Abstract
Background.
The role of vascular endothelial (VE) components in dengue infection with plasma leakage is unknown. Therefore, we conducted a study to determine the adjusted association of the endothelial glycocalyx layer (EGL) and tight and adherens junction markers with plasma leakage.
Methods.
A prospective observational study was conducted at Cipto Mangunkusumo Hospital and Persahabatan Hospital, Jakarta, Indonesia. Adult dengue patients admitted to the hospital on the third day of fever from November 2013 through August 2015 were included in the study. Multiple regression analysis was used to determine the adjusted association of the VE biomarkers with the severity of the plasma leakage.
Results.
A total of 103 dengue-infected patients participated in the study. In the critical phase, levels of syndecan-1 (odds ratio [OR] = 1.004; 95% confidence interval [CI] = 1.001–1.007) and chondroitin sulfate (OR = 1.157; 95% CI = 1.025–1.307) had an adjusted association with plasma leakage, whereas levels of syndecan-1 (OR = 1.004; 95% CI = 1.000–1.008) and claudin-5 (OR = 1.038; 95% CI = 1.004–1.074) had an adjusted association with severe plasma leakage.
Conclusions.
In dengue-infected patients, elevated levels of syndecan-1 and chondroitin sulfate are strongly associated with plasma leakage, and elevated levels of syndecan-1 and claudin-5 are strongly associated with severe plasma leakage.
Keywords: chondroitin sulfate, claudin-5, dengue infection, plasma leakage, syndecan-1.
Plasma leakage is the pathological hallmark of dengue hemorrhagic fever (DHF), which is responsible for the development of severe dengue and dengue shock syndrome (DSS) [1]. This leakage can occur in the febrile phase within 3 days after fever onset but is most commonly detected in the critical phase (ie, 1 day after defervescence) [2, 3]. Plasma leakage is primarily caused by alteration of the permeability of the microvascular endothelium, resulting in movement of plasma albumin and intravascular fluid toward the extravascular space [4, 5]. This movement causes hypoalbuminemia and hypovolemia [4, 6]. The degree of hypoalbuminemia can be used as a marker of vascular endothelial (VE) permeability alteration and the severity of intravascular volume depletion in dengue-infected patients [7, 8]. Previous studies have reported that severe intravascular volume depletion caused by a severe increase in vascular permeability, which can be associated with a serum albumin level <3 g/dL, is associated with complications and more severe dengue compared with dengue without plasma leakage or dengue fever (DF) [9, 10].
Plasma protein and solute transport across the endothelium can occur through both transcellular and paracellular pathways [11, 12]. Under normal conditions, plasma proteins such as albumin are transported through the transcellular pathway by the process of transcytosis, whereas small molecules, including urea and glucose, are transported through the paracellular pathway. The transcellular pathway is highly associated with the endothelial glycocalyx layer (EGL), which plays an important role in maintaining vascular integrity. The 4 major components of the EGL are syndecan-1, hyaluronic acid, chondroitin sulfate, and heparan sulfate [13]. By contrast, the paracellular pathway is highly associated with interendothelial junctions. Tight junctions and adherens junctions are the 2 most important components in the control of endothelial permeability [14]. Claudins appear to be a major structural component of tight junctions, and among the various types of claudins, claudin-5 is predominantly expressed in the VE cells [15]. Meanwhile, VE cadherin–catenin complexes are the primary component of adherens junction [16].
In vitro studies using endothelial cell models have reported that dengue virus (DENV) is able to bind to the EGL, decreasing the expression of and redistributing VE-cadherin and the tight junction protein ZO-1, which in turn result in increased endothelial permeability [17, 18]. Studies in pediatric patients showed that the serum level of hyaluronan in DSS was significantly higher than that in DF in the acute phase and that urinary heparan sulfate was significantly higher in individuals with DSS compared with healthy subjects [19, 20]. These in vivo studies indicate roles for hyaluronan and heparan sulfate in the pathogenesis of vascular leakage. In addition, a recent study reported that the anti-inflammatory cytokine interleukin 10 (IL-10) and chemokine (C-X-C motif) ligand 10/interferon γ-inducible protein 10 (CXCL10/IP10) play important roles in the induction of vascular leakage [21]. However, existing publications have not assessed the strength of the association of the VE components (ie, EGL and tight and adherens junction markers) with the severity of plasma leakage.
Knowledge of the association of the VE components, cytokines, and chemokines with the severity of plasma leakage is essential to be able to assess the mechanisms underlying microvascular leakage. Therefore, we conducted a study to investigate the adjusted association of the VE components in DF, DHF, and dengue with severe plasma leakage in the febrile and critical phases. We also investigated the association of IL-10 and CXCL10/IP10 with the severity of plasma leakage in the critical phase. These findings provide new insight into the pathophysiology of plasma leakage in dengue-infected patients.
METHODS
Study Design and Population
We performed a prospective observational study at Cipto Mangunkusumo and Persahabatan Hospitals, Jakarta, Indonesia. Patients with dengue infection who were aged >14 years who were admitted to the hospital on the third day of fever from November 2013 through August 2015 were included in the study. Patients with dengue infection were diagnosed based on clinical manifestation of acute fever and a positive result for dengue nonstructural protein 1 (NS1) antigen, as confirmed by both conventional reverse-transcriptase polymerase chain reaction (RT-PCR) and Simplexa Dengue real-time RT-PCR assay [22, 23]. The exclusion criteria included patients with comorbidities and pregnant women.
