Abstract
Vasculogenesis is a complex process by which endothelial stem and progenitor cells undergo de novo vessel formation. Quantitative assessment of vasculogenesis is a central readout of endothelial progenitor cell functionality. However, current assays lack kinetic measurements. To address this issue, new approaches were developed to quantitatively assess in vitro endothelial colony-forming cell (ECFC) network formation in real time. Eight parameters of network structure were quantified using novel Kinetic Analysis of Vasculogenesis (KAV) software. KAV assessment of structure complexity identified two phases of network formation. This observation guided the development of additional vasculogenic readouts. A tissue cytometry approach was established to quantify the frequency and localization of dividing ECFCs. Additionally, Fiji TrackMate was used to quantify ECFC displacement and speed at the single-cell level during network formation. These novel approaches were then implemented to identify how intrauterine exposure to maternal diabetes mellitus (DM) impairs fetal ECFC vasculogenesis. Fetal ECFCs exposed to maternal DM form fewer initial network structures, which are not stable over time. Correlation analyses demonstrated that ECFC samples with greater division in branches form fewer closed network structures. Additionally, reductions in average ECFC movement over time decrease structural connectivity. Identification of these novel phenotypes utilizing the newly established methodologies provides evidence for the cellular mechanisms contributing to aberrant ECFC vasculogenesis.
Keywords: endothelial, vasculogenesis, migration, diabetes, proliferation
vasculogenesis is the process by which endothelial progenitor cells (EPCs) undergo de novo vessel formation to establish embryonic vasculature. Some progenitor cells are also capable of “postnatal vasculogenesis,” an important mechanism for vascular homeostasis and for new vessel formation in response to disease (6, 7, 20, 28). Increasing investigation is ongoing to understand EPC function during vascular development as well as their potential therapeutic use for postnatal vascular repair (5, 22, 29, 39, 41).
Asahara et al. discovered that circulating EPCs incorporate into active sites of angiogenesis and suggested EPC infusion as an angiogenic therapy (6). The term “endothelial progenitor cell” describes diverse cell types defined by colony formation or cell surface markers (8, 25, 30, 32, 33). Endothelial colony-forming cells (ECFCs) are a well-defined cell population that displays key characteristics of an immature EPC, including high proliferative potential, self-renewal capacity, and de novo vessel formation in vivo (24, 25, 46). Moreover, ECFCs reside in endothelium to maintain vascular integrity and circulate in blood to facilitate vessel formation or repair (24, 45). Although few studies evaluate ECFCs as a cellular therapy for tissue regeneration, the potential applications are numerous because of their unique proliferative and vasculogenic properties (18, 32). In addition, ECFCs are enriched in cord blood and may provide insight into the impact of abnormal intrauterine environments on increased risk for children to develop vascular morbidities such as hypertension and cardiovascular disease (11, 23, 26). Therefore, ECFCs are a unique vasculogenic population with therapeutic potential that may prove useful in predicting the long-term impact of abnormal intrauterine environment exposure.
Individuals exposed to both gestational and pregestational diabetic in utero environments are at an increased risk for developing chronic disease, such as high blood pressure, later in life (10, 13, 21). Exposure to the diabetic intrauterine environment has a negative impact on endothelial progenitor cell number and function (7, 9). ECFCs exposed to maternal diabetes in utero undergo premature senescence and have reduced proliferative potential, decreased clonogenic capacity, and impaired vasculogenesis (23). Thus neonatal ECFC exposure to the diabetic intrauterine environment results in significant functional impairment, the mechanisms of which are unknown. Therefore, in-depth analyses of ECFC vasculogenesis are needed to identify how and why functional deficits are incurred in utero.
Modeling vasculogenesis by seeding cells in extracellular matrix is a standard quantitative measurement to assess function in vitro (4, 27, 31), allowing for rapid analysis of cells to perform basic tasks of network or vessel formation (15, 17, 38). However, standard methods are limited in scope and fail to capture many aspects of this highly dynamic process. Though attempts have been made to increase quantitative analyses, current methods are insufficient for dynamic studies (27, 36, 40). In this study, novel techniques were developed to provide kinetic quantification of vasculogenesis in vitro. With the use of three imaging techniques, multiple quantitative measurements of vasculogenesis were developed. These novel methods allow for an in-depth dissection of the vasculogenic process to identify functional differences between ECFCs from uncomplicated pregnancies and those complicated by type 2 diabetes mellitus.
MATERIALS AND METHODS
Umbilical cord blood sample acquisition.
Umbilical cord blood samples were collected at the time of birth following written informed consent from the mothers. Samples were obtained from women with uncomplicated (UC) pregnancies and from women diagnosed with type 2 diabetes mellitus (T2DM) before pregnancy. All pregnancies were single gestation. Infants with known chromosomal abnormalities were excluded. Women with preeclampsia or hypertension, women with other illnesses known to affect glucose metabolism, and women taking medications known to affect glucose metabolism were excluded. The Institutional Review Board at the Indiana University School of Medicine approved this protocol. Clinical data from women and infants are summarized in Tables 1 and 2, respectively. No significant differences were identified between the UC and T2DM groups for the following clinical values: maternal age, prepregnancy body mass index, gestational age, infant weight, infant weight percentile, infant weight/length percentile, and ponderal index (all P values >0.05).
Table 1.
Clinical data for maternal subjects
| Sample | Maternal Age, yr | Medication | Maternal Prepregnancy BMI | Hb A1C |
|---|---|---|---|---|
| Uncomplicated 1 | 25 | None | 37.2 | ND |
| Uncomplicated 2 | 32 | None | 27.4 | ND |
| Uncomplicated 3 | 26 | None | 22.8 | ND |
| Uncomplicated 4 | 27 | None | 27.6 | ND |
| Uncomplicated 5 | 25 | None | 22.0 | ND |
| Uncomplicated 6 | 37 | None | 33.5 | ND |
| Uncomplicated 7 | 27 | None | 42.6 | ND |
| Uncomplicated 8 | 27 | None | 31.7 | ND |
| Uncomplicated 9 | 25 | None | 23.8 | ND |
| Uncomplicated 10 | 42 | None | 34.6 | 5.6 |
| T2DM 1 | 31 | Insulin | 25.6 | 6.8, 6.5 |
| T2DM 2 | 25 | Insulin, lisinopril, metformin | 34.3 | 12.5, 13.5 |
| T2DM 3 | 26 | Insulin | 30.5 | 15.2, 12.4, 11.7, 12, 11.9, 14.4 |
| T2DM 4 | 38 | Insulin | 36.8 | 6.8, 5.8, 6.0 |
| T2DM 5 | 35 | Glyburide | 32.9 | 6.5, 6.0, 5.5 |
| T2DM 6 | 38 | Insulin | 38.7 | 9.2, 6.7, 5.9 |
| T2DM 7 | 30 | Metformin, glyburide | 29.1 | 5.5, 5.3 |
| T2DM 8 | 30 | Metformin, insulin | 48.4 | 6.1, 5.7 |
| T2DM 9 | 31 | Insulin | 25.6 | 6.7, 5.8 |
| T2DM 10 | 38 | Insulin | 29.9 | ND |
BMI, body mass index. ND, not done; T2DM, type 2 diabetes mellitus.
Table 2.
Clinical data for infant subjects
| Sample | Sex | Gestational Age | Infant Weight, kg | Infant Weight Percentile, % | Infant Weight/Length, Percentile, % | Ponderal Index, kg/m3 |
|---|---|---|---|---|---|---|
| Uncomplicated 1 | Female | 39 | 3.7 | 84.6 | 55.2 | 27.2 |
| Uncomplicated 2 | Female | 37 | 3.6 | 79.4 | 5.8 | 23.6 |
| Uncomplicated 3 | Male | 39 | 3.6 | 70.2 | 31.9 | 25.7 |
| Uncomplicated 4 | Female | 40 | 2.9 | 24.2 | 1.8 | 22.3 |
| Uncomplicated 5 | Male | 38 | 3.4 | 58.3 | 38.6 | 26.0 |
| Uncomplicated 6 | Male | 40 | 3.6 | 68.1 | 26.2 | 25.3 |
| Uncomplicated 7 | Female | 39 | 4.3 | 98.6 | 74.4 | 28.9 |
| Uncomplicated 8 | Female | 41 | 3.3 | 55.2 | 78.2 | 28.8 |
| Uncomplicated 9 | Male | 39 | 3.3 | 42.1 | 83.1 | 26.8 |
| Uncomplicated 10 | Female | 38 | 2.9 | 23.3 | 1.0 | 21.9 |
| T2DM 1 | Male | 37 | 4.5 | 98.6 | 91.6 | 30.4 |
| T2DM 2 | Female | 39 | 3.4 | 61.8 | 76.4 | 28.7 |
| T2DM 3 | Male | 38 | 3.7 | 81.1 | 7.1 | 23.9 |
| T2DM 4 | Female | 39 | 3.6 | 80.0 | 80.8 | 29.0 |
| T2DM 5 | Female | 38 | 3.2 | 50.0 | 13.6 | 24.3 |
| T2DM 6 | Male | 38 | 3.3 | 45.2 | 88.5 | 29.7 |
| T2DM 7 | Male | 39 | 3.9 | 84.6 | 34.5 | 26.0 |
| T2DM 8 | Female | 37 | 2.7 | 11.9 | 37.1 | 26.2 |
| T2DM 9 | Male | 36 | 3.1 | 32.6 | 94.7 | 31.1 |
| T2DM 10 | Female | 39 | 3.7 | 84.6 | 41.7 | 26.5 |
ND, not done; T2DM, type 2 diabetes mellitus.
