Abstract
Connexins (Cxs) are a group of integral membrane proteins that can form gap junctions between adjacent cells. Recently, it was reported that Cx43 is expressed not only in the plasma membrane but also in the inner mitochondrial membrane and that it regulates mitochondrial functions. Cx40 is predominantly expressed in vascular endothelial cells (ECs) and plays an important role in the electrical propagation between ECs and endothelial/smooth muscle cells. However, it is unknown whether Cx40 is expressed in the mitochondria and what the role of mitochondrial Cx40 is in endothelial functions. We observed in coronary ECs that Cx40 protein was expressed in the mitochondria, as determined by Western blot and immunofluorescence studies. We found that mouse coronary ECs (MCECs) isolated from Cx40 knockout (Cx40 KO) mice exhibited significantly lower resting mitochondrial calcium concentration ([Ca2+]mito) than MCECs from wild-type (WT) mice. After increase in cytosolic Ca2+ concentration ([Ca2+]cyto) with cyclopiazonic acid, calcium uptake into the mitochondria was significantly attenuated in MCECs from Cx40 KO mice compared with WT MCECs. There was no difference in resting [Ca2+]cyto and store-operated calcium entry in MCECs from WT and Cx40 KO mice. We also detected a significant decrease in the concentration of mitochondrial reactive oxygen species (ROS) in Cx40 KO MCECs. Cx40 overexpression in ECs significantly increased resting [Ca2+]mito level and calcium uptake by mitochondria in response to increased [Ca2+]cyto and augmented mitochondrial ROS production. These data suggest that mitochondrial Cx40 contributes to the regulation of mitochondrial calcium homeostasis.
Keywords: connexin, mitochondria, reactive oxygen species, endothelial cell, calcium imaging
gap junctions are cell membrane channels, and gap junction intercellular communication (GJIC) is a pathway for the intercellular electrical propagation and transportation of small molecules (e.g., ATP) (50). One gap junction is composed of two hemichannels (i.e., connexon), and each hemichannel is made of six connexins (Cxs) (17). It has been reported that some hemichannels can also pass small molecules between the cytoplasm and extracellular space (18, 45). To date, 21 Cx genes have been identified in the human genome and 20 in the mouse. Cx37, Cx40, Cx43, and Cx45 and Cx32 (in some cases) are expressed in the vascular wall, and Cx40 is highly expressed in endothelial cells (ECs) (7, 9, 15, 19, 26, 27, 44, 56, 63). It should be noted that levels of these Cxs might be slightly different in ECs from different organs. Cx40 knockdown or inhibition of Cx40 attenuates vascular relaxation (13) and inhibits EC proliferation and migration (3, 21). Cx40 knockout (Cx40 KO) mice develop hypertension due to enhanced renin secretion (31). Although endothelium-specific Cx40 KO mice are normotensive (10), previous studies demonstrated that the loss of functional Cx40 in ECs is associated with accelerated atherosclerosis and exercise-induced hypertension (10, 41). We have also demonstrated that decreased Cx40 expression in mouse coronary endothelial cells (MCECs) is responsible for impaired endothelium-derived hyperpolarization-dependent vascular relaxation in coronary arteries and decreased capillary density in the left ventricle in diabetic mice (36). These data indicate the critical role of Cx40 in the plasma membrane in endothelial functions.
In addition to the role of Cxs in the regulation of GJIC, there is increasing evidence that some Cxs are expressed and function in the mitochondrial membranes. Mitochondrial Cx43 has been reported in ECs (33, 40, 62) and cardiac myocytes (4–6, 23, 30, 39, 48, 58). Inhibition of Cx43 attenuates K+ uptake (6, 39), Ca2+ uptake (58), and ADP-stimulated complex I respiration (5) in the mitochondria of cardiac myocytes. Stress stimulation (e.g., ischemia-reperfusion) increases the translocation of Cx43 to the mitochondria, and, moreover, Cx43 reduces the damage induced by stress (23, 30, 33, 48, 60). These data suggest that mitochondrial Cx43 exerts a cardioprotective effect upon stress stimulation. Fowler et al. (20) demonstrated that Cx32 is expressed in the inner mitochondrial membrane of mouse hepatocytes; however, its function is not clear.
In the present study, we demonstrate for the first time that Cx40 is expressed in the mitochondria of coronary ECs and functions as a regulator of mitochondrial Ca2+ homeostasis.
MATERIALS AND METHODS
Reagents.
Medium 199 (M199), streptomycin-penicillin, trypsin-EDTA, EC growth supplement, FBS, anti-Cx40 (for immunofluorescent study), anti-GAPDH, MitoTracker Green, MitoSOX Red, fura-2 AM, rhod-2 AM, Dynabeads sheep anti-rat IgG, and paraformaldehyde were obtained from Thermo Fisher Scientific. Collagenase II and dispase II were purchased from Worthington Biochemical. The Qproteome Mitochondria Isolation Kit was from Qiagen. Anti-Cx40 [for Western blot (WB)], anti- ATP synthase subunit α (ATP5A), and anti-Actin were from Santa Cruz Biotechnology. Rat anti-mouse CD31 was obtained from BD Biosciences. All other chemicals were from Sigma-Aldrich.
Animals and cell lines.
This study was conducted in accordance with the guidelines established by the Institutional Animal Care and Use Committee at the University of Arizona. All animal use was in compliance with all current US government regulations concerning the care and use of laboratory animals. The University of Arizona has been certified (Animal Welfare Assurance no. A3248-01), and the approved protocol number for this study is 14-520. All surgery was performed under anesthesia with a mixture of ketamine (100 mg/kg ip) and xylazine (5 mg/kg ip), and all efforts were made to minimize suffering. Male C57BL/6 mice were purchased from The Jackson Laboratory. Cx40 KO and Cx37 KO mice were kindly provided by Dr. Janis Burt (Univ. of Arizona) and bred in the animal facility of the University of Arizona. KO mice were used for the experiments starting at 5 wk of age (age range: 5–24 wk), and age-matched control mice were used for each time experiment. Human coronary endothelial cells (HCECs) were obtained from Lonza Group and cultured in EC medium composed of M199 supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 20 μg/ml EC growth supplement, and 16 U/ml heparin.
Isolation of mouse coronary endothelial cells.
MCECs were isolated as described previously (34–36). Briefly, dissected mouse heart was minced and incubated with M199 containing 1 mg/ml collagenase II and 0.6 U/ml dispase II for 1 h at 37°C. The digested material was collected and incubated with magnetic beads that were prepared as follows: Dynabeads sheep anti-rat IgG was incubated with rat anti-mouse CD31 monoclonal antibody (1 μg/ml) at 4°C overnight. The cell suspension was incubated with beads for 1 h at 4°C, and then MCECs were captured and isolated by the Dynal magnet.
Mitochondria isolation.
Mitochondria were isolated from HCECs with the Qproteome Mitochondria Isolation Kit according to the manufacturer’s instructions.
Western blot.
