Abstract
Primary cilia sense environmental conditions, including osmolality, but whether cilia participate in the osmotic response in renal epithelial cells is not known. The transient receptor potential (TRP) channels TRPV4 and TRPM3 are osmoresponsive. TRPV4 localizes to cilia in certain cell types, while renal subcellular localization of TRPM3 is not known. We hypothesized that primary cilia are required for maximal activation of the osmotic response of renal epithelial cells and that ciliary TRPM3 and TRPV4 mediate that response. Ciliated [murine epithelial cells from the renal inner medullary collecting duct (mIMCD-3) and 176-5] and nonciliated (176-5Δ) renal cells expressed Trpv4 and Trpm3. Ciliary expression of TRPM3 was observed in mIMCD-3 and 176-5 cells and in wild-type mouse kidney tissue. TRPV4 was identified in cilia and apical membrane of mIMCD-3 cells by electrophysiology and in the cell body by immunofluorescence. Hyperosmolal stress at 500 mOsm/kg (via NaCl addition) induced the osmotic response genes betaine/GABA transporter (Bgt1) and aldose reductase (Akr1b3) in all ciliated cell lines. This induction was attenuated in nonciliated cells. A TRPV4 agonist abrogated Bgt1 and Akr1b3 induction in ciliated and nonciliated cells. A TRPM3 agonist attenuated Bgt1 and Akr1b3 induction in ciliated cells only. TRPM3 knockout attenuated Akr1b3 induction. Viability under osmotic stress was greater in ciliated than nonciliated cells. Akr1b3 induction was also less in nonciliated than ciliated cells when mannitol was used to induce hyperosmolal stress. These findings suggest that primary cilia are required for the maximal osmotic response in renal epithelial cells and that TRPM3 is involved in this mechanism. TRPV4 appears to modulate the osmotic response independent of cilia.
Keywords: osmoregulation, TRPM3, TRPV4, primary cilium
the osmolality of the inner medulla of the mammalian kidney is highly variable, owing to its role in the urinary concentrating mechanism, and cells in this region can be exposed to high levels of NaCl and urea. In an antidiuretic state, osmolality of the medullary interstitium and lumen of the inner medullary collecting duct (IMCD) can reach 1,200 mOsm/kg in humans and even higher levels (~2,500–3,000 mOsm/kg) in rodent species. Cells in this environment must adapt to withstand such hostile conditions. Adaptation occurs through accumulation of organic osmolytes to avoid osmotic water loss, upregulation of molecular chaperones to protect against protein denaturation, and activation of cellular stress response mechanisms. This process is directed at the transcriptional level by tonicity element-binding protein (TonEBP, also known as NFAT5) binding to an osmotic response element (ORE) in the promoter region of osmotic response genes. This action upregulates expression of proteins responsible for organic osmolyte synthesis [such as aldose reductase (Akr1b3)] or transport [such as betaine/GABA transporter (Bgt1), sodium/myo-inositol transporter, and taurine transporter], as well as numerous heat shock proteins, DNA damage response proteins, stress response proteins, and antiapoptotic mediators (5). Failure to properly activate this process may lead to cell destruction through apoptosis or crenation. Osmosensation is critical to activate the osmotic response, but the proximate biochemical sensors of osmolality, particularly those directly employed by renal epithelial cells in the inner medulla, have not been expressly identified or described.
Primary cilia serve as sensory organelles of the cell; they detect mechanical and chemical stimuli in the cell’s surrounding environment and transduce these stimuli to a myriad of signaling pathways influencing cellular differentiation, proliferation, survival, and migration (3, 33, 37). One chemical stimulus detected by primary cilia is osmolality. This was shown in the nematode Caenorhabditis elegans, as disruption of either sensory cilia or OSM-9 [an ion channel that is homologous to the transient receptor potential (TRP) channel vanilloid subfamily (TRPV) in mammals] confers defects in olfaction, mechanosensation, and avoidance of hyperosmolal media (22).
Two osmosensitive TRP channels have been identified in the mammalian kidney. TRPV4 responds to changes in osmolality, and it has been reported that TRPV4 is expressed throughout the nephron, but primarily in the distal nephron and on the apical cell surface (34). TRPV4 is activated by hyposmolality and inhibited by hyperosmolality (16). A role for TRPV4 in osmotic regulation is supported by studies reporting abnormal peripheral osmotic regulation in Trpv4−/− mice, likely due to TRPV4 deficiency in sensory neurons that detect physiologically relevant changes in blood osmolality (26). Hypotonicity has been shown to stimulate endogenous TRPV4 in M-1 murine renal cortical collecting duct cells (48), and its position in the nephron suggests a role in nephrogenic osmosensation. Response to hypotonicity by TRPV4 has also been demonstrated in cholangiocytes, where it localizes to primary cilia (17). TRPV4 associates with TRPP2 (polycystin-2) in renal primary cilia and contributes to mechanosensitivity in an exogenous expression system (24). A channel that depends on TRPV4 and TRPP2 is expressed in the collecting duct of mouse kidney (54). TRP melastatin-3 (TRPM3) is also osmosensitive and, like TRPV4, is activated by hyposmolality and inhibited by hyperosmolality (32). TRPM3 is expressed in collecting duct epithelium in the medulla, medullary rays, and periglomerular regions (18). Less is known regarding the subcellular localization of TRPM3, and its expression on renal primary cilia has not been determined.
In the present study we tested two hypotheses: 1) primary cilia participate in nephrogenic osmosensation through the functions of the osmoresponsive TRPV4 and TRPM3 channels and 2) cilia are required for a maximal and appropriate osmotic response in renal epithelial cells.
MATERIALS AND METHODS
Materials.
All reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Cell lines.
An immortalized renal epithelial cell line with a conditional allele of Kif3atm2Gsn (hereafter referred to as 176-5 cells) was generated from mice carrying both the H-2Kb-tsA58 transgene (ImmortoMouse) and the ubiquitously expressed tamoxifen-inducible CAGG-cre/Esr1/5Amc/J, as previously described (4, 51). These cells were generously provided by Dr. Bradley Yoder. In some experiments, these cells were cultured in the presence of tamoxifen to delete Kif3a and cause abrogated cilium formation (hereafter referred to as 176-5Δ cells). The 176-5 and 176-5Δ cells were cultured in phenol red-free Dulbecco’s modified Eagle’s medium (DMEM)/F12 (catalog no. 11330, Thermo Fisher Scientific, Waltham, MA) containing 10% FBS (Atlanta Biologicals, Norcross, GA), 100 U/ml penicillin, 100 µg/ml streptomycin (Thermo Fisher Scientific), ITS liquid medium supplement, 1.3 μg/l 3,3′,5-triiodothyronine, and 200 ng/ml dexamethasone. Cells were cultured at 33°C in the presence of 20 U/ml interferon-γ (IFNγ; Thermo Fisher Scientific) to promote expression of the temperature-sensitive SV40 large-T antigen and maintain immortalization. Cells were cultured at 37°C in the absence of IFNγ while FBS concentration was reduced to 5% to promote differentiation.
Oak Ridge polycystic kidney (Orpk) cilia(+) and cilia(−) cell lines (a generous gift of Dr. P. Darwin Bell) were generated as described previously (39, 51). Cells were cultured under permissive conditions for SV40 large-T antigen expression (33°C, 10 U/ml IFNγ) in defined collecting duct medium [DMEM/F12 (catalog no. 11330, Thermo Fisher Scientific), 10% FBS (Atlanta Biologicals), 100 U/ml penicillin, 100 µg/ml streptomycin (Thermo Fisher Scientific), 1.3 μg/l sodium selenite, 1.3 μg/l 3,3′,5-triiodothyronine, 5 mg/l insulin, 5 mg/l transferrin, 2.5 mM glutamine, and 5 μM dexamethasone] and cultured at 37°C in the absence of IFNγ to promote differentiation.
Murine epithelial cells from the renal IMCD (mIMCD-3 cells; catalog no. CRL-2123, American Type Culture Collection, Manassas, VA) (35) were cultured in DMEM/F12 [catalog nos. MT10092CV (for electrophysiology) and 11330 (for all other experiments), Thermo Fisher Scientific] with 10% FBS (Thermo Fisher Scientific), 100 U/ml penicillin, and 100 µg/ml streptomycin (Thermo Fisher Scientific) at 37°C.
The osmolality of DMEM/F12 base medium with the indicated supplements is 300 mOsm/kg, as measured previously in our laboratory (9) and by others using this medium (12, 52).
No tests for mycoplasmal contamination were performed.
CRISPR/Cas9 knockout of TRPM3.
To confirm that we were manipulating the effects of a TRPM3-dependent channel, we generated a clonal cell line in which TRPM3 expression was knocked out by CRISPR/Cas9 genome editing of the Trpm3 gene. A CRISPR plasmid with constitutive green fluorescent protein (GFP) expression and containing the guide RNA sequence CTTCTCATCTCCGTGCACGG was made by the Cincinnati Children’s Hospital Medical Center (CCHMC) Transgenic Animal Genome Editing Core Facility. The guide RNA sequence was selected using algorithms in Benchling.com for on-target (13, 14) and off-target (21) sites. This sequence is present in an early exon (exon 4) common to all six protein-coding forms of Trpm3 in the Ensembl database (8). We used exon 4, because two forms (Trpm3-204 and Trpm3-205) use an alternative translation start site (ATG) at the end of exon 3. We used Lipofectamine 3000 (Thermo Fisher Scientific) to transfect mIMCD-3 cells with this plasmid. At 2 days after transfection, single GFP-positive cells were sorted into separate wells for expansion (FACSAria II, BD Biosciences, San Jose, CA, located at the CCHMC Research Flow Cytometry Core). After expansion, genomic DNA extracted from the clones (GeneJET Genomic DNA Purification Kit, catalog no. K0721, Thermo Fisher Scientific) was used in a polymerase chain reaction (PCR; Phusion Hot Start II DNA polymerase, catalog no. F549, Thermo Fisher Scientific) to amplify the targeted region. The resulting PCR products were screened for loss of the Alw44I restriction site. PCR products of restriction site mutants were purified (GeneJet PCR Purification Kit, Thermo Fisher Scientific) and sequenced (CCHMC DNA Sequencing and Genotyping Core). We selected a clonal line that had, in both alleles, a frame-shifting mutation that resulted in an early (exon 4) stop codon. Potential off-target sites were identified and scored using an algorithm provided by the Zhang laboratory (http://crispr.mit.edu/) (21). Scores were ≤0.7 for all off-target sites, which indicates a low chance of mutation by the guide RNA. The four top-scoring sites with a standard PAM (protospacer adjacent motif) sequence were amplified via PCR with genomic DNA and sequenced (CCHMC DNA Sequencing and Genotyping Core and GENEWIZ, South Plainfield, NJ) to confirm that they were unchanged in the TRPM3 knockout (KO) cell line.
Immunofluorescence.
The 176-5, 176-5Δ, and mIMCD-3 cells were grown on coverslips, washed with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA), and permeabilized/blocked with SuperBlock (Thermo Fisher Scientific) plus 0.2% Triton X-100. The cells were then incubated with rabbit polyclonal anti-TRPM3 (catalog no. ab63016, Abcam, Cambridge, MA) or rabbit polyclonal anti-TRPV4 (catalog no. ab39260, Abcam), diluted 1:200 in blocking buffer, overnight at 4°C. Then the cells were washed with PBS and incubated with mouse monoclonal anti-acetylated α-tubulin (catalog no. T6793, Sigma-Aldrich), diluted 1:5,000 in blocking buffer, for 1 h at room temperature. The cells were washed again with PBS and then incubated with chicken anti-mouse Alexa Fluor 488 and chicken anti-rabbit Alexa Fluor 594 (Thermo Fisher Scientific), diluted 1:500 in blocking buffer, for 30 min at room temperature. The cells were washed with PBS and mounted on slides with ProLong Gold with 4′,6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific).
Kidneys were removed from wild-type (WT) C57BL/6 mice in accordance with the policies and procedures stipulated by the Institutional Animal Care and Use Committee as described in our approved protocol (no. 2013-0178) and acclimated in OCT embedding compound (Electron Microscopy Sciences) with two 5-min washes. Kidneys were snap-frozen in cryomolds (made with aluminum foil) using liquid nitrogen-chilled isopentane. Frozen samples were stored at −80°C until they were cryosectioned (model HM 550, Thermo Fisher Scientific) at −15°C, acclimated to cryostat temperatures for 30 min, and then sectioned at 10 µm for immunofluorescence staining. Sections were melted onto room-temperature polylysine-coated slides (Erie Scientific, Portsmouth, NH).
