Abstract
Background:
In vitro experiments have revealed that toll-like receptor 4 (TLR4) pathway is involved in the progression of immunoglobulin A nephropathy (IgAN) by induction of proinflammatory cytokines. Evidence showed that, in other disease models, peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists have been shown to exert anti-inflammatory effects through suppression of the expression and activity of TLR4. However, the interaction between PPAR-γ and TLR4 in IgAN has not been fully studied both in vitro and in vivo. In this study, we explored whether TLR4 pathway attributed to the progression of IgAN in experimental rats.
Methods:
Bovine gamma globulin was used to establish IgAN model. Fifty-four Lewis rats were randomly divided into six groups: ControlTAK242, IgANTAK242, toll-like receptor 4 inhibitor (TAK242) groups (rats were administrated with TLR4 inhibitor, TAK242) and ControlPio, IgANPio, Pio groups (rats were administrated with PPAR-γ agonist, pioglitazone). Urinary albumin-to-creatinine ratio (ACR), serum creatinine, and blood urea nitrogen were detected by automatic biochemical analyzer. Renal histopathological changes were observed after hematoxylin-eosin staining, and the IgA deposition in glomeruli was measured by immunofluorescence staining. Real-time polymerase chain reaction and Western blotting were used to detect TLR4 and interleukin-1 beta (IL-1β) message ribonucleic acid (mRNA) and protein expression in renal tissues. Results were presented as mean ± standard deviation. Differences between groups were analyzed by one-way analysis of variance.
Results:
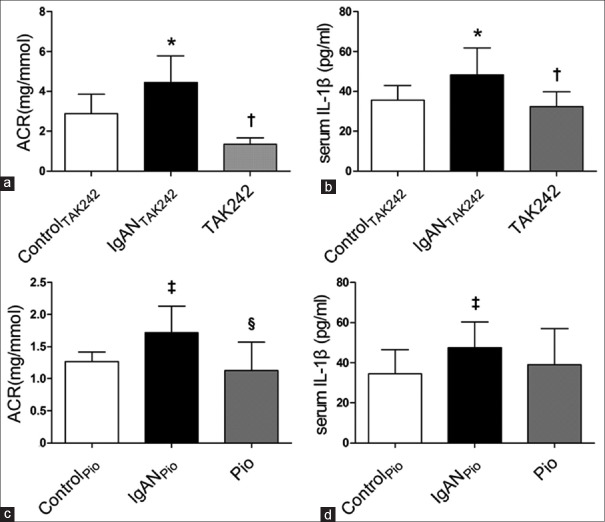
Compared to normal rats, experimental rats showed higher ACR (4.45 ± 1.33 mg/mmol vs. 2.89 ± 0.96 mg/mmol, P < 0.05), obvious IgA deposition with mesangial hypercellularity, hyperplasia of mesangial matrix accompanied by increased serum IL-1β (48.28 ± 13.49 pg/ml vs. 35.56 ± 7.41pg/ml, P < 0.05), and renal expression of IL-1β and TLR4. The biochemical parameters and renal pathological injury were relieved in both TAK242 group and Pio group. The expressions of renal tissue TLR4, IL-1β, and serum IL-1β were decreased in rats treated with TAK242, and the expression of TLR4 mRNA and protein was significantly reduced in Pio group compared to IgANPio group (1.22 ± 0.28 vs. 1.72 ± 0.45, P < 0.01, and 0.12 ± 0.03 vs. 0.21 ± 0.05, P < 0.01).
Conclusions:
Our study proves that inflammation mediated by TLR4 signaling pathway is involved in the progression of IgAN in rat models. Moreover, pioglitazone can inhibit the expression of TLR4 in IgAN.
Keywords: Glomerulonephritis, Immunoglobulin A, Peroxisome Proliferator-activated Receptor-γ, Toll-like Receptor 4
Introduction
Immunoglobulin A (IgA) nephropathy is characterized by mesangial IgA deposits and expansion of glomerular mesangial matrix with proliferation of mesangial cells. Its clinical manifestation is extremely variable, ranging from asymptomatic microscopic hematuria to rapidly progressive renal failure. It is considered as the most common primary glomerulonephritis worldwide[1] and 36% of adult patients with IgA nephropathy (IgAN) progress to end-stage renal failure within 20 years in China.[2] However, the pathogenesis of IgAN is obscure and its current therapy remains unsatisfactory.
Activation of inflammatory and immune system is one of the important causal factors in the pathogenesis of chronic renal disease.[3] It is becoming clear that toll-like receptors (TLRs) are directly involved in the pathogenesis of chronic kidney diseases related to inflammatory responses.[4,5] TLRs are evolutionarily conserved receptors that bind to pathogens and initiate inflammatory responses which might trigger renal injuries. In humans, TLRs are expressed on a wide range of cell types including renal mesangial cells and tubular epithelial cells.[6,7] Among the eleven human TLRs, TLR4 has been demonstrated to be related to mesangial cell injury[8] and renal fibrosis[9] by induction of proinflammatory cytokines, profibrotic molecules, and transforming growth factor-β1 in chronic kidney diseases,[9,10] including IgAN, renal ischemia-reperfusion injury,[11] acute kidney injury,[12] and transplantation rejection.[13] In vitro experiments have confirmed that TLR4 expression is increased in circulating mononuclear cells of patients with IgAN[14,15,16] and TLR4 signaling is implicated in the activation of IgA-stimulated mesangial cells.[8] However, at present, there are few in vivo researches concerning TLR4 and IgAN. Coppo et al.[15] revealed that the upregulation of TLR4 in circulating mononuclear cells of patients with IgAN was associated with heavy microscopic hematuria and proteinuria. He et al.[17] observed that the expression of TLR4 message ribonucleic acid (mRNA) and protein in renal tissue was significantly increased in IgAN rats established by oral and intravenous immunization with bovine serum albumin (BSA) for 12 weeks. Although these researches mentioned above suggested that the expression of TLR4 was increased in the serum or kidney of patients or rats with IgAN, they could not demonstrate what role TLR4 might play in the progression of IgAN.