ECFC cell culture.
ECFCs were isolated from umbilical cord blood samples as previously described (9, 23). Umbilical cord blood was diluted 1:1 with phosphate-buffered saline (PBS) and underlaid with Ficoll-Paque PLUS (GE Healthcare, Piscataway, NJ). The blood was centrifuged for 30 min at 740 g. Mononuclear cells were isolated from the buffy coat and washed with Endothelial Growth Medium 2 (EGM2; Lonza, Walkersville, MD) containing 10% Hyclone defined fetal bovine serum (FBS; ThermoFisher, Waltham, MA), antibiotic-antimycotic solution (Corning, Manassas, VA), and MycoZap PR antibiotic (Lonza) (EGM2 + 10% FBS). Mononuclear cells were resuspended in EGM2 + 10% FBS and plated in six-well tissue culture plates precoated with type 1 collagen (Corning). After 24 h in culture, all wells were washed with EGM2 + 10% FBS to remove nonadherent cells, and medium was changed daily for 7 days and then on alternate days until first passage. ECFC colonies appeared between 5 and 8 days of culture. After reaching confluence, cells were detached with 0.25% trypsin-EDTA (Invitrogen, Grand Island, NY) and frozen in 5% dimethyl sulfoxide (ThermoFisher) in FBS (Atlanta Biologicals, Flowery Branch, GA). ECFC aliquots were thawed, resuspended in EGM2 + 10% FBS, and plated on type 1 collagen-coated flasks for culture. ECFCs used in these studies were passaged two to five times.
Microscopy settings and equipment.
All imaging was performed on a fully automated Nikon TiE microscope equipped with a ProScan II motorized stage (Prior Scientific, Rockland, MA), xenon lamp source, Lambda LS, and Lambda 10-3 filter wheel controller (Sutter Instrument, Novato, CA), fitted with an ORCA-ER or ORCA-Flash 4.0 (Hamamatsu, Japan) controlled by Elements 4.20 (Nikon Instruments, Melville, NY). Fluorescent filters were from the Quad Sedat v89000 (Chroma Technology, Bellows Falls, VT). Experiments with phase-contrast imaging used a chrome-free infinity (CFI) Plan Fluor dark low low (DLL) ×10 objective, and those with fluorescence imaging for contrast used a CFI S Plan Fluor extralong-working distance apodized dark medium (ELWD ADM) ×20 objective (Nikon Instruments). Multiple images and Z-positions were collected to cover the sample wells and stitched together with Elements as required. For live imaging, the microscope was fitted with a stage top incubator with humidity (75–85%), temperature (37°C), and CO2 regulation (5%; OkoLab, Burlingame, CA).
Matrigel assay.
ECFCs (passage 3–4) were plated at 400,000 cells per 100-mm dish and incubated overnight. The following day, ECFCs were trypsinized (ThermoFisher), counted on a hemocytometer, and plated at equal densities in EGM2 supplemented with 10% FBS. ECFCs were plated on 10 µl of Matrigel lot no. 4209014 (Corning) in 15-well μ-slides (Ibidi USA). The Matrigel slide was placed in the microscope stage top incubator to maintain temperature, CO2, and humidity, for overnight live cell imaging. Images of entire wells were collected with the ORCA-ER every 15 min for 15 h for a total of 60 data points per ECFC sample in each experiment.
Kinetic Analysis of Vasculogenesis.
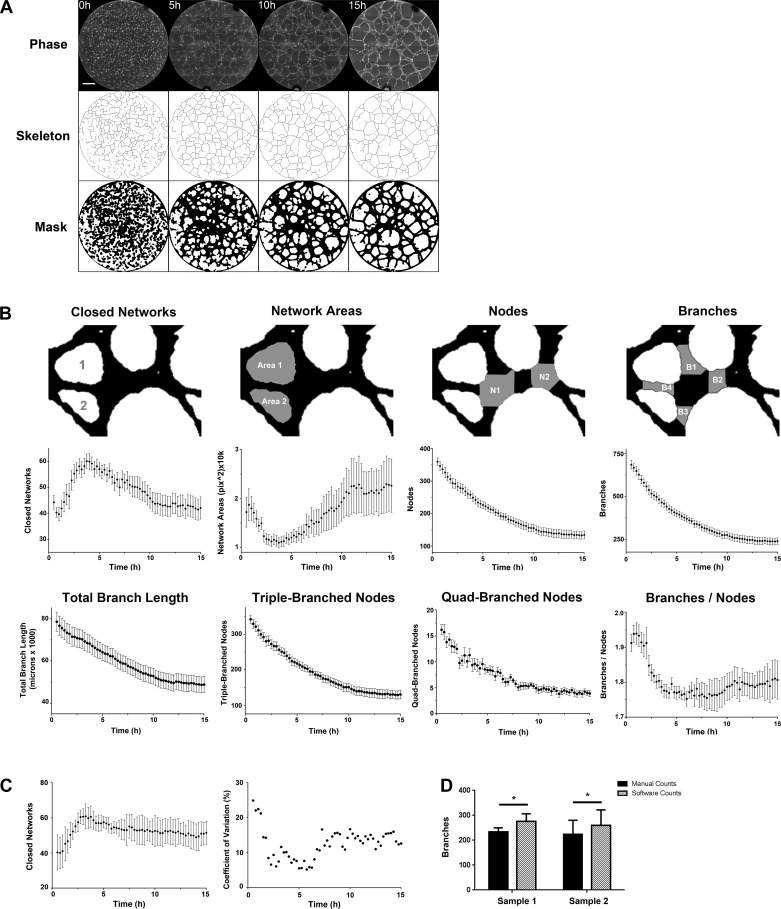
Phase-contrast images were compiled, processed, and analyzed using a custom Fiji is Just ImageJ (Fiji) plug-in called Kinetic Analysis of Vasculogenesis (KAV). KAV is reliant on the Skeletonize 2D/3D and Analyze Skeleton plug-ins in Fiji (3). The software allowed for quantification of eight distinct parameters of vasculogenesis over time including closed networks, network area, nodes, branches, the ratio of branches to nodes, branch length, nodes with three branch extensions, and nodes with four branch extensions. A closed network was defined in KAV as an enclosed area surrounded on all sides by cells. Network area is the size of the closed network openings. Definitions for nodes and branches were created to differentiate the structures in KAV. Nodes were defined as points within the network that have three to four branch extensions. Nodes with more than four branch extensions were not observed. A structure connected on at least one end by a node was defined as a branch. However, branches could be connected on both ends as indicated by the branches in Fig. 1B. KAV calculated the ratio of branches to nodes by dividing the total number of branches by the total number of nodes detected in a well. The values generated from the software analysis were graphed in Prism (GraphPad, San Diego, CA) and R (version 3.1.1; 34).
Fig. 1.
Kinetic Analysis of Vasculogenesis (KAV) quantitates network metrics. A: images of ECFC network formation were captured every 15 min for 15 h by phase-contrast microscopy. Representative phase-contrast images at 5-h increments, starting at the time of plating (t = 0), are shown. The phase-contrast images were analyzed using KAV to produce both “skeleton” and “mask” renditions of the network structure. The scale bar represents 500 µm. B: mean data of four parameters of network structure are illustrated. Data were quantitated using the newly developed KAV Fiji plug-in over a 15-h period. The top row is a magnified region of mask and depicts, in gray, the parameter analyzed in the line graphs below. Line graphs for the following parameters are shown: closed networks, network area, nodes, branches, total branch length, triple-branched nodes, quadruple-branches nodes, and the ratio of branches to nodes. The line graphs represent the mean ± SE data for 10 separate patient samples from uncomplicated pregnancies. C: to determine day-to-day variation of the assay design, KAV analysis was run for a single ECFC sample on 3 different days. Data shown represent means ± SD. Coefficient of variation (%) is shown for the data in C. D: to validate the KAV software, manual scoring of network formation was compared with data generated by KAV for two individual ECFC samples on 4 different experimental days. The number of branches detected was significantly increased when using KAV compared with manual counting for both samples (*P < 0.05). Data are shown as means ± SD.
Matrigel immunofluorescence technique.