Slides containing sectioned mouse kidney were blocked for 1 h in 10% normal goat serum with sodium azide (Thermo Fisher Scientific) containing 1% bovine serum albumin, 0.2% Triton X-100, and 0.05% Tween 20 (Thermo Fisher Scientific). Primary antibodies were applied sequentially. Rabbit polyclonal anti-TRPM3 (catalog no. ab63016, Abcam; 1:300 dilution) was applied overnight at 4°C. The second primary antibodies [mouse polyclonal anti-γ-tubulin (catalog no. ab11317, Abcam; 1:500 dilution) or mouse monoclonal anti-acetylated α-tubulin (catalog no. T6793, Sigma-Aldrich; 1:1,000 dilution)] were applied for 1 h at room temperature. The secondary antibodies, Alexa Fluor 594-conjugated goat anti-rabbit IgG and either Alexa Fluor 488-conjugated goat anti-rabbit IgG or Alexa Fluor 488-conjugated goat anti-mouse IgG (all from Jackson ImmunoResearch, West Grove, PA), diluted 1:500, were applied for 1 h at room temperature. Slides were then mounted with ProLong Gold with DAPI mounting medium (Thermo Fisher Scientific).
Images were obtained using a Zeiss LSM710 inverted confocal microscope with a ×63 oil immersion objective (numerical aperture 1.4) with band-pass filters of 493–630 and 638–747 nm for the Alexa 488 and Alexa 594 fluorochromes, respectively, or a Nikon A1 inverted confocal microscope with a ×60 water immersion objective (numerical aperture 1.2) with band-pass filters of 425–475, 500–550, and 570–620 nm for the DAPI, Alexa 488, and Alexa 594 fluorochromes, respectively. Each channel was acquired sequentially to prevent signal overlap. Images were analyzed and three-dimensional reconstructions were prepared using Imaris Scientific 3D/4D Image Analysis software (Bitplane, Zürich, Switzerland).
RT-quantitative PCR.
After reaching confluence, cultures were maintained for 5–7 days to increase ciliation. For some experiments [mIMCD-3 WT vs. TRPM3 KO (see Fig. 10) and 176-5 and 176-5Δ (see Figs. 1B, Fig. 5B, and Fig. 9, A and B) cells], FBS was reduced from 10% to 5% after confluence to further increase ciliation. Then 176-5, 176-5Δ, Orpk cilia(+), Orpk cilia(−), WT mIMCD-3, and TRPM3 KO mIMCD-3 cells were exposed to hyperosmolality at 500 mOsm/kg (by supplementation of culture medium with NaCl to raise osmolality an additional 200 mOsm/kg) or maintained at basal osmolality for 16 h. The 176-5 and 176-5Δ cells were additionally treated with a TRPV4 agonist (GSK1016790A, 100 nM), a TRPM3 agonist (pregnenolone sulfate sodium salt, 100 µM), or vehicle control (0.1% DMSO) under isosmolal and hyperosmolal conditions. WT mIMCD-3 and TRPM3 KO mIMCD-3 cells were additionally treated with pregnenolone sulfate sodium salt (100 µM) or vehicle control (0.1% DMSO) under isosmolal and hyperosmolal conditions. In a separate experiment, Orpk cilia(+) and cilia(−) cells were exposed to hyperosmolality at 500 mOsm/kg (by supplementation of culture medium with mannitol to raise osmolality by 200 mOsm/kg) or maintained at basal osmolality for 16 h.
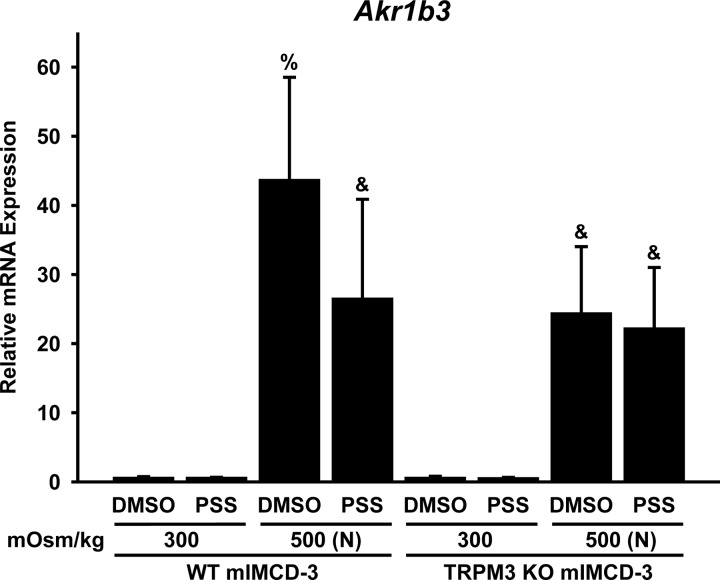
Fig. 10.
Hyperosmolality-induced expression of Akr1b3 (aldose reductase) mRNA is affected by activation or expression of TRPM3. mIMCD-3 cells in which Trpm3 was deleted by CRISPR/Cas9 genome editing [TRPM3 knockout (KO)], as well as wild-type (WT) mIMCD-3 cells, were treated for 16 h with 0.1% DMSO or a TRPM3 agonist [100 μM pregnenolone sulfate (PSS)] at 300 mOsm/kg or 500 mOsm/kg [adjusted with NaCl (N)]. RT-qPCR was performed to determine mRNA expression relative to the reference gene B2m. Hyperosmolal stress induced Akr1b3 expression in both cell lines, but the response was greater in WT than TRPM3 KO cells. The TRPM3 agonist attenuated hyperosmolality-induced Akr1b3 expression in WT, but not TRPM3 KO, cells. %Significantly different (P < 0.05, by Student-Newman-Keuls method) from all other conditions. &Significantly different from all conditions except each other. No other comparisons were significantly different. n = 5 experiments.
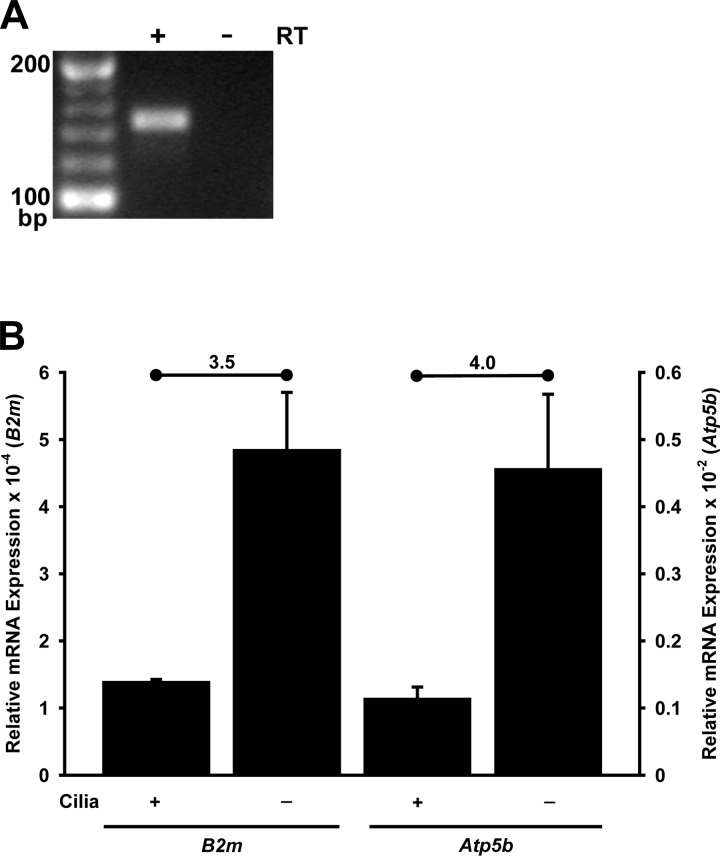
Fig. 1.
Transient receptor potential (TRP) melastatin-3 (Trpm3) mRNA expression in renal cells. A: Trpm3 mRNA is detected by RT-PCR in a murine inner medullary collecting duct (mIMCD-3) RNA sample treated with reverse transcriptase (RT), but not in an untreated sample. PCR products were subjected to electrophoresis on a 4% agarose gel stained with ethidium bromide. Expected size of the Trpm3 quantitative PCR (qPCR) product is 145 bp. Trpm3 mRNA was also detected in RT reactions from 3 additional, independent mIMCD-3 cultures (data not shown). B: as measured by RT-qPCR, expression of Trpm3 mRNA was significantly less (P < 0.008, t-test) in ciliated (176-5) than nonciliated (176-5Δ) renal cells, regardless of which reference gene was used: β2-microglobulin (B2m) or ATP synthase subunit β (Atp5b). Fold change (nonciliated/ciliated) is noted above each pair of conditions; average change was 3.8-fold. For B, n = 3 experiments.
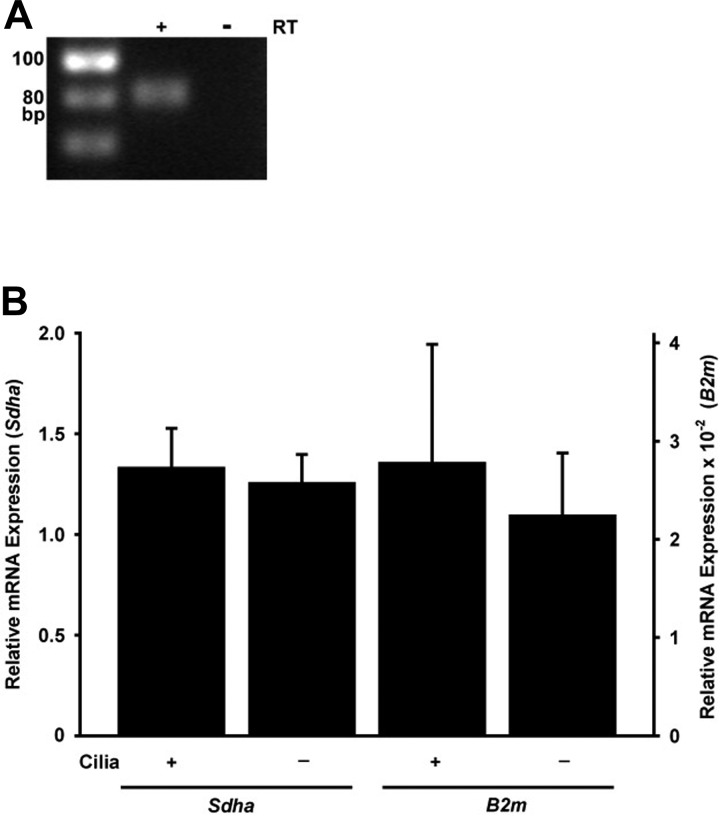
Fig. 5.
Trpv4 mRNA expression in renal cells. A: Trpv4 mRNA was detected by RT-PCR in a mIMCD-3 RNA sample treated with reverse transcriptase (RT) but not in an untreated sample. PCR products were subjected to electrophoresis on a 4% agarose gel stained with ethidium bromide. The 79-bp PCR product migrated a bit more slowly than the 80-bp marker, but its identity was confirmed with sequencing. Trpv4 mRNA was also detected in RT reactions from 3 additional, independent mIMCD-3 cultures (data not shown). B: as measured by RT-qPCR, expression of Trpv4 mRNA was similar in ciliated (176-5) and nonciliated (176-5Δ) renal cells, regardless of which reference gene was used: succinate dehydrogenase complex flavoprotein subunit A (Sdha) or B2m (P > 0.5, t-test). For B, n = 3 experiments.
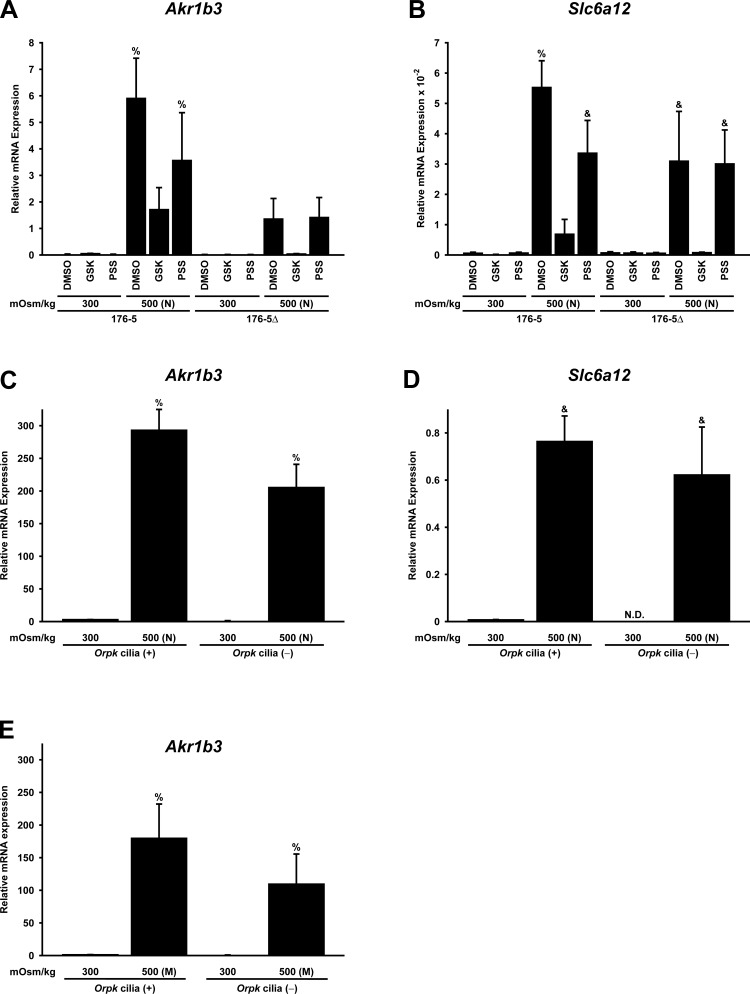
Fig. 9.
