Abstract
In critical illness, such as sepsis or the acute respiratory distress syndrome, acute kidney injury (AKI) is common and associated with increased morbidity and mortality. Mechanical ventilation in critical illnesses is also a risk factor for AKI, but it is potentially modifiable. Injurious ventilation strategies may lead to the systemic release of inflammatory mediators from the lung due to ventilator induced lung injury (VILI). The systemic consequences of VILI are difficult to differentiate clinically from other systemic inflammatory syndromes, such as sepsis. The purpose of this study was to identify unique changes in the expression of inflammatory mediators in kidney tissue in response to VILI compared with systemic sepsis to gain insight into direct effects of VILI on the kidney. Four groups of mice were compared—mice with sepsis from cecal ligation and puncture (CLP), mice subjected to injurious mechanical ventilation with high tidal volumes (VILI), mice exposed to CLP followed by VILI (CLP+VILI), and sham controls. Protein expression of common inflammatory mediators in kidneys was analyzed using a proteome array and confirmed by Western blot analysis or ELISA. VEGF and VCAM-1 were found to be significantly elevated in kidneys from VILI mice compared with sham and CLP. Angiopoietin-2 was significantly increased in CLP+VILI compared with CLP alone and was also correlated with higher levels of AKI biomarker, neutrophil gelatinase-associated lipocalin. These results suggest that VILI alters the renal expression of VEGF, VCAM-1, and angiopoietin-2, and these proteins warrant further investigation as potential biomarkers and therapeutic targets.
Keywords: lung-kidney crosstalk
the kidney is highly susceptible to injury in critical illness, as evidenced by the occurrence of acute kidney injury (AKI) in 30–50% of intensive care unit admissions (34). Acute respiratory distress syndrome (ARDS) is characterized by acute onset of diffuse lung infiltrates, profound disturbance in gas exchange, and high mortality. Interestingly, respiratory failure accounts for only 16% of deaths in ARDS, and the leading cause of death is actually multiple organ failure (MOF) (32). The kidney is the most common organ affected, and ARDS that leads to AKI carries a mortality of 58% compared with 28% from ARDS alone (7, 21).
Mechanical ventilation (MV) is required in nearly all patients with ARDS as a life-saving intervention. Of note, MV also is associated with a threefold increase in the risk of AKI (36). Renal consequences of MV have been known for several decades, and hemodynamic alterations and neurohormonal activation were implicated, as mechanisms leading to reduced renal function (9, 27, 28). Recently, inflammatory crosstalk from lungs to kidneys via systemic cytokines generated by ventilator-induced lung injury (VILI), due to high tidal volume MV, has been suggested to contribute to AKI in preclinical and clinical studies (16–18, 31). VILI occurs when lung stress created by MV leads to alveolar-capillary barrier damage. This process triggers injurious cytokine and chemokine release from injured pulmonary epithelial and endothelial cells i.e., biotrauma (18, 31). Importantly, biotrauma due to MV may be a modifiable risk factor for AKI. A large multicenter trial showed that a ventilation strategy aimed at preventing VILI also attenuated AKI and improved mortality in patients with ARDS (1). However, the understanding of specific mediators of this lung-kidney crosstalk is limited. Recognition of VILI clinically is difficult due to lack of diagnostic biomarkers and the similarity between clinical manifestations of VILI and other diffuse inflammatory syndromes, such as sepsis. AKI is among the most common complications in patients with sepsis. Incidence of AKI rises with the increasing severity of sepsis (2, 30), and sepsis accounts for nearly 50% of episodes of AKI in critically ill patients (35). Moreover, sepsis and VILI often occur simultaneously, as sepsis is the leading cause of both ARDS and MOF (13, 15). These challenges are barriers to understanding better the pathophysiology of AKI due to VILI and to the development of novel strategies aimed at mitigating AKI due to VILI in critical illness.
We hypothesized that the renal expression of inflammatory markers due to VILI differs from sepsis and may be used to differentiate VILI from sepsis. Herein, we attempt to identify mediators of injury that are more specific to VILI-induced AKI compared with other injury patterns by evaluating the renal expression pattern of inflammatory markers in mice exposed to VILI compared with sepsis.