Peroxisome proliferator-activated receptor-γ (PPAR-γ) is a member of the nuclear hormone receptor family which expresses in many tissues including the kidney. In addition to regulating the metabolism of glucose and lipid, PPAR-γ also exerts anti-inflammatory effects on kidney diseases[18] including IgAN.[19,20] Our groups and others have noted that thiazolidinediones, a PPAR-γ agonist, could attenuate inflammatory responses through the angiotensin II signaling pathway by suppressing the activation of angiotensin receptor 1, extracellular regulated protein kinases ½, and nuclear factor-kappa B (NF-κB) in the in vitro experiments.[19,20,21] As it has been proved, NF-κB is the key factor of the downstream of TLR4 signaling pathway. It has been reported that pioglitazone exerted its anti-inflammatory effect through suppression of the expression and activity of TLR4 in many in vitro experiments of other disease models.[22,23,24,25] So far, the interaction between PPAR-γ and TLR4 and the plausible mechanism in IgAN have not been fully studied.
The current study was conducted to assess whether TLR4 signaling pathway was involved in the pathogenesis and progression of IgAN in vivo. We hypothesized that the PPAR-γ agonist exerted its role on alleviating the severity of IgAN by interfering with TLR4-dependent signaling pathway and impressed the increase of inflammatory mediators. With this aim, we evaluated the renal expression of inflammatory cytokine interleukin-1 beta (IL-1β), and TLR4 mRNA and protein in rats before and after the establishment of IgAN and the changes of these factors after treated with TLR4 inhibitor, toll-like receptor 4 inhibitor (TAK242). Moreover, we tested the alterations of renal TLR4 mRNA and protein in rats after gavaged by pioglitazone with a focus on the interactive roles between TLR4 and PPAR-γ and how this interaction on the severity of experimental IgAN.
Methods
Antibodies and reagents
Bovine gamma globulin (BGG) was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Antibodies were rabbit anti-rat TLR4, rabbit anti-rat IL-1β (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA), and goat anti-rat IgA (LifeSpan BioSciences, Seattle, CA, USA). Pioglitazone was a gift from Taiyang Pharmaceutical Industry Co., Ltd., in Beijing, China. TLR4 inhibitor TAK242 was purchased from MedChem Express Co., Ltd. (New Jersey, USA).
Experimental design
All procedures performed in studies involving animals were in accordance with the ethical standards of the Ethics Committee of Huadong Hospital for Animal Studies and the National Institute of Health Guidelines for the care and use of laboratory animals. A total of 54 male, 3-week-old Lewis rats[26] weighing 45 ± 5 g were purchased from Weitonglihua Animal Technical Co., Ltd., in Beijing, China, and housed in an specific pathogen-free room at the Animal Experimental Center of Fudan University with a room temperature of 25°C and a 12-h light-dark cycle. The animals were provided standard rodent chow and water to drink ad libitum for 1 week before the initiation of the study.[27] Because of the different treating time of TAK242 and pioglitazone, the animals were sacrificed at different time intervals. Hence, 54 Lewis rats were randomly divided into six groups with the method of simple randomization: ControlTAK242, IgANTAK242, TAK242, ControlPio, IgANPio, and Pio groups. Experimental IgAN was induced in 4-week-old Lewis rats by continuous oral immunization with 0.1% BGG in 6 mmol/L HCl as drinking water for 9 weeks, followed by intravenous injection of 1 mg BGG (dissolved in 0.3 ml 6 mmol/L HCl) into the tail vein daily for 3 successive days.[26,28] TAK242, dissolved in 20% lipovenoes at 3 mg∙kg−2∙d−1 (7.5 ml/kg), was injected into rats with IgAN for 8 days after completing the model-creation (TAK242 group). Experimental IgAN rats receiving intravenous injection of the same amount of lipovenoes for 8 days by tail veins were named IgANTAK242 group. ControlTAK242 group was normal rats who drank 6 mmol/L HCl for 9 weeks, and then injected with 1mg 6 mmol/L HCl into the tail vein daily for three successive days followed by injection with 20% lipovenoes at 7.5 ml∙kg−2∙d−1 for 8 days. Pioglitazone, dissolved in saline at 1 mg/ml, was administered to rats after the model-creation by intragastric injection at 10 mg∙kg−2∙d−1 for 4 weeks until the rats were killed (Pio group). Rats of experimental IgAN receiving intragastric injection of saline at 10 ml∙kg−2∙d−1 for 4 weeks were named IgANPio group and Controlpio group referred to normal rats who drank 6 mmol/L HCl for 9 weeks, and then were given intravenous injection of 1 mg 6 mmol/L HCl into the tail vein daily for three successive days followed by intragastric injection of saline at 10 ml∙kg−2∙d−1 for 4 weeks.
Biochemical analyses of renal function
Twenty-four hours urine was collected by metabolic cages for urinary analysis. Albumin-to-creatinine ratio (ACR) was used to express the degree of albuminuria. The urine was centrifuged at 600 revolutions per minute for 5 min and the sediment was examined microscopically for erythrocytes. A diagnostic criterion for hematuria was more than 10 red cells on average per high-power field.[17,29] Serum creatinine (Scr) and blood urea nitrogen (BUN) were measured at the clinical laboratory at Huadong Hospital affiliated to Fudan University.
Enzyme-linked immunosorbent assay
Blood samples were drawn through the abdominal aorta after intraperitoneal anesthesia with 2% pentobarbital sodium to detect serum IL-1β by enzyme-linked immunosorbent assay kits (MultiSciences Biotech Co., Ltd., China) according to the manufacturer's instructions. Each sample was analyzed in duplicates.
Histological and immunofluorescence examination
The left kidney was rapidly harvested and bisected transversely. The cortex from half of the kidney was fixed in 4% paraformaldehyde and paraffin-embedded for histological observation. The other half was snap frozen in liquid nitrogen and stored at −80°C for total RNA and protein extraction. Three-micrometer thick paraffin-embedded kidney sections were deparaffinized with xylene and then rehydrated through a descending gradient of ethanol. The sections were stained with H and E. Glomerular damage and mesangial hypercellularity were determined by examining 25 glomeruli in each sample. The results were expressed as average number of sectioned glomerular nucleated cells. All sections were evaluated by an investigator who was blinded to the samples.
Immunofluorescence examination of the paraffin-embedded renal sections was performed by staining with fluorescing isothiocyanate-labeled, specific antibodies against rat IgA (1:16) at 4°C overnight. The anti-rat IgA antibody was visualized as bright green color using the Dako Envision Plus System (Dako, Carpinteria, CA, USA). The nephrologist who examined the specimens was unaware of the group assignments of the individual animals.