Hyperosmolality-induced expression of Akr1b3 (aldose reductase) and Slc6a12 [betaine/GABA transporter (Bgt1)] mRNA is affected by the presence of cilia and by stimulation of TRPV4 or TRPM3. A and B: ciliated (176-5) and nonciliated (176-5Δ) renal epithelial cells were treated for 16 h with 0.1% DMSO, a TRPV4 agonist [100 nM GSK1016790A (GSK)], or a TRPM3 agonist [100 μM pregnenolone sulfate (PSS)] at 300 mOsm/kg or 500 mOsm/kg [adjusted with NaCl (N)]. RT-qPCR was performed to determine mRNA expression relative to the reference gene B2m. Hyperosmolal stress induced Akr1b3 and Slc6a12 expression in both cell lines, but the response was greater in ciliated than nonciliated cells. The TRPV4 agonist abrogated hyperosmolality-induced Akr1b3 and Slc6a12 expression in both cell lines (although abrogation of Akr1b3 was not significant for the nonciliated cells). The TRPM3 agonist attenuated hyperosmolality-induced Akr1b3 and Slc6a12 expression only in ciliated cells. C and D: to examine a second cell line, expression of Akr1b3 and Slc6a12 was measured in Orpk cilia(+) and cilia(−) renal epithelial cells exposed to isosmolal (300 mOsm/kg) and hyperosmolal [500 mOsm/kg; adjusted with NaCl (N)] conditions. Hyperosmolality-induced expression of Akr1b3 was enhanced by the presence of cilia. A trend toward increased hyperosmolality-induced Slc6a12 expression was observed in Orpk cilia(+) cells compared with cilia(−) cells, but the increase did not reach statistical significance. Expression is relative to the reference gene Sdha. ND, not detectable. E: to examine another osmolyte, expression of Akr1b3 was measured in Orpk cilia(+) and cilia(−) renal epithelial cells with osmolality increased to 500 mOsm/kg by addition of mannitol (M), rather than NaCl. Mannitol-induced expression of Akr1b3 was enhanced by the presence of cilia. Expression is relative to the reference gene Sdha. %Significantly different (P < 0.05, by Student-Newman-Keuls method) from all other conditions. &Significantly different from all conditions except each other. No other comparisons were significantly different. n = 3 (A, B, and E) and 4 (C and D) experiments.
Total RNA was extracted from these cells using the RNeasy Mini Kit (Qiagen, Valencia, CA), treated with RNase-free DNase I (Thermo Fisher Scientific), and reverse-transcribed to cDNA with random hexamers (Maxima reverse transcriptase, Thermo Fisher Scientific). For reverse transcription (RT), DNase-treated RNA was used at a final concentration of 0.03 µg/µl. The amount of RNA in the RT reaction was within the linear range of the efficiency of the RT. The RT reaction product was then diluted 25-fold in water. For quantitative PCR (qPCR), each 15-µl reaction contained diluted RT reaction product as follows: 3.63 µl for betaine/GABA transporter (Slc6a12) and reference gene for 176-5/176-5Δ cells, 4 µl for Trpm3 and reference genes for 176-5/176-5Δ and mIMCD-3 cells, and 0.66 µl for all other reactions. qPCR was done with HotStart-IT SYBR Green qPCR Master Mix (catalog no. 75762, Affymetrix, Santa Clara, CA) on a StepOnePlus thermal cycler (Thermo Fisher Scientific) with an annealing temperature of 64°C. Primer pairs were selected using Primer-BLAST (National Center for Biotechnology Information, Database: Mus musculus refseq_rna). Primers were designed to cross at least one intron, to amplify several protein-coding variants, and to be specific for the gene of interest (Table 1). Using LinRegPCR software (36), we determined the starting amount of mRNA (N0, in arbitrary fluorescence units) for each qPCR per well in a multiwell plate. The efficiency of the PCR amplification was calculated for each well, and an average efficiency for a given primer pair was calculated for each plate. (Rare efficiencies that varied from the median by >2.5% were excluded from the calculation of the mean efficiency.) This mean efficiency was taken into account when the starting amount of mRNA (N0) was calculated. Relative expression of mRNA (the y-axis on all RT-qPCR graphs) was calculated by dividing the N0 of the target mRNA by the N0 of the reference mRNA. For experiments with more than two conditions, the data were examined with a one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls (SNK; Sigma Plot, Systat software, San Jose, CA) all-pairwise multiple comparison procedure. For experiments with two conditions, Student’s t-test was used. P < 0.05 was considered significant. Three to five independent cultures were analyzed per experiment. Two to three wells per condition were used as technical replicates. The reference genes [β2-microglobulin (B2m), succinate dehydrogenase complex flavoprotein subunit A (Sdha), and ATP synthase subunit β (Atp5b) for 176-5/176-5Δ cells, Sdha for Orpk cells, and B2m for mIMCD-3 cells] were selected, because we found that equal amounts of RNA from several samples of a given cell line yielded a similar starting amount of mRNA (N0) for these genes. Moreover, Student’s t-test (for experiments with 2 conditions) and one-way ANOVA (for experiments with >2 conditions) with the N0 of the reference gene showed no significant differences for any of the conditions compared here, with one inconsequential exception. The Sdha N0 used in the ratio of aldose reductase (Akr1b3) N0 to Sdha N0 for Orpk cells (see Fig. 9C) showed slight significant differences under the conditions used for the second and third bars (P = 0.046, SNK method) and also the third and fourth bars (P = 0.041). These small differences do not meaningfully affect the large difference in Akr1b3 N0 for these same comparisons (P < 0.001, SNK method). As negative controls, we used samples processed without RT or reactions with water in place of cDNA. Melt curves were used to confirm that only the intended qPCR product was made. Sequencing confirmed the identity of the qPCR products. Error bars indicate SD. The PCR products for the expression of Trpm3 and Trpv4 in mIMCD-3 cells came from cultures that had been at isosmolal conditions with 0.1% DMSO for 16 h; they were subjected to electrophoresis on a 4% agarose gel stained with ethidium bromide.
Table 1.
qPCR primers
| P-C Variants |
|||||
|---|---|---|---|---|---|
| Gene | Product Size, bp | Primer Sequence (5′–3′) | NCBI No. | Recognized | Not recognized |
| Akr1b3 | 142 | TCTACTCAGCTACAACAGGAACTG | NM_009658.3 | 001 | 002 |
| GAAAGAACAGGTGCAAGCCA | |||||
| Atp5b | 140 | CCTTATTGGGCAGAATCCCTTC | NM_016774.3 | 001 | |
| GTCATCAGCAGGCACATAGATAG | |||||
| B2m | 211 | CACAGTTCCACCCGCCTCACA | NM_009735.3 | 001 | |
| TCTCGATCCCAGTAGACGGTCTTGG | |||||
| Slc6a12 | 150 | CAACCTGCACCAACTCGTGGAACA | NM_133661.3 | 001, 002, 007 | 003 |
| CGTGGATGCCCGATGTAATACCCAA | |||||
| Sdha | 75 | AAGAAGGCATCAGCTAAAGTTTCA | NM_023281.1 | 001 | |
| CACAGCATCAAATTCATGATCCAC | |||||
| Trpm3 | 145 | ATCCAGTCTGAGAAGTGGTCTATC | NM_177341.4 | 201–206 | |
| GCAAGAGATCAGGTTTCGTATCAA | |||||
| Trpv4 | 79 | GCTTCCTGCTTGTGTACCTGCTCT | NM_022017.3 | 001–002, 005–007 | |
| ATGTTGGTGCACGGATTCAGGAGG | |||||
Protein-coding (P-C) variants are noted with Ensembl nomenclature (8).
RT2 profiler PCR array.
As a control to compare the osmotic responses in cells cultured on plastic with those in cells cultured on a permeable membrane, mIMCD-3 cells were seeded onto tissue culture-treated plastic dishes or 24-mm Corning Transwell permeable tissue culture supports (polycarbonate, 0.4-µm pore size). After reaching confluence, cultures were maintained for 6 days with FBS concentration reduced to 2.5% to maximize ciliation. Cells were exposed to hyperosmolality at 500 mOsm/kg (by supplementation of culture medium with NaCl to raise osmolality an additional 200 mOsm/kg) or maintained at basal osmolality for 16 h. For cells cultured on permeable supports, the treatment was applied to the upper chamber only. Total RNA was extracted from these cells using the RNeasy Mini Kit (Qiagen), treated with RNase-free DNase I (Thermo Fisher Scientific), and reverse-transcribed to cDNA with random hexamers using the RT2 First Strand kit (Qiagen). For RT, DNase-treated RNA was used at a final concentration of 0.05 µg/µl. The 10-µl RT reaction product was then diluted by addition of 91 µl of water. For qPCR, we mixed 1,350 µl of 2× RT2 SYBR Green ROX qPCR Mastermix (catalog no. 330523, Qiagen, Hilden, Germany), 102 µl of RT product, and 1,248 µl of water to yield 2,700 µl of master mix. Twenty-five microliters of this master mix were added to each well in the Mouse Osmotic Stress RT2 Profiler PCR Array (PAMM-151Z, Qiagen), which evaluates expression of 84 osmotically regulated genes. qPCR was performed with a StepOnePlus thermal cycler with an annealing temperature of 64°C. Using LinRegPCR software (36), we determined the starting amount of mRNA (N0, in arbitrary fluorescence units) for each qPCR per well of the 96-well array plate, as described above. Relative expression of mRNA was calculated by dividing the N0 of the target mRNA by the N0 of the average of the five reference mRNAs included in the array [β-actin (Actb), B2m, Gapdh, β-glucuronidase (Gusb), and heat shock protein 90 alpha, class B, member 1 (Hsp90ab1)]. If the reactions did not pass quality-control checks or if targeted cDNA was not detected, data for those genes were excluded from analysis.
Western blotting.
After confluence for 1 wk with FBS concentration reduced to 5% to maximize ciliation, whole cell lysates of 176-5 and 176-5Δ cells maintained under isosmolal conditions were prepared by scraping into radioimmunoprecipitation assay lysis buffer plus protease and phosphatase inhibitors. Protein content was quantified using the bicinchoninic acid assay (Pierce BCA Kit, Thermo Fisher Scientific), and equal amounts of protein were separated by SDS-PAGE and transferred to PVDF membranes. Membranes were blocked with SuperBlock (Thermo Fisher Scientific) plus 0.05% Tween 20 (Thermo Fisher Scientific) and blotted with rabbit polyclonal anti-TRPV4 (1:5,000 dilution; catalog no. ab39260, Abcam) or rabbit polyclonal anti-GAPDH (1:50,000 dilution; Trevigen, Gaithersburg, MD) in blocking buffer overnight at 4°C. Membranes were washed with Tris-buffered saline plus 0.05% Tween 20 (TBST) and then blotted with horseradish peroxidase-conjugated donkey anti-rabbit IgG (1:30,000 dilution; GE Healthcare Life Sciences, Pittsburgh, PA) in blocking buffer for 1 h at room temperature. Membranes were again washed with TBST, resolved with chemiluminescence (Pierce ECL 2 Western Blotting Substrate, Thermo Fisher Scientific), and imaged with autoradiography film. Blot images were digitized using a gel imaging station (Alpha Innotech, San Leandro, CA), and the density of each band of interest was quantified with National Institutes of Health ImageJ 1.37a software. Densitometric analysis was performed by normalization of TRPV4 band density (nonglycoslyated form only) to GAPDH band density for each cell line in each experiment, and the TRPV4-to-GAPDH densitometric ratio was compared between 176-5 and 176-5Δ cells using a t-test (n = 5 experiments).
Electrophysiology.
As previously described (23), mIMCD-3 cells were grown on beads that were free to move in the recording chamber. Macroscopic currents due to TRPV4 channels were recorded by patch-clamp recording of excised, inside-out membrane patches from the apical membrane. In some cases, a single primary cilium was excised for recording, as described previously (23). To record from a cilium, suction was applied to a recording pipette, so that a single primary cilium entered the pipette. After a resistance of ≥1 GΩ formed between the membrane and the pipette, the cilium was excised from the cell. This left the cilium inside the recording pipette in the inside-out configuration. The pipette containing the cilium could then be transferred among different solutions that bathed the cytoplasmic face of the membrane. Recordings were made under voltage clamp at room temperature (24°C). During recording, the cells were stored in a standard extracellular Ringer solution containing (in mM) 140 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 2 sodium pyruvate, 5 HEPES, and 9.4 d-glucose, with pH adjusted to 7.4 with NaOH. The recording pipettes also contained this solution. After excision of the apical membrane patch or cilium, the solution used to bathe the cytoplasmic face of the excised cilium contained (in mM) 140 KCl, 5 NaCl, 0.76 CaCl2, 2 MgCl2, 5 HEPES, 2 BAPTA, and 5 d-glucose, with pH adjusted to 7.4 with KOH. Free Ca2+ concentration in this solution was 0.1 μM. As noted, we added the TRPV4 agonist GSK1016790A (46), the TRPV4 antagonist HC-067047 (Tocris Biosciences, Minneapolis, MN) (15), or the TRPM3 agonist CIM0216 (Tocris Biosciences) (19) to the cytoplasmic solution. In some experiments, the TRPM3 agonist pregnenolone sulfate was included in the pipette (external) solution; it is only effective from the outside (19, 45). All four of these reagents were diluted from stock solutions in DMSO (100 µM for GSK1016790A and HC-067047, 100 mM for pregnenolone sulfate, and 5 mM for CIM0216).