HCECs, MCECs, and isolated mitochondria from HCECs were lysed in lysate buffer (in mM: 25 HEPES, 150 NaCl, 10 MgCl2, and 1 EDTA, with 1% IGEPAL, 1% protease inhibitor, and 1% phosphate inhibitor, pH 7.5). Proteins were separated with a 10% SDS-polyacrylamide gel and transferred to nitrocellulose membranes. Blots were incubated with the following primary antibodies: anti-Cx40 (Santa Cruz, sc-20466; 1:2,000), anti-ATP5A (Santa Cruz, sc-136178; 1:2,000), anti-Actin (Santa Cruz, sc-1616; 1:4,000), anti-Cx37 (Thermo Fisher Scientific, 42-4400; 1:1,000), anti-Cx43 (Thermo Fisher Scientific, 71-0700; 1:2,000), anti-Cx45 (Thermo Fisher Scientific, 41-5800; 1:2,000), or anti-GAPDH (Thermo Fisher Scientific, MA5-15738; 1:4,000), followed by horseradish peroxidase-linked secondary antibody incubation. The immunoblots were identified with the SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, 34087). Band intensity was calculated with ImageJ 1.48v (National Institutes of Health), and the intensity data from focus proteins were normalized to GAPDH or Actin (for whole cell and cytosolic samples) or ATP5A (for mitochondrial samples) and are expressed in arbitrary units.
Detection of Cx40 localization in mitochondria by superresolution microscopy.
MCECs on coverslips were fixed with 100% methanol for 10 min at −20°C. Cells were blocked with 5% iron-fortified calf serum (IFCS) in PBS-Tween for 1 h followed by incubation with primary antibody against Cx40 (Thermo Fisher Scientific, 36-4900; 1:200) and ATP5A (Santa Cruz, sc-136178; 1:200) overnight. Anti-rabbit Alexa Fluor 488 and anti-mouse Alexa Fluor 594 antibodies (Thermo Fisher Scientific, A-21441 and A-21201; 1:4,000) and Hoechst 33342 (Thermo Fisher Scientific, H1399; 1 μg/ml) in 1% IFCS were used in secondary probing. Images were taken with a ×63 oil lens (numerical aperture: 1.40) and a Zeiss Elyra S.1 Structured Illumination Microscope and processed with Structured Illumination using ZEN 2 software (Carl Zeiss Microscopy).
Cytosolic Ca2+ concentration measurement with fura-2 AM.
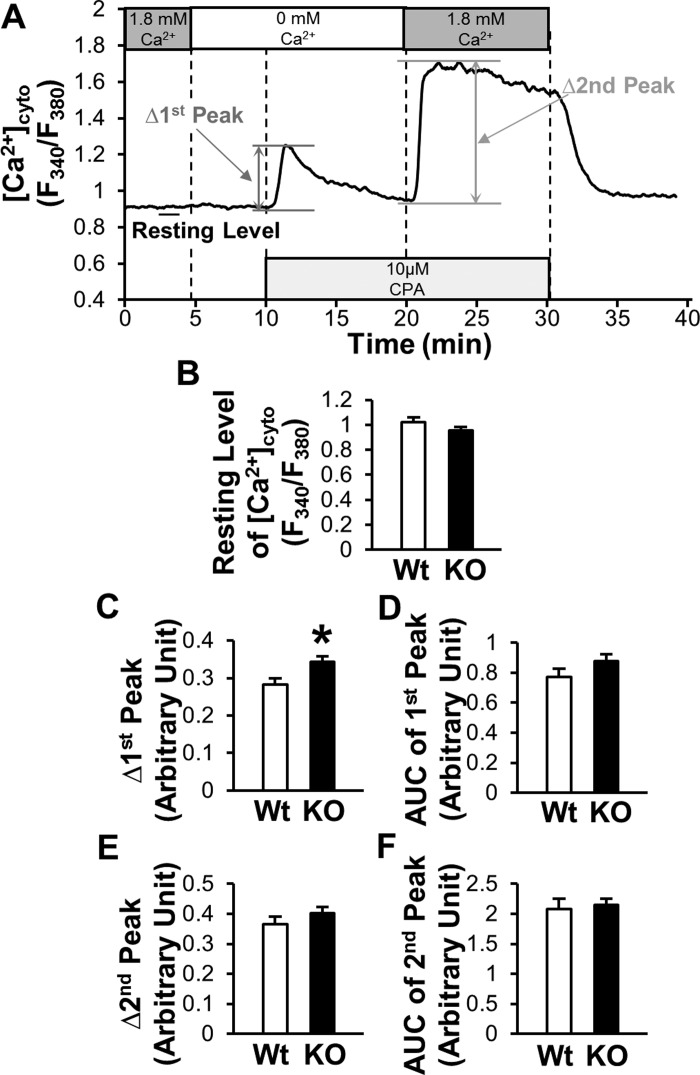
Cytosolic Ca2+ concentration ([Ca2+]cyto) in MCECs was measured with a modification of previously described methods (16). Cells on coverslips were loaded with fura-2 AM (4 µmol/l) for 1 h in the dark at room temperature. The dye was washed for 30 min with Ca2+-physiological salt solution (PSS) (in mM: 140 NaCl, 4.7 KCl, 1.8 CaCl2, 1.2 MgCl2, 10 HEPES, 10 glucose). The fura-2 fluorescence (340- and 380-nm excitation; 510-nm emission) from the cells and background fluorescence were imaged with an Eclipse Ti-E inverted fluorescence microscope (Nikon, Tokyo, Japan). Data are described as the ratio F340/F380. The peak [Ca2+]cyto increase (Δpeak) and the area under the curve (AUC) of the peak were calculated (see Fig. 3A). Cells were isolated from at least three mice.
Fig. 3.
Effects of Cx40 deletion on [Ca2+]cyto in MCECs. A: typical record of the change in [Ca2+]cyto in MCECs. 1st peak describes the rise in [Ca2+]cyto by CPA treatment in the absence of extracellular Ca2+ (indirect indicator of [Ca2+]ER), and 2nd peak indicates store-operated Ca2+ entry (SOCE). Data are described as a ratio (F340/F380). B–F: summarized data of resting level of [Ca2+]cyto (B), Δ1st peak (C), AUC of 1st peak (D), Δ2nd peak (E), and AUC of 2nd peak (F) in MCECs of WT mice (n = 74 cells) and Cx40 KO mice (n = 96 cells). Data are means ± SE. *P < 0.05 vs. WT.
Mitochondrial calcium concentration measurement with rhod-2 AM.