METHODS
Animals.
Experiments were performed in accordance with international guidelines and approved by the local animal care and use committees. C57BL/6 male mice (n = 16) underwent either cecal ligation and puncture (CLP) (one double puncture with a 23-gauge needle and 100% cecal ligation) or sham operation (6). At 24 h, they were randomized to injurious MV with a tidal volume of 20 ml/kg and 2.5 cmH2O PEEP for 4 h (Flexivent; SciReq) or spontaneously breathing control. Animals were euthanized and kidneys frozen in liquid nitrogen.
Sample preparation.
Whole kidneys were submerged in RIPA buffer with protease inhibitor and pulverized via homogenizer. Samples were centrifuged at 15,000 rpm at 4°C for 15 min. Pooled supernatants were used for cytokine array, and individual supernatant samples were used for Western blot analysis and ELISA.
Multiplex cytokine array.
The Mouse XL cytokine array (R&D Systems) was used to determine protein levels of 111 different cytokines and chemokines, as per the manufacturer’s instructions. After total protein quantification, pooled samples using equal amounts of protein from each of the four animals in each group (Sham, CLP, VILI, and CLP+ VILI) were added to the appropriate buffers and array membranes and incubated overnight at 4°C. Membranes were washed, incubated with the detection antibody cocktail before incubation with streptavidin-horseradish peroxidase, and were followed by incubation with chemiluminescent reagent mix. An X-ray exposure time of 3 min was used for analysis. Intensity was evaluated using the “Proteome Array Analyzer” macro-programmed for ImageJ. Analysis and interpretation were carried out in a blinded fashion.
Western blot analysis.
Lysates at 100 μg/lane were loaded on NuPage gels in MOPS buffer. Gel proteins were transferred to nitrocellulose membranes and immunoblotted with the appropriate primary antibodies, followed by appropriate secondary antibodies. Antibodies to mouse neutrophil gelatinase-associated lipocalin (NGAL; ab63929; Abcam), VEGF (ab46154; Abcam), endostatin (AF570; R&D Systems), and VCAM-1 (AF643; R&D Systems) were used. Samples were compared with β-actin or GAPDH control.
ELISA.
We used a quantikine ELISA kit (R&D Systems) to quantify angiopoietin 2 levels, according to manufacturer’s guidelines. Samples were diluted and incubated at room temperature for 2 h. Incubation and wash cycles were repeated with the addition of conjugate and substrate solution. Finally, stop solution was added, and intensity was read using a Li-Cor spectrophotometer.
Statistical analysis.
Data were analyzed by one-way ANOVA, as appropriate, using commercial software (SigmaPlot) with appropriate post hoc tests. Unless stated otherwise, results are presented as group means ± SE.
RESULTS
Mediators of vascular endothelial inflammation are increased in the kidney after VILI.
To evaluate changes in renal inflammatory mediator expression between VILI and sepsis, we identified protein levels of different cytokines and chemokines in renal tissue from sham, injurious MV (VILI), CLP, or CLP followed by injurious MV (CLP+VILI) mice. We specifically assessed for mediators that were more robustly expressed in the VILI groups relative to sham and CLP. Recent papers have also suggested that VILI in the setting of sepsis is a perpetuator of MOF via a “second hit” that leads to an overwhelming immune response (38). Therefore, we also evaluated mediators that showed at least a twofold increase in CLP+VILI. Proteins that fit either of these criteria were considered for further quantification.