TonEBP reporter assay.
The 176-5 and 176-5Δ, as well as mIMCD-3, cells were cotransfected at a 20:1 ratio with the TonEBP reporter construct pSEAP2TonE (generous gift of Dr. Wolfgang Neuhofer) in which two tandem copies of the ORE drive expression of a secreted alkaline phosphatase and with pCDNA3.1 to provide a neomycin resistance gene (31). Cells harboring the reporter construct were then seeded into tissue culture-treated 96-well plates (Becton Dickinson, Franklin Lakes, NJ), and, after confluence for 1 wk with FBS concentration reduced to 5% to maximize ciliation, cells were exposed to hyperosmolality (500 mOsm/kg, achieved by supplementation of the culture medium with NaCl or mannitol to raise osmolality an additional 200 mOsm/kg) or maintained at basal osmolality (300 mOsm/kg) for 12 h. Cells were concurrently treated with a TRPV4 agonist (GSK1016790A), a TRPM3 agonist (pregnenolone sulfate sodium salt), or vehicle control (0.1% DMSO) under isosmolal and hyperosmolal conditions. mIMCD-3 cells were also treated concurrently for 12 h under hyperosmolal conditions with the TRPV4 antagonist HC-067047 and the TRPM3 antagonist isosakuranetin (Extrasynthese, Lyon, France), in combination with their respective agonists.
After 12 h of exposure to hyperosmolal or isosmolal conditions, the conditioned culture medium was removed and assayed for alkaline phosphatase activity by addition of p-nitrophenyl phosphate substrate (Thermo Fisher Scientific) and measurement of absorbance at 405 nm with a microplate reader (Synergy H4, BioTek, Winooski, VT). Background absorbance was subtracted from measurements, and absorbances were averaged from four wells for each treatment condition per experiment. Averaged absorbances for each treatment condition were normalized to that at 300 mOsm/kg with DMSO treatment for single-treatment experiments and 500 mOsm/kg with DMSO treatment for the agonist-plus-antagonist experiments. Experiments were repeated 10 times for 176-5 and 176-5Δ cells and six times for mIMCD-3 cells. Each treatment group was compared with control using Friedman’s test with Dunn’s correction for multiple comparisons with Prism 6 statistical software (GraphPad, La Jolla, CA).
Crystal violet cell quantitation assay.
The 176-5 and 176-5Δ, as well as Orpk cilia(+) and cilia(−), cells were seeded into tissue culture-treated 96-well plates (Becton Dickinson) at a density of 8 × 103 cells/well. After confluence for 1 wk, cells were exposed to hyperosmolal NaCl at 600 mOsm/kg (by supplementation of culture medium with NaCl to raise osmolality an additional 300 mOsm/kg) or maintained at basal osmolality for 24 h. At the completion of treatment, wells were washed twice with PBS and fixed in 4% paraformaldehyde (Electron Microscopy Sciences)-PBS for 10 min at room temperature. Wells were washed twice with distilled water and incubated in 0.1% crystal violet (Becton Dickinson)-water for 30 min at room temperature. After the wells were washed three more times, the stain was dissolved in 10% acetic acid, and the plates were agitated on a plate shaker until the solution was uniform in color. Absorbance was quantified at 540 nm. Background absorbance was measured in unstained cells from each group and subtracted from measurements. Absorbances from eight wells were averaged for each treatment condition per experiment, and average absorbance of the wells under hyperosmolal conditions (600 mOsm/kg) was normalized to the average of wells maintained at isosmolal conditions. Experiments with 176-5 and 176-5Δ cells were performed nine times, and experiments with Orpk cilia(+) and cilia(−) cells were performed four times. Each treatment group was compared with control using Friedman’s test with Dunn’s correction for multiple comparisons with Prism 6 statistical software (GraphPad).
RESULTS
TRPM3 expression in cilia of renal epithelial cells and tissue.
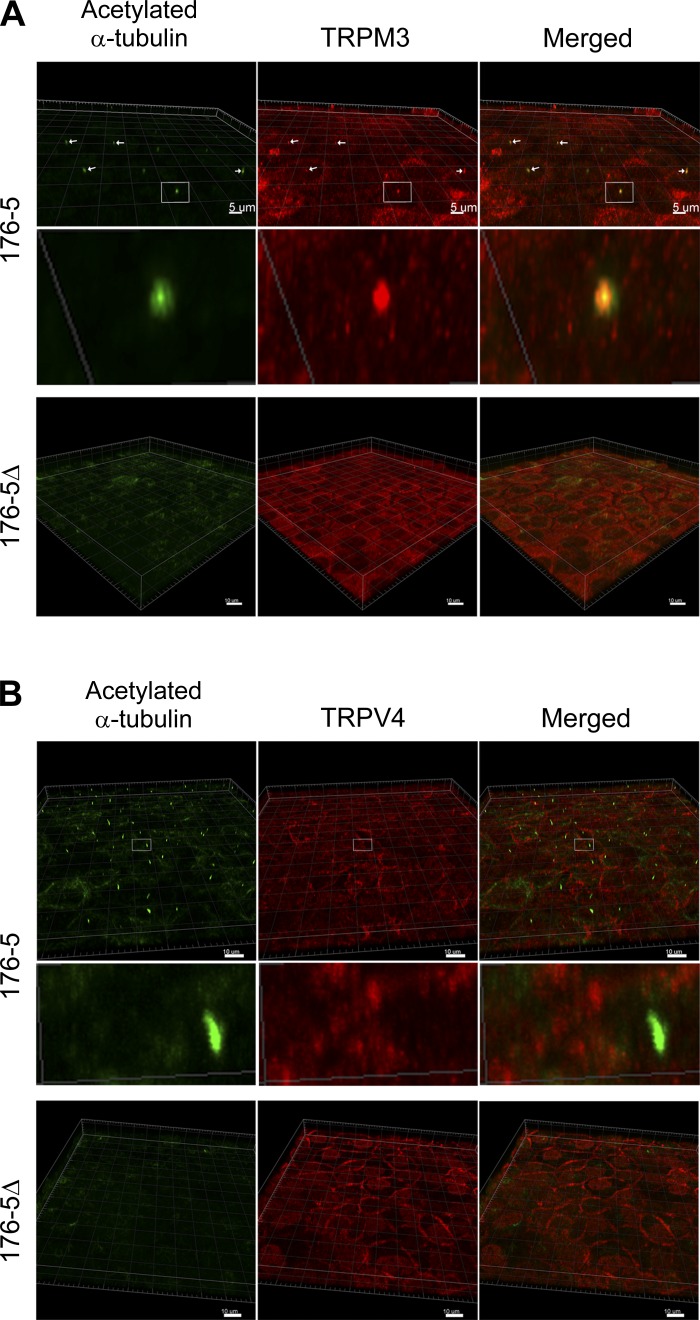
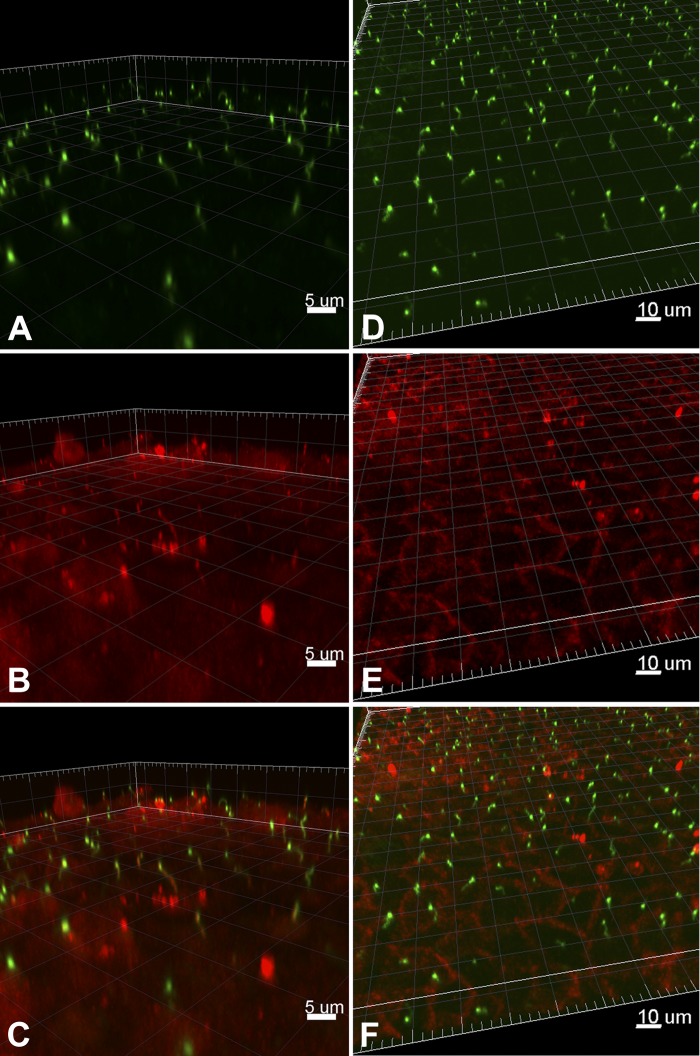
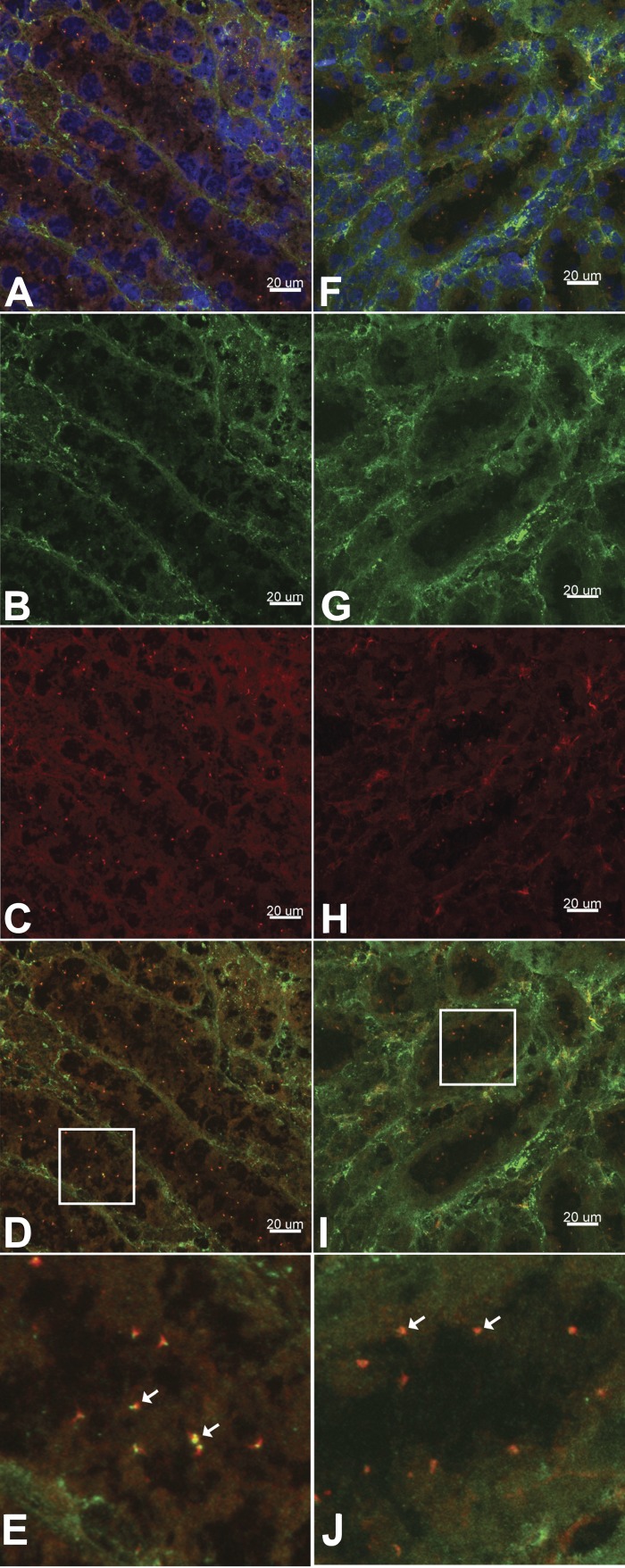
As the TRPM3 channel is responsive to changes in extracellular osmolality, we sought to characterize the expression and subcellular localization of TRPM3 in renal epithelial cells to explore its possible contribution to the nephrogenic osmotic response. Trpm3 mRNA expression was identified in mIMCD-3 cells (Fig. 1A), as well as in the 176-5 collecting duct cell line (Fig. 1B). Interestingly, RT-qPCR revealed that Trpm3 expression was elevated by an average of 3.8-fold in nonciliated 176-5Δ cells compared with the ciliated 176-5 cells (Fig. 1B; P < 0.008, Student’s t-test). Utilizing an antibody that has been previously described to reliably detect TRPM3 (7, 18), we observed TRPM3 coexpressed with acetylated α-tubulin, a marker for the primary cilium (27), in both 176–5 and mIMCD-3 cells (Fig. 2A and Fig. 3, A–C), indicating ciliary localization of this channel. We also examined TRPM3 expression in WT mouse kidney tissue and observed coexpression with acetylated α-tubulin and γ-tubulin (a marker of basal bodies), indicating ciliary expression of this channel (Fig. 4). With electrical recording, we were unable to observe ciliary currents with the single-channel conductance (18) and ionic selectivities (44) reported for TRPM3. These channels can be spontaneously active (18, 44), but we did not observe them. We also failed to detect them in the presence of the TRPM3 agonists pregnenolone sulfate (external, 100 µM; n = 57) and CIM0216 (cytoplasmic, 5 mM; n = 55).
