Abstract
Caveolins (Cavs) are ~20 kDa scaffolding proteins that assemble as oligomeric complexes in lipid raft domains to form caveolae, flask-shaped plasma membrane (PM) invaginations. Caveolae (“little caves”) require lipid-lipid, protein-lipid, and protein-protein interactions that can modulate the localization, conformational stability, ligand affinity, effector specificity, and other functions of proteins that are partners of Cavs. Cavs are assembled into small oligomers in the endoplasmic reticulum (ER), transported to the Golgi for assembly with cholesterol and other oligomers, and then exported to the PM as an intact coat complex. At the PM, cavins, ~50 kDa adapter proteins, oligomerize into an outer coat complex that remodels the membrane into caveolae. The structure of caveolae protects their contents (i.e., lipids and proteins) from degradation. Cellular changes, including signal transduction effects, can destabilize caveolae and produce cavin dissociation, restructuring of Cav oligomers, ubiquitination, internalization, and degradation. In this review, we provide a perspective of the life cycle (biogenesis, degradation), composition, and physiologic roles of Cavs and caveolae and identify unanswered questions regarding the roles of Cavs and cavins in caveolae and in regulating cell physiology.1
Keywords: caveolin, cavin, lipid raft, caveolae
caveolins (Cavs) are ~20 kDa oligomeric proteins required for the formation of caveolae, which are lipid raft plasma membrane (PM) domains enriched in proteins and lipids, including cholesterol and sphingolipids (154). Caveolae are flask-shaped membrane invaginations, formed by cis/medial-Golgi-PM transport of oligomeric Cavs embedded in cholesterol-rich membranes. Once at the cell surface, these complexes interact with cavins, adapter proteins that form oligomers and assist in membrane curvature (reviewed in refs 85, 144). Based, at least in part, on the ability of Cavs to scaffold protein partners, caveolae play key roles in a variety of cellular responses, including: signal transduction (Table 1); transport of nutrients into and out of cells [e.g., insulin receptor activation stimulates translocation of the GLUT4 glucose transporter to caveolae in adipocytes (185)]; and cellular entry of certain pathogens, toxins, and endocytic cargo [e.g., simian virus 40 (133), cholera toxin (136), albumin (120)]. Caveolae and the functions they regulate have been recently reviewed: e.g., caveolae as membrane sensors and organizers (144, 164), in cytoskeletal interactions (70), endo/exocytosis (25), and signal transduction (186). Moreover, mutations in Cavs and cavins are associated with a variety of diseases (Table 2).1
Table 1.
Examples of caveolae-associated signal transduction proteins
| Signal Transduction Protein | Mechanism(s) of Association | References |
|---|---|---|
| Receptors | ||
| G protein-coupled receptors (GPCRs, e.g., adrenergic, adenosine, angiotensin-1, opioid, serotonin) | Transmembrane (TM), fatty acylation (FA), Cav association (Cav) | (47, 49, 71, 139, 226) |
| Steroid hormone acceptors (e.g., estrogen receptor-α) | TM, FA, Cav | (2, 23, 99) |
| Transforming growth factor-β receptors (e.g., bone morphogenic receptor II) | TM, Cav | (163) |
| Tyrosine kinase (e.g., insulin, EGFR, NGF, IGF, PDGF) | TM, FA, Cav | (28, 75, 152) |
| Inositol 1,4,5-triphosphate receptor (IP3R) | TM, FA, Cav | (48, 189) |
| Ion channels, reporters, and exchangers | ||
| Ca2+-ATPase | TM, Cav | (189) |
| Ca2+ pumps (e.g., Na+/Ca2+ exchanger type 1) | TM, Cav | (31) |
| L-type Ca2+ | TM, Cav | (31, 110) |
| Large-conductance Ca2+-activated K+ | TM, Cav | (222) |
| Transient receptor potential cation (TRPC) | TM, Cav | (90, 109, 129, 192) |
| Na+/K+-ATPase | TM, Cav | (63, 107) |
| Voltage-gated K+ | TM, Cav | (1, 26, 115) |
| KATP | TM, Cav | (180) |
| Kinases | ||
| Src-family | FA, Cav | (35, 94, 95, 101, 104, 148, 197, 200, 207, 218) |
| Protein kinase A | Cav | (73, 97, 166, 167, 180) |
| Protein kinase Cα, ζ | Cav | (45, 159, 179) |
| p42/p44 Mitogen-activated protein kinase | Cav | (10, 37, 52) |
| p38 Mitogen-activated protein kinase | Cav | (10) |
| Phosphatidylinositol-4,5-bisphosphate 3-kinase | PI(4,5)P2 binding | (191, 198) |
| Protein kinase B | PI(3,4,5)P3 binding | (62, 82, 100, 135, 190, 191, 198, 230, 231) |
| Other postreceptor components | ||
| Heterotrimeric GTP binding (G) protein α-subunits | FA, Cav | (15, 26, 49, 71, 77, 101, 103, 130, 134) |
| Heterotrimeric GTP binding (G) protein βγ-subunits | FA | (77) |
| Ras | FA, Cav | (160, 176, 199) |
| Adenylyl cyclases (AC, e.g., AC3, 5, 6) | TM, Cav | (39, 71, 72, 138, 139) |
| Cyclic nucleotide phosphodiesterase (PDE3B) | Cav | (132) |
| Endothelial nitric oxide synthase (eNOS/NOS3) | Cav | (22, 24, 43, 57, 58, 73, 98, 165, 183, 215) |
| Neuronal nitric oxide synthase (nNOS/NOS1) | Cav | (14, 22, 30, 58, 182, 215) |
Adapted from Patel et al. (147).
Table 2.
Examples of diseases associated with Cav or Cavin mutations
| Disease | Clinical Features | Mutated Protein | References |
|---|---|---|---|
| Berardinelli-Seip Congenital Generalized Lipodystrophy types 3 (Cav1) and 4 (Cavin1) | Lack of adipose tissue, hypertriglyceridemia, insulin resistance, diabetes mellitus, hypertrophic cardiomyopathy, hepatic steatosis | Cav1, Cavin1 | (19, 161) |
| Pulmonary arterial hypertension | Pulmonary vascular remodeling and proliferation, high pulmonary arterial blood pressure, right ventricular failure | Cav1 | (7) |
| Limb-girdle muscular dystrophy type 1C | Symmetric, progressive, proximal weakness of the limb-girdle muscles, myoglobinuria, myotonia, elevated serum creatine kinase | Cav3 | (119) |
| Rippling muscle disease | Mechanically triggered contractions of skeletal muscle; subsequent electrically silent muscle contraction cascades | Cav3 | (89) |
| Long QT syndrome | Extended Q–T interval on electrocardiogram, arrhythmias, ventricular fibrillation | Cav3, Cavin1 | (161) |
| Sudden infant death syndrome | Sudden death of an infant unexplained by medical history or autopsy | Cav3 | (92) |
| Hypertrophic or dilated cardiomyopathy | Thickened myocardium, nonischemic cardiomyopathy, reduced cardiac function | Cav3, Cavin1, Cavin4 | (92, 161, 171) |
| Cancer (implicated) | Breast, prostate, ovarian, and pancreatic | Cav1 | (65, 105, 146) |
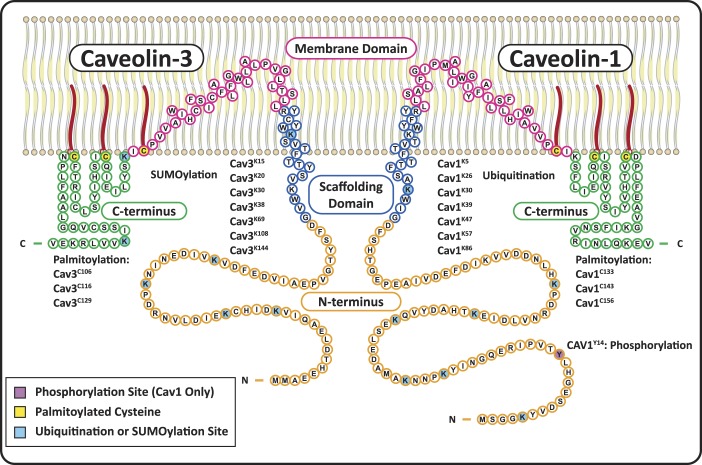
There are three Cav isoforms: Cav1, Cav2, and Cav3. Cav1 and Cav3 are necessary and sufficient for caveolae formation in most tissues and striated muscle, respectively. Cav2 associates with Cav1 in hetero-oligomers and does not independently form caveolae (102, 201). Cavs have four domains (Fig. 1): 1) an NH2-terminal domain, which includes a Cav1 phosphorylation site (Cav1Y14), ubiquitination sites in Cav1, and SUMOylation sites in Cav3; 2) an α-helical caveolin scaffolding domain (CSD) (implicated in protein-protein interactions, oligomerization, and inhibition of signaling proteins) with a cholesterol recognition/interaction consensus sequence (CRAC) and ubiquitination or SUMOylation sites; 3) a membrane domain that interacts with PM lipids through an atypical helix-turn-helix motif that exposes both the NH2- and COOH-termini of Cav to the cytoplasm; and 4) a COOH-terminal domain with three palmitoylated cysteines involved in oligomerization, protein binding, and Cav stability.
Fig. 1.
Structure and posttranslational modifications of caveolin-1 (Cav1) and caveolin-3 (Cav3). Cav1 and Cav3 have four primary domains: NH2-terminal domains (orange) with multiple ubiquitination/SUMOylation sites on both Cavs, as well as a phosphorylation site on Cav1; scaffolding domains (blue) that form α-helices and are inserted into the membrane, with a cholesterol recognition/interaction amino acid consensus (CRAC) composed of the eight residues proximal to the membrane domain; helix-turn-helix membrane domains (fuchsia) that exit the membrane at a palmitoylation site; and COOH-terminal domains (green) with two more palmitoylated cysteines and two SUMOylation sites on Cav3.
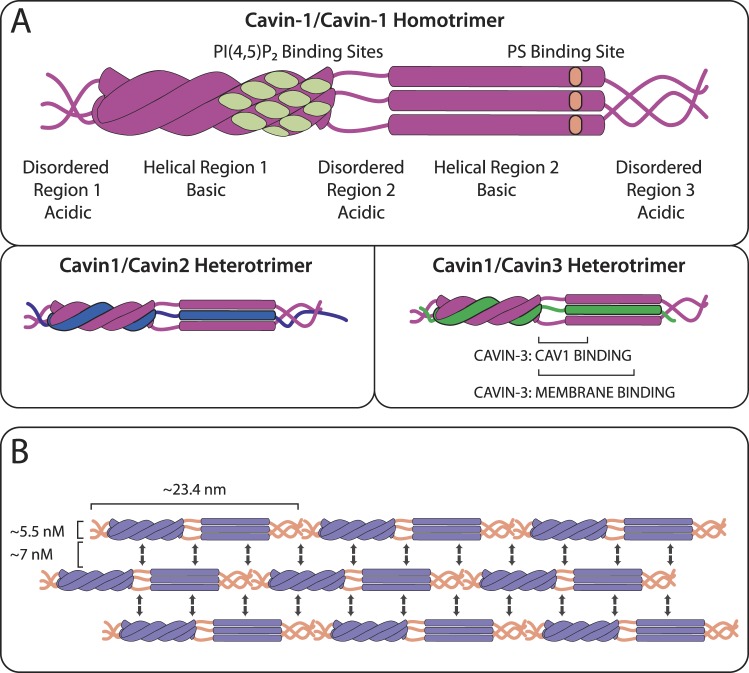
Cavin proteins aid oligomerized Cavs to form flask-shaped caveolae. Four cavin isoforms have been identified: Cavin1 [also known as (aka) polymerase I and transcript release factor (PTRF)], Cavin2 [aka serum deprivation response protein (SDPR)], Cavin3 [aka serum deprivation response factor-related gene product that binds to C-kinase (SRBC)], and Cavin4 [aka muscle-restricted coiled-coil (MURC)]. Cavins homo- and hetero-trimerize with two Cavin1 proteins and one Cavin1, Cavin2, or Cavin3 via an α-helical domain, bind to caveolae membranes by basic regions on the helical domains, and assist Cavs in the membrane curvature that forms caveolae (Fig. 2).
Fig. 2.
Structure of cavins. A: cavins are cytosolic proteins with three disordered regions (DR1, DR2, DR3) alternating with two helical regions (HR1 and HR2). Two Cavin-1s homo- and hetero-trimerize with a Cavin1, Cavin2, or Cavin3 monomer via an extended coiled-coil structure in HR1, which drives HR2 to generate an α-helical structure. Cavin1 has membrane-binding sites, including a phosphatidylinositol(4,5)-bisphosphate [PI(4,5)P2]-binding site composed of four basic regions in close proximity on HR1 and a phosphatidylserine (PS)-binding basic site on HR2. B: cavins detected on cellular membranes with electron microscopy form rod-like structures that are 5.5 nm wide and 23.4 nm long and associate laterally in 7 nM intervals. One idea, as described in the text and shown here, proposes that electrostatic interactions between alternating acidic DRs (orange) and basic HRs (purple) may facilitate these lateral associations while permitting flexibility in the cavin coat complex.
Interactions of Cavs with binding partners during trafficking, at the PM, and as part of signal transduction events modulate the behavior of caveolae as structural and functional microdomains. This review follows the life cycle of Cavs from the ER to the lysosome and explores the roles of lipid and protein interactions within caveolae that contribute to aspects of cell physiology.
Trafficking of Cavs and Cavins for the Formation of Caveolae
Studies of caveolae formation have used primary cultures of epithelial cells (e.g., human lung microvascular endothelial cells) or fibroblasts (e.g., human skin fibroblasts, primary mouse embryonic fibroblasts), various epitheloid (e.g., Chinese hamster ovary, MDCK, Fischer rat thyroid), fibroblast-like (e.g., CV-1 and COS-7 from African green monkey kidney, NIH mouse 3T3), adipocyte (e.g., 3T3-L1), cancer (e.g., MCF-7 and MDA-MB-231 breast cancer, PC3 prostate cancer, A431 epidermoid carcinoma) cells, and/or other commonly used systems (e.g., HeLa, HEK293, Escherichia coli cells). Cav3, Cavin1, and Cavin4 studies have also been performed in primary skeletal or cardiac muscle isolates or in the immortalized cardiac myoblastic cell lines H9C2 (rat) or HL-1 (mouse). Some of these cells provide null (or near-null) backgrounds for Cav expression; for example, MCF-7 cells have very low Cav1 expression, do not express cavins, and do not form caveolae and PC3 cells express Cav1 at high levels but do not express cavins (74, 93, 210). MDCK, A431, and MEF cells all express Cav1, Cavins 1–3, and form caveolae at the PM (13, 74, 193, 210). The diversity of cellular backgrounds in research on caveolae can contribute to differences in results; for example, in studies of polarized epithelial cells, apical and basolateral membranes perform distinct roles in endo/exocytosis, signal transduction, mechanosensation, adherence, junction formation, and/or migration compared with studies of caveolae in a sessile adipocyte, contractile primary skeletal muscle cell, or HEK293 cell (70). Cav1 and Cav3 may differ in their biogenesis of caveolae. However, most data on caveolae formation are based on studies of Cav1-expressing systems, in which the basic components and pathway of caveolae formation appear to be similar among different cell types and thus are the focus of this review.
Early steps in caveolae assembly: membrane insertion, 8S-Cav oligomerization, and endoplasmic reticulum to Golgi transition.
