Abstract
Background:
Primary anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) is a chronic autoimmune disease associated with multisystem dysfunction. Renal involvement is common and closely associated with outcome. The purpose of this study was to investigate the clinical determinants of mortality of patients with AAV-related renal injury in the first 2 years after diagnosis in a single West Chinese center.
Methods:
Demographic and laboratory parameters of 123 consecutive patients with AAV-related renal injury diagnosed in Renal Division and Institute of Nephrology, Sichuan Provincial People's Hospital between 2004 and 2012 were collected retrospectively. All patients were followed up for 2 years after diagnosis. Survivors were compared with nonsurvivors to identify the clinical baseline variables associated with mortality. Multivariate Cox regression model was used to determine the independent predictors of mortality.
Results:
Of the 123 patients, 46 (37.4%) died by the end of 2 years after diagnosis, with 41 (33.3%) patients dying within the first 12 months. In comparison with the survivors, Birmingham Vasculitis Activity Score (BVAS), the incidence of pulmonary hemorrhage and digestive system (DS) involvement, serum creatinine, and erythrocyte sedimentation rate were significantly higher in nonsurvivors, whereas lymphocyte counts, hemoglobin, and complement 3 (C3) were significantly lower. Renal replacement therapy was more common in nonsurvivors. High BVAS (hazard ratio [HR] = 1.058, 95% confidence interval [CI]: 1.002–1.117; P = 0.042), pulmonary hemorrhage (HR = 1.970, 95% CI: 1.033–3.757; P = 0.04), DS involvement (HR = 2.911, 95% CI: 1.212–6.911; P = 0.017), and serum creatinine >400 μmol/L (HR = 2.910, 95% CI: 1.271–6.664; P = 0.012) were independent predictors of death in patients with AAV-related renal injury.
Conclusions:
Patients with AAV-related renal injury have high early mortality. Those with high BVAS (particularly with pulmonary or DS involvement) and serious renal dysfunction should receive aggressive therapy and careful monitoring to reduce the occurrence of adverse events and improve prognosis.
Keywords: Anti-neutrophil Cytoplasmic Autoantibody-associated Vasculitis, Mortality, Predictors, Renal Involvement
Introduction
Primary anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a chronic multisystem autoimmune disease that includes microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA; Wegener granulomatosis), eosinophilic granulomatosis with polyangiitis (EPGA; Churg-Strauss syndrome), and localized forms of these diseases. AAV is a life-threatening disease. After the introduction of corticosteroids and immunosuppressants, early remission has been favorably improved in most patients, but patients with AAV are still at increasing risk of death compared with age- and sex-matched population despite immunosuppressive therapy.[1]
Renal involvement is one of the most common manifestations of vasculitis and is closely associated with the outcomes.[2,3] The incidence of AAV-associated renal injury is not different between Asian and Western populations.[4] However, whereas GPA is more common than MPA in Europe, MPA is approximately two to three times more common than GPA in China and Japan.[5,6,7,8] The clinical phenotype of AAV is also different between Asian and Western populations.[8,9] Fujimoto et al.[4] found that the ratio of serum myeloperoxidase ANCA (MPO-ANCA) to proteinase 3-ANCA (PR3-ANCA) was much higher in Japanese patients with ANCA-associated primary renal vasculitis than that in European and American patients. A study in Chinese patients has demonstrated that the prevalence of renal involvement is significantly higher in patients with MPO-ANCA than in patients with PR3-ANCA.[2] In addition, renal injury secondary to AAV is often associated with MPA or MPO-ANCA in China, but this is not so in the Western countries. Renal prognosis of ANCA-associated crescentic glomerulonephritis in a Chinese cohort has been reported to be different from that of the European Vasculitis Study Group cohort.[10,11] Therefore, it is important to investigate the different clinical manifestations and outcomes of AAV-associated renal injury according to different geographic location and ethnicity.
Mortality is high in AAV patients with renal involvement. Some studies on the outcome of subgroup with renal involvement showed that in addition to the decreased renal function, many other factors were associated with early death, including advanced age, high Birmingham Vasculitis Activity Score (BVAS), therapy-related adverse events, pulmonary hemorrhage, renal insufficiency, etc.[12,13,14,15,16,17] However, patient selection criteria and the choice of predictors of mortality have not been uniform in the studies conducted to date. A few studies have been conducted on the predictors of mortality in AAV patients in China. The purpose of this study was to determine the baseline clinical factors that predict early mortality in Chinese patients with AAV-related renal injury.
