Abstract
Borrelia burgdorferi genome harbors several paralogous gene families (pgf) that can encode immunogenic proteins of unknown function. Protein–protein interaction assays using a transmission-blocking vaccine candidate, BBA52, as bait identified an interacting partner in spirochetes—a member of pgf 54, annotated as BBI39. We show that BBI39 is a surface-exposed membrane antigen that is immunogenic during spirochete infection, despite the gene being primarily transcribed in the vector with a transient expression in the host only at tick-bite sites. Immunization of rodents with BBI39, or a diverse paralog, BBI36, or their combination impaired pathogen acquisition by the vector, transmission from ticks to hosts, or induction of disease. High-titer BBI39 immunoglobulin G antibodies, which have borreliacidal properties, could be generated through routine subcutaneous or oral immunization, further highlighting use of BBI39 proteins as novel Lyme disease vaccines that can target pathogens in the host or in ticks.
Keywords: Borrelia burgdorferi, BBI39, paralogous gene family, pathogen persistence, vaccine.
Lyme disease, caused by Borrelia burgdorferi, is a prevalent tick-borne illness. Along with increasing number of cases, emergence of new and more virulent Lyme disease–causing spirochetes is also reported [1–3]. The pathogen persists in a complex enzootic life cycle involving Ixodes scapularis ticks and mammalian hosts. The bacteria adapts to diverse host environments by altering its proteome, including surface antigens, some of which are involved in dynamic host–pathogen interactions [4, 5]. Infected ticks can engorge on accidental hosts like humans, where B. burgdorferi induces serious multisystem disorders such as arthritis, carditis, and a variety of neurological complications [6, 7]. Although the antibiotic treatment is usually effective, a minority of treated patients show relapsing nonobjective symptoms of variable intensity, termed as chronic Lyme disease or posttreatment Lyme disease syndrome that remains nonresponsive to further antimicrobial therapy [8].
Borrelia burgdorferi produces many antigens that are surface exposed, for example, OspA, OspB, OspC, BBK32, BBA52, BB0405, BBA57, BBK50, DbpA, Lmp1, Elp, and OspF, some of which have shown promising results as vaccine candidates [9–17]. However, these antigens have their limitations; for example, immunization with OspA or BBA52, which are expressed in ticks, can block pathogen transmission from the vector but cannot eliminate spirochetes from already infected mammals [10, 18]. Similarly, vaccination with antigens that are primarily expressed in the host, such as DbpA, is protective only during experimental syringe-borne infection [19] but cannot protect the hosts when challenged by tick infestation [20]. In other cases, the protection is only partial, such as BmpA [21, 22] or BBI16 [23], or vaccination only induces strain-specific immunity, such as OspC [14]. As a result, despite progress in early vaccine discovery, only one candidate, OspA, advanced through clinical trials and was finally licensed as LYMErix by SmithKline. However, within 2 years of commercialization and FDA approval, the vaccine was withdrawn from the market due to poor sales. Therefore, the search for an effective Lyme disease vaccine remains a public health priority [14, 24].
One of the unique features of B. burgdorferi genome is its genetic redundancy [25–27]. Many plasmid-encoded genes, including paralogous gene families (pgf), such as pgf 54, are differentially expressed and regulated by temperature, pH, or factors intrinsic to ticks or mammalian hosts [28], although their functions remain unknown. This is partly due to their lack of homology to known proteins as well as their presence in several discrete plasmids that limits targeted gene deletion efforts. For example, six genes of pgf 54 are contained in the plasmid lp54 (bba64, bba65, bba66, bba68, bba69, and bba73) and 4 homologous genes (bbi36, bbi38, bbi39, and bbj41) are distributed in 2 additional plasmids (lp28-4 and lp38) [25]. These latter 4 genes encode proteins of similar molecular weight (approximately 32 kDa) with amino-acid sequence divergence ranging 1%–26%. These antigens, referred to herein as BBI39 paralogs, are differentially regulated by environmental cues, such as pH [29] or temperature [30], and are abundantly expressed by cultured spirochetes [31], although their biological significance remains enigmatic. Herein we report a complex in vivo expression profile of these antigens, which is likely to serve important functions in supporting the B. burgdorferi infectious cycle. Vaccination studies suggested that these proteins are the target of host immunity, interfering with pathogen survival either in the hosts or in the vector.
MATERIALS AND METHODS
Borrelia burgdorferi, Ticks, and Mice
Borrelia burgdorferi infectious isolates B31, N40, and 297 grown in Barbour-Stoenner-Kelly-H (BSK-H) medium were used in this study [32, 13]. Four- to six-week-old C3H/HeN mice were purchased from the National Institutes of Health. Ixodes scapularis ticks used in this study originated from a colony that is maintained in the laboratory [32]. All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee and Institutional Bio-safety Committee of the University of Maryland, College Park.
Generation of Recombinant BBI36 and BBI39 Proteins and Antisera
Because sequences of bbi36 and bbi39 are highly similar, we initially amplified the region surrounding the respective genes using the specific primers (Supplementary Table 1). The respective amplicon containing either bbi36 or bbi39 was then used as template to amplify bbi36 and bbi39 genes, which were cloned into pGEX-6P-1. The recombinant proteins without the N-terminal leader sequence were produced in Escherichia coli and purified as described previously [16]. Polyclonal antisera against recombinant BBI39 (without the GST tag) were generated in mice and assessed for titer and specificity as described [16]. Expression of BBI39 in E. coli for use in mouse oral immunization studies and determination of titers for immunoglobulin G (IgG) subtypes were performed as detailed previously [33].
Polymerase Chain Reaction
The primers used in polymerase chain reaction (PCR) and quantitative reverse transcription-PCR (qRT-PCR) are shown in Supplementary Table 1. For qRT-PCR, RNA was isolated and analyzed as described elsewhere [16, 18]. Expression of bbi39 was analyzed in various tissues of C3H/HeN mice (n = 3 animals/group), which were infected with spirochetes via ticks, or in naive or infected nymphal ticks that parasitized infected mice or naive mice (n = 10 ticks/mouse), respectively, as detailed [18]. The bbi39 transcript levels in tick and mouse samples were normalized against flaB transcripts. For analysis of gene expression at the tick-bite sites, 1 mm skin biopsy around the tick hypostome were excised and processed for qRT-PCR. Selective amplification of murine β-actin but absence of tick β-actin further confirmed the specific expression of bbi39 in the host (data not shown).
Western Blotting and Far-Western Blotting
These assays were performed as detailed [34]. The following antibody dilutions were used: anti-BBI39 (1:5000), anti-FlaB (1:4000), anti-BBA52 (1:5000), anti-OspA (1:500), anti-GST (1:5000), followed by horseradish peroxidase conjugated secondary antibodies (1:5000–10000). For far-Western studies, protein preparations (0.5–1 µg per lane) were subjected to 12% SDS-PAGE and probed with bait proteins and secondary antibodies as described [34].
Assays for Subcellular Localization
BBI39 paralog proteins were assessed for their association with borrelial outer membrane and their exposure to extracellular surface using a variety of assays, including proteinase K accessibility assay, Triton-X-114 phase partitioning, purification of B. burgdorferi outer membrane, and salt and detergent treatment, as described elsewhere [16, 18].
GST Pull-down Assay
The GST-BBI36 or GST-BBI39 proteins were used for pull-down studies using B. burgdorferi lysate as described earlier [18]. Briefly, GST or GST-fused recombinant proteins were incubated with glutathione-sepharose beads in Tris-buffered saline pH 7.5 with 0.05% Tween 20 (TBST), washed with TBST, centrifuged at 1000 × g for 5 minutes, and finally analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting.
Growth-Inhibition Assay
In vitro bacteriostatic activities of specific antibodies against B. burgdorferi were determined using published procedures. Briefly, spirochetes (106/mL) were incubated with serum from vaccinated mice for 48 hours, after which aliquots of the culture were tested for spirochete regrowth, as detailed previously [13]. After antibody incubation, the culture was dispersed using a pipette to minimize spirochete clumping and checked under the microscope. One microliter of medium containing spirochetes was added to 1 ml of fresh BSK-H medium to assess their ability to regrow in the culture using a dark-field microscope as detailed previously [16].
Immunization and Infection Experiments
Subcutaneous immunization and spirochete infection studies were performed as previously described [13, 18]. Briefly, groups of C3H mice (n = 3 animals/group) were immunized with BBI36, BBI39, or combination of both or phosphate-buffered saline (PBS; control) using Freund’s adjuvant, and antibody development including titer and specificity were assessed by enzyme-linked immunosorbent assay (ELISA) and immunoblotting. Oral immunization studies were performed as described [33]. Briefly, separate groups of 4 C3H mice were immunized twice by oral gavage either with E. coli expressing BBI39 or empty vector (control), and serum samples were assessed for antibody development by ELISA. Ten days after final immunization, groups of mice were challenged with infected nymphs (n = 5 ticks/mouse). After 1 or 3 weeks of infection, mice were euthanized, and B. burgdorferi burden in tissues was assessed by qRT-PCR [13, 18]. Portions of skin and heart were cultured in BSK-H medium. For tick acquisition study, immunized mice after 10 days of final boost were infected with spirochetes (105 cells/mouse) by needle inoculum. After 12 days of infection, naive nymphal ticks (n = 10 ticks/mouse) were fed on each group. Ticks that had fed for 48 hours as well as fully engorged nymphs were collected, and pathogen burden was measured by qRT-PCR.
Histological Analysis
For histological evaluations of arthritis, at least 3 ankle joints were collected from each group of vaccinated mice at day 21 after B. burgdorferi infection and processed for hematoxylin-eosin staining. Signs of arthritis were evaluated in a double-blinded manner as described elsewhere [17].
Statistical Analysis
Results are expressed as the mean ± standard error (SEM). The significance of the difference between the mean values of the groups was evaluated by 2-tailed Student’s t test.
RESULTS
Interaction of BBI39 With a Known Transmission-Blocking Vaccine Target, BBA52
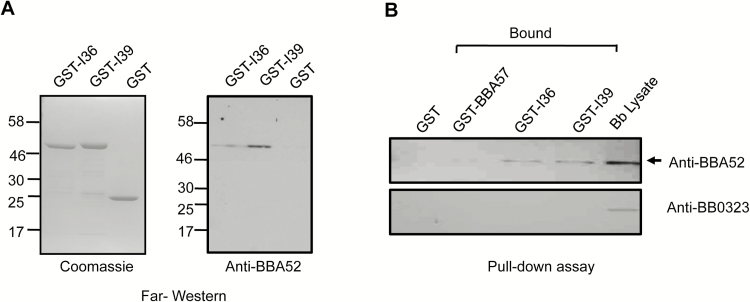
We have previously shown that a surface antigen of B. burgdorferi, BBA52, is selectively expressed in the vector and supports spirochete transmission from ticks to mammals [32]. The antigen also forms protein complexes in the outer membrane and is a target of protective host immunity [18, 35]. To identify other proteins functionally associated with BBA52 that are also potential vaccine targets, we sought to determine its interacting protein(s) in spirochetes. Borrelia burgdorferi lysates were resolved in 2-dimensional gels and probed with recombinant BBA52, and bound proteins were identified using mass spectrometry. A 32-kDa protein was identified as BBA52-bound protein, encoded by bbi39 locus, which is closely related to 3 other paralogous genes: bbi36, bbi38, and bbj41 (data not shown), referred to herein as BBI39 paralogs. Amino-acid alignment indicated that, although BBI39 is 99% identical to BBJ41, it reflects 74%–75% identity to BBI36 and BBI38, both of which are 99% similar (Supplementary Figure 1). To further verify their interaction with BBA52, we produced recombinant forms of 2 of the most divergent members of this gene family, BBI36 and BBI39, using a bacterial expression system and generated polyclonal antiserum in mice. Two independent assays, far-Western and GST pull-down analysis, further confirmed the interaction of BBA52 with both BBI36 and BBI39 (Figure 1).
Figure 1.
BBI39 interacts with BBA52. A, Far-Western blot analysis demonstrates specific binding of BBI36 or BBI39 to BBA52. Glutathione S-transferase fused recombinant proteins (either BBI36 or BBI39) or GST (control) were separated by SDS-PAGE, transferred onto nitrocellulose membrane, and incubated with rBBA52 protein. Bound proteins were detected by anti-BBA52 antibody. Migration of protein standards is shown to the left in kilodaltons. B, Interaction of BBI36 or BBI39 and BBA52 in a GST pull-down assay. GST-fused BBI36 or BBI39 or BBA57 (negative control) were bound to glutathione-sepharose beads, washed, and incubated with Borrelia cell lysate. The bound proteins were separated by SDS-PAGE and immunoblotted with anti-BBA52 or anti-BB0323 C-terminal (negative control) antibodies.
Localization of BBI39 as Surface-Exposed and Tightly Associated Outer Membrane Antigens
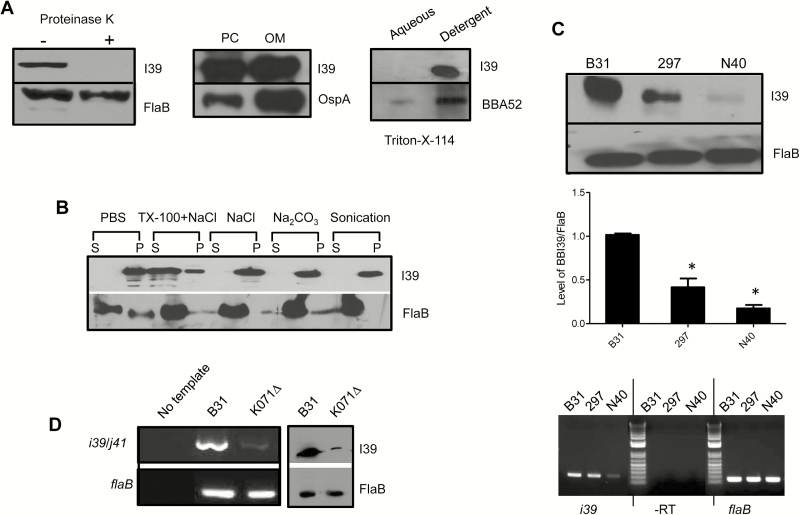
To assess whether BBI39 paralogs contribute in microbial virulence and are a target of protective host immunity, we first characterized their subcellular localization. Because BBI39 antisera would recognize all 4 paralog members, we referred to the group of proteins as BBI39. A variety of established assays [16, 18], such as limited proteolytic digestion of surface proteins in intact spirochetes, isolation of borrelial outer membrane vesicles, Triton X-114 phase partitioning studies, and salt and detergent treatments that release membrane proteins, collectively suggested that BBI39 is a surface-exposed and outer membrane antigen that is tightly associated with the cellular membrane (Figure 2A and B). We further showed that, although detectable, the level of BBI39 expression is variable among various isolates of B. burgdorferi (Figure 2C). We also determined the relative transcript levels of paralog members on “I” plasmid (lp28-4), which encodes bbi36/38/39, versus “J” plasmid (lp38), which encodes bbj41, using an isogenic B. burgdorferi B31 isolate that lost I plasmid (designated K071∆). A dramatic reduction of bbi39 mRNA and protein levels in K071∆ isolate, as compared with isogenic wild type, highlighted their predominant expression, including a basal level of expression of bbj41 (Figure 2D).
Figure 2.
BBI39 encodes for an outer membrane surface-exposed protein. A, BBI39 is exposed on the spirochete surface. Borrelia burgdorferi cells were treated with (+) or without (−) proteinase K and processed for immunoblot analysis using either BBI39 or subsurface (FlaB) protein antibodies (left panel). BBI39 is distributed in the outer membrane of spirochetes. Borrelia burgdorferi lysates were separated into protoplasmic cylinder (PC) and outer membrane (OM) fraction and assessed by immunoblotting (middle panel). BBI39 is partitioned in the detergent phases of B. burgdorferi proteins. Spirochete lysates were subjected to Triton X-114 phase partitioning and immunoblotted (right panel). B, Association of BBI39 with borrelial membranes. Borrelia burgdorferi lysates were treated with salt and detergent, and supernatant (S) and pellet (P) fractions were assessed by immunoblotting. C, Production of BBI39 in representative infectious isolates of B. burgdorferi. Different isolates of B. burgdorferi were grown to an equal cell density (108 cells/mL). Spirochete lysates were prepared and immunoblotted with BBI39 antibody generated against B. burgdorferi B31 isolate (upper panel) and subjected to quantitative densitometry (middle panel). The levels of BBI39 are significantly reduced in 297 and N40 strains (*P < .05). The bars represent the mean values from 3 independent experiments, and the error bars represent the SEM. The lower panel shows reverse transcription-polymerase chain reaction (RT-PCR) analysis of bbi39 transcripts. Levels of flaB served as loading control, and absence of DNA contamination was ensured by RT-PCR reaction in the absence of reverse transcriptase (-RT). D, Assessment of relative transcript abundance of bbi39 and its paralogs. The mRNA levels of bbi39 or its paralogous genes in B. burgdorferi and an isogenic B31 isolate that is missing lp28 (hence missing bbi36, bbi38, and bbi39), designated K071∆, were assessed by RT-PCR. The primer information is presented in Supplementary Table 1.
Vector- and Tick-bite site-specific expression of bbi39 in the host
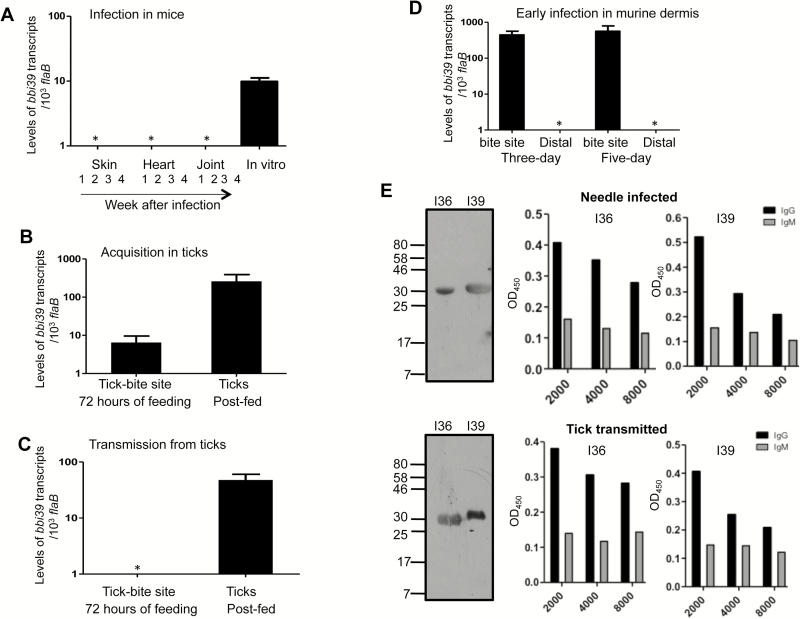
To further characterize BBI39, we next assessed its expression in vivo, in representative phases of borrelial infection cycle. Because of extensive nucleotide sequence homology (up to 99%; Supplementary Figure 2), we were unable to design paralog-specific primers and thus used a common primer pair to detect expression of all bbi39 paralogs. The qRT-PCR analysis suggested that, unlike cultured spirochetes, bbi39 expression is rapidly shut down in the murine hosts infected by tick bite (Figure 3A). Although bbi39 transcripts remain undetectable in most murine tissues examined, the gene is upregulated at tick-bite sites within the host skin and in the vector during spirochete acquisition by larval ticks (Figure 3B). The gene remained highly expressed in ticks, including fed nymphs, during pathogen transmission to naive hosts (Figure 3C). Although bbi39 transcripts are generally undetectable in murine dermis, a transient expression was recorded at the tick-bite sites within the host skin during early infection, between 3–5 days of infection (Figure 3D). Immunoblot and ELISA analyses showed that antibodies against BBI39, which are predominantly IgG subclasses, are detectable in infected murine hosts, either during experimental or natural infection with B. burgdorferi, suggesting that a transient expression of bbi39 expression at murine dermis leads to the antibody development in the host (Figure 3E).
Figure 3.
bbi39 expression and immunogenicity in representative stages of Borrelia burgdorferi infectious cycle. The transcript levels were measured using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and are presented as number of copies of bbi39 transcript relative to flaB. Error bars represent the mean ± standard error of 4 qRT-PCR analyses of at least 2 independent murine-tick infection experiments. A, bbi39 expression in rodent hosts. Mice (n = 3 animals/group) were parasitized with B. burgdorferi–infected ticks, and tissues were collected at weekly intervals after infection and pooled by tissue type. The transcript level was also measured in in vitro–grown spirochetes. B, bbi39 expression in fed ticks during pathogen acquisition from infected hosts. Nymphs (n = 10 ticks/mouse) were allowed to engorge on B. burgdorferi–infected mice 2 weeks after inoculation with cultured spirochetes. At 72 hours of tick feeding, transcript levels were measured at the tick-bite sites of the mice and in the fully engorged nymphs. C, Transcript levels during B. burgdorferi transmission from infected ticks to naive hosts. Borrelia burgdorferi–infected ticks were allowed to feed on naive mice, and expression of bbi39 was measured during tick feeding, at 72 hours of the engorgement process. Transcripts levels were measured within host skin surrounding the tick-bite sites (left bar), as well as in the post-fed ticks (right bar). D, bbi39 expression during early murine infection. Transcript level was measured in murine dermis at the tick-bite sites or at distant sites within the host dermis after tick feeding, following 3 or 5 days of tick engorgement. E, BBI36 and BBI39 are immunogenic in mammalian hosts and predominantly induce immunoglobulin G (IgG) antibody responses. Mice (n = 3 animals/group) were infected either with ticks harboring B. burgdorferi or by needle inoculation. After 28 days, sera were collected, and development of BBI36 and BBI39 specific antibodies was assessed using Western blotting assays (left panels). The middle and right panels represent enzyme-linked immunosorbent assay showing development of IgG antibodies at day 21 of infection. The wells were coated with either BBI36 or BBI39 and probed with serially diluted serum from mice infected by either needle inoculum or ticks.
Reduction of Borrelia burgdorferi Persistence in Murine Tissues and Acquisition in Ticks via Active Immunization of Mice With BBI36, BBI39, or Their Combination
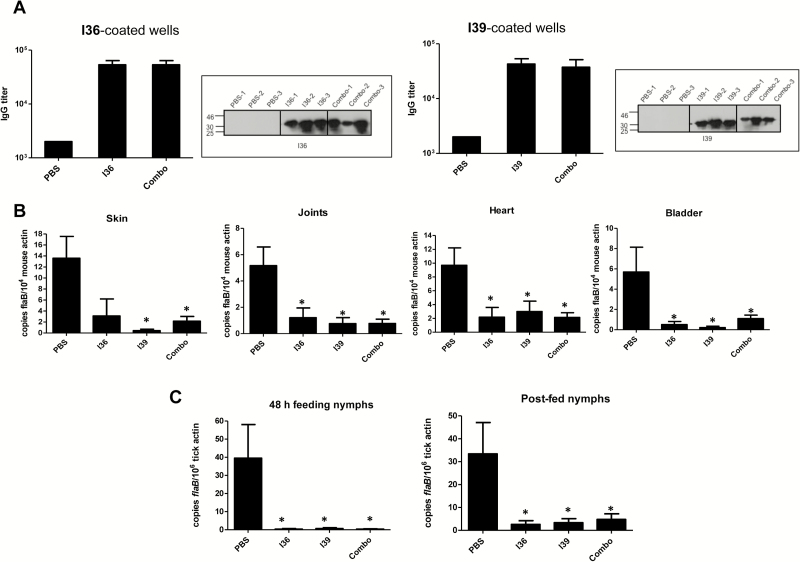
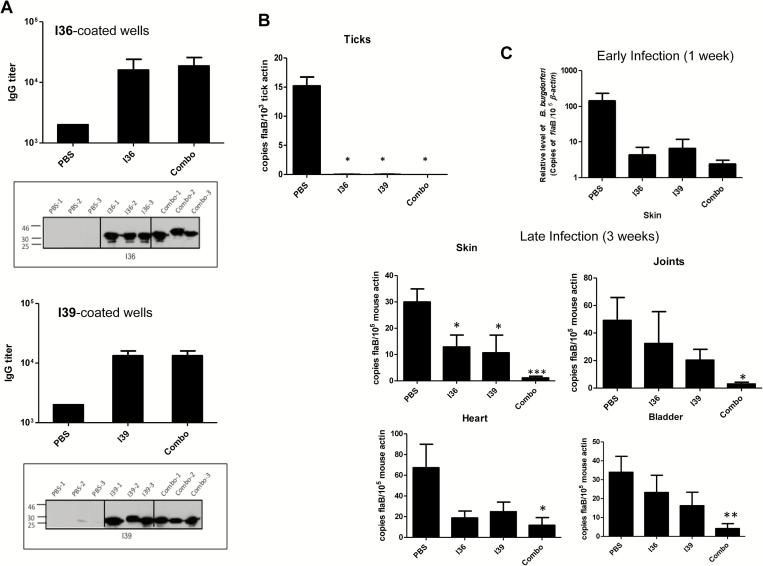
Next we sought to determine whether BBI39 immunization induces protective immunity, interfering with B. burgdorferi infectivity in mice and in ticks. Because BBI36 and BBI39 are the most divergent paralog members of this family with overall 26% amino-acid divergence (Supplementary Figure 1), we separately assessed the effect of immunization using BBI36, BBI39, or a combination of both. To accomplish this, separate groups of C3H mice (n = 3 animals/group) were immunized with recombinant proteins or controls (PBS mixed with an equal volume of adjuvant). Enzyme-linked immunosorbent assay and immunoblot analysis using serum samples collected from immunized hosts confirmed that all mice had developed antibody responses (Figure 4A). Groups of mice were infected with the equal numbers of spirochetes (105 cells/animal) by needle inoculation, and after 12 days, naive nymphal ticks (10 ticks/mouse) were allowed to parasitize infected host. Samples were collected as partially fed ticks at 48 hours of feeding, as well as fully engorged nymphs, along with various murine tissues. The B. burgdorferi burden was determined using qRT-PCR. The results showed a significant reduction in pathogen levels in all tissues of mice (Figure 4B), as well as in ticks that had fed for 48 hours and fully engorged nymphs (Figure 4C) that had fed on animals vaccinated with BBI36, BBI39, or their combination, as compared with control mice.
Figure 4.
Immunization with BBI36, BBI39, or their combination impairs the ability of spirochete to persist in mice or in feeding vectors. Groups of C3H mice (n = 3 animals/group) were immunized with BBI36, BBI39, or a combination of both proteins (Combo) and then challenged with Borrelia burgdorferi by needle (105 spirochetes/mouse). In parallel, a group of mice was immunized with phosphate-buffered saline (PBS)–containing adjuvant and served as the control. After 12 days of infection, naive nymphs (n = 10 ticks/mouse) were allowed to feed on infected mice. A, Enzyme-linked immunosorbent assay (ELISA) showing development of high-titer serum antibodies in individual immunized mice. Ten days after final immunization, serum was collected and subjected to ELISA. The wells were coated with either recombinant BBI36 (left panel) or BBI39 (right panel) and probed with serially diluted serum collected from mice immunized with PBS, BBI36 or BBI39, or a combination of BBI36 and BBI39 (Combo). Similarly, BBI36 (left panel) or BBI39 (right panel) or combo proteins were also immunoblotted (as indicated by a box) with 1:1000 dilution of serum. B, Spirochete burden in mice. Animals were infected with B. burgdorferi for 12 days, as detailed in panel A. Total RNA was isolated from murine tissue samples, and flaB levels were measured using quantitative polymerase chain reaction (PCR), which were normalized with murine β-actin. Bars represent the mean ± SEM of 3 quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analyses derived from 3 animals that were independently processed. Spirochetes were either undetectable or persisted at levels significantly lower in all of the tissues of mice immunized with proteins compared with control mice (*P < .05). C, Borrelia burgdorferi burdens in ticks. Naive nymphal ticks were allowed to become engorged on B. burgdorferi–infected mice, as detailed in panel A. Spirochete levels were analyzed by qRT-PCR in ticks that fed 48 hours or fully engorged nymphs, and flaB levels were normalized against tick β-actin. Bars represent the mean ± SEM of 3 qRT-PCR analyses derived from 5 partially fed nymphs (for 48 hours) or 8 fully engorged nymphs that were independently processed. Borrelia burgdorferi burden was significantly less in ticks that fed on mice immunized with proteins compared with ticks that fed on PBS-immunized mice (*P < .05).
Reduction of Borrelia burgdorferi Transmission From Ticks to mice via Active Immunization of Mice With BBI36, BBI39, or Their Combination
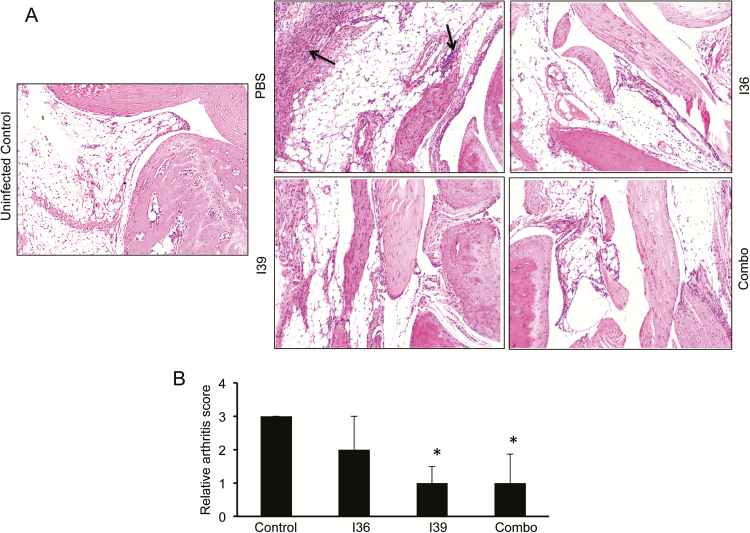
Because vaccination with BBI39 proteins reduced pathogen persistence when mice were inoculated by syringe injection, we next assessed whether similar protection also occurs during tick-transmitted infection. Separate groups of C3H mice (n = 3 animals/group) were immunized as detailed (Figure 4), and development of antibody response was confirmed (Figure 5A). Immunized mice were then challenged with B. burgdorferi–infected nymphs (n = 5 ticks/mouse). Spirochete burden was determined by qRT-PCR in fully engorged nymphs and in murine tissues after 1 week and 3 weeks of tick engorgement. A portion of murine heart and skin samples was also cultured in BSK media for reisolation of spirochetes. Results showed that unlike the control, BBI36 or BBI39 immunization decreases the level of B. burgdorferi in the ticks (Figure 5B) and in the host, either during early (1 week) or at late (3 weeks) infection, with the most dramatic effects recorded with use of the antigen combinations (Figure 5C). Culture data using a limited set of tissue samples tested until 4 weeks of inoculation also indicated the mice immunized with combination of both proteins were negative for the presence of spirochetes (Supplementary Table 2). Assessment of the disease in the host suggested that compared with controls, mice immunized with BBI39 or the combination of both proteins displayed less obvious histopathological signs of inflammation in joints (Figure 6). Collectively, these studies suggest that immunization with BBI39 proteins not only reduces pathogen levels in the hosts but also influences the outcome of disease.
Figure 5.
Immunization with BBI36, BBI39, or their combination interferes with spirochete transmission from infected ticks to naive rodent hosts. Mice (n = 3 animals/group) were immunized with either BBI36 or BBI39 or their combination (combo) or phosphate-buffered saline (PBS) with adjuvant (control). Ten days after final immunization, mice were bled to check development of specific antibodies and allowed to be parasitized by Borrelia burgdorferi–infected nymphs (n = 5 ticks/mouse). A, Enzyme-linked immunosorbent assay (ELISA) and immunoblot analysis showing high-titer serum antibodies induced in individual immunized mouse, as detailed in Figure 4A. B, Borrelia burgdorferi burden in fully engorged nymphs assessed by measuring copies of the B. burgdorferi flaB RNA and normalized against tick β-actin levels. Bars represent the mean ± SEM of 3 quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analyses derived from 3 independent infection experiments (*P < .05). C, Murine tissues were assessed for spirochete transmission during early (1 week) or late (3 weeks) infection. Total RNA was isolated from murine samples, and B. burgdorferi flaB was measured using qRT-PCR, normalized against corresponding murine β-actin levels. The animal studies were repeated 3 times, and the bars represent the mean measurements ± SEM of 3 experiments. Mice immunized with BBI36, BBI39, or their combination had significantly less B. burgdorferi burden (*P < .05) than control mice.
Figure 6.
Histopathological examination of the Borrelia burgdorferi–infected joints in vaccinated animals. Mice were immunized with recombinant BBI36 and BBI39 and then challenged with B. burgdorferi–infected nymphs. Joint samples were isolated after 21 days of infection and subjected to histological analysis. A, Cellular infiltration in the joint tissue is indicated by arrows (right panels). As a comparator, the joint of an uninfected mouse is also similarly processed for histology (left panel). B, Quantitative representation of the data shown in part A. At least 10 random sections from each group were scored for severity of arthritis in blinded fashion on a scale of 0–3.
Immunogenicity of BBI39 and Bacteriostatic Activity of Anti-BBI39 Antibodies
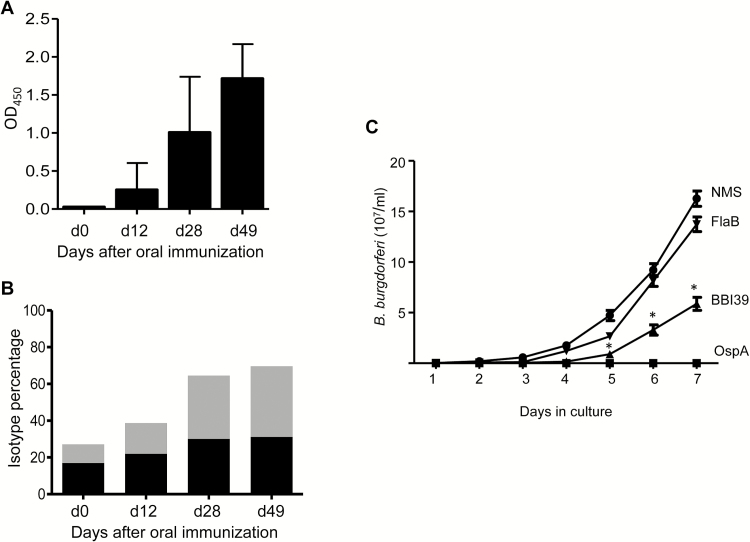
Immunization with recombinant BBI39 affected B. burgdorferi burden in fed ticks (Figures 4C and 5B), highlighting its potential utility as reservoir-targeted vaccines. Because these vaccine targets should induce high-titer antibody responses, especially when delivered orally, we next assessed murine serological responses when animals were immunized orally, such as with recombinant E. coli expressing BBI39. The results showed that BBI39-immunized mice, but not the control (empty vector–immunized mice), developed high levels of IgG1 and IgG2a antibodies in the serum that peaked within 28 days after immunization and remained elevated until day 49 when the animals were euthanized (Figure 7A and 7B).
Figure 7.
BBI39 is immunogenic by oral immunization and induces borreliacidal antibody responses. A, Serological response in mice immunized orally with recombinant Escherichia coli expressing BBI39. Mice were immunized orally with live recombinant E. coli expressing BBI39. Blood samples were collected on days 0, 12, 28, and 49, and antibody levels were measured by enzyme-linked immunosorbent assay (ELISA) using 1:100 serum dilutions. The average of triplicate readings per mouse/per group was determined, and the error bar indicates standard deviation. B, Immunoglobulin G (IgG) isotypes for orally immunized BBI39 antibodies. BBI39 antibodies measured by ELISA were subtyped into IgG1 (black bars) and IgG2a (gray bars). C, In vitro bacteriostatic activities of BBI39 antibodies. Spirochetes were incubated with normal mouse serum (NMS), OspA antibodies (positive control), FlaB antibodies (negative control), and BBI39 antibodies in the absence of active complement. The sensitivity of spirochetes to the bactericidal effects of the antibodies was assessed by a regrowth assay after 48 hours of antibody incubation. Growth of the spirochetes was determined by dark-field microscopy every 24 hours. BBI39 antisera showed significant difference in borreliacidal activities compared with that of normal mouse serum or FlaB antibodies (*P < .001).
Because BBI39 proteins are surface-exposed membrane proteins that could induce protective immunity, we next assessed the borreliacidal activities of the BBI39 antisera. Antibody generated against OspA was used as a positive control, and antibody against FlaB served as the negative control. Significant inhibition of spirochete growth was recorded when B. burgdorferi cells were exposed to antibodies generated against recombinant BBI39 in vaccination studies (Figures 4 and 5). The spirochete growth was either absent or slower in cultures exposed to OspA or BBI39 antibodies, as compared with cultures with FlaB antibodies or normal mouse sera (Figure 7C).
DISCUSSION
Lyme disease is considered a highly prevalent tick-borne illness [1, 36]. A vaccine for human Lyme disease is currently unavailable. Owing to the monophyletic nature of evolution of spirochetes [37], the biology and infectivity of spirochetes reflect distinctive features [38]. The genome also features notable redundancy, as highlighted by a significant number of paralogous gene clusters as well as unique genes [25, 27] that serve indispensable roles in pathogen survival [4, 5, 38, 39]. Here we report identification of a new class of vaccine candidates, selected members of pgf 54 [25], referred to herein as BBI39 paralogs that are mainly expressed in the vector, including a transient expression at the tick–host interface. These proteins are remarkably immunogenic and constitute a vaccine target to inhibit B. burgdorferi both in the host and in the vector.
The bbi39 paralog genes are predominantly expressed within ticks and remain downregulated in mammals. In agreement with our data, an environmental dependency of expression of bbi39 or related genes was also previously reported [29, 30, 40, 41, 42]. Although bbi39 paralog genes are retained in the same or different plasmids, it remains unknown whether they are part of a multicistronic operon or their expression is under the control of similar or different regulatory mechanisms [30, 42]. Even though bbi39 and bbj41 are 99% homologous, there is heterogeneity in the upstream regions, such as an inverted repeat sequence selectively present in the 5′ of bbi39 but absent in bbj41 [29]. Similarly, although bbi36 and bbi38 also display 99% homology, strikingly, the expression of the latter was undetectable in cultured spirochetes [42]. Nevertheless, our studies suggest that these antigens may have important roles in pathogen persistence in vivo. Although BBI39 proteins could support spirochete persistence in ticks, their roles in mammalian infection are likely to be limited during the early stages because bbi39 expression is evidenced at the tick-bite sites of the dermis but undetectable in distant skin or any of the internal organs tested. Such downregulation of BBI39 proteins in mammals might be necessary, because despite their transient expression, hosts develop robust antibody responses that have bacteriostatic properties that are sustained during chronic stages of the infection, as previously seen for other antigens like OspC [43].
Vaccination studies suggested that immunization with BBI39 paralog proteins induces host immunity, although the mechanism of protection remains unknown. Although bbi39 expression is downregulated within the first week of host infection, the bacterial levels in immunized hosts failed to recover, at least a few weeks after downregulation of the bbi39 paralogs. It is possible that BBI39 antibodies induce greater immune recognition of spirochetes in immunized hosts and help generate higher titers of antibodies against other Borrelia antigens, although immunoblot analysis using sera from previously immunized and challenged mice did not reveal notable differences (data not shown). Other than bacteriostatic properties of BBI39 antibodies, it is also possible that these antibodies bind to surface- exposed epitopes and thus block the interaction with other host or borrelial proteins, which may have a role in protective immunity. In fact, we show that BBI39 proteins interact with another vector-induced, borrelial surface protein, BBA52, which is critical for B. burgdorferi transmission [32]. Nevertheless, unlike most other antigens that can induce protective immunity by clearing spirochetes in the hosts or by targeting pathogens in the tick [5, 14, 38, 44], BBI39 immunization was able to interfere not only with pathogen transmission from ticks to mice but also with the establishment of infection in mammals. Notably, combination of the 2 proteins in the immunizing dose is more effective for blocking tick-borne transmission versus needle-borne infection, likely due to more natural regulation of both antigens and antibody accessibility in tick-transmitted pathogens, as compared with cultured spirochetes. Although BBI39 orthologs are conserved in diverse isolates and strains of Lyme disease pathogens (data not shown), whether BBI39 vaccination induces strain-specific or broader immunity against diverse strains warrants future investigation because their expression varies among infectious isolates, at least by cultured spirochetes, as also previously documented [31]. Nevertheless, multivalent vaccines derived from combinations of multiple antigens, including OspA, DbpA, OspC, and BBK32 antigens, have been shown to evoke more potent and broadly protective immunity against Lyme borreliosis as compared with single-component vaccines [9, 14]. Because BBI39 antibodies can target spirochetes both in the ticks and in the murine hosts, they are useful as novel components of transmission-blocking as well as reservoir-targeted vaccines against Lyme disease.
Supplementary Material
Notes
Acknowledgments. The authors thank John Anderson for his assistance with this study.
Financial support. This work was supported by funding from the National Institute of Allergy and Infectious Diseases (R01AI080615 to U. P.).
Potential conflicts of interest. M. G. S. is Founder of Immuno Technologies, Inc, the company recipient of the NIH SBIR grant which supported this work, grant number R44AI096551. M. G. S. is Co-Founder of US BIOLOGIC, Inc., which holds the license to commercialize a reservoir targeted vaccine against Lyme borreliosis using the oral delivery system described in this paper.
References
- 1. Mead PS. Epidemiology of Lyme disease. Infect Dis Clin North Am 2015; 29:187–210. [DOI] [PubMed] [Google Scholar]
- 2. Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis 2016; 16:556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rudenko N, Golovchenko M, Vancova M, Clark K, Grubhoffer L, Oliver JH., Jr Isolation of live Borrelia burgdorferi sensu lato spirochaetes from patients with undefined disorders and symptoms not typical for Lyme borreliosis. Clin Microbiol Infect 2016; 22:267.e9–15. [DOI] [PubMed] [Google Scholar]
- 4. Caimano MJ, Drecktrah D, Kung F, Samuels DS. Interaction of the Lyme disease spirochete with its tick vector. Cell Microbiol 2016; 18:919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pal U, Fikrig E. Tick interactions. In: Samuels DS, Radolf JD, eds. Borrelia, molecular biology, host interaction and pathogenesis. Norfolk, UK: Caister Academic Press, 2010:279–98. [Google Scholar]
- 6. Radolf JD, Salazar JC, Dattwyler RJ. Lyme disease in humans. In: Samuels DS, Radolf JD, eds. Borrelia, molecular biology, host interaction and pathogenesis. Norfolk, UK: Caister Academic Press, 2010:487–533. [Google Scholar]
- 7. Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest 2004; 113:1093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marques A. Chronic Lyme disease: a review. Infect Dis Clin North Am 2008; 22:341–60, vii–viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown EL, Kim JH, Reisenbichler ES, Höök M. Multicomponent Lyme vaccine: three is not a crowd. Vaccine 2005; 23:3687–96. [DOI] [PubMed] [Google Scholar]
- 10. de Silva AM, Telford SR, 3rd, Brunet LR, Barthold SW, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med 1996; 183:271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fikrig E, Barthold SW, Kantor FS, Flavell RA. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science 1990; 250:553–6. [DOI] [PubMed] [Google Scholar]
- 12. Hefty PS, Jolliff SE, Caimano MJ, Wikel SK, Akins DR. Changes in temporal and spatial patterns of outer surface lipoprotein expression generate population heterogeneity and antigenic diversity in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun 2002; 70:3468–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kung F, Kaur S, Smith AA, et al. A Borrelia burgdorferi surface-exposed transmembrane protein lacking detectable immune responses supports pathogen persistence and constitutes a vaccine target. J Infect Dis 2016; 213:1786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marconi RT, Earnhart CG. Lyme disease vaccines. In: Samuels DS, Radolf JD, eds. Borrelia, molecular biology, host interaction and pathogenesis. Norfolk, UK: Caister Academic Press, 2010:467–86. [Google Scholar]
- 15. Sigal LH, Zahradnik JM, Lavin P, et al. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. N Engl J Med 1998; 339:216–22. [DOI] [PubMed] [Google Scholar]
- 16. Yang X, Lenhart TR, Kariu T, Anguita J, Akins DR, Pal U. Characterization of unique regions of Borrelia burgdorferi surface-located membrane protein 1. Infect Immun 2010; 78:4477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang X, Qin J, Promnares K, Kariu T, Anderson JF, Pal U. Novel microbial virulence factor triggers murine lyme arthritis. J Infect Dis 2013; 207:907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar M, Kaur S, Kariu T, et al. Borrelia burgdorferi BBA52 is a potential target for transmission blocking Lyme disease vaccine. Vaccine 2011; 29:9012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanson MS, Cassatt DR, Guo BP, et al. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun 1998; 66:2143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hagman KE, Yang X, Wikel SK, et al. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect Immun 2000; 68:4759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pal U, Wang P, Bao F, et al. Borrelia burgdorferi basic membrane proteins A and B participate in the genesis of Lyme arthritis. J Exp Med 2008; 205:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiao S, Kumar M, Yang X, et al. A host-restricted viral vector for antigen-specific immunization against Lyme disease pathogen. Vaccine 2011; 29:5294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Labandeira-Rey M, Baker EA, Skare JT. VraA (BBI16) protein of Borrelia burgdorferi is a surface-exposed antigen with a repetitive motif that confers partial protection against experimental Lyme borreliosis. Infect Immun 2001; 69:1409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Šmit R, Postma MJ. Lyme borreliosis: reviewing potential vaccines, clinical aspects and health economics. Expert Rev Vaccines 2015; 14:1549–61. [DOI] [PubMed] [Google Scholar]
- 25. Casjens S, Palmer N, van Vugt R, et al. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol 2000; 35:490–516. [DOI] [PubMed] [Google Scholar]
- 26. Casjens SR, Mongodin EF, Qiu WG, et al. Genome stability of Lyme disease spirochetes: comparative genomics of Borrelia burgdorferi plasmids. PLoS One 2012; 7:e33280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fraser CM, Casjens S, Huang WM, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 1997; 390:580–6. [DOI] [PubMed] [Google Scholar]
- 28. Samuels DS. Gene regulation in Borrelia burgdorferi. Annu Rev Microbiol 2011; 65:479–99. [DOI] [PubMed] [Google Scholar]
- 29. Carroll JA, Cordova RM, Garon CF. Identification of 11 pH-regulated genes in Borrelia burgdorferi localizing to linear plasmids. Infect Immun 2000; 68:6677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ojaimi C, Brooks C, Casjens S, et al. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect Immun 2003; 71:1689–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Norris SJ. The dynamic proteome of Lyme disease Borrelia. Genome Biol 2006; 7:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kumar M, Yang X, Coleman AS, Pal U. BBA52 facilitates Borrelia burgdorferi transmission from feeding ticks to murine hosts. J Infect Dis 2010; 201:1084–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meirelles Richer L, Aroso M, Contente-Cuomo T, Ivanova L, Gomes-Solecki M. Reservoir targeted vaccine for lyme borreliosis induces a yearlong, neutralizing antibody response to OspA in white-footed mice. Clin Vaccine Immunol 2011; 18:1809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kariu T, Yang X, Marks CB, Zhang X, Pal U. Proteolysis of BB0323 results in two polypeptides that impact physiologic and infectious phenotypes in Borrelia burgdorferi. Mol Microbiol 2013; 88:510–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang X, Promnares K, Qin J, et al. Characterization of multiprotein complexes of the Borrelia burgdorferi outer membrane vesicles. J Proteome Res 2011; 10:4556–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Piesman J, Gern L. Lyme borreliosis in Europe and North America. Parasitology 2004; 129:S191–220. [DOI] [PubMed] [Google Scholar]
- 37. Paster BJ, Dewhirst FE, Weisburg WG, et al. Phylogenetic analysis of the spirochetes. J Bacteriol 1991; 173:6101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol 2012; 10:87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosa PA, Tilly K, Stewart PE. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat Rev Microbiol 2005; 3:129–43. [DOI] [PubMed] [Google Scholar]
- 40. Ojaimi C, Brooks C, Akins D, et al. Borrelia burgdorferi gene expression profiling with membrane-based arrays. Methods Enzymol 2002; 358:165–77. [DOI] [PubMed] [Google Scholar]
- 41. Brooks CS, Hefty PS, Jolliff SE, Akins DR. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect Immun 2003; 71:3371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tokarz R, Anderton JM, Katona LI, Benach JL. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect Immun 2004; 72:5419–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liang FT, Jacobs MB, Bowers LC, Philipp MT. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J Exp Med 2002; 195:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kung F, Anguita J, Pal U. Borrelia burgdorferi and tick proteins supporting pathogen persistence in the vector. Future Microbiol 2013; 8:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.