Abstract
Zebrafish provide an excellent model in which to assess the role of the renin-angiotensin system in renal development, injury, and repair. In contrast to mammals, zebrafish kidney organogenesis terminates with the mesonephros. Despite this, the basic functional structure of the nephron is conserved across vertebrates. The relevance of teleosts for studies relating to the regulation of the renin-angiotensin system was established by assessing the phenotype and functional regulation of renin-expressing cells in zebrafish. Transgenic fluorescent reporters for renin (ren), smooth muscle actin (acta2), and platelet-derived growth factor receptor-beta (pdgfrb) were studied to determine the phenotype and secretory ultrastructure of perivascular renin-expressing cells. Whole kidney ren transcription responded to altered salinity, pharmacological renin-angiotensin system inhibition, and renal injury. Mesonephric ren-expressing cells occupied niches at the preglomerular arteries and afferent arterioles, forming intermittent epithelioid-like multicellular clusters exhibiting a granular secretory ultrastructure. In contrast, renin cells of the efferent arterioles were thin bodied and lacked secretory granules. Renin cells expressed the perivascular cell markers acta2 and pdgfrb. Transcriptional responses of ren to physiological challenge support the presence of a functional renin-angiotensin system and are consistent with the production of active renin. The reparative capability of the zebrafish kidney was harnessed to demonstrate that ren transcription is a marker for renal injury and repair. Our studies demonstrate substantive conservation of renin regulation across vertebrates, and ultrastructural studies of renin cells reveal at least two distinct morphologies of mesonephric perivascular ren-expressing cells.
Keywords: renin, perivascular, renin-angiotensin system, kidney injury, zebrafish
renin-expressing cells are anatomically restricted to the juxtaglomerular apparatus (JGA) of the adult mammalian metanephros and secrete active renin, the initiating enzyme of the renin-angiotensin system (RAS). The RAS principally functions to maintain cardiovascular homeostasis. In humans, overstimulation of RAS is associated with clinical hypertension and an increased risk of chronic kidney disease (76). Overproduction of angiotensin II (ANG II), the effector of the RAS, may be pharmacologically targeted by angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs; 78). Renin-expressing cells of the developing metanephros are widespread throughout the nascent renal vasculature, yet their role remains poorly understood (19, 20, 62). In the embryo, renin cells may secrete active renin for RAS-mediated homeostasis or developmental pathways (42) and, as activated pericytes, may be required for renal angiogenesis (56, 58, 77).
The canonical RAS first appeared in teleosts, and perivascular ren-expressing cells are conserved in larval zebrafish (14, 38). A complete understanding of their functional relationship across vertebrates is, however, lacking. The intracellular granules of renin cells, only partially characterized in teleosts (8, 32, 33, 50), are fundamental to the synthesis and release of active renin. Fully differentiated renin cells of the mammalian JGA contain a large number of acidic secretory granules that process prorenin into its active form for regulated secretion (71). Mammalian renin cells present during development (72), or those recruited in response to homeostatic challenge (66), exhibit an intermediate phenotype with smaller and sparser renin granules.
The teleost kidney allows modeling of both nephron repair and regeneration postinjury (13, 43, 86). The final-stage kidney of adult zebrafish, the mesonephros, retains a nephron progenitor population throughout life and continually undergoes de novo nephrogenesis (61, 86). Tubular injury is expected to activate the RAS as a result of impaired solute uptake or nephron filtration (31). Post-renal injury, the RAS and renin pericytes may be activated for tubular repair and neonephrogenesis (21, 45, 65). Current evidence suggests that renin cells share a lineage with pericytes, but their differentiation pathways remain to be fully elucidated (7, 70). In mammalian experimental disease models, cells of renin lineage (CoRL) are multipotent and capable of repopulating multiple glomerular cell niches, including pdgfrb-expressing mesangial cells (54, 55, 68).
The aims of the present study were to determine the phenotypes and functions of mesonephric renin-expressing cells in zebrafish. We report that as seen in the developing mammalian metanephros, perivascular renin cells are widespread in the fish mesonephric vascular tree (62). Mesonephric renin-expressing cells display two distinct morphologies and express markers of smooth muscle and pericytes. Consistent with an endocrine function, ren transcription in granulated renin cells is modulated by RAS inhibition, ambient salinity, and renal injury. Our data demonstrating the functional conservation of renin cells across vertebrates establish the zebrafish model for studies of the RAS and its cognate cells.