Clinical and Laboratory Parameters
The clinical characteristics and laboratory parameters of each patient were recorded at the ward before enrollment. The subjects were classified as having plasma leakage based on World Health Organization criteria (ie, an increase in hematocrit >20%, hypoalbuminemia, or the presence of pleural effusions/ascites detected by ultrasonography) [24]. Subjects without plasma leakage were categorized as having DF (group 1). Subjects with plasma leakage and a serum albumin level >3 g/dL were categorized as having DHF (group 2). Subjects with plasma leakage and a serum albumin level ≤3 g/dL were categorized as having severe plasma leakage (group 3). Albumin measurement and abdominal ultrasonography were performed 24 hours after defervescence [2, 4].
Detection of the Biomarkers Using Enzyme-Linked Immunosorbent Assay
Biomarkers of the VE components were measured in duplicate using the following commercially available enzyme-linked immunosorbent assays (ELISAs), performed according to the manufacturers’ recommendations: human sCD138 (Syndecan-1) ELISA (Diaclone), human heparan sulfate ELISA (MyBioSource), human chondroitin sulfate ELISA (MyBioSource), human hyaluronan quantikine ELISA (R&D Systems), human claudin 5 ELISA (MyBioSource), and human VE-Cadherin quantikine ELISA (R&D Systems). Serum samples were diluted according to the manufacturers’ recommendation to obtain the best dilution factors to generate an optical density within the range of detection of the ELISA reader. The measurements were performed using a Multiskan microplate reader with a wavelength set to 450 nm. Biomarkers of VE components in all 3 groups were measured twice: on the third day of fever (febrile phase) and 24 hours after defervescence (critical phase). Interleukin 10 and CXCL10/IP10 detection using ELISA kits (both from R&D Systems).
Ethics
The study was approved by the Faculty of Medicine Universitas Indonesia Ethics Committee, and written informed consent was obtained from all patients.
Statistical Analysis
The sample size calculation was performed by estimation of the heparan sulfate population variance as 3.24 [25], which was the highest population variance among all of the VE biomarkers studied. Assuming a difference in the population mean of 1.25, with a confidence level of 95% and a power of 80%, the minimal total sample size required for the 3 groups was 99 patients.
A bivariate analysis with a Mann–Whitney test for nonparametric data regarding the VE biomarkers and the cytokine and chemokine studied was performed between pairs among the 3 groups. The VE biomarker variables that significantly differed based on the severity of plasma leakage in the bivariate analysis were then entered into a multivariate analysis to determine the adjusted association. The statistical analyses were performed using SPSS Version 20.0 and GraphPad Prism version 7.00 for Windows.
RESULTS
Clinical Characteristics
This study enrolled 103 patients with dengue infection. A total of 30 patients (29.1%) were classified as having DF, 50 patients (48.5%) were classified as having DHF, and 23 patients (22.3%) were classified as having dengue with severe plasma leakage. Table 1 presents the clinical characteristics of each study group.
Table 1.
Dengue-Infected Patients: Comparison of Patients With Dengue Fever, Dengue Hemorrhagic Fever, and Severe Plasma Leakage
| Variable | DF group (n = 30) | DHF group (n = 50) | Severe plasma leakage group (n = 23) |
|---|---|---|---|
| Sex, no. male/female | 14/16 | 25/25 | 9/14 |
| Age, y, median (IQR) | 21 (19–28) | 22 (18–29) | 22 (18–30) |
| Dengue serotype, no. (%) | |||
| DENV-1 | 6 (20) | 13 (26) | 5 (21.7) |
| DENV-2 | 11 (36.7) | 15 (30) | 9 (39.1) |
| DENV-3 | 8 (26.7) | 14 (28) | 7 (30.4) |
| DENV-4 | 5 (16.7) | 8 (16) | 2 (8.7) |
| Degree of hemoconcentration, %, median (IQR) | 10.67 (7.26–12.89) | 19.20 (13.77–24.39) | 20.59 (16.67–31.25) |
| Albumin concentration, g/dL, median (IQR) | 3.69 (3.52–3.75) | 3.4 (3.3–3.61) | 2.9 (2.8–2.99) |
| Platelet count × 1,000/µL, median (IQR) | 63 (46.5–95.5) | 38 (24–60.5) | 19 (13–30) |
| Pleural effusion or ascites, no. (%) | 0 | 34 (68) | 20 (87) |
Abbreviations: DENV, dengue virus; DF, dengue fever; DHF, dengue hemorrhagic fever; IQR, interquartile range.
Plasma Concentrations of the Vascular Endothelial Biomarkers
In the bivariate analysis in the febrile phase, there were significantly increased levels of syndecan-1 (P = .002), chondroitin sulfate (P = .007), and hyaluronan (P = .03) in the DHF group compared with the DF group. Levels of syndecan-1 (P < .001), chondroitin sulfate (P = .02), and hyaluronan (P = .002) were significantly higher in the group with severe plasma leakage compared with the DF group as well. We also found a significantly increased level of claudin-5 (P = .002) in the severe plasma leakage group. Between the DHF and severe plasma leakage groups, there were significant differences in levels of syndecan-1 (P = .04) and claudin-5 (P = .04) (Table 2). In the multivariate analysis of the biomarkers, there was an adjusted association for syndecan-1 between the DF and DHF groups as well as between the DF and severe plasma leakage groups. There was also an adjusted association for claudin-5 between the DHF and severe plasma leakage groups (Table 3).