Methods
Study design and participants
One hundred and twenty-three patients with ANCA-associated renal vasculitis, who were newly diagnosed in Renal Division and Institute of Nephrology, Sichuan Provincial People's Hospital between 2004 and 2012, were enrolled in the current study retrospectively. Disease diagnosis complied with the Chapel Hill Consensus Conference criteria for AAV.[18] Inclusion criteria for this study were: (1) positive serology for MPO-ANCA or PR3-ANCA; (2) renal involvement: rapidly elevated serum creatinine, and/or the presence of hematuria ≥10 red blood cells per high power field, and/or red cell casts, and/or proteinuria >0.5 g/24 h; (3) for negative serology of MPO-ANCA or PR3-ANCA, there is histological evidence of vasculitis. In brief, the histopathologic characteristics of the renal specimen are pauci-immune staining on immunofluorescence microscopy, the absence of immune deposits on electron microscopy, and necrotizing and crescentic glomerulonephritis on light microscopy. Patients with secondary vasculitis were excluded, including propylthiouracil-induced AAV,[19] or lupus nephritis, or other connective tissue diseases. It is a retrospective study focusing on the case data analysis, there is no intervention study, and hence there is no signed consent with the patients. In the article, the information on the identity of the subject, including the name, abbreviation, and hospital number, are avoided. The study did not violate the Helsinki Declaration.
Data collection
Clinical and laboratory data were collected from the case records retrospectively; the variables recorded included age at diagnosis, time from onset of symptoms to admission, organs involved, urinary red blood cell count, 24-h urinary protein, white blood cell count, lymphocyte count, hemoglobin level, platelet count, initial serum creatinine, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and complement 3 (C3). MPO-ANCA and PR3-ANCA were assessed by both antigen-specific enzyme-linked immunosorbent assay and indirect immunofluorescence assay at the time of diagnosis. Disease activity was scored using the BVAS.[20]
All patients were followed up for 2 years after diagnosis, and any major events were recorded. These events included disease relapse, treatment resistance, rehospitalization, and death. Relapse was defined as an increase in serum creatinine level or worsening/new extrarenal manifestations attributable to active vasculitis, with accompanying increase in ANCA titers after remission. Treatment resistance was defined as progressive decline in kidney function with persistent active urine sediment, or persistent or new appearance of any extrarenal manifestations of active vasculitis despite optimal immunosuppressive therapy.[12] Hospitalization was recorded only if it was for intensive treatment for progression of vasculitis or complications. Death due to any cause was recorded as an end event.
Treatment
All patients received induction therapy with various combinations of corticosteroid and immunosuppressants. Oral prednisone was prescribed at an initial dosage of 0.80 mg·kg−1·d−1 in all cases. Three patients received mycophenolate mofetil; the others received cyclophosphamide (CTX). CTX was administered by intravenous infusion every ½ month, the total dose of a month was 0.60 g/m2. Fifty-five patients with rapidly progressive renal failure and/or pulmonary hemorrhage received three intravenous pulses of methylprednisolone (0.25–0.50 g/day) before the induction therapy. Nine patients with severe pulmonary hemorrhage underwent plasma exchanges. Forty-two patients received renal replacement therapy after diagnosis. Maintenance therapy was with oral leflunomide or azathioprine.
Statistical analysis
Consecutive variables were expressed as the mean ± standard deviation (for normally distributed data) or median (interquartile range [IQR]; for nonnormally distributed data). Categorical variables were summarized as percentages. The differences between groups were compared using the t-test (for normally distributed data) or a nonparametric test (for nonnormally distributed data); the Chi-square test was used for categorical variables. Kaplan-Meier analysis was used to study survival. Multivariate Cox regression model was used for identifying the predictors of mortality, and the results were expressed as hazard ratios (HRs) (with 95% confidence interval [CI]). A P ≤ 0.05 indicated statistical significance; all tests were two-sided. SPSS statistical software version 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Demographic and clinical features
A total of 123 patients (including 59 females) with AAV-related renal vasculitis were included in the study. Mean age at diagnosis was 61.86 ± 12.25 years (range, 29–85 years). Median time from onset of symptoms to admission was 2 months (IQR, 1,6 months). Table 1 shows the clinical features of the patients at the time of diagnosis. MPO-ANCA was present in 104 (84.6%) patients. Mean BVAS was 19.76 ± 5.47. At diagnosis, mean serum creatinine was 442.38 ± 338.57 μmol/L, and the median 24-h urinary protein was 1.50 g (IQR, 1.0, 3.05 g); 97 (78.9%) patients had hematuria. Mean hemoglobin was 83.03 ± 21.16 g/L, and mean ESR was 90.00 ± 41.37 mm/h; the median CRP was 34.80 mg/L (IQR, 11.0, 76.72 mg/L). The pulmonary system was the most commonly involved system at diagnosis (67.5%); 29 patients (23.6%) had pulmonary hemorrhage. The ear, nose, and throat were the next most commonly involved organs (21.1%). The cardiovascular system was affected in 13% of patients [Figure 1].
Table 1.