Most of the proteins analyzed, such as TNF-α, IL-1β, and myeloperoxidase, had more robust levels in CLP compared with VILI alone (Fig. 1). However, we identified IGFBP-1, IL-33, endostatin, VEGF, and VCAM-1 with higher levels after VILI alone compared with sham and CLP (Fig. 1, highlighted in yellow). We chose to focus on the vascular effector proteins, VEGF, VCAM-1, and endostatin (Fig. 2), since vascular endothelial inflammation is a key feature of MOF syndromes (20, 23, 40). Protein levels of VEGF were found to be significantly elevated in both VILI and CLP+VILI compared with sham (P < 0.001 and P < 0.05, respectively) and CLP kidneys (P < 0.001 and P < 0.01, respectively) by Western blot analysis. VCAM-1 levels were significantly elevated in VILI compared with sham (P < 0.05) and CLP (P < 0.05). VCAM-1 levels in the CLP+VILI group were also higher than in both sham (P < 0.05) and CLP (P = 0.08). Both VEGF and VCAM-1 levels were unchanged in CLP compared with sham kidneys. Differences in endostatin were not significant.
Fig. 1.
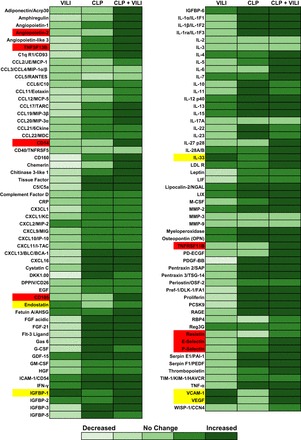
Proteome array analysis of inflammatory mediator levels in different injury models. Pooled kidney tissue lysates from mice exposed to Sham operation, cecal ligation and puncture (CLP), ventilator induced lung injury (VILI), and CLP+VILI (n = 4 for each group) were evaluated by Mouse XL cytokine array (R&D Systems). Relative expression of 111 different mediators are shown relative to Sham. Mediators with higher levels in VILI and VILI+CLP groups compared with CLP and Sham are highlighted in yellow. Mediators with twofold increases with dual injury (CLP+VILI) are highlighted in red.
Fig. 2.
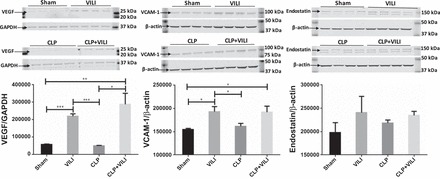
Renal VCAM-1, VEGF, and endostatin levels following VILI, CLP, or CLP+VILI. In kidney homogenates, protein levels of VCAM-1, VEGF, and endostatin were determined by Western blot analysis. All groups were run on the same gel. n = 4 mice for each group. VCAM-1 level in CLP+VILI vs. CLP (P = 0.08). (*P < 0.05, **P < 0.01, ***P < 0.001).
Renal angiopoietin-2 is significantly elevated in CLP+VILI.
Of the proteins analyzed in the proteome array, only eight had at least a twofold increase in the CLP+VILI group compared with other groups (Fig. 1, highlighted in red). Of these, angiopoietin-2 (Angpt2) had the greatest increase in expression compared with other endothelial proteins. Angpt2 was significantly elevated in both CLP and CLP+VILI compared with sham (P < 0.01 and P < 0.001, respectively) with ELISA (Fig. 3). Levels were also significantly elevated in CLP+VILI compared with CLP alone (P < 0.01).
Fig. 3.
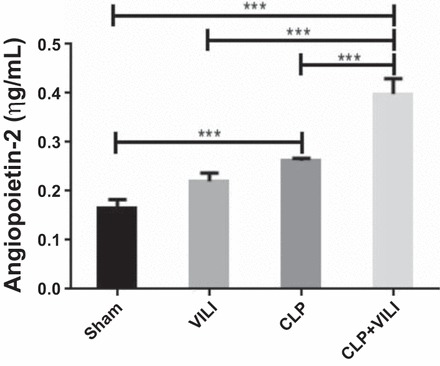
Renal angiopoietin-2 levels following VILI, CLP, or CLP+VILI. In total kidney homogenates, angiopoietin-2 levels were determined by ELISA. n = 4 mice for each group. ***P < 0.001).
To assess renal injury in response to these conditions, we measured renal NGAL expression, which is a known biomarker for renal tubular injury (8, 26). NGAL was significantly elevated in the CLP+VILI group compared with other groups (P < 0.01), and in CLP and VILI groups compared with sham (P < 0.01 and P < 0.05, respectively) (Fig. 4). These results correlated with the Angpt2 results described above.