Table 2.
Plasma Concentration of the Endothelial Glycocalyx Layer and Tight and Adherens Junction Markers Measured in the Febrile and Critical Phases
| Phase of dengue | Biomarker | DF group (n = 30) |
DHF group (n = 50) |
Severe plasma leakage group (n = 23) |
|---|---|---|---|---|
| Febrile phase | ||||
| Syndecan-1, ng/mL | 30.26 (13.65–159.45) | 122.81 (32.46–313.62)a | 256.42 (100.73–414.09)a, b | |
| Heparan sulfate, pg/mL | 148.34 (85.14–399.51) | 147 (104.53–523.72) | 200.93 (112.88–300.71) | |
| Chondroitin sulfate, ng/mL | 24.56 (20.21–34.58) | 34.77 (24.93–44.05)a | 34.62 (24.42–42.62)a | |
| Hyaluronan, ng/mL | 207.43 (56.35–553.1) | 842.91 (105.48–3025.74)a | 1216 (320.2–4229)a | |
| Claudin-5, pg/mL | 17.42 (11.71–34.56) | 28.77 (11.87–52.98) | 50.1 (22.7–78.7)a, b | |
| VE-cadherin, ng/mL | 23.01 (19.29–38.69) | 22.81 (19.48–30.01) | 22.36 (18.85–29.14) | |
| Critical phase | ||||
| Syndecan-1, ng/mL | 35.91 (15.15–160.21) | 264.67 (110.58–471.81)a | 468.87 (278.7–538.06)a, b | |
| Heparan sulfate, pg/mL | 146.65 (89.15–402.73) | 131.42 (86.67–309.58) | 148.05 (111.45–217.62) | |
| Chondroitin sulfate, ng/mL | 21.07 (19.07–24.33) | 25.91 (22.89–29.04)a | 28.62 (25.78–31.15)a | |
| Hyaluronan, ng/mL | 218.71 (67.07–1792.90) | 2720.50 (911.62–4790.03)a | 3230.31 (2361.29–6617.7)a | |
| Claudin-5, pg/mL | 23.77 (11.66–35.31) | 20.01 (10.77–48.13) | 81.12 (39.12–118.47)a, b | |
| VE-cadherin, ng/mL | 23.51 (18.09–40.05) | 25.06 (20.58–33.42) | 31.49 (22.34–41.1) |
Data presented as median (interquartile range) for each group. The Mann–Whitney test was used to assess differences between two groups.
Abbreviations: DF, dengue fever; DHF, dengue hemorrhagic fever.
aSignificant difference from the DF group (P < .05).
bSignificant difference from the DHF group (P < .05).
Table 3.
Multivariate Analysis of the Plasma Concentrations of the Endothelial Glycocalyx Layer and Tight and Adherens Junction Markers Measured in the Febrile Phase Between Groups
| Groups | Biomarker | Odds ratio (95% CI) |
P value |
|---|---|---|---|
| DF vs DHF | Syndecan-1 | 1.005 (1.001–1.009) | .01 |
| DF vs severe plasma leakage | Syndecan-1 | 1.009 (1.004–1.015) | .001 |
| DHF vs severe plasma leakage | Claudin-5 | 1.019 (1.001–1.037) | .04 |
Abbreviations: CI, confidence interval; DF, dengue fever; DHF, dengue hemorrhagic fever.
In the critical phase, we found significantly different levels of syndecan-1, chondroitin sulfate, and hyaluronan (P < .001 for each comparison) between the DF and DHF groups. Between the DF and severe plasma leakage groups, there were significantly different levels of syndecan-1, chondroitin sulfate, hyaluronan, and claudin-5 (P < .001 for each comparison). Furthermore, between the DHF and severe plasma leakage groups, we found significantly different levels of syndecan-1 (P = .03) and claudin-5 (P < .001) (Table 2). In the multivariate analysis between the DF and DHF groups, we found an adjusted association for syndecan-1 and chondroitin sulfate. Between the DF and severe plasma leakage groups, we found an adjusted association for syndecan-1 and claudin-5. Finally, between the DHF and severe plasma leakage groups, we found an adjusted association for claudin-5 (Table 4).
Table 4.
Multivariate Analysis of the Plasma Concentrations of the Endothelial Glycocalyx Layer and Tight and Adherens Junction Markers Measured in the Critical Phase Between Groups
| Groups | Biomarker | Odds ratio (95% CI) | P value |
|---|---|---|---|
| DF vs DHF | Syndecan-1 | 1.004 (1.001–1.007) | .02 |
| Chondroitin sulfate | 1.157 (1.025–1.307) | .02 | |
| DF vs severe plasma leakage | Syndecan-1 | 1.004 (1.000–1.008) | .047 |
| Claudin-5 | 1.038 (1.004–1.074) | .03 | |
| DHF vs severe plasma leakage | Claudin-5 | 1.022 (1.009–1.036) | .001 |
Abbreviations: CI, confidence interval; DF, dengue fever; DHF, dengue hemorrhagic fever.