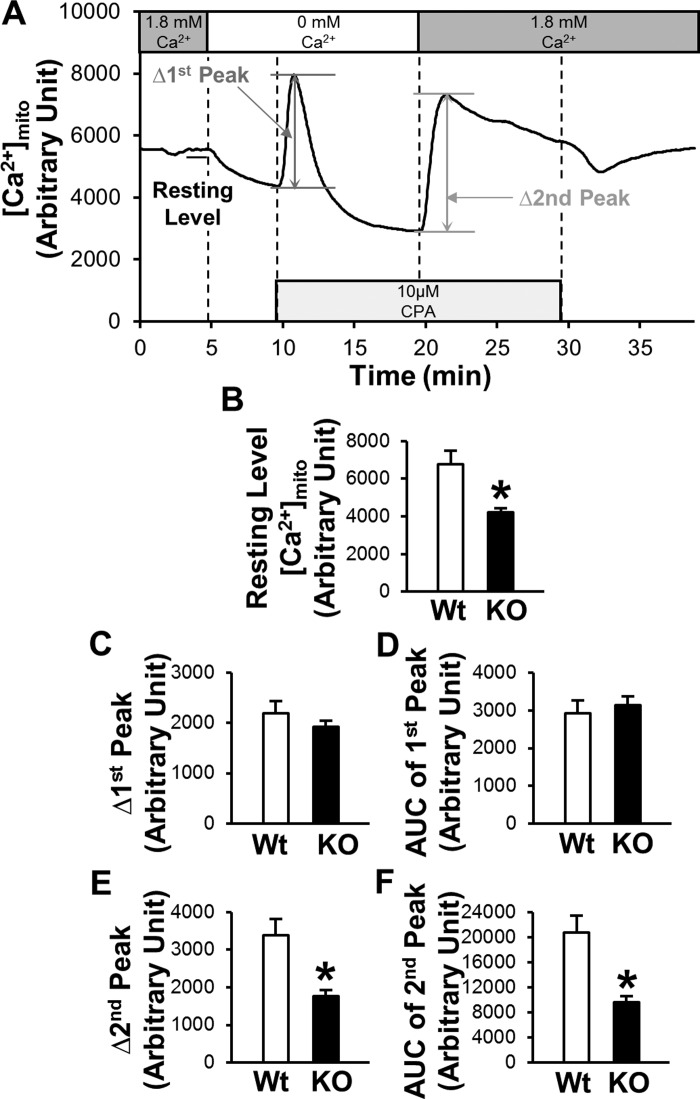
Mitochondrial calcium concentration ([Ca2+]mito) in MCECs was measured with a method described in our previous publication (52). Cells on coverslips were loaded with rhod-2 AM (2 µmol) for 20 min in the dark at 37°C and washed with Ca2+-PSS for 70 min at 32°C. Rhod-2 fluorescence (530-nm excitation; 580-nm emission) from the cells and background fluorescence were imaged with a Nikon Eclipse Ti-E inverted fluorescence microscope with a ×20 objective lens. The background intensity was subtracted from the cell intensity. The peak [Ca2+]mito increase and the AUC of the peak were calculated (see Fig. 4A). Cells were isolated from at least three mice.
Fig. 4.
Deletion of Cx40 decreases the resting level of [Ca2+]mito, Δ2nd peak, and AUC of 2nd peak in MCECs. A: typical record of the change in [Ca2+]mito in MCECs. B–F: summarized data of resting level of [Ca2+]mito (B), Δ1st peak (C), AUC of 1st peak (D), Δ2nd peak (E), and AUC of 2nd peak (F) in MCECs of WT mice (n = 50 cells) or Cx40 KO mice (n = 100 cells). Data are means ± SE. *P < 0.05 vs. WT.
[Ca2+]mito measurement with mitocameleon.
We first generated Tie2-mitocameleon-adenovirus (Tie2-mtcam-Adv). The CMV-mtcam plasmid was kindly provided by Dr. Roger Tsien from the University of California, San Diego (46). Mtcam encodes duplicated mitochondria-targeting sequences and the cameleon sequence [enhanced cyan fluorescent protein (ECFP)-calmodulin-enhanced yellow fluorescent protein (EYFP) that generates a fluorescence resonance energy transfer (FRET) signal when Ca2+ binds to calmodulin]. Cx40-Adv was generated as described in our previous report (36). HCECs were transduced with Tie2-mtcam-Adv (100 pfu/cell) with Cx40-Adv or control-Adv (100 pfu/cell). Two days after transduction, the experiments for [Ca2+]mito were conducted. FRET (excitation 430 nm, emission 535 nm) and CFP (excitation 430 nm, emission 470 nm) fluorescence were imaged with a Nikon Eclipse Ti-E inverted fluorescence microscope with a ×20 objective lens. Data are described as the ratio FRET/CFP. The peak [Ca2+]mito increase and the AUC of the peak were calculated for individual cells. We used rhod-2 in MCECs instead of Tie2-mtcam-Adv because a high titer of Tie2-mtcam-Adv (1,000 pfu/cell) was required to achieve sufficient signal in MCECs and this titer was cytotoxic.
Measurement of mitochondrial reactive oxygen species.
Mitochondrial reactive oxygen species ([ROS]mito) was measured as described previously (11, 37). Briefly, MCECs were stained with 5 µM MitoSOX Red (a mitochondrial ROS indicator; excitation 510 nm, emission 580 nm) and 100 nM MitoTracker Green (excitation 490 nm, emission 516 nm) for 30 min at 37°C. Images were captured with a Nikon Eclipse Ti-E inverted fluorescence microscope with a ×60 objective lens (numerical aperture: 1.4). The fluorescence intensities were calculated with ImagePro-PLUS 7.0 software (Media Cybernetics). MitoTracker Green signal was used to define the mitochondrial area, and the intensity of MitoSOX Red was measured in the mitochondria. The background intensity was subtracted from the cell intensity.
Statistical analysis.
Statistical analysis was performed with SigmaPlot 12.5 (Systat Software). Data are expressed as means ± SE. Student’s t-test for unpaired samples was used to identify significant differences. Differences were considered to be statistically significant when P < 0.05.
RESULTS
Evidence of Cx40 existence in mitochondria of coronary ECs.
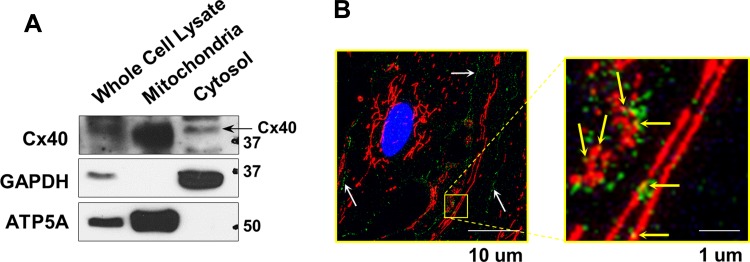
HCECs were used and mitochondria and cytosol were subfractionated from whole cell lysate. We used mitochondrial ATP5A as a mitochondria-specific marker and GAPDH as a marker for the cytosolic fraction for the WB study. The strong immunoreactivity of ATP5A and a lack of GAPDH signal in the mitochondrial fraction demonstrate the high purity of our mitochondrial preparation. Strong Cx40 signal was detected in the mitochondrial fraction isolated from HCECs (Fig. 1A). In addition, superresolution microscopy images showed the colocalization of Cx40 with Tom20 (a mitochondrial marker) in ECs isolated from mice (MCECs) (Fig. 1B).
Fig. 1.
Mitochondrial Cx40 expression in coronary ECs. A: Western blots showing Cx40, GAPDH, and ATP5A expression in whole cell lysate, mitochondrial fraction, and cytosolic fraction of HCECs. ATP5A was used as a specific marker for the mitochondria and GAPDH as a marker for cytosolic proteins. B: photomicrographs show typical image of Cx40 localization in MCECs. MCECs were stained with Cx40 (green) and Tom20 (a marker of mitochondria; red). Left: white arrows indicate the lining of Cx40 in the plasma membrane. Right: yellow arrows indicate the Cx40 in (or on) the mitochondria.
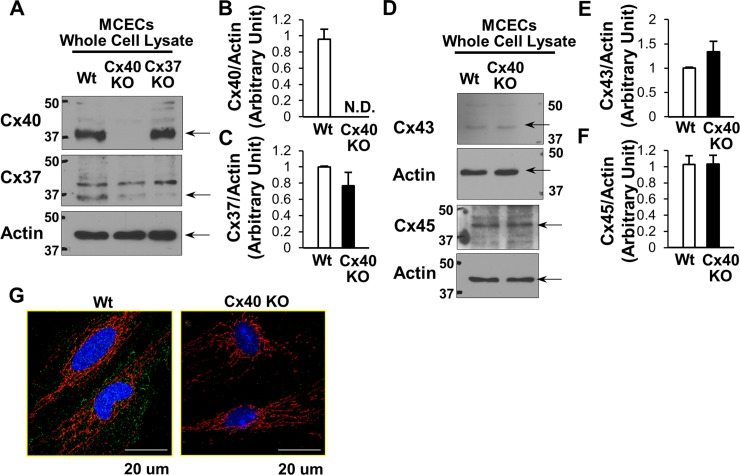
Cx protein levels in MCECs from Cx40 KO mice.