Quantitative real-time polymerase chain reaction and Western blotting
Total RNA was extracted from renal tissues using the Trizol reagent kit (Life Technologies, USA) according to the method described in the manufacturer's protocol. Thereafter, 1 ng of total RNA was reverse transcribed using the PrimeScript Reverse Transcription Kit and random primers (Takara Biotechnology Co., Ltd., Japan) to cDNA. Real-time polymerase chain reaction amplification was carried out in an ABI Prism 7500 sequence detection system. Primer sequences for rat TLR4 are forward 5’-TGACAGACCTCAGGCAGATTGT-3’, reverse 5’-AATAGTGCAATCGATAGAAGGAACA-3’; IL-1β forward 5’-CCGTGGCACATTCTGGTCA-3’, reverse 5’-GCTGTGCACTGGTCCAAATTC-3’; and β-actin forward 5’-AGATTACTGCCCTGGCTCCTAG-3’, reverse 5’-CATCGTACTCCTGCTTGCTGA-3’. β-actin was used as an endogenous control. Data obtained were calculated by the comparative cycle threshold method conformed to the introduction.[19]
Protein extracted from the renal tissues was analyzed by Western blotting for TLR4 and IL-1β in the following manners. Total protein was homogenized in protein lysis buffer. After homogenization, the solution was centrifuged at 10,800 ×g at 4°C for 5 min. The supernatant was used for Western blotting. Protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. The membrane was incubated with BSA, and then with following primary antibodies including rabbit antibody to IL-1β and TLR4. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was used for loading controls on stripped membranes. Horseradish peroxidase-conjugated secondary antibodies were applied. Detection was used by enhanced chemiluminescence, and the light signals were exposed to X-ray film. The results of protein expression were normalized to GAPDH in all figures.
Statistical analysis
Data were analyzed using SPSS software version 20 (IBM, New York, USA). Results were presented as mean ± standard deviation (SD). Differences between groups were analyzed by one-way analysis of variances (ANOVA). A value of P < 0.05 was considered statistically significant.
Results
Biochemical parameters
As shown in Figure 1a and 1b, urinary ACR and serum IL-1β level were significantly increased in IgANTAK242 rats compared to ControlTAK242 rats (4.45 ± 1.33 mg/mmol vs. 2.89 ± 0.96 mg/mmol, P < 0.05; 48.28 ± 13.49 pg/ml vs. 35.56 ± 7.41pg/ml, P < 0.05). Furthermore, treatment with TAK242 significantly reversed the elevations of ACR and serum IL-1β (1.34 ± 0.33 mg/mmol vs. 4.45 ± 1.33 mg/mmol, P < 0.05; 32.43 ± 7.42 pg/ml vs. 48.28 ± 13.49 pg/ml, P < 0.05). Similarly, urinary ACR was significantly increased in IgANpio rats compared to Controlpio rats (1.72 ± 0.41 mg/mmol vs. 1.27 ± 0.15 mg/mmol, P < 0.05), which was reversed by pioglitazone (1.13 ± 0.44 mg/mmol vs. 1.72 ± 0.41 mg/mmol, P < 0.05) [Figure 1c]. Compared to IgANPio group, there was a tendency of reduction of serum IL-1β levels in Pio group, but the difference did not reach the statistical difference (39.06 ± 17.92 pg/ml vs. 47.45 ± 12.91 pg/ml, P > 0.05) [Figure 1d]. However, urine from normal rats, IgAN rats, rats received treatment with TAK242 or pioglitazone all revealed sporadic hematuria and the difference among groups did not reach the statistical significance. There was no significant difference in BUN or Scr between various groups (data not shown).
Figure 1.
Urine and serum biochemical parameters (n=9 in each group). (a) ACR of all groups detected by automatic biochemical analyzer. (b) Serum concentration of IL-1β of all groups detected by ELISA kits. (c) ACR of all groups detected by automatic biochemical analyzer. (d) Serum concentration of IL-1β of all groups detected by ELISA kits. *Compared to ControlTAK242, P < 0.05; †Compared to IgANTAK242, P < 0.05; ‡Compared to ControlPio, P < 0.05; §Compared to IgANPio, P < 0.05. ACR: Urinary albumin-to-creatinine ratio; IgAN: Immunoglobulin A nephropathy; Pio: Pioglitazone; TAK242: Toll-like receptor 4 inhibitor; IL-1β: Interleukin-1 beta; ELISA: Enzyme-linked immunosorbent assay.
Histology examination
The IgA deposits in the glomeruli were observed by immunofluorescent staining. Figure 2a indicates that there is hardly no clear green fluorescence seen in the glomeruli of ControlTAK242 and ControlPio groups. In contrast, bright mass-like green fluorescence was found in the glomeruli of IgANTAK242 and IgANPio rats. These results indicated that the IgAN animal models were established successfully.
Figure 2.