Cytokine and Chemokine Plasma Concentrations
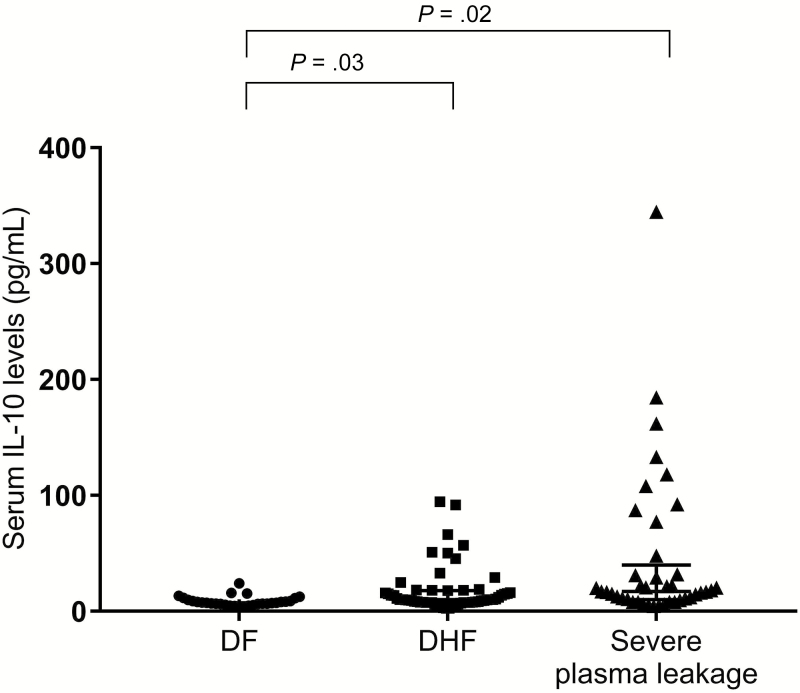
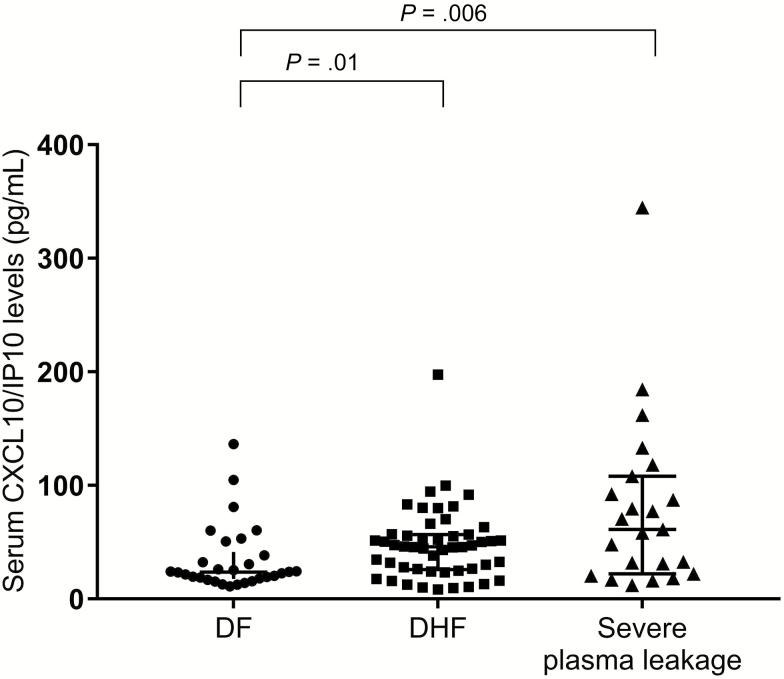
We found a significantly increased (P = .03) IL-10 in the DHF group (median = 9.47 pg/mL; interquartile range [IQR] = 7.65-13.83) compared with the DF group (median = 7.29 pg/mL; IQR = 6.29-10.36) and also in the severe plasma leakage group (median = 11.23 pg/mL; IQR = 8.06-17.06) compared with the DF group (P = .02) (Figure 1). Moreover, there was a significantly increased (P = .01) level of CXCL10/IP10 in the DHF group (median = 46.07 pg/mL; IQR = 26.02-56.84) compared with the DF group (median = 23.69 pg/mL; IQR = 17.78-41.51). Finally, there was a significantly increased (P = .006) level of CXCL10/IP10 in the severe plasma leakage group (median = 61.39 pg/mL; IQR = 22.24-108.13) compared with the DF group (Figure 2).
Figure 1.
Scatter plot of the serum interleuking 10 (IL-10) levels in the dengue fever (DF), dengue hemorrhagic fever (DHF), and severe plasma leakage groups. The horizontal bars represent the median serum IL-10 levels. Differences in the median between pairs of groups were tested using the Mann–Whitney test (P = .03 for the difference between the DF and the DHF groups; P = .02 for the difference between the DF and the severe plasma leakage groups).
Figure 2.
Scatter plot of the serum chemokine (C-X-C motif) ligand 10/interferon γ-inducible protein 10 (CXCL10/IP10) levels in the dengue fever (DF), dengue hemorrhagic fever (DHF), and severe plasma leakage groups. The horizontal bars represent the median serum CXCL10/IP10 levels. Differences in the median between pairs of groups were tested using the Mann–Whitney test (P = .01 for the difference between the DF and the DHF groups; P = .006 for the difference between the DF and the severe plasma leakage groups).