METHODS
Fish lines and husbandry.
Experiments were approved by the local ethics committee and conducted in accordance with the Animals (Scientific Procedures) Act 1986 in a United Kingdom Home Office-approved establishment. Zebrafish (Danio rerio) were maintained at 28.5°C, as described by Rider et al. (58) and Westerfield (80). Established lines used included WIK, casper (81), Tg(ren:LifeAct-RFP) (58), Tg(kdrl:EGFP) (11), Tg(wt1b:EGFP) (52), and Tg(acta2:EGFP) (82), where RFP is red fluorescent protein and EGFP is enhanced green fluorescent protein. Adult fish were anesthetized with 40 μg/ml tricaine methanesulfonate (MS-222). All fish used in this study were 10–12 mo of age. For all experiments, fish were individually housed in 1-liter tanks in solutions adjusted to pH 7.6. For experiments longer than 24 h, fish were fed daily; otherwise, feed was withheld.
Generation of Tg(pdgfrb:EGFP) fish.
A 7.16-kb region upstream of the pdgfrb translational initiation site was isolated from WIK genomic DNA using the following primer sequences with attB sites for gateway recombination into pDONR P4-P1R (Invitrogen); pdgfrb forward 5′-GGGGACAACTTTGTATAGAAAAGTTGCTTCTCAGGCTCTATCAAGTTGGATGG; pdgfrb reverse 3′-GGGGACTGCTTTTTTGTACAAACTTGCTCAACACTGCAGACGGAGAGAAAAC. The DNA fragment was recombined upstream of EGFP and simian virus 40 (SV40) polyA sequences by three-way gateway cloning into pDestTol2CG2 (containing minimal tol2 ends and cardiac myosin light chain:EGFP) of the tol2 system (37). Plasmid DNA was coinjected with transposase mRNA transcribed in house. Fish with visible pdgfrb-EGFP fluorescence displayed similar expression patterns to those previously reported (3, 79, 83).
In situ hybridization.
Whole adult fish were fixed in 4% paraformaldehyde (PFA) and processed into paraffin-embedded sagittal sections. In situ hybridization (ISH) was conducted using standard protocols (58, 75). Briefly, a 500-bp digoxigenin (DIG)-labeled RNA probe was synthesized from ren cDNA. Embryos were rehydrated, permeabilized, and incubated at 65°C for 16 h in hybridization buffer. Following hybridization, DIG-labeled RNA probes were detected with an alkaline phosphatase-conjugated anti-DIG antibody (Roche) visualized by reaction with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (NBT). Sections were counterstained with methyl green, and ren mRNA was only detected in the renal tissue.
Intracellular acidic granule staining.
Whole kidneys from Tg(ren:LifeAct-RFP, casper) were excised into Dulbecco's modified Eagle's medium (DMEM) with 5 µM Lysotracker Green DND-26 (Molecular Probes) for 1 h at room temperature. Kidney tissue was then prepared by kidney squash for immediate confocal imaging of ren:LifeAct-RFP-expressing cells.
Electron microscopy and ren:LifeAct-RFP immunogold.
Prior to fixation, renal tissue was dissociated from hematopoietic cells by trituration in DMEM. Renal tissue was recovered with a 40-µm cell strainer. Samples were prepared for standard and ren:LifeAct-RFP immunogold electron microscopy (EM) by standard methods (1). Briefly, for immunogold EM, segments were stained with uranyl acetate (2% wt/vol in 0.1 M sodium acetate buffer), dehydrated through increasing concentrations of methanol (70–100%) at −20°C, and embedded in LR Gold (London Resin, Reading, United Kingdom). Ultrathin sections (50–80 nm) were prepared using a Reichert Ultracut S microtome and mounted on 200-mesh nickel grids. Sections were incubated at room temperature for 2 h with anti-RFP (dilution 1:1,000; Clontech, Mountain View, CA) and for 1 h with anti-rabbit IgG-15-nm gold complex (dilution 1:50; BBI, Cardiff, United Kingdom). All antisera were diluted in 0.1 M phosphate buffer containing 0.1% egg albumin. As a secondary antibody negative control the primary antibody was replaced by phosphate buffer/egg albumin, and no labeling was observed. After immunolabelling, sections were lightly counterstained with lead citrate and uranyl acetate and examined with a JEOL transmission electron microscope (JEM-1010; JEOL, Peabody, MA) fitted with an Orius digital camera (Gatan, Pleasanton, CA).