Matrigel assays were conducted and analyzed at time points indicated using immunofluorescence staining (19). Briefly, the samples were fixed with 4% paraformaldehyde at 5-h postplating, permeabilized with 0.5% Triton X-100 in PBS, quenched with 100 mM glycine, and blocked with 0.1% bovine serum albumin, 0.2% Triton X-100, 0.05% Tween-20, and 10% goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA) in PBS. Samples were incubated with alpha-tubulin primary antibody at a 1:1,000 dilution (no. T6199, clone DM1A; Sigma-Aldrich, St. Louis, MO) overnight at room temperature. The following day, Alexa568-conjugated goat anti-mouse secondary antibody (no. A11031; ThermoFisher Scientific) was added at 1:400 for 40 min at room temperature. Samples were imaged in PBS containing NucBlue Fixed Cell Stain Ready Probes reagent (no. R37605; ThermoFisher Scientific) using the recommended dilution on the Nikon TiE inverted microscope with a xenon lamp source and fluorescent filters (Chroma Technology). Negative controls lacking primary antibody did not show nonspecific secondary antibody staining. The α-tubulin antibody clone DM1A was selected because of its specificity. By Western blotting, the antibody detects a single band at 50 kDa in ECFCs (data not shown). Also, immunofluorescence staining for α-tubulin by DM1A yields identical staining to a separate clone that binds a different epitope on α-tubulin (1). To ensure full coverage of the samples, six Z-positions were collected. For high-resolution images, cells were imaged using a ×20 objective on a Leica SP8 MP microscope.
Tissue cytometry to identify mitotic cells.
Immunofluorescence images were processed and analyzed using tissue cytometry (TC) software, Volumetric Tissue Exploration and Analysis (VTEA; 43). The software allows for single-cell quantification of both nuclear and cytoplasmic fluorescence intensities in three dimensions. A spatial measurement to assess the localization of cells within the network structures (i.e., branches and nodes) is accomplished using a combination of the original image volume and a scatterplot of cell-associated signals. Populations of cells on the scatterplot were interrogated with a “gating” tool similar to flow cytometry, enabling quantitative analysis. Cells were identified by their nuclei following nuclear staining with NucBlue Fixed Cell Stain Ready Probes reagent (no. R37605; ThermoFisher Scientific). The intensity of NucBlue staining was used to assess for mitotic cells. In some cases, a well-characterized antibody against phospho-histone H3 was used to confirm accuracy of this approach [phospho-histone H3 staining (Ser10) conjugated to Alexa488 at a 1:50 dilution (no. 9708; Cell Signaling Technology; 16)]. The accuracy of NucBlue intensity as an indicator of mitotic vs. nonmitotic cells and intensity thresholds for each experiment were assessed manually on a subregion of one volume from each data set. Between all experiments the specificity, precision, and accuracy were 96.8 ± 1.3, 56 ± 5.2, and 96.5 ± 1.2%, respectively (means ± SD). Branch and node thickness were determined with the Local Thickness tool in Fiji, which generated a distance map and hence thickness of a mask derived from microtubule staining of the network. This distance map was added as a channel to facilitate image analysis with VTEA.
Flow cytometric proliferation analysis.
Baseline ECFC proliferation was evaluated by flow cytometry on cells in standard culture conditions. Cells were washed with 2% FBS in PBS, resuspended in cytofix/cytoperm buffer (BD Biosciences, San Jose, CA), and incubated for 20 min on ice. Cell suspensions were washed twice with perm/wash buffer (BD Biosciences), followed by incubation with phospho-histone H3 (Ser10) conjugated to Alexa488 (no. 9708; Cell Signaling Technology) for 45 min in the dark at 37°C. The suspensions were washed again with perm/wash buffer and resuspended in 2% FBS in PBS for flow cytometric analysis. Samples were analyzed using an LSRII flow cytometer (Becton Dickinson, San Jose, CA) with at least 10,000 events collected per sample. FlowJo software (TreeStar, Ashland, OR) was used in analysis.
Baculoviral system for nuclear green fluorescent protein expression.
ECFCs (passage 3–4) were split and incubated using standard culture conditions overnight. The following day, CellLight nucleus-green fluorescent protein (Nucleus-GFP) BacMam2.0 (ThermoFisher Scientific) was added at a multiplicity of infection of 30. The cells were incubated overnight and then processed for Matrigel assays as described.
TrackMate motility analysis.
Images were processed in Elements (Nikon Instruments) and Fiji (35). Fiji TrackMate was used to analyze cell motility parameters including displacement and speed. In TrackMate, the difference of Gaussian (DoG) detector was used with an estimated spot diameter of 25.0 pixels and a threshold of 2.0 for individual cell nuclei detection. The Simple Linear Assignment Problem (LAP) tracker with a linking maximum distance of 50 µm, a gap-closing maximum distance of 90 µm, and a gap-closing maximum frame gap of 4 was used for tracking the cells through the time course images. Data from the TrackMate analysis were then analyzed using R (34).
Statistical analysis.
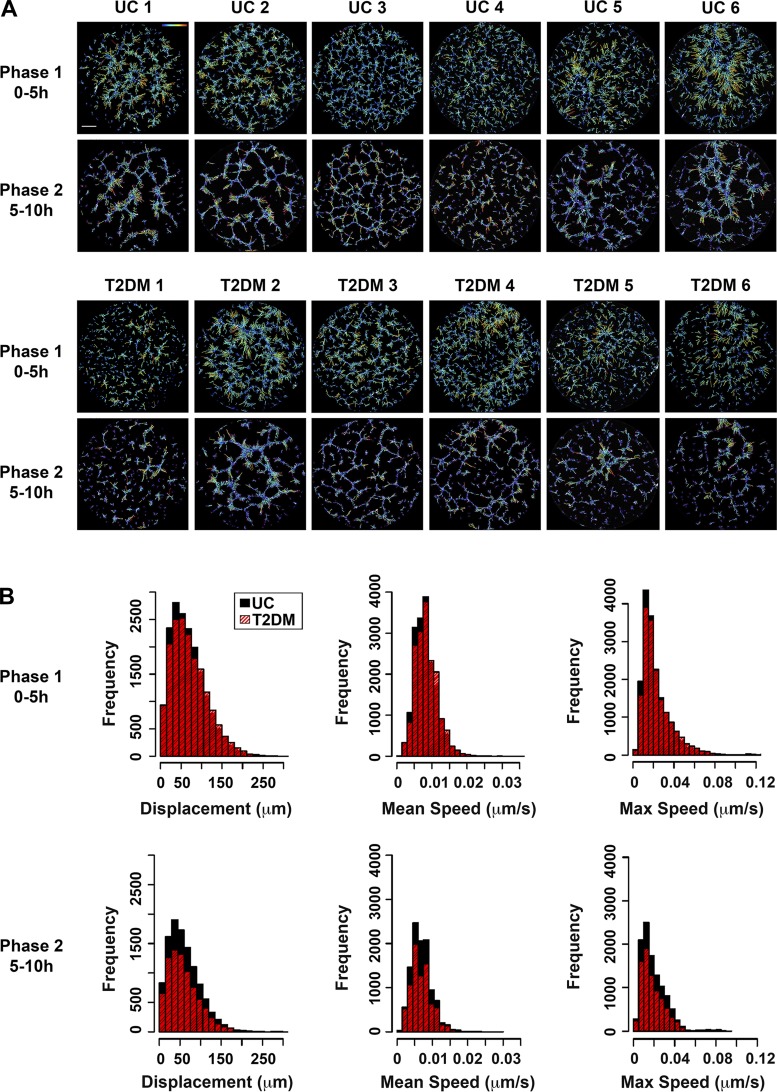
The kinetic network formation graphs for KAV data were created in Prism 6. The graphs represent the mean ± SE values at each time point for the sample sizes indicated in the figure legends. To statistically analyze the differences between the entire kinetic curves, individual time point data were integrated to produce estimated mean difference curves and the corresponding 95% confidence intervals. These curves were generated using the spline smoothing technique, and the analysis was performed using the Mixed Generalized Additive Model (GAM) Computation Vehicle (MGCV) package in R (34, 44). For histogram generation to analyze ECFC motility, the frequency of phenotype distribution was plotted such that x- and y-axis ranges were kept constant for each variable between phases 1 and 2. Data were binned on the basis of fixed values, with bin sizes remaining consistent across phases. The bin sizes were as follows: displacement, 15; mean speed, 0.005; and maximum speed, 0.0015. Correlations of displacement, mean speed, and maximum speed were calculated using phase 1 and phase 2 motility data from both UC and T2DM samples. The Pearson product-moment correlation coefficient and the 95% confidence interval were computed in R. For models of kinetic and motility data correction, a multilevel model was built to test linear relationships between KAV and motility variables. The models incorporated phase 1 motility data from all 20 ECFC samples (10 UC and 10 T2DM). Log transformations were applied to all three motility variables to normalize the distributions. Because of the sample size, simple linear regression models were utilized for analysis of kinetic data associations. P values <0.05 were considered significant.
Code availability.
Codes for KAV and image processing can be found at https://github.com/icbm-iupui/kinetic-analysis-vasculogenesis.
RESULTS
Dynamic assessment of neonatal ECFC vasculogenesis.