DISCUSSION
This is the first Indonesian study to assess the role of EGL and endothelial cell junction components in determining the severity of plasma leakage, which is responsible for the life-threatening nature of DHF. Hypoalbuminemia is objective evidence of plasma leakage due to vascular permeability alteration [24]. Previous studies reported that the level of serum albumin was associated with the severity of dengue infection and could be used as a surrogate indicator of severe plasma leakage [8, 9].
Bivariate analysis of EGL components in the febrile phase showed significantly increased levels of syndecan-1, chondroitin sulfate, and hyaluronan in the DHF and severe plasma leakage groups compared with the DF group. An explanation for these findings is that there are several possible mechanisms of vascular permeability alteration due to EGL degradation in dengue infection based on previous in vitro studies. The first mechanism is release of cytokines and chemokines by peripheral blood mononuclear cells through direct activation of Toll-like receptor 4 by NS1 [26]. The second mechanism is complement activation induced by either attachment of NS1 to the glycosaminoglycans heparan sulfate and chondroitin sulfate E or binding of DENV to a specific syndecan-2 proteoglycan receptor [27–29]. The third mechanism is activation of endothelial cell-intrinsic pathways caused by binding of DENV NS1 to the glycocalyx [26]. These 3 possible mechanisms may explain the degradation and shedding of the EGL components in the circulation [26, 29]. However, we found no significant increase in the level of heparan sulfate within the 3 groups. Furthermore, in the multivariate analysis, the only EGL component that had a strong association with the severity of plasma leakage was syndecan-1. Our finding may be explained based on the mechanism that regulates synthesis of the syndecan-1 core protein by heparan sulfate. In endothelial cells, DENV NS1 induces lysosomal cysteine proteinase and activates heparanase through enzymatic cleavage [26]. Heparanase then cleaves heparan sulfate chains, resulting in degradation of the heparan sulfate attached to syndecan-1. Diminished levels of heparan sulfate chains on the syndecan-1 core protein cause a significant increase in shedding of syndecan-1 [30]. This effect is specifically induced by NS1 from DENV-1–4, and not by proteins from other flaviviruses [26].
In this study, both bivariate and multivariate analyses of tight junction markers showed an adjusted association for claudin-5 between the DHF and severe plasma leakage groups. This finding is consistent with an in vitro study that showed disrupted tight junctions among human VE cells after exposure to monocyte chemoattractant protein-1 (MCP-1) from the sera of DSS patients [31]. Bivariate analysis of adherens junction markers in the febrile phase showed there was no difference in VE-cadherin levels within the subject groups. VE-cadherin has been proposed as a biomarker reflecting endothelial damage. In fact, in patients with hemolytic uremic syndrome (HUS) caused by Shiga toxin–producing Escherichia coli (STEC) infection, soluble VE-cadherin levels are significantly higher than in non-HUS patients; this finding indicates that VE-cadherin is released when membrane damage occurs [16]. In contrast with HUS, plasma leakage in dengue infection occurs without morphological damage to the vascular endothelium [29]. This feature may explain the lack of a difference in VE-cadherin levels within the groups of dengue patients with different severities of plasma leakage in this study. However, further studies are needed to determine the role of VE-cadherin in the most severe dengue infections, such as in DSS. Our findings regarding biomarker levels in the febrile phase suggest that syndecan-1 plays a dual role as a risk factor for plasma leakage occurring at 24 days after defervescence: first, as a risk factor for plasma leakage in general, and second, as a risk factor for severe plasma leakage together with claudin-5.
As has been discussed previously, syndecan-1 plays a vital role in the early phase, serving as a risk factor for plasma leakage, and this role has also been confirmed in the critical phase. We specifically found an adjusted association for syndecan-1 between the DF and DHF groups. We also found significantly elevated chondroitin sulfate in the DHF group compared with the DF group. However, based on the multivariate analysis, the adjusted association of elevated chondroitin sulfate only persisted in the critical phase. This finding can be explained by the fact that plasma leakage is most commonly detected in the critical phase [2, 3]. In addition, the median level of chondroitin sulfate was lower in the critical phase compared with the febrile phase. A previous study characterizing circulating glycosaminoglycans during critical illness reported a decline in circulating chondroitin sulfate 72 hours after study enrollment; this finding may reflect glycosaminoglycan clearance by the liver or kidney [32].
Physiologically, syndecan-1 contains 3 attachment sites for heparan sulfate and 2 additional attachment sites for chondroitin sulfate [33]. We postulate that the peak shedding of syndecan-1 is followed by a significant increase in the level of chondroitin sulfate. In contrast, due to heparanase activity, there is no increase in the level of heparan sulfate [30]. Although hyaluronan showed a significant association in the bivariate analysis in both the febrile and the critical phases, the association did not persist in the multivariate analysis. This finding may indicate a less significant role for hyaluronan, which is located on the apical surface of endothelial cells [33], compared with syndecan-1 and chondroitin sulfate in terms of the process of plasma leakage in dengue infection. In vitro studies have reported that hyaluronidase treatment to remove hyaluronan results only in minimal changes in permeability. It appears that the components of the EGL other than hyaluronan can stabilize the properties of the glycocalyx [34].