Demographics and clinical features of 123 AAV-related renal involvement patients according to outcome
| Characteristics | Total (N = 123) | Survivors (n = 77) | Nonsurvivors (n = 46) | P |
|---|---|---|---|---|
| Age (years) | 61.86 ± 12.25 | 61.25 ± 12.14 | 62.89 ± 12.50 | 0.470 |
| Gender (male/female, n) | 64/59 | 38/39 | 26/20 | 0.440 |
| Time from symptom onset to admission (months) | 2.0 (1.0–6.0) | 2.0 (1.0–5.0) | 2.0 (0.7–6.0) | 0.570 |
| BVAS | 19.76 ± 5.47 | 18.66 ± 4.80 | 21.59 ± 6.06 | 0.004 |
| Nonspecific symptoms, n (%) | ||||
| Fever | 56 (45.5) | 34 (44.2) | 22 (47.8) | 0.690 |
| Weight loss | 41 (33.3) | 27 (35.1) | 14 (30.4) | 0.600 |
| Arthralgia | 23 (18.3) | 15 (19.5) | 8 (17.4) | 0.770 |
| Muscle pain | 22 (17.9) | 15 (19.5) | 7 (15.2) | 0.550 |
| Systems involvement, n (%) | ||||
| Skin | 14 (11.4) | 9 (11.7) | 5 (10.9) | 0.890 |
| Ophthalmic and mucocutaneous | 7 (5.7) | 3 (3.9) | 4 (8.7) | 0.460 |
| ENT | 26 (21.1) | 18 (23.4) | 8 (17.4) | 0.430 |
| Pulmonary system | 83 (67.5) | 43 (55.8) | 40 (87.0) | <0.001 |
| Pulmonary hemorrhage | 29 (23.6) | 12 (15.6) | 17 (37) | 0.007 |
| Pulmonary interstitial fibrosis | 31 (25.2) | 15 (19.5) | 16 (34.8) | 0.060 |
| Digestive system | 8 (6.5) | 1 (1.3) | 7 (15.2) | 0.004 |
| Cardiovascular system | 16 (13.0) | 10 (13.0) | 6 (13.0) | 0.990 |
| Nervous system | 12 (9.8) | 7 (9.1) | 5 (10.9) | 0.750 |
| Lymphocyte count (×109/L) (range) | 1.02 (0.69–1.31) | 1.04 (0.74–1.40) | 0.88 (0.64–1.14) | 0.020 |
| Hemoglobin (g/L) | 83.04 ± 21.16 | 87.55 ± 21.98 | 75.59 ± 17.56 | 0.002 |
| Initial serum creatinine (µmol/L) | 442.38 ± 338.56 | 359.95 ± 304.44 | 580.37 ± 350.93 | <0.001 |
| 24-h urinary protein (g/24 h) (range) | 1.50 (1.00–3.05) | 1.55 (0.92–1.27) | 1.42 (1.00–3.31) | 0.980 |
| Hematuria, n (%) | 97 (78.9) | 58 (75.3) | 39 (84.8) | 0.210 |
| ESR (mm/h) | 90.00 ± 41.37 | 83.30 ± 38.86 | 101.27 ± 43.42 | 0.020 |
| CRP (mg/L) (range) | 34.80 (11.00–76.72) | 28.55 (9.28–69.82) | 41.40 (16.85–106.80) | 0.060 |
| Serum C3 (C3) (mg/L) | 0.92 ± 0.27 | 0.97 ± 0.29 | 0.84 ± 0.23 | 0.020 |
| MPO-ANCA (+)/PR3-ANCA (−), n (%) | 104 (84.6) | 66 (85.7) | 38 (82.6) | 0.583 |
| PR3-ANCA (+)/MPO-ANCA (−), n (%) | 12 (9.8) | 6 (7.8) | 6 (13.0) | 0.583 |
| MPO-AMCA (+)/MPO-ANCA (+), n (%) | 7 (5.7) | 5 (6.5) | 2 (4.3) | 0.583 |
| Renal replacement therapy, n (%) | 42 (34.1) | 20 (26.0) | 22 (47.8) | 0.010 |
ENT: Ear, nose, and throat; BVAS: Birmingham Vasculitis Activity Score; AAV: ANCA-associated vasculitis; ANCA: Anti-neutrophil cytoplasmic autoantibody; CRP: C-reactive protein; MPO: Myeloperoxidase; PR3: Proteinase 3; C3: Complement 3.
Figure 1.
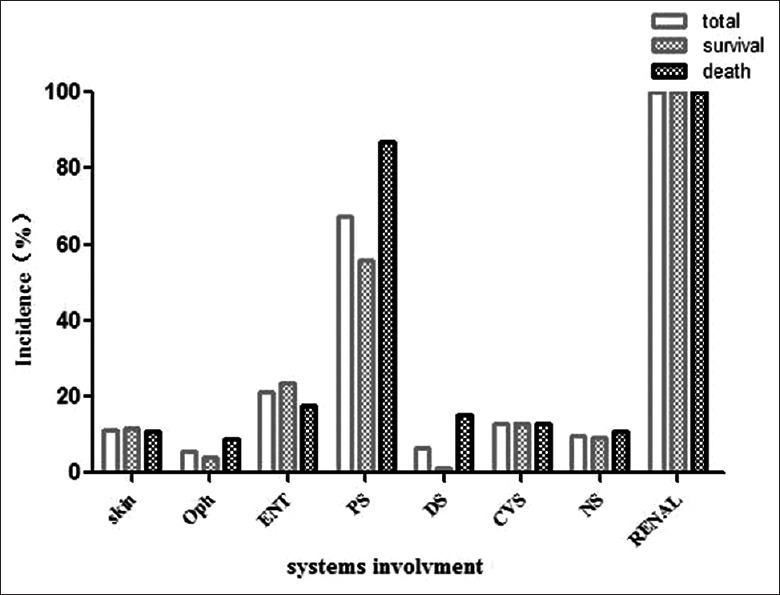
Organ involvement of patients with ANCA-related renal vasculitis at diagnosis. Oph: Ophthalmic and mucocutaneous; ENT: Ear, nose, and throat; PS: Pulmonary system; DS: Digestive system; CVS: Cardiovascular system; NS: Nervous system; ANCA: Anti-neutrophil cytoplasmic autoantibody.