Kidney tissue RNA analysis.
Kidney samples were excised either whole or with the head kidney separated from the trunk and tail regions (Fig. 1D). Tissue was immediately frozen on dry ice and stored at −80°C until analysis. Total RNA was extracted from kidney tissue in TRIzol (Ambion) reagent using a Qiagen RNeasy kit (Qiagen) according to the manufacturer's instructions. RNA samples were quantified using a NanoDrop (Thermo Scientific) and integrity analyzed by gel electrophoresis. RNA (500 ng) was reverse transcribed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems), and real-time PCR was performed with the Universal Probe Library (UPL) system from Roche Diagnostics, using a Roche Lightcycler 480 (Roche, West Sussex, United Kingdom). Primers were designed and utilized with the Roche Universal Probe Library (Table 1). Gene expression was normalized to the mean expression of ribosomal protein S18 (rps18) and elongation factor 1 alpha (eef1a1).
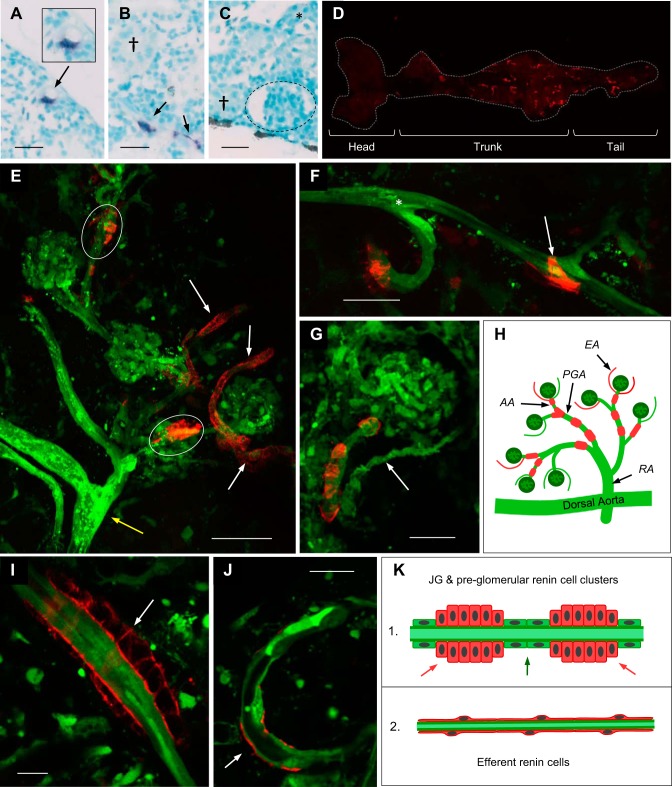
Fig. 1.
