Abstract
In the healthy lung the opportunistic pathogen, Pseudomonas aeruginosa, is rapidly eliminated by mucociliary clearance, a process that is dependent on the activity of the CFTR anion channel that, in concert with a number of other transport proteins, regulates the volume and composition of the periciliary surface liquid. This fluid layer is essential to enable cilia to clear pathogens from the lungs. However, in cystic fibrosis (CF), mutations in the CFTR gene reduce Cl− and secretion, thereby decreasing periciliary surface liquid volume and mucociliary clearance of bacteria. In CF this leads to persistent infection with the opportunistic pathogen, P. aeruginosa, which is the cause of reduced lung function and death in ~95% of CF patients. Others and we have conducted studies to elucidate the effects of P. aeruginosa on wild-type and Phe508del-CFTR Cl− secretion as well as on the host immune response. These studies have demonstrated that Cif (CFTR inhibitory factor), a virulence factor secreted by P. aeruginosa, is associated with reduced lung function in CF and induces the ubiquitination and degradation of wt-CFTR as well as TAP1, which plays a key role in viral and bacterial antigen presentation. Cif also enhances the degradation of Phe508del-CFTR that has been rescued by ORKAMBI, a drug approved for CF patients homozygous for the Phe508del-CFTR mutation, thereby reducing drug efficacy. This review is based on the Hans Ussing Distinguished Lecture at the 2016 Experimental Biology Meeting given by the author.
Keywords: Pseudomonas aeruginosa, outer membrane vesicles, CFTR, cystic fibrosis, ORKAMBI
Tribute to Professor Ussing
The development of the “short circuit” technique to measure electrogenic sodium (Na+) transport across frog skin, published in 1951 by Professor Hans Ussing, led to the first comprehensive and mechanistic understanding of Na+ transport across frog skin (39, 81). This approach to measure electrogenic Na+ as well as chloride (Cl−) transport across epithelial cell monolayers is highly relevant 66 years after its development. It has enabled numerous investigators to elucidate the ion transport properties of a number of epithelia, including epithelial cells in the lung in health and disease including cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD). Two excellent and comprehensive reviews of Professor Ussing’s career and the development of the short circuit current technique have been published (44, 45).
Introduction
This article will first review what is currently known about electrogenic Na+ and anion (Cl− and ) transport by airway epithelial cells, and how salt and water transport plays an essential role in mucociliary clearance of bacteria from the lungs. This is followed by a discussion of how mutations in the CFTR anion channel cause CF, which leads to chronic, unremitting colonization of the lungs by bacteria, the major cause of reduced lung function in CF patients. Subsequent sections of this review will focus on studies describing how the virulence factor Cif (CFTR inhibitory factor), secreted by Pseudomonas aeruginosa, the dominant pathogen in late stage CF, inhibits wild-type and ORKAMBI-rescued Phe508del-CFTR anion secretion, reduces bacterial and viral antigen presentation, a key component of the adaptive immune response, and inhibits the generation of host proresolving lipids.1
Overview of Cl− and Secretion by Airway Epithelial Cells: Role of wt-CFTR
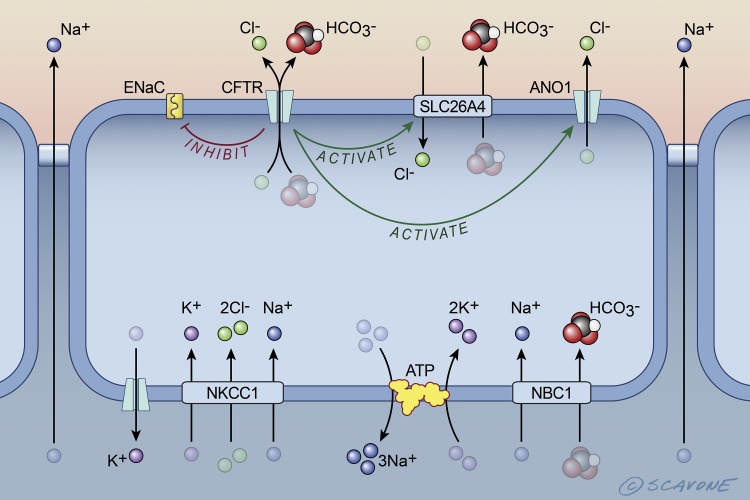
Ussing chamber and patch-clamp studies have demonstrated that Cl− and secretion by wt-CFTR plays an essential role in the secretion of isotonic fluid onto the airway surface, which is essential for the mucociliary clearance from the lungs of inhaled bacterial and other pathogenic organisms as well as environmental contaminants (8, 21, 22, 32, 34, 46, 61, 69, 72, 73). A model of the transport proteins that mediate isotonic fluid secretion by airway epithelia cells is depicted in Fig. 1. Cl− enters airway epithelial cells across the basolateral membrane via the NKCC1 transporter that has a stoichiometry of 1K+/2Cl−/1Na+. The K+ that enters the cell via NKCC1 recycles across the basolateral membrane into blood via a K+ channel. The Na+ that enters the cell via NKCC1 also recycles across the basolateral membrane back into the blood via the Na+-K+-ATPase. Cl− exits the cell across the apical membrane via the CFTR anion channel, as well as anoctamine 1 (ANO1, also called TMEM16A) a calcium-activated Cl− channel. Cl− secretion by CFTR and ANO1 generates an apical-negative transepithelial voltage that provides the driving force for the paracellular diffusion of Na+ across the tight junctions between cells. Although the epithelial Na+ channel, ENaC, is present in the apical membrane, several studies, but not all, have shown that CFTR suppresses ENaC activity, such that normally little Na+ enters the cell across the apical membrane (21, 34, 67).
Fig. 1.
Cell model of the transport proteins in airway epithelial cells that mediate NaCl and water secretion into the periciliary layer in cells expressing wt-CFTR (cystic fibrosis transmembrane conductance regulator). wt-CFTR activates SLC26A4 (Cl−/ exchanger, also called pendrin) and ANO1 (anoctamine 1, also called TMEM16A) and inhibits ENaC (epithelial Na+ channel). NKCC1 (Na+-K+-2 Cl− cotransporter, also known as SLC12A2), ATP (Na+-K+-ATPase), and NBC1 (Na+- cotransporter, also known as SLC4A2) are located in the basolateral membrane. Na+ diffuses across the tight junctions down an electrochemical gradient into the pericilary space. Water is secreted into the periciliary space across the tight junctions and across cells via aquaporin water channels (not shown), due to the osmotic gradient across epithelial cells established by NaCl secretion into the periciliary fluid. [Printed with permission from William Scavone.]
Airway epithelial cells also secrete (Fig. 1). NBC1 (SLC4A2), a Na+- cotransporter, mediates uptake across the basolateral membrane. The that enters the cell is secreted across the apical membrane by CFTR and SLC26A4 (pendrin), a Cl−/ exchanger (34, 69). The Na+ that enters the cell across the basolateral membrane with is recycled across the membrane by the Na+-K+-ATPase. Some Cl− that is secreted by CFTR recycles across the apical membrane back into the cells to drive secretion by SLC26A4. secretion by CFTR and SLC26A4 plays a key role in establishing a pH of the periciliary fluid of ~7.4, a value that is important for the optimal antimicrobial properties of the periciliary fluid (34, 60, 69).
