11) by an RGS domain in the G protein-coupled receptor kinase, GRK2. J Biol Chem 274: 34483–34492, 1999. doi: 10.1074/jbc.274.48.34483. [DOI] [PubMed] [Google Scholar]
- 7.Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA 103: 15463–15468, 2006. doi: 10.1073/pnas.0607224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clausson CM, Allalou A, Weibrecht I, Mahmoudi S, Farnebo M, Landegren U, Wählby C, Söderberg O. Increasing the dynamic range of in situ PLA. Nat Methods 8: 892–893, 2011. doi: 10.1038/nmeth.1743. [DOI] [PubMed] [Google Scholar]
- 9.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6: 16–26, 2009. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Koning W, van Dam K. A method for the determination of changes of glycolytic metabolites in yeast on a subsecond time scale using extraction at neutral pH. Anal Biochem 204: 118–123, 1992. doi: 10.1016/0003-2697(92)90149-2. [DOI] [PubMed] [Google Scholar]
- 11.dela Paz NG, Melchior B, Shayo FY, Frangos JA. Heparan sulfates mediate the interaction between platelet endothelial cell adhesion molecule-1 (PECAM-1) and the Gαq/11 subunits of heterotrimeric G proteins. J Biol Chem 289: 7413–7424, 2014. doi: 10.1074/jbc.M113.542514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher AM. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ Res 83: 334–341, 1998. doi: 10.1161/01.RES.83.3.334. [DOI] [PubMed] [Google Scholar]
- 13.Feng W, Gu YF, Nie L, Guo DY, Xiang LX, Shao JZ. Characterization of SIGIRR/IL-1R8 Homolog from Zebrafish Provides New Insights into Its Inhibitory Role in Hepatic Inflammation. J Immunol 197: 151–167, 2016. doi: 10.4049/jimmunol.1502334. [DOI] [PubMed] [Google Scholar]
- 14.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science 227: 1477–1479, 1985. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 15.Goldsmith ZG, Dhanasekaran DN. G protein regulation of MAPK networks. Oncogene 26: 3122–3142, 2007. doi: 10.1038/sj.onc.1210407. [DOI] [PubMed] [Google Scholar]
- 16.Gudi S, Huvar I, White CR, McKnight NL, Dusserre N, Boss GR, Frangos JA. Rapid activation of Ras by fluid flow is mediated by Galpha(q) and Gbetagamma subunits of heterotrimeric G proteins in human endothelial cells. Arterioscler Thromb Vasc Biol 23: 994–1000, 2003. doi: 10.1161/01.ATV.0000073314.51987.84. [DOI] [PubMed] [Google Scholar]
- 17.Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci USA 95: 2515–2519, 1998. doi: 10.1073/pnas.95.5.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gudi SR, Clark CB, Frangos JA. Fluid flow rapidly activates G proteins in human endothelial cells. Involvement of G proteins in mechanochemical signal transduction. Circ Res 79: 834–839, 1996. doi: 10.1161/01.RES.79.4.834. [DOI] [PubMed] [Google Scholar]
- 19.Harris GL, Creason MB, Brulte GB, Herr DR. In vitro and in vivo antagonism of a G protein-coupled receptor (S1P3) with a novel blocking monoclonal antibody. PLoS One 7: e35129, 2012. doi: 10.1371/journal.pone.0035129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Igarashi J, Bernier SG, Michel T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. Differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J Biol Chem 276: 12420–12426, 2001. doi: 10.1074/jbc.M008375200. [DOI] [PubMed] [Google Scholar]
- 21.Jung B, Obinata H, Galvani S, Mendelson K, Ding BS, Skoura A, Kinzel B, Brinkmann V, Rafii S, Evans T, Hla T. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev Cell 23: 600–610, 2012. doi: 10.1016/j.devcel.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Licht T, Tsirulnikov L, Reuveni H, Yarnitzky T, Ben-Sasson SA. Induction of pro-angiogenic signaling by a synthetic peptide derived from the second intracellular loop of S1P3 (EDG3). Blood 102: 2099–2107, 2003. doi: 10.1182/blood-2002-12-3634. [DOI] [PubMed] [Google Scholar]
- 23.Lin FT, Chen W, Shenoy S, Cong M, Exum ST, Lefkowitz RJ. Phosphorylation of beta-arrestin2 regulates its function in internalization of beta(2)-adrenergic receptors. Biochemistry 41: 10692–10699, 2002. doi: 10.1021/bi025705n. [DOI] [PubMed] [Google Scholar]
- 24.Lin FT, Krueger KM, Kendall HE, Daaka Y, Fredericks ZL, Pitcher JA, Lefkowitz RJ. Clathrin-mediated endocytosis of the beta-adrenergic receptor is regulated by phosphorylation/dephosphorylation of beta-arrestin1. J Biol Chem 272: 31051–31057, 1997. doi: 10.1074/jbc.272.49.31051. [DOI] [PubMed] [Google Scholar]
- 25.Makino A, Prossnitz ER, Bünemann M, Wang JM, Yao W, Schmid-Schönbein GW. G protein-coupled receptors serve as mechanosensors for fluid shear stress in neutrophils. Am J Physiol Cell Physiol 290: C1633–C1639, 2006. doi: 10.1152/ajpcell.00576.2005. [DOI] [PubMed] [Google Scholar]
- 26.Marion S, Kara E, Crepieux P, Piketty V, Martinat N, Guillou F, Reiter E. G protein-coupled receptor kinase 2 and beta-arrestins are recruited to FSH receptor in stimulated rat primary Sertoli cells. J Endocrinol 190: 341–350, 2006. doi: 10.1677/joe.1.06857. [DOI] [PubMed] [Google Scholar]