Fig. 4.
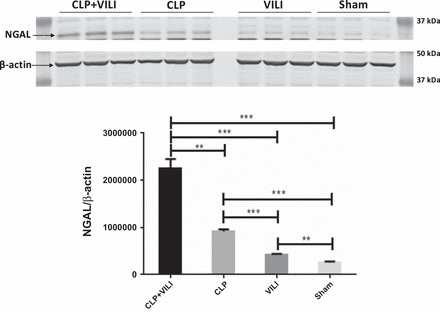
Neutrophil gelatinase-associated lipocalin (NGAL) levels in renal tissue following VILI, CLP, or CLP+VILI. In total kidney homogenates, NGAL levels were determined by Western blot analysis. All groups were run on the same gel. n = 3 mice for each group. **P < 0.01, ***P < 0.001).
DISCUSSION
In critical illnesses, the lungs and kidneys often sustain injury, and we believe that there are specific signals released from each organ that impact the other. We also believe these signals differ from other forms of systemic injury, such as sepsis. The purpose of this study was to identify novel and unique patterns of renal inflammation in mice exposed to VILI with or without sepsis. We identified two unique inflammatory mediators, VEGF and VCAM-1, that showed significantly increased levels in renal tissue after VILI, as compared with sepsis. We also showed that Angpt2, a potential mediator of MOF, was significantly increased by VILI in the setting of sepsis due to CLP. Angpt2 levels also correlated with greater renal injury as demonstrated by increased renal NGAL expression.
VEGF is an angiogenic protein known to cause endothelial activation and increase microvascular permeability in diffuse inflammatory conditions (10, 24). Microvascular dysfunction is a key element in most multiple organ dysfunction syndromes. Remarkably, we observed increased VEGF levels in the kidney, even in the setting of remote lung injury (VILI). Our findings are consistent with previous work by Choi et al. (5), in which microvascular leak in the kidney was demonstrated in a rat model of VILI. This was associated with increased serum VEGF levels, but renal VEGF levels were not reported. We also observed increased VEGF levels in the kidney in VILI compared with systemic inflammation in CLP. It is possible that cytokines and damage-associated molecular patterns (DAMPs) released during VILI trigger de novo VEGF production in the kidney. Another possibility is that the VEGF in the kidney is of lung origin, as lung tissue has among the highest levels of VEGF in the body (22, 24). Damage to the alveolar capillary membrane during VILI could lead to a massive release of VEGF into systemic circulation and reach the kidney through this mechanism. However, corresponding levels of other vascular effector proteins (discussed below) increased in the VILI groups suggest de novo effects in the kidney. Further research into these possible mechanisms is required to elucidate pathophysiology of biotrauma. Nonetheless, our findings suggest that renal VEGF may be a useful biomarker of distant organ injury in the kidney due to VILI.
VCAM-1 and other adhesion molecules are increasingly expressed following endothelial activation by circulating cytokines (including VEGF) (37, 40). They are known to facilitate leukocyte binding to the endothelium in areas of inflammation and promote microvascular dysfunction and tissue injury (37). We observed increased VCAM-1 protein levels in the kidney after VILI compared with sham and CLP kidneys. Increased mRNA expression of adhesion molecules has been demonstrated in the lungs and other organs in VILI (14). Similar to VEGF levels, we also observed increased renal VCAM-1 levels in VILI compared with sepsis. Interestingly, other adhesion molecules in the proteome array did not show this pattern. This may be due to differences in temporal expression of adhesion molecules in sepsis. After LPS administration in mice, the greatest increase in renal ICAM-1 and VCAM-1 mRNA expression was seen at 2 h, but VCAM-1 expression decreased rapidly and reached control levels at 18 h, while ICAM-1 was still increased at 20-fold (39). There may also be cell-specific differences in constitutive vs. inducible upregulation of adhesion molecules during VILI and sepsis, as has been demonstrated in renal biopsies from patients with allograft rejection (3). Finally, binding to VCAM-1 is much more specific for integrin ligands produced by monocytes and lymphocytes compared with neutrophils, and perhaps the sterile inflammation of VILI affects these leukocytes to a greater extent (25). All of these potential mechanisms warrant further investigation, as does VCAM-1 as a potential biomarker of VILI-mediated AKI.