In addition to the standard induction therapy, 55 (44.7%) patients received three intravenous pulses of methylprednisolone. Furthermore, 42 (34.1%) patients received renal replacement treatment and 9 (7.3%) patients underwent plasma exchanges immediately after diagnosis.
To identify the candidate predictors of mortality, the patients were divided into two groups: survivors and nonsurviors. Table 1 shows the clinical features at diagnosis in the two groups. The nonsurvivor group comprised 46 patients (26 males and 20 females). The nonsurvivors had significantly higher BVAS, higher prevalence of pulmonary hemorrhage, higher proportion of patients with pulmonary and digestive system (DS) involvement, higher initial serum creatinine and ESR, lower blood lymphocyte count, lower hemoglobin, lower complement C3, and higher proportion of patients receiving renal replacement therapy. The other variables were not significantly different between the two groups.
Events during follow-up
Mean duration of follow-up was for 16.6 ± 10.3 months. Of the 123 patients, 88 achieved remission after initial induction therapy. Of these 88 patients, 20 (22.7%) experienced relapse during follow-up, and 6 died after relapse. The median time from remission to relapse was 12.3 months (IQR, 3.0, 24.0 months). Figure 2 shows the survival probability of relapse-free patients.
Figure 2.
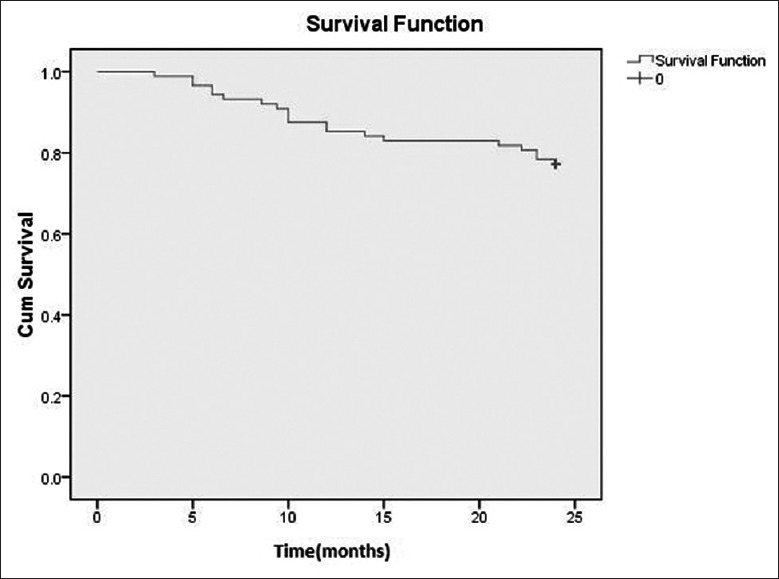
Kaplan-Meier analysis of the probability of Relapse-free survival of patients with ANCA-related renal vasculitis achieving remission. ANCA: Anti-neutrophil cytoplasmic autoantibody.
Over the 2 years of follow-up, 33 patients were rehospitalized; in all, there were 39 rehospitalized. The reasons for rehospitalization were relapse (20/39; 51.3%), major infectious episode (13/39; 33.3%), and therapy resistance (6/39; 15.4%).
Predictors of mortality
In all, 46/123 (37.4%) patients died during follow-up. The most common causes of death were a major infectious episode (21/46; 45.7%) and active vasculitis (16/46; 34.8%). The cumulative mortality at 6 months, 1 year, and 2 years were 30.1%, 34.1%, and 37.4%, respectively. The peak mortality was within the first 6 months after diagnosis [Figure 3].
Figure 3.