Fig. 2.
TRPM3 and TRP vanilloid-4 (TRPV4) cellular localization in 176-5Δ and 176-5 cells. A: immunofluorescence was performed using primary antibodies directed against acetylated α-tubulin (green) and TRPM3 (red), and images were overlaid (Merged). Images were obtained using a Zeiss LSM710 confocal microscope and analyzed using Imaris Scientific 3D/4D Image Analysis software. Top: in 176-5 cells, arrows indicate the presence of cilia (left), ciliary TRPM3 (middle), and colocalization (right). Scale bars = 5 µm. Middle: magnified view of the area within the white box in top row. Bottom: 176-5Δ cells were devoid of primary cilia (green, left), and TRPM3 expression was observed diffusely throughout the cells (middle and right). Scale bars = 10 µm. B: immunofluorescence was performed using primary antibodies directed against acetylated α-tubulin (green) and TRPV4 (red), and images were overlaid (Merged). Images were obtained and analyzed as described in A. Top: primary cilia were observed in 176-5 cells (green, left); cell body (possibly plasma membrane) expression of TRPV4 expression was apparent (red, middle), but no ciliary expression of TRPV4 was observed (right). Middle: magnified view of the area within the white box in top row. Bottom: 176-5Δ cells were devoid of primary cilia (green, left); cell body expression of TRPV4 resembled that of 176-5 cells (middle and right). Scale bars = 10 µm.
Fig. 3.
TRPM3 (A–C) and TRPV4 (D–F) cellular localization in mIMCD-3 cells. A and B: immunofluorescence was performed using primary antibodies directed against acetylated α-tubulin (A, green) and TRPM3 (B, red). Images were obtained using a Nikon A1 inverted confocal microscope and analyzed using Imaris Scientific 3D/4D Image Analysis software. C: overlaid image of A and B demonstrates TRPM3 coexpression with acetylated α-tubulin (yellow), indicating localization on primary cilia. D and E: immunofluorescence was performed using primary antibodies directed against acetylated α-tubulin (D, green) and TRPV4 (E, red). Images were obtained and analyzed as described in A and B. F: overlaid image of D and E demonstrating no coexpression of TRPV4 with acetylated α-tubulin.
Fig. 4.
TRPM3 cellular localization in wild-type mouse kidney tissue. A–E: frozen tissue sections were subjected to immunofluorescence using primary antibodies directed against acetylated α-tubulin (green, A, B, D, and E) and TRPM3 (red, A, C, D, and E). Nuclei were labeled with DAPI (blue, A). TRPM3 coexpression with acetylated α-tubulin (yellow, D and E) indicates localization on primary cilia. F–J: frozen tissue sections were subjected to immunofluorescence using primary antibodies directed against γ-tubulin (green, F, G, I, and J) and TRPM3 (red, F, H, I, and J). Nuclei were labeled with DAPI (blue, F). TRPM3 coexpression with γ-tubulin (yellow, I and J) supports ciliary localization of the channel. Images were obtained using a Nikon A1 inverted confocal microscope and analyzed using Imaris Scientific 3D/4D Image Analysis software. E and J: magnified views of areas in white boxes in D and I, respectively. White arrows indicate coexpression.
TRPV4 expression and activity in cilia of renal epithelial cells.
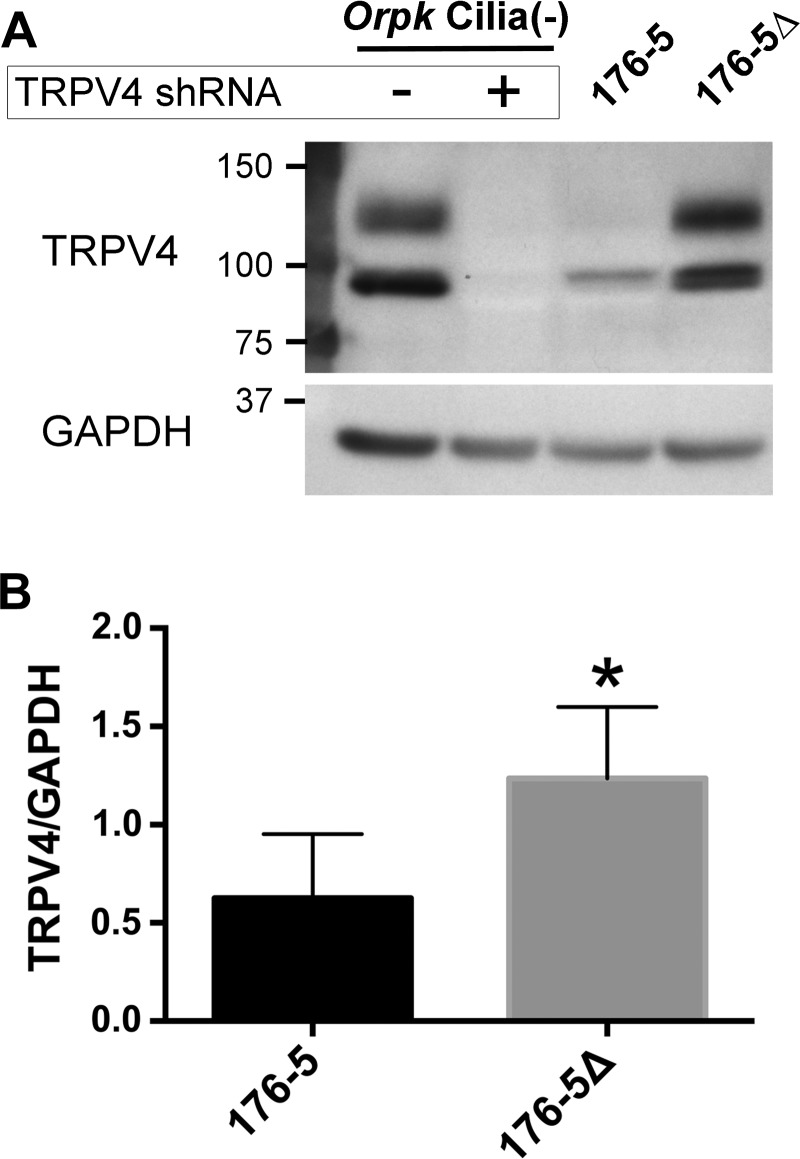
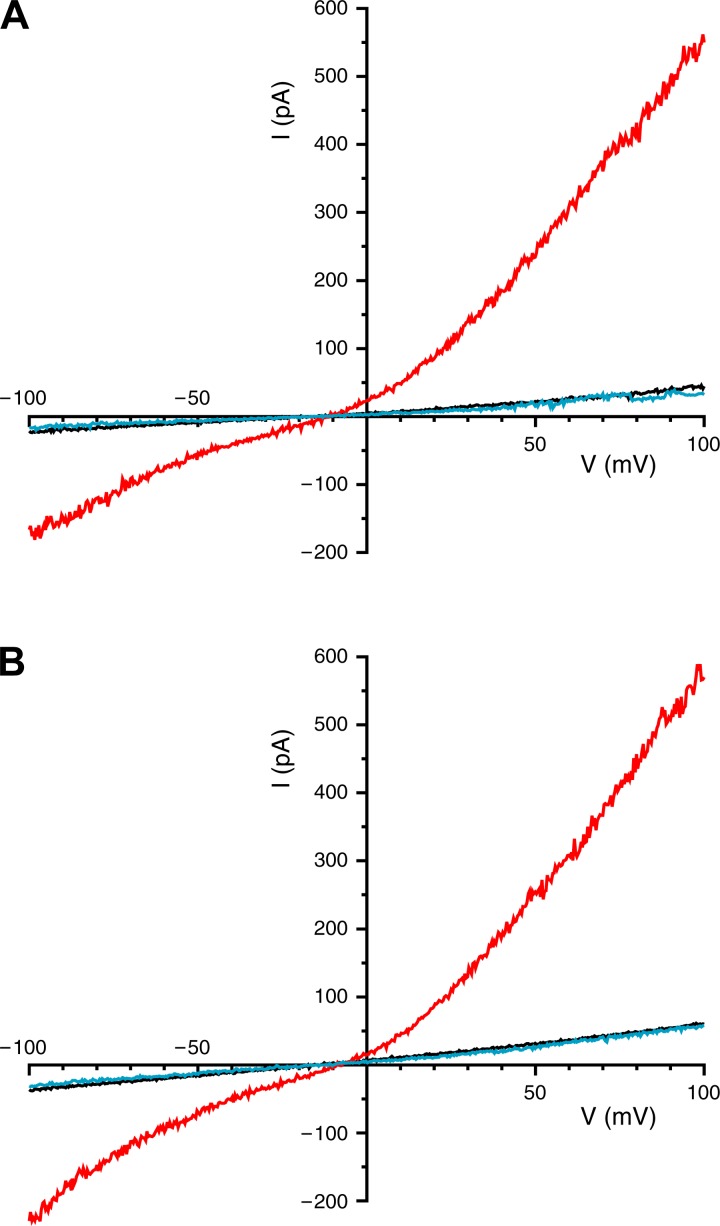
Trpv4 mRNA expression was identified in mIMCD-3 cells (Fig. 5A), as well as in the 176-5 collecting duct cell line (Fig. 5B). RT-qPCR revealed that Trpv4 mRNA expression in nonciliated 176-5Δ cells was not statistically different from that in ciliated 176-5 cells (Fig. 5B; P > 0.5, Student’s t-test). TRPV4 protein expression was detected by immunoblot analysis of whole cell lysates in 176-5 cells. Densitometric analysis revealed that TRPV4 expression was twofold higher in 176-5Δ than 176-5 cells (Fig. 6; P = 0.01, n = 5). In contrast to findings by Köttgen et al. (24) and Zhang et al. (54) in Madin-Darby canine kidney (MDCK) and Orpk collecting duct cells, respectively, we did not observe TRPV4 in the primary cilium in either mIMCD-3 or 176-5 cells by immunofluorescence, although we did observe expression in the cell body (Fig. 2B and Fig. 3, D–F). However, a macroscopic current consistent with TRPV4 was detected in mIMCD-3 cells, both in patches from the apical membrane (Fig. 7A) and in intact, excised primary cilia (Fig. 7B). The current was detected on addition of the specific TRPV4 agonist GSK1016790A (100 nM) (46) (Fig. 7, A and B, red traces). The current showed outward rectification that was relieved at very negative potentials, characteristic of TRPV4 in the presence of extracellular Ca2+ (43). Of 16 apical membrane patches tested, 15 showed this current, which averaged 401 ± 445 pA at +100 mV (range 16–1,581 pA). Each of seven cilia tested also showed the current, which averaged 280 ± 156 pA (range 136-510 pA). The mean reversal potential of the current was −9.3 ± 3.0 mV (n = 10) in apical membrane patches and −9.3 ± 4.1 mV (n = 7) in the cilia. Given the physiological solutions used (23) and the relative ionic permeabilities of TRPV4 (43), the predicted reversal potential was −4.6 mV. In the presence of the agonist, simultaneous addition of the specific TRPV4 antagonist HC-067047 (1 µM) (15) eliminated the current (Fig. 7, A and B, blue traces). The fraction of current remaining after addition of the antagonist was 0.05 ± 0.06 in the apical patches (n = 6) and 0.01 ± 0.01 in the cilia (n = 6).
Fig. 6.
TRPV4 protein expression in 176-5Δ and 176-5 cells. A: Western blot of TRPV4 expression in ciliated 176-5 and nonciliated 176-5Δ cells (lanes 3 and 4) and in Orpk cilia(−) cells with and without stable expression of a TRPV4-targeted shRNA construct (lanes 1 and 2) as an antibody control (54). A band for TRPV4 was observed just below 100 kDa, which is consistent with the predicted molecular weight (48). A higher-molecular-weight band was observed between 100 and 150 kDa, which is consistent with a glycosylated form of TRPV4 (49). Expression of both TRPV4 bands was eliminated in Orpk cilia(−) cells expressing the TRPV4-targeted construct. B: densitometric analysis of TRPV4 expression in 176-5 and 176-5Δ cells normalized to GAPDH levels. *P < 0.05. n = 5 experiments.