Distribution and morphology of mesonephric renin cells. The location and morphology of mesonephric renin cells was assessed across the whole kidney by in situ hybridization and Tg(ren:LifeAct-RFP, kdrl:EGFP). A–C: in situ hybridizations of renal tissue with background structures stained by methyl green and ren mRNA detected by NBT (blue). Perivascular ren is associated with intrarenal vessels (A and B) and not detected in †proximal or *distal tubules or inside glomeruli (dashed outline). Scale bars = 20 µm. GFP and RFP fluorescently label endothelial and renin cells, respectively. D: ventral view of the whole adult kidney in Tg(ren:LifeAct-RFP) showing prominent and sparse expression of ren:LifeAct-RFP in the trunk and tail regions compared with the head kidney, respectively. E–G: maximum-intensity projections of Tg(ren:LifeAct-RFP, kdrl:EGFP). E: group of glomeruli and associated vasculature showing ren:LifeAct-RFP at the afferent arterioles (white ovals) and weaker ren:LifeAct-RFP at the efferent arterioles (white arrows). A larger preglomerular artery is indicated by the yellow arrow. Scale bar = 50 µm. F: expression of ren:LifeAct-RFP at preglomerular arteries. The white arrow shows a branch to an efferent arteriole with ren:LifeAct-RFP, and the asterisk shows a branch without ren:LifeAct-RFP. G: juxtaglomerular (JG) ren:LifeAct-RFP at the afferent arteriole. Scale bars = 25 µm. H: schematic showing localization of renin cells (red) in the renal vasculature (green); RA, renal artery; PGA, preglomerular artery; AA, afferent arteriole; EA, efferent arteriole. I and J: single 1-µm optical sections of Tg(ren:LifeAct-RFP, kdrl:EGFP). I: cross section of multicell epithelioid renin cluster at a preglomerular artery. Boundaries of cuboidal-shaped renin cells are demarcated by ren:LifeAct-RFP. J: cross section of an efferent arteriole showing the thin and small cell body (arrow) of an efferent perivascular renin-expressing cell. Scale bars = 10 µm. K: schematic showing the cross sections of 1) JG and preglomerular renin cell clusters (red arrows) with intermediate smooth muscle cells (green arrow) and 2) efferent arteriolar renin cells. JG and preglomerular renin cells are present as multicellular clusters. Efferent renin cells surround the endothelium with thin-bodied cells that have a low cytoplasmic volume.
Table 1.
Primer sequences and Roche UPL probe number for quantitative PCR analysis
| Gene | Forward | Reverse | UPL Probe |
|---|---|---|---|
| rps18 | GATGGGAAATACAGCCAGGTC | CCAGAAGTGACGGAGACCAC | 41 |
| eef1a1l1 (ef1a) | CCTTCGTCCCAATTTCAGG | CCTTGAACCAGCCCATGTT | 67 |
| ren | AGGCAAGTGGGAGGTCATC | CCATCCTTGCAAAACAGGAT | 43 |
| wt1a | TTACCTGTCCAACTGCATGG | GCGTGTGGCCATAGTTTGA | 1 |
| wt1b | GGCCTGGAATCCTGTTAGC | CAGAGGAGGTGCTCCTGAAG | 107 |
| slc20a1a | GACTCCCAGTCAGCACTACTCA | CGGAAAAGATGCCAATCG | 36 |
| havcr1 (kim1) | AAACCAGAGCCTCGCTAGAA | CCACAGCCATCTCTCTGTTGT | 74 |
| lhx1a | AGTCCGAGAAGAATGCGAAC | GGCCGTAGTACTCGCTTTGA | 80 |
Salinity challenge and captopril treatment.
Fish were exposed to various saline solutions for a 24-h period (n = 8). As similarly described (25, 59), 1× conditioned water (CW) contained 60 mg/l marine salts (Tropic Marin), 1/10 CW contained 6 mg/l marine salts, and 10× Na and K contained 1× CW supplemented with 365 µM KCl and 171 mM NaCl. Waterborne captopril (0.05 mM; C4042; Sigma-Aldrich) was administered in 1× CW for 4 days (n = 10).
Kidney injury and regeneration with captopril treatment.
For the induction of tubular injury, fish were intraperitoneally injected with either 65 mg/kg gentamicin or PBS for sham fish using a 10-µl NanoFil syringe with a 35-gauge needle. Transcriptional responses to kidney injury were determined at 48 h postinjection. For the analysis of regeneration, fish were sampled 8 days postinjection. The effects of RAS inhibition on regeneration were determined with 0.05 mM waterborne captopril from 24 h post-gentamicin injection until sampling. Only fish that responded significantly to captopril treatment were included for further analysis, i.e., those with a mean relative ren expression at or above that of the control fish. For all other groups, n = 8.
Kidney regeneration and spatial analysis of kidney ren.
Fish were subjected to an intraperitoneal dose of either 75 mg/kg gentamicin or PBS for sham fish. Postrecovery, fish were individually housed in 1-liter tanks and fed daily. Head, or tail and trunk kidney regions were sampled for RNA analysis 9 days post-gentamicin injection. Fish not responding significantly to gentamicin, i.e., those with a mean relative wt1 expression at or below that of the mean of sham fish, were excluded from further analysis. Control group n = 7.