Current quantification methods of network formation are largely static, providing limited insight into potential mechanisms that contribute to functional deficits. To ascertain a realistic depiction of the dynamic process of ECFC network formation, longitudinal data were analyzed from phase-contrast images over 15 h. To derive quantitative data, an analysis plug-in was created for the Fiji image-processing package (35), which will be referred to as Kinetic Analysis of Vasculogenesis (KAV). KAV uses image segmentation followed by skeletonization to analyze network components (Fig. 1A). Parameters measured were closed networks, network areas, nodes, branches, branch length, triple-branched nodes, and quadruple-branched nodes (Fig. 1B). These parameters were chosen to evaluate network structure and connectivity because of use in previous studies or their descriptive nature (27). Using time-lapse imaging coupled with KAV, several interesting observations were made. Closed networks increased for the first 4–5 h and decreased thereafter (Fig. 1B), suggesting that network formation occurs in two distinct phases. Concurrently, average network area initially decreased followed by increased network area (Fig. 1B). Total nodes and branches in the overall network structure steadily decreased in the first 10 h and then stabilized. Quantitated KAV parameters provided a detailed summary of network components; however, these measurements do not assess node connectivity. Given the observation of unstable or “broken” branch connections in some ECFC samples, KAV software was programmed to calculate a branch-to-node ratio, as an assessment of network connectivity. KAV identified that the branch-to-node ratio decreased in the first 5 h as connectivity of the network increased and then remained unchanged from 5 to 10 h as the network structure became stable (Fig. 1B). After 10 h the ratio of branches to nodes increased slightly, indicative of node condensation and branch destabilization. In total, 5-h postplating was identified as a critical time point in network formation and represented the time of maximal network connectivity. By 5 h, the essential components of the network were established with subsequent changes in structure being indicative of structure stability and/or remodeling. Identification of this critical time point guided later studies, such that the first 5 h is referred to as phase 1 and 5–10 h is referred to as phase 2.
Reproducibility was validated by evaluating a single ECFC sample on multiple days (Fig. 1C). To confirm accuracy, a comparison was made between the traditional method of manual scoring and KAV. Total networks and nodes identified by KAV were comparable with manual counts (data not shown). KAV detected slightly more branches than identified manually (Fig. 1D). Given the difficulty in scoring >200 branches per well, this observation suggests that KAV may be more sensitive. Therefore this novel, validated analytic method provides a longitudinal assessment of vasculogenic potential that is currently lacking in traditional, static quantification methods. Furthermore, this approach identified dynamic changes in network structure with a transition at 5 h that may provide new mechanistic insights into vasculogenesis.
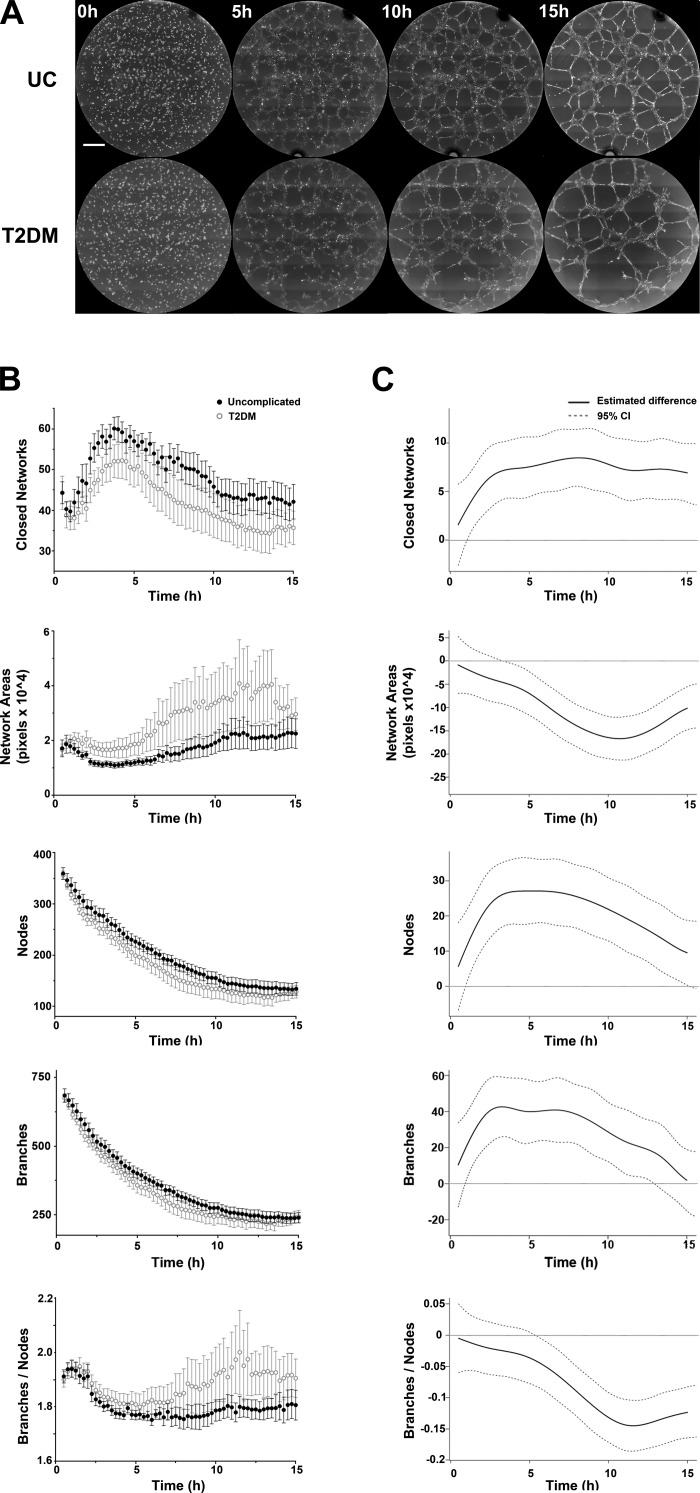
After establishing advanced assessments of ECFC function, KAV was applied to ECFCs exposed to maternal T2DM in utero to further elucidate functional deficits (23). KAV demonstrated that the complexity of network structures is qualitatively and quantitatively different between ECFCs from uncomplicated and T2DM pregnancies (Fig. 2 and Supplemental Video S1; Supplemental Material for this article is available online at the Journal website). Qualitatively, ECFCs from T2DM pregnancies form fewer closed networks and have larger network areas compared with ECFCs from uncomplicated pregnancies (Fig. 2A). These observations were confirmed by quantitative KAV analyses (Fig. 2B). Reductions in more subtle phenotypes, such as total nodes and total branches, were also identified by KAV for T2DM-exposed ECFCs (Fig. 2B). Additionally, decreased network stability, or increased broken branch connections, was observed in T2DM-exposed ECFCs during phase 2. Quantitation by KAV confirmed that the ratio of branches to nodes, a connectivity measure, is increased in T2DM-exposed ECFCs, with the greatest differences occurring in phase 2. This suggests that not only do ECFCs from T2DM pregnancies form fewer networks in phase 1 but also the network connections formed are not maintained. For example, UC ECFC samples exhibited the highest branch-to-node ratio in phase 1, just before maximal network formation. Conversely, ECFCs from T2DM pregnancies achieved maximal branch-to-node ratio more frequently in phase 2 (P = 0.005), suggesting a higher frequency of broken connections and condensing networks.
Fig. 2.
ECFCs from T2DM pregnancies exhibit impaired network formation. A: representative phase-contrast images of ECFCs plated on Matrigel at 5-h increments, starting at the time of plating (t = 0), are shown. ECFCs were obtained from uncomplicated pregnancies (UC) and pregnancies complicated by T2DM. The scale bar represents 500 µm. B: Kinetic Analysis of Vasculogenesis (KAV) software quantitated closed networks, network areas, nodes, branches, and the ratio of total branches divided by total nodes for both UC (●) and T2DM pregnancies (○). The data illustrated represent the means ± SE of 10 separate ECFC samples from each experimental group. C: differences between the mean kinetic curves of the two experimental groups for each parameter are shown as solid black lines with the 95% confidence interval (CI) represented by dotted lines. A significant difference between the curves for a specific time point is detected if the CI of the difference curve does not cross the reference line (y = 0) at that time point.
To rigorously evaluate for alterations of T2DM samples, estimated differences between time-lapse data were calculated (Fig. 2C). Statistical significance exists between uncomplicated and T2DM groups when the estimated difference (±95% confidence limit) does not cross the reference line (y = 0). These analyses indicate that for the majority of the 15-h time course, significant differences were detected between uncomplicated and T2DM data in all KAV parameters. Similar to qualitative observations, the greatest difference between the curves (farthest point from reference line) for network area and branch-to-node ratio occurs in phase 2. Total closed networks, nodes, and branches initially peak near 5 h and then level off, suggesting that differences in KAV parameters are phase specific. Together, these results confirm that T2DM-exposed ECFCs form fewer closed networks, nodes, and branches but develop larger network areas and an increased branch-to-node ratio compared with ECFCs from uncomplicated pregnancies.
Tissue cytometry quantitates dividing ECFC frequency and localization.