We found significantly increased levels of the cytokine IL-10 and the chemokine CXCL10/IP10 in the DHF group compared with the DF group in the critical phase. A previous study in pediatric patients reported that circulating levels of IL-10 and CXCL10/IP10 significantly increased in dengue patients with vascular leakage [21]. A study in hemorrhagic shock patients reported a significantly increased level of syndecan-1 after injury and a positive correlation between the IL-10 level and syndecan-1 shedding. Furthermore, an in vitro model of endothelial injury showed that syndecan-1 shedding correlated with changes in endothelial permeability [35], and another in vitro study revealed that chemokines stimulate cell metalloproteinases, which are involved in syndecan shedding [36].
We found no significant association between DF and DHF and either tight or adherens junction components in the critical phase. The glycocalyx plays an important role as an endothelial barrier because of its net negative charge. This role is supported by experiments that characterized the effect of disrupting the glycocalyx or neutralizing its negative charge [11]. In particular, treatment with neuraminidase, causing removal of the majority of the glycocalyx, increased albumin flux across the endothelium [37]. Moreover, neutralization of the apical endothelial negative charge with cationic agents (eg, ferritin or protamine) increased the transendothelial permeability of albumin [11]. The transcellular pathway is initiated by binding of albumin to the protein gp60, which induces the phosphorylation of the Src protein tyrosine kinases (PTKs), leading to phosphorylation of caveolin-1 and dynamin-2. The activation of these proteins is required for caveolar fission and the internalization of albumin within caveolae [38], and inflammatory cytokines such as TNF-ɑ may directly increase the phosphorylation of the Src PTKs [39]. Moreover, an in vitro study using human dendritic cells showed that chemokines could stimulate the activation of Src PTKs in both immature and mature dendritic cells [40]. Another in vitro study found that DENV infection enhances caveolae-mediated albumin transcytosis, which consequently induces vascular leakage [41]. These mechanisms may explain the increase in albumin leakage through transcytosis in DHF, independent of the paracellular pathway.
Consistent with our findings in the febrile phase, in the critical phase, there was an adjusted association for syndecan-1 and claudin-5 between the DF and severe plasma leakage groups. We also found that claudin-5 had an adjusted association between the DHF and the severe plasma leakage groups. In addition, there were increased levels of IL-10 and CXCL10/IP10 in the severe plasma leakage group compared with the DF group. The disruption of tight junctions has been elucidated by in vitro studies that have determined that the mechanism of severe dengue is based on excessive release of various cytokines and chemokines, known as cytokine storm, as observed in U937-derived macrophages infected with a humanized monoclonal antibody recognizing protein E [42].
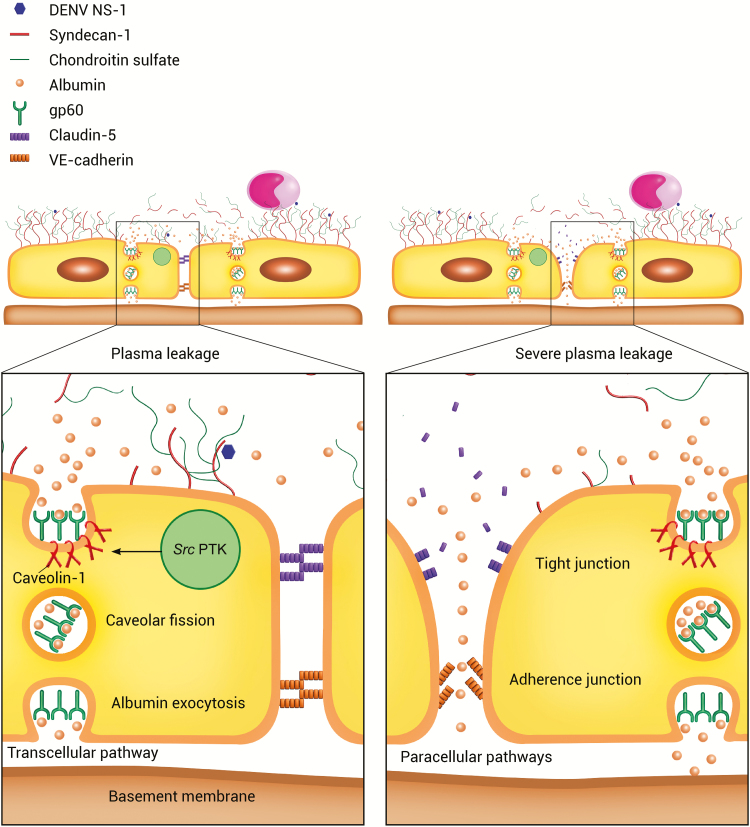
This degradation of tight junctions allows the passage of albumin across the endothelial barrier, causing hypoalbuminemia, even without degradation of adherens junctions [11]. The potential explanation for this paracellular pathway is that endothelial-cell retraction may lead to opening of intercellular gaps. This reversible effect may be caused by histamine, thrombin, and VE growth factors, without affecting vascular damage [11, 43]. In sum, the results reflect major roles for syndecan-1 and claudin-5 in the mechanism of albumin leakage through transcellular and paracellular pathways during severe plasma leakage. A proposed mechanism of microvascular leakage in dengue-infected patients based on the overall results of our study is shown in Figure 3.