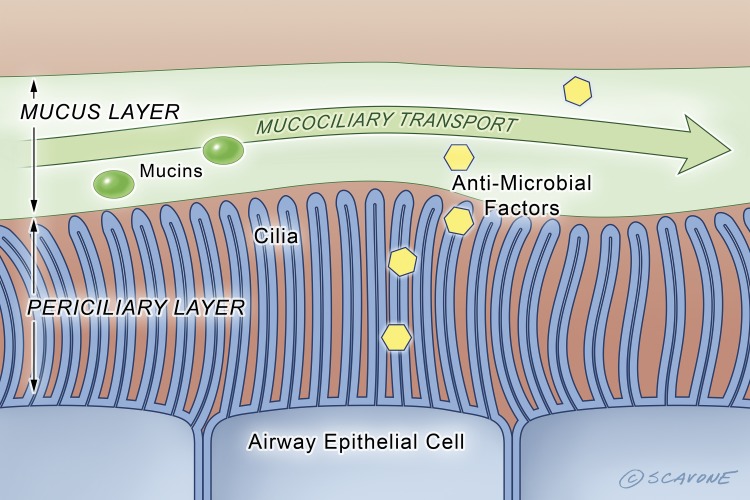
The secretion of NaCl and NaHCO3 into the airway surface establishes a small osmotic gradient that drives the paracellular and transcellular movement of water into the airway surface via aquaporin water channels, which hydrates the overlying mucus, thereby decreasing its viscosity. In the presence of wt-CFTR the transport processes depicted in Fig. 1 maintain the periciliary fluid depth at ~7 μm, which is the length of the cilia, and is the optimal depth to allow cilia to beat (52). This clears bacteria from the airways that have been trapped in the mucus overlying the periciliary fluid (Fig. 2).
Fig. 2.
In the non-CF airway, wt-CFTR mediates anion secretion that results in a periciliary layer that is ~7 μm deep, which allows cilia to beat and clear bacteria and environmental contaminants from the lungs (i.e., mucociliary transport). The yellow hexagons represent antimicrobial factors secreted by epithelial cells. The green circles represent mucins. [Printed with permission from William Scavone.]
Mutations in the CFTR Gene Cause Cystic Fibrosis
Mutations in the CFTR gene cause CF, the most common fatal genetic disease in Caucasians (34, 69). Worldwide, ~80,000 people have CF and ~95% die from respiratory failure subsequent to chronic bacterial infections of the lungs at a mean age of 38 years (24, 26, 75). However, recent studies in the United States based on a 10-year period from 2000 to 2010 have shown that survival has improved by 1.8%/year due to advances in treatment (49). If this rate continues it would predict a mean age of survival for children born in 2016 at 56 years (49).
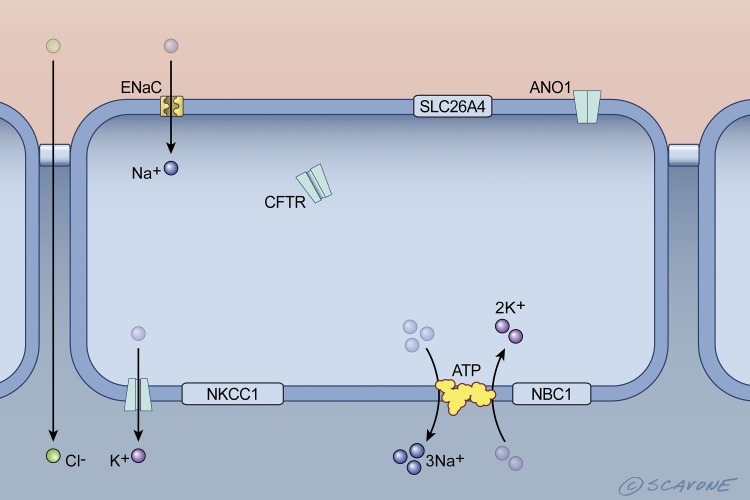
Mutations in the CFTR gene reduce the abundance of functional CFTR anion channels in the apical membrane of airway epithelial cells and submucosal glands, leading to a dramatic reduction in Cl− and secretion, a decrease in periciliary depth and pH, and an increase in the amount of mucus secreted by submucosal glands, resulting in a layer of thick mucus that sticks to cilia, inducing ciliastasis and an inability to clear bacteria from the airways (52)(Figs. 3 and 4). At the time this review was written, 2,008 mutations had been identified in the CFTR gene (http://www.genet.sickkids.on.ca/StatisticsPage.html). However, only ~150 mutations are known to cause CF, and these mutations have been divided into six classes based on the effects that the mutations have on CFTR location and function (23, 25, 26, 63, 75). Class I mutations lead to an absence of CFTR protein due to lack of mRNA transcripts and stop-codon mutations that lead to degradation of mRNA. Recently, it has been suggested that class I mutations can be subdivided into two categories based on the effect on CFTR mRNA (24, 25, 51, 76). Class II mutations, the most common (e.g., Phe508del), lead to premature degradation or incomplete maturation of CFTR such that the channel is degraded in the proteasome. As a result it does not reach the apical plasma membrane, resulting in a dramatic reduction of CFTR-mediated Cl− and secretion. The Phe508del mutation also reduces channel activity and the residence time of chemically rescued CFTR in the apical plasma membrane (82, 83). Approximately fifty percent of CF patients are homozygous for the Phe508del mutation. Class III mutations lead to defects in CFTR channel regulation and gating. The most common mutation in this category is Gly551Asp, which is present in ~4–5% of CF patients. Although Gly551Asp-CFTR is present in the apical membrane of airway epithelial cells, it does not secrete anions. Class IV mutations, present in 1–2% of CF patients, lead to defective Cl− and conductance of CFTR. Class V mutations cause a major reduction in the levels of functionally normal CFTR protein due to mutations in the promoter region of CFTR or abnormal mRNA splicing. Finally, Class VI mutations reduce the stability, and therefore the amount, of CFTR in the apical plasma membrane, thereby reducing the amount of CFTR-mediated Cl− and secretion.
Fig. 3.
Cell model of the transport proteins in CF airway epithelial cells. Mutations in CFTR, in particular Phe508del, cause the retention of Phe508del-CFTR in the endoplasmic reticulum, and its degradation in the proteasome (this is indicated by CFTR inside the cell and its absence in the apical plasma membrane). Because CFTR, which inactivates ENaC, is not present in the apical plasma membrane, ENaC is active and mediates Na+ reabsorption. Moreover, the absence of CFTR, which activates ANO1 and SLC26A4, results in inactivation of ANO1 and SLC26A4. This is illustrated by the lack of ion movement across the apical plasma membrane via these transporters. NKCC1 and NBC1 do not contribute to NaCl reabsorption. ATP, Na+-K+-ATPase. [Printed with permission from William Scavone.]
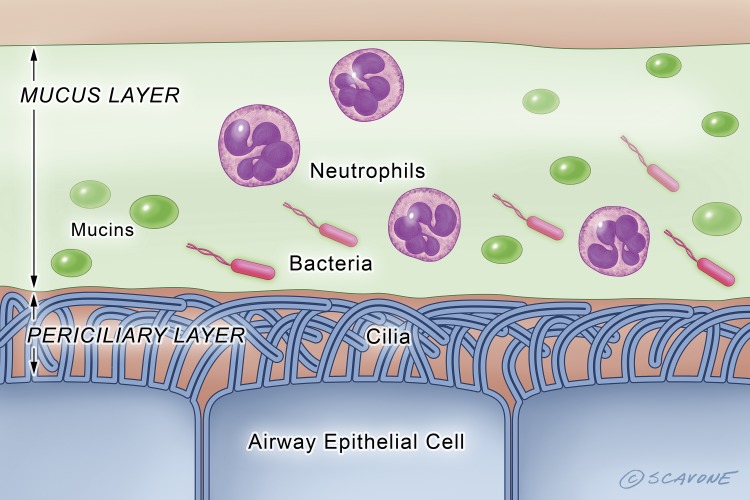
Fig. 4.
In the CF airway the lack of CFTR anion secretion results in a dramatic reduction in the depth of the periciliary fluid layer, which reduces the ability of cilia to beat and clear bacteria, viruses, and environmental contaminants from the lungs (i.e., mucociliary transport). Moreover, increased mucin secretion and the presence of DNA in mucus due to lysis of neutrophils increases mucus viscosity, which also decreases mucociliary transport. The green circles represent mucins. The red oblongs with tails (flagella) represent P. aeruginosa. [Printed with permission from William Scavone.]
In addition to a progressive decline in lung function, assessed by measuring FEV1 (percentage of the predicted value for the forced expiratory volume in 1 s), CF is also characterized by exocrine pancreatic insufficiency resulting in diabetes, gastrointestinal malabsorption that results in malnutrition and impaired growth, sinusitis, and male infertility due to a congenital bilateral malformation of the vas deferens (63). Mutations in CFTR also severely reduce the ability of neutrophils and macrophages to kill bacteria and dramatically increase the levels of proinflammatory cytokines in the lungs (63).