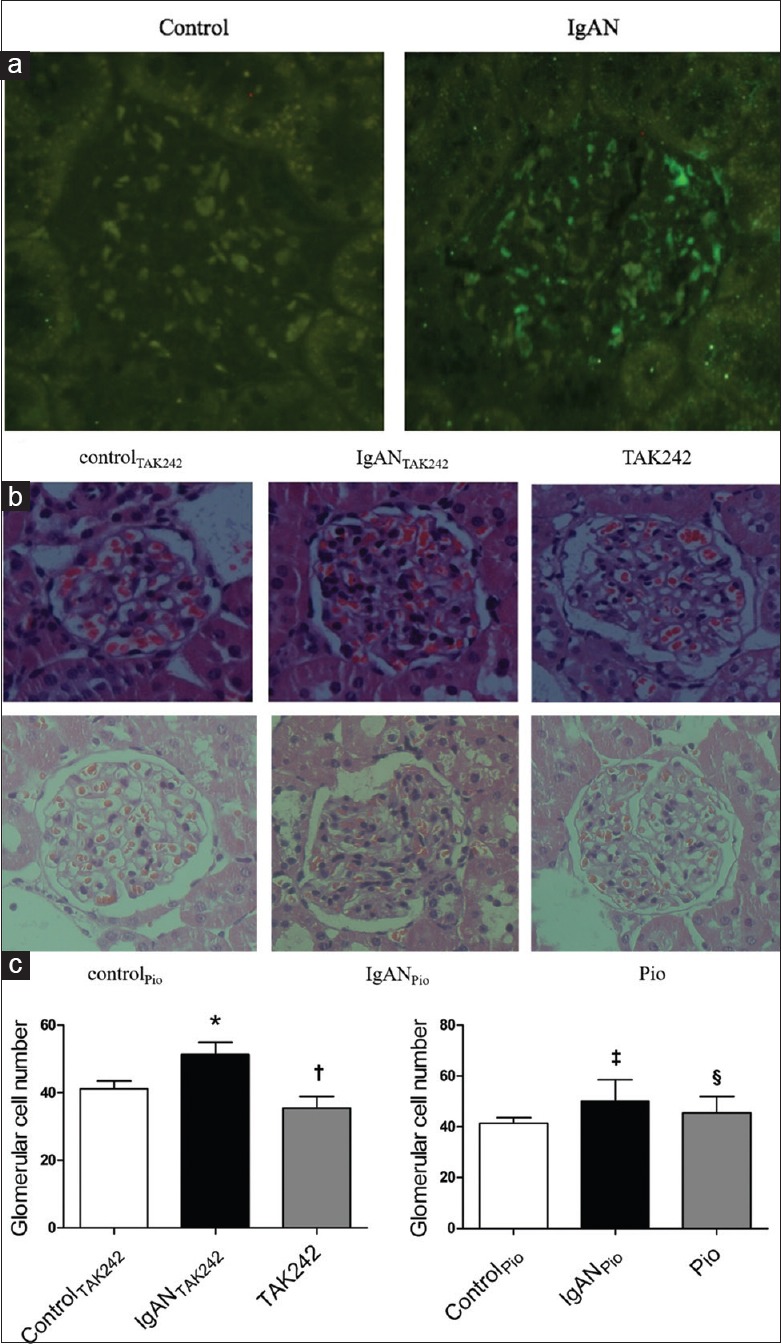
Morphological changes observed by immunofluorescence and H and E staining (n=9 in each group). (a) Contrastive immunofluorescence of IgA deposition in the glomeruli of kidney sections from normal rats and rats with IgA nephropathy (×450). (b) Representative H and E staining of glomeruli in kidney sections from rat of each group (×400). (c) Glomerular cell number expressed as cells per glomerular cross-section of all groups stained by HE. *Compared to ControlTAK242, P < 0.05; †Compared to IgANTAK242, P < 0.05; ‡Compared to ControlPio, P < 0.05; §Compared to IgANPio, P < 0.05. IgAN: Immunoglobulin A nephropathy; Pio: Pioglitazone; TAK242: Toll-like receptor 4 inhibitor.
The kidney sections were stained with H and E. Renal pathologic structure of ControlTAK242 and ControlPio groups was found without any glomerular or tubular injuries. The histological changes of rats in IgANTAK242 and IgANPio groups were both characterized by glomerular hypercellularity, moderate proliferation of the mesangial cells, and hyperplasia of mesangial matrix. Some renal tubular epithelial cells were swelling and necrotic. The changes mentioned above were apparently ameliorated in rats of TAK242 and Pio groups [Figure 2b]. The number of glomerular cells of cross section in experimental rats was higher than those in normal rats (51.39 ± 3.58 vs.41.24 ± 2.24, P < 0.01). Compared to IgANTAK242 group, the number of glomerular cells of cross section was much lower in TAK242 group (35.47 ± 3.38 vs.51.39 ± 3.58, P < 0.01). In addition, there was a significant decrease of glomerular cells in Pio group compared to IgANPio group (45.53 ± 6.37 vs.50.12 ± 8.43, P < 0.05) [Figure 2c].
Renal expression of interleukin-1 beta and toll-like receptor 4 at the message ribonucleic acid and protein levels
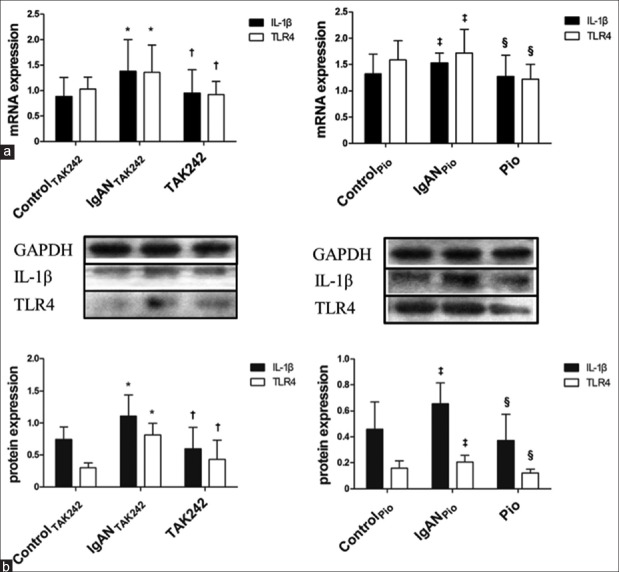
We studied the expression of IL-1β and TLR4 mRNA as well as protein of each sample from renal tissues. Our findings illustrated that TLR4, IL-1β mRNA, and protein in IgANTAK242 group were dramatically higher than those in ControlTAK242 group (TLR4 mRNA: 1.36 ± 0.54 vs. 1.03 ± 0.24, P < 0.05; IL-1β mRNA: 1.38 ± 0.62 vs. 0.88 ± 0.37, P < 0.01; TLR4 protein: 0.81 ± 0.18 vs. 0.30 ± 0.07, P < 0.01; IL-1β protein: 1.10 ± 0.33 vs. 0.74 ± 0.19, P < 0.05). In contrast, the levels of TLR4, IL-1β mRNA, and protein in TAK242 group were much lower than those in IgANTAK242 group (TLR4 mRNA: 0.92 ± 0.26 vs. 1.36 ± 0.54, P < 0.01; IL-1β mRNA: 0.95 ± 0.45 vs. 1.38 ± 0.62, P < 0.01; TLR4 protein: 0.43 ± 0.30 vs. 0.81 ± 0.18, P < 0.01; IL-1β protein: 0.60 ± 0.33 vs. 1.10 ± 0.33, P < 0.01). Likewise, there was a significant increase of TLR4, IL-1β mRNA, and protein in IgANPio group compared to ControlPio group (TLR4 mRNA: 1.72 ± 0.45 vs. 1.58 ± 0.37, P < 0.05; IL-1β mRNA: 1.53 ± 0.19 vs. 1.32 ± 0.37, P < 0.05; TLR4 protein: 0.21 ± 0.05 vs. 0.16 ± 0.06, P < 0.05; IL-1β protein: 0.66 ± 0.16 vs. 0.46 ± 0.21, P < 0.05). In addition, the increase was reversed in Pio group: the expression of TLR4, IL-1β mRNA, and protein in Pio group was dramatically decreased compared to IgANPio group (TLR4 mRNA: 1.22 ± 0.28 vs. 1.72 ± 0.45, P < 0.05; IL-1β mRNA: 1.27 ± 0.41 vs. 1.53 ± 0.19, P < 0.01; TLR4 protein: 0.12 ± 0.03 vs. 0.21 ± 0.05, P < 0.05; IL-1β protein: 0.37 ± 0.20 vs. 0.66 ± 0.16, P < 0.05) [Figure 3a and 3b].