Previous studies found that the loss of Cx40 alters the expression of other Cxs in ECs (29, 55). To confirm the expression profile of Cxs in Cx40 KO mice, MCECs were isolated from Cx40 KO and wild-type (WT) mice. As shown in Fig. 2, A and B, Cx40 protein was not detectable in MCECs from Cx40 KO mice. We found that there is no significant change in Cx37, Cx43, and Cx45 levels (Fig. 2. C–F) in MCECs from these mice. Systemic Cx37 deletion did not affect Cx40 level in MCECs. Immunofluorescent images demonstrate Cx40 expression in the plasma membrane, mitochondria, and cytosol in WT MCECs and diminished signals of Cx40 in Cx40 KO MCECs (Fig. 2G).
Fig. 2.
Protein levels of Cx40, Cx37, Cx43, and Cx45 in MCECs. A and D: typical WB images of Cx40, Cx37, Cx43, and Cx45 protein levels in the whole lysate of MCECs isolated from WT, Cx40 KO, and Cx37 KO mice. Actin was used as a loading control for samples of whole lysate from MCECs. B: Cx40 protein level normalized to actin: WT (n = 5) and Cx40 KO (n = 5). Data are means ± SE. C: Cx37 protein levels in MCECs of WT and Cx40 KO mice (n = 4 in each group). Data are means ± SE. E: Cx43 protein levels in MCECs of WT and Cx40 KO mice (n = 5 in each group). Data are means ± SE. F: Cx45 protein levels in MCECs of WT and Cx40 KO mice (n = 6 in each group). Data are means ± SE. G: photomicrographs show typical image of Cx40 expression in WT MCECs (left) and Cx40 KO MCECs (right). MCECs were stained with Cx40 (green) and Tom20 (a marker of mitochondria; red).
Effects of Cx40 deletion on cytosolic Ca2+ concentration.
Figure 3A illustrates the time course of [Ca2+]cyto measured with fura-2 AM. After the resting level of [Ca2+]cyto was obtained in Ca2+-PSS, perfusate was changed to Ca2+-free-PSS for 5 min. Then, we added 10 µM cyclopiazonic acid [CPA, a sarco(endo)plasmic reticulum Ca2+-ATPase blocker] to Ca2+ free-PSS. This “1st peak” of [Ca2+]cyto is representative of the Ca2+ leak from the endoplasmic reticulum (ER), and it is an indirect indicator of Ca2+ concentration in the ER ([Ca2+]ER). Ten minutes after the application of CPA, the perfusate was changed to Ca2+-PSS supplemented with CPA to initiate the “2nd peak” of [Ca2+]cyto. This 2nd peak results from store-operated calcium entry (SOCE). As shown in Fig. 3B, the resting level of [Ca2+]cyto was not different between MCECs from WT and Cx40 KO mice. Interestingly, we observed that Δ1st peak was slightly, but significantly, increased in MCECs from Cx40 KO mice compared with WT MCECs (Fig. 3C). However, Δ2nd peak and AUC of 1st peak and 2nd peak did not show any difference between WT MCECs and Cx40 KO MCECs (Fig. 3, D–F).
[Ca2+]mito was decreased in Cx40 KO MCECs.
Next, we used rhod-2 AM to measure [Ca2+]mito in MCECs. Figure 4 demonstrates that the resting level of [Ca2+]mito was significantly decreased in Cx40 KO MCECs compared with MCECs from WT mice. Although there was no difference in Δ1st peak and AUC of 1st peak, Δ2nd peak and AUC of 2nd peak were significantly decreased in Cx40 KO MCECs compared with the control.
Cx40 deletion decreased the level of [ROS]mito.
It has been reported that change of [Ca2+]mito influences [ROS]mito (1, 8, 22, 52). Therefore, we examined whether [ROS]mito would be altered in MCECs isolated from Cx40 KO mice. MitoSOX was used to measure [ROS]mito, and MitoTracker Green was used to label mitochondria. As shown in Fig. 5, [ROS]mito was significantly decreased in MCECs from Cx40 KO mice compared with the control.
Fig. 5.
MCECs isolated from Cx40 KO mice exhibit decreased mitochondrial ROS ([ROS]mito) formation. A: representative images showing [ROS]mito in MCECs isolated from WT (left) and Cx40 KO mice (right). Bars, 10 μm. B: summarized data of [ROS]mito. Ic, intensity of the cell; Ib, intensity of background. n = 66 cells (WT) and 106 cells (Cx40 KO). Data are means ± SE. *P < 0.05 vs. WT.
Effect of Cx40 overexpression on [Ca2+]mito.
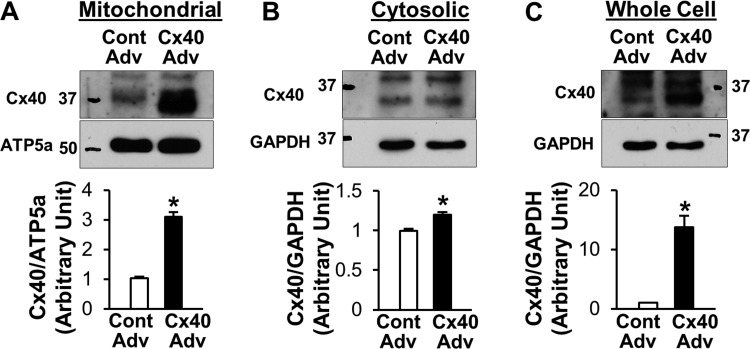
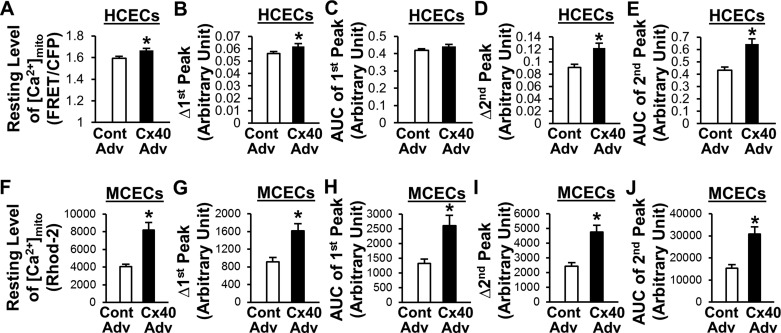
To further confirm the effect of Cx40 on [Ca2+]mito, we overexpressed Cx40 in HCECs with an adenovirus carrying the Cx40 gene (Cx40-Adv). As shown in Fig. 6, A–C, Cx40-Adv transduction significantly increased the level of Cx40 protein in mitochondrial, cytosolic, and whole cell samples, respectively. [Ca2+]mito was measured with mtcam in this study. mtcam consists of YFP and CFP that are linked by calmodulin (46) and includes a mitochondria-targeting sequence. When [Ca2+]mito increases, the ratiometric FRET signal to CFP signal also increases. In contrast to the results from Cx40 KO MCECs, overexpression of Cx40 significantly increased the resting level of [Ca2+]mito, Δ1st peak, Δ2nd peak, and AUC of 2nd peak (Fig. 7, A–E). We also repeated the experiments using MCECs (Fig. 7, F–J). After Cx40-Adv transduction, [Ca2+]mito in MCECs was measured with rhod-2 AM. In line with the results in HCECs, Cx40 overexpression in MCECs significantly increased all parameters of [Ca2+]mito.