Although CF is caused by mutations in the CFTR gene, there is considerable phenotypic variation even among individuals with the same mutation (63). Although some of the variation has been attributed to modifier genes, including SLC26A9, SLC9A3, SLC6A14, TNF, and TGFβ1, environmental factors including second-hand smoke, pollutants, and infectious agents such as bacteria, fungi, and viruses also influence disease severity (30, 63).
Drug Discovery and Cystic Fibrosis: KALYDECO and ORKAMBI
In 2012, KALYDECO (Ivacaftor, VX-770) was approved by the FDA for CF patients who have at least one Gly551Asp allele, and more recently was approved for other mutations in class III (2, 62, 82, 83). KALYDECO increases the open probability of Gly551Asp-CFTR channels and increases Cl− and secretion. KALYDECO also decreases mucus viscosity (9). Importantly, KALYDECO in the short term also reduces the number of Gly551Asp-CFTR patients who are culture positive for P. aeruginosa and Aspergillus, but not Staphylococcus aureus or other common CF pathogens (35, 66). In 2015 the FDA approved ORKAMBI, a combination of Ivacaftor (VX-770) and Lumicaftor (VX-809), which increases the export of misfolded Phe508del-CFTR from the endoplasmic reticulum to the apical plasma membrane, for patients homozygous for the Phe508del mutation (82, 83, 85). Although ORKAMBI does not reduce the number of patients infected with P. aeruginosa and only increases FEV1 by 2.6 to 4.0%, it reduces the rate of pulmonary exacerbations by 30–39% (85). It has been suggested that the modest effect of ORKAMBI on FEV1 is due to an inhibitory effect of Ivacaftor (VX-770) on the ability of Lumicaftor (VX-809) to increase plasma membrane Phe508del-CFTR (20, 77, 84). However, a recent study suggests that the inhibitory effect on Lumicaftor only occurs at levels of Ivacaftor above pharmacologically relevant levels (53).
Chronic Lung Infections in Cystic Fibrosis
The lungs of individuals with CF become chronically colonized with bacteria early in life, in part due to decreased mucociliary clearance and increased mucus viscosity due to periciliary dehydration, the release of DNA by neutrophils, acidification of the periciliary layer that reduces the activity of antimicrobial peptides secreted by airway epithelial cells, and defects in the ability of macrophages and neutrophils to clear pathogens (7, 34, 60, 63) (Fig. 4). Although there is considerable variability among CF patients regarding the composition of the lung microbiome, the most common pathogens include P. aeruginosa, Staphylococcus aureus, and Aspergillus species. Respiratory exacerbations caused by P. aeruginosa are associated with an elevated inflammatory response in CF that leads to precipitous declines in FEV1, which often does not recover following hospitalization and antibiotic treatment (7, 63). In addition, methicillin-resistant S. aureus (MRSA) lung infections are present in as many as 30% of CF patients in some populations. Acute viral infections also induce pulmonary exacerbations and decrease FEV1 (7, 63).
A variety of virulence factors secreted by P. aeruginosa have adverse effects on mucociliary clearance. Rhamnolipids promote ciliastasis (64), and alginate increases mucus production, thereby reducing immune recognition and mucociliary clearance of bacteria (7). Ciliary beating and mucociliary transport are also reduced by pyocyanin, which also decreases CFTR Cl− secretion (71). Alkaline proteases also contribute to lung infection by proteolytically activating ENaC, which increases Na+ absorption, thereby reducing periciliary fluid volume and mucociliary clearance (18, 19).
P. aeruginosa Inhibits CFTR Cl− Secretion and Mucociliary Clearance
In a series of studies, others and we have demonstrated that P. aeruginosa (PA14 and six clinical isolates, including three mucoid and three nonmucoid isolates) reduces both wt-CFTR and ORKAMBI-rescued Phe508del-CFTR Cl− secretion by airway epithelial cells (3, 6, 16, 48, 68, 77, 79, 80). This work began several years ago when we initiated a series of studies examining the effect of P. aeruginosa on CFTR Cl− secretion by human airway epithelial cells. The initial hypothesis was that P. aeruginosa would stimulate wt-CFTR Cl− secretion and thereby enhance NaCl and fluid secretion and promote mucociliary clearance of bacteria to protect the lungs from infection. Intuitively, this hypothesis made sense from a homeostasis perspective. However, to our surprise the opposite was observed. Addition of P. aeruginosa to the apical side of a variety of polarized airway epithelial cells lines as well as primary airway epithelia cells revealed that P. aeruginosa dramatically reduced wt-CFTR Cl− secretion (3, 6, 16, 48, 77, 79). In addition, P. aeruginosa also inhibited ORKAMBI (VX-809+VX-770)-rescued Phe508del-CFTR Cl− secretion (3, 6, 16, 48, 77, 79).
By contrast, P. aeruginosa (PAK) has been shown to stimulate fluid secretion by pig submucosal glands (47). The discrepancy between this observation and those by others and us may reflect a difference in the pig versus human, a difference in the effects of P. aeruginosa on surface epithelial cells versus submucosal glands and/or a unique feature of PAK, a laboratory strain of Pseudomonas. Since LPS, homoserine lactone, and flagellin stimulate CFTR Cl− secretion whereas phospholipase C (PLcH), sphingomyelinases, and β-lactamase inhibit CFTR Cl− secretion by airway epithelia cells, it is possible that the effect of variants of P. aeruginosa on CFTR Cl− secretion may also depend on the relative abundance of factors that stimulate versus those that inhibit CFTR Cl− secretion (6, 15–17, 36, 77).
The inhibition of wt-CFTR and ORKAMBI-rescued Phe508del-CFTR Cl− secretion by P. aeruginosa is mediated by outer membrane vesicles (OMVs) (6, 15, 16, 77). P. aeruginosa resides primarily in the mucus layer, and secreted OMVs diffuse through the mucus layer and fuse with lipid rafts in the apical membrane of airway epithelial cells. OMVs, spheroid proteoliposomes 10 to 300 nm in diameter secreted by Gram-negative bacteria, including P. aeruginosa, contain a number of toxins and virulence factors and deliver virulence factors directly into host cell cytoplasm by fusion with lipid rafts in the plasma membrane (17, 36, 43). Others and we have shown that OMVs secreted by P. aeruginosa contain alkaline phosphatase, hemolytic phospholipase C (PLcH), sphingomyelinases (SMAse), and β-lactamase (6, 15–17, 36, 77). SMAse and PlcH hydrolyze sphingomyelin, which reduces the phosphorylation of the regulatory domain of CFTR, thereby decreasing Cl− secretion. P. aeruginosa packages β-lactamase into the lumen of OMVs, which degrades β-lactam antibiotics including tobramycin, the most commonly prescribed antibiotic for CF patients, and thereby increases antibiotic resistance of P. aeruginosa (42).
By Western blotting of P. aeruginosa OMVs and a mass spectrometry, proteomic approach, we demonstrated that P. aeruginosa OMVs also contain a protein, PA2934, which codes for a novel 29-kDa epoxide hydrolase that enhances the ubiquitination and degradation of wt-CFTR (3, 5, 6, 13, 15, 16, 48, 77, 79, 87). Accordingly, we named PA2934 Cif, for CFTR inhibitory factor. Cif reduces wt-CFTR Cl− secretion in a variety of airway epithelial cell lines and primary airway epithelial cells (3, 6, 15, 16). Importantly, Cif is expressed in both mucoid and nonmucoid clinical isolates of P. aeruginosa obtained from CF lungs, and Cif homologs are expressed in Acinetobacter nosocomialis and Burkholderia cepacia that also infect CF lungs (4). Moreover, Cif also enhances the ubiquitination and degradation of Phe508del-CFTR (68). These observations led us to the conclusion that Cif, as well as the other virulence factors noted above, could facilitate the colonization of the CF lung by P. aeruginosa by reducing ORKAMBI-stimulated Phe508del-CFTR abundance and activity, thereby reducing mucociliary clearance of bacteria. If so, Cif may play a major role in reducing the efficacy of ORKAMBI, resulting in the inability to reduce the P. aeruginosa load in the CF lung (85).