Figure 3.
Expression of IL-1β and TLR4 in renal tissues of all groups of rats (n=9 in each group). (a) The levels of IL-1β and TLR4 in the kidney tissues determined by reverse transcription polymerase chain reaction. (b) The levels of IL-1β and TLR4 in the kidney tissues determined by Western blots. Representative results are shown at the top. Values are expressed as the mean ± standard deviation. *Compared to ControlTAK242, P < 0.05; †Compared to IgANTAK242, P < 0.05; ‡Compared to ControlPio, P < 0.05; §Compared to IgANPio, P < 0.05. IgAN: Immunoglobulin A nephropathy; Pio: Pioglitazone; TAK242: Toll-like receptor 4 inhibitor; IL-1β: Interleukin-1 beta; mRNA: Message ribonucleic acid; TLR4: Toll-like receptor 4; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
Discussion
The present study provided evidence that inflammatory responses and TLR4 signaling pathway are involved in the progression of IgAN. Pioglitazone exerted its anti-inflammatory effects by interfering with TLR4 signaling pathway and reducing inflammatory cytokines to alleviate the progression of IgAN.
Research of IgAN has been handicapped due to the lack of a good animal model of IgAN for many years. Since Rifai et al.[30] established IgAN model with BALB/c mice for the first time in 1979, many scholars have made various IgAN models based on the known pathogenesis. However, there were wide gaps in pathologic changes between the animal models and human IgAN. Recently, the ddY strain of mouse was used as a spontaneous animal model for human IgAN. However, these mice showed mild proteinuria without hematuria at more than 40 weeks of age and the incidence of IgAN was highly variable.[31] Some investigators used BSA gavage and hypodermic injection of staphylococcal enterotoxin B plus lipopolysaccharides (LPS) to establish IgAN models.[32,33] However, LPS itself is a high potent agonist of TLR4 which is apt to confound the interpretation of the subsequent inflammatory pathway.[34,35] In 1983, Emancipator et al.[36] for the first time made IgAN models with BALB/c mice by orally immunizing BGG. Only part of the mice showed proteinuria and hematuria was not obvious. In 1992, Gesualdo et al.[26] induced IgAN in different rat strains by oral BGG immunization and found that mesangial IgA deposition was most severe in Lewis rats. Due to the mild histopathology even after prolonged observation of this model, Lai et al.[19] improved the model with Lewis rats by immunization of BGG and followed by unilateral nephrectomy. This model had diffuse glomerular IgA deposition, mesangial hypercellularity, high ACR and obvious hematuria. Although unilateral nephrectomy hastened the development of renal abnormalities, it may lead to acute kidney injury and other injuries not characteristic to IgAN which could confound the findings. Taken together, our establishment of IgAN models with Lewis rats by oral immunization of BGG for 9 weeks and then injection of BGG through tail vein for 3 successive days[29,36] might be able to avoid those disadvantages mentioned above. Although our models did not show obvious hematuria which was consistent with other studies, immunofluorescent assay showed granular and massive IgA depositions and pathological observation revealed diffuse proliferation of mesangial cells and matrix. Furthermore, our models showed obvious proteinuria. Above all, IgAN rat model were successfully established and can be used to reflect the pathophysiological findings in human IgAN.
IgAN is a complex multifactorial disease which relates to chronic inflammatory process. Inflammation is increasingly recognized as a key driving force of IgAN.[37] IL-1 is one of the most powerful moderators of inflammation and is closely involved in the development of mesangial cell proliferation and extracellular matrix production.[38] In IgAN patients, IL-1β has been shown to be produced locally in the glomeruli and interstitial.[39] In our study, induction of IgAN is associated with increased renal expression of IL-1β mRNA and protein as well as serum IL-1β which suggests that inflammatory responses participate in the pathogenesis of IgAN.
TLR4 expressed within the kidney is a potential mediator of innate activation and inflammation.[40] There has been circumstantial evidence for the involvement of TLRs, notably TLR4, in the pathogenesis of renal diseases. However, the reports about the effects of TLR4 on IgAN in vivo are incompletely elucidated. In our study, there was increased renal expression of TLR4 mRNA and protein in rats with IgAN. TAK242, a selective inhibitor of TLR4 intracellular signaling pathway, had been used in the current study to unravel whether the mechanisms of inflammatory response in IgAN were TLR4 dependent and, in such a case, to ascertain the specific role of TLR4. We found that the expression of TLR4 mRNA and protein significantly was decreased in rats treated with TAK242. This finding further supported the role of activated TLR4 signaling pathway in the pathophysiology of IgAN. In keeping with our findings, the study conducted by Kwon et al.[41] proved that in IgAN patients, the expression of renal TLR4 mRNA was significantly elevated. The transcriptional level of TLR4 mRNA in circulating mononuclear cells was significantly higher in patients with IgAN than those in healthy controls.[15] What's more, the increased expression of TLR4 in circulating mononuclear cells was significantly correlated with proteinuria or phases of clinical activity in patients with IgAN.[16,15] Binding with their ligands, TLRs initiate an intracellular signaling cascade, activate protein kinases, release cytokines, and enhance the expression of transcription factors[16] which results in the generation of mediators including adhesion molecules, chemokines, cytokines, and inflammatory responses.[42] The nuclear translocation of the NF-κB favored the hyperplasia of B-cell and therefore increased IgA synthesis.[43] TLRs have been conformed to be involved in the switch from IgM to IgA production in B-cells in experimental animals.[43] In addition, TLR4 was involved in the injuries of mesangial cells by induction of proinflammatory cytokines in IgAN[8] and was constitutively expressed in podocytes to mediate glomerular injuries by modulating the expression of chemokines.[44] All of the results mentioned above suggested that renal TLR4 signaling pathway may be a therapeutic target for the alleviation of IgAN.