Fig. 6.
Cx40 adenovirus transduction in HCECs increases Cx40 levels in mitochondrial fraction. A: mitochondrial fraction. B: cytosolic fraction. C: whole cell lysate. Graphs show the summarized data of Cx40 protein level normalized to respective loading control. n = 5 (A), 5 (B), and 8 (C). Cont Adv, control adenovirus; Cx40 Adv, Cx40-adenovirus. Data are means ± SE. *P < 0.05 vs. Cont Adv.
Fig. 7.
Overexpression of Cx40 increases resting level of [Ca2+]mito, Δ1st peak, Δ2nd peak, and AUC of 2nd peak in HCECs and MCECs. A–E: summarized data of resting [Ca2+]mito level (A), Δ1st peak (B), AUC of 1st peak (C), Δ2nd peak (D), and AUC of 2nd peak (E) in HCECs transduced with Cont Adv (n = 75) or Cx40 Adv (n = 76). Data are means ± SE. *P < 0.05 vs. Cont Adv. F–J: summarized data of resting [Ca2+]mito level (F), Δ1st peak (G), AUC of 1st peak (H), Δ2nd peak (I), and AUC of 2nd peak (J) in MCECs transduced with Cont Adv (n = 68) or Cx40 Adv (n = 69). Data are means ± SE. *P < 0.05 vs. Cont Adv.
Cx40 overexpression increased the level of [ROS]mito.
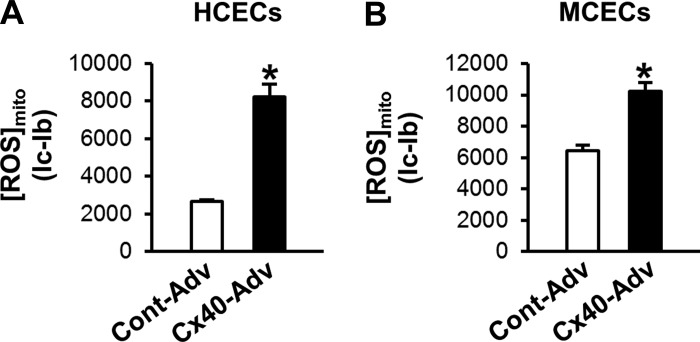
Cx40 deletion decreased [Ca2+]mito and [ROS]mito (Fig. 4 and Fig. 5). Because Cx40 overexpression significantly increased [Ca2+]mito (Fig. 7), we examined whether [ROS]mito was altered by Cx40 overexpression. Cx40-Adv was used to transduce ECs, and [ROS]mito was measured with MitoSOX. As shown in Fig. 8, Cx40 overexpression significantly increased the level of [ROS]mito in HCECs and MCECs. These data suggest that the increase in Cx40 in mitochondrial might be harmful to the cells.
Fig. 8.
Cx40 overexpression augments mitochondrial ROS ([ROS]mito) formation in HCECs and MCECs. [ROS]mito was measured 48 h after Cx40 gene transduction. A: summarized data of [ROS]mito in HCECs. n = 63 (Cont Adv) and 52 (Cx40 Adv). Data are means ± SE. *P < 0.05 vs. Cont Adv. B: summarized data of [ROS]mito in MCECs. n = 81 (Cont Adv) and n = 93 (Cx40 Adv). Data are means ± SE. *P < 0.05 vs. Cont Adv.
DISCUSSION
The role of Cxs located at the plasma membrane in the regulation of hemichannel activity has been well studied for many decades, whereas the functional role of mitochondrial Cxs in cellular functions has been less well explored. The first publication that evidenced the existence of a mitochondrial Cx was in 2002 by Li et al. (33). They identified the expression of Cx43 in the mitochondrial fraction with WB and measured the signal of Cx43 in the mitochondria with immunofluorescence in human umbilical vein endothelial cells. Later, two groups reported that retinal ECs also expressed mitochondrial Cx43 and demonstrated that high-glucose treatment decreased mitochondrial Cx43 expression, which resulted in altered mitochondrial function (40) and mitochondrial morphology (62). Most studies on mitochondrial Cx43 have been conducted in cardiac myocytes, perhaps because Cx43 is strongly expressed in these cells, and the localization and function of mitochondrial Cx43 are therefore better understood in cardiac myocytes (4–6, 23, 30, 39, 48, 58).
In vascular ECs, Cx40 is strongly expressed and plays an important role in the regulation of GJIC (7, 9, 15, 19, 24, 26, 27, 56, 63). There are, however, no reports investigating mitochondrial Cx40 in ECs. Therefore, we examined in this study whether Cx40 is expressed in the mitochondria from ECs. First, we confirmed that the mitochondrial isolation yielded highly purified mitochondria, as determined by WB (Fig. 1A). With this method, we observed a strong Cx40 signal in the mitochondrial fraction. It should be noted that the same amount of protein (8 μg/lane) was loaded for whole cell, mitochondrial, and cytosolic samples. From WB it appears that Cx40 is most abundant in the mitochondrial fraction; however, this may not be the case. The pool of mitochondrial proteins is much smaller than the pool of cytosolic and whole cell proteins, and Cx40 constitutes a higher percentage of the protein found in mitochondria than in the cytosol or whole cell. By loading an equal amount of protein for each sample type, a larger relative abundance of Cx40 is therefore expected in mitochondria, as we observed.
As shown in Fig. 1B, left, the majority of Cx40 is located at the plasma membrane, where it forms hemichannels and gap junctions (indicated with white arrows). However, at higher magnification (Fig. 1B, right) Cx40 is shown to colocalize with a mitochondrial marker, suggesting that Cx40 also exists on (or in) the mitochondria. In this study we do not identify where Cx40 is in the mitochondria (e.g., inner or outer mitochondrial membrane) because the low concentration of mitochondria that was obtained from coronary ECs precluded the further separation of inner and outer membrane fractions. It has been shown that Cx43 is expressed in the inner mitochondrial membrane in cardiac myocytes (48), and it will be of interest to determine the mitochondrial membrane localization of Cx40 in future studies.
To examine the functional role of mitochondrial Cx40 in ECs, we used systemic Cx40 KO mice to isolate MCECs. In Fig. 2, we confirmed the expression of Cx40, Cx37, Cx43, and Cx45 in WT MCECs and the absence of detectable Cx40 in MCECs of Cx40 KO mice. Cx45 expression levels were not altered in Cx40 KO MCECs, while Cx40 deletion markedly decreased Cx37 levels and increased Cx43 levels. This finding is in line with previous reports (2, 54), and there is as yet no definitive explanation as to why Cx40 KO decreases Cx37 expression. It may be possible that Cx40 somehow acts as a regulator of gene expression for other Cxs, but further experiments are needed to evaluate this hypothesis. We also tested Cx32 expression level in MCECs and found that Cx32 was not detectable in MCECs (data not shown).