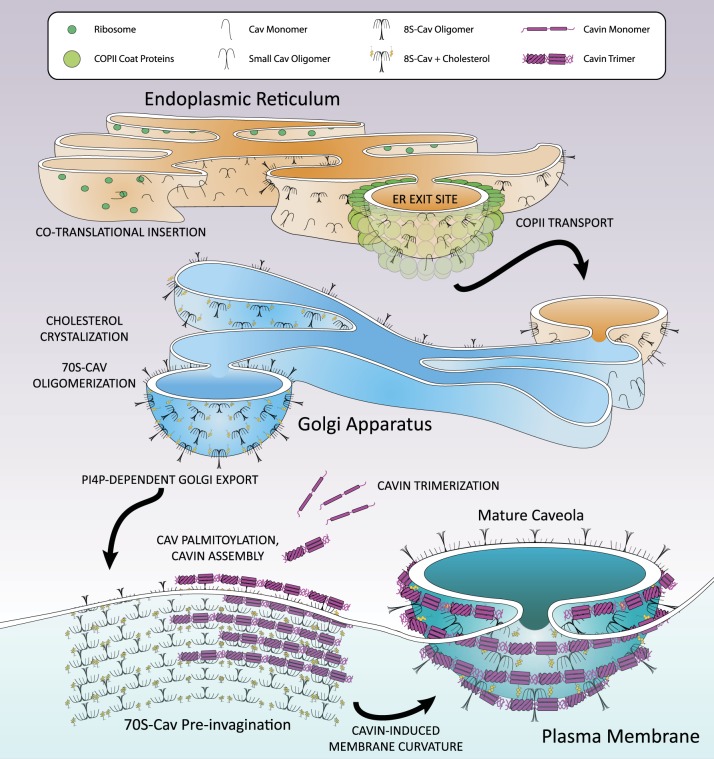
Caveolae are constructed in a complex, stepwise assembly process that involves endoplasmic reticulum (ER) membrane insertion, oligomerization, and export of Cav; Golgi Cav-cholesterol association, oligomerization, and export to the PM; palmitoylation near the PM; and addition of cavins at the PM to form invaginated structures (reviewed in refs 144, 173) (Fig. 3). The timeline from Cav translation to cavin addition at the PM in CV1 cells includes ER exit site (ERES) localization and initial oligomer (8S-Cav) formation within 5 min; Golgi localization within 15 min; secondary oligomer (70S-Cav) formation, cholesterol addition, and PM translocation by ~60 min; and accumulation of cavin at PM 70S-Cav oligomers that occurs over >25 min (68). Cavs are the primary protein components of caveolae, influencing membrane composition and protein content from translation to degradation. Thus, the translation and early oligomerization of Cavs begin the caveolae biogenesis pathway.
Fig. 3.
Maturation of caveolae. Cav1 and Cav2 monomers are cotranslationally inserted into the endoplasmic reticulum (ER) membrane and swiftly oligomerized into 8S-Cav oligomers containing 7–14 Cavs. These oligomers are transported to ER exit sites (ERES) within 5 min of synthesis for COPII-dependent transport to the Golgi apparatus 15 min postsynthesis. In the Golgi, cholesterol crystalizes in the membrane and assists in the formation of 70S-Cav complexes composed of 18–25 8S-Cav subunits by ~60 min after synthesis, whereupon 70S-Cav is transported to the PM by four phosphate-adapter protein (FAPP-1, -2)-dependent secretory vesicles. Near or on the PM, palmitoyl acyltransferases palmitoylate 70S-Cav oligomers. Also on the PM, cavin proteins that trimerize in the cytosol gradually aggregate on the 70S-Cav membrane over the course of more than 25 min and assist in membrane curvature. Mature caveolae consist of three layers: a cholesterol and anionic lipid-rich membrane embedded with a palmitoylated 70S-Cav coat, which is surrounded by a striated oligomerized 60S-Cavin coat.
Formation of caveolae is initiated by the cotranslational ER membrane insertion of monomeric Cav1 in a signal recognition particle-dependent manner (68, 123). As quickly as 5 min after synthesis in the ER, Cav1 forms 8S20,w oligomers (8S-Cav, 150–200 kDa with a minor species around 443–669 kDa) that is estimated to contain 7–14 Cavs in a Cav1:Cav2 ratio of 2–4:1 (41, 68, 111, 123, 181, 184). The formation of 8S-Cav oligomers depends on aa residues Cav166–70, Cav181–100 (i.e., the CSD), and Cav1134–178 (i.e., the COOH-terminus) (41, 113, 188). No molecular chaperone has been identified for the formation of 8S-Cav.
8S-Cav complexes translocate to the ERES, where recognition of a NH2-terminal diacidic sequence motif, Asp-X-Glu (Cav167DFE69), results in COPII vesicle-dependent export to the Golgi by ~15 min after synthesis (68, 124, 137). Disruption of this signal (e.g., Cav1D67G) delays ER export and leads to accumulation in tubular-reticular ER structures and lipid droplets (68, 137). Experimental overexpression of Cav1 exhibits similar trafficking errors, implying that this export pathway may be a potentially saturable step in Cav maturation (64, 67). 8S oligomer formation is a prerequisite for caveolae formation (124) but Cav1 monomers and small oligomers reach the ER and may be subject to the same COP II-dependent export pathway as 8S complexes (68).
Intermediary steps in caveolae assembly: 70S-Cav oligomerization, cholesterol incorporation, and PM insertion.
Golgi-localized 8S-Cav oligomerizes by ~60 min after synthesis into 70S20,w complexes (70S-Cav) of ~3.3 MDa, predicted to comprise ~160 Cav1 and Cav2 molecules in 15–25 8S-Cav subunits (68, 150). Recent work suggests that the mature caveolae coat has a single 70S-Cav unit; thus, 70S-Cav formation is the penultimate step of Cav maturation (5, 68, 111, 150, 219). 70S-Cav oligomerization is restricted to the Golgi: treatment with brefeldin A (which inhibits protein transport from the ER to the Golgi) prevents 70S-Cav oligomerization (68).
During the oligomerization step, 70S-Cav-associated membranes attain a high content of cholesterol, which is thought to involve a cholesterol recognition/interaction amino acid consensus of Cavs (CRAC: -Leu/Val-X1–5-Tyr-X1–5-Arg/Lys- where X1–5 represents one to five residues of any aa). The CRAC of Cav1 (94VTKYWFYR101) induces cholesterol crystallization in the membrane and results in deeper Cav1 peptide insertion (38). Cholesterol depletion reduces 70S-Cav complex formation, implying that oligomerization depends on membrane integration of cholesterol (68). Cavs that lack the CRAC allow 8S-Cav oligomerization but can prevent Cav association with lipid rafts in the Golgi and 70S-Cav formation, suggesting that 8S-Cav oligomers are not sufficient to establish the cholesterol-rich environment of caveolae (113, 168). The properties of the Golgi that allow 70S-Cav formation and cholesterol aggregation are not well defined, nor are the chaperones or cofactors involved in assembly of 70S-Cav oligomers.
After oligomerization, 70S-Cav is exported to the PM from the cis/medial-Golgi network in carrier-dependent, dyanamin-2-independent secretory vesicles within 60 min of synthesis (68). Transport is directed by four phosphate adapter protein 1 and 2 (FAPP-1, -2) that interact with phosphatidylinositol-4-phosphate (PI4P), the primary phosphoinositide (PI) species in the Golgi, to generate post-Golgi vesicles for transport (59, 68, 217). These small, uniform 70S-Cav-cholesterol vesicles can transport cargo, including glycosylphosphatidyl inositol (GPI)-linked proteins that associate with lipid rafts (68, 203). Vesicle fusion with the PM allows GPI-linked proteins to diffuse laterally while the Cav coat remains at the point of integration (68).
70S-Cav complexes encounter palmitoyl acyltransferases near the PM, which palmitoylate Cavs on three cysteines in the late membrane/early COOH-terminal domains (Cav1C133, C143, C156, Cav3C106, C116, C124) (Fig. 1) (8, 32, 36, 141). Palmitoylation in many proteins is dynamic, but Cav1 palmitoyl modifications are stable for at least 24 h in endothelial cells (141). Palmitoylation is not essential for membrane localization of Cavs. Although palmitoylation-deficient Cav1 mutants (Cav1Cys−) form high-molecular-weight oligomers in detergent-resistant membranes (DRMs, i.e., lipid raft domains) at the PM, they exhibit reduced stability (122, 142). The lipid affinities of Cav domains and palmitoylation sites can influence the oligomerization and stability of Cavs at the PM. The composition of the lipids in caveolae stabilizes proteins and influences protein function, as we will discuss subsequently.
Terminal steps in caveolae assembly: cavin association, invagination, and stabilization.
Once 70S-Cav oligomers and their cholesterol-rich membranes reach the PM, ~60–80 cavin proteins accumulate in caveolae over >25 min and create a striated coat [detected by electron microscopy (68, 86, 112, 174)]. Cavins can assemble into cytoplasmic complexes in the absence of Cav1 but cavins are gradually added to 70S-Cav at the PM (56, 68). Cavins are not primarily bound to Cavs at the membrane: Cavin3 is the only isoform known to directly interact with Cav1 (in the CSD and middle domains of Cav1 and Cavin3, respectively) (Fig. 2) (121). However, cavins have binding sites for the anionic phospholipids phosphatidylserine (PS) and phosphatidylinositol (4, 5)-bisphosphate [PI(4,5)P2] that are locally concentrated in caveolae membranes (40, 56, 74, 111, 155, 220). All four cavin proteins have a COOH-terminal basic domain putatively involved in membrane association (66). Cavin1 also has an NH2-terminal leucine zipper domain (aa 53–75) that enables membrane association, while Cavin4 uses its NH2-terminal coiled-coil domain (aa 44–77) to localize to the PM (131, 224).
The basic oligomeric unit of cavin proteins is a trimer composed of two Cavin1 units with a monomer of Cavin1, Cavin2, or Cavin3 (Cavin4 has not been studied), interacting through helical region 1 (HR1); after HR1 trimerization, helical region 2 (HR2) undergoes conformational changes and forms an α-helical secondary structure, enabling further oligomerization (56, 111) (Fig. 2). A nine-cavin complex forms from identical cavin trimers: Cavin1/Cavin2 and Cavin1/Cavin3 are mutually excluded from such trimeric-trimer-cavin complexes and segregate separately on the caveolae surface, implying that Cavin1/Cavin2 or Cavin1/Cavin3 oligomers may have distinct roles (56). Cavin-cavin interactions yield 60S20,w complexes (60S-Cavin, predicted ~2.5 MDa) which, with addition of 70S-Cav, forms an 80S complex (68, 85).
Membrane invagination in a nascent caveola depends on localized biophysical properties of the lipid bilayer and the intrinsic membrane-remodeling capacity of Cavs, cavins, and lipids (66, 86, 219) (Fig. 3). Cav1 or Cavin1 expressed alone can independently influence membrane curvature and in mammalian cells can form tubular membrane structures (66, 216). Cavs interact with phospholipid headgroups of the membrane through a helix-turn-helix membrane domain that enters and exits from the cytosol (Fig. 1) (178). Additionally, the three palmitoylated cysteines of Cavs stabilize Cav oligomers in the caveolae membrane (122, 142) and may participate in membrane curvature (178). Membrane lipids, such as PI4P in Golgi export vesicles, may also contribute to membrane curvature and caveolae invagination (50, 51).
A proposed mechanism of cavin-driven membrane remodeling is that alternation between basic HR1 and HR2 domains interspersed with acidic disordered regions 1, 2, and 3 (DR1–3) (Fig. 2) allows DRs of one cavin trimer to recognize HRs of a trimer in the adjacent parallel striation and vice versa, thereby creating a flexible lattice of lateral cavin-cavin interactions around caveolae that exert curve-inducing forces on the membrane (85). Support for this idea includes a zigzag appearance of cavin striations around the caveolae coat, with cavin subunits alternating in proximity to adjacent striations (112). Details of membrane invagination are not well defined, for example, the roles of other binding partners of Cavs and cavins and of cytoskeletal components.
70S-Cav and 60S-Cavin together form mature, stable caveolae that have three layers: a cholesterol- and negatively charged phospholipid-enriched membrane, 8S-Cav subunits organized into a palmitoylated 70S-Cav coat, and a 60S-Cavin complex that spirals around the outside of the 70S-Cav coat. These mature caveolae (Figs. 3 and 4) stabilize and protect Cavs from degradation by preventing lateral movement and exchange of Cavs and cavins (68, 208, 209). Cavs and caveolae proteins are also protected from ubiquitination and depalmitoylation. An example of this is Gα protein (GTP-binding proteins in αβγ heterotrimers): palmitoyl turnover of Gα proteins is increased by receptor activation and Gα dissociation from Gβγ and caveolae, implying that caveolae may exclude acyl thioesterases (81, 103, 126, 223). Cavins are also protected from degradation within caveolae: a PI-binding site on HR1 of Cavin1 is a major ubiquitination site that is obscured by membrane association (210).
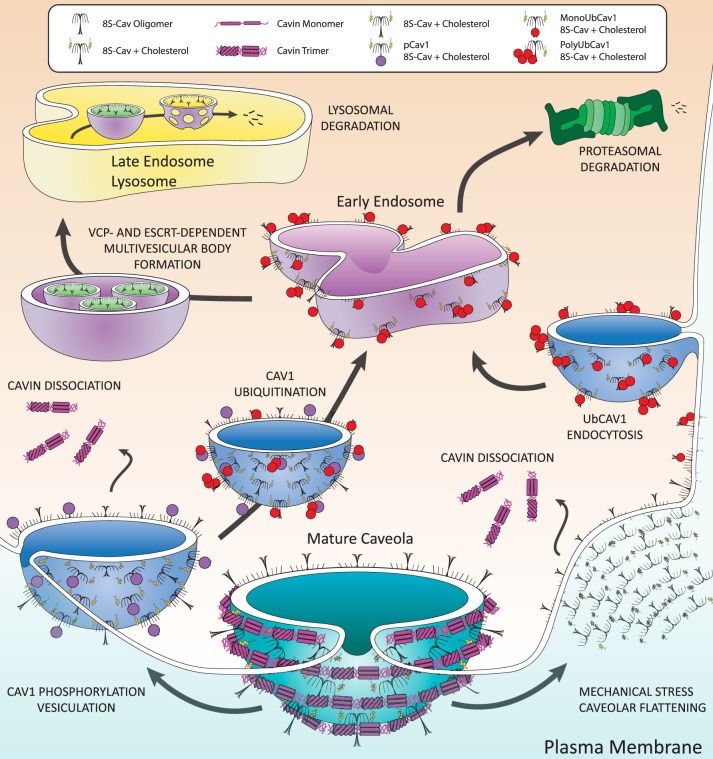
Fig. 4.
Disassembly and degradation of caveolae. Caveolae disassembly begins at the plasma membrane. Examples shown here are Src-dependent phosphorylation of Cav1 and membrane stretch. Cav1 phosphorylation initiates dynamin-dependent vesiculation that distorts caveolae, dislodging cavin trimers, and leads to ubiquitination of Cavs (UbCav) during vesicular transport to the early endosome. Mechanical stress displaces cavins from the membrane, followed by ubiquitination and subsequent endocytosis of Cav1 to the early endosome. Endosomal UbCav and cholesterol associate with valosin-containing protein (VCP) and are packaged in multivesicular bodies by endosomal sorting complexes required for transport (ESCRT) complex proteins for transport to and degradation in the late endosome/lysosome. Some polyUbCav is degraded by the proteasome.
Cav1 has a reported half-life (t1/2) of >24 h (69, 106, 141), although some studies report other values (27, 44, 76). Cavin1 knockdown destabilizes caveolae and reduces the t1/2 of Cav1 to 7 h (74). Similarly, truncation constructs of Cav1 that cannot oligomerize have a decreased half-life and do not form stable caveolae (113). Cav3 appears to have a shorter t1/2 than Cav1 (5.5–7 h), and mutant forms of Cav3 can have an even shorter t1/2 (45–60 min) as a result of ubiquitination and proteosomal degradation (54, 55, 204). Cavin1 has a t1/2 that ranges between 5 and 8 h; however, in the absence of Cav1, the t1/2 decreases by almost half (210).
Thus, in the process of caveolae biogenesis, unstructured 18–21 kDa Cav monomers assemble in a tightly controlled fashion to form 8S-Cav, 70S-Cav-cholesterol, and finally, 80S-Cav-cholesterol-cavin complexes that are protected from degradation.