Imaging.
Kidney squashes were prepared using fresh tissue in DMEM or fixed tissue (4% PFA in PBS). For fixed tissue, 300 nM 4′,6-diamidino-2-phenylindole (DAPI) was diluted in PBS for nuclear staining. Confocal images were taken with a Leica SP5 using a ×63 or ×100 objective, 3 times averaging, and a 0.5–1-µm z-step size for z-stacks. Optical thickness ranged between 0.5 and 1 µm. Maximal-intensity projections were created with Fiji. Bright-field ISH images were taken using an Olympus Provis AX70 microscope.
Statistical analysis.
Statistical analyses were performed with GraphPad Prism 6 (La Jolla, CA). Differences in means between two treatments were analyzed by an independent samples t-test. Means between three or more groups were subject to a one-way ANOVA and where appropriate followed by a post hoc Bonferroni test for comparisons between predetermined treatment groups. Values are reported as means ± SE, and P values <0.05 were considered significant.
RESULTS
Renin cell localization and morphology.
Visualization of ren:LifeAct-RFP and ren ISH confirmed the location of renin-expressing cells in adult zebrafish. ISH showed that ren mRNA is specifically associated with the mesonephric vasculature and not detectable in glomeruli, tubular epithelium, or hematopoietic cells (Fig. 1, A–C), as characteristic of the developing mouse metanephros (26). The ren:LifeAct-RFP transgene is bona fide for endogenous ren in adults and larval fish (58). Despite an even distribution of nephrons across the mesonephros, ren:LifeAct-RFP (Fig. 1D) and ren mRNA transcripts are spatially varied across the kidney, being markedly reduced in the head kidney compared with the trunk and tail regions (Fig. 7).
Fig. 7.
Effect of kidney regeneration on ren expression in head kidney vs. tail and trunk regions. To determine any involvement of renin in kidney regeneration, ren mRNA was assessed in separate regions of the regenerating kidney. Differences in mRNA transcripts were tested between the head kidney and trunk and tail region. Regenerating kidneys were selected on the basis of the upregulation of the nephron progenitor marker, Wilm's tumor. Renal damage and the subsequent regenerative response were induced by 75 mg/kg ip injected gentamicin. Expression of mRNA was analyzed 9 days postinjection (dpi). A and B: both homologs of Wilm's tumor are increased at 9 dpi, but differences between regions are not significant. C: the tail and trunk kidney region has significantly more ren mRNA than the head kidney. Slight increases in ren mRNA with regeneration were not significant at 9 dpi. P value summary: ns, P ≥ 0.123; **P ≤ 0.002; and ***P ≤ 0.001.
Crossing Tg(ren:LifeAct-RFP) to Tg(kdrl:EGFP), which have endothelial cells labeled with EGFP (11), confirmed the perivascular location of renin cells. Renin is not detectable in the endothelial cells, which is consistent with the distinct lineage of larval zebrafish renin cells to hemangioblasts and endothelial cells (58). Renin is also not detectable in the endothelium of adult fish. ren:LifeAct-RFP was detected 1) in afferent arterioles, at or close to the vascular pole entering the glomerulus, termed the juxtaglomerular (JG) region; 2) in the preglomerular arteries; and 3) in the efferent arterioles (Fig. 1, E–H). Branches of preglomerular arteries were present both with and without ren:LifeAct-RFP (Fig. 1F). As similarly reported in the developing mammalian metanephros (26, 62), renin reporter expression at the afferent arterioles and preglomerular arteries was circumferential and discontinuous (Fig. 1, E, H, and K). In contrast, expression at efferent arterioles was continuous (Fig. 1, E, H, and K).
The use of LifeAct to direct RFP to filamentous actin (F-actin) allowed for the visualization of intracellular myofilaments (60) and increased RFP labeling in thin-bodied perivascular cells. Renin-expressing cells of the preglomerular arteries and JG cells formed multicellular epithelioid-like cell clusters composed of tens of cuboidal-shaped cells (Figs. 1, I and K, and 2K). Conversely, efferent ren:LifeAct-RFP-expressing cells displayed flattened cell bodies thinly covering arterioles (Figs. 1, J and K, and 2J), as similarly observed in the pectoral arteries of larval fish (58). Regardless of morphology, as reported in other fish species, perivascular renin cells were always composed of a single cell layer (Figs. 1I and 2K) (33).