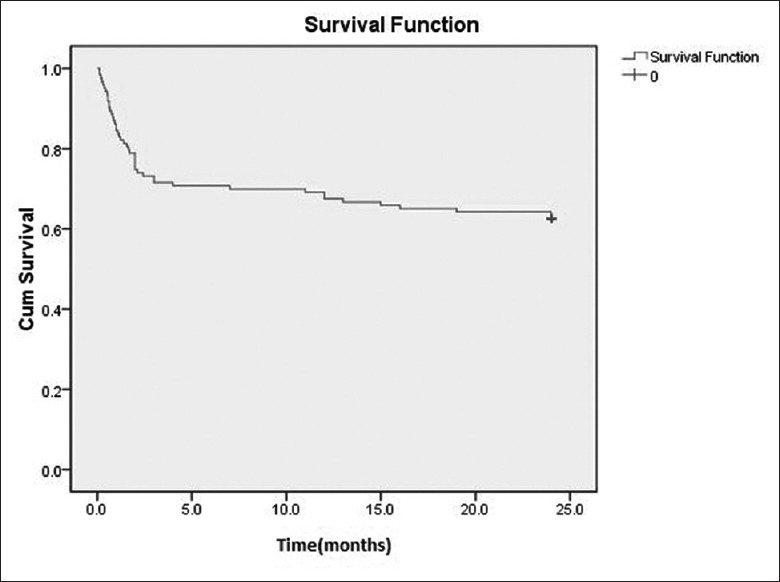
Kaplan-Meier survival curve of 123 patients with ANCA-related renal vasculitis. ANCA: Anti-neutrophil cytoplasmic autoantibody.
The value of P < 0.05 in univariate analysis; thus, the predictor was allowed to be entered into the multivariable regression model. Age and renal function, which had been identified as confounding factors in previous studies, were also included in the multivariable analysis. Due to the close relationship between the BVAS and organ-specific involvement, two multivariate regression models were used to identify the predictors of all-cause mortality. In model A, initial serum creatinine >400 μmol/L (HR = 2.910, 95% CI: 1.271–6.664; P = 0.012), pulmonary hemorrhage (HR = 1.970, 95% CI: 1.033–3.757; P = 0.040), and digestive involvement by AAV (HR = 2.911, 95% CI:1.212–6.911; P = 0.017) were independent predictors of all-cause mortality. In model B, initial serum creatinine >400 μmol/L (HR = 3.754, 95% CI:1.651–8.537; P = 0.002) and BVAS (HR = 1.058, 95% CI: 1.002–1.117; P = 0.042) were independent predictors of all-cause mortality in ANCA-related renal vasculitis patients [Table 2].
Table 2.
Predictors of all-cause mortality in the 123 AAV-related renal involvement patients
| Variable | Model A | Model B | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (years) | 1.010 (0.982–1.038) | 0.494 | 1.006 (0.979–1.033) | 0.683 |
| Pulmonary hemorrhage | 1.970 (1.033–3.757) | 0.040 | – | – |
| Digestive system involvement | 2.911 (1.212–6.991) | 0.017 | – | – |
| Initial serum creatinine >400 (µmol/L) | 2.910 (1.271–6.664) | 0.012 | 3.754 (1.651–8.537) | 0.002 |
| BVAS | – | – | 1.058 (1.002–1.117) | 0.042 |
| Lymphocyte counts (×109/L) | 0.612 (0.279–1.343) | 0.221 | 0.555 (0.244–1.260) | 0.159 |
| Hemoglobin (g/L) | 1.002 (0.982–1.022) | 0.857 | 1.005 (0.984–1.028) | 0.631 |
| ESR (mm/h) | 1.007 (0.998–1.016) | 0.109 | 1.008 (1.000–1.016) | 0.059 |
| Serum C3 (mg/L) | 0.445 (0.094–2.108) | 0.307 | 0.426 (0.099–1.836) | 0.252 |
Model A: Adjusted for age, pulmonary hemorrhage, digestive system involvement, initial serum creatinine, lymphocyte counts, hemoglobin, ESR, serum C3. Model B: Adjusted for age, Initial serum creatinine, BVAS, lymphocyte counts, hemoglobin, ESR, serum C3. BVAS: Birmingham Vasculitis Activity Score; ESR: Erythrocyte sedimentation rate; AAV: ANCA-associated vasculitis; ANCA: Anti-neutrophil cytoplasmic autoantibody; C3: Complement 3; HR: Hazard ratio; CI: Confidence interval.
Discussion
This retrospective study was conducted to identify the factors associated with mortality in Chinese patients presenting with AAV-related renal injury. The results identified high BVAS – especially with pulmonary or DS involvement – and high serum creatinine as independent predictors of mortality in these patients.
The high mortality of AAV remains a challenge for physicians. Renal dysfunction is known to be a risk factor for early death in AAV patients,[1] and attempts to improve prognosis should, therefore, focus on the treatment of renal vasculitis. Better understanding of the factors affecting prognosis will help in the choice of the appropriate interventions in individual patients.
In this study, the primary causes of death were major infectious episodes and active vasculitis; furthermore, mortality was concentrated in the first 6 months after diagnosis. Other cohort studies have also identified severe infection and active vasculitis as major causes of early mortality of AAV patients.[15,21,22,23] While many studies found that severe infection was the first cause of death of AAV, fewer than 50% of deaths were related to the vasculitis activity.[3] Our results suggest that in addition to severe infection, active vasculitis also contributed to a significant proportion death of patients with AAV-related renal vasculitis and therefore warrants more attention. Bourgarit et al.[22] showed that active vasculitis was the leading cause of deaths in the 1st year after diagnosis and that treatment with corticosteroid alone was associated with early death, suggesting that such treatment is insufficient and inappropriate.