Processes of Caveolae Disassembly and Degradation
Stability of caveolae at the PM requires that Cav and cavin oligomers remain tightly associated. Disruption of that structural stability by mechanical stress, endocytic activity, or signal transduction pathways dissociates cavins from caveolae and leads to endocytosis of Cavs (Fig. 4). In contrast to the maturation and stability of 70S-Cav, which requires constitutive, stable palmitoylation, caveolae disassembly and 70S-Cav dissociation involve dynamic posttranslational modifications (PTMs) of Cavs that include phosphorylation, S-nitrosylation, and ubiquitination.
Cavs are subject to multiple endocytic pathways, two of which are depicted in Fig. 4: Caveolae, including the 70S-Cav complex, can be endocytosed as a result of Src-dependent phosphorylation (3, 21, 128, 206) or the PM can undergo mechanical stresses that flatten caveolae and dissociate 60S-Cavin complexes, after which Cav is ubiquitinated (UbCav1) and endocytosed (9, 84). The pathways are likely more complex than described below and in Fig. 4. Key to both pathways are cavin dissociation, endocytosis, Cav ubiquitination, and endosomal processing of UbCav1, in which mono- and poly-UbCav1 is directed to intraluminal vesicles for lysosomal degradation (68, 84) and poly-UbCav1 undergoes proteosomal processing (9).
Cavin dissociation occurs in both pathways portrayed in Fig. 4. If caveolae lose structural integrity in response to mechanical stress or vesiculation, 60S-Cavin dissociates into the cytosol and separates into trimeric-trimer-cavin units (56, 85, 108, 196). Because cavin associates with the lipids of caveolae, its departure does not directly affect 70S-Cav localization to the PM (40, 69, 74, 155). For example, although Cav3, Cavin1, and Cavin4 colocalize at the PM of myocytes, during hypo-osmotic stress, Cav3, but not Cavin1 or Cavin4, is present in membrane blebs, suggesting that membrane stress leads to dissociation of the cavins but not Cav3 (108). Cholesterol depletion with U18666A (which inhibits cholesterol trafficking in cells) also results in 60S-Cavin dissociation from the PM (69).
Cytosolic Cavin1 is susceptible to ubiquitination on Lys residues within PI-binding sites (i.e., groupings of surface-exposed basic residues) in the HR1 region that are occluded by membrane contact (Fig. 5) (210). Mutation of the five Lys and Arg residues in this region reduces Cavin1 ubiquitination and turnover by the proteasome (210) without substantially influencing Cav1 colocalization or caveolae formation (86). Such data imply that the colocalization of the PI-binding/ubiquitination site selectively protects Cavin1 from ubiquitination while bound to caveolae and leads to the ubiquitination of cytosolic Cavin1 (210).
Fig. 5.
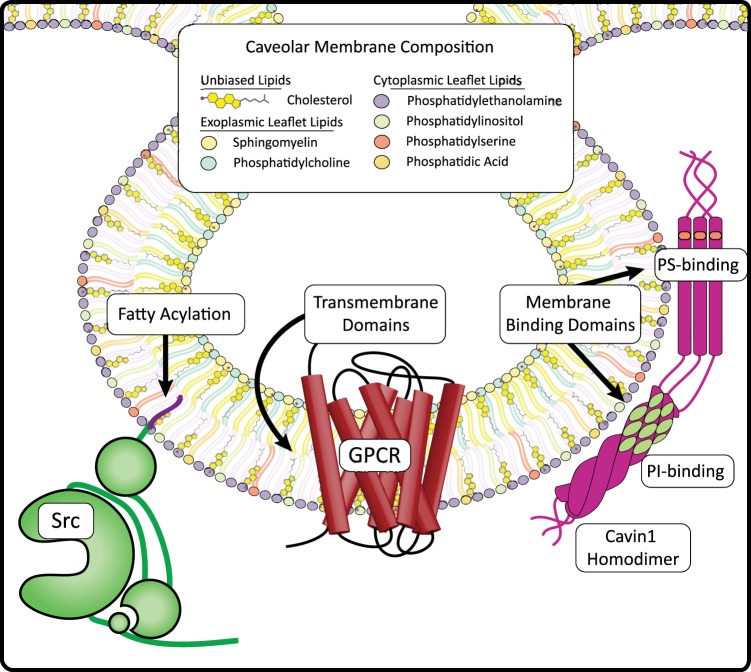
Caveolae membrane composition and caveolae-targeting signals. Caveolae membranes are enriched in phospholipids, sphingolipids, and cholesterol. This diagram depicts the distribution of membrane lipid species within a caveolae bilayer. Cholesterol is concentrated within caveolae, representing a third of all lipids, and is present in both leaflets of the bilayer. Sphingomyelin is slightly more prevalent than PC in the exofacial leaflet of caveolae, while PE composes more than half of the cytoplasmic leaflet. Also in the cytoplasmic leaflet, the anionic phospholipids PI, PS, and PA compose the minor fraction of lipid content with PI roughly twice as prevalent as PS and PA at half the concentration of PS. Proteins can be targeted to caveolae through transmembrane domains (e.g., GPCRs, Cavs), fatty acylations (e.g., Src myristoylation) and membrane binding domains (e.g., Cavin1 PI- and PS-binding domains).
Src-dependent phosphorylation of Cav1 is implicated as a control point of clathrin-independent membrane protein internalization and macromolecular cargo endocytosis (80, 120, 145, 158). Phosphorylation of Cav1 by Src kinases on an NH2-terminal tyrosine (p-Cav1Y14) is an interaction that requires COOH-terminal palmitoylation of Cav1C156 and the myristoyl plus basic motif of Src (95, 104, 120, 149, 195, 205, 211, 221). p-Cav1Y14 also adds a binding site on Cav1 to the SH2 domain of Src, thereby scaffolding this kinase more closely to Cav1 after Src activation (61).
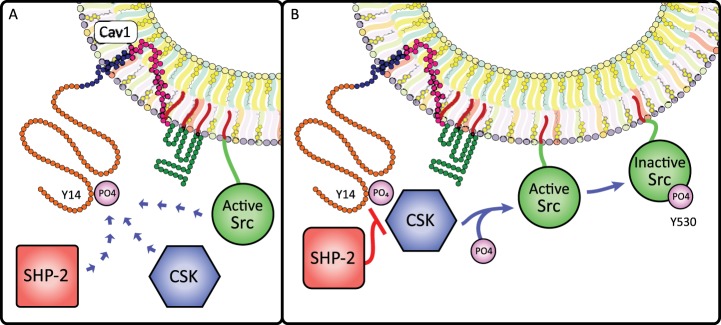
Cav1 phosphorylation and sustained Src signaling initiates endocytosis by phosphorylating dynamin-2 to close off and detach caveolae from the membrane, recruiting actin and the actin regulator cortactin, and cross-linking p-Cav1Y14 to filamin A (3, 20, 88, 117, 128, 177, 206). p-Cav1Y14 regulates the duration of Src activity by binding kinases and phosphatases: p-Cav1Y14 recruits c-Src tyrosine kinase (Csk) to inhibit Src via SrcY527 phosphorylation, while recruitment of Src homology 2 domain-containing protein tyrosine phosphatase 2 (SHP-2) interferes with Csk-p-Cav1Y14 complex formation and preserves Src activity (Fig. 6) (79, 169). The balance between Csk and SHP-2 action on p-CavY14 thus may control Src-mediated caveolae endocytosis.
Fig. 6.
Cav1 phosphorylation influences Src signaling. A: Cav1Y14 phosphorylation creates a Src homology 2 (SH2)-binding site that regulates Src activity through binding of Src and recruitment of two cytosolic Src-regulating enzymes: COOH-terminal Src kinase (CSK) and SH2 domain-containing non-transmembrane protein tyrosine phosphatase (SHP-2). B: when recruited to caveolae by pCav1Y14, CSK deactivates Src by phosphorylating SrcY530; however, pCav1Y14 binding to SHP-2 blocks CSK recruitment and prevents SrcY530 phosphorylation, increasing the duration of Src activity. Thus, Cav1 phosphorylation permits a rheostat-like control of Src signaling.
Cav1Y14 phosphorylation also destabilizes 70S-Cav oligomers by altering the relationships between oligomerized Cav1: pCav1Y14 or phosphomimetic Cav1Y14D increases the intermolecular distance between Cavs in caveolae vesicles, resulting in dissociation of higher-order Cav1 oligomers (232). Phosphorylation-induced loss of 70S-Cav integrity may be a mechanism by which the previously stable structure is made accessible to ubiquitination enzymes, acyl-protein thioesterases, and other degradative influences. Addition of a cell-permeable CSD peptide, which directly blocks Src-dependent Cav1Y14 phosphorylation, stabilizes large oligomers (232).
Mechanical effects on the PM that flatten caveolae initiate another type of caveolae disassembly. Flattening can result from cholesterol depletion (87, 214), hypo-osmotic stress (108), and substrate stretch (196, 210). The flattening of caveolae after mechanical stress may be a protective mechanism that provides a reservoir of PM to prevent membrane tension and injury by increasing PM surface area (108). Caveolae in skeletal muscle cluster in PM surface-connected rosettes; under hypo-osmotic stress, caveolae density decreases and reduces the ratio of rosettes to independent caveolae (108). Treatment of mouse lung endothelial cells with methyl-β-cyclodextrin, which depletes the PM of cholesterol, increases membrane tension during hypotonic stress (196).
The flattening of caveolae is ATP- and actin-independent. During recovery from nonlethal hypotonic stress, caveolae re-form by integrating the PM-localized pool of Cav1 with Cavin1 within 10 min to form an equal number of caveolae; this requires ATP but not actin remodeling (196). Chronic and repetitive shear stress, such as at the luminal PM of endothelial cells exposed to laminar flow, can increase PM caveolae number without increasing mRNA, suggesting that signaling induced by shear stress increases Golgi processing and export of Cav1 (16, 143). The number of caveolae increases only at the shear-exposed membrane, where they may play roles in translocating proteins to caveolae and activation of signaling cascades (16, 143, 227). Caveolae flattening thus protects the PM from rupture by reducing PM tension during transient stress.
Absence of cavins from caveolae through mechanical detachment, cholesterol depletion, or knockdown disrupts 70S-Cav oligomers into their 8S-Cav subunits and induces Cav1 ubiquitination, caveolae-independent packaging, and endocytosis of UbCav1 to the early endosome (69). The cholesterol-rich membranes surrounding 8S-Cav units are transported to the endosome and lysosome, indicating that Cavs can maintain their membrane localization even with loss of the 70S-Cav structure (127).
Cav1 separated from mature caveolae can be monoubiquitinated on six NH2-terminal lysines (Cav1K5, K26, K30, K39, K47, K57) (84), polyubiquitinated on Cav1K86 (9), and may have other states of ubiquitination. The most prevalent ubiquitination is monoUbCav1. PolyUbCav1 is divided into two groups: 64% Lys-63-linked and 28% Lys-48-linked (18, 69, 170). p-Cav1Y14 undergoes Cav1K86 polyubiquitination and proteosomal degradation (9). Additionally, Cav1 and DRMs are implicated in the inhibition of lysosomal function, which may influence Cav1 degradation (193). Cav3 ubiquitination of specific lysines has not been shown; however, a small ubiquitin-like modifier (SUMO) can be added by the E3 ligase PIASγ to lysines on Cav3 that are homologous to the ubiquitin sites on Cav1, suggesting that such sites may be loci for ubiquitination (47).
After ubiquitination and translocation of Cav to endosomes, it is degraded by proteosomal and lysosomal pathways (9, 69). Mono- and polyUbCav1 are packaged within multivesicular bodies (MVB) by endosomal sorting complexes required for transport (ESCRT) and an AAA+-type ATPase, valosin-containing protein (VCP; aka p97, Cdc48) (18, 69, 84, 170). VCP uses conformational changes from ATP hydrolysis to extract polypeptides from larger assemblies of oligomers or membranes, regulates endosome size and sorting, and with the cofactor UBXD1 enables MVB formation (17, 29, 33, 162, 170, 175, 228). Two mutually exclusive VCP cofactors, UBXD1 and Ankrd13, regulate VCP interaction with monoUbCav1 and mono/polyUbCav1, respectively, and can drive Cav segregation to intraluminal vesicles (17, 18, 84, 170). VCP preferentially interacts with higher-order Cav oligomers [8S-Cav, ~150–200 kDa major species and 443–669 kDa minor species (68)]. but it is unknown whether VCP also interacts with 70S-Cav (18, 170, 181). 8S-Cav and VCP interaction is disrupted by the depletion of membrane cholesterol, suggesting that VCP-dependent regulation of Cav oligomers occurs after cholesterol membrane integration in the Golgi and that cholesterol remains in the local membrane environment of Cavs during endocytosis and endosomal sorting (170).
PolyUb chains on residue Cav1K86 direct it to proteasomes for degradation. The proteasomal inhibitor MG132 reduces Cav1 degradation but also prevents acidification of Cav1-containing endosomes; thus, Cav1 may be packaged in MVB before proteasomal and endosomal processing (Fig. 4) (69). Because VCP is typically involved in membrane protein extraction and proteasomal transport (17) and UbCav1 interacts with VCP, VCP complexes may also be involved in the proteasomal pathway of UbCav1 degradation; however, the process by which polyUbCav1 is trafficked to the proteasome is not known.
Importantly, tagged, overexpressed, and/or mutant Cav constructs are subject to defective 8S-Cav and 70S-Cav oligomerization, lipid raft exclusion, intracellular accumulation/aggregation, and increased turnover (18, 64, 67, 69, 84, 170). However, many studies use tagged Cavs to assess turnover, intracellular Cavs, or interaction of Cavs with partners. Cav1 oligomerization is also influenced by experimental tagging: overexpression of GFP-, mCherry- or C-myc-tagged Cav1 yields aberrant Cav1 oligomers that do not interact with endogenous Cav1 or Cav2 (64). Tags and overexpression can also affect localization to lipid rafts: tagged mutant Cav1P132L (a mutation in multiple diseases) is more sensitive to oligomer and lipid raft disruption than is Cav1WT (64). Differences in the reported t1/2 of Cav1 can at least partially be attributed to the use of tagged proteins: in one study, endogenous Cav1 and Cav1-HA had t1/2's of >24 h and 13.6 h, respectively (69). Because of the role of VCP in ER-associated degradation [ERAD, in which misfolded or incomplete protein assemblies are dislocated from the ER and degraded by the ubiquitin-proteasome system (228, 229)], tagged/overexpressed Cav1 in the biogenesis pathway may be a target of VCP and yield nonphysiologic results. Careful attention thus must be paid in Cav trafficking and signaling studies to identify possible artifacts created by expression constructs with abnormal trafficking characteristics.
Composition and Roles of the Caveolae Lipid Microenvironment
The protein complex of caveolae comprises 80S-Cav multimers: ~160 membrane-bound, palmitoylated Cavs bound in close proximity to cholesterol-rich membranes and complexed with ~50 cavin oligomers. Interactions between Cavs, cavins, lipids, and other proteins create an unique protein, lipid, and lipoprotein microenvironment. Cavs shape this environment not only through cholesterol binding and palmitoylation but also by interaction with the inner leaflet of membrane lipids by a hairpin helix-turn-helix membrane domain. This domain includes Cav1T91, K96, Y97, R101, Y118, which preferentially interact with phospholipid headgroups of the cytosolic PM leaflet, and Cav1G108, which interacts with the exofacial leaflet (5, 96, 178). Cavins are dependent on the lipid composition of caveolae because they have affinities for negatively charged phospholipids [PS, PI(4,5)P2] and cholesterol in caveolae membranes and their lipid binding may occlude ubiquitination sites, thereby protecting them from degradation (40, 56, 74, 111, 155). Other proteins localize to caveolae by association with Cavs, the caveolae membrane, and/or scaffolding proteins that associate with caveolae. Major factors that influence these interactions are caveolae lipid composition, PTMs, and activation state changes that exchange proteins between caveolae and cytosolic or PM domains.