Cellular Mechanism of Cif Inhibition of CFTR Cl− Secretion
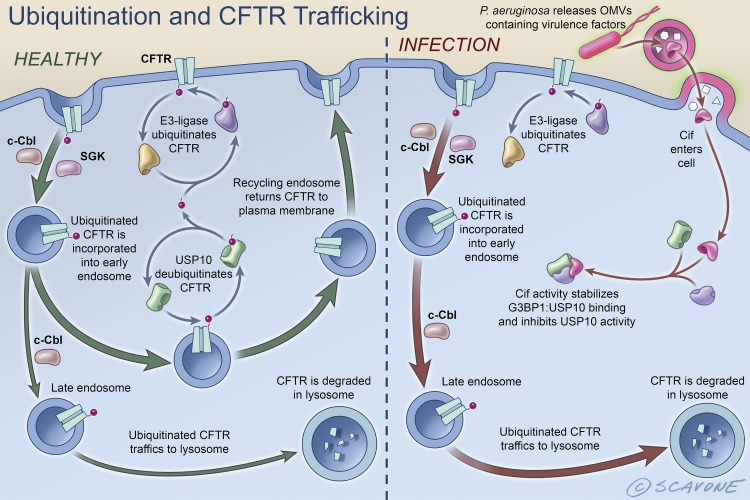
The primary mechanism of action of Cif is that it disrupts the endocytic trafficking of CFTR and thereby reduces the amount of CFTR in the apical plasma membrane (1, 3, 5–7, 10, 15, 16, 28, 38, 40, 48, 50, 78, 86, 87)(Fig. 5). In noninfected airway epithelial cells, CFTR in the apical plasma membrane is endocytosed from the plasma membrane by a process that is regulated by c-Cbl (named after casitas B-lineage lymphoma), SGK1 (serum glucocorticoid kinase 1), and a variety of other adaptor proteins (11, 27, 29, 50, 70, 74, 87). Whereas SGK1 inhibits endocytosis of CFTR (11), c-Cbl facilitates CFTR endocytosis by two mechanisms (12, 29, 87). First, c-Cbl is an adaptor protein that facilitates CFTR endocytosis by a ubiquitin-independent mechanism, and second, c-Cbl ubiquitinates CFTR in early endosomes thereby facilitating its lysosomal degradation (12, 87). If CFTR in endosomes is deubiquitinated by the deubiquitinating enzyme, USP10 (ubiquitin-specific peptidase 10), CFTR returns to the apical cell membrane in recycling endosomes, thereby maintaining CFTR anion secretion (12, 16). By contrast, if CFTR is not deubiquitinated by USP10, it is not recycled back to the apical membrane and is delivered to the lysosome for degradation, resulting in a reduction in CFTR abundance and thus, anion secretion. During infection with P. aeruginosa, secreted OMVs fuse with the apical cell membrane of airway epithelia cells and deliver the virulence factors described above, as well as Cif, into the cytoplasm of airway epithelial cells where Cif’s activity stabilizes G3BP1:USP10 binding, thereby inhibiting USP10 activity (12, 13, 16). This in turn decreases the USP10-mediated deubiquitination of CFTR and increases the degradation of ubiquitinated CFTR in lysosomes (12, 13, 16).
Fig. 5.
Schematic representation of the intracellular trafficking of wt-CFTR in healthy, noninfected airway epithelial cells (left) and in airway epithelial cells infected with P. aeruginosa (right). In noninfected cells, CFTR is ubiquitinated and removed from the plasma membrane by endocytosis by a process that is regulated by c-Cbl and SGK1. Whereas SGK1 inhibits the endocytosis of CFTR, c-Cbl increases the endocytosis of CFTR. If deubiquitinated by USP10, CFTR recycles back to the apical cell membrane. By contrast, if CFTR is not deubiquitinated, it is delivered to the lysosome for degradation. During infections with P. aeruginosa, OMVs secreted by the bacterium fuse with lipid rafts in the apical cell membrane of airway epithelia cells and deliver Cif to the cytoplasm, where Cif activity stabilizes G3BP1:USP10 interaction. This inhibits USP10 activity and reduces USP10-mediated deubiquitination of CFTR. This increases the degradation of CFTR in lysosomes and reduces the amount of CFTR in the apical plasma membrane. [Printed with permission from William Scavone.]
P. aeruginosa Reduces Viral Antigen Presentation by MHC Class I
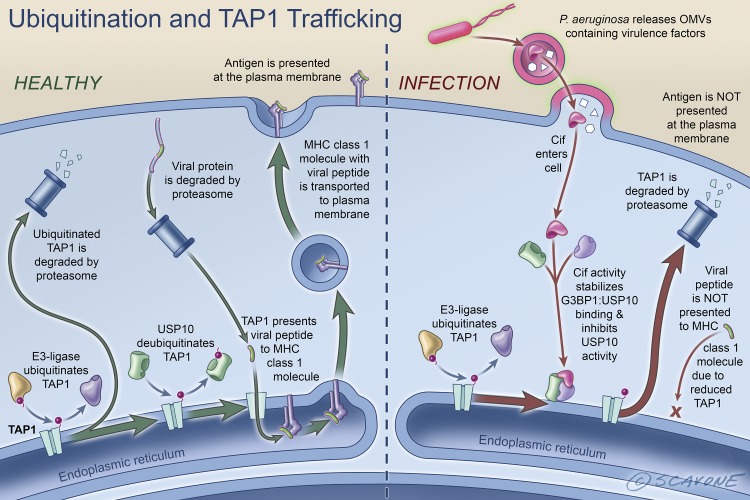
Cif also has a negative effect on bacterial and viral antigen presentation by airway epithelial cells (13, 33) (Fig. 6). TAP1, like CFTR, is a member of the ABC transporter family. The TAP1/TAP2 complex transports antigens across the membrane of the endoplasmic reticulum where they combine with MHC class I, whereupon the antigen-loaded MHC class I complex is transported to the plasma membrane where the complex is recognized by CD8+ T lymphocytes, the activation of which is a key component of the adaptive immune response (13, 33). Like CFTR, TAP1 is ubiquitinated by an E3-ligase and deubiquitinated by USP10. When the lungs are infected with P. aeruginosa, Cif activity stabilizes G3BP1:USP10 binding and inhibits the deubiquitinating activity of USP10, thereby increasing the amount of polyubiquitinated TAP1, which is degraded in the proteasome. The reduction in TAP1 abundance decreases antigen translocation into the endoplasmic reticulum, an effect that reduces antigen available to MHC class I molecules for presentation at the plasma membrane of airway epithelial cells and recognition by CD8+ T lymphocytes. Cif is the first bacterial factor identified that inhibits TAP function and MHC class I antigen presentation (13).
Fig. 6.
Schematic representation of antigen presentation by MHC class I in airway epithelial cells and the effect of Cif on antigen presentation. Left: viral and bacterial proteins in the cytoplasm of airway epithelial cells are degraded in the lysosome and transported into the lumen of the endoplasmic reticulum by the TAP1/TAP2 complex whereupon antigen is presented to MHC class I, which transports the antigen from the endoplasmic reticulum to the plasma membrane. The antigen is recognized by CD8+ T lymphocytes, an important component of the adaptive immune response to infection. When the lungs are infected with P. aeruginosa, secreted OMVs fuse with epithelial cells and deliver Cif into the cytoplasm (right side of figure). Cif activity stabilizes G3BP1:USP10 interaction. This inhibits USP10 activity and reduces USP10-mediated deubiquitination of TAP1. This results in increased degradation of ubiquitinated TAP1 in the proteasome, thereby reducing the ability of the TAP1/TAP2 complex to transport antigen into the lumen of the endoplasmic reticulum for antigen presentation by MHC class I. This effect of Cif attenuates the adaptive immune response to infection by P. aeruginosa. [Printed with permission from William Scavone.]