In this study, a small number of patients received corticosteroid alone in the induction stage. This may decrease the infection while at the same time result in uncontrolled vasculitis. We found that infection, in combination with the pulmonary involvement of active vasculitis was an important cause of death, this is consistent with earlier studies.[16,23] Previous studies on severe infection and therapy-related death in AAV patients have reported that pulmonary involvement (especially pulmonary hemorrhage) was an independent predictor of mortality.[15,21] Vasculitis activity may, therefore, be partly responsible for the infection-related deaths. Bourgarit et al.[22] showed that most first-year nonsurvivors with GPA or EPGA die of vasculitis, whereas MPA patients mostly die of infection. Therefore, patients with different subtypes of AAV should receive individual immunosuppressive therapy, so as to balance the control of vasculitis and therapy-related infection.
In our cohort, an initial serum creatinine level >400 μmol/L was an independent predictor of all-cause mortality. Severe renal dysfunction was also identified a predictor of mortality in patients with AAV in other similar studies.[1,12,24] However, the mean initial serum creatinine was much higher in our cohort than that of renal involvement subgroup in Western studies (442 vs. 221 μmol/L).[12] This was likely because our cohort had far more MPO-ANCA patients than PR3-ANCA patients (84.6%MPO-ANCA vs. 9.8%PR3-ANCA). MPO-ANCA patients have more extensive and chronic lesions of renal histology and shorter mean duration from disease onset to renal involvement and therefore tend to have severe renal involvement at diagnosis.[24,25,26,27,28]
In this study, high BVAS was a predictor of mortality. The BVAS was used for evaluating the symptoms of nine systems. Patients with pulmonary hemorrhage or DS involvement had a relatively high risk of mortality. Pulmonary hemorrhage was not only a predictor of all-cause mortality but also a predictor of infection.[15] Pulmonary hemorrhage could increase mortality by increasing susceptibility to pulmonary infections. Special care should be taken, therefore, to prevent pulmonary infection in patients with pulmonary involvement. Digestive tract involvement, as a predictor of mortality in AAV, has only been reported in a few studies. One previous study has shown that, in AAV patients, gastrointestinal (GI) involvement is more frequent in the first-year nonsurvivors than that in first-year survivors. For organ-specific involvement related death, GI involvement (16/33, 48.5%) was the most common organ. GI involvement in AAV was associated with the mortality.[22] For the patients with unreasonably-explained severe GI manifestations (i.e., GI bleeding, pancreatitis), AAV should be considered and screened. Right treatment in time may change the prognosis dramatically.
Low initial blood lymphocyte count was associated with mortality in this study; however, it was not an independent predictor of mortality. Lymphocyte count reduction was known to be independently associated with infectious complication,[22,29,30] and therefore, it could be used in the clinic as a monitoring index for identifying patients at increased risk of infection.
This study has some limitations. First, this was a retrospective study and so the treatment and follow-up protocols were not standardized. Second, the patients enrolled in this study were all from a single center and therefore may not be representative of all vasculitis patients. Third, detailed records of the total amount of corticosteroids and immunosuppressive drugs used and the laboratory indices during follow-up were not available, and as a result, the effect of the therapy on prognosis could not be analyzed.
To conclude, early mortality is high in patients with AAV-related renal injury. Patients with high BVAS (particularly with pulmonary or DS involvement) and serious renal dysfunction need aggressive therapy and careful monitoring to reduce adverse events and improve prognosis.