The lipidome of caveolae appears to depend on the cell type studied, the method used to isolate caveolae, and physiological “state” (e.g., mechanical stress or signal transduction events). Cavs are inserted and exit through the cytoplasmic leaflet of the PM, which is enriched in PS, phosphatidylethanolamine (PE), phosphatidylinositol (PI), and phosphatidylcholine (PC), while the outer leaflet is enriched in sphingolipids (including glycolipids) and phosphatidylcholine (PC) (116, 212). Caveolae isolated without detergents have more than twice the molar concentration of lipid per mg of protein than do PM membranes (6, 156). These isolates are highly enriched in phospholipids, cholesterol, and sphingomyelin (SM) when compared with non-caveolae membranes: caveolae contain 3–5 times more PS, 2–5 times more cholesterol and SM, 2–4 times more phosphatidic acid (PA), and ~2-fold more PE (which composes around 40% of all membrane phospholipids) (Fig. 5) (6, 156). However, studies disagree about PC and PI concentrations: a concanavalin-A affinity chromatography method of caveolae isolation in murine L-cell fibroblasts identified a 15-fold increase in PC and 3-fold increase in PI within caveolae, whereas an Optiprep density gradient centrifugation method in KB human epidermal cancer cells expressing exogenous Cav1 found a minimal increase in PC or PI species (6, 156). Caveolae membranes contain higher concentrations of unsaturated, saturated, and monounsaturated fatty acid species (6). Caveolae isolated with 1% Triton X-100 are enriched in cholesterol and SM without the glycerophospholipid enrichment seen in nondetergent-isolated lipid rafts (156). Thus, detergent treatment of PMs may preferentially extract exoplasmic leaflet lipids but not anionic inner leaflet glycerophospholipids (153). However, when Cav1 is not present in cells, nondetergent lipid rafts contain similar proportions of lipids as do those isolated from Cav1-expressing cells except that cholesterol is reduced (156). Thus, caveolae are distinct from other PM lipid rafts primarily due to caveolin and its interaction with cholesterol.
Cholesterol has a large role in the formation and structure of caveolae. Because the integration of cholesterol is necessary for 70S-Cav assembly from 8S-Cav subunits, cholesterol is essential for caveolae formation; however, it is also necessary for the stability, structure, and function of caveolae at the PM. Cholesterol increases the ordering and packing of lipid membranes and has numerous effects, for example, increasing resistance to disruption by mechanical stress (42, 194), membrane thickness (172), and depth of Cav CSD peptide insertion into membranes (38). Cholesterol depletion distorts caveolae, implying that PMs do not maintain an invaginated morphology without cholesterol (151, 174). Substitution of cholesterol with its precursor, desmosterol, reduces Cav affinity for sterol and results in enlarged caveolae with increased Cav1Y14 phosphorylation (78).
Proteins associate with PM lipids through transmembrane domains, membrane-interacting sites, and/or fatty acid PTMs; however, conformational changes, binding site obstruction, and de-lipidation can alter lipid affinities, which may influence movement of proteins into and out of caveolae. Protein-protein, protein-lipid, and lipid-lipid associations can reciprocally stabilize and concentrate binding partners (83). For instance, membrane cholesterol and other lipids within caveolae influence conformational plasticity of the β2AR, receptor ligand affinity, activation/inhibition, and G protein affinity and activation; importantly, lipids that stabilize the β2AR concentrate in its local membrane (34, 91, 114, 157). Certain lipids can also be allosteric modifiers of β2AR activity (34). Membrane lipid composition thus influences the stability and behavior of the receptor, which can influence the lipid composition in its local environment. In conjunction with evidence that agonist-stimulated β2AR dissociates laterally from lipid rafts to undergo β-arrestin-mediated clathrin endocytosis and degradation (34, 60, 233), localization within or outside caveolae may influence receptor behavior independent of protein scaffolds, activators, and downstream effectors. Therefore, by aggregating certain lipid species within caveolae, Cavs may stabilize proteins in conformations that can regulate ligand affinity, protein-protein interactions and signal transduction; in addition, conformational changes and/or PTMs that change lipid affinities may alter protein localization in caveolae (91, 103, 114, 157, 199, 223).
Stable membrane-bound homeostasis and stimulus-dependent dynamic behavior or modification occur with other caveolae-resident proteins. For example, nitric oxide (NO) production in endothelial cells is a key regulator of vascular smooth muscle relaxation. Within caveolae, eNOS is constitutively bound and inhibited by Cav1 (58, 118). Src activity enabled by Cav1 binding can lead to eNOS dissociation from Cav1 and eNOS activation: Src phosphorylates Cav1Y14, which causes dissociation of eNOS; Src phosphorylates PI3K, which phosphorylates Akt, which phosphorylates uninhibited cytosolic eNOS at the activating Ser1177 site; and p-eNOSS1177 produces more NO to stimulate smooth muscle relaxation (Fig. 7) (11). Each component of this pathway has membrane and/or protein affinities that change upon activation or modification. Proteins can cycle out of caveolae and be activated; others may cycle into them upon stimulation.
Fig. 7.
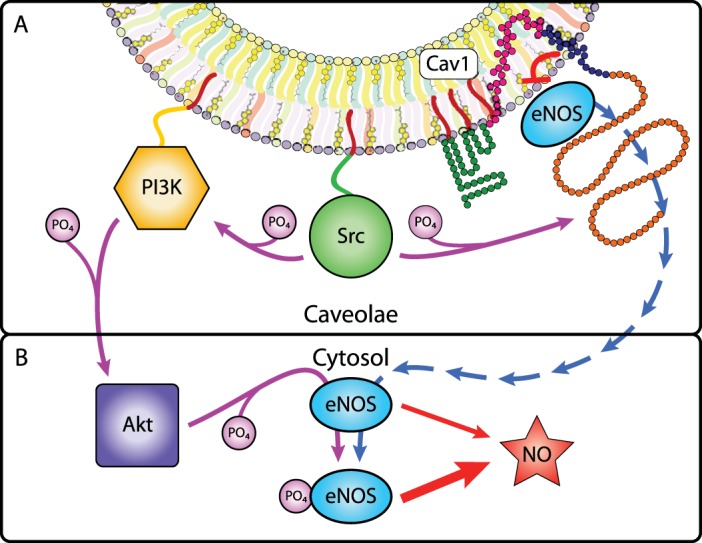
Caveolae-cytosol exchange of eNOS increases NO production. A: within caveolae, Src phosphorylates Cav1, leading to the release of eNOS from Cav1 inhibition (blue arrows). Src also phosphorylates and activates PI3K, which phosphorylates and activates Akt. B: in the cytosol, free eNOS is phosphorylated by Akt, which increases NO production.
Fatty acylations (e.g., palmitoylation, myristoylation, GPI-link formation) influence caveolae localization and protein association in caveolae. Palmitoylation does not influence Cav1 stabilization in cholesterol-enriched membranes but interacts with other lipid components of the membrane and perturbs conformational dynamics (46). Cavs are palmitoylated near the PM after oligomerization, implying that palmitoylation has a limited role in lipid species selection or Golgi-localized oligomerization (32, 36, 141). However, the caveolae lipid environment can aggregate proteins (e.g., GPCRs, Gα’s, Ras, c-Src), with fatty modifications (53, 199, 213). In some cases, acylation is required for protein-protein associations: c-Src is localized to caveolae as a consequence of its myristoylation and binds to Cav1 through CavC156 palmitoylation (95, 203, 213). CavC156 can be S-nitrosylated secondary to eNOS activation, acting as potent negative regulator of Cav palmitoylation and thus palmitoylation-dependent binding activity.
Much is unknown regarding the facilitation by fatty acylation of membrane, Cav1 binding, and caveolae localization. The complexity of these interactions is illustrated by the behavior of Gα isoforms. Gα isoforms are CSD-associated and subject to dynamic palmitoylation but Gαi/o/z possess an additional myristoylation site that localizes them to caveolae (53, 125, 213). Palmitoylation of Gα and all three Cav1 cysteines is required for binding (53). Palmitoylation-deficient, but not myristoylation-deficient, Gαi is trafficked to caveolae but binding to Cav1 is abrogated, implying that caveolae localization requires myristoylation but Cav1 binding requires palmitoylation and thus suggesting a two-step mechanism for Gαi localization and association with Cav1 (53). By contrast, Gαs has no myristoylation site and thus its PM localization and subsequent palmitoylation depend on its association with isoprenylated Gβγ subunits (125). Localization of Gα is also dependent on its activation state: GPCR-mediated activation of Gα subunits induces Gβγ dissociation, separation from Cav1, and accelerates Gα depalmitoylation, thus delaying reassociation of Gα and Cav1 (103, 223). Cav1 expression also modifies the palmitoylation state of certain proteins (12), and palmitoylation of Cav1C143+C156 is required for the efficient transport of GPI-anchored proteins to the cell surface (203). Taken together, these data demonstrate that fatty acylations may induce differential localization and Cav binding behavior between protein isoforms, thus altering the stoichiometry or function of signal transduction proteins.
In summary, the microenvironment of caveolae represents a multifaceted, interrelated, and dynamic collaboration between Cavs, cholesterol, cavins, membrane lipids, and other membrane-interacting proteins. Forces within and outside caveolae can influence signal transduction paradigms in a variety of ways. Prior research has only begun to address this complexity.
Discussion and Unanswered Questions
This review focuses on the biogenesis and degradation of caveolae microdomains and the roles of the lipid and protein components in those microdomains and in aspects of cell physiology. Much is known regarding caveolae biogenesis and degradation but many questions remain.
Caveolae biogenesis is sensitive to changes in protein structure, oligomerization, cholesterol, and PTMs. For example, numerous mutations in Cav1, Cav3, Cavin1, and Cavin4 have been associated with human diseases (Table 2). Most of these mutations cause defects in Cav localization and loss of morphological caveolae and result in diseases in adipose tissue (Cav1, Cavin1) (19, 161), pulmonary endothelium and smooth muscle (Cav1) (7), skeletal muscle (Cav3) (89, 119), and cardiac muscle (Cav3, Cavin1, Cavin4) (92, 161, 171). Some Cav1 mutations occur in certain cancers (65, 105, 146). Cav biogenesis can be disrupted by epitope tags, Cav constructs, and Cav overexpression that recapitulate some disease-related defects (4, 64, 67, 113, 187, 188, 202, 225). The disease-related mutations in these proteins reinforce the idea that the caveolae biogenesis system is physiologically relevant in numerous tissues. Normal Cav function may be tightly controlled by factors and/or chaperones that require unaltered Cav subunits to properly oligomerize, traffic to and function in the PM, and be degraded.
8S-Cav must be formed before COP II-dependent ER export to generate 70S-Cav in the Golgi. As Cav proteins localize to ERES within 5 min of synthesis and reach the Golgi by 15 min, 8S-Cav oligomerization is a relatively rapid process (68). The lack of substantial secondary or tertiary structural stability in Cav may make this initial oligomerization step slower if Cav is modified or mutated. Indeed, if given more time, conditions that facilitate protein folding (e.g., incubation at 30°C or 10% glycerol supplementation), or inhibition of proteasomal degradation (e.g., with MG-132), some trafficking-deficient Cav3 is competent to reach the PM, accrue in lipid rafts, and be protected from premature degradation (54). Even so, chaperones and information regarding the requirements for 8S-Cav oligomerization remain unknown.
Tagged, mutant, and/or overexpressed Cavs can form aggregates larger than 8S-Cav (64, 67, 204) and raise the question: how do 8S-Cav oligomers assemble into immobile and protected 70S-Cav structures with cholesterol in the Golgi? A mixture of incomplete Cav oligomers and monomers can be transported to the Golgi, but 8S-Cav does not form outside of the ER (68). Might unknown factors facilitate 70S-Cav assembly from 8S-Cav subunits (38, 64)? Perhaps long-lived caveolae structures require strict steric precision to prevent degradation, such that sequence changes lead to premature loss of oligomers, e.g., when Src-dependent Cav1Y14 phosphorylation wedges apart 8S-Cav clusters at the PM (232). Without 70S-Cav-cholesterol and 60S-Cavin complexed at the PM, “exposed” ubiquitination, palmitoylation, and/or phosphorylation sites may direct Cavs to degradation (54).
Another poorly understood aspect is the recognition pathway for 70S-Cav export from the Golgi. 8S-Cav is partially competent to reach the PM, but aggregates substantially larger than 8S-Cav are unable to reach the PM and may accumulate insufficient cholesterol to become buoyant lipid rafts (168). Are 70S-Cav-driven changes in Golgi lipid membrane composition a prerequisite for efficient export? Although FAPP-1 and -2 are components of the secretory pathway, their binding target PI4P is the most prevalent Golgi phospholipid and not unique to 70S-Cav (59, 217). Is there a cofactor that recognizes cholesterol, another enriched lipid, or Cav oligomers and drives the 70S-Cav Golgi export pathway?
Alternating acidic/basic domains in neighboring striations of the outer cavin coat have been proposed to exert forces for membrane curvature, but more information is needed regarding cavin recruitment, the role of cavins in caveolae curvature, determinants of cavin stability, and detachment of the cavin complex (68, 108, 196). Tomographic reconstruction of caveolae complexes has yielded promising data on the ultrastructure of the complete caveolae coat (111, 112) and the crystal structure of cavin (86) has helped advance understanding of cavins. By contrast, the lack of a crystal structure for Cavs has hampered the accrual of more precise information of aspects of their structure and function.
The degradation pathways of caveolae and their resident proteins are less well defined than those of biogenesis. In view of the trafficking and aggregation problems of tagged Cav constructs, their use may reveal information that identifies the sequelae of structural instability. Such “artifacts” can be useful, e.g., HA-Cav1 has a shorter t1/2 than wild-type Cav1, thus facilitating cell culture experiments (18). However, most studies of Cav degradation cited in this review used tagged Cav1 to draw conclusions about the endocytic, endosomal, lysosomal, and/or proteasomal degradation pathway of Cavs (18, 69, 84, 170). The lack of data on the degradation of endogenous Cavs and cavins (85) is thus an important gap in terms of normal cell physiology.
In summary, sixty years after their discovery (140), caveolae remain enigmatic dark caves. Our understanding of Cavs, cavins, their maturation and degradation processes, and the dynamic complexity of their lipid and protein components remains a work in progress.
GRANTS
Work in the authors’ laboratory on the topic of this review is supported by grants from National Institutes of Health, T32GM007752 (A. R. Busija, trainee), AG053568 (P. A. Insel), HL091071 (H. H. Patel), HL107200 (H. H. Patel), and the Veterans Administration, BX001963 (H. H. Patel).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.R.B. prepared figures; A.R.B. drafted manuscript; A.R.B., H.H.P., and P.A.I. edited and revised manuscript; A.R.B., H.H.P., and P.A.I. approved final version of manuscript.
ACKNOWLEDGMENTS
P. A. Insel was the 2016 Davson lecturer of the Cell and Molecular Physiology Section of the American Physiological Society.