Figure 3.
Proposed mechanism of microvascular leakage in dengue infection. Plasma leakage: dengue virus (DENV) nonstructural protein 1 (NS1)–induced degradation of syndecan-1 and chondroitin sulfate results in gp60 activation by albumin. Gp60 activation leads to phosphorylation of the Src protein tyrosine kinases (PTKs) and caveolin-1, which is responsible for regulation of caveolar fission. Cytokines and chemokines can also stimulate activation of Src PTKs. This process increases albumin exocytosis through the transcellular pathway. Severe plasma leakage: Claudin-5 degradation causes retraction of endothelial cells and opening of intercellular gaps, allowing albumin movement into the extravascular space through paracellular pathways. Albumin leakage that occurs through both paracellular and transcellular pathways results in severe plasma leakage.
A limitation of our study is the measurement of EGL components and tight and adherens junction markers in the plasma. In particular, the predominant source of these components is endothelial cells, but these components are also present on epithelial tissues and macrophages, so the levels of these components in the circulation might not be solely influenced by endothelial cells.
In conclusion, in patients with dengue infection, elevated levels of syndecan-1 and chondroitin sulfate have a strong association with plasma leakage, and elevated levels of syndecan-1 and claudin-5 have a strong association with severe plasma leakage.
Notes
Acknowledgments. The authors would like to thank the patients, clinicians, and other medical staff involved in this study. The authors also thank Bonita Effendi, MD, and Kresna Dharma Suryana, MD, for sample collection and data management. The technical assistance of Eleanor Louana Urfa, MBiomed; Beti Ernawati Dewi, PhD; Rahma F. Hayati; and Benediktus Yohan is greatly appreciated as well.
Disclaimer. The funders had no role in the study design, the data collection or analysis, the decision to publish, or the preparation of this manuscript. The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the funding agencies.
Financial support. This work was supported by the Teladan Utama Foundation and the Ministry of Research, Technology, and Higher Education of the Republic of Indonesia.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rajapakse S, Rodrigo C, Maduranga S, Rajapakse AC. Corticosteroids in the treatment of dengue shock syndrome. Infect Drug Resist 2014; 7:137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Srikiatkhachorn A, Krautrachue A, Ratanaprakarn W, et al. Natural history of plasma leakage in dengue hemorrhagic fever: a serial ultrasonographic study. Pediatr Infect Dis J 2007; 26:283–90; discussion 91–2. [DOI] [PubMed] [Google Scholar]
- 3. Suwarto S, Nainggolan L, Sinto R, et al. Dengue score: a proposed diagnostic predictor for pleural effusion and/or ascites in adults with dengue infection. BMC Infect Dis 2016; 16:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Srikiatkhachorn A. Plasma leakage in dengue haemorrhagic fever. Thromb Haemost 2009; 102:1042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Azeredo EL, Monteiro RQ, de-Oliveira Pinto LM. Thrombocytopenia in dengue: interrelationship between virus and the imbalance between coagulation and fibrinolysis and inflammatory mediators. Mediators Inflamm 2015; 2015:313842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heilman JM, De Wolff J, Beards GM, Basden BJ. Dengue fever: a Wikipedia clinical review. Open Med 2014; 8:e105–15. [PMC free article] [PubMed] [Google Scholar]
- 7. Wills BA, Oragui EE, Stephens AC, et al. Coagulation abnormalities in dengue hemorrhagic fever: serial investigations in 167 Vietnamese children with dengue shock syndrome. Clin Infect Dis 2002; 35:277–85. [DOI] [PubMed] [Google Scholar]
- 8. Villar-Centeno LA, Díaz-Quijano FA, Martínez-Vega RA. Biochemical alterations as markers of dengue hemorrhagic fever. Am J Trop Med Hyg 2008; 78:370–4. [PubMed] [Google Scholar]
- 9. Wang CC, Liu SF, Liao SC, et al. Acute respiratory failure in adult patients with dengue virus infection. Am J Trop Med Hyg 2007; 77:151–8. [PubMed] [Google Scholar]
- 10. Fariz-Safhan MN, Tee HP, Abu Dzarr GA, Sapari S, Lee YY. Bleeding outcome during a dengue outbreak in 2005 in the east-coast region of Peninsular Malaysia: a prospective study. Trop Biomed 2014; 31:270–80. [PubMed] [Google Scholar]
- 11. Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 2006; 86:279–367. [DOI] [PubMed] [Google Scholar]
- 12. Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol 2010; 72:463–93. [DOI] [PubMed] [Google Scholar]
- 13. Rahbar E, Cardenas JC, Baimukanova G, et al. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med 2015; 13:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell 2009; 16:209–21. [DOI] [PubMed] [Google Scholar]
- 15. Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev 2013; 93:525–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doulgere J, Otto B, Nassour M, et al. Soluble plasma VE-cadherin concentrations are elevated in patients with STEC infection and haemolytic uraemic syndrome: a case-control study. BMJ Open 2015; 5:e005659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yacoub S, Mongkolsapaya J, Screaton G. Recent advances in understanding dengue. F1000Res 2016; 5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanlaya R, Pattanakitsakul SN, Sinchaikul S, Chen ST, Thongboonkerd V. Alterations in actin cytoskeletal assembly and junctional protein complexes in human endothelial cells induced by dengue virus infection and mimicry of leukocyte transendothelial migration. J Proteome Res 2009; 8:2551–62. [DOI] [PubMed] [Google Scholar]