Financial support and sponsorship
This work was supported by a grant from the Youth Science and Technology Creative Research Groups of Sichuan Province, China (No. 2015TD0013).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
References
- 1.Flossmann O, Berden A, de Groot K, Hagen C, Harper L, Heijl C, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. 2011;70:488–94. doi: 10.1136/ard.2010.137778. doi: 10.1136/ard.2010.137778. [DOI] [PubMed] [Google Scholar]
- 2.Chen M, Yu F, Zhang Y, Zhao MH. Clinical [corrected] and pathological characteristics of Chinese patients with antineutrophil cytoplasmic autoantibody associated systemic vasculitides: A study of 426 patients from a single centre. Postgrad Med J. 2005;81:723–7. doi: 10.1136/pgmj.2005.034215. doi: 10.1136/pgmj.2005.034215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corral-Gudino L, Borao-Cengotita-Bengoa M, Del Pino-Montes J, Lerma-Márquez JL. Overall survival, renal survival and relapse in patients with microscopic polyangiitis: A systematic review of current evidence. Rheumatology (Oxford) 2011;50:1414–23. doi: 10.1093/rheumatology/ker112. doi: 10.1093/rheumatology/ker112. [DOI] [PubMed] [Google Scholar]
- 4.Fujimoto S, Uezono S, Hisanaga S, Fukudome K, Kobayashi S, Suzuki K, et al. Incidence of ANCA-associated primary renal vasculitis in the Miyazaki Prefecture: The first population-based, retrospective, epidemiologic survey in Japan. Clin J Am Soc Nephrol. 2006;1:1016–22. doi: 10.2215/CJN.01461005. doi: 10.2215/CJN.01461005. [DOI] [PubMed] [Google Scholar]
- 5.Watts RA, Lane SE, Scott DG, Koldingsnes W, Nossent H, Gonzalez-Gay MA, et al. Epidemiology of vasculitis in Europe. Ann Rheum Dis. 2001;60:1156–7. doi: 10.1136/ard.60.12.1156a. doi: 10.1136/ard.60.12.1156a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu LJ, Chen M, Yu F, Zhao MH, Wang HY. Evaluation of a new algorithm in classification of systemic vasculitis. Rheumatology (Oxford) 2008;47:708–12. doi: 10.1093/rheumatology/ken079. doi: 10.1093/rheumatology/ken079. [DOI] [PubMed] [Google Scholar]
- 7.Kamali S, Artim-Esen B, Erer B, Ozdener L, Gul A, Ocal L, et al. Re-evaluation of 129 patients with systemic necrotizing vasculitides by using classification algorithm according to consensus methodology. Clin Rheumatol. 2012;31:325–8. doi: 10.1007/s10067-011-1793-3. doi: 10.1007/s10067-011-1793-3. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto S, Watts RA, Kobayashi S, Suzuki K, Jayne DR, Scott DG, et al. Comparison of the epidemiology of anti-neutrophil cytoplasmic antibody-associated vasculitis between Japan and the U.K. Rheumatology (Oxford) 2011;50:1916–20. doi: 10.1093/rheumatology/ker205. doi: 10.1093/rheumatology/ker205. [DOI] [PubMed] [Google Scholar]
- 9.Watts RA, Scott DG, Jayne DR, Ito-Ihara T, Muso E, Fujimoto S, et al. Renal vasculitis in Japan and the UK – Are there differences in epidemiology and clinical phenotype? Nephrol Dial Transplant. 2008;23:3928–31. doi: 10.1093/ndt/gfn354. doi: 10.1093/ndt/gfn354. [DOI] [PubMed] [Google Scholar]
- 10.Chang DY, Wu LH, Liu G, Chen M, Kallenberg CG, Zhao MH. Re-evaluation of the histopathologic classification of ANCA-associated glomerulonephritis: A study of 121 patients in a single center. Nephrol Dial Transplant. 2012;27:2343–9. doi: 10.1093/ndt/gfr643. doi: 10.1093/ndt/gfr643. [DOI] [PubMed] [Google Scholar]
- 11.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21:1628–36. doi: 10.1681/ASN.2010050477. doi: 10.1681/ASN.2010050477. [DOI] [PubMed] [Google Scholar]
- 12.Pagnoux C, Hogan SL, Chin H, Jennette JC, Falk RJ, Guillevin L, et al. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis: Comparison of two independent cohorts. Arthritis Rheum. 2008;58:2908–18. doi: 10.1002/art.23800. doi: 10.1002/art.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Booth AD, Almond MK, Burns A, Ellis P, Gaskin G, Neild GH, et al. Outcome of ANCA-associated renal vasculitis: A 5-year retrospective study. Am J Kidney Dis. 2003;41:776–84. doi: 10.1016/s0272-6386(03)00025-8. doi: 10.1016/S0272-6386(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe K, Tani Y, Kimura H, Tanaka K, Hayashi Y, Asahi K, et al. Clinical outcomes of Japanese MPO-ANCA-related nephritis: Significance of initial renal death for survival. Intern Med. 2012;51:1969–76. doi: 10.2169/internalmedicine.51.7727. doi: 10.2169/internalmedicine.51.7727. [DOI] [PubMed] [Google Scholar]