Glossary
- 8S-Cav
8S20,w oligomer of Cavs
- 60S-Cavin
60S20,w oligomer of cavins
- 70S-Cav
70S20,w oligomer of Cavs
- 80S
80S20,w complex of 70S20,w-Cav and 6020,w-Cavin
- Ankrd13
Ankyrin repeat domain-containing protein 13
- β2AR
β2-adrenergic receptor
- Cav
Caveolin
- COPII
Coat protein complex II
- CSD
Caveolin scaffolding domain
- Csk
c-Src tyrosine kinase
- CRAC
Cholesterol recognition/interaction consensus sequence
- DR1, DR2, DR3
Disordered regions of cavins
- DRMs
Detergent-resistant membranes (lipid raft membranes)
- eNOS
Endothelial nitric oxide synthase (NOS3)
- ER
Endoplasmic reticulum
- ERAD
ER-associated degradation
- ERES
ER exit site
- ESCRT
Endosomal sorting complexes required for transport
- FAPP-1, FAPP-2
Four phosphate adapter protein 1 and 2
- Gα, Gβγ
Subunits of heterotrimeric G proteins
- GPCR
G protein-coupled receptor
- GPI
Glycosylphosphatidyl inositol
- HR1, HR2
Helical regions 1 and 2 of cavins
- MDCK
Madin-Darby canine kidney
- MVB
Multivesicular bodies
- nNOS
Neuronal nitric oxide synthase (NOS1)
- PM
Plasma membrane
- PA
Phosphatidic acid
- PC
Phosphatidylcholine
- pCav1Y14
Cav1 phosphorylated at tyrosine 14
- PE
Phosphatidylethanolamine
- PG
Phosphatidylglycerol
- PI3K
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- PI4P
Phosphatidylinositol 4-phosphate
- PI(4,5)P2
Phosphatidylinositol 4,5-bisphosphate
- PI
Phosphatidylinositol
- PS
Phosphatidylserine
- PTM
Posttranslational modification
- SHP-2
Src homology 2 domain-containing protein tyrosine phosphatase 2
- SM
Sphingomyelin
- Src
Proto-oncogene tyrosine-protein kinase Src
- t1/2
Half-life
- UbCav
Ubiquitinated Cav
- monoUbCav
Monoubiquitinated Cav
- polyUbCav
Polyubiquitinated Cav
- UBXD1
Ubiquitin regulatory X (UBX) domain-containing protein 1
- VCP
Valosin-containing protein
Footnotes
Glossary of abbreviations appears at the end of the article.
REFERENCES
- 1.Abi-Char J, Maguy A, Coulombe A, Balse E, Ratajczak P, Samuel JL, Nattel S, Hatem SN. Membrane cholesterol modulates Kv1.5 potassium channel distribution and function in rat cardiomyocytes. J Physiol 582: 1205–1217, 2007. doi: 10.1113/jphysiol.2007.134809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acconcia F, Bocedi A, Ascenzi P, Marino M. Does palmitoylation target estrogen receptors to plasma membrane caveolae? IUBMB Life 55: 33–35, 2003. doi: 10.1080/1521654031000081256. [DOI] [PubMed] [Google Scholar]
- 3.Ahn S, Maudsley S, Luttrell LM, Lefkowitz RJ, Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem 274: 1185–1188, 1999. doi: 10.1074/jbc.274.3.1185. [DOI] [PubMed] [Google Scholar]
- 4.Aoki S, Thomas A, Decaffmeyer M, Brasseur R, Epand RM. The role of proline in the membrane re-entrant helix of caveolin-1. J Biol Chem 285: 33371–33380, 2010. doi: 10.1074/jbc.M110.153569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ariotti N, Rae J, Leneva N, Ferguson C, Loo D, Okano S, Hill MM, Walser P, Collins BM, Parton RG. Molecular characterization of caveolin-induced membrane curvature. J Biol Chem 290: 24875–24890, 2015. doi: 10.1074/jbc.M115.644336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atshaves BP, Gallegos AM, McIntosh AL, Kier AB, Schroeder F. Sterol carrier protein-2 selectively alters lipid composition and cholesterol dynamics of caveolae/lipid raft vs nonraft domains in L-cell fibroblast plasma membranes. Biochemistry 42: 14583–14598, 2003. doi: 10.1021/bi034966+. [DOI] [PubMed] [Google Scholar]
- 7.Austin ED, Ma L, LeDuc C, Berman Rosenzweig E, Borczuk A, Phillips JA III, Palomero T, Sumazin P, Kim HR, Talati MH, West J, Loyd JE, Chung WK. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet 5: 336–343, 2012. doi: 10.1161/CIRCGENETICS.111.961888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker TL, Booden MA, Buss JE. S-nitrosocysteine increases palmitate turnover on Ha-Ras in NIH 3T3 cells. J Biol Chem 275: 22037–22047, 2000. doi: 10.1074/jbc.M001813200. [DOI] [PubMed] [Google Scholar]
- 9.Bakhshi FR, Mao M, Shajahan AN, Piegeler T, Chen Z, Chernaya O, Sharma T, Elliott WM, Szulcek R, Bogaard HJ, Comhair S, Erzurum S, van Nieuw Amerongen GP, Bonini MG, Minshall RD. Nitrosation-dependent caveolin 1 phosphorylation, ubiquitination, and degradation and its association with idiopathic pulmonary arterial hypertension. Pulm Circ 3: 816–830, 2013. doi: 10.1086/674753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballard-Croft C, Locklar AC, Kristo G, Lasley RD. Regional myocardial ischemia-induced activation of MAPKs is associated with subcellular redistribution of caveolin and cholesterol. Am J Physiol Heart Circ Physiol 291: H658–H667, 2006. doi: 10.1152/ajpheart.01354.2005. [DOI] [PubMed] [Google Scholar]
- 11.Banquet S, Delannoy E, Agouni A, Dessy C, Lacomme S, Hubert F, Richard V, Muller B, Leblais V. Role of G(i/o)-Src kinase-PI3K/Akt pathway and caveolin-1 in β2-adrenoceptor coupling to endothelial NO synthase in mouse pulmonary artery. Cell Signal 23: 1136–1143, 2011. doi: 10.1016/j.cellsig.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Baran J, Mundy DI, Vasanji A, Parat MO. Altered localization of H-Ras in caveolin-1-null cells is palmitoylation-independent. J Cell Commun Signal 1: 195–204, 2007. doi: 10.1007/s12079-008-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastiani M, Liu L, Hill MM, Jedrychowski MP, Nixon SJ, Lo HP, Abankwa D, Luetterforst R, Fernandez-Rojo M, Breen MR, Gygi SP, Vinten J, Walser PJ, North KN, Hancock JF, Pilch PF, Parton RG. MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes. J Cell Biol 185: 1259–1273, 2009. doi: 10.1083/jcb.200903053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beigi F, Oskouei BN, Zheng M, Cooke CA, Lamirault G, Hare JM. Cardiac nitric oxide synthase-1 localization within the cardiomyocyte is accompanied by the adaptor protein, CAPON. Nitric Oxide 21: 226–233, 2009. doi: 10.1016/j.niox.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth B, Roth BL. Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq-coupled protein receptors. J Biol Chem 279: 34614–34623, 2004. doi: 10.1074/jbc.M404673200. [DOI] [PubMed] [Google Scholar]
- 16.Boyd NL, Park H, Yi H, Boo YC, Sorescu GP, Sykes M, Jo H. Chronic shear induces caveolae formation and alters ERK and Akt responses in endothelial cells. Am J Physiol Heart Circ Physiol 285: H1113–H1122, 2003. doi: 10.1152/ajpheart.00302.2003. [DOI] [PubMed] [Google Scholar]
- 17.Buchberger A, Schindelin H, Hänzelmann P. Control of p97 function by cofactor binding. FEBS Lett 589: 2578–2589, 2015. doi: 10.1016/j.febslet.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Burana D, Yoshihara H, Tanno H, Yamamoto A, Saeki Y, Tanaka K, Komada M. The Ankrd13 family of ubiquitin-interacting motif-bearing proteins regulates valosin-containing protein/p97 protein-mediated lysosomal trafficking of caveolin 1. J Biol Chem 291: 6218–6231, 2016. doi: 10.1074/jbc.M115.710707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao H, Alston L, Ruschman J, Hegele RA. Heterozygous CAV1 frameshift mutations (MIM 601047) in patients with atypical partial lipodystrophy and hypertriglyceridemia. Lipids Health Dis 7: 3, 2008. doi: 10.1186/1476-511X-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao H, Courchesne WE, Mastick CC. A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: recruitment of C-terminal Src kinase. J Biol Chem 277: 8771–8774, 2002. doi: 10.1074/jbc.C100661200. [DOI] [PubMed] [Google Scholar]
- 21.Cao H, Orth JD, Chen J, Weller SG, Heuser JE, McNiven MA. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol Cell Biol 23: 2162–2170, 2003. doi: 10.1128/MCB.23.6.2162-2170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carnicer R, Crabtree MJ, Sivakumaran V, Casadei B, Kass DA. Nitric oxide synthases in heart failure. Antioxid Redox Signal 18: 1078–1099, 2013. doi: 10.1089/ars.2012.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERbeta has nongenomic action in caveolae. Mol Endocrinol 16: 938–946, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Bakhshi FR, Shajahan AN, Sharma T, Mao M, Trane A, Bernatchez P, van Nieuw Amerongen GP, Bonini MG, Skidgel RA, Malik AB, Minshall RD. Nitric oxide-dependent Src activation and resultant caveolin-1 phosphorylation promote eNOS/caveolin-1 binding and eNOS inhibition. Mol Biol Cell 23: 1388–1398, 2012. doi: 10.1091/mbc.E11-09-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng JP, Nichols BJ. Caveolae: one function or many? Trends Cell Biol 26: 177–189, 2016. doi: 10.1016/j.tcb.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Cogolludo A, Moreno L, Lodi F, Frazziano G, Cobeño L, Tamargo J, Perez-Vizcaino F. Serotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells: role of 5-HT2A receptors, caveolin-1, and KV1.5 channel internalization. Circ Res 98: 931–938, 2006. doi: 10.1161/01.RES.0000216858.04599.e1. [DOI] [PubMed] [Google Scholar]
- 27.Conrad PA, Smart EJ, Ying YS, Anderson RG, Bloom GS. Caveolin cycles between plasma membrane caveolae and the Golgi complex by microtubule-dependent and microtubule-independent steps. J Cell Biol 131: 1421–1433, 1995. doi: 10.1083/jcb.131.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couet J, Sargiacomo M, Lisanti MP. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem 272: 30429–30438, 1997. doi: 10.1074/jbc.272.48.30429. [DOI] [PubMed] [Google Scholar]
- 29.Dai RM, Li CC. Valosin-containing protein is a multi-ubiquitin chain-targeting factor required in ubiquitin-proteasome degradation. Nat Cell Biol 3: 740–744, 2001. doi: 10.1038/35087056. [DOI] [PubMed] [Google Scholar]
- 30.Damy T, Ratajczak P, Robidel E, Bendall JK, Oliviéro P, Boczkowski J, Ebrahimian T, Marotte F, Samuel JL, Heymes C. Up-regulation of cardiac nitric oxide synthase 1-derived nitric oxide after myocardial infarction in senescent rats. FASEB J 17: 1934–1936, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Darby PJ, Kwan CY, Daniel EE. Caveolae from canine airway smooth muscle contain the necessary components for a role in Ca2+ handling. Am J Physiol Lung Cell Mol Physiol 279: L1226–L1235, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Das AK, Dasgupta B, Bhattacharya R, Basu J. Purification and biochemical characterization of a protein-palmitoyl acyltransferase from human erythrocytes. J Biol Chem 272: 11021–11025, 1997. doi: 10.1074/jbc.272.17.11021. [DOI] [PubMed] [Google Scholar]
- 33.Davies JM, Tsuruta H, May AP, Weis WI. Conformational changes of p97 during nucleotide hydrolysis determined by small-angle X-ray scattering. Structure 13: 183–195, 2005. doi: 10.1016/j.str.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Dawaliby R, Trubbia C, Delporte C, Masureel M, Van Antwerpen P, Kobilka BK, Govaerts C. Allosteric regulation of G protein-coupled receptor activity by phospholipids. Nat Chem Biol 12: 35–39, 2016. doi: 10.1038/nchembio.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Pozo MA, Schwartz MA. Rac, membrane heterogeneity, caveolin and regulation of growth by integrins. Trends Cell Biol 17: 246–250, 2007. doi: 10.1016/j.tcb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Dunphy JT, Greentree WK, Manahan CL, Linder ME. G-protein palmitoyltransferase activity is enriched in plasma membranes. J Biol Chem 271: 7154–7159, 1996. doi: 10.1074/jbc.271.12.7154. [DOI] [PubMed] [Google Scholar]
- 37.Engelman JA, Chu C, Lin A, Jo H, Ikezu T, Okamoto T, Kohtz DS, Lisanti MP. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett 428: 205–211, 1998. doi: 10.1016/S0014-5793(98)00470-0. [DOI] [PubMed] [Google Scholar]
- 38.Epand RM, Sayer BG, Epand RF. Caveolin scaffolding region and cholesterol-rich domains in membranes. J Mol Biol 345: 339–350, 2005. doi: 10.1016/j.jmb.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 39.Fagan KA, Smith KE, Cooper DM. Regulation of the Ca2+-inhibitable adenylyl cyclase type VI by capacitative Ca2+ entry requires localization in cholesterol-rich domains. J Biol Chem 275: 26530–26537, 2000. doi: 10.1074/jbc.M001369200. [DOI] [PubMed] [Google Scholar]
- 40.Fairn GD, Schieber NL, Ariotti N, Murphy S, Kuerschner L, Webb RI, Grinstein S, Parton RG. High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. J Cell Biol 194: 257–275, 2011. doi: 10.1083/jcb.201012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez I, Ying Y, Albanesi J, Anderson RG. Mechanism of caveolin filament assembly. Proc Natl Acad Sci USA 99: 11193–11198, 2002. doi: 10.1073/pnas.172196599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernández-Hernando C, Yu J, Dávalos A, Prendergast J, Sessa WC. Endothelial-specific overexpression of caveolin-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol 177: 998–1003, 2010. doi: 10.2353/ajpath.2010.091287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feron O, Kelly RA. The caveolar paradox: suppressing, inducing, and terminating eNOS signaling. Circ Res 88: 129–131, 2001. doi: 10.1161/01.RES.88.2.129. [DOI] [PubMed] [Google Scholar]
- 44.Forbes A, Wadehra M, Mareninov S, Morales S, Shimazaki K, Gordon LK, Braun J. The tetraspan protein EMP2 regulates expression of caveolin-1. J Biol Chem 282: 26542–26551, 2007. doi: 10.1074/jbc.M702117200. [DOI] [PubMed] [Google Scholar]
- 45.Fox TE, Houck KL, O’Neill SM, Nagarajan M, Stover TC, Pomianowski PT, Unal O, Yun JK, Naides SJ, Kester M. Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem 282: 12450–12457, 2007. doi: 10.1074/jbc.M700082200. [DOI] [PubMed] [Google Scholar]
- 46.Frank PG, Marcel YL, Connelly MA, Lublin DM, Franklin V, Williams DL, Lisanti MP. Stabilization of caveolin-1 by cellular cholesterol and scavenger receptor class B type I. Biochemistry 41: 11931–11940, 2002. doi: 10.1021/bi0257078. [DOI] [PubMed] [Google Scholar]
- 47.Fuhs SR, Insel PA. Caveolin-3 undergoes SUMOylation by the SUMO E3 ligase PIASy: sumoylation affects G-protein-coupled receptor desensitization. J Biol Chem 286: 14830–14841, 2011. doi: 10.1074/jbc.M110.214270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujimoto T, Miyawaki A, Mikoshiba K. Inositol 1,4,5-trisphosphate receptor-like protein in plasmalemmal caveolae is linked to actin filaments. J Cell Sci 108: 7–15, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Fujita T, Toya Y, Iwatsubo K, Onda T, Kimura K, Umemura S, Ishikawa Y. Accumulation of molecules involved in alpha1-adrenergic signal within caveolae: caveolin expression and the development of cardiac hypertrophy. Cardiovasc Res 51: 709–716, 2001. doi: 10.1016/S0008-6363(01)00348-0. [DOI] [PubMed] [Google Scholar]
- 50.Furse S, Brooks NJ, Seddon AM, Woscholski R, Templer RH, Tate EW, Gaffney PRJ, Ces O. Lipid membrane curvature induced by distearoyl phosphatidylinositol 4-phosphate. Soft Matter 8: 3090–3093, 2012. doi: 10.1039/c2sm07358g. [DOI] [Google Scholar]
- 51.Furse S, Brooks NJ, Woscholski R, Gaffney PRJ, Templer RH. Pressure-dependent inverse bicontinuous cubic phase formation in a phosphatidylinositol 4-phosphate/phosphatidylcholine system. Chemical Data Collections 3–4: 15–20, 2016. doi: 10.1016/j.cdc.2016.08.001. [DOI] [Google Scholar]