The available information about the functional role of mitochondrial Cxs comes only from the studies on Cx43 in cardiac myocytes. Miro-Casas et al. (39) demonstrated that Cx43 hemichannels on the mitochondrial membrane gate K+ and that inhibition of Cx43 decreases K+ uptake into mitochondria. Boengler et al. (6) used Cx43 mimetic peptide to inhibit Cx43 and showed an inhibition of K+ influx in the mitochondria. Mitochondrial K+ channels are important regulators of inner mitochondrial membrane integrity by balancing potassium concentration in the intermembrane space and matrix and maintaining the mitochondrial membrane potential (12, 14, 28, 49, 53, 59, 61). It is known that the opening of mitochondrial K+ channels leads to the depolarization of the mitochondrial membrane, reduces mitochondrial Ca2+ overload, and results in cardioprotection (38, 42, 43, 57). There is increasing evidence that mitochondrial Cx43 reduces mitochondrial damage induced by pathophysiological stress (23, 30, 33, 48, 60).
Srisakuldee et al. (58) demonstrated that phosphorylation of Cx43 attenuates Ca2+-induced mitochondrial swelling; however, there is no evidence showing that mitochondrial Cx43 directly regulates [Ca2+]mito. In most cells, including ECs, mitochondrial Ca2+ overload leads to mitochondria-induced cell apoptosis (25, 32). Therefore, inhibition of mitochondrial Ca2+ overload is a beneficial approach to decrease cell apoptosis under pathophysiological conditions (52). Our data show that the loss of Cx40 decreased the resting [Ca2+]mito and attenuated Ca2+ influx into the mitochondria after SOCE without changing the levels in [Ca2+]cyto, suggesting that mitochondrial Cx40 directly regulates mitochondrial Ca2+ uptake. In addition, overexpression of Cx40 increased the resting level of [Ca2+]mito and the peak of Ca2+ influx in the mitochondria. These data suggest that pathophysiological increase of mitochondrial Cx40 leads to augmented Ca2+ uptake into the mitochondria and might result in enhanced mitochondria-induced cell apoptosis. In contrast to the result from mitochondrial Cx40, the loss of Cx40 in the plasma membrane has maladaptive effects on endothelial functions (10, 31, 35, 36, 41). We have demonstrated that restoring Cx40 levels in diabetic MCECs had a beneficial effect on diabetes-induced endothelial dysfunction (35, 36). The result of the present study was therefore contrary to our expectations, and we will examine the role of mitochondrial Cx40 in endothelial functions under pathophysiological conditions (e.g., diabetes) in future experiments.
In the present study, Δ1st peak of [Ca2+]cyto was slightly enhanced in MCECs from Cx40 KO mice compared with WT MCECs. The increase of [Ca2+]cyto indicates that 1) Ca2+ leak from the ER is enhanced and/or 2) [Ca2+]ER is increased. Since AUC of 1st peak was not significantly changed, the cause of the increase of Δ1st peak is likely the upregulation of Ca2+ leak from the ER. Ca2+ leak from the ER in ECs is mediated by the inositol 1,4,5-trisphosphate receptor on the ER. One possible explanation for the increase in Δ1st peak in Cx40 KO MCECs is that Cx40 deletion influences inositol 1,4,5-trisphosphate expression as shown in other proteins (2, 54).
It has been reported that Cx40 inhibition did not affect stimulation-induced [Ca2+]cyto increase in uterine artery ECs (64). The cause of these contradictory results might be the difference in the tissue of origin. The increase of [Ca2+]mito induced by SOCE was prominently altered by the deletion of Cx40, but no change in Δ1st peak of [Ca2+]mito, suggesting that Cx40-mediated Ca2+ uptake into the mitochondria might not be mediated through the direct Ca2+ transport from the ER.
Mitochondria are constantly generating ROS as a by-product of the activity of the electron transport chain. However, excess ROS production is harmful to cellular functions and potentially leads to apoptosis. We and other investigators have shown that mitochondrial ROS formation is also controlled by [Ca2+]mito (1, 47, 51, 52, 65). We found that Cx40 deletion decreased mitochondrial ROS formation while Cx40 overexpression increased [ROS]mito, implying that mitochondrial Cx40 might regulate the level of [ROS]mito via altering [Ca2+]mito.
In this study, we do not identify whether mitochondrial Cx40 forms gap junctions or acts as hemichannels in the mitochondrial membrane. Miro-Casas et al. (39) implied that mitochondrial Cx43 in cardiac myocytes might potentially form hemichannels; however, it is methodologically difficult to confirm the form of connexin channels (i.e., hemichannel or gap junctions) in mitochondria. It has been reported that hemichannels in the plasma membrane can also transfer small molecules like gap junctions (18, 45).
In conclusion, coronary ECs express Cx40 not only in the plasma membrane but also in the mitochondria. Mitochondrial Cx40 functions as a Ca2+ gate, regulating Ca2+ uptake into the mitochondria and mitochondrial ROS formation. The findings in this study provide important and useful information that can be used to further study mitochondrial Cx40 in ECs under pathophysiological conditions.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-115578 to A. Makino.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.G., R.S., and A.M. performed experiments; R.G., R.S., and A.M. analyzed data; R.G. and A.M. interpreted results of experiments; R.G. and A.M. prepared figures; R.G. drafted manuscript; R.G., R.S., B.T.S., and A.M. approved final version of manuscript; B.T.S. and A.M. edited and revised manuscript.
REFERENCES
- 1.Adam-Vizi V, Starkov AA. Calcium and mitochondrial reactive oxygen species generation: how to read the facts. J Alzheimers Dis 20, Suppl 2: S413–S426, 2010. doi: 10.3233/JAD-2010-100465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso F, Boittin FX, Bény JL, Haefliger JA. Loss of connexin40 is associated with decreased endothelium-dependent relaxations and eNOS levels in the mouse aorta. Am J Physiol Heart Circ Physiol 299: H1365–H1373, 2010. doi: 10.1152/ajpheart.00029.2010. [DOI] [PubMed] [Google Scholar]
- 3.Alonso F, Domingos-Pereira S, Le Gal L, Derré L, Meda P, Jichlinski P, Nardelli-Haefliger D, Haefliger JA. Targeting endothelial connexin40 inhibits tumor growth by reducing angiogenesis and improving vessel perfusion. Oncotarget 7: 14015–14028, 2016. doi: 10.18632/oncotarget.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boengler K, Dodoni G, Rodriguez-Sinovas A, Cabestrero A, Ruiz-Meana M, Gres P, Konietzka I, Lopez-Iglesias C, Garcia-Dorado D, Di Lisa F, Heusch G, Schulz R. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc Res 67: 234–244, 2005. doi: 10.1016/j.cardiores.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Boengler K, Ruiz-Meana M, Gent S, Ungefug E, Soetkamp D, Miro-Casas E, Cabestrero A, Fernandez-Sanz C, Semenzato M, Di Lisa F, Rohrbach S, Garcia-Dorado D, Heusch G, Schulz R. Mitochondrial connexin 43 impacts on respiratory complex I activity and mitochondrial oxygen consumption. J Cell Mol Med 16: 1649–1655, 2012. doi: 10.1111/j.1582-4934.2011.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boengler K, Ungefug E, Heusch G, Leybaert L, Schulz R. Connexin 43 impacts on mitochondrial potassium uptake. Front Pharmacol 4: 73, 2013. doi: 10.3389/fphar.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brisset AC, Isakson BE, Kwak BR. Connexins in vascular physiology and pathology. Antioxid Redox Signal 11: 267–282, 2009. doi: 10.1089/ars.2008.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287: C817–C833, 2004. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 9.Cai WJ, Koltai S, Kocsis E, Scholz D, Schaper W, Schaper J. Connexin37, not Cx40 and Cx43, is induced in vascular smooth muscle cells during coronary arteriogenesis. J Mol Cell Cardiol 33: 957–967, 2001. doi: 10.1006/jmcc.2001.1360. [DOI] [PubMed] [Google Scholar]