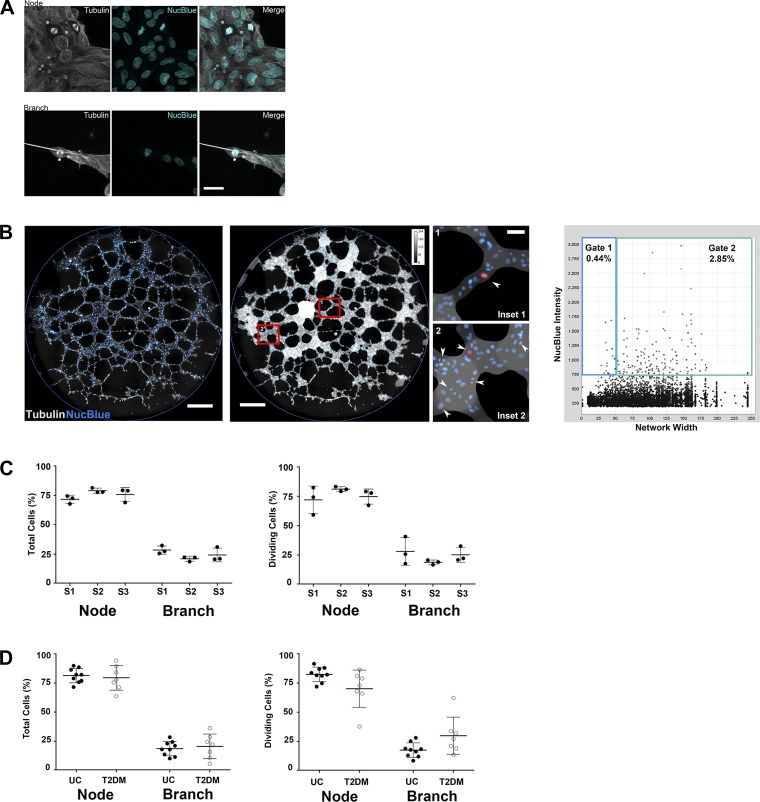
Disrupted vasculogenic potential may be due to altered proliferation, survival, and/or migration, which are challenging phenotypes to assess in situ because of the semisolid nature of the matrix used for in vitro studies. To address this technical obstacle, immunofluorescence methods were developed to evaluate proliferation and migration. Initially, to enhance visualization of key cellular components (i.e., cytoskeleton and nucleus), ECFC networks were fixed, permeabilized, and stained with anti-α-tubulin and NucBlue for high-resolution imaging using confocal microscopy. Interestingly, dividing ECFCs were observed in both nodes and branches (Fig. 3A). To quantitate the frequency and localization of dividing ECFCs, a TC software was developed. In this application, tubulin immunofluorescence generated a Euclidean distance map, which represented network structure width (Fig. 3B). High NucBlue intensity was programmed to recognize mitotic cells. The distance map, in combination with NucBlue intensity, allowed for identification of mitotic cells localized within a branch (thin <60 pixels) or node (thick >60 pixels; Fig. 3B, insets 1 and 2). TC allowed for gating on specific populations within the sample based on localization and NucBlue fluorescence. The TC methodology was validated across 3 days using multiple ECFC samples (Fig. 3C). Importantly, these analyses were conducted on entire wells, enabling simultaneous, large-scale quantitation of thousands of cells in situ across the whole network.
Fig. 3.
Tissue cytometry quantifies the frequency and localization of proliferating ECFCs on Matrigel. A: ECFCs were plated on Matrigel for 5 h, then fixed and stained for α-tubulin (gray) and NucBlue (cyan). Representative photomicrographs of dividing cells in a node and branch are shown. The scale bar represents 30 µm. B: immunofluorescence imaging was performed on entire wells (left, representative image, scale bar = 500 µm). Tissue cytometry (TC) analysis generated an overlay based on α-tubulin immunofluorescence (middle), which was used to quantitate network width and discriminate between node and branch structures. Mitotic cells, highlighted in red, were in branches (inset 1) and nodes (inset 2). TC integrated network width and NucBlue fluorescence intensity to generate scatterplots (right) that included all cells detected in the network (n = 4,733 cells). Gating identified mitotic cells within branches (gate 1) and nodes (gate 2). Scale bars represent 500 µm (panels at left and in middle) and 50 µm (inset 1). C: percentage of total ECFCs (left) and percentage of mitotic ECFCs (right) located in nodes or branches was determined by TC for three independent ECFC samples on 3 days (S1, sample 1; S2, sample 2; S3, sample 3). Individual points represent data from different days. Means ± SD are shown. D: percentage of total ECFCs (left) and percentage of mitotic ECFCs (right) located in nodes or branches was determined for UC and T2DM experimental groups. Graphs represent means ± SD (individual points represent a unique ECFC sample).
After establishing the TC method to assess dividing cells, proliferation of ECFCs from T2DM pregnancies was assessed. A 5-h time point was chosen on the basis of KAV results indicating the time for maximal closed networks. The majority of ECFCs from both experimental groups were localized in nodes (~75% of cells, including dividing and nondividing; Fig. 3D) and divided at similar rates (UC, 4.1 ± 2.4%; T2DM, 4.5 ± 2.8%; n = 9, UC; n = 7, T2DM). Dividing ECFCs in both groups were primarily located within nodes (75%) compared with branches (25%), indicating an equal propensity to divide irrespective of network location. Similar to TC data, ECFC proliferation was not different between T2DM and uncomplicated samples determined by flow cytometry (data not shown). Therefore neither ECFC localization nor intrauterine T2DM exposure altered the likelihood of ECFCs to divide during early network formation.
Fiji TrackMate assesses ECFC motility at the single-cell level.
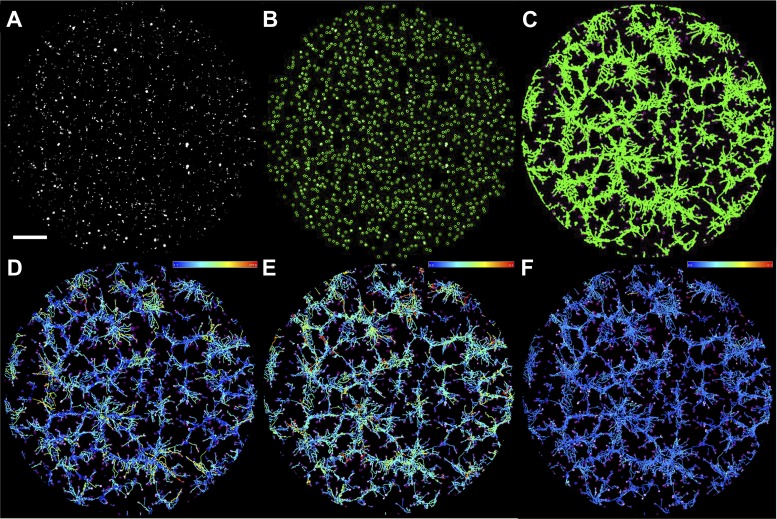
To kinetically assess ECFC motility, a time-lapse imaging approach was established to track cell movement during in vitro vasculogenesis. A baculoviral delivery system labeled ECFC nuclei with GFP to allow for individual cell tracking by Fiji TrackMate (Fig. 4; 35). Qualitatively, individual cells assembled into primitive network structures in phase 1, while more coordinated cell movements were visualized in phase 2 as ECFCs comprised larger network structures. Cumulative cell movement is summarized in tracks representing individual cell displacement or speed (Fig. 4 and Supplemental Video S2). Cell numbers identified by TrackMate were equivalent in phase 1 (P = 0.75) and phase 2 (P = 0.28). Additionally, TrackMate analyzed a similar number of tracks for both the uncomplicated and T2DM groups in phase 1 and phase 2 (P = 0.79 and P = 0.23, respectively). Together, these observations suggest equal plating densities and survival of ECFCs between experimental groups. Moreover, the number of individual ECFCs analyzed between the uncomplicated and T2DM groups was comparable.
Fig. 4.
ECFC motility was assessed during network formation using Fiji TrackMate. A: representative photomicrograph of nuclear GFP-labeled ECFCs immediately following plating on Matrigel. GFP fluorescence is shown in gray. B: GFP signal was used to segment individual ECFCs in TrackMate. ECFCs identified by the system are highlighted in green circles. C: the series of fluorescence images acquired over time were analyzed as stacks using Fiji TrackMate. TrackMate produces paths, shown in green, representative of total cell movement over the imaging interval (10 h shown). D–F: individual ECFC paths are color coded by the software on the basis of variables such as displacement (D), maximum speed (E), and mean speed (F). The color scales indicate the displacement or speed ranges, with high values depicted by orange/red and low values represented by blue. The scale bar represents 500 µm.
Using this approach, three parameters of motility were assessed: displacement, mean speed, and maximum speed. Each parameter positively correlated with the others in the uncomplicated as well as T2DM-exposed ECFCs (Table 3; all P values <0.05). Positive correlations between displacement and speed demonstrate that faster moving ECFCs also move farther, suggesting that ECFC movement is coordinated during network formation.
Table 3.