- 52.Galbiati F, Volonte D, Engelman JA, Watanabe G, Burk R, Pestell RG, Lisanti MP. Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J 17: 6633–6648, 1998. doi: 10.1093/emboj/17.22.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galbiati F, Volonte D, Meani D, Milligan G, Lublin DM, Lisanti MP, Parenti M. The dually acylated NH2-terminal domain of gi1α is sufficient to target a green fluorescent protein reporter to caveolin-enriched plasma membrane domains. Palmitoylation of caveolin-1 is required for the recognition of dually acylated g-protein alpha subunits in vivo. J Biol Chem 274: 5843–5850, 1999. doi: 10.1074/jbc.274.9.5843. [DOI] [PubMed] [Google Scholar]
- 54.Galbiati F, Volonte D, Minetti C, Bregman DB, Lisanti MP. Limb-girdle muscular dystrophy (LGMD-1C) mutants of caveolin-3 undergo ubiquitination and proteasomal degradation. Treatment with proteasomal inhibitors blocks the dominant negative effect of LGMD-1C mutanta and rescues wild-type caveolin-3. J Biol Chem 275: 37702–37711, 2000. doi: 10.1074/jbc.M006657200. [DOI] [PubMed] [Google Scholar]
- 55.Galbiati F, Volonte D, Minetti C, Chu JB, Lisanti MP. Phenotypic behavior of caveolin-3 mutations that cause autosomal dominant limb girdle muscular dystrophy (LGMD-1C). Retention of LGMD-1C caveolin-3 mutants within the golgi complex. J Biol Chem 274: 25632–25641, 1999. doi: 10.1074/jbc.274.36.25632. [DOI] [PubMed] [Google Scholar]
- 56.Gambin Y, Ariotti N, McMahon KA, Bastiani M, Sierecki E, Kovtun O, Polinkovsky ME, Magenau A, Jung W, Okano S, Zhou Y, Leneva N, Mureev S, Johnston W, Gaus K, Hancock JF, Collins BM, Alexandrov K, Parton RG. Single-molecule analysis reveals self assembly and nanoscale segregation of two distinct cavin subcomplexes on caveolae. Elife 3: e01434, 2013. doi: 10.7554/eLife.01434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.García-Cardeña G, Fan R, Stern DF, Liu J, Sessa WC. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J Biol Chem 271: 27237–27240, 1996. doi: 10.1074/jbc.271.44.27237. [DOI] [PubMed] [Google Scholar]
- 58.García-Cardeña G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem 272: 25437–25440, 1997. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 59.Godi A, Di Campli A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, De Matteis MA. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol 6: 393–404, 2004. doi: 10.1038/ncb1119. [DOI] [PubMed] [Google Scholar]
- 60.Goodman OB Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature 383: 447–450, 1996. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 61.Gottlieb-Abraham E, Shvartsman DE, Donaldson JC, Ehrlich M, Gutman O, Martin GS, Henis YI. Src-mediated caveolin-1 phosphorylation affects the targeting of active Src to specific membrane sites. Mol Biol Cell 24: 3881–3895, 2013. doi: 10.1091/mbc.E13-03-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ha H, Pak Y. Modulation of the caveolin-3 and Akt status in caveolae by insulin resistance in H9c2 cardiomyoblasts. Exp Mol Med 37: 169–178, 2005. doi: 10.1038/emm.2005.23. [DOI] [PubMed] [Google Scholar]
- 63.Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem 275: 27832–27837, 2000. [DOI] [PubMed] [Google Scholar]
- 64.Han B, Tiwari A, Kenworthy AK. Tagging strategies strongly affect the fate of overexpressed caveolin-1. Traffic 16: 417–438, 2015. doi: 10.1111/tra.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han SE, Park KH, Lee G, Huh YJ, Min BM. Mutation and aberrant expression of Caveolin-1 in human oral squamous cell carcinomas and oral cancer cell lines. Int J Oncol 24: 435–440, 2004. [PubMed] [Google Scholar]
- 66.Hansen CG, Bright NA, Howard G, Nichols BJ. SDPR induces membrane curvature and functions in the formation of caveolae. Nat Cell Biol 11: 807–814, 2009. doi: 10.1038/ncb1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanson CA, Drake KR, Baird MA, Han B, Kraft LJ, Davidson MW, Kenworthy AK. Overexpression of caveolin-1 is sufficient to phenocopy the behavior of a disease-associated mutant. Traffic 14: 663–677, 2013. doi: 10.1111/tra.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayer A, Stoeber M, Bissig C, Helenius A. Biogenesis of caveolae: stepwise assembly of large caveolin and cavin complexes. Traffic 11: 361–382, 2010. doi: 10.1111/j.1600-0854.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- 69.Hayer A, Stoeber M, Ritz D, Engel S, Meyer HH, Helenius A. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J Cell Biol 191: 615–629, 2010. doi: 10.1083/jcb.201003086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Head BP, Patel HH, Insel PA. Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta 1838: 532–545, 2014. doi: 10.1016/j.bbamem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Head BP, Patel HH, Roth DM, Lai NC, Niesman IR, Farquhar MG, Insel PA. G-protein-coupled receptor signaling components localize in both sarcolemmal and intracellular caveolin-3-associated microdomains in adult cardiac myocytes. J Biol Chem 280: 31036–31044, 2005. doi: 10.1074/jbc.M502540200. [DOI] [PubMed] [Google Scholar]
- 72.Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem 281: 26391–26399, 2006. doi: 10.1074/jbc.M602577200. [DOI] [PubMed] [Google Scholar]
- 73.Heijnen HF, Waaijenborg S, Crapo JD, Bowler RP, Akkerman JW, Slot JW. Colocalization of eNOS and the catalytic subunit of PKA in endothelial cell junctions: a clue for regulated NO production. J Histochem Cytochem 52: 1277–1285, 2004. doi: 10.1177/002215540405201004. [DOI] [PubMed] [Google Scholar]
- 74.Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S, Hancock JF, Parton RG. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell 132: 113–124, 2008. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang CS, Zhou J, Feng AK, Lynch CC, Klumperman J, DeArmond SJ, Mobley WC. Nerve growth factor signaling in caveolae-like domains at the plasma membrane. J Biol Chem 274: 36707–36714, 1999. doi: 10.1074/jbc.274.51.36707. [DOI] [PubMed] [Google Scholar]
- 76.Hung MJ, Cherng WJ, Hung MY, Wu HT, Pang JH. Interleukin-6 inhibits endothelial nitric oxide synthase activation and increases endothelial nitric oxide synthase binding to stabilized caveolin-1 in human vascular endothelial cells. J Hypertens 28: 940–951, 2010. doi: 10.1097/HJH.0b013e32833992ef. [DOI] [PubMed] [Google Scholar]
- 77.Iiri T, Backlund PS Jr, Jones TL, Wedegaertner PB, Bourne HR. Reciprocal regulation of Gsα by palmitate and the βγ subunit. Proc Natl Acad Sci USA 93: 14592–14597, 1996. doi: 10.1073/pnas.93.25.14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jansen M, Pietiaïnen VM, Pölönen H, Rasilainen L, Koivusalo M, Ruotsalainen U, Jokitalo E, Ikonen E. Cholesterol substitution increases the structural heterogeneity of caveolae. J Biol Chem 283: 14610–14618, 2008. doi: 10.1074/jbc.M710355200. [DOI] [PubMed] [Google Scholar]
- 79.Jo A, Park H, Lee SH, Ahn SH, Kim HJ, Park EM, Choi YH. SHP-2 binds to caveolin-1 and regulates Src activity via competitive inhibition of CSK in response to H2O2 in astrocytes. PLoS One 9: e91582, 2014. doi: 10.1371/journal.pone.0091582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.John TA, Vogel SM, Tiruppathi C, Malik AB, Minshall RD. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol Lung Cell Mol Physiol 284: L187–L196, 2003. doi: 10.1152/ajplung.00152.2002. [DOI] [PubMed] [Google Scholar]
- 81.Jones TL, Degtyarev MY, Backlund PS Jr. The stoichiometry of G alpha(s) palmitoylation in its basal and activated states. Biochemistry 36: 7185–7191, 1997. doi: 10.1021/bi9628376. [DOI] [PubMed] [Google Scholar]
- 82.Kim HA, Kim KH, Lee RA. Expression of caveolin-1 is correlated with Akt-1 in colorectal cancer tissues. Exp Mol Pathol 80: 165–170, 2006. doi: 10.1016/j.yexmp.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 83.Kim JH, Peng D, Schlebach JP, Hadziselimovic A, Sanders CR. Modest effects of lipid modifications on the structure of caveolin-3. Biochemistry 53: 4320–4322, 2014. doi: 10.1021/bi5005238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kirchner P, Bug M, Meyer H. Ubiquitination of the N-terminal region of caveolin-1 regulates endosomal sorting by the VCP/p97 AAA-ATPase. J Biol Chem 288: 7363–7372, 2013. doi: 10.1074/jbc.M112.429076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kovtun O, Tillu VA, Ariotti N, Parton RG, Collins BM. Cavin family proteins and the assembly of caveolae. J Cell Sci 128: 1269–1278, 2015. doi: 10.1242/jcs.167866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kovtun O, Tillu VA, Jung W, Leneva N, Ariotti N, Chaudhary N, Mandyam RA, Ferguson C, Morgan GP, Johnston WA, Harrop SJ, Alexandrov K, Parton RG, Collins BM. Structural insights into the organization of the cavin membrane coat complex. Dev Cell 31: 405–419, 2014. doi: 10.1016/j.devcel.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 87.Kozera L, White E, Calaghan S. Caveolae act as membrane reserves which limit mechanosensitive I(Cl,swell) channel activation during swelling in the rat ventricular myocyte. PLoS One 4: e8312, 2009. doi: 10.1371/journal.pone.0008312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krueger EW, Orth JD, Cao H, McNiven MA. A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol Biol Cell 14: 1085–1096, 2003. doi: 10.1091/mbc.E02-08-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kubisch C, Schoser BG, von Düring M, Betz RC, Goebel HH, Zahn S, Ehrbrecht A, Aasly J, Schroers A, Popovic N, Lochmüller H, Schröder JM, Brüning T, Malin JP, Fricke B, Meinck HM, Torbergsen T, Engels H, Voss B, Vorgerd M. Homozygous mutations in caveolin-3 cause a severe form of rippling muscle disease. Ann Neurol 53: 512–520, 2003. doi: 10.1002/ana.10501. [DOI] [PubMed] [Google Scholar]
- 90.Kwiatek AM, Minshall RD, Cool DR, Skidgel RA, Malik AB, Tiruppathi C. Caveolin-1 regulates store-operated Ca2+ influx by binding of its scaffolding domain to transient receptor potential channel-1 in endothelial cells. Mol Pharmacol 70: 1174–1183, 2006. doi: 10.1124/mol.105.021741. [DOI] [PubMed] [Google Scholar]
- 91.Laganowsky A, Reading E, Allison TM, Ulmschneider MB, Degiacomi MT, Baldwin AJ, Robinson CV. Membrane proteins bind lipids selectively to modulate their structure and function. Nature 510: 172–175, 2014. doi: 10.1038/nature13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lariccia V, Nasti AA, Alessandrini F, Pesaresi M, Gratteri S, Tagliabracci A, Amoroso S. Identification and functional analysis of a new putative caveolin-3 variant found in a patient with sudden unexplained death. J Biomed Sci 21: 58, 2014. doi: 10.1186/1423-0127-21-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lavie Y, Fiucci G, Liscovitch M. Up-regulation of caveolae and caveolar constituents in multidrug-resistant cancer cells. J Biol Chem 273: 32380–32383, 1998. doi: 10.1074/jbc.273.49.32380. [DOI] [PubMed] [Google Scholar]
- 94.Lee H, Volonte D, Galbiati F, Iyengar P, Lublin DM, Bregman DB, Wilson MT, Campos-Gonzalez R, Bouzahzah B, Pestell RG, Scherer PE, Lisanti MP. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol 14: 1750–1775, 2000. doi: 10.1210/mend.14.11.0553. [DOI] [PubMed] [Google Scholar]
- 95.Lee H, Woodman SE, Engelman JA, Volonté D, Galbiati F, Kaufman HL, Lublin DM, Lisanti MP. Palmitoylation of caveolin-1 at a single site (Cys-156) controls its coupling to the c-Src tyrosine kinase: targeting of dually acylated molecules (GPI-linked, transmembrane, or cytoplasmic) to caveolae effectively uncouples c-Src and caveolin-1 (TYR-14). J Biol Chem 276: 35150–35158, 2001. doi: 10.1074/jbc.M104530200. [DOI] [PubMed] [Google Scholar]
- 96.Lee J, Glover KJ. The transmembrane domain of caveolin-1 exhibits a helix-break-helix structure. Biochim Biophys Acta 1818: 1158–1164, 2012. doi: 10.1016/j.bbamem.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Levin AM, Coroneus JG, Cocco MJ, Weiss GA. Exploring the interaction between the protein kinase A catalytic subunit and caveolin-1 scaffolding domain with shotgun scanning, oligomer complementation, NMR, and docking. Protein Sci 15: 478–486, 2006. doi: 10.1110/ps.051911706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Levin AM, Murase K, Jackson PJ, Flinspach ML, Poulos TL, Weiss GA. Double barrel shotgun scanning of the caveolin-1 scaffolding domain. ACS Chem Biol 2: 493–500, 2007. doi: 10.1021/cb700055t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci USA 100: 4807–4812, 2003. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol 23: 9389–9404, 2003. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li S, Couet J, Lisanti MP. Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem 271: 29182–29190, 1996. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li S, Galbiati F, Volonte D, Sargiacomo M, Engelman JA, Das K, Scherer PE, Lisanti MP. Mutational analysis of caveolin-induced vesicle formation. Expression of caveolin-1 recruits caveolin-2 to caveolae membranes. FEBS Lett 434: 127–134, 1998. doi: 10.1016/S0014-5793(98)00945-4. [DOI] [PubMed] [Google Scholar]
- 103.Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti MP. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem 270: 15693–15701, 1995. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- 104.Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem 271: 3863–3868, 1996. doi: 10.1074/jbc.271.7.3863. [DOI] [PubMed] [Google Scholar]
- 105.Li T, Sotgia F, Vuolo MA, Li M, Yang WC, Pestell RG, Sparano JA, Lisanti MP. Caveolin-1 mutations in human breast cancer: functional association with estrogen receptor alpha-positive status. Am J Pathol 168: 1998–2013, 2006. doi: 10.2353/ajpath.2006.051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lim JS, Nguyen KC, Han JM, Jang IS, Fabian C, Cho KA. Direct regulation of TLR5 expression by caveolin-1. Mol Cells 38: 1111–1117, 2015. doi: 10.14348/molcells.2015.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu L, Askari A. β-Subunit of cardiac Na+-K+-ATPase dictates the concentration of the functional enzyme in caveolae. Am J Physiol Cell Physiol 291: C569–C578, 2006. doi: 10.1152/ajpcell.00002.2006. [DOI] [PubMed] [Google Scholar]
- 108.Lo HP, Nixon SJ, Hall TE, Cowling BS, Ferguson C, Morgan GP, Schieber NL, Fernandez-Rojo MA, Bastiani M, Floetenmeyer M, Martel N, Laporte J, Pilch PF, Parton RG. The caveolin-cavin system plays a conserved and critical role in mechanoprotection of skeletal muscle. J Cell Biol 210: 833–849, 2015. doi: 10.1083/jcb.201501046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lockwich TP, Liu X, Singh BB, Jadlowiec J, Weiland S, Ambudkar IS. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J Biol Chem 275: 11934–11942, 2000. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- 110.Löhn M, Fürstenau M, Sagach V, Elger M, Schulze W, Luft FC, Haller H, Gollasch M. Ignition of calcium sparks in arterial and cardiac muscle through caveolae. Circ Res 87: 1034–1039, 2000. doi: 10.1161/01.RES.87.11.1034. [DOI] [PubMed] [Google Scholar]