Angpt2 is another mediator of endothelial inflammation that is rapidly released from Weibel-Palade bodies following endothelial stimulation by cytokines, leukocytes, platelets, alterations in blood flow, and other factors (12, 19). After release, Angpt2 is known to destabilize quiescent endothelium via binding to its cognate Tie-2 receptor, with receptor phosphorylation (12). This process leads to microvascular leak and propagation of inflammation similar to VEGF. The action of Angpt2 is typically rapid, as it is preformed and immediately released. Prolonged elevations in Angpt2 levels in patients are associated with a higher rate of MOF and poor prognosis (4). Recent studies have identified Angpt2 as a potential mediator of MOF in sepsis and ARDS (4, 19, 33, 41). However, the role of Angpt2 in remote organ injury due to VILI has not been previously described. We show here that VILI in the setting of sepsis may be a source of sustained Angpt2 elevation and correlates with greater renal injury, as demonstrated by increased NGAL expression. NGAL is among the most highly induced proteins in the kidney tissue after AKI in animal models and also accepted as a AKI biomarker in clinical studies (8, 26). It has also been shown to play a physiological role in tubular epithelial development and differentiation. Dual injury from VILI and CLP could trigger increased transcription of renal Angpt2, or the activation of a greater number of renal endothelial cells, which requires further investigation.
There are important limitations of our study. Normal tidal volume ventilation may be a more appropriate control for the VILI group to demonstrate that ventilation at normal tidal volume does not alter renal expression of VEGF, VCAM-1, Angpt2, and NGAL. However, our choice of spontaneous breathing control reflects the need to use an appropriate control group for both experimental injury groups (CLP and VILI). In our subsequent detailed investigations into lung-kidney crosstalk in VILI, normal tidal volume ventilation will be utilized as the control group. Also, our findings, although novel, particularly for Angpt2, are preliminary and demonstrate correlation rather than causation, and they warrant further detailed investigations.
In conclusion, we have demonstrated that injurious MV remotely in the lungs led to significantly elevated VEGF and VCAM-1 levels in the kidney compared with systemic inflammation in sepsis. Injurious MV also led to elevated kidney Angpt2 levels after sepsis, correlating with increased renal injury. Further research into these proteins as biomarkers and potential mediators of VILI-induced AKI and distant organ injury is warranted, especially as biotrauma with VILI may be modifiable. A better understanding of the mechanisms underlying biotrauma in lung-kidney interactions is essential to develop innovative therapies aimed at improving the high morbidity and mortality associated with these common disorders.
GRANTS
This work was supported by Veterans Affairs (VA) Merit BX002175 (PS), National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK-107852 (PS) and R03 DK-101841 (to P. Singh), T32 DK-104717 (supporting M. Hepokoski), VA CDA-2 1IK2BX001313 (to L. C. Alexander), Pilot and Feasibility Grant (to L. C. Alexander) from UAB-UCSD O’Brien Center [National Institutes of Health (NIH) P30-DK 079337], NIH K08 GM102695 (J. A. Englert), NIH R01-HL-091957 (to R. M. Baron), NIH R01-HL107652 (to M. M. Fuster), NIH RO1 HL-085188 (to A. Malhotra), K24 HL-132105 (to A. Malhotra).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.H. and J.A.E. performed experiments; M.H., L.E.C.A., M.M.F., and P.S. analyzed data; M.H., J.A.E., R.M.B., L.E.C.A., M.M.F., and P.S. interpreted results of experiments; M.H. prepared figures; M.H. and P.S. drafted manuscript; M.H., J.A.E., R.M.B., L.E.C.A., M.M.F., J.R.B., A.M., and P.S. edited and revised manuscript; M.H. and P.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Some of the results have been presented as abstracts at National Kidney Foundation and American Thoracic Society Annual Meetings.