- 10.Chadjichristos CE, Scheckenbach KE, van Veen TA, Richani Sarieddine MZ, de Wit C, Yang Z, Roth I, Bacchetta M, Viswambharan H, Foglia B, Dudez T, van Kempen MJ, Coenjaerts FE, Miquerol L, Deutsch U, Jongsma HJ, Chanson M, Kwak BR. Endothelial-specific deletion of connexin40 promotes atherosclerosis by increasing CD73-dependent leukocyte adhesion. Circulation 121: 123–131, 2010. doi: 10.1161/CIRCULATIONAHA.109.867176. [DOI] [PubMed] [Google Scholar]
- 11.Cho YE, Basu A, Dai A, Heldak M, Makino A. Coronary endothelial dysfunction and mitochondrial reactive oxygen species in type 2 diabetic mice. Am J Physiol Cell Physiol 305: C1033–C1040, 2013. doi: 10.1152/ajpcell.00234.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Marchi U, Sassi N, Fioretti B, Catacuzzeno L, Cereghetti GM, Szabò I, Zoratti M. Intermediate conductance Ca2+-activated potassium channel (KCa3.1) in the inner mitochondrial membrane of human colon cancer cells. Cell Calcium 45: 509–516, 2009. doi: 10.1016/j.ceca.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 13.de Wit C, Roos F, Bolz SS, Kirchhoff S, Krüger O, Willecke K, Pohl U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res 86: 649–655, 2000. doi: 10.1161/01.RES.86.6.649. [DOI] [PubMed] [Google Scholar]
- 14.Dolga AM, Netter MF, Perocchi F, Doti N, Meissner L, Tobaben S, Grohm J, Zischka H, Plesnila N, Decher N, Culmsee C. Mitochondrial small conductance SK2 channels prevent glutamate-induced oxytosis and mitochondrial dysfunction. J Biol Chem 288: 10792–10804, 2013. doi: 10.1074/jbc.M113.453522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esseltine JL, Laird DW. Next-generation connexin and pannexin cell biology. Trends Cell Biol 26: 944–955, 2016. doi: 10.1016/j.tcb.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Estrada IA, Donthamsetty R, Debski P, Zhou MH, Zhang SL, Yuan JX, Han W, Makino A. STIM1 restores coronary endothelial function in type 1 diabetic mice. Circ Res 111: 1166–1175, 2012. doi: 10.1161/CIRCRESAHA.112.275743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans WH. Cell communication across gap junctions: a historical perspective and current developments. Biochem Soc Trans 43: 450–459, 2015. doi: 10.1042/BST20150056. [DOI] [PubMed] [Google Scholar]
- 18.Fasciani I, Temperán A, Pérez-Atencio LF, Escudero A, Martínez-Montero P, Molano J, Gómez-Hernández JM, Paino CL, González-Nieto D, Barrio LC. Regulation of connexin hemichannel activity by membrane potential and the extracellular calcium in health and disease. Neuropharmacology 75: 479–490, 2013. doi: 10.1016/j.neuropharm.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 19.Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid Redox Signal 11: 251–266, 2009. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fowler SL, Akins M, Zhou H, Figeys D, Bennett SA. The liver connexin32 interactome is a novel plasma membrane-mitochondrial signaling nexus. J Proteome Res 12: 2597–2610, 2013. doi: 10.1021/pr301166p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gärtner C, Ziegelhöffer B, Kostelka M, Stepan H, Mohr FW, Dhein S. Knock-down of endothelial connexins impairs angiogenesis. Pharmacol Res 65: 347–357, 2012. doi: 10.1016/j.phrs.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol 6: 260–271, 2015. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goubaeva F, Mikami M, Giardina S, Ding B, Abe J, Yang J. Cardiac mitochondrial connexin 43 regulates apoptosis. Biochem Biophys Res Commun 352: 97–103, 2007. doi: 10.1016/j.bbrc.2006.10.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haefliger JA, Nicod P, Meda P. Contribution of connexins to the function of the vascular wall. Cardiovasc Res 62: 345–356, 2004. doi: 10.1016/j.cardiores.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Hajnóczky G, Csordás G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, Yi M. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium 40: 553–560, 2006. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill CE, Rummery N, Hickey H, Sandow SL. Heterogeneity in the distribution of vascular gap junctions and connexins: implications for function. Clin Exp Pharmacol Physiol 29: 620–625, 2002. doi: 10.1046/j.1440-1681.2002.03699.x. [DOI] [PubMed] [Google Scholar]
- 27.Hong T, Hill CE. Restricted expression of the gap junctional protein connexin 43 in the arterial system of the rat. J Anat 192: 583–593, 1998. doi: 10.1046/j.1469-7580.1998.19240583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature 352: 244–247, 1991. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- 29.Isakson BE, Damon DN, Day KH, Liao Y, Duling BR. Connexin40 and connexin43 in mouse aortic endothelium: evidence for coordinated regulation. Am J Physiol Heart Circ Physiol 290: H1199–H1205, 2006. doi: 10.1152/ajpheart.00945.2005. [DOI] [PubMed] [Google Scholar]
- 30.Jeyaraman MM, Srisakuldee W, Nickel BE, Kardami E. Connexin43 phosphorylation and cytoprotection in the heart. Biochim Biophys Acta 1818: 2009–2013, 2012. doi: 10.1016/j.bbamem.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 31.Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. Connexin40 regulates renin production and blood pressure. Kidney Int 72: 814–822, 2007. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- 32.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 1787: 1395–1401, 2009. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Brodsky S, Kumari S, Valiunas V, Brink P, Kaide J, Nasjletti A, Goligorsky MS. Paradoxical overexpression and translocation of connexin43 in homocysteine-treated endothelial cells. Am J Physiol Heart Circ Physiol 282: H2124–H2133, 2002. doi: 10.1152/ajpheart.01028.2001. [DOI] [PubMed] [Google Scholar]
- 34.Luo S, Truong AH, Makino A. Isolation of mouse coronary endothelial cells. J Vis Exp 113: e53985, 2016. doi: 10.3791/53985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makino A, Dai A, Han Y, Youssef KD, Wang W, Donthamsetty R, Scott BT, Wang H, Dillmann WH. O-GlcNAcase overexpression reverses coronary endothelial cell dysfunction in type 1 diabetic mice. Am J Physiol Cell Physiol 309: C593–C599, 2015. doi: 10.1152/ajpcell.00069.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makino A, Platoshyn O, Suarez J, Yuan JX, Dillmann WH. Downregulation of connexin40 is associated with coronary endothelial cell dysfunction in streptozotocin-induced diabetic mice. Am J Physiol Cell Physiol 295: C221–C230, 2008. doi: 10.1152/ajpcell.00433.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makino A, Scott BT, Dillmann WH. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia 53: 1783–1794, 2010. doi: 10.1007/s00125-010-1770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCully JD, Levitsky S. The mitochondrial KATP channel and cardioprotection. Ann Thorac Surg 75: S667–S673, 2003. doi: 10.1016/S0003-4975(02)04689-1. [DOI] [PubMed] [Google Scholar]