Recently, increasing attention has been paid to the role of PPAR-γ agonists in regulating inflammatory responses in kidney diseases.[45,46] However, there were few reports on whether PPAR-γ agonists exerted anti-inflammatory effects by interfering with TLR4 signaling pathway to improve IgAN in vivo. In the present study, activation of PPAR-γ pathway with pioglitazone significantly repressed the expression of TLR4 and its downstream inflammatory factors. This confirmed that TLR4 pathway was involved in the renal protective effects of PPAR-γ in vivo. Our findings are consistent with that of Dasu et al,[47] who demonstrated that pioglitazone decreased the expression of TLR4 mRNA and protein in a concentration-dependent manner and lowered the activity of NF-κB, IL-1β, TNF-alpha, and other inflammatory factors in human monocytes. In addition, in peritoneal macrophages of db/db mice, pioglitazone markedly suppressed the expression of MyD88 (myeloid differentiation factor 88) and TRIF (TIR-domain-containing adaptor inducing interferon-β) protein in the cytoplasm and reduced the phosphorylation of interleukin receptor-associated kinase and p38, which subsequently inhibited the activation of TLR4 signaling pathway and NF-κB.[47] Ji et al.[48] revealed that PPAR-γ agonist, rosiglitazone, could concentration dependently downregulate the expression of TLR4 mRNA and protein in vascular smooth muscle cells of Sprague–Dawley rats. The beneficial effects of rosiglitazone on inflammation were mediated through interference with TLR4 and its downstream signaling components such as TRIF, interferon regulatory factor 3 (IRF3), and inducible protein-10.[49] A recent study confirmed that PPAR-γ negatively regulated the downstream of TLR4 pathway – IFN-β production and IRF3 transcriptional activation in a PPAR-γ-dependent manner in macrophages.[49]
There are some possible limitations in our study. First, we do not provide additional evidence that the alterations of TLR4 mRNA and protein as well as inflammatory factors after using TLR4 agonist. Second, our research is lack of data using specific PPAR-γ antagonists for IgAN rats. It is unclear whether pioglitazone-induced anti-inflammatory effects are direct or indirect in in vivo experiment. One possible scenario is that pioglitazone as a ligand binds to the PPAR-γ receptor and directly binds to the PPRE of IL-1β gene itself or genes which codes for its upstream mediators and inactivates them such as TLR4. Another possible scenario is whether pioglitazone itself or its metabolites are the active compound responsible for anti-inflammatory effects. In vitro researches with renal cells are recommended to dissect the precise mechanism of pioglitazone-induced anti-inflammatory effects in TLR4 signaling pathway in IgAN.
In summary, our study indicated that TLR4 and inflammatory responses played a key role in the progression of IgAN. Pioglitazone improved biochemical parameters, ameliorated renal histological damage, and alleviated IgA deposits in glomeruli, at least in part, by negatively regulating the expression of TLR4 and blunted the activation of its downstream signal pathways involved in the expression of inflammatory factors. It suggested that either drug antagonizing TLR4 or PPAR-γ agonists could have potently beneficial effects in the treatment of IgAN.
Financial support and sponsorship
This study was supported by a grant from the National Natural Science Foundation of China (No. 81170646).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
References
- 1.Fang J, Li W, Li D, Tan Z. Baseline proteinuria, urinary osmotic pressure, and renal function as positive predictors of corticosteroids plus cyclophosphamide treatment efficacy in IgA nephropathy. Chin Med J. 2014;127:1710–4. doi: 10.3760/cma.j.issn.0366-6999.20132318. [PubMed] [Google Scholar]
- 2.Le W, Liang S, Hu Y, Deng K, Bao H, Zeng C, et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: Results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant. 2012;27:1479–85. doi: 10.1093/ndt/gfr527. doi: 10.1093/ndt/gfr527. [DOI] [PubMed] [Google Scholar]
- 3.Imig JD, Ryan MJ. Immune and inflammatory role in renal disease. Compr Physiol. 2013;3:957–76. doi: 10.1002/cphy.c120028. doi: 10.1002/cphy.c120028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gluba A, Banach M, Hannam S, Mikhailidis DP, Sakowicz A, Rysz J. The role of Toll-like receptors in renal diseases. Nat Rev Nephrol. 2010;6:224–35. doi: 10.1038/nrneph.2010.16. doi: 10.1038/nrneph.2010.16. [DOI] [PubMed] [Google Scholar]
- 5.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 6.Zager RA, Johnson AC, Lund S, Randolph-Habecker J. Toll-like receptor (TLR4) shedding and depletion: Acute proximal tubular cell responses to hypoxic and toxic injury. Am J Physiol Renal Physiol. 2007;292:F304–12. doi: 10.1152/ajprenal.00237.2006. doi: 10.1152/ajprenal.00237.2006. [DOI] [PubMed] [Google Scholar]
- 7.Anders HJ, Schlöndorff D. Toll-like receptors: Emerging concepts in kidney disease. Curr Opin Nephrol Hypertens. 2007;16:177–83. doi: 10.1097/MNH.0b013e32803fb767. doi: 10.1097/MNH.0b013e32803fb767. [DOI] [PubMed] [Google Scholar]