REFERENCES
- 1.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Brockmeyer C, Ulbrecht M, Schendel DJ, Weiss EH, Hillebrand G, Burkhardt K, Land W, Gokel MJ, Riethmüller G, Feucht HE. Distribution of cell adhesion molecules (ICAM-1, VCAM-1, ELAM-1) in renal tissue during allograft rejection. Transplantation 55: 610–615, 1993. doi: 10.1097/00007890-199303000-00027. [DOI] [PubMed] [Google Scholar]
- 4.Calfee CS, Gallagher D, Abbott J, Thompson BT, Matthay MA, NHLBI ARDS Network . Plasma angiopoietin-2 in clinical acute lung injury: prognostic and pathogenetic significance. Crit Care Med 40: 1731–1737, 2012. doi: 10.1097/CCM.0b013e3182451c87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi WI, Quinn DA, Park KM, Moufarrej RK, Jafari B, Syrkina O, Bonventre JV, Hales CA. Systemic microvascular leak in an in vivo rat model of ventilator-induced lung injury. Am J Respir Crit Care Med 167: 1627–1632, 2003. doi: 10.1164/rccm.200210-1216OC. [DOI] [PubMed] [Google Scholar]
- 6.Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest 118: 239–247, 2008. doi: 10.1172/JCI32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darmon M, Clec’h C, Adrie C, Argaud L, Allaouchiche B, Azoulay E, Bouadma L, Garrouste-Orgeas M, Haouache H, Schwebel C, Goldgran-Toledano D, Khallel H, Dumenil AS, Jamali S, Souweine B, Zeni F, Cohen Y, Timsit JF. Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin J Am Soc Nephrol 9: 1347–1353, 2014. doi: 10.2215/CJN.08300813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devarajan P. NGAL in acute kidney injury: from serendipity to utility. Am J Kidney Dis 52: 395–399, 2008. doi: 10.1053/j.ajkd.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Drury DR, Henry JP, Goodman J. The effects of continuous pressure breathing on kidney function. J Clin Invest 26: 945–951, 1947. doi: 10.1172/JCI101889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146: 1029–1039, 1995. [PMC free article] [PubMed] [Google Scholar]
- 12.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103: 4150–4156, 2004. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 13.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, Kellum JA. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 41: 3–11, 2014. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hegeman MA, Hennus MP, Heijnen CJ, Specht PA, Lachmann B, Jansen NJ, van Vught AJ, Cobelens PM. Ventilator-induced endothelial activation and inflammation in the lung and distal organs. Crit Care 13: R182, 2009. doi: 10.1186/cc8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med 151: 293–301, 1995. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 16.Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V, Cutz E, Liu M, Keshavjee S, Martin TR, Marshall JC, Ranieri VM, Slutsky AS. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 289: 2104–2112, 2003. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- 17.Koyner JL, Murray PT. Mechanical ventilation and lung-kidney interactions. Clin J Am Soc Nephrol 3: 562–570, 2008. doi: 10.2215/CJN.03090707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuiper JW, Vaschetto R, Della Corte F, Plötz FB, Groeneveld AB. Bench-to-bedside review: Ventilation-induced renal injury through systemic mediator release–just theory or a causal relationship? Crit Care 15: 228, 2011. doi: 10.1186/cc10282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kümpers P, Lukasz A, David S, Horn R, Hafer C, Faulhaber-Walter R, Fliser D, Haller H, Kielstein JT. Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care 12: R147, 2008. doi: 10.1186/cc7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kung CT, Su CM, Chang HW, Cheng HH, Hsiao SY, Tsai TC, Chang WN, Tsai NW, Wang HC, Su YJ, Huang CC, Lin WC, Cheng BC, Chang YT, Chiang YF, Lu CH. Serum adhesion molecules as outcome predictors in adult severe sepsis patients requiring mechanical ventilation in the emergency department. Clin Biochem 47: 38–43, 2014. doi: 10.1016/j.clinbiochem.