Motility parameter correlations
| UC | T2DM | |
|---|---|---|
| Phase 1 | ||
| Mean speed vs. max speed | 0.64 (0.634–0.651) | 0.65 (0.645–0.662) |
| Displacement vs. mean speed | 0.51 (0.495–0.516) | 0.50 (0.486–0.509) |
| Displacement vs. max speed | 0.26 (0.251–0.278) | 0.25 (0.235–0.263) |
| Phase 2 | ||
| Mean speed vs. max speed | 0.69 (0.682–0.702) | 0.67 (0.657–0.681) |
| Displacement vs. mean speed | 0.46 (0.449–0.479) | 0.53 (0.512–0.544) |
| Displacement vs. max speed | 0.27 (0.256–0.291) | 0.33 (0.306–0.345) |
Values are given as r (confidence interval). Max, maximum.
In phase 1, ECFCs exhibit increased displacement and speed compared with ECFCs in phase 2 (P = 0.0001 and P = 0.0002, respectively), suggesting that greater cell movement occurs in early network formation. Linear models were generated to determine if mean ECFC motility in phase 1 is maintained in phase 2. Assessments using linear models indicate that the amount of displacement, mean speed, and maximum speed were all positively correlated between phase 1 and phase 2 (Table 4). These analyses confirm that samples with greater displacement and speed in phase 1 continue to exhibit higher displacement and speed in phase 2.
Table 4.
Phase 1 and 2 motility parameters
| Phase 1 | Phase 2 | Slope | Standard Error | P Value |
|---|---|---|---|---|
| Displacement | Displacement | 0.4747 | 0.1279 | 0.0026 |
| Mean speed | Displacement | 4326.2 | 1506.3 | 0.0131 |
| Mean speed | Mean speed | 0.5741 | 0.2312 | 0.0275 |
| Mean speed | Max speed | 1.5891 | 0.6615 | 0.0319 |
| Max speed | Displacement | 1772.7 | 785.03 | 0.0418 |
| Max speed | Mean speed | 0.3745 | 0.0863 | 0.0008 |
| Max speed | Max speed | 1.0939 | 0.2326 | 0.0004 |
Overall, ECFC motility in the uncomplicated and T2DM groups was heterogeneous. Some ECFCs exhibited decreased motility indicated by reduced displacement (Fig. 5A). However, this phenotype was not consistently representative of a single clinical group. Following statistical analysis, no differences were detected in displacement, mean speed, or maximum speed between the uncomplicated and T2DM groups in phase 1 or phase 2 (Fig. 5B).
Fig. 5.
ECFCs exhibit a wide range of motility. A: time-lapse images of network formation were captured over 10 h. ECFC displacement was assessed separately for phase 1 (0–5 h) and phase 2 (5–10 h). Data from six UC and six T2DM-exposed ECFC samples are shown. A colored path represents total displacement, with greater displacement indicated by orange/red and less displacement indicated in blue. A color scale is located in uncomplicated 1 (UC 1), upper right corner. The scale bar represents 500 µm. B: histograms represent the frequency of ECFC displacement, mean speed, and maximum (max) speed in phases 1 and 2 for UC (black) and T2DM (red) samples. Approximately 35,000 individual ECFCs were analyzed for phase 1 (n = 20 samples, 10 UC and 10 T2DM), and 20,000 individual ECFCs were analyzed for phase 2 (n = 15, 8 UC and 7 T2DM).
Data integration to enhance mechanistic insights of vasculogenesis.
KAV, TC, and motility assays assess ECFC function within the same context of network formation. The power of this approach is the identification of correlated phenotypes that promote disrupted ECFC vasculogenic function and the potential discovery of mechanisms involved. Correlating TC data (Fig. 3D) with KAV data (Fig. 2B) revealed that ECFC samples with increased cell division in branches formed fewer closed networks (r = −0.50, P = 0.048). KAV revealed that some ECFC samples achieved maximal network number quickly while other samples displayed delayed maximal network formation. Therefore, to assess whether localized cell division impacted the rate of network formation, the time to maximum networks was analyzed. ECFCs with increased branch division reached maximal networks more quickly than samples with lower branch division (r = −0.51, P = 0.044). These data suggest that increased branch division and faster network formation result in an overall reduction in total network number. In addition, associations were discovered between KAV and motility data. Decreased ECFC displacement and mean speed in phase 1 were associated with a higher branch-to-node ratio (r = −0.85, P = 0.039 and r = −0.64, P = 0.035, respectively). Similarly, ECFCs with reduced displacement in phase 2 also had a higher ratio of branches to nodes, though not statistically significant (r = −0.27, P = 0.11). Importantly, these results indicate that ECFCs that move a shorter distance or at a slower speed have higher ratios of branches to nodes, which is indicative of reduced network connectivity (Fig. 6).
Fig. 6.
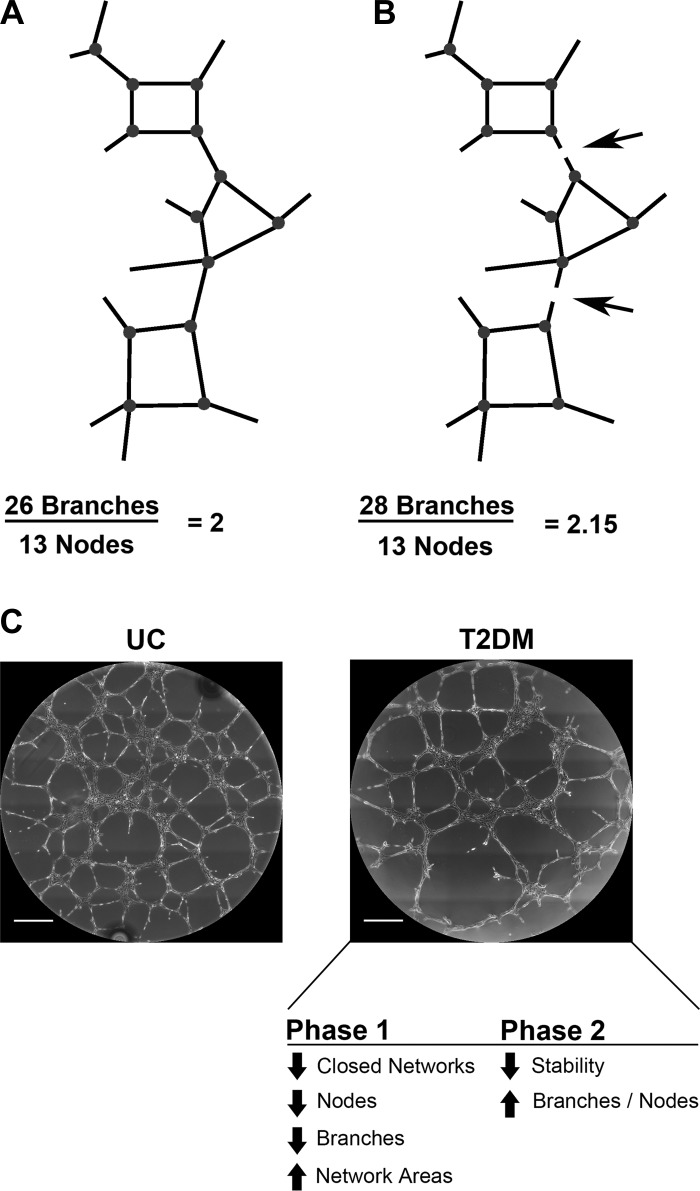
Schematic of network connectivity models of T2DM ECFC phenotypes. A: a model network structure composed of 26 branches and 13 nodes is completely connected to create 1 continuous structure. Because of structure continuity, even though each node has three to four branches, the ratio of branches to nodes equals 2. B: the same network structure is shown, but with breaks in two branches (arrows). No longer a continuous structure, the number of branches identified by KAV increases by 2, resulting in an increase in the branch-to-node ratio to 2.15. Decreased network connectivity results in discontinuous structures, which increases the ratio of branches to nodes. Network structures not to scale. C: representative phase-contrast images from UC and T2DM experimental groups are shown. In summary, our data support a model whereby, in phase 1, T2DM samples exhibit decreased closed networks, nodes, and branches resulting in increased network areas. These changes in early network formation contribute to an overall decrease in network stability leading to an increase in the branch-to-node ratio in phase 2, changes indicative of decreased network connectivity. Scale bars represent 500 µm.
DISCUSSION
Advancements in imaging technology have created new challenges for image processing and quantitative analysis. These challenges include managing large volumes of data and deriving new biologically meaningful results that are not dependent on manual analyses (14). Therefore development of automated image analysis is crucial for maximizing information obtained from independent experiments and providing opportunities for discovery of novel pathologic phenotypes. Automation enables multiparametric investigation of a broad range of quantitative measures that are too difficult to detect either manually or through previously established methods (2, 12, 14). Additionally, automation can increase assay sensitivity and reproducibility while minimizing time, labor, and bias. In the study of vasculogenesis, automation of image analysis allows for greater mechanistic understanding of the complex, kinetic process of network formation. Although some automated vasculogenic image analyses are commercially available, many technologies are cost prohibitive for large, kinetic studies (42). Therefore, a primary limitation in the field is an open-source platform for assessing kinetic measurements of vasculogenesis in vitro.