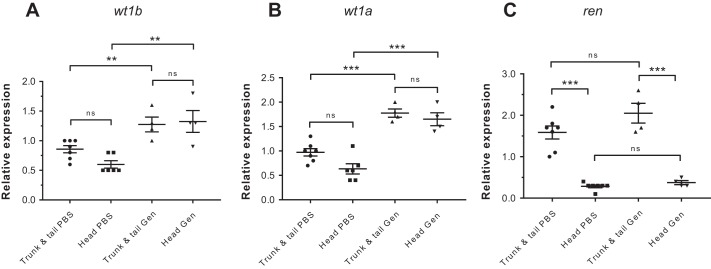
Fig. 2.
Expression of smooth muscle and pericyte markers in mesonephric renin cells. To determine the relationship of renin-expressing cells with smooth muscle cells and pericytes, ren:LifeAct-RFP fish were crossed to transgenic lines for established mural cell markers. In mammals and fish, pdgfrb is an early marker of pericytes and smooth muscle actin (acta2) is a marker of mature pericytes and smooth muscle cells. Expression of perivascular cell markers was assessed in JG, preglomerular, and efferent ren:LifeAct-RFP-expressing cells. A: maximum-intensity projection of Tg(ren:LifeAct-RFP, acta2:EGFP) shows coexpression of acta2-EGFP and ren:LifeAct-RFP in the juxtaglomerular (JG) afferent and efferent cells (glomerulus, white outline; efferent arteriole, white arrow; afferent arteriole, yellow arrow). Scale bar = 50 µm. B–E: single 0.5-µm optical sections of Tg(ren:LifeAct-RFP, acta2:EGFP). B: JG renin cell clusters strongly express acta2. Filamentous actin is highest in density at the luminal region of renin-expressing cells. Scale bar = 50 µm. C: both acta2-EGFP and ren:LifeAct-RFP are also coexpressed in preglomerular arteriolar renin clusters (white arrows) in Tg(ren:LifeAct-RFP, acta2:EGFP). Scale bar = 25 µm. D: clusters of ren:LifeAct-RFP express acta2-EGFP at a preglomerular branch point in Tg(ren:LifeAct-RFP, acta2:EGFP). Scale bar = 25 µm. E: detail of preglomerular arteriolar cells from D showing renin cells have a cuboidal shape in contrast to neighboring and thinner-bodied smooth muscle cells (white arrow). Scale bar = 10 µm. F: expression of acta2:EGFP is always present but variable as shown by the projection of weaker expression in some preglomerular renin cell clusters. Scale bar = 25 µm. G and H: 0.5-µm optical sections of Tg(ren:LifeAct-RFP, pdgfrb:EGFP) showing detail of pdgfrb-EGFP expression both at a preglomerular artery (G) and in JG cells (H). Scale bars = 10 µm. I: a single 0.5-µm optical section of Tg(ren:LifeAct-RFP, pdgfrb:EGFP) showing coexpression of ren:LifeAct-RFP and pdgfrb-EGFP in the afferent JG cells (yellow arrow). The efferent arteriole (white arrow) also expresses pdgfrb-EGFP; glomerulus, white outline. Scale bar = 25 µm. J and K: Confocal projections of nuclei stained with DAPI (gray) inside ren:LifeAct-RFP-expressing cells (yellow outlines). In renin cells of the efferent arterioles, nuclei are flattened occupying a thin-bodied cell (J). Epithelioid renin cells are present as multicellular clusters (K). Nuclei not outlined within ren:LifeAct-RFP regions are endothelial. Scale bars = 10 µm. L and M: Tg(ren:LifeAct-RFP, wt1b:GFP) showing early-stage (L) and late-stage (M) nascent nephron clusters expressing Wilm's tumor (wt1b:GFP). Expression of ren:LifeAct-RFP is only detected in the latter stages of nephron development and is associated with the juxtaglomerular cells of the afferent arteriole. *Glomeruli. Scale bars = 25 µm.