- 8.Lim BJ, Lee D, Hong SW, Jeong HJ. Toll-like receptor 4 signaling is involved in IgA-stimulated mesangial cell activation. Yonsei Med J. 2011;52:610–5. doi: 10.3349/ymj.2011.52.4.610. doi: 10.3349/ymj.2011.52.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulskens WP, Rampanelli E, Teske GJ, Butter LM, Claessen N, Luirink IK, et al. TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. J Am Soc Nephrol. 2010;21:1299–308. doi: 10.1681/ASN.2009070722. doi: 10.1681/ASN.2009070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lepenies J, Eardley KS, Kienitz T, Hewison M, Ihl T, Stewart PM, et al. Renal TLR4 mRNA expression correlates with inflammatory marker MCP-1 and profibrotic molecule TGF-ß1 in patients with chronic kidney disease. Nephron Clin Pract. 2011;119:c97–104. doi: 10.1159/000324765. doi: 10.1159/000324765. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, John R, Richardson JA, Shelton JM, Zhou XJ, Wang Y, et al. Toll-like receptor 4 regulates early endothelial activation during ischemic acute kidney injury. Kidney Int. 2011;79:288–99. doi: 10.1038/ki.2010.381. doi: 10.1038/ki.2010.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham PN, Wang Y, Guo R, He G, Quigg RJ. Role of Toll-like receptor 4 in endotoxin-induced acute renal failure. J Immunol. 2004;172:2629–35. doi: 10.4049/jimmunol.172.4.2629. doi: 10.4049/jimmunol.172.4.2629. [DOI] [PubMed] [Google Scholar]
- 13.Ducloux D, Deschamps M, Yannaraki M, Ferrand C, Bamoulid J, Saas P, et al. Relevance of Toll-like receptor-4 polymorphisms in renal transplantation. Kidney Int. 2005;67:2454–61. doi: 10.1111/j.1523-1755.2005.00354.x. doi: 10.1111/j.1523-1755.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 14.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone clinical trial in macrovascular events): A randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 15.Coppo R, Camilla R, Amore A, Peruzzi L, Daprà V, Loiacono E, et al. Toll-like receptor 4 expression is increased in circulating mononuclear cells of patients with immunoglobulin A nephropathy. Clin Exp Immunol. 2010;159:73–81. doi: 10.1111/j.1365-2249.2009.04045.x. doi: 10.1111/j.1365-2249.2009.04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coppo R, Amore A, Peruzzi L, Vergano L, Camilla R. Innate immunity and IgA nephropathy. J Nephrol. 2010;23:626–32. [PubMed] [Google Scholar]
- 17.He L, Peng X, Liu G, Tang C, Liu H, Liu F, et al. Anti-inflammatory effects of triptolide on IgA nephropathy in rats. Immunopharmacol Immunotoxicol. 2015;37:421–7. doi: 10.3109/08923973.2015.1080265. doi: 10.3109/08923973.2015.1080265. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Zhou Y, Guan Y. PPARγ as a therapeutic target in diabetic nephropathy and other renal diseases. Curr Opin Nephrol Hypertens. 2012;21:97–105. doi: 10.1097/MNH.0b013e32834de526. doi: 10.1097/MNH.0b013e32834de526. [DOI] [PubMed] [Google Scholar]
- 19.Lai KN, Chan LY, Guo H, Tang SC, Leung JC. Additive effect of PPAR-γ agonist and ARB in treatment of experimental IgA nephropathy. Pediatr Nephrol. 2011;26:257–66. doi: 10.1007/s00467-010-1703-y. doi: 10.1007/s00467-010-1703-y. [DOI] [PubMed] [Google Scholar]
- 20.Xiao J, Leung JC, Chan LY, Guo H, Lai KN. Protective effect of peroxisome proliferator-activated receptor-gamma agonists on activated renal proximal tubular epithelial cells in IgA nephropathy. Nephrol Dial Transplant. 2009;24:2067–77. doi: 10.1093/ndt/gfn746. doi: 10.1093/ndt/gfn746. [DOI] [PubMed] [Google Scholar]
- 21.Xiao J, Leung JC, Chan LY, Tang SC, Lai KN. Crosstalk between peroxisome proliferator-activated receptor-gamma and angiotensin II in renal tubular epithelial cells in IgA nephropathy. Clin Immunol. 2009;132:266–76. doi: 10.1016/j.clim.2009.04.004. doi: 10.1016/j.clim.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Darehgazani R, Peymani M, Hashemi MS, Omrani MD, Movafagh A, Ghaedi K, et al. PPARγ ameliorated LPS induced inflammation of HEK cell line expressing both human Toll-like receptor 4 (TLR4) and MD2. Cytotechnology. 2016;68:1337–48. doi: 10.1007/s10616-015-9893-6. doi: 10.1007/s10616-015-9893-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Liu C, Zhang L, Liu Y, Guo A, Shi H, et al. Intensive atorvastatin therapy attenuates the inflammatory responses in monocytes of patients with unstable angina undergoing percutaneous coronary intervention via peroxisome proliferator-activated receptor γ activation. Inflammation. 2015;38:1415–23. doi: 10.1007/s10753-015-0116-2. doi: 10.1007/s10753-015-0116-2. [DOI] [PubMed] [Google Scholar]
- 24.Yin Y, Hou G, Li E, Wang Q, Kang J. PPARγ agonists regulate tobacco smoke-induced Toll like receptor 4 expression in alveolar macrophages. Respir Res. 2014;15:28. doi: 10.1186/1465-9921-15-28. doi: 10.1186/1465-9921-15-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mudaliar H, Pollock C, Komala MG, Chadban S, Wu H, Panchapakesan U. The role of Toll-like receptor proteins (TLR) 2 and 4 in mediating inflammation in proximal tubules. Am J Physiol Renal Physiol. 2013;305:F143–54. doi: 10.1152/ajprenal.00398.2012. doi: 10.1152/ajprenal.00398.2012. [DOI] [PubMed] [Google Scholar]
- 26.Gesualdo L, Emancipator SN, Kesselheim C, Lamm ME. Glomerular hemodynamics and eicosanoid synthesis in a rat model of IgA nephropathy. Kidney Int. 1992;42:106–14. doi: 10.1038/ki.1992.268. doi: 10.1038/ki.1992.268. [DOI] [PubMed] [Google Scholar]
- 27.Trachtman H, Chan JC, Chan W, Valderrama E, Brandt R, Wakely P, et al. Vitamin E ameliorates renal injury in an experimental model of immunoglobulin A nephropathy. Pediatr Res. 1996;40:620–6. doi: 10.1203/00006450-199610000-00018. doi: 10.1203/00006450-199610000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Kuemmerle NB, Krieg RJ, Jr, Chan W, Trachtman H, Norkus EP, Chan JC. Influence of alpha-tocopherol over the time course of experimental IgA nephropathy. Pediatr Nephrol. 1999;13:108–12. doi: 10.1007/s004670050573. doi: 10.1007/s004670050573. [DOI] [PubMed] [Google Scholar]