New techniques were optimized to kinetically assess ECFC function and identify correlative vasculogenic phenotypes. KAV measured numerous structural phenotypes, identifying two phases of network formation. The transition point between phases was associated with maximal network connectivity. Additionally, KAV calculated a novel and direct measure of connectivity, the branch-to-node ratio. Kinetic motility analyses determined how individual cell movement impacted network formation. Greater movement occurred during early network formation, and movement in phase 1 directly correlated with phase 2 movement. Future studies that assess directionality of cell movement are important to determine if ECFCs respond to promigratory stimuli with coordinated movements. Overall, these improvements to standard imaging and quantitation strategies provide insight into potential mechanisms contributing to altered vasculogenesis and can be applied to any vascular system or cell type.
Assessment of proliferation by nuclear markers, such as histone H3 phosphorylation, is a standard technique. However, assessing proliferation during network formation is novel. TC enabled identification of distinct cell populations based on fluorescence and localization. Thus TC is an innovative and powerful tool to assess a variety of cellular events, such as proliferation, apoptosis, and senescence in a large number of cells. Specifically, we applied this technique to quantitate localization of proliferating ECFCs within networks at a single-cell resolution. TC revealed that few ECFCs divide (2–8%) in early network formation. Dividing ECFCs are primarily in nodes where the majority of cells reside; however, ECFCs in branches have a similar propensity to divide. Importantly, this TC approach could be applied to any cellular event or protein expression that can be fluorescently labeled. Thus the potential impact of applying this newly developed methodology to unique biological systems is yet to be realized.
Assessing multiple phenotypes within the same biological system provides an opportunity to identify correlative phenotypes. In our system, negative correlations were identified between KAV parameters and TC data. Samples with higher branch proliferation formed maximal closed networks quickly, though fewer total closed networks overall. KAV parameters also correlated with ECFC motility. Specifically, ECFCs that move shorter distances or at slower speeds during early network formation have a higher branch-to-node ratio, or reduced connectivity. We speculate that ECFCs that move less form fewer nodes, resulting in longer branches and decreased network stability. Fewer nodes and decreased network stability (i.e., reduced connectivity) would result in an increased ratio of branches to nodes.
Though in vivo models provide a more complete view of vasculogenic function (37), the new approaches outlined for in vitro studies are informative without being cost prohibitive and can guide future in vivo studies. A concrete example is that the ratio of branches to nodes is not generally evaluated. However, it was the phenotype most strongly correlated with ECFC motility and one of the most informative phenotypes in differentiating ECFC vasculogenic function. On the basis of these findings, future studies that focus on understanding the basis for increased branch-to-node ratio could be highly instructive and promote mechanistic insights into altered vasculogenesis. Furthermore, the automated imaging analyses reported could be applied to in vivo models, broadening the potential impact of having these analytic, open-source resources available.
The techniques developed were implemented to identify how exposure to a maternal T2DM environment alters ECFC network formation. These new data confirm previous findings that ECFCs exposed to intrauterine T2DM have impaired vasculogenesis (23). However, time-lapse imaging identified two phases of vasculogenesis, which was previously unappreciated. KAV methodology demonstrated that ECFCs exposed to T2DM in utero form (phase 1) and maintain fewer networks (phase 2), resulting in increased average network areas. In T2DM ECFCs, the onset of a pathologic increase in the branch-to-node ratio in phase 2 is speculated to indicate network instability, or an inability to maintain branch connections. Interestingly, two T2DM ECFC samples (T2DM 2 and T2DM 3) were from pregnancies in which the mother had elevated hemoglobin A1C, indicating poor glucose control (Table 1). These ECFC samples had exacerbated KAV phenotypes compared with T2DM averages (data not shown). These observations suggest that measurements obtained by KAV may correlate with the severity of T2DM exposure in utero.
Surprisingly, ECFC proliferation and motility were not different between the uncomplicated and T2DM ECFC groups, suggesting that differences in network structure may not be attributable to these phenotypes. On the other hand, these unexpected results could be due to T2DM sample variability, method resolution, or a relatively low sample size. Additionally, differences between population-based functional assessments vs. single ECFC measurements may account for these findings. For instance, KAV, a population-based analysis, identified differences between clinical groups. Subsequent ECFC studies (i.e., proliferation and motility) were at a single-cell resolution. However, as discussed above, single-cell phenotypes correlated with measures of network formation. Therefore, subtle alterations in single ECFC function may impact overall cell coordination required for optimal network formation. Future studies that correlate individual cell functions with population-based readouts are predicted to reveal a better understanding of the complex process of vasculogenesis.
Studies using primary human stem/progenitors provide the opportunity to produce clinically relevant data. However, human samples introduce variability, which can introduce a barrier in identifying differences between groups with small sample sizes. Overall, greater variation was observed in T2DM samples, consistent with the hypothesis that ECFC samples from T2DM mothers are heterogeneous with varying functionality because of factors such as disease severity and therapeutics used during pregnancy (9, 23). Thus more sensitive readouts are necessary to delineate meaningful differences in a disease with significant heterogeneity.
Novel microscopic approaches were optimized to provide greater mechanistic insight into the dynamic process of vasculogenesis. KAV identified two phases of network formation and guided further mechanistic studies of proliferation and motility, which correlated with network structure parameters. These correlations provide new insight into how neonatal ECFC vasculogenesis may be altered from intrauterine T2DM exposure. Importantly, the methods outlined have broad implications beyond the scope reported here. Implementation of these approaches will enhance mechanistic assessment and improve functional readouts of vasculogenesis in numerous cell types or disease states.
GRANTS
This work was supported by National Institutes of Health Grants R01-HL-094725, P30-CA-82709, and U10-HD-063094 and the Riley Children's Foundation. Additionally, this publication was made possible with partial support from National Heart, Lung, and Blood Institute Award T32-HL-007910. Contract grant sponsors are National Institutes of Health, Contract Grants R01-HL-094725 (L. S. Haneline), P30-CA-82709 (Indiana University Simon Cancer Center), U10-HD-063094 (L. S. Haneline), UL1-TR-001108 (C. R. Gohn), and T32-HL-007910-15 (K. M. Varberg), and Riley Children's Foundation, Indianapolis, Indiana (L. S. Haneline).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.M.V., E.K.B., and L.S.H. conceived and designed the research; K.M.V. and S.W. performed experiments; K.M.V., S.W., C.C., W.T., E.K.B., and L.S.H. analyzed data; K.M.V., S.W., E.K.B., C.R.G., K.W.D., and L.S.H. interpreted results of experiments; K.M.V., S.W., and C.C. prepared figures; K.M.V. and S.W. drafted manuscript; K.M.V., S.W., C.C., W.T., E.K.B., C.R.G., and L.S.H. edited and revised manuscript; K.M.V., S.W., C.C., W.T., E.K.B., C.R.G., K.W.D., and L.S.H. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Lucy Miller, Leanne Hernandez, Dr. David Haas, and Brittany Yeley (Indiana University School of Medicine), Dr. Karen Pollok, Julie Mund, Matthew Repass, and Emily Sims (Angio BioCore at the Indiana University Simon Cancer Center), and Riddhi Shukla and Lauren Kneller (Indiana University School of Medicine) for excellent technical assistance. The authors also thank Drs. Maureen Harrington, Edward Srour, Richard Day, Mervin Yoder, and Matthias Clauss (Indiana University School of Medicine) for scholarly discussion as well as Janice Walls (Indiana University School of Medicine) for administrative support. All imaging was performed at the Indiana Center for Biological Microscopy, Indiana University School of Medicine.
REFERENCES
- 1.Abcam Anti-alpha tubulin antibody (DM1A) [Online]. http://www.abcam.com/alpha-tubulin-antibody-dm1a-loading-control-ab7291.html. [2017].