Smooth muscle and pericyte markers in renin cells.
The relationship of renin-expressing cells to smooth muscle cells (SMC) expressing (acta2) and pericytes expressing (pdgfrb) was analyzed using Tg(ren:LifeAct-RFP, acta2:EGFP) and Tg(ren:LifeAct-RFP, pdgfrb:EGFP) fish, respectively. With an inverse relationship to smooth muscle actin (SMA), mammalian renin expression increases along metanephric afferent arterioles with decreasing distance from the glomerulus (35, 36, 48, 62). In zebrafish, all renin-expressing cells coexpressed acta2:EGFP (Fig. 2, A–F), and no inverse relationship between acta2 and ren was evident. Occasional cell clusters at nonspecific vascular locations exhibited a lower acta2:EGFP expression (Fig. 2F), which may represent nascent renin cells acquiring a SMC phenotype during maturation (40). SMCs between renin cell clusters were observed to have thinner cell bodies than epithelioid ren-expressing cells (Fig. 2, C–E). Expression of mammalian pericyte markers NG2 and CD146 precedes αSMA during embryonic renin cell differentiation (69). The expression of pdgfrb:EGFP in epithelioid renin cells (Fig. 2, G–I) suggests that as observed in mammals, zebrafish mesonephric renin cells maintain a functional relationship with pericytes (69).
Renin cell intracellular structure.
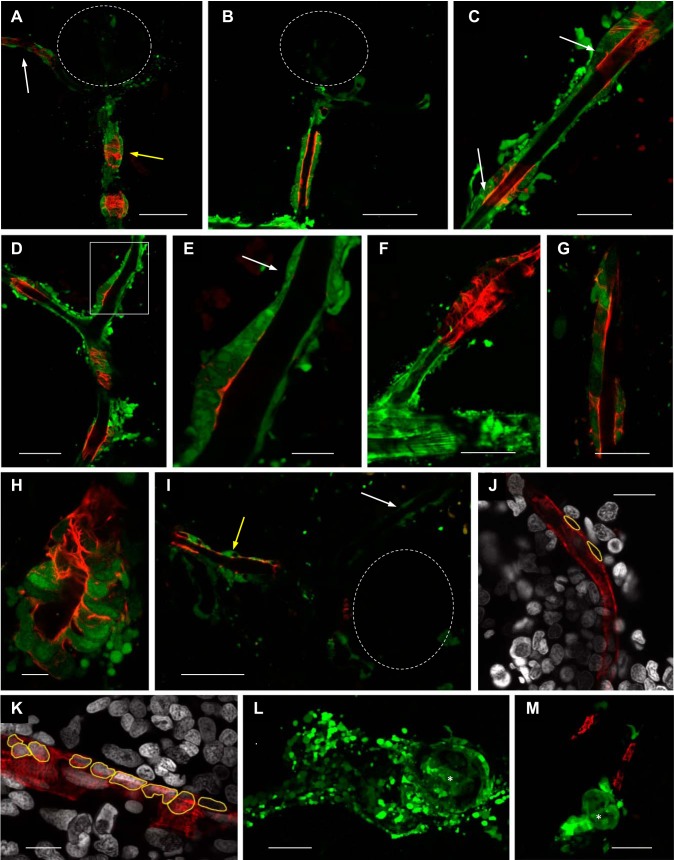
The intracellular structure of ren:LifeAct-RFP-expressing cells was assessed by immunogold electron microscopy and LysoTracker Green. The acidic milieu of mammalian renin granules is thought to be required for the activation of prorenin by cleavage of its pro segment to active renin (84). To test for the presence of acidic granules in fish, ren:LifeAct-RFP-expressing cells were stained with the acidotrophic dye LysoTracker Green, which stains lysosomes and other intracellular acidic organelles. Acidic granules were present in renin cell clusters at both the afferent arterioles and preglomerular arteries (Fig. 3, A and B). By comparison, very few acidic organelles were observed in the efferent arterioles (Fig. 3C).
Fig. 3.
Presence of acidic intracellular vesicles in renin cells. To test for the presence of acidic granules in renin cells across the mesonephric kidney, whole kidney squashes of Tg(ren:LifeAct-RFP) were stained with LysoTracker Green. Single