- 111.Ludwig A, Howard G, Mendoza-Topaz C, Deerinck T, Mackey M, Sandin S, Ellisman MH, Nichols BJ. Molecular composition and ultrastructure of the caveolar coat complex. PLoS Biol 11: e1001640, 2013. doi: 10.1371/journal.pbio.1001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ludwig A, Nichols BJ, Sandin S. Architecture of the caveolar coat complex. J Cell Sci 129: 3077–3083, 2016. doi: 10.1242/jcs.191262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Machleidt T, Li WP, Liu P, Anderson RG. Multiple domains in caveolin-1 control its intracellular traffic. J Cell Biol 148: 17–28, 2000. doi: 10.1083/jcb.148.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mancia F, Assur Z, Herman AG, Siegel R, Hendrickson WA. Ligand sensitivity in dimeric associations of the serotonin 5HT2c receptor. EMBO Rep 9: 363–369, 2008. doi: 10.1038/embor.2008.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martens JR, Sakamoto N, Sullivan SA, Grobaski TD, Tamkun MM. Isoform-specific localization of voltage-gated K+ channels to distinct lipid raft populations. Targeting of Kv1.5 to caveolae. J Biol Chem 276: 8409–8414, 2001. doi: 10.1074/jbc.M009948200. [DOI] [PubMed] [Google Scholar]
- 116.Maxfield FR, Mondal M. Sterol and lipid trafficking in mammalian cells. Biochem Soc Trans 34: 335–339, 2006. doi: 10.1042/BST0340335. [DOI] [PubMed] [Google Scholar]
- 117.McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, Wong TW. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J Cell Biol 151: 187–198, 2000. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem 272: 15583–15586, 1997. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 119.Minetti C, Sotgia F, Bruno C, Scartezzini P, Broda P, Bado M, Masetti E, Mazzocco M, Egeo A, Donati MA, Volonte D, Galbiati F, Cordone G, Bricarelli FD, Lisanti MP, Zara F. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat Genet 18: 365–368, 1998. doi: 10.1038/ng0498-365. [DOI] [PubMed] [Google Scholar]
- 120.Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, Malik AB. Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J Cell Biol 150: 1057–1070, 2000. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mohan J, Morén B, Larsson E, Holst MR, Lundmark R. Cavin3 interacts with cavin1 and caveolin1 to increase surface dynamics of caveolae. J Cell Sci 128: 979–991, 2015. doi: 10.1242/jcs.161463. [DOI] [PubMed] [Google Scholar]
- 122.Monier S, Dietzen DJ, Hastings WR, Lublin DM, Kurzchalia TV. Oligomerization of VIP21-caveolin in vitro is stabilized by long chain fatty acylation or cholesterol. FEBS Lett 388: 143–149, 1996. doi: 10.1016/0014-5793(96)00519-4. [DOI] [PubMed] [Google Scholar]
- 123.Monier S, Parton RG, Vogel F, Behlke J, Henske A, Kurzchalia TV. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell 6: 911–927, 1995. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mora R, Bonilha VL, Marmorstein A, Scherer PE, Brown D, Lisanti MP, Rodriguez-Boulan E. Caveolin-2 localizes to the golgi complex but redistributes to plasma membrane, caveolae, and rafts when co-expressed with caveolin-1. J Biol Chem 274: 25708–25717, 1999. doi: 10.1074/jbc.274.36.25708. [DOI] [PubMed] [Google Scholar]
- 125.Morales J, Fishburn CS, Wilson PT, Bourne HR. Plasma membrane localization of G alpha z requires two signals. Mol Biol Cell 9: 1–14, 1998. doi: 10.1091/mbc.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mumby SM, Kleuss C, Gilman AG. Receptor regulation of G-protein palmitoylation. Proc Natl Acad Sci USA 91: 2800–2804, 1994. doi: 10.1073/pnas.91.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mundy DI, Li WP, Luby-Phelps K, Anderson RG. Caveolin targeting to late endosome/lysosomal membranes is induced by perturbations of lysosomal pH and cholesterol content. Mol Biol Cell 23: 864–880, 2012. doi: 10.1091/mbc.E11-07-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mundy DI, Machleidt T, Ying YS, Anderson RG, Bloom GS. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J Cell Sci 115: 4327–4339, 2002. doi: 10.1242/jcs.00117. [DOI] [PubMed] [Google Scholar]
- 129.Murata T, Lin MI, Stan RV, Bauer PM, Yu J, Sessa WC. Genetic evidence supporting caveolae microdomain regulation of calcium entry in endothelial cells. J Biol Chem 282: 16631–16643, 2007. doi: 10.1074/jbc.M607948200. [DOI] [PubMed] [Google Scholar]
- 130.Murthy KS, Makhlouf GM. Heterologous desensitization mediated by G protein-specific binding to caveolin. J Biol Chem 275: 30211–30219, 2000. doi: 10.1074/jbc.M002194200. [DOI] [PubMed] [Google Scholar]
- 131.Naito D, Ogata T, Hamaoka T, Nakanishi N, Miyagawa K, Maruyama N, Kasahara T, Taniguchi T, Nishi M, Matoba S, Ueyama T. The coiled-coil domain of MURC/cavin-4 is involved in membrane trafficking of caveolin-3 in cardiomyocytes. Am J Physiol Heart Circ Physiol 309: H2127–H2136, 2015. doi: 10.1152/ajpheart.00446.2015. [DOI] [PubMed] [Google Scholar]
- 132.Nilsson R, Ahmad F, Swärd K, Andersson U, Weston M, Manganiello V, Degerman E. Plasma membrane cyclic nucleotide phosphodiesterase 3B (PDE3B) is associated with caveolae in primary adipocytes. Cell Signal 18: 1713–1721, 2006. doi: 10.1016/j.cellsig.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 133.Norkin LC. Simian virus 40 infection via MHC class I molecules and caveolae. Immunol Rev 168: 13–22, 1999. doi: 10.1111/j.1600-065X.1999.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 134.Oh P, Schnitzer JE. Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default. Mol Biol Cell 12: 685–698, 2001. doi: 10.1091/mbc.12.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ono K, Iwanaga Y, Hirayama M, Kawamura T, Sowa N, Hasegawa K. Contribution of caveolin-1α and Akt to TNF-α-induced cell death. Am J Physiol Lung Cell Mol Physiol 287: L201–L209, 2004. doi: 10.1152/ajplung.00293.2003. [DOI] [PubMed] [Google Scholar]
- 136.Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol 141: 905–915, 1998. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ostermeyer AG, Paci JM, Zeng Y, Lublin DM, Munro S, Brown DA. Accumulation of caveolin in the endoplasmic reticulum redirects the protein to lipid storage droplets. J Cell Biol 152: 1071–1078, 2001. doi: 10.1083/jcb.152.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ostrom RS, Bundey RA, Insel PA. Nitric oxide inhibition of adenylyl cyclase type 6 activity is dependent upon lipid rafts and caveolin signaling complexes. J Biol Chem 279: 19846–19853, 2004. doi: 10.1074/jbc.M313440200. [DOI] [PubMed] [Google Scholar]
- 139.Ostrom RS, Gregorian C, Drenan RM, Xiang Y, Regan JW, Insel PA. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J Biol Chem 276: 42063–42069, 2001. doi: 10.1074/jbc.M105348200. [DOI] [PubMed] [Google Scholar]
- 140.Palade GE. Fine structure of blood capillaries. J Appl Phys 24: 1424, 1953. [Google Scholar]
- 141.Parat MO, Fox PL. Palmitoylation of caveolin-1 in endothelial cells is post-translational but irreversible. J Biol Chem 276: 15776–15782, 2001. doi: 10.1074/jbc.M006722200. [DOI] [PubMed] [Google Scholar]
- 142.Parat MO, Stachowicz RZ, Fox PL. Oxidative stress inhibits caveolin-1 palmitoylation and trafficking in endothelial cells. Biochem J 361: 681–688, 2002. doi: 10.1042/bj3610681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Park H, Go YM, St John PL, Maland MC, Lisanti MP, Abrahamson DR, Jo H. Plasma membrane cholesterol is a key molecule in shear stress-dependent activation of extracellular signal-regulated kinase. J Biol Chem 273: 32304–32311, 1998. doi: 10.1074/jbc.273.48.32304. [DOI] [PubMed] [Google Scholar]
- 144.Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol 14: 98–112, 2013. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- 145.Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol 127: 1199–1215, 1994. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Patani N, Lambros MB, Natrajan R, Dedes KJ, Geyer FC, Ward E, Martin LA, Dowsett M, Reis-Filho JS. Non-existence of caveolin-1 gene mutations in human breast cancer. Breast Cancer Res Treat 131: 307–310, 2012. doi: 10.1007/s10549-011-1761-2. [DOI] [PubMed] [Google Scholar]
- 147.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol 48: 359–391, 2008. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, Huang D, Moreno AL, Patel PM, Insel PA, Roth DM. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J 21: 1565–1574, 2007. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- 149.Pelkmans L, Püntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296: 535–539, 2002. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 150.Pelkmans L, Zerial M. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature 436: 128–133, 2005. doi: 10.1038/nature03866. [DOI] [PubMed] [Google Scholar]
- 151.Pike LJ. The challenge of lipid rafts. J Lipid Res 50, Suppl: S323–S328, 2009. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pike LJ. Growth factor receptors, lipid rafts and caveolae: an evolving story. Biochim Biophys Acta 1746: 260–273, 2005. doi: 10.1016/j.bbamcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 153.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res 44: 655–667, 2003. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 154.Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res 47: 1597–1598, 2006. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 155.Pike LJ, Casey L. Localization and turnover of phosphatidylinositol 4,5-bisphosphate in caveolin-enriched membrane domains. J Biol Chem 271: 26453–26456, 1996. doi: 10.1074/jbc.271.43.26453. [DOI] [PubMed] [Google Scholar]
- 156.Pike LJ, Han X, Chung KN, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry 41: 2075–2088, 2002. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- 157.Prasanna X, Sengupta D, Chattopadhyay A. Cholesterol-dependent conformational plasticity in GPCR dimers. Sci Rep 6: 31858, 2016. doi: 10.1038/srep31858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Predescu SA, Predescu DN, Timblin BK, Stan RV, Malik AB. Intersectin regulates fission and internalization of caveolae in endothelial cells. Mol Biol Cell 14: 4997–5010, 2003. doi: 10.1091/mbc.E03-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Prevostel C, Alice V, Joubert D, Parker PJ. Protein kinase C(alpha) actively downregulates through caveolae-dependent traffic to an endosomal compartment. J Cell Sci 113: 2575–2584, 2000. [DOI] [PubMed] [Google Scholar]
- 160.Prior IA, Harding A, Yan J, Sluimer J, Parton RG, Hancock JF. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat Cell Biol 3: 368–375, 2001. doi: 10.1038/35070050. [DOI] [PubMed] [Google Scholar]
- 161.Rajab A, Straub V, McCann LJ, Seelow D, Varon R, Barresi R, Schulze A, Lucke B, Lützkendorf S, Karbasiyan M, Bachmann S, Spuler S, Schuelke M. Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet 6: e1000874, 2010. doi: 10.1371/journal.pgen.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ramanathan HN, Ye Y. The p97 ATPase associates with EEA1 to regulate the size of early endosomes. Cell Res 22: 346–359, 2012. doi: 10.1038/cr.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ramos M, Lamé MW, Segall HJ, Wilson DW. The BMP type II receptor is located in lipid rafts, including caveolae, of pulmonary endothelium in vivo and in vitro. Vascul Pharmacol 44: 50–59, 2006. doi: 10.1016/j.vph.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 164.Ray S, Kassan A, Busija AR, Rangamani P, Patel HH. The plasma membrane as a capacitor for energy and metabolism. Am J Physiol Cell Physiol 310: C181–C192, 2016. doi: 10.1152/ajpcell.00087.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem 276: 38121–38138, 2001. [DOI] [PubMed] [Google Scholar]
- 166.Razani B, Lisanti MP. Two distinct caveolin-1 domains mediate the functional interaction of caveolin-1 with protein kinase A. Am J Physiol Cell Physiol 281: C1241–C1250, 2001. [DOI] [PubMed] [Google Scholar]
- 167.Razani B, Rubin CS, Lisanti MP. Regulation of cAMP-mediated signal transduction via interaction of caveolins with the catalytic subunit of protein kinase A. J Biol Chem 274: 26353–26360, 1999. doi: 10.1074/jbc.274.37.26353. [DOI] [PubMed] [Google Scholar]
- 168.Ren X, Ostermeyer AG, Ramcharan LT, Zeng Y, Lublin DM, Brown DA. Conformational defects slow Golgi exit, block oligomerization, and reduce raft affinity of caveolin-1 mutant proteins. Mol Biol Cell 15: 4556–4567, 2004. doi: 10.1091/mbc.E04-06-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ren Y, Meng S, Mei L, Zhao ZJ, Jove R, Wu J. Roles of Gab1 and SHP2 in paxillin tyrosine dephosphorylation and Src activation in response to epidermal growth factor. J Biol Chem 279: 8497–8505, 2004. doi: 10.1074/jbc.M312575200. [DOI] [PubMed] [Google Scholar]
- 170.Ritz D, Vuk M, Kirchner P, Bug M, Schütz S, Hayer A, Bremer S, Lusk C, Baloh RH, Lee H, Glatter T, Gstaiger M, Aebersold R, Weihl CC, Meyer H. Endolysosomal sorting of ubiquitylated caveolin-1 is regulated by VCP and UBXD1 and impaired by VCP disease mutations. Nat Cell Biol 13: 1116–1123, 2011. doi: 10.1038/ncb2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rodriguez G, Ueyama T, Ogata T, Czernuszewicz G, Tan Y, Dorn GW II, Bogaev R, Amano K, Oh H, Matsubara H, Willerson JT, Marian AJ. Molecular genetic and functional characterization implicate muscle-restricted coiled-coil gene (MURC) as a causal gene for familial dilated cardiomyopathy. Circ Cardiovasc Genet 4: 349–358, 2011. doi: 10.1161/CIRCGENETICS.111.959866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Róg T, Murzyn K, Karttunen M, Pasenkiewicz-Gierula M. Nonpolar interactions between trans-membrane helical EGF peptide and phosphatidylcholines, sphingomyelins and cholesterol. Molecular dynamics simulation studies. J Pept Sci 14: 374–382, 2008. doi: 10.1002/psc.936. [DOI] [PubMed] [Google Scholar]
- 173.Root KT, Plucinsky SM, Glover KJ. Recent progress in the topology, structure, and oligomerization of caveolin: a building block of caveolae. Curr Top Membr 75: 305–336, 2015. doi: 10.1016/bs.ctm.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 174.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell 68: 673–682, 1992. doi: 10.1016/0092-8674(92)90143-Z. [DOI] [PubMed] [Google Scholar]
- 175.Rouiller I, Butel VM, Latterich M, Milligan RA, Wilson-Kubalek EM. A major conformational change in p97 AAA ATPase upon ATP binding. Mol Cell 6: 1485–1490, 2000. doi: 10.1016/S1097-2765(00)00144-1. [DOI] [PubMed] [Google Scholar]