- 19. Honsawek S, Kongtawelert P, Pothacharoen P, Khongphatthanayothin A, Chongsrisawat V, Poovorawan Y. Increased levels of serum hyaluronan in patients with dengue infection. J Infect 2007; 54:225–9. [DOI] [PubMed] [Google Scholar]
- 20. Wills BA, Oragui EE, Dung NM, et al. Size and charge characteristics of the protein leak in dengue shock syndrome. J Infect Dis 2004; 190:810–8. [DOI] [PubMed] [Google Scholar]
- 21. Ferreira RA, de Oliveira SA, Gandini M, et al. Circulating cytokines and chemokines associated with plasma leakage and hepatic dysfunction in Brazilian children with dengue fever. Acta Trop 2015; 149:138–47. [DOI] [PubMed] [Google Scholar]
- 22. Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol 1992; 30:545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sasmono RT, Aryati A, Wardhani P, et al. Performance of Simplexa dengue molecular assay compared to conventional and SYBR green RT-PCR for detection of dengue infection in Indonesia. PLoS One 2014; 9:e103815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization South-East Asia Regional Office. Comprehensive guidelines for prevention and control of dengue and dengue haemorrhagic fever. Revised and expanded edition New Delhi:World Health Organization, 2011. [Google Scholar]
- 25. Guven FM, Aydin H, Kaya A, et al. The role of heparan sulphate in pathogenesis of Crimean-Congo hemorrhagic fever disease. J Vector Borne Dis 2013; 50:133–6. [PubMed] [Google Scholar]
- 26. Puerta-Guardo H, Glasner DR, Harris E. Dengue virus NS1 disrupts the endothelial glycocalyx, leading to hyperpermeability. PLoS Pathog 2016; 12:e1005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okamoto K, Kinoshita H, Parquet Mdel C, et al. Dengue virus strain DEN2 16681 utilizes a specific glycochain of syndecan-2 proteoglycan as a receptor. J Gen Virol 2012; 93(Pt 4):761–70. [DOI] [PubMed] [Google Scholar]
- 28. Avirutnan P, Zhang L, Punyadee N, et al. Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E. PLoS Pathog 2007; 3:e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chuang YC, Wang SY, Lin YS, Chen HR, Yeh TM. Re-evaluation of the pathogenic roles of nonstructural protein 1 and its antibodies during dengue virus infection. J Biomed Sci 2013; 20:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramani VC, Pruett PS, Thompson CA, DeLucas LD, Sanderson RD. Heparan sulfate chains of syndecan-1 regulate ectodomain shedding. J Biol Chem 2012; 287:9952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee YR, Liu MT, Lei HY, et al. MCP-1, a highly expressed chemokine in dengue haemorrhagic fever/dengue shock syndrome patients, may cause permeability change, possibly through reduced tight junctions of vascular endothelium cells. J Gen Virol 2006; 87(Pt 12):3623–30. [DOI] [PubMed] [Google Scholar]
- 32. Schmidt EP, Li G, Li L, et al. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J Biol Chem 2014; 289:8194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 2007; 9:121–67. [DOI] [PubMed] [Google Scholar]
- 34. Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol 1999; 277(2 Pt 2):H508–14. [DOI] [PubMed] [Google Scholar]
- 35. Haywood-Watson RJ, Holcomb JB, Gonzalez EA, et al. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS One 2011; 6:e23530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Charnaux N, Sutton A, Brule S, Gattegno L. Regulated shedding of syndecan ectodomains by chemokines. ScientificWorldJournal 2006; 6:1037–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singh A, Satchell SC, Neal CR, McKenzie EA, Tooke JE, Mathieson PW. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J Am Soc Nephrol 2007; 18:2885–93. [DOI] [PubMed] [Google Scholar]
- 38. Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol 2003; 285:L1179–83. [DOI] [PubMed] [Google Scholar]
- 39. Okutani D, Lodyga M, Han B, Liu M. Src protein tyrosine kinase family and acute inflammatory responses. Am J Physiol Lung Cell Mol Physiol 2006; 291:L129–41. [DOI] [PubMed] [Google Scholar]
- 40. Sato K, Kawasaki H, Nagayama H, et al. Signaling events following chemokine receptor ligation in human dendritic cells at different developmental stages. Int Immunol 2001; 13:167–79. [DOI] [PubMed] [Google Scholar]
- 41. Chanthick C, Kanlaya R, Kiatbumrung R, Pattanakitsakul SN, Thongboonkerd V. Caveolae-mediated albumin transcytosis is enhanced in dengue-infected human endothelial cells: a model of vascular leakage in dengue hemorrhagic fever. Sci Rep 2016; 6:31855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Puerta-Guardo H, Raya-Sandino A, González-Mariscal L, et al. The cytokine response of U937-derived macrophages infected through antibody-dependent enhancement of dengue virus disrupts cell apical-junction complexes and increases vascular permeability. J Virol 2013; 87:7486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 2008; 121(Pt 13): 2115–22. [DOI] [PubMed] [Google Scholar]