Cif inhibits Host Proresolving Lipid Mediators
A recent study has identified an unexpected effect of Cif (28). Cif promotes the hydrolysis of airway epithelial cell-derived 14,15-epoxyeicosatrienoic acid, thereby disrupting the production of the proresolving lipid 15-epi lipoxin A4, which suppresses IL-8-stimulated transmigration of neutrophils. Thus, Cif promotes excessive transmigration into the lungs of neutrophils, which release elastase and DNA, the proximate cause of lung damage and reduced lung function in CF (63). Importantly, it was also shown that levels of Cif in brochoalveolar lavage fluid obtained from CF patients correlated with reduced levels of 15-epi lipoxin A4, elevated IL-8, and reduced lung function (28). These exciting observations suggest that inhibition of Cif activity with a small molecule may be an effective therapy to reduce excessive inflammation and neutrophil transmigration in the CF lungs, and will also block the adverse effects of Cif to reduce ORKAMBI-stimulated Phe508del-CFTR anion secretion (3, 28, 38, 77).
Mutations in the CFTR Gene and Viral Infection Enhance the Formation of Antibiotic Resistant P. aeruginosa Biofilms by Increasing Iron Availability
In addition to Cif, several other factors enhance the ability of P. aeruginosa to chronically infect the CF lung. In CF, increased levels of iron in the airway surface fluid correlates with the frequency of bacterial exacerbations; thus, increased iron in the airway surface liquid has been proposed to play a role in the development and maintenance of chronic antibiotic resistant bacterial lung infections in CF (31, 65). Several years ago we developed a coculture model of P. aeruginosa growing on the apical side of polarized CF human airway epithelial cells to elucidate the mechanism(s) whereby the Phe508del mutation in CFTR enhances the formation of antibiotic resistance biofilms (14, 55–59). We observed that CF airway cells, compared with cells expressing wt-CFTR, enhance the development of tobramycin-resistant P. aeruginosa biofilms, in part, by releasing iron into the apical fluid. Chelation of iron reduced biofilm formation on airway cells, whereas iron supplementation enhanced the formation of P. aeruginosa biofilms (14, 55–59).
Because pulmonary exacerbations caused by viral infections in CF are associated with increased severity of bacterial infections, a recent study was conducted to elucidate the mechanism whereby viral infection increases bacterial infection by P. aeruginosa (37). Infection of airway epithelial cells with respiratory syncytial virus (RSV) increased biofilm formation via an increase in antiviral interferon (IFN) secretion, which stimulates the release of the iron-binding protein transferrin in to the airway surface fluid. Addition of an iron chelator blocked the effect of RSV on the formation of antibiotic-resistant P. aeruginosa biofilms, and addition of excess iron reversed the effect of the chelator. The effect of RSV on biofilm formation by P. aeruginosa was also observed in a mouse lung infection model (37, 54). Thus, in CF, disruption of iron homeostasis and viral stimulated iron secretion into the periciliary fluid enhances the development of antibiotic-resistant biofilms of P. aeruginosa.
OMVs Contain sRNAs That Mitigate the Immune Response to P. aeruginosa, Thereby Allowing P. aeruginosa to Thrive
P. aeruginosa also enhances its ability to chronically infect CF lungs by suppressing the innate immune response to infection (41). Several recent studies have shown that OMVs contain short interfering RNAs that have the potential to target host mRNA function and/or stability (41). To examine the role of sRNAs in OMVs secreted by P. aeruginosa, we used RNA-Seq to identify sRNAs in OMVs, and we observed that many sRNAs are selectively packaged in OMVs and that OMVs deliver sRNAs into the cytoplasm of airway epithelial cells (41). One sRNA (sRNA52320) was abundant in OMVs and reduced LPS-mediated activation of the MAPK pathway and IL-8 secretion by primary human airway epithelial cells. Deletion of sRNA52320 enhanced both LPS and OMV-induced IL-8 secretion. sRNA52320 also reduced OMV-stimulated secretion of KC (the mouse analog of IL-8) and neutrophil infiltration in mouse lung. Taken together, these findings demonstrate that P. aeruginosa secretes OMVs that deliver sRNAs into lung epithelial cells to reduce the host innate immune response, and thereby reduces the ability of the host to reduce and/or eliminate P. aeruginosa from the lungs.
Future Prospects
This review has described a number of approaches that are utilized by P. aeruginosa to establish and maintain a chronic infection in the CF lungs. These include secretion of a number of virulence factors that have adverse effects on CFTR function, including Cif packaged in OMVs that reduces ORKAMBI stimulated Phe508del-CFTR Cl− and secretion. Cif also suppresses MHC class I bacterial and viral antigen presentation, a key component of the adaptive immune response. In addition, Cif promotes the hydrolysis of airway epithelial cell-derived 14,15-epoxyeicosatrienoic acid, thereby disrupting the production of the proresolving lipid 15-epi lipoxin A4. This results in excessive neutrophil migration into the CF lungs and release of DNA, which increases mucus viscosity, and elastase that causes lung damage. Moreover, P. aeruginosa also secretes OMVs containing sRNA52320, which suppresses the innate immune response to bacterial infection. Thus, identification of novel strategies to block the fusion of OMVs with lipid drafts in host cells to block the delivery of Cif into CF epithelial cells, and/or a small molecule to inhibit Cif activity, are likely to lead to an increase in the efficacy of ORKAMBI and a dramatic improvement in lung function in CF.
GRANTS
The research described by the author in this article was supported by the National Institutes of Health (R01 HL074175 and P30 GM106394), a Cystic Fibrosis Foundation Research Development Program grant (STANTO19R0), and a Cystic Fibrosis Foundation research grant (STANTO16GO).
DISCLOSURES
The author was a consultant to Vertex Pharmaceuticals from 2000 to 2003.
AUTHOR CONTRIBUTIONS
B.A.S. drafted manuscript; edited and revised manuscript; approved final version of manuscript.
ACKNOWLEDGMENTS
I thank my colleagues who have contributed to the intellectual and technical development of our research on CF. I also thank the members of the Epithelial Transport Group committee for the kind invitation to present the Hans Ussing Distinguished Lecture at the 2016 Experimental Biology Meeting. I also thank Dr. Dean Madden for comments on the manuscript. William Scavone of Kestrel Studio designed the figures. This review had a limit on the number of references, thus, it was not possible to include all of the relevant publications on this topic.
Glossary
- c-Cbl
Casitas B-lineage lymphoma
- Cif
CFTR inhibitory factor
- COPD
Chronic obstructive pulmonary disease
- ENaC
Epithelial sodium channel
- FEV1
Percentage of the predicted value for the forced expiratory volume in 1 second
- G3BP1
Ras GTPase-activating protein-binding protein 1
- Gly551Asp
Substitution of a glycine with an aspartic acid at the 551 amino acid position
- IFN
Interferon
- KC
Mouse homolog of the cytokine IL-8
- MHC
Major histocompatibility complex
- NKCC1
Na+-K+-2 Cl− cotransporter
- OMV
Outer membrane vesicle
- P. aeruginosa
Pseudomonas aeruginosa
- Phe508del-CFTR
Deletion of the phenylalanine at the 508 amino acid position
- RSV
Respiratory syncytial virus
- SGK1
Serum glucocorticoid kinase 1
- sRNA
Short interfering RNA
- TAP1/TAP2
Transporter associated with antigen processing 1 and 2
- USP10
Ubiquitin-specific peptidase 10
Footnotes
Glossary of abbreviations appears at the end of the article.