- 176.Roy S, Plowman S, Rotblat B, Prior IA, Muncke C, Grainger S, Parton RG, Henis YI, Kloog Y, Hancock JF. Individual palmitoyl residues serve distinct roles in H-ras trafficking, microlocalization, and signaling. Mol Cell Biol 25: 6722–6733, 2005. doi: 10.1128/MCB.25.15.6722-6733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rozelle AL, Machesky LM, Yamamoto M, Driessens MH, Insall RH, Roth MG, Luby-Phelps K, Marriott G, Hall A, Yin HL. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr Biol 10: 311–320, 2000. doi: 10.1016/S0960-9822(00)00384-5. [DOI] [PubMed] [Google Scholar]
- 178.Rui H, Root KT, Lee J, Glover KJ, Im W. Probing the U-shaped conformation of caveolin-1 in a bilayer. Biophys J 106: 1371–1380, 2014. doi: 10.1016/j.bpj.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rybin VO, Xu X, Steinberg SF. Activated protein kinase C isoforms target to cardiomyocyte caveolae: stimulation of local protein phosphorylation. Circ Res 84: 980–988, 1999. doi: 10.1161/01.RES.84.9.980. [DOI] [PubMed] [Google Scholar]
- 180.Sampson LJ, Hayabuchi Y, Standen NB, Dart C. Caveolae localize protein kinase A signaling to arterial ATP-sensitive potassium channels. Circ Res 95: 1012–1018, 2004. doi: 10.1161/01.RES.0000148634.47095.ab. [DOI] [PubMed] [Google Scholar]
- 181.Sargiacomo M, Scherer PE, Tang Z, Kübler E, Song KS, Sanders MC, Lisanti MP. Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci USA 92: 9407–9411, 1995. doi: 10.1073/pnas.92.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sato Y, Sagami I, Shimizu T. Identification of caveolin-1-interacting sites in neuronal nitric-oxide synthase. Molecular mechanism for inhibition of NO formation. J Biol Chem 279: 8827–8836, 2004. doi: 10.1074/jbc.M310327200. [DOI] [PubMed] [Google Scholar]
- 183.Sbaa E, Frérart F, Feron O. The double regulation of endothelial nitric oxide synthase by caveolae and caveolin: a paradox solved through the study of angiogenesis. Trends Cardiovasc Med 15: 157–162, 2005. doi: 10.1016/j.tcm.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 184.Scheiffele P, Verkade P, Fra AM, Virta H, Simons K, Ikonen E. Caveolin-1 and -2 in the exocytic pathway of MDCK cells. J Cell Biol 140: 795–806, 1998. doi: 10.1083/jcb.140.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Scherer PE, Lisanti MP, Baldini G, Sargiacomo M, Mastick CC, Lodish HF. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J Cell Biol 127: 1233–1243, 1994. doi: 10.1083/jcb.127.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Schilling JM, Roth DM, Patel HH. Caveolins in cardioprotection - translatability and mechanisms. Br J Pharmacol 172: 2114–2125, 2015. doi: 10.1111/bph.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schlegel A, Arvan P, Lisanti MP. Caveolin-1 binding to endoplasmic reticulum membranes and entry into the regulated secretory pathway are regulated by serine phosphorylation. Protein sorting at the level of the endoplasmic reticulum. J Biol Chem 276: 4398–4408, 2001. doi: 10.1074/jbc.M005448200. [DOI] [PubMed] [Google Scholar]
- 188.Schlegel A, Lisanti MP. A molecular dissection of caveolin-1 membrane attachment and oligomerization. Two separate regions of the caveolin-1 C-terminal domain mediate membrane binding and oligomer/oligomer interactions in vivo. J Biol Chem 275: 21605–21617, 2000. doi: 10.1074/jbc.M002558200. [DOI] [PubMed] [Google Scholar]
- 189.Schnitzer JE, Oh P, Jacobson BS, Dvorak AM. Caveolae from luminal plasmalemma of rat lung endothelium: microdomains enriched in caveolin, Ca(2+)-ATPase, and inositol trisphosphate receptor. Proc Natl Acad Sci USA 92: 1759–1763, 1995. doi: 10.1073/pnas.92.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sedding DG, Hermsen J, Seay U, Eickelberg O, Kummer W, Schwencke C, Strasser RH, Tillmanns H, Braun-Dullaeus RC. Caveolin-1 facilitates mechanosensitive protein kinase B (Akt) signaling in vitro and in vivo. Circ Res 96: 635–642, 2005. doi: 10.1161/01.RES.0000160610.61306.0f. [DOI] [PubMed] [Google Scholar]
- 191.Shack S, Wang XT, Kokkonen GC, Gorospe M, Longo DL, Holbrook NJ. Caveolin-induced activation of the phosphatidylinositol 3-kinase/Akt pathway increases arsenite cytotoxicity. Mol Cell Biol 23: 2407–2414, 2003. doi: 10.1128/MCB.23.7.2407-2414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shaw L, Sweeney MA, O’Neill SC, Jones CJ, Austin C, Taggart MJ. Caveolae and sarcoplasmic reticular coupling in smooth muscle cells of pressurised arteries: the relevance for Ca2+ oscillations and tone. Cardiovasc Res 69: 825–835, 2006. doi: 10.1016/j.cardiores.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 193.Shi Y, Tan SH, Ng S, Zhou J, Yang ND, Koo GB, McMahon KA, Parton RG, Hill MM, Del Pozo MA, Kim YS, Shen HM. Critical role of CAV1/caveolin-1 in cell stress responses in human breast cancer cells via modulation of lysosomal function and autophagy. Autophagy 11: 769–784, 2015. doi: 10.1080/15548627.2015.1034411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shigematsu T, Koshiyama K, Wada S. Molecular dynamics simulations of pore formation in stretched phospholipid/cholesterol bilayers. Chem Phys Lipids 183: 43–49, 2014. doi: 10.1016/j.chemphyslip.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 195.Singh RD, Puri V, Valiyaveettil JT, Marks DL, Bittman R, Pagano RE. Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol Biol Cell 14: 3254–3265, 2003. doi: 10.1091/mbc.E02-12-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sinha B, Köster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C, Nassoy P. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144: 402–413, 2011. doi: 10.1016/j.cell.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Smythe GM, Eby JC, Disatnik MH, Rando TA. A caveolin-3 mutant that causes limb girdle muscular dystrophy type 1C disrupts Src localization and activity and induces apoptosis in skeletal myotubes. J Cell Sci 116: 4739–4749, 2003. doi: 10.1242/jcs.00806. [DOI] [PubMed] [Google Scholar]
- 198.Smythe GM, Rando TA. Altered caveolin-3 expression disrupts PI(3) kinase signaling leading to death of cultured muscle cells. Exp Cell Res 312: 2816–2825, 2006. doi: 10.1016/j.yexcr.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 199.Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem 271: 9690–9697, 1996. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- 200.Song KS, Sargiacomo M, Galbiati F, Parenti M, Lisanti MP. Targeting of a G alpha subunit (Gi1 alpha) and c-Src tyrosine kinase to caveolae membranes: clarifying the role of N-myristoylation. Cell Mol Biol (Noisy-le-grand) 43: 293–303, 1997. [PubMed] [Google Scholar]
- 201.Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem 271: 15160–15165, 1996. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- 202.Song KS, Tang Z, Li S, Lisanti MP. Mutational analysis of the properties of caveolin-1. A novel role for the C-terminal domain in mediating homo-typic caveolin-caveolin interactions. J Biol Chem 272: 4398–4403, 1997. doi: 10.1074/jbc.272.7.4398. [DOI] [PubMed] [Google Scholar]
- 203.Sotgia F, Razani B, Bonuccelli G, Schubert W, Battista M, Lee H, Capozza F, Schubert AL, Minetti C, Buckley JT, Lisanti MP. Intracellular retention of glycosylphosphatidyl inositol-linked proteins in caveolin-deficient cells. Mol Cell Biol 22: 3905–3926, 2002. doi: 10.1128/MCB.22.11.3905-3926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sotgia F, Woodman SE, Bonuccelli G, Capozza F, Minetti C, Scherer PE, Lisanti MP. Phenotypic behavior of caveolin-3 R26Q, a mutant associated with hyperCKemia, distal myopathy, and rippling muscle disease. Am J Physiol Cell Physiol 285: C1150–C1160, 2003. doi: 10.1152/ajpcell.00166.2003. [DOI] [PubMed] [Google Scholar]
- 205.Sun Y, Hu G, Zhang X, Minshall RD. Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary vascular permeability via paracellular and transcellular pathways. Circ Res 105: 676–685, 2009. doi: 10.1161/CIRCRESAHA.109.201673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sverdlov M, Shinin V, Place AT, Castellon M, Minshall RD. Filamin A regulates caveolae internalization and trafficking in endothelial cells. Mol Biol Cell 20: 4531–4540, 2009. doi: 10.1091/mbc.E08-10-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Swaney JS, Patel HH, Yokoyama U, Head BP, Roth DM, Insel PA. Focal adhesions in (myo)fibroblasts scaffold adenylyl cyclase with phosphorylated caveolin. J Biol Chem 281: 17173–17179, 2006. doi: 10.1074/jbc.M513097200. [DOI] [PubMed] [Google Scholar]
- 208.Tagawa A, Mezzacasa A, Hayer A, Longatti A, Pelkmans L, Helenius A. Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J Cell Biol 170: 769–779, 2005. doi: 10.1083/jcb.200506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Thomsen P, Roepstorff K, Stahlhut M, van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol Cell 13: 238–250, 2002. doi: 10.1091/mbc.01-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tillu VA, Kovtun O, McMahon KA, Collins BM, Parton RG. A phosphoinositide-binding cluster in cavin1 acts as a molecular sensor for cavin1 degradation. Mol Biol Cell 26: 3561–3569, 2015. doi: 10.1091/mbc.E15-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem 272: 25968–25975, 1997. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- 212.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9: 112–124, 2008. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.van’t Hof W, Resh MD. Targeting proteins to plasma membrane and membrane microdomains by N-terminal myristoylation and palmitoylation. Methods Enzymol 327: 317–330, 2000. doi: 10.1016/S0076-6879(00)27287-X. [DOI] [PubMed] [Google Scholar]
- 214.Vega-Moreno J, Tirado-Cortes A, Álvarez R, Irles C, Mas-Oliva J, Ortega A. Cholesterol depletion uncouples β-dystroglycans from discrete sarcolemmal domains, reducing the mechanical activity of skeletal muscle. Cell Physiol Biochem 29: 905–918, 2012. doi: 10.1159/000186933. [DOI] [PubMed] [Google Scholar]
- 215.Venema VJ, Ju H, Zou R, Venema RC. Interaction of neuronal nitric-oxide synthase with caveolin-3 in skeletal muscle. Identification of a novel caveolin scaffolding/inhibitory domain. J Biol Chem 272: 28187–28190, 1997. doi: 10.1074/jbc.272.45.28187. [DOI] [PubMed] [Google Scholar]
- 216.Verma P, Ostermeyer-Fay AG, Brown DA. Caveolin-1 induces formation of membrane tubules that sense actomyosin tension and are inhibited by polymerase I and transcript release factor/cavin-1. Mol Biol Cell 21: 2226–2240, 2010. doi: 10.1091/mbc.E09-05-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Vieira OV, Verkade P, Manninen A, Simons K. FAPP2 is involved in the transport of apical cargo in polarized MDCK cells. J Cell Biol 170: 521–526, 2005. doi: 10.1083/jcb.200503078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Volonté D, Galbiati F, Pestell RG, Lisanti MP. Cellular stress induces the tyrosine phosphorylation of caveolin-1 (Tyr(14)) via activation of p38 mitogen-activated protein kinase and c-Src kinase. Evidence for caveolae, the actin cytoskeleton, and focal adhesions as mechanical sensors of osmotic stress. J Biol Chem 276: 8094–8103, 2001. doi: 10.1074/jbc.M009245200. [DOI] [PubMed] [Google Scholar]
- 219.Walser PJ, Ariotti N, Howes M, Ferguson C, Webb R, Schwudke D, Leneva N, Cho KJ, Cooper L, Rae J, Floetenmeyer M, Oorschot VM, Skoglund U, Simons K, Hancock JF, Parton RG. Constitutive formation of caveolae in a bacterium. Cell 150: 752–763, 2012. doi: 10.1016/j.cell.2012.06.042. [DOI] [PubMed] [Google Scholar]
- 220.Wanaski SP, Ng BK, Glaser M. Caveolin scaffolding region and the membrane binding region of SRC form lateral membrane domains. Biochemistry 42: 42–56, 2003. doi: 10.1021/bi012097n. [DOI] [PubMed] [Google Scholar]
- 221.Wang H, Wang AX, Aylor K, Barrett EJ. Caveolin-1 phosphorylation regulates vascular endothelial insulin uptake and is impaired by insulin resistance in rats. Diabetologia 58: 1344–1353, 2015. doi: 10.1007/s00125-015-3546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang XL, Ye D, Peterson TE, Cao S, Shah VH, Katusic ZS, Sieck GC, Lee HC. Caveolae targeting and regulation of large conductance Ca(2+)-activated K+ channels in vascular endothelial cells. J Biol Chem 280: 11656–11664, 2005. doi: 10.1074/jbc.M410987200. [DOI] [PubMed] [Google Scholar]
- 223.Wedegaertner PB, Bourne HR. Activation and depalmitoylation of Gs alpha. Cell 77: 1063–1070, 1994. doi: 10.1016/0092-8674(94)90445-6. [DOI] [PubMed] [Google Scholar]
- 224.Wei Z, Zou X, Wang H, Lei J, Wu Y, Liao K. The N-terminal leucine-zipper motif in PTRF/cavin-1 is essential and sufficient for its caveolae-association. Biochem Biophys Res Commun 456: 750–756, 2015. doi: 10.1016/j.bbrc.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 225.Woodman SE, Schlegel A, Cohen AW, Lisanti MP. Mutational analysis identifies a short atypical membrane attachment sequence (KYWFYR) within caveolin-1. Biochemistry 41: 3790–3795, 2002. doi: 10.1021/bi0120751. [DOI] [PubMed] [Google Scholar]
- 226.Wyse BD, Prior IA, Qian H, Morrow IC, Nixon S, Muncke C, Kurzchalia TV, Thomas WG, Parton RG, Hancock JF. Caveolin interacts with the angiotensin II type 1 receptor during exocytic transport but not at the plasma membrane. J Biol Chem 278: 23738–23746, 2003. doi: 10.1074/jbc.M212892200. [DOI] [PubMed] [Google Scholar]
- 227.Yang B, Rizzo V. Shear stress activates eNOS at the endothelial apical surface through β1 containing integrins and caveolae. Cell Mol Bioeng 6: 346–354, 2013. doi: 10.1007/s12195-013-0276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ye Y, Meyer HH, Rapoport TA. Function of the p97-Ufd1-Npl4 complex in retrotranslocation from the ER to the cytosol: dual recognition of nonubiquitinated polypeptide segments and polyubiquitin chains. J Cell Biol 162: 71–84, 2003. doi: 10.1083/jcb.200302169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ye Y, Shibata Y, Kikkert M, van Voorden S, Wiertz E, Rapoport TA. Recruitment of the p97 ATPase and ubiquitin ligases to the site of retrotranslocation at the endoplasmic reticulum membrane. Proc Natl Acad Sci U S A 102: 14132–14138, 2005. doi: 10.1073/pnas.0505006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhang B, Peng F, Wu D, Ingram AJ, Gao B, Krepinsky JC. Caveolin-1 phosphorylation is required for stretch-induced EGFR and Akt activation in mesangial cells. Cell Signal 19: 1690–1700, 2007. doi: 10.1016/j.cellsig.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 231.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res 62: 2227–2231, 2002. [PubMed] [Google Scholar]
- 232.Zimnicka AM, Husain YS, Shajahan AN, Sverdlov M, Chaga O, Chen Z, Toth PT, Klomp J, Karginov AV, Tiruppathi C, Malik AB, Minshall RD. Src-dependent phosphorylation of caveolin-1 Tyr-14 promotes swelling and release of caveolae. Mol Biol Cell 27: 2090–2106, 2016. doi: 10.1091/mbc.E15-11-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zocher M, Zhang C, Rasmussen SG, Kobilka BK, Müller DJ. Cholesterol increases kinetic, energetic, and mechanical stability of the human β2-adrenergic receptor. Proc Natl Acad Sci USA 109: E3463–E3472, 2012. doi: 10.1073/pnas.1210373109. [DOI] [PMC free article] [PubMed] [Google Scholar]