- 39.Miro-Casas E, Ruiz-Meana M, Agullo E, Stahlhofen S, Rodríguez-Sinovas A, Cabestrero A, Jorge I, Torre I, Vazquez J, Boengler K, Schulz R, Heusch G, Garcia-Dorado D. Connexin43 in cardiomyocyte mitochondria contributes to mitochondrial potassium uptake. Cardiovasc Res 83: 747–756, 2009. doi: 10.1093/cvr/cvp157. [DOI] [PubMed] [Google Scholar]
- 40.Mohammad G, Kowluru RA. Novel role of mitochondrial matrix metalloproteinase-2 in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci 52: 3832–3841, 2011. doi: 10.1167/iovs.10-6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morton SK, Chaston DJ, Howitt L, Heisler J, Nicholson BJ, Fairweather S, Bröer S, Ashton AW, Matthaei KI, Hill CE. Loss of functional endothelial connexin40 results in exercise-induced hypertension in mice. Hypertension 65: 662–669, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04578. [DOI] [PubMed] [Google Scholar]
- 42.O’Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res 94: 420–432, 2004. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Rourke B. Mitochondrial ion channels. Annu Rev Physiol 69: 19–49, 2007. doi: 10.1146/annurev.physiol.69.031905.163804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamoto T, Akiyama M, Takeda M, Gabazza EC, Hayashi T, Suzuki K. Connexin32 is expressed in vascular endothelial cells and participates in gap-junction intercellular communication. Biochem Biophys Res Commun 382: 264–268, 2009. doi: 10.1016/j.bbrc.2009.02.148. [DOI] [PubMed] [Google Scholar]
- 45.Palacios-Prado N, Huetteroth W, Pereda AE. Hemichannel composition and electrical synaptic transmission: molecular diversity and its implications for electrical rectification. Front Cell Neurosci 8: 324, 2014. doi: 10.3389/fncel.2014.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer AE, Giacomello M, Kortemme T, Hires SA, Lev-Ram V, Baker D, Tsien RY. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem Biol 13: 521–530, 2006. doi: 10.1016/j.chembiol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Peng TI, Jou MJ. Oxidative stress caused by mitochondrial calcium overload. Ann NY Acad Sci 1201: 183–188, 2010. doi: 10.1111/j.1749-6632.2010.05634.x. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Sinovas A, Boengler K, Cabestrero A, Gres P, Morente M, Ruiz-Meana M, Konietzka I, Miró E, Totzeck A, Heusch G, Schulz R, Garcia-Dorado D. Translocation of connexin 43 to the inner mitochondrial membrane of cardiomyocytes through the heat shock protein 90-dependent TOM pathway and its importance for cardioprotection. Circ Res 99: 93–101, 2006. doi: 10.1161/01.RES.0000230315.56904.de. [DOI] [PubMed] [Google Scholar]
- 49.Rusznák Z, Bakondi G, Kosztka L, Pocsai K, Dienes B, Fodor J, Telek A, Gönczi M, Szucs G, Csernoch L. Mitochondrial expression of the two-pore domain TASK-3 channels in malignantly transformed and non-malignant human cells. Virchows Arch 452: 415–426, 2008. doi: 10.1007/s00428-007-0545-x. [DOI] [PubMed] [Google Scholar]
- 50.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83: 1359–1400, 2003. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 51.Santo-Domingo J, Wiederkehr A, De Marchi U. Modulation of the matrix redox signaling by mitochondrial Ca(2.). World J Biol Chem 6: 310–323, 2015. doi: 10.4331/wjbc.v6.i4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sasaki K, Donthamsetty R, Heldak M, Cho YE, Scott BT, Makino A. VDAC: old protein with new roles in diabetes. Am J Physiol Cell Physiol 303: C1055–C1060, 2012. doi: 10.1152/ajpcell.00087.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siemen D, Loupatatzis C, Borecky J, Gulbins E, Lang F. Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem Biophys Res Commun 257: 549–554, 1999. doi: 10.1006/bbrc.1999.0496. [DOI] [PubMed] [Google Scholar]
- 54.Simon AM, McWhorter AR. Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J Cell Sci 116: 2223–2236, 2003. doi: 10.1242/jcs.00429. [DOI] [PubMed] [Google Scholar]
- 55.Simon AM, McWhorter AR. Role of connexin37 and connexin40 in vascular development. Cell Commun Adhes 10: 379–385, 2003. doi: 10.1080/cac.10.4-6.379.385. [DOI] [PubMed] [Google Scholar]
- 56.Söhl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res 62: 228–232, 2004. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 57.Soltysinska E, Bentzen BH, Barthmes M, Hattel H, Thrush AB, Harper ME, Qvortrup K, Larsen FJ, Schiffer TA, Losa-Reyna J, Straubinger J, Kniess A, Thomsen MB, Brüggemann A, Fenske S, Biel M, Ruth P, Wahl-Schott C, Boushel RC, Olesen SP, Lukowski R. KCNMA1 encoded cardiac BK channels afford protection against ischemia-reperfusion injury. PLoS One 9: e103402, 2014. doi: 10.1371/journal.pone.0103402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Srisakuldee W, Makazan Z, Nickel BE, Zhang F, Thliveris JA, Pasumarthi KB, Kardami E. The FGF-2-triggered protection of cardiac subsarcolemmal mitochondria from calcium overload is mitochondrial connexin 43-dependent. Cardiovasc Res 103: 72–80, 2014. doi: 10.1093/cvr/cvu066. [DOI] [PubMed] [Google Scholar]
- 59.Stowe DF, Gadicherla AK, Zhou Y, Aldakkak M, Cheng Q, Kwok WM, Jiang MT, Heisner JS, Yang M, Camara AK. Protection against cardiac injury by small Ca2+-sensitive K+ channels identified in guinea pig cardiac inner mitochondrial membrane. Biochim Biophys Acta 1828: 427–442, 2013. doi: 10.1016/j.bbamem.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun Y, Zhao X, Yao Y, Qi X, Yuan Y, Hu Y. Connexin 43 interacts with Bax to regulate apoptosis of pancreatic cancer through a gap junction-independent pathway. Int J Oncol 41: 941–948, 2012. doi: 10.3892/ijo.2012.1524. [DOI] [PubMed] [Google Scholar]
- 61.Szabò I, Bock J, Jekle A, Soddemann M, Adams C, Lang F, Zoratti M, Gulbins E. A novel potassium channel in lymphocyte mitochondria. J Biol Chem 280: 12790–12798, 2005. doi: 10.1074/jbc.M413548200. [DOI] [PubMed] [Google Scholar]
- 62.Trudeau K, Muto T, Roy S. Downregulation of mitochondrial connexin 43 by high glucose triggers mitochondrial shape change and cytochrome C release in retinal endothelial cells. Invest Ophthalmol Vis Sci 53: 6675–6681, 2012. doi: 10.1167/iovs.12-9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yeh HI, Dupont E, Coppen S, Rothery S, Severs NJ. Gap junction localization and connexin expression in cytochemically identified endothelial cells of arterial tissue. J Histochem Cytochem 45: 539–550, 1997. doi: 10.1177/002215549704500406. [DOI] [PubMed] [Google Scholar]
- 64.Yi FX, Boeldt DS, Gifford SM, Sullivan JA, Grummer MA, Magness RR, Bird IM. Pregnancy enhances sustained Ca2+ bursts and endothelial nitric oxide synthase activation in ovine uterine artery endothelial cells through increased connexin 43 function. Biol Reprod 82: 66–75, 2010. doi: 10.1095/biolreprod.109.078253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol 292: H2023–H2031, 2007. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]