- 27.Melchior B, Frangos JA. Gαq/11-mediated intracellular calcium responses to retrograde flow in endothelial cells. Am J Physiol Cell Physiol 303: C467–C473, 2012. doi: 10.1152/ajpcell.00117.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreno JL, Miranda-Azpiazu P, García-Bea A, Younkin J, Cui M, Kozlenkov A, Ben-Ezra A, Voloudakis G, Fakira AK, Baki L, Ge Y, Georgakopoulos A, Morón JA, Milligan G, López-Giménez JF, Robakis NK, Logothetis DE, Meana JJ, González-Maeso J. Allosteric signaling through an mGlu2 and 5-HT2A heteromeric receptor complex and its potential contribution to schizophrenia. Sci Signal 9: ra5, 2016. doi: 10.1126/scisignal.aab0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni X, Epshtein Y, Chen W, Zhou T, Xie L, Garcia JGN, Jacobson JR. Interaction of integrin β4 with S1P receptors in S1P- and HGF-induced endothelial barrier enhancement. J Cell Biochem 115: 1187–1195, 2014. doi: 10.1002/jcb.24770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otte LA, Bell KS, Loufrani L, Yeh JC, Melchior B, Dao DN, Stevens HY, White CR, Frangos JA. Rapid changes in shear stress induce dissociation of a G alpha(q/11)-platelet endothelial cell adhesion molecule-1 complex. J Physiol 587: 2365–2373, 2009. doi: 10.1113/jphysiol.2009.172643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650, 2002. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 32.Rakesh K, Yoo B, Kim IM, Salazar N, Kim KS, Rockman HA. beta-Arrestin-biased agonism of the angiotensin receptor induced by mechanical stress. Sci Signal 3: ra46, 2010. doi: 10.1126/scisignal.2000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rishi G, Crampton EM, Wallace DF, Subramaniam VN. In situ proximity ligation assays indicate that hemochromatosis proteins Hfe and transferrin receptor 2 (Tfr2) do not interact. PLoS One 8: e77267, 2013. doi: 10.1371/journal.pone.0077267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson SD, Reynolds LE, Kostourou V, Reynolds AR, da Silva RG, Tavora B, Baker M, Marshall JF, Hodivala-Dilke KM. Alphav beta3 integrin limits the contribution of neuropilin-1 to vascular endothelial growth factor-induced angiogenesis. J Biol Chem 284: 33966–33981, 2009. doi: 10.1074/jbc.M109.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem 78: 743–768, 2009. doi: 10.1146/annurev.biochem.78.072407.103733. [DOI] [PubMed] [Google Scholar]
- 36.Ryu JM, Baek YB, Shin MS, Park JH, Park SH, Lee JH, Han HJ. Sphingosine-1-phosphate-induced Flk-1 transactivation stimulates mouse embryonic stem cell proliferation through S1P1/S1P3-dependent β-arrestin/c-Src pathways. Stem Cell Res (Amst) 12: 69–85, 2014. doi: 10.1016/j.scr.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4: 397–407, 2003. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 38.Storch U, Schnitzler MM, Gudermann T. G protein-mediated stretch reception. Am J Physiol Heart Circ Physiol 302: H1241–H1249, 2012. doi: 10.1152/ajpheart.00818.2011. [DOI] [PubMed] [Google Scholar]
- 39.Thymiakou E, Episkopou V. Detection of signaling effector-complexes downstream of bmp4 using PLA, a proximity ligation assay. J Vis Exp 3: 49, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res 102: 669–676, 2008. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vilardaga JP, Bünemann M, Krasel C, Castro M, Lohse MJ. Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat Biotechnol 21: 807–812, 2003. doi: 10.1038/nbt838. [DOI] [PubMed] [Google Scholar]
- 42.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci 28: 416–422, 2007. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Wu J, Spiegel S, Sturgill TW. Sphingosine 1-phosphate rapidly activates the mitogen-activated protein kinase pathway by a G protein-dependent mechanism. J Biol Chem 270: 11484–11488, 1995. doi: 10.1074/jbc.270.19.11484. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto K, Ando J. Vascular endothelial cell membranes differentiate between stretch and shear stress through transitions in their lipid phases. Am J Physiol Heart Circ Physiol 309: H1178–H1185, 2015. doi: 10.1152/ajpheart.00241.2015. [DOI] [PubMed] [Google Scholar]
- 45.Yamazaki T, Yoshimatsu Y, Morishita Y, Miyazono K, Watabe T. COUP-TFII regulates the functions of Prox1 in lymphatic endothelial cells through direct interaction. Genes Cells 14: 425–434, 2009. doi: 10.1111/j.1365-2443.2008.01279.x. [DOI] [PubMed] [Google Scholar]
- 46.Yeh JC, Otte LA, Frangos JA. Regulation of G protein-coupled receptor activities by the platelet-endothelial cell adhesion molecule, PECAM-1. Biochemistry 47: 9029–9039, 2008. doi: 10.1021/bi8003846. [DOI] [PubMed] [Google Scholar]
- 47.Zhang YL, Frangos JA, Chachisvilis M. Mechanical stimulus alters conformation of type 1 parathyroid hormone receptor in bone cells. Am J Physiol Cell Physiol 296: C1391–C1399, 2009. doi: 10.1152/ajpcell.00549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol 6: 499–506, 2004. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]