REFERENCES
- 1.Anderson GG, Moreau-Marquis S, Stanton BA, O’Toole GA. In vitro analysis of tobramycin-treated Pseudomonas aeruginosa biofilms on cystic fibrosis-derived airway epithelial cells. Infect Immun 76: 1423–1433, 2008. doi: 10.1128/IAI.01373-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora K, Naren AP. Pharmacological correction of cystic fibrosis: molecular mechanisms at the plasma membrane to augment mutant CFTR function. Curr Drug Targets 17: 1275–1281, 2016. doi: 10.2174/1389450117666151209114343. [DOI] [PubMed] [Google Scholar]
- 3.Bahl CD, Hvorecny KL, Bomberger JM, Stanton BA, Hammock BD, Morisseau C, Madden DR. Inhibiting an epoxide hydrolase virulence factor from Pseudomonas aeruginosa protects CFTR. Angew Chem Int Ed Engl 54: 9881–9885, 2015. doi: 10.1002/anie.201503983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahl CD, Hvorecny KL, Bridges AA, Ballok AE, Bomberger JM, Cady KC, O’Toole GA, Madden DR. Signature motifs identify an Acinetobacter Cif virulence factor with epoxide hydrolase activity. J Biol Chem 289: 7460–7469, 2014. doi: 10.1074/jbc.M113.518092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahl CD, Morisseau C, Bomberger JM, Stanton BA, Hammock BD, O’Toole GA, Madden DR. Crystal structure of the cystic fibrosis transmembrane conductance regulator inhibitory factor Cif reveals novel active-site features of an epoxide hydrolase virulence factor. J Bacteriol 192: 1785–1795, 2010. doi: 10.1128/JB.01348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballok AE, Filkins LM, Bomberger JM, Stanton BA, O’Toole GA. Epoxide-mediated differential packaging of Cif and other virulence factors into outer membrane vesicles. J Bacteriol 196: 3633–3642, 2014. doi: 10.1128/JB.01760-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballok AE, O’Toole GA. Pouring salt on a wound: Pseudomonas aeruginosa virulence factors alter Na+ and Cl− flux in the lung. J Bacteriol 195: 4013–4019, 2013. doi: 10.1128/JB.00339-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bangel N, Dahlhoff C, Sobczak K, Weber WM, Kusche-Vihrog K. Upregulated expression of ENaC in human CF nasal epithelium. J Cyst Fibros 7: 197–205, 2008. doi: 10.1016/j.jcf.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Birket SE, Chu KK, Houser GH, Liu L, Fernandez CM, Solomon GM, Lin V, Shastry S, Mazur M, Sloane PA, Hanes J, Grizzle WE, Sorscher EJ, Tearney GJ, Rowe SM. Combination therapy with cystic fibrosis transmembrane conductance regulator modulators augment the airway functional microanatomy. Am J Physiol Lung Cell Mol Physiol 310: L928–L939, 2016. doi: 10.1152/ajplung.00395.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bomberger JM, Barnaby RL, Stanton BA. The deubiquitinating enzyme USP10 regulates the endocytic recycling of CFTR in airway epithelial cells. Channels (Austin) 4: 150–154, 2010. doi: 10.4161/chan.4.3.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bomberger JM, Coutermarsh BA, Barnaby RL, Sato JD, Chapline MC, Stanton BA. Serum and glucocorticoid-inducible kinase1 increases plasma membrane wt-CFTR in human airway epithelial cells by inhibiting its endocytic retrieval. PLoS One 9: e89599, 2014. doi: 10.1371/journal.pone.0089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bomberger JM, Coutermarsh BA, Barnaby RL, Stanton BA. Arsenic promotes ubiquitinylation and lysosomal degradation of cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels in human airway epithelial cells. J Biol Chem 287: 17130–17139, 2012. doi: 10.1074/jbc.M111.338855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bomberger JM, Ely KH, Bangia N, Ye S, Green KA, Green WR, Enelow RI, Stanton BA. Pseudomonas aeruginosa Cif protein enhances the ubiquitination and proteasomal degradation of the transporter associated with antigen processing (TAP) and reduces major histocompatibility complex (MHC) class I antigen presentation. J Biol Chem 289: 152–162, 2014. doi: 10.1074/jbc.M113.459271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bomberger JM, Guggino WB, Stanton BA. Methods to monitor cell surface expression and endocytic trafficking of CFTR in polarized epithelial cells. Methods Mol Biol 741: 271–283, 2011. doi: 10.1007/978-1-61779-117-8_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bomberger JM, Maceachran DP, Coutermarsh BA, Ye S, O’Toole GA, Stanton BA. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog 5: e1000382, 2009. doi: 10.1371/journal.ppat.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bomberger JM, Ye S, Maceachran DP, Koeppen K, Barnaby RL, O’Toole GA, Stanton BA. A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system. PLoS Pathog 7: e1001325, 2011. doi: 10.1371/journal.ppat.1001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnington KE, Kuehn MJ. Protein selection and export via outer membrane vesicles. Biochim Biophys Acta 1843: 1612–1619, 2014. doi: 10.1016/j.bbamcr.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butterworth MB, Zhang L, Heidrich EM, Myerburg MM, Thibodeau PH. Activation of the epithelial sodium channel (ENaC) by the alkaline protease from Pseudomonas aeruginosa. J Biol Chem 287: 32556–32565, 2012. doi: 10.1074/jbc.M112.369520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butterworth MB, Zhang L, Liu X, Shanks RM, Thibodeau PH. Modulation of the epithelial sodium channel (ENaC) by bacterial metalloproteases and protease inhibitors. PLoS One 9: e100313, 2014. doi: 10.1371/journal.pone.0100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cholon DM, Quinney NL, Fulcher ML, Esther CR Jr, Das J, Dokholyan NV, Randell SH, Boucher RC, Gentzsch M. Potentiator ivacaftor abrogates pharmacological correction of ΔF508 CFTR in cystic fibrosis. Sci Transl Med 6: 246ra96, 2014. doi: 10.1126/scitranslmed.3008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collawn JF, Lazrak A, Bebok Z, Matalon S. The CFTR and ENaC debate: how important is ENaC in CF lung disease? Am J Physiol Lung Cell Mol Physiol 302: L1141–L1146, 2012. doi: 10.1152/ajplung.00036.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collawn JF, Matalon S. CFTR and lung homeostasis. Am J Physiol Lung Cell Mol Physiol 307: L917–L923, 2014. doi: 10.1152/ajplung.00326.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet 16: 45–56, 2015. doi: 10.1038/nrg3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Boeck K, Amaral MD. Progress in therapies for cystic fibrosis. Lancet Respir Med 4: 662–674, 2016. doi: 10.1016/S2213-2600(16)00023-0. [DOI] [PubMed] [Google Scholar]
- 25.Dekkers JF, Berkers G, Kruisselbrink E, Vonk A, de Jonge HR, Janssens HM, Bronsveld I, van de Graaf EA, Nieuwenhuis EE, Houwen RH, Vleggaar FP, Escher JC, de Rijke YB, Majoor CJ, Heijerman HG, de Winter-de Groot KM, Clevers H, van der Ent CK, Beekman JM. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med 8: 344ra84, 2016. doi: 10.1126/scitranslmed.aad8278. [DOI] [PubMed] [Google Scholar]