- 2.Allier CP, Kesavan SV, Coutard JG, Cioni O, Momey F, Navarro F, Menneteau M, Chalmond B, Obeid P, Haguet V, David-Watine B, Dubrulle N, Shorte S, van der Sanden B, Di Natale C, Hamard L, Wion D, Dolega ME, Picollet-D'hahan N, Gidrol X, Dinten JM. Video lensfree microscopy of 2D and 3D cultures of cells. In: Proceedings of SPIE, Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues XII, edited by Farkas DL, Nicolau DV, and Leif RC. San Francisco, CA: SPIE, 2014, vol. 8947, p. 89471H. doi: 10.1117/12.2038098. [DOI] [Google Scholar]
- 3.Arganda-Carreras I, Fernández-González R, Muñoz-Barrutia A, Ortiz-De-Solorzano C. 3D reconstruction of histological sections: application to mammary gland tissue. Microsc Res Tech 73: 1019–1029, 2010. doi: 10.1002/jemt.20829. [DOI] [PubMed] [Google Scholar]
- 4.Arnaoutova I, Kleinman HK. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc 5: 628–635, 2010. doi: 10.1038/nprot.2010.6. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T. Cell therapy and gene therapy using endothelial progenitor cells for vascular regeneration. Handb Exp Pharmacol 180: 181–194, 2007. doi: 10.1007/978-3-540-68976-8_8. [DOI] [PubMed] [Google Scholar]
- 6.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 7.Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care 34, Suppl 2: S285–S290, 2011. doi: 10.2337/dc11-s239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basile DP, Yoder MC. Circulating and tissue resident endothelial progenitor cells. J Cell Physiol 229: 10–16, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blue EK, DiGiuseppe R, Derr-Yellin E, Acosta JC, Pay SL, Hanenberg H, Schellinger MM, Quinney SK, Mund JA, Case J, Haneline LS. Gestational diabetes induces alterations in the function of neonatal endothelial colony-forming cells. Pediatr Res 75: 266–272, 2014. doi: 10.1038/pr.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115: e290–e296, 2005. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 11.Borghesi A, Garofoli F, Cabano R, Tzialla C, Bollani L, Stronati M. Circulating endothelial progenitor cells and diseases of the preterm infant. Minerva Pediatr 62, Suppl 1: 21–23, 2010. [PubMed] [Google Scholar]
- 12.Bray MA, Carpenter AE. CellProfiler Tracer: exploring and validating high-throughput, time-lapse microscopy image data. BMC Bioinformatics 16: 368, 2015. doi: 10.1186/s12859-015-0759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunt JC, Tataranni PA, Salbe AD. Intrauterine exposure to diabetes is a determinant of hemoglobin A(1)c and systolic blood pressure in pima Indian children. J Clin Endocrinol Metab 90: 3225–3229, 2005. doi: 10.1210/jc.2005-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol 7: R100, 2006. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpentier G. Angiogenesis Analyzer for ImageJ [Online]. Univ. Paris Est Créteil Val-de-Marne; http://image.bio.methods.free.fr/ImageJ/?Angiogenesis-Analyzer-for-ImageJ [20 Oct. 2012]. [Google Scholar]
- 16.Cell Signaling Technology Phospho-Histone H3 (Ser10) Antibody #9701 [Online]. https://www.cellsignal.com/products/primary-antibodies/phospho-histone-h3-ser10-antibody/9701 [2017].
- 17.Crabtree B, Subramanian V. Behavior of endothelial cells on Matrigel and development of a method for a rapid and reproducible in vitro angiogenesis assay. In Vitro Cell Dev Biol Anim 43: 87–94, 2007. doi: 10.1007/s11626-007-9012-x. [DOI] [PubMed] [Google Scholar]
- 18.Critser PJ, Yoder MC. Endothelial colony-forming cell role in neoangiogenesis and tissue repair. Curr Opin Organ Transplant 15: 68–72, 2010. doi: 10.1097/MOT.0b013e32833454b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30: 256–268, 2003. doi: 10.1016/S1046-2023(03)00032-X. [DOI] [PubMed] [Google Scholar]
- 20.Eguchi M, Masuda H, Asahara T. Endothelial progenitor cells for postnatal vasculogenesis. Clin Exp Nephrol 11: 18–25, 2007. doi: 10.1007/s10157-006-0448-1. [DOI] [PubMed] [Google Scholar]
- 21.Franks PW, Looker HC, Kobes S, Touger L, Tataranni PA, Hanson RL, Knowler WC. Gestational glucose tolerance and risk of type 2 diabetes in young Pima Indian offspring. Diabetes 55: 460–465, 2006. doi: 10.2337/diabetes.55.02.06.db05-0823. [DOI] [PubMed] [Google Scholar]
- 22.Georgescu A. Vascular dysfunction in diabetes: the endothelial progenitor cells as new therapeutic strategy. World J Diabetes 2: 92–97, 2011. doi: 10.4239/wjd.v2.i6.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingram DA, Lien IZ, Mead LE, Estes M, Prater DN, Derr-Yellin E, DiMeglio LA, Haneline LS. In vitro hyperglycemia or a diabetic intrauterine environment reduces neonatal endothelial colony-forming cell numbers and function. Diabetes 57: 724–731, 2008. doi: 10.2337/db07-1507. [DOI] [PubMed] [Google Scholar]
- 24.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 105: 2783–2786, 2005. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 25.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104: 2752–2760, 2004. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 26.Javed MJ, Mead LE, Prater D, Bessler WK, Foster D, Case J, Goebel WS, Yoder MC, Haneline LS, Ingram DA. Endothelial colony forming cells and mesenchymal stem cells are enriched at different gestational ages in human umbilical cord blood. Pediatr Res 64: 68–73, 2008. doi: 10.1203/PDR.0b013e31817445e9. [DOI] [PubMed] [Google Scholar]
- 27.Khoo CP, Micklem K, Watt SM. A comparison of methods for quantifying angiogenesis in the Matrigel assay in vitro. Tissue Eng Part C Methods 17: 895–906, 2011. doi: 10.1089/ten.tec.2011.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoo CP, Pozzilli P, Alison MR. Endothelial progenitor cells and their potential therapeutic applications. Regen Med 3: 863–876, 2008. doi: 10.2217/17460751.3.6.863. [DOI] [PubMed] [Google Scholar]
- 29.Kirton JP, Xu Q. Endothelial precursors in vascular repair. Microvasc Res 79: 193–199, 2010. doi: 10.1016/j.mvr.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Mead LE, Prater D, Yoder MC, Ingram DA. Isolation and characterization of endothelial progenitor cells from human blood. In: Current Protocols in Stem Cell Biology, chapt. 2, unit 2C.1, Hoboken, NJ: Wiley, 2008. doi: 10.1002/9780470151808.sc02c01s6. [DOI] [PubMed] [Google Scholar]
- 31.Mukai N, Akahori T, Komaki M, Li Q, Kanayasu-Toyoda T, Ishii-Watabe A, Kobayashi A, Yamaguchi T, Abe M, Amagasa T, Morita I. A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp Cell Res 314: 430–440, 2008. doi: 10.1016/j.yexcr.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Prasain N, Lee MR, Vemula S, Meador JL, Yoshimoto M, Ferkowicz MJ, Fett A, Gupta M, Rapp BM, Saadatzadeh MR, Ginsberg M, Elemento O, Lee Y, Voytik-Harbin SL, Chung HM, Hong KS, Reid E, O’Neill CL, Medina RJ, Stitt AW, Murphy MP, Rafii S, Broxmeyer HE, Yoder MC. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat Biotechnol 32: 1151–1157, 2014. doi: 10.1038/nbt.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia 21: 1141–1149, 2007. doi: 10.1038/sj.leu.2404676. [DOI] [PubMed] [Google Scholar]
- 34.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2014. http://www.R-project.org/. [Google Scholar]
- 35.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sievert W, Tapio S, Breuninger S, Gaipl U, Andratschke N, Trott KR, Multhoff G. Adhesion molecule expression and function of primary endothelial cells in benign and malignant tissues correlates with proliferation. PLoS One 9: e91808, 2014. doi: 10.1371/journal.pone.0091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simons M, Alitalo K, Annex BH, Augustin HG, Beam C, Berk BC, Byzova T, Carmeliet P, Chilian W, Cooke JP, Davis GE, Eichmann A, Iruela-Arispe ML, Keshet E, Sinusas AJ, Ruhrberg C, Woo YJ, Dimmeler S; American Heart Association Council on Basic Cardiovascular Sciences and Council on Cardiovascular Surgery and Anesthesia . State-of-the-art methods for evaluation of angiogenesis and tissue vascularization: a Scientific Statement from the American Heart Association. Circ Res 116: e99–e132, 2015. doi: 10.1161/RES.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon I, O'Reilly M, Ionescu L, Alphonse RS, Rajabali S, Zhong S, Vadivel A, Shelley WC, Yoder MC, Thébaud B. Functional differences between placental micro- and macrovascular endothelial colony-forming cells. Stem Cells Transl Med 5: 291–300, 2016. doi: 10.5966/sctm.2014-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89: E1–E7, 2001. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Chen Q, Zhang Z, Jiang F, Meng X, Yan H. Interleukin-10 overexpression improves the function of endothelial progenitor cells stimulated with TNF-α through the activation of the STAT3 signaling pathway. Int J Mol Med 35: 471–477, 2015. [DOI] [PubMed] [Google Scholar]
- 41.Watt SM, Athanassopoulos A, Harris AL, Tsaknakis G. Human endothelial stem/progenitor cells, angiogenic factors and vascular repair. J R Soc Interface 7: Suppl 6: S731–S751, 2010. doi: 10.1098/rsif.2010.0377.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wimasis WimTube [Online]. http://www.wimasis.com/en/products/13/WimTube [2014].
- 43.Winfree S, Khan S, Micanovic R, Eadon MT, Kelly KJ, Sutton TA, Phillips CL, Dunn KW, El-Achkar TM. Quantitative three-dimensional tissue cytometry to study kidney tissue and resident immune cells. J Am Soc Nephrol (Feb. 2, 2017). doi: 10.1681/ASN.2016091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood SN. Generalized Additive Models: An Introduction with R. Boca Raton, FL: CRC, 2006. [Google Scholar]
- 45.Yoder MC. Is endothelium the origin of endothelial progenitor cells? Arterioscler Thromb Vasc Biol 30: 1094–1103, 2010. doi: 10.1161/ATVBAHA.109.191635. [DOI] [PubMed] [Google Scholar]
- 46.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109: 1801–1809, 2007. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.