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Liu KD, Matthay MA. Advances in critical care for the nephrologist: acute lung injury/ARDS. Clin J Am Soc Nephrol 3: 578–586, 2008. doi: 10.2215/CJN.01630407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maharaj AS, Saint-Geniez M, Maldonado AE, D’Amore PA. Vascular endothelial growth factor localization in the adult. Am J Pathol 168: 639–648, 2006. doi: 10.2353/ajpath.2006.050834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mårtensson J, Jonsson N, Glassford NJ, Bell M, Martling CR, Bellomo R, Larsson A. Plasma endostatin may improve acute kidney injury risk prediction in critically ill patients. Ann Intensive Care 6: 6, 2016. doi: 10.1186/s13613-016-0108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medford AR, Millar AB. Vascular endothelial growth factor (VEGF) in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): paradox or paradigm? Thorax 61: 621–626, 2006. doi: 10.1136/thx.2005.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyao N, Suzuki Y, Takeshita K, Kudo H, Ishii M, Hiraoka R, Nishio K, Tamatani T, Sakamoto S, Suematsu M, Tsumura H, Ishizaka A, Yamaguchi K. Various adhesion molecules impair microvascular leukocyte kinetics in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 290: L1059–L1068, 2006. doi: 10.1152/ajplung.00365.2005. [DOI] [PubMed] [Google Scholar]
- 26.Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, Viltard M, Yu W, Forster CS, Gong G, Liu Y, Kulkarni R, Mori K, Kalandadze A, Ratner AJ, Devarajan P, Landry DW, D’Agati V, Lin CS, Barasch J. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med 17: 216–222, 2011. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Priebe HJ, Heimann JC, Hedley-Whyte J. Mechanisms of renal dysfunction during positive end-expiratory pressure ventilation. J Appl Physiol 50: 643–649, 1981. [DOI] [PubMed] [Google Scholar]
- 28.Qvist J, Pontoppidan H, Wilson RS, Lowenstein E, Laver MB. Hemodynamic responses to mechanical ventilation with PEEP: the effect of hypervolemia. Anesthesiology 42: 45–55, 1975. doi: 10.1097/00000542-197501000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 273: 117–123, 1995. doi: 10.1001/jama.1995.03520260039030. [DOI] [PubMed] [Google Scholar]
- 31.Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med 157: 1721–1725, 1998. doi: 10.1164/ajrccm.157.6.9709092. [DOI] [PubMed] [Google Scholar]
- 32.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest 128: 525–532, 2005. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 33.Stiehl T, Thamm K, Kaufmann J, Schaeper U, Kirsch T, Haller H, Santel A, Ghosh CC, Parikh SM, David S. Lung-targeted RNA interference against angiopoietin-2 ameliorates multiple organ dysfunction and death in sepsis. Crit Care Med 42: e654–e662, 2014. doi: 10.1097/CCM.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 34.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 34: 1913–1917, 2006. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 35.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators . Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294: 813–818, 2005. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 36.van den Akker JP, Egal M, Groeneveld AB. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care 17: R98, 2013. doi: 10.1186/cc12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verma SK, Molitoris BA. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin Nephrol 35: 96–107, 2015. doi: 10.1016/j.semnephrol.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villar J, Blanco J, Zhang H, Slutsky AS. Ventilator-induced lung injury and sepsis: two sides of the same coin? Minerva Anestesiol 77: 647–653, 2011. [PubMed] [Google Scholar]
- 39.Wu L, Tiwari MM, Messer KJ, Holthoff JH, Gokden N, Brock RW, Mayeux PR. Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiol Renal Physiol 292: F261–F268, 2007. doi: 10.1152/ajprenal.00263.2006. [DOI] [PubMed] [Google Scholar]
- 40.Xing K, Murthy S, Liles WC, Singh JM. Clinical utility of biomarkers of endothelial activation in sepsis–a systematic review. Crit Care 16: R7, 2012. doi: 10.1186/cc11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zinter MS, Spicer A, Orwoll BO, Alkhouli M, Dvorak CC, Calfee CS, Matthay MA, Sapru A. Plasma angiopoietin-2 outperforms other markers of endothelial injury in prognosticating pediatric ARDS mortality. Am J Physiol Lung Cell Mol Physiol 310: L224–L231, 2016. doi: 10.1152/ajplung.00336.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