- 26.Elborn JS. Cystic fibrosis. Lancet 388: 2519–2531, 2016. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 27.Farinha CM, Canato S. From the endoplasmic reticulum to the plasma membrane: mechanisms of CFTR folding and trafficking. Cell Mol Life Sci 74: 39–55, 2017. doi: 10.1007/s00018-016-2387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flitter BA, Hvorecny KL, Ono E, Eddens T, Yang J, Kwak DH, Bahl CD, Hampton TH, Morisseau C, Hammock BD, Liu X, Lee JS, Kolls JK, Levy BD, Madden DR, Bomberger JM. Pseudomonas aeruginosa sabotages the generation of host proresolving lipid mediators. Proc Natl Acad Sci USA 114: 136–141, 2017. doi: 10.1073/pnas.1610242114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu L, Rab A, Tang LP, Rowe SM, Bebok Z, Collawn JF. Dab2 is a key regulator of endocytosis and post-endocytic trafficking of the cystic fibrosis transmembrane conductance regulator. Biochem J 441: 633–643, 2012. doi: 10.1042/BJ20111566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh A, Boucher RC, Tarran R. Airway hydration and COPD. Cell Mol Life Sci 72: 3637–3652, 2015. doi: 10.1007/s00018-015-1946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gifford AH, Moulton LA, Dorman DB, Olbina G, Westerman M, Parker HW, Stanton BA, O’Toole GA. Iron homeostasis during cystic fibrosis pulmonary exacerbation. Clin Transl Sci 5: 368–373, 2012. doi: 10.1111/j.1752-8062.2012.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol 7: 426–436, 2006. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- 33.Hampton TH, Stanton BA. A novel approach to analyze gene expression data demonstrates that the ΔF508 mutation in CFTR downregulates the antigen presentation pathway. Am J Physiol Lung Cell Mol Physiol 298: L473–L482, 2010. doi: 10.1152/ajplung.00379.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haq IJ, Gray MA, Garnett JP, Ward C, Brodlie M. Airway surface liquid homeostasis in cystic fibrosis: pathophysiology and therapeutic targets. Thorax 71: 284–287, 2016. doi: 10.1136/thoraxjnl-2015-207588. [DOI] [PubMed] [Google Scholar]
- 35.Heltshe SL, Mayer-Hamblett N, Burns JL, Khan U, Baines A, Ramsey BW, Rowe SM; GOAL (the G551D Observation-AL) Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network . Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor. Clin Infect Dis 60: 703–712, 2015. doi: 10.1093/cid/ciu944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendricks MR, Bomberger JM. Who’s really in control: microbial regulation of protein trafficking in the epithelium. Am J Physiol Cell Physiol 306: C187–C197, 2014. doi: 10.1152/ajpcell.00277.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hendricks MR, Lashua LP, Fischer DK, Flitter BA, Eichinger KM, Durbin JE, Sarkar SN, Coyne CB, Empey KM, Bomberger JM. Respiratory syncytial virus infection enhances Pseudomonas aeruginosa biofilm growth through dysregulation of nutritional immunity. Proc Natl Acad Sci USA 113: 1642–1647, 2016. doi: 10.1073/pnas.1516979113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitamura S, Hvorecny KL, Niu J, Hammock BD, Madden DR, Morisseau C. Rational design of potent and selective inhibitors of an epoxide hydrolase virulence factor from Pseudomonas aeruginosa. J Med Chem 59: 4790–4799, 2016. doi: 10.1021/acs.jmedchem.6b00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koefoed-Johnsen V, Ussing HH, Zerahn K. The origin of the short-circuit current in the adrenaline stimulated frog skin. Acta Physiol Scand 27: 38–48, 1953. doi: 10.1111/j.1748-1716.1953.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 40.Koeppen K, Coutermarsh BA, Madden DR, Stanton BA. Serum- and glucocorticoid-induced protein kinase 1 (SGK1) increases the cystic fibrosis transmembrane conductance regulator (CFTR) in airway epithelial cells by phosphorylating Shank2E protein. J Biol Chem 289: 17142–17150, 2014. doi: 10.1074/jbc.M114.555599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koeppen K, Hampton TH, Jarek M, Scharfe M, Gerber SA, Mielcarz DW, Demers EG, Dolben EL, Hammond JH, Hogan DA, Stanton BA. A novel mechanism of host-pathogen interaction through sRNA in bacterial outer membrane vesicles. PLoS Pathog 12: e1005672, 2016. doi: 10.1371/journal.ppat.1005672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kokai-Kun JF, Bristol JA, Setser J, Schlosser M. Nonclinical safety assessment of syn-004: an oral β-lactamase for the protection of the gut microbiome from disruption by biliary-excreted, intravenously administered antibiotics. Int J Toxicol 35: 309–316, 2016. doi: 10.1177/1091581815623236. [DOI] [PubMed] [Google Scholar]
- 43.Kulkarni HM, Jagannadham MV. Biogenesis and multifaceted roles of outer membrane vesicles from Gram-negative bacteria. Microbiology 160: 2109–2121, 2014. doi: 10.1099/mic.0.079400-0. [DOI] [PubMed] [Google Scholar]
- 44.Larsen EH. Hans H. Ussing—scientific work: contemporary significance and perspectives. Biochim Biophys Acta 1566: 2–15, 2002. doi: 10.1016/S0005-2736(02)00592-8. [DOI] [PubMed] [Google Scholar]
- 45.Larsen EH. Hans Henriksen Ussing. 30 December 1911–22 December 2000. Biogr Mem Fellows R Soc 55: 305–335, 2009. doi: 10.1098/rsbm.2009.0002. [DOI] [Google Scholar]
- 46.Li X, Tang XX, Vargas Buonfiglio LG, Comellas AP, Thornell IM, Ramachandran S, Karp PH, Taft PJ, Sheets K, Abou Alaiwa MH, Welsh MJ, Meyerholz DK, Stoltz DA, Zabner J. Electrolyte transport properties in distal small airways from cystic fibrosis pigs with implications for host defense. Am J Physiol Lung Cell Mol Physiol 310: L670–L679, 2016. doi: 10.1152/ajplung.00422.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luan X, Campanucci VA, Nair M, Yilmaz O, Belev G, Machen TE, Chapman D, Ianowski JP. Pseudomonas aeruginosa triggers CFTR-mediated airway surface liquid secretion in swine trachea. Proc Natl Acad Sci USA 111: 12930–12935, 2014. doi: 10.1073/pnas.1406414111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacEachran DP, Ye S, Bomberger JM, Hogan DA, Swiatecka-Urban A, Stanton BA, O’Toole GA. The Pseudomonas aeruginosa secreted protein PA2934 decreases apical membrane expression of the cystic fibrosis transmembrane conductance regulator. Infect Immun 75: 3902–3912, 2007. doi: 10.1128/IAI.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacKenzie T, Gifford AH, Sabadosa KA, Quinton HB, Knapp EA, Goss CH, Marshall BC. Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the Cystic Fibrosis Foundation patient registry. Ann Intern Med 161: 233–241, 2014. doi: 10.7326/M13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madden DR, Swiatecka-Urban A. Tissue-specific control of CFTR endocytosis by Dab2: cargo recruitment as a therapeutic target. Commun Integr Biol 5: 473–476, 2012. doi: 10.4161/cib.21375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marson FA, Bertuzzo CS, Ribeiro JD. Classification of CFTR mutation classes. Lancet Respir Med 4: e37–e38, 2016. doi: 10.1016/S2213-2600(16)30188-6. [DOI] [PubMed] [Google Scholar]
- 52.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95: 1005–1015, 1998. doi: 10.1016/S0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 53.Matthes E, Goepp J, Carlile GW, Luo Y, Dejgaard K, Billet A, Robert R, Thomas DY, Hanrahan JW. Low free drug concentration prevents inhibition of F508del CFTR functional expression by the potentiator VX-770 (ivacaftor). Br J Pharmacol 173: 459–470, 2016. doi: 10.1111/bph.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melvin JA, Bomberger JM. Compromised defenses: exploitation of epithelial responses during viral-bacterial co-infection of the respiratory tract. PLoS Pathog 12: e1005797, 2016. doi: 10.1371/journal.ppat.1005797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreau-Marquis S, Bomberger JM, Anderson GG, Swiatecka-Urban A, Ye S, O’Toole GA, Stanton BA. The DeltaF508-CFTR mutation results in increased biofilm formation by Pseudomonas aeruginosa by increasing iron availability. Am J Physiol Lung Cell Mol Physiol 295: L25–L37, 2008. doi: 10.1152/ajplung.00391.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moreau-Marquis S, Coutermarsh B, Stanton BA. Combination of hypothiocyanite and lactoferrin (ALX-109) enhances the ability of tobramycin and aztreonam to eliminate Pseudomonas aeruginosa biofilms growing on cystic fibrosis airway epithelial cells. J Antimicrob Chemother 70: 160–166, 2015. doi: 10.1093/jac/dku357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreau-Marquis S, O’Toole GA, Stanton BA. Tobramycin and FDA-approved iron chelators eliminate Pseudomonas aeruginosa biofilms on cystic fibrosis cells. Am J Respir Cell Mol Biol 41: 305–313, 2009. doi: 10.1165/rcmb.2008-0299OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moreau-Marquis S, Redelman CV, Stanton BA, Anderson GG. Co-culture models of Pseudomonas aeruginosa biofilms grown on live human airway cells. J Vis Exp 44: 2186, 2010. doi: 10.3791/2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moreau-Marquis S, Stanton BA, O’Toole GA. Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm Pharmacol Ther 21: 595–599, 2008. doi: 10.1016/j.pupt.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, Bánfi B, Horswill AR, Stoltz DA, McCray PB Jr, Welsh MJ, Zabner J. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487: 109–113, 2012. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quinton PM. The neglected ion: HCO3−. Nat Med 7: 292–293, 2001. doi: 10.1038/85429. [DOI] [PubMed] [Google Scholar]