- 15.Li ZY, Gou SJ, Chen M, Zhao MH. Predictors for outcomes in patients with severe ANCA-associated glomerulonephritis who were dialysis-dependent at presentation: A study of 89 cases in a single Chinese center. Semin Arthritis Rheum. 2013;42:515–21. doi: 10.1016/j.semarthrit.2012.09.005. doi: 10.1016/j.semarthrit.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Chen YX, Yu HJ, Zhang W, Ren H, Chen XN, Shen PY, et al. Analyzing fatal cases of Chinese patients with primary antineutrophil cytoplasmic antibodies-associated renal vasculitis: A 10-year retrospective study. Kidney Blood Press Res. 2008;31:343–9. doi: 10.1159/000165117. doi: 10.1159/000165117. [DOI] [PubMed] [Google Scholar]
- 17.Hu WX, Liu ZH, Liu CB, Tang Z, Wang QW, Chen HP, et al. Prognosis of microscopic polyangiitis with renal involvement: Report of 60 Chinese patients. Chin Med J. 2005;118:2089–92. [PubMed] [Google Scholar]
- 18.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 19.Liu HL, Gao MJ, Zheng YA, Liu GH. Propylthiouracil induced antineutrophil cytoplasmic antibodies-associated vasculitis. Chin Med J. 2013;126:4814. doi: 10.3760/cma.j.issn. 0366-6999.20131487. [PubMed] [Google Scholar]
- 20.Flossmann O, Bacon P, de Groot K, Jayne D, Rasmussen N, Seo P, et al. Development of comprehensive disease assessment in systemic vasculitis. Ann Rheum Dis. 2007;66:283–92. doi: 10.1136/ard.2005.051078. doi: 10.1136/ard. 2005.051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai QY, Ma TT, Li ZY, Chang DY, Zhao MH, Chen M. Predictors for mortality in patients with antineutrophil cytoplasmic autoantibody-associated vasculitis: A study of 398 Chinese patients. J Rheumatol. 2014;41:1849–55. doi: 10.3899/jrheum.131426. doi: 10.3899/jrheum.131426. [DOI] [PubMed] [Google Scholar]
- 22.Bourgarit A, Le Toumelin P, Pagnoux C, Cohen P, Mahr A, Le Guern V, et al. Deaths occurring during the first year after treatment onset for polyarteritis nodosa, microscopic polyangiitis, and Churg-Strauss syndrome: A retrospective analysis of causes and factors predictive of mortality based on 595 patients. Medicine (Baltimore) 2005;84:323–30. doi: 10.1097/01.md.0000180793.80212.17. doi: 10.1097/01.md.0000180793.80212.17. [DOI] [PubMed] [Google Scholar]
- 23.Little MA, Nightingale P, Verburgh CA, Hauser T, De Groot K, Savage C, et al. Early mortality in systemic vasculitis: Relative contribution of adverse events and active vasculitis. Ann Rheum Dis. 2010;69:1036–43. doi: 10.1136/ard.2009.109389. doi: 10.1136/ard.2009.109389. [DOI] [PubMed] [Google Scholar]
- 24.de Joode AA, Sanders JS, Stegeman CA. Renal survival in proteinase 3 and myeloperoxidase ANCA-associated systemic vasculitis. Clin J Am Soc Nephrol. 2013;8:1709–17. doi: 10.2215/CJN.01020113. doi: 10.2215/CJN.01020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vizjak A, Rott T, Koselj-Kajtna M, Rozman B, Kaplan-Pavlovcic S, Ferluga D. Histologic and immunohistologic study and clinical presentation of ANCA-associated glomerulonephritis with correlation to ANCA antigen specificity. Am J Kidney Dis. 2003;41:539–49. doi: 10.1053/ajkd.2003.50142. doi: 10.1053/ajkd.2003.50142. [DOI] [PubMed] [Google Scholar]
- 26.Hauer HA, Bajema IM, van Houwelingen HC, Ferrario F, Noël LH, Waldherr R, et al. Renal histology in ANCA-associated vasculitis: differences between diagnostic and serologic subgroups. Kidney Int. 2002;61:80–9. doi: 10.1046/j.1523-1755.2002.00089.x. doi: 10.1046/j.1523-1755.2002.00089.x. [DOI] [PubMed] [Google Scholar]
- 27.Ono N, Niiro H, Ueda A, Sawabe T, Nishizaka H, Furugo I, et al. Characteristics of MPO-ANCA-positive granulomatosis with polyangiitis: a retrospective multi-center study in Japan. Rheumatol Int. 2015;35:555–9. doi: 10.1007/s00296-014-3106-z. [DOI] [PubMed] [Google Scholar]
- 28.Chen M, Yu F, Wang SX, Zou WZ, Zhang Y, Zhao MH, et al. Renal histology in Chinese patients with anti-myeloperoxidase autoantibody-positive Wegener's granulomatosis. Nephrol Dial Transplant. 2007;22:139–45. doi: 10.1093/ndt/gfl509. doi: 10.1093/ndt/gfl509. [DOI] [PubMed] [Google Scholar]
- 29.Goupil R, Brachemi S, Nadeau-Fredette AC, Déziel C, Troyanov Y, Lavergne V, et al. Lymphopenia and treatment-related infectious complications in ANCA-associated vasculitis. Clin J Am Soc Nephrol. 2013;8:416–23. doi: 10.2215/CJN.07300712. doi: 10.2215/CJN.07300712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi YY, Li ZY, Zhao MH, Chen M. The CD4 Lymphocyte Count is a Better Predictor of Overall Infection Than the Total Lymphocyte Count in ANCA-Associated Vasculitis Under a Corticosteroid and Cyclophosphamide Regimen: A Retrospective Cohort. Medicine (Baltimore) 2015;94:e843. doi: 10.1097/MD.0000000000000843. doi: 10.1097/MD.0000000000000843. [DOI] [PMC free article] [PubMed] [Google Scholar]