- 29.Lai KN. Recent Advances in IgA Nephropathy. Singapore: World Scientific; 2009. [Google Scholar]
- 30.Rifai A, Small PA, Jr, Teague PO, Ayoub EM. Experimental IgA nephropathy. J Exp Med. 1979;150:1161–73. doi: 10.1084/jem.150.5.1161. doi: 10.1084/jem.150.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomino Y. IgA nephropathy: Lessons from an animal model, the ddY mouse. J Nephrol. 2008;21:463–7. [PubMed] [Google Scholar]
- 32.Zhang DW, Qiu H, Mei YM, Fu H, Zheng HG. Repair effects of umbilical cord mesenchymal stem cells on podocyte damage of IgA nephropathy. J Biol Regul Homeost Agents. 2015;29:609–17. [PubMed] [Google Scholar]
- 33.Lu H, Chen LL, Jiang XY, Mo Y, Ling YH, Sun LZ. Temporal and spatial expression of podocyte-associated molecules are accompanied by proteinuria in IgA nephropathy rat model. Physiol Res. 2013;62:35–45. doi: 10.33549/physiolres.932380. [DOI] [PubMed] [Google Scholar]
- 34.Menon D, Coll R, O’Neill LA, Board PG. GSTO1-1 modulates metabolism in macrophages activated through the LPS and TLR4 pathway. J Cell Sci. 2015;128:1982–90. doi: 10.1242/jcs.167858. doi: 10.1242/jcs.167858. [DOI] [PubMed] [Google Scholar]
- 35.Piazza M, Colombo M, Zanoni I, Granucci F, Tortora P, Weiss J, et al. Uniform lipopolysaccharide (LPS)-loaded magnetic nanoparticles for the investigation of LPS-TLR4 signaling. Angew Chem Int Ed Engl. 2011;50:622–6. doi: 10.1002/anie.201004655. doi: 10.1002/anie.201004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emancipator SN, Gallo GR, Lamm ME. Experimental IgA nephropathy induced by oral immunization. J Exp Med. 1983;157:572–82. doi: 10.1084/jem.157.2.572. doi: 10.1084/jem.157.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan LY, Leung JC, Lai KN. Novel mechanisms of tubulointerstitial injury in IgA nephropathy: A new therapeutic paradigm in the prevention of progressive renal failure. Clin Exp Nephrol. 2004;8:297–303. doi: 10.1007/s10157-004-0324-9. doi: 10.1007/s10157-004-0324-9. [DOI] [PubMed] [Google Scholar]
- 38.Chen WP, Chen A, Lin CY. In vitro effects of interleukins on human mesangial cells: Implications for glomerulonephritis. J Pathol. 1995;175:327–37. doi: 10.1002/path.1711750311. doi: 10.1002/path.1711750311. [DOI] [PubMed] [Google Scholar]
- 39.Yoshioka K, Takemura T, Murakami K, Okada M, Yagi K, Miyazato H, et al. In situ expression of cytokines in IgA nephritis. Kidney Int. 1993;44:825–33. doi: 10.1038/ki.1993.317. doi: 10.1038/ki.1993.317. [DOI] [PubMed] [Google Scholar]
- 40.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–59. doi: 10.1172/JCI31008. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon J, Park J, Lee D, Kim YS, Jeong HJ. Toll-like receptor expression in patients with renal allograft dysfunction. Transplant Proc. 2008;40:3479–80. doi: 10.1016/j.transproceed.2008.06.073. doi: 10.1016/j.transproceed.2008.06.073. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Peng S, Zeng H, Fu A, Zhu Q. Toll-like receptor 4 is involved in a protective effect of rhein on immunoglobulin A nephropathy. Indian J Pharmacol. 2015;47:27–33. doi: 10.4103/0253-7613.150319. doi: 10.4103/0253-7613.150319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarthy DD, Chiu S, Gao Y, Summers-deLuca LE, Gommerman JL. BAFF induces a hyper-IgA syndrome in the intestinal lamina propria concomitant with IgA deposition in the kidney independent of LIGHT. Cell Immunol. 2006;241:85–94. doi: 10.1016/j.cellimm.2006.08.002. doi: 10.1016/j.cellimm.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Banas MC, Banas B, Hudkins KL, Wietecha TA, Iyoda M, Bock E, et al. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol. 2008;19:704–13. doi: 10.1681/ASN.2007040395. doi: 10.1681/ASN.2007040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gurley C, Nichols J, Liu S, Phulwani NK, Esen N, Kielian T. Microglia and astrocyte activation by Toll-Like receptor ligands: Modulation by PPAR-gamma agonists. PPAR Res 2008. 2008 doi: 10.1155/2008/453120. 453120. doi: 10.1155/2008/453120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y, Zhao XD, Zhuang Z, Xue YJ, Cheng HL, Yin HX, et al. Peroxisome proliferator-activated receptor gamma agonist rosiglitazone attenuates oxyhemoglobin-induced Toll-like receptor 4 expression in vascular smooth muscle cells. Brain Res. 2010;1322:102–8. doi: 10.1016/j.brainres.2010.01.073. doi: 10.1016/j.brainres.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 47.Dasu MR, Park S, Devaraj S, Jialal I. Pioglitazone inhibits Toll-like receptor expression and activity in human monocytes and db/db mice. Endocrinology. 2009;150:3457–64. doi: 10.1210/en.2008-1757. doi: 10.1210/en.2008-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji Y, Liu J, Wang Z, Li Z. PPARγ agonist rosiglitazone ameliorates LPS-induced inflammation in vascular smooth muscle cells via the TLR4/TRIF/IRF3/IP-10 signaling pathway. Cytokine. 2011;55:409–19. doi: 10.1016/j.cyto.2011.05.020. doi: 10.1016/j.cyto.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 49.Zhao W, Wang L, Zhang M, Wang P, Zhang L, Yuan C, et al. Peroxisome proliferator-activated receptor gamma negatively regulates IFN-beta production in Toll-like receptor (TLR) 3- and TLR4-stimulated macrophages by preventing interferon regulatory factor 3 binding to the IFN-beta promoter. J Biol Chem. 2011;286:5519–28. doi: 10.1074/jbc.M110.149823. doi: 10.1074/jbc.M110.149823. [DOI] [PMC free article] [PubMed] [Google Scholar]