- 62.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermet-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordoñez C, Elborn JS; VX08-770-102 Study Group . A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 365: 1663–1672, 2011. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ratjen F, Bell SC, Rowe SM, Goss CH, Quittner AL, Bush A. Cystic fibrosis. Nat Rev Dis Primers 1: 15010, 2015. doi: 10.1038/nrdp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Read RC, Roberts P, Munro N, Rutman A, Hastie A, Shryock T, Hall R, McDonald-Gibson W, Lund V, Taylor G, Cole PJ, Wilson R. Effect of Pseudomonas aeruginosa rhamnolipids on mucociliary transport and ciliary beating. J Appl Physiol (1985) 72: 2271–2277, 1992. [DOI] [PubMed] [Google Scholar]
- 65.Reid DW, Lam QT, Schneider H, Walters EH. Airway iron and iron-regulatory cytokines in cystic fibrosis. Eur Respir J 24: 286–291, 2004. doi: 10.1183/09031936.04.00104803. [DOI] [PubMed] [Google Scholar]
- 66.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, Sagel SD, Khan U, Mayer-Hamblett N, Van Dalfsen JM, Joseloff E, Ramsey BW; GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network . Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med 190: 175–184, 2014. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rubenstein RC, Lockwood SR, Lide E, Bauer R, Suaud L, Grumbach Y. Regulation of endogenous ENaC functional expression by CFTR and ΔF508-CFTR in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L88–L101, 2011. doi: 10.1152/ajplung.00142.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rubino R, Bezzerri V, Favia M, Facchini M, Tebon M, Singh AK, Riederer B, Seidler U, Iannucci A, Bragonzi A, Cabrini G, Reshkin SJ, Tamanini A. Pseudomonas aeruginosa reduces the expression of CFTR via post-translational modification of NHERF1. Pflugers Arch 466: 2269–2278, 2014. doi: 10.1007/s00424-014-1474-6. [DOI] [PubMed] [Google Scholar]
- 69.Saint-Criq V, Gray MA. Role of CFTR in epithelial physiology. Cell Mol Life Sci 74: 93–115, 2017. doi: 10.1007/s00018-016-2391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sato JD, Chapline MC, Thibodeau R, Frizzell RA, Stanton BA. Regulation of human cystic fibrosis transmembrane conductance regulator (CFTR) by serum- and glucocorticoid-inducible kinase (SGK1). Cell Physiol Biochem 20: 91–98, 2007. doi: 10.1159/000104157. [DOI] [PubMed] [Google Scholar]
- 71.Schwarzer C, Fischer H, Kim EJ, Barber KJ, Mills AD, Kurth MJ, Gruenert DC, Suh JH, Machen TE, Illek B. Oxidative stress caused by pyocyanin impairs CFTR Cl(−) transport in human bronchial epithelial cells. Free Radic Biol Med 45: 1653–1662, 2008. doi: 10.1016/j.freeradbiomed.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shah VS, Meyerholz DK, Tang XX, Reznikov L, Abou Alaiwa M, Ernst SE, Karp PH, Wohlford-Lenane CL, Heilmann KP, Leidinger MR, Allen PD, Zabner J, McCray PB Jr, Ostedgaard LS, Stoltz DA, Randak CO, Welsh MJ. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science 351: 503–507, 2016. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shamsuddin AK, Quinton PM. Native small airways secrete bicarbonate. Am J Respir Cell Mol Biol 50: 796–804, 2014. doi: 10.1165/rcmb.2013-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shaw JR, Sato JD, VanderHeide J, LaCasse T, Stanton CR, Lankowski A, Stanton SE, Chapline C, Coutermarsh B, Barnaby R, Karlson K, Stanton BA. The role of SGK and CFTR in acute adaptation to seawater in Fundulus heteroclitus. Cell Physiol Biochem 22: 69–78, 2008. doi: 10.1159/000149784. [DOI] [PubMed] [Google Scholar]
- 75.Solomon GM, Marshall SG, Ramsey BW, Rowe SM. Breakthrough therapies: cystic fibrosis (CF) potentiators and correctors. Pediatr Pulmonol 50, Suppl 40: S3–S13, 2015. doi: 10.1002/ppul.23240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stanke F, Tümmler B. Classification of CFTR mutation classes. Lancet Respir Med 4: e36, 2016. doi: 10.1016/S2213-2600(16)30147-3. [DOI] [PubMed] [Google Scholar]
- 77.Stanton BA, Coutermarsh B, Barnaby R, Hogan D. Pseudomonas aeruginosa reduces VX-809 stimulated F508del-CFTR. PLoS One 10: e0127742, 2015. doi: 10.1371/journal.pone.0127742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swiatecka-Urban A, Brown A, Moreau-Marquis S, Renuka J, Coutermarsh B, Barnaby R, Karlson KH, Flotte TR, Fukuda M, Langford GM, Stanton BA. The short apical membrane half-life of rescued ΔF508-cystic fibrosis transmembrane conductance regulator (CFTR) results from accelerated endocytosis of ΔF508-CFTR in polarized human airway epithelial cells. J Biol Chem 280: 36762–36772, 2005. doi: 10.1074/jbc.M508944200. [DOI] [PubMed] [Google Scholar]
- 79.Swiatecka-Urban A, Moreau-Marquis S, Maceachran DP, Connolly JP, Stanton CR, Su JR, Barnaby R, O’toole GA, Stanton BA. Pseudomonas aeruginosa inhibits endocytic recycling of CFTR in polarized human airway epithelial cells. Am J Physiol Cell Physiol 290: C862–C872, 2006. doi: 10.1152/ajpcell.00108.2005. [DOI] [PubMed] [Google Scholar]
- 80.Trinh NT, Bilodeau C, Maillé É, Ruffin M, Quintal MC, Desrosiers MY, Rousseau S, Brochiero E. Deleterious impact of Pseudomonas aeruginosa on cystic fibrosis transmembrane conductance regulator function and rescue in airway epithelial cells. Eur Respir J 45: 1590–1602, 2015. doi: 10.1183/09031936.00076214. [DOI] [PubMed] [Google Scholar]
- 81.Ussing HH, Zerahn K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand 23: 110–127, 1951. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 82.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, Zhou J, McCartney J, Arumugam V, Decker C, Yang J, Young C, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu P. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA 106: 18825–18830, 2009. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu PA. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci USA 108: 18843–18848, 2011. doi: 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Veit G, Avramescu RG, Perdomo D, Phuan PW, Bagdany M, Apaja PM, Borot F, Szollosi D, Wu YS, Finkbeiner WE, Hegedus T, Verkman AS, Lukacs GL. Some gating potentiators, including VX-770, diminish ΔF508-CFTR functional expression. Sci Transl Med 6: 246ra97, 2014. doi: 10.1126/scitranslmed.3008889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, Colombo C, Davies JC, De Boeck K, Flume PA, Konstan MW, McColley SA, McCoy K, McKone EF, Munck A, Ratjen F, Rowe SM, Waltz D, Boyle MP; TRAFFIC Study Group; TRANSPORT Study Group . Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 373: 220–231, 2015. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolde M, Fellows A, Cheng J, Kivenson A, Coutermarsh B, Talebian L, Karlson K, Piserchio A, Mierke DF, Stanton BA, Guggino WB, Madden DR. Targeting CAL as a negative regulator of DeltaF508-CFTR cell-surface expression: an RNA interference and structure-based mutagenetic approach. J Biol Chem 282: 8099–8109, 2007. doi: 10.1074/jbc.M611049200. [DOI] [PubMed] [Google Scholar]
- 87.Ye S, Cihil K, Stolz DB, Pilewski JM, Stanton BA, Swiatecka-Urban A. c-Cbl facilitates endocytosis and lysosomal degradation of cystic fibrosis transmembrane conductance regulator in human airway epithelial cells. J Biol Chem 285: 27008–27018, 2010. doi: 10.1074/jbc.M110.139881. [DOI] [PMC free article] [PubMed] [Google Scholar]