Fig. 7.
TRPV4-dependent currents (I) in an excised inside-out patch from the apical membrane (A) and in an excised single primary cilium (B), both from mIMCD-3 cells. Recordings were obtained under each of 3 conditions: control (black trace), in the presence of the TRPV4 agonist (GSK1016790A, 100 nM; red trace), and in the presence of both the agonist (100 nM) and a TRPV4 antagonist (HC-067047, 1 µM; blue trace). Reagents were applied to the cytoplasmic face of the membrane.
Cilia, TRPM3, and TRPV4 modulate the response of renal epithelial cells to osmotic stress.
We hypothesized that primary cilia function as osmosensors in the kidney by virtue of ciliary expression of osmosensitive TRP channels. As both TRPV4 and TRPM3 were identified in the primary cilia of renal epithelial cell models, the dependence of the osmotic stress response on the presence of primary cilia was assessed in two collecting duct cell lines. Both the cilium-deficient (176-5Δ) and cilium-expressing (176-5) cell lines were stably transfected with a TonEBP reporter construct. Exposure of cells to hyperosmolal conditions, achieved by increasing the osmolality of culture medium to 500 mOsm/kg with NaCl, produced a significant increase in TonEBP activity in the ciliated 176-5 cells only (Fig. 8A, DMSO). The mIMCD-3 cells were also transfected with the TonEBP reporter construct. Similarly, exposure of these cells, cultured under conditions known to promote formation of cilia, to hyperosmolal stress produced a significant increase in TonEBP activity (Fig. 8B, DMSO).
Fig. 8.
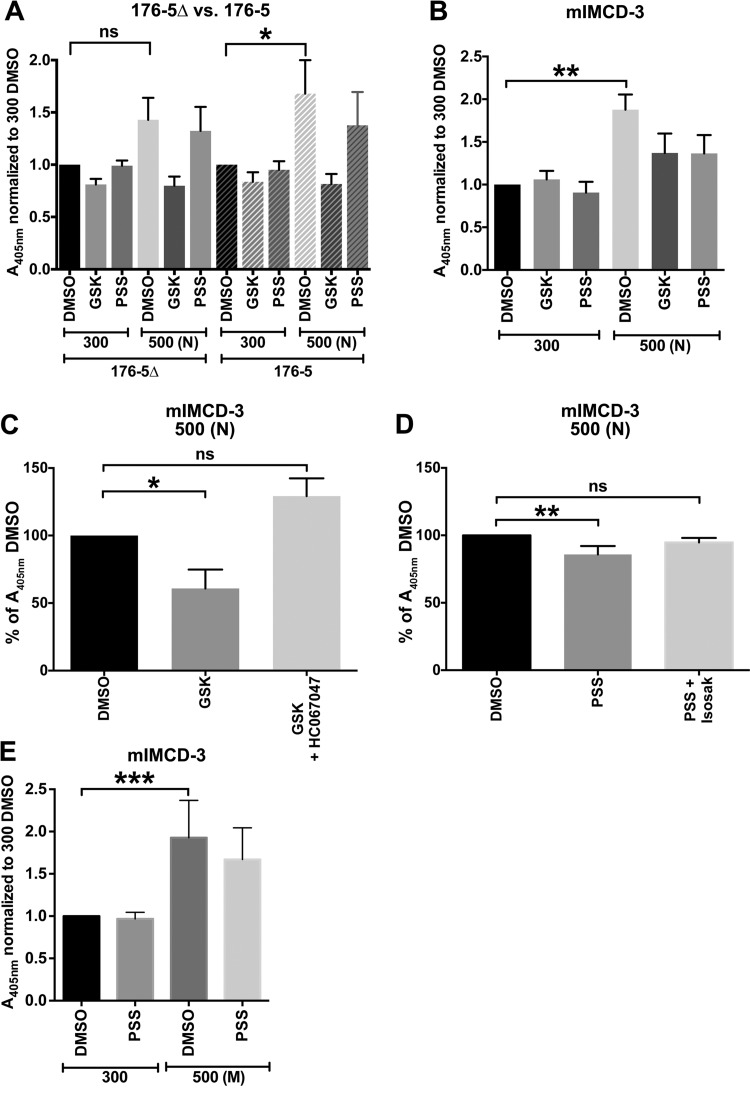
Hyperosmolality-induced tonicity element-binding protein (TonEBP) reporter activity is affected by expression of cilia and stimulation of TRPV4 or TRPM3. A: ciliated (176-5) and nonciliated (176-5Δ) pSEAP2TonE/pcDNA3.1-transfected cells in 96-well plates were treated with a TRPV4 agonist [100 nM GSK1016790A (GSK)] or a TRPM3 agonist [100 μM pregnenolone sulfate (PSS)] at 300 mOsm/kg or 500 mOsm/kg [adjusted with NaCl (N)]. Vehicle control groups were treated with 0.1% DMSO. TonEBP activity (normalized to control) was significantly induced in 176-5 cells only. This significant induction was not observed in groups treated with the TRPV4 agonist and the TRPM3 agonist in these cells. A405, absorbance at 405 nm. B: mIMCD-3 cells transfected with pSEAP2TonE/pcDNA3.1 were treated with a TRPV4 agonist (25 nM GSK1016790A) or a TRPM3 agonist (100 μM pregnenolone sulfate) at 300 mOsm/kg or 500 mOsm/kg (adjusted with NaCl). Vehicle control groups were treated with 0.1% DMSO. Hyperosmolality significantly induced TonEBP reporter activity, which was not observed in groups treated with the TRPV4 agonist and the TRPM3 agonist. C and D: agonist-mediated attenuation of hyperosmolality-induced TonEBP reporter activity was competed away by cotreatment with the TRPV4 antagonist HC-067047 and by the TRPM3 antagonist isosakuranetin (Isosak). E: in mIMCD-3 cells, increasing osmolality to 500 mOsm/kg [adjusted with mannitol (M)] produced a statistically significant increase in TonEBP reporter activity that was not significant with concurrent treatment with the TRPM3 agonist. Treatment groups were compared with controls using Friedman’s test with Dunn’s correction for multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001. ns, Not significant. n = 10 (A), 6 (B–D), and 9 (E) experiments.
We also measured expression of two osmotic response genes by RT-qPCR following hyperosmolal stress in two independent ciliated-nonciliated cell line pairs. We noted robust induction of expression of Akr1b3 and Slc6a12 in the ciliated 176-5 cells following exposure to hyperosmolality (500 mOsm/kg with NaCl). The induction was attenuated in cilium-deficient 176-5Δ cells (Fig. 9, A and B, DMSO). In the cortical collecting duct cell line with abrogated formation of primary cilia due to hypomorphic expression of intraflagellar transport 88 [Ift88; Orpk cilia(−)], we noted an attenuated induction of Akr1b3 following exposure to hyperosmolality (500 mOsm/kg with NaCl) compared with the robust Akr1b3 induction in cells rescued by reexpression of a WT Ift88 construct [Orpk cilia(+)] and exposed to this hyperosmolal stress (Fig. 9C). A trend toward attenuated expression of Slc6a12 was noted in Orpk cilia(−) cells exposed to hyperosmolal conditions (Fig. 9D), but this did not reach statistical significance.
Seminal work in the field of osmoregulation was performed using similar methods with renal epithelial cells cultured on plastic (10, 11). However, cells cultured on permeable supports, to more closely mimic in vivo conditions, may respond differently to increased osmolality. We performed experiments to compare the osmotic response to increased NaCl in mIMCD-3 cells cultured on plastic with mIMCD-3 cells cultured on permeable supports. We used PCR arrays to measure the relative mRNA amounts (relative to an average of 5 reference genes) of 84 genes that are known to respond to changes in osmolality. The comparison of the osmotic responses of cells cultured on plastic with cells cultured on permeable supports is presented in Tables 2 and 3. We observed considerable overlap in up- and downregulation of osmotic response genes between cells exposed to hyperosmolal stress in the two culture methodologies.
Table 2.
Genes upregulated in mIMCD-3 cells by exposure to 500 mOsm/kg vs. isosmolal conditions
| Gene | Fold Change |
|---|---|
| Cultured on tissue culture-treated plastic | |
| Akr1b3 | 65.27 |
| Aqp1 | 26.04 |
| Plat | 18.54 |
| Pax2 | 11.45 |
| Sgk1 | 10.23 |
| Hspa4l | 10.19 |
| Slc5a3 | 9.81 |
| Hspa1a | 6.72 |
| Cryab | 5.71 |
| Adm | 4.83 |
| Dusp1 | 3.94 |
| Atp1b1 | 2.84 |
| Vegfa | 2.83 |
| Slc14a2 | 2.59 |
| Atp1a1 | 2.56 |
| Slc2a1 | 2.36 |
| Nfkbia | 2.25 |
| Slc6a6 | 2.25 |
| Tnf | 2.21 |
| Slc38a2 | 1.76 |
| Cd9 | 1.53 |
| Cultured on Transwell tissue culture-treated semipermeable polycarbonate membrane | |
| Aqp1 | 4.65 |
| Akr1b3 | 4.56 |
| Atp1b1 | 1.99 |
| Hspa1a | 1.89 |
| Cryab | 1.84 |
| Plat | 1.74 |
| Slc5a3 | 1.73 |
| Dusp1 | 1.72 |
| Pax2 | 1.56 |
| Slc38a2 | 1.56 |
Hyperosmolal stress (500 mOsm/kg) was achieved by supplementation of culture medium with NaCl. Genes shown in boldface are common between cells cultured on plastic and permeable supports. Each condition was repeated 3 times, and results were determined on the basis of the average of these 3 replicates. Change ≥1.5-fold was considered an increase.
Table 3.
Genes downregulated in mIMCD-3 cells by exposure to 500 mOsm/kg vs. isosmolal conditions
| Gene | Fold Change |
|---|---|
| Cultured on tissue culture-treated plastic | |
| Tgfa | 0.48 |
| Ctfg | 0.45 |
| Vim | 0.36 |
| Egr1 | 0.31 |
| Gadd45a | 0.27 |
| Egr3 | 0.25 |
| Ptk2b | 0.23 |
| Lcn2 | 0.18 |
| Agt | 0.18 |
| Cultured on Transwell tissue culture-treated semipermeable polycarbonate membrane | |
| Tnf | 0.46 |
| Ptk2b | 0.45 |
| Vim | 0.28 |
Hyperosmolal stress (500 mOsm/kg) was achieved by supplementation of culture medium with NaCl. Genes shown in boldface are common between cells cultured on plastic and permeable supports. Each condition was repeated 3 times, and results were determined on the basis of the average of these 3 replicates. Change ≤0.5-fold was considered a decrease.
Because TRPV4 and TRPM3 are expressed in primary cilia of renal epithelial cells, we tested whether pharmacological manipulation of these channels would impact the cilium-dependent response to hyperosmolal stress. TRPV4 (16) and TRPM3 (32) are activated by hyposmolality and inhibited by hyperosmolality. To counter the reduction in channel activity by hyperosmolal stress, cells were treated with the specific TRPV4 agonist GSK1016790A or the specific TRPM3 agonist pregnenolone sulfate. In 176-5 cells expressing the TonEBP reporter construct, the significant increase in TonEBP activity with hyperosmolal stress was not observed in groups treated with GSK1016790A (100 nM) or pregnenolone sulfate (100 µM) (Fig. 8A).
We similarly assessed the effects of TRPV4 and TRPM3 manipulation in mIMCD-3 cells expressing the TonEBP reporter construct. Acute hyperosmolal stress of 500 mOsm/kg (with NaCl) produced a significant increase in TonEBP reporter activity that was not observed in groups treated with GSK1016790A (25 nM) or pregnenolone sulfate (100 µM) (Fig. 8B). To demonstrate that these effects were due specifically to the action of the agonists on their respective targets, we competed away the effect of GSK1016790A with addition of the TRPV4 antagonist HC-067047 (100 nM) (46) (Fig. 8C). Similarly, the attenuation of hyperosmolal TonEBP induction by pregnenolone sulfate was mitigated by the specific TRPM3 antagonist isosakuranetin (200 nM) (40) (Fig. 8D).
In 176-5 and 176-5Δ cells, GSK1016790A (100 nM) blunted the induction of both Akr1b3 and Slc6a12 mRNA (Fig. 9, A and B). Interestingly, pregnenolone sulfate (100 µM) attenuated the induction of the osmotic response genes in ciliated 176-5, but not nonciliated 176-5Δ, cells (Fig. 9, A and B). Knocking out TRPM3 in mIMCD-3 cells reduced the induction of Akr1b3 by hyperosmolality (Fig. 10). Pregnenolone sulfate (100 µM) attenuated the induction of Akr1b3 in WT mIMCD-3, but not TRPM3 KO mIMCD-3, cells (Fig. 10).
To determine whether the cilium-dependent effect arises from a specific chemical or ionic property of NaCl, we also tested a second, nonionic osmolyte, mannitol. We increased osmolality in Orpk cells from 300 to 500 mOsm/kg with mannitol. As with NaCl, the hyperosmolality-induced increase in Akr1b3 expression in Orpk cilia(−) cells was again less than that in Orpk cilia(+) cells (Fig. 9E). We also increased osmolality in ciliated mIMCD-3 cells from 300 to 500 mOsm/kg with mannitol and observed a significant increase TonEBP reporter activity compared with control (Fig. 8E). The osmolality-induced increase was not significant when a TRPM3 agonist, pregnenolone sulfate (100 µM), was concurrently applied (Fig. 8E).
Cilia are important for survival of renal epithelial cells exposed to osmotic stress.
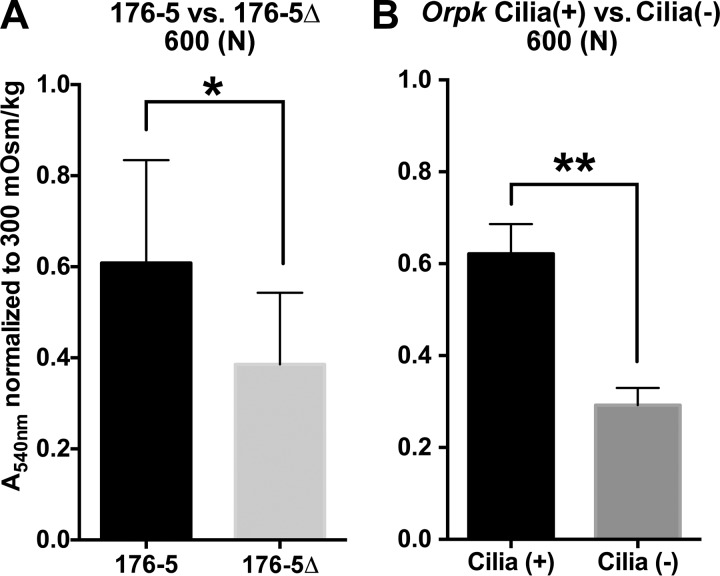
Survival after exposure to hyperosmolal conditions of 600 mOsm/kg with NaCl was reduced in 176-5Δ compared with 176-5 cells (Fig. 11A). Similarly, survival after hyperosmolal shock at 600 mOsm/kg with NaCl was reduced in Orpk cilia(−) compared with Orpk cilia(+) cells (Fig. 11B).
Fig. 11.
Crystal violet cell quantitation of 176-5 and 176-5Δ (A) and Orpk cilia(+) and cilia(−) cells (B). Greater absorbance (A540) values indicate greater number of cells. 176-5 and 176-5Δ and Orpk cilia(+) and cilia(−) cells were acutely exposed to isosmolal (300 mOsm/kg) and hyperosmolal [600 mOsm/kg, with addition of NaCl (N) to basal medium] conditions for 24 h. Absorbance values were normalized to isosmolal control (300 mOsm/kg) groups. *P < 0.05, **P < 0.01. n = 9 (A) and 4 (B) experiments.
DISCUSSION
In the present study we provide evidence for a novel physiological function, osmosensation, attributed to primary cilia in renal tubular epithelial cells. Specifically, structurally intact renal primary cilia are required for normal osmosensation and maximal activation of the osmotic response. Systemically, osmoregulation is controlled by specialized nuclei within the hypothalamus that detect changes in plasma osmolality and initiate appropriate responses, such as signaling the release of vasopressin from the posterior pituitary. Vasopressin regulates the body's water balance by acting to increase water reabsorption in the collecting ducts of the nephron. This process effectively maintains the blood osmolality at physiologically normal levels (~290 mOsm/kg in mammals). However, peripheral variations in osmolality, such as the vast changes that can occur in the inner medulla of the kidney between the antidiuretic state, which can reach ~1,200 mOsm/kg in humans, and a maximally dilute urine (~50 mOsm/kg in humans), require that cells in this environment sense and adapt to these variations in order to survive and function normally. The osmotic response in the renal inner medulla has been well described (1, 5). However, the present study is the first to provide clear evidence that structurally intact cilia are required for maximal activation of the osmotic response, supporting a role for primary cilia in nephrogenic osmosensation.
For most of the present studies, we used NaCl as the osmotically active particle to induce hyperosmolal stress. This method has been used effectively by others to study the osmotic stress response of renal epithelial cells in vitro and in vivo (10, 11). Abundance of NaCl is high in the inner medulla of the mammalian kidney, and NaCl concentration in urine can vary widely, depending on the sodium content of the diet and the action of several mineralotropic hormones, including aldosterone (30). Urea is also a primary solute of urine; however, because of its (albeit low) membrane permeability, hyperosmolal urea does not activate the TonEBP-regulated osmotic stress response (28). Rather, it activates the phosphoinositide 3-kinase and MAP kinase pathways as an alternative cellular stress response mechanism, leading to Egr1 transcription (42, 53). Therefore, hyperosmolal urea in the microenvironment of the IMCD is detected through mechanisms that could be cilium-mediated but are almost certainly distinct from that of NaCl. To determine whether the cilium-dependent effect observed in the present studies arises from a specific chemical or ionic property of NaCl, we increased osmolality in mIMCD-3 and Orpk cells with mannitol, a nonionic osmolyte that has been used to induce hypertonic stress in IMCD cells (6). As with NaCl, mannitol induced a TonEBP-mediated osmotic response in mIMCD-3 cells that was dampened by exposure to the TRPM3 agonist. The osmotic response to mannitol was less in Orpk cilia(−) than cilia(+) cells, supporting a role of cilia in the mechanism.
One technical consideration for these studies is that the osmotic response was evaluated in cells cultured on plastic, which does not recapitulate the physiological conditions of renal epithelial cells in the nephron. Extensive work defining the osmotic response in renal epithelial cells has been conducted using this technique (10, 11), but to test the validity of this method, we conducted experiments to evaluate the expression of 84 osmotically regulated genes by qPCR array in cells cultured on permeable supports compared with cells cultured on plastic. We found substantial overlap of genes up- and downregulated by increased osmolality between cells cultured on plastic and those cultured on permeable supports, supporting the validity of our current findings of cells cultured on plastic. In particular, the most robustly upregulated gene in both conditions was Akr1b3 (aldose reductase), which was the primary gene used to evaluate the osmotic response in the present studies. Slc6a12 [betaine/GABA transporter (BGT1)] expression was not evaluated in these qPCR assays, as amplification of this gene did not pass quality-control measures.
We show that knocking out TRPM3 reduces the osmotic response in a ciliated, renal epithelial cell line. In addition, we report for the first time that TRPM3 is expressed in the primary cilia of renal epithelium. [Zhao et al. recently detected TRPM3 expression in the base of primary cilia of human fetal retinal pigment epithelial cells (55).] Previous studies have suggested that TRPM3 is expressed in human renal tissue but either is not detected (18; reviewed in 32) or is only weakly expressed (25) in mouse kidney. Here we demonstrate expression of TRPM3 in tissue from WT mouse kidney and in two murine renal epithelial cell models. Our ability to detect TRPM3 in murine models by qPCR may be explained if the detection of Trpm3 transcripts is more sensitive in collecting duct cell lines than in whole kidney tissue preparations. In addition, our study of protein expression by immunofluorescence focused specifically on the primary cilium of the renal tubule, a relatively small cellular component. With electrical recording, we were unable to observe ciliary currents consistent with the reported characteristics for TRPM3. There are ≥24 identified splice variants of TRPM3, and the channel properties of only 2 variants have been studied (32). It may be that the ciliary TRPM3 protein is one of the many variants for which biophysical properties have not been determined.
In contrast to published reports of ciliary localization of TRPV4 in Orpk and MDCK renal epithelial cells (24, 54) and our electrophysiological identification of ciliary TRPV4 activity, we did not detect expression of TRPV4 in renal primary cilia of mIMCD-3 or 176-5 cells by immunofluorescence. This may be the result of low channel abundance within primary cilia in these cells compared with Orpk cells, which are cortical collecting duct-derived, and MDCK cells, which are likely of distal tubule origin (thick ascending limb and distal convoluted tubule) (20). It has been reported that TRPV4 is absent from the proximal tubule and descending limb, most highly expressed in the water-impermeant nephron segments (thick ascending limb and distal convoluted tubule), and moderately expressed in the collecting ducts (34). There is evidence of apical and basolateral expression, and TRPV4 has been localized to primary cilia, although it is possible that TRPV4 is not expressed at a high level in all primary cilia of all nephron segments (34).
The present findings support a role for primary cilia and the cilium-expressed TRPM3 and TRPV4 channels in the nephrogenic osmotic response. Mechanical modulation of TRPV4 caused by membrane deformation from fluctuations in cell volume has been proposed as a mechanism by which TRPV4 senses hyposmolality (2), although other investigators have identified TRPV4 activation by hyposmolality independent of positive pressure applied (41). Cell volume changes have been proposed as one mechanism by which TRPM3 is regulated (32). Importantly, TRPM3 and TRPV4 contribute to cellular Ca2+ entry, and constitutive channel activity is linked to increased intracellular Ca2+ levels. As the activity of both channels is directly inhibited by hyperosmolality, a reduction in overall cellular abundance of Ca2+ or Ca2+ concentration in certain microdomains may transmit the signal of hyperosmolal stress to activation of the TonEBP-regulated osmotic stress response by one of several Ca2+-dependent signaling pathways. Reduced Ca2+ entry is hypothesized to contribute to altered cellular behavior in another disease process, polycystic kidney disease, by affecting MAPK signaling (50). TonEBP activity is regulated by MAPK signaling, particularly by p38, and the MAP kinases are known to be affected by changes in cytosolic Ca2+ (38). Importantly, the entirety of mechanism(s) controlling the TonEBP-mediated osmotic response is not known, and more work is required to elucidate the link(s) between ciliary TRPV4 and TRPM3 activity and regulation of TonEBP.
One important finding from the present studies is that while attenuation of the osmotic response was observed with a TRPV4 agonist in ciliated and nonciliated cells, the effect of the TRPM3 agonist was observed only in ciliated cells. This suggests that the role of TRPV4 in renal epithelial osmosensation may extend beyond the scope of the primary cilium, perhaps functioning as an overall plasma membrane osmosensor, but the role of TRPM3 may be specific to the cilium. The upregulation of TRPM3 in nonciliated 176-5Δ cells may represent a type of compensatory mechanism due to impairment of ciliary osmosensation similar to that observed with TRPP2, or polycystin-2, in Orpk cilia(−) cells (39).
Renal tubular atrophy with increased apoptosis in the inner medulla has been described in TonEBP KO mice (29). This histological finding is particularly prominent in the inner medulla, where osmolality can reach the highest levels in the kidney. Renal tubular atrophy is also a prominent histological finding of nephronophthisis, an inherited disease characterized by corticomedullary kidney cysts and tubulointerstitial sclerosis leading to end-stage renal disease (47). Importantly, the pathogenic mechanism of nephronophthisis is related to structural and/or functional defects in primary cilia in the renal epithelium. In light of this association, it is interesting to speculate that the tubular atrophy observed in ciliopathies may result, at least in part, from failure to maximally activate the osmotic response.
We conclude from the findings of this study that primary cilia of renal epithelial cells are a critical component of their response to hyperosmolality. TRPM3 seems to participate in the cilium-dependent response to hyperosmolality, as suggested by localization of TRPM3 to the primary cilium and the attenuating effect of pregnenolone only on the osmotic response of ciliated cells. TRPV4, however, modulates the osmotic response independent of the presence of a primary cilium, which may owe to its presence in the cilia and on the apical cell membrane. By pharmacological manipulation of the activity of TRPM3 and TRPV4 to augment the osmotic response in cells with structural or functional defects in primary cilia, such as those seen in the large group of renal cystic diseases, it may be possible to use these channels as novel therapeutic targets to ameliorate cystogenesis in patients with these diseases.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R21 DK-091917 (to S. J. Kleene) and K08 DK-081737 (to B. P. Dixon).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.J.S., N.K.K., S.J.K., C.D.V., R.G.C., J.L., L.L., N.W.P., and B.P.D. performed the experiments; B.J.S., N.K.K., S.J.K., C.D.V., R.G.C., J.L., L.L., N.W.P., and B.P.D. analyzed the data; B.J.S., N.K.K., S.J.K., C.D.V., R.G.C., J.L., L.L., N.W.P., J.J.B., and B.P.D. interpreted the results of the experiments; B.J.S., N.K.K., S.J.K., and B.P.D. prepared the figures; B.J.S., N.K.K., S.J.K., and B.P.D. drafted the manuscript; B.J.S., N.K.K., S.J.K., J.J.B., and B.P.D. edited and revised the manuscript; B.J.S., N.K.K., S.J.K., C.D.V., R.G.C., J.L., L.L., N.W.P., J.J.B., and B.P.D. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Priscilla Amofa for excellent technical assistance. The authors also thank the Cincinnati Children’s Hospital Medical Center Transgenic Animal and Genome Editing Core Facility and Dr. Yueh-Chiang Hu for manufacture of the Trpm3 CRISPR plasmid and advice in screening the clones.
REFERENCES
- 1.Bagnasco S, Balaban R, Fales HM, Yang YM, Burg M. Predominant osmotically active organic solutes in rat and rabbit renal medullas. J Biol Chem 261: 5872–5877, 1986. [PubMed] [Google Scholar]
- 2.Becker D, Blase C, Bereiter-Hahn J, Jendrach M. TRPV4 exhibits a functional role in cell-volume regulation. J Cell Sci 118: 2435–2440, 2005. doi: 10.1242/jcs.02372. [DOI] [PubMed] [Google Scholar]
- 3.Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol 19: R526–R535, 2009. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berbari NF, Sharma N, Malarkey EB, Pieczynski JN, Boddu R, Gaertig J, Guay-Woodford L, Yoder BK. Microtubule modifications and stability are altered by cilia perturbation and in cystic kidney disease. Cytoskeleton 70: 24–31, 2013. doi: 10.1002/cm.21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev 87: 1441–1474, 2007. doi: 10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- 6.Chakravarty D, Cai Q, Ferraris JD, Michea L, Burg MB, Kültz D. Three GADD45 isoforms contribute to hypertonic stress phenotype of murine renal inner medullary cells. Am J Physiol Renal Physiol 283: F1020–F1029, 2002. doi: 10.1152/ajprenal.00118.2002. [DOI] [PubMed] [Google Scholar]
- 7.Cost NG, Czyzyk-Krzeska MF. Regulation of autophagy by two products of one gene: TRPM3 and miR-204. Mol Cell Oncol 2: e1002712, 2015. doi: 10.1080/23723556.2014.1002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham F, Amode MR, Barrell D, Beal K, Billis K, Brent S, Carvalho-Silva D, Clapham P, Coates G, Fitzgerald S, Gil L, Girón CG, Gordon L, Hourlier T, Hunt SE, Janacek SH, Johnson N, Juettemann T, Kähäri AK, Keenan S, Martin FJ, Maurel T, McLaren W, Murphy DN, Nag R, Overduin B, Parker A, Patricio M, Perry E, Pignatelli M, Riat HS, Sheppard D, Taylor K, Thormann A, Vullo A, Wilder SP, Zadissa A, Aken BL, Birney E, Harrow J, Kinsella R, Muffato M, Ruffier M, Searle SM, Spudich G, Trevanion SJ, Yates A, Zerbino DR, Flicek P. Ensembl 2015. Nucleic Acids Res 43, D1: D662–D669, 2015. doi: 10.1093/nar/gku1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon BP, Chu A, Henry J, Kim R, Bissler JJ. Increased cancer risk of augmentation cystoplasty: possible role for hyperosmolal microenvironment on DNA damage recognition. Mutat Res 670: 88–95, 2009. doi: 10.1016/j.mrfmmm.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dmitrieva NI, Bulavin DV, Burg MB. High NaCl causes Mre11 to leave the nucleus, disrupting DNA damage signaling and repair. Am J Physiol Renal Physiol 285: F266–F274, 2003. doi: 10.1152/ajprenal.00060.2003. [DOI] [PubMed] [Google Scholar]
- 11.Dmitrieva NI, Cai Q, Burg MB. Cells adapted to high NaCl have many DNA breaks and impaired DNA repair both in cell culture and in vivo. Proc Natl Acad Sci USA 101: 2317–2322, 2004. doi: 10.1073/pnas.0308463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dmitrieva NI, Celeste A, Nussenzweig A, Burg MB. Ku86 preserves chromatin integrity in cells adapted to high NaCl. Proc Natl Acad Sci USA 102: 10730–10735, 2005. doi: 10.1073/pnas.0504870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doench JG, Fusi N, Sullender M, Hegde M, Vaimberg EW, Donovan KF, Smith I, Tothova Z, Wilen C, Orchard R, Virgin HW, Listgarten J, Root DE. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol 34: 184–191, 2016. doi: 10.1038/nbt.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doench JG, Hartenian E, Graham DB, Tothova Z, Hegde M, Smith I, Sullender M, Ebert BL, Xavier RJ, Root DE. Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat Biotechnol 32: 1262–1267, 2014. doi: 10.1038/nbt.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, Strassmaier T, Uykal E, Owsianik G, Vennekens R, De Ridder D, Nilius B, Fanger CM, Voets T. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci USA 107: 19084–19089, 2010. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Elias A, Mrkonjić S, Jung C, Pardo-Pastor C, Vicente R, Valverde MA. The TRPV4 channel. Handb Exp Pharmacol 222: 293–319, 2014. doi: 10.1007/978-3-642-54215-2_12. [DOI] [PubMed] [Google Scholar]
- 17.Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, Masyuk TV, Larusso NF. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci USA 104: 19138–19143, 2007. doi: 10.1073/pnas.0705964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem 278: 21493–21501, 2003. doi: 10.1074/jbc.M300945200. [DOI] [PubMed] [Google Scholar]
- 19.Held K, Kichko T, De Clercq K, Klaassen H, Van Bree R, Vanherck JC, Marchand A, Reeh PW, Chaltin P, Voets T, Vriens J. Activation of TRPM3 by a potent synthetic ligand reveals a role in peptide release. Proc Natl Acad Sci USA 112: E1363–E1372, 2015. doi: 10.1073/pnas.1419845112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herzlinger DA, Easton TG, Ojakian GK. The MDCK epithelial cell line expresses a cell surface antigen of the kidney distal tubule. J Cell Biol 93: 269–277, 1982. doi: 10.1083/jcb.93.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 31: 827–832, 2013. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn-Kirby AH, Bargmann CI. TRP channels in C. elegans. Annu Rev Physiol 68: 719–736, 2006. doi: 10.1146/annurev.physiol.68.040204.100715. [DOI] [PubMed] [Google Scholar]
- 23.Kleene NK, Kleene SJ. A method for measuring electrical signals in a primary cilium. Cilia 1: 17, 2012. doi: 10.1186/2046-2530-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Köttgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 182: 437–447, 2008. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunert-Keil C, Bisping F, Krüger J, Brinkmeier H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics 7: 159, 2006. doi: 10.1186/1471-2164-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lechner SG, Markworth S, Poole K, Smith ES, Lapatsina L, Frahm S, May M, Pischke S, Suzuki M, Ibañez-Tallon I, Luft FC, Jordan J, Lewin GR. The molecular and cellular identity of peripheral osmoreceptors. Neuron 69: 332–344, 2011. doi: 10.1016/j.neuron.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 27.LeDizet M, Piperno G. Detection of acetylated α-tubulin by specific antibodies. Methods Enzymol 196: 264–274, 1991. doi: 10.1016/0076-6879(91)96025-M. [DOI] [PubMed] [Google Scholar]
- 28.Lee SD, Choi SY, Kwon HM. Distinct cellular pathways for resistance to urea stress and hypertonic stress. Am J Physiol Cell Physiol 300: C692–C696, 2011. doi: 10.1152/ajpcell.00150.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.López-Rodríguez C, Antos CL, Shelton JM, Richardson JA, Lin F, Novobrantseva TI, Bronson RT, Igarashi P, Rao A, Olson EN. Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc Natl Acad Sci USA 101: 2392–2397, 2004. doi: 10.1073/pnas.0308703100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meneton P, Loffing J, Warnock DG. Sodium and potassium handling by the aldosterone-sensitive distal nephron: the pivotal role of the distal and connecting tubule. Am J Physiol Renal Physiol 287: F593–F601, 2004. doi: 10.1152/ajprenal.00454.2003. [DOI] [PubMed] [Google Scholar]
- 31.Neuhofer W, Fraek ML, Beck FX. Nitric oxide decreases expression of osmoprotective genes via direct inhibition of TonEBP transcriptional activity. Pflügers Arch 457: 831–843, 2009. doi: 10.1007/s00424-008-0540-3. [DOI] [PubMed] [Google Scholar]
- 32.Oberwinkler J, Philipp SE. TRPM3. Handb Exp Pharmacol 222: 427–459, 2014. doi: 10.1007/978-3-642-54215-2_17. [DOI] [PubMed] [Google Scholar]
- 33.Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol 15: 105–110, 2003. doi: 10.1016/S0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 34.Pochynyuk O, Zaika O, O’Neil RG, Mamenko M. Novel insights into TRPV4 function in the kidney. Pflügers Arch 465: 177–186, 2013. doi: 10.1007/s00424-012-1190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rauchman MI, Nigam SK, Delpire E, Gullans SR. An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am J Physiol Renal Physiol 265: F416–F424, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, Moorman AF. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37: e45, 2009. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci 123: 499–503, 2010. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheikh-Hamad D, Di Mari J, Suki WN, Safirstein R, Watts BA 3rd, Rouse D. p38 kinase activity is essential for osmotic induction of mRNAs for HSP70 and transporter for organic solute betaine in Madin-Darby canine kidney cells. J Biol Chem 273: 1832–1837, 1998. doi: 10.1074/jbc.273.3.1832. [DOI] [PubMed] [Google Scholar]
- 39.Siroky BJ, Ferguson WB, Fuson AL, Xie Y, Fintha A, Komlosi P, Yoder BK, Schwiebert EM, Guay-Woodford LM, Bell PD. Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am J Physiol Renal Physiol 290: F1320–F1328, 2006. doi: 10.1152/ajprenal.00463.2005. [DOI] [PubMed] [Google Scholar]
- 40.Straub I, Krügel U, Mohr F, Teichert J, Rizun O, Konrad M, Oberwinkler J, Schaefer M. Flavanones that selectively inhibit TRPM3 attenuate thermal nociception in vivo. Mol Pharmacol 84: 736–750, 2013. doi: 10.1124/mol.113.086843. [DOI] [PubMed] [Google Scholar]
- 41.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2: 695–702, 2000. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 42.Tian W, Cohen DM. Urea stress is more akin to EGF exposure than to hypertonic stress in renal medullary cells. Am J Physiol Renal Physiol 283: F388–F398, 2002. doi: 10.1152/ajprenal.00031.2002. [DOI] [PubMed] [Google Scholar]
- 43.Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, Bödding M, Droogmans G, Nilius B. Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem 277: 33704–33710, 2002. doi: 10.1074/jbc.M204828200. [DOI] [PubMed] [Google Scholar]
- 44.Wagner TF, Drews A, Loch S, Mohr F, Philipp SE, Lambert S, Oberwinkler J. TRPM3 channels provide a regulated influx pathway for zinc in pancreatic beta cells. Pflügers Arch 460: 755–765, 2010. doi: 10.1007/s00424-010-0838-9. [DOI] [PubMed] [Google Scholar]
- 45.Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, Düfer M, Lis A, Flockerzi V, Philipp SE, Oberwinkler J. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol 10: 1421–1430, 2008. doi: 10.1038/ncb1801. [DOI] [PubMed] [Google Scholar]
- 46.Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, Sulpizio A, Behm DJ, Hoffman S, Lin Z, Lozinskaya I, Casillas LN, Lin M, Trout RE, Votta BJ, Thorneloe K, Lashinger ES, Figueroa DJ, Marquis R, Xu X. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J Pharmacol Exp Ther 326: 443–452, 2008. doi: 10.1124/jpet.107.134551. [DOI] [PubMed] [Google Scholar]
- 47.Wolf MT, Hildebrandt F. Nephronophthisis. Pediatr Nephrol 26: 181–194, 2011. doi: 10.1007/s00467-010-1585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu L, Gao X, Brown RC, Heller S, O’Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol 293: F1699–F1713, 2007. doi: 10.1152/ajprenal.00462.2006. [DOI] [PubMed] [Google Scholar]
- 49.Xu H, Fu Y, Tian W, Cohen DM. Glycosylation of the osmoresponsive transient receptor potential channel TRPV4 on Asn-651 influences membrane trafficking. Am J Physiol Renal Physiol 290: F1103–F1109, 2006. doi: 10.1152/ajprenal.00245.2005. [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 279: 40419–40430, 2004. doi: 10.1074/jbc.M405079200. [DOI] [PubMed] [Google Scholar]
- 51.Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE Jr, Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol 282: F541–F552, 2002. doi: 10.1152/ajprenal.00273.2001. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, Dmitrieva NI, Park JH, Levine RL, Burg MB. High urea and NaCl carbonylate proteins in renal cells in culture and in vivo, and high urea causes 8-oxoguanine lesions in their DNA. Proc Natl Acad Sci USA 101: 9491–9496, 2004. doi: 10.1073/pnas.0402961101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Z, Yang XY, Soltoff SP, Cohen DM. PI3K signaling in the murine kidney inner medullary cell response to urea. Am J Physiol Renal Physiol 278: F155–F164, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Zhang ZR, Chu WF, Song B, Gooz M, Zhang JN, Yu CJ, Jiang S, Baldys A, Gooz P, Steele S, Owsianik G, Nilius B, Komlosi P, Bell PD. TRPP2 and TRPV4 form an EGF-activated calcium permeable channel at the apical membrane of renal collecting duct cells. PLoS One 8: e73424, 2013. doi: 10.1371/journal.pone.0073424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao PY, Gan G, Peng S, Wang SB, Chen B, Adelman RA, Rizzolo LJ. TRP channels localize to subdomains of the apical plasma membrane in human fetal retinal pigment epithelium. Invest Ophthalmol Vis Sci 56: 1916–1923, 2015. doi: 10.1167/iovs.14-15738. [DOI] [PMC free article] [PubMed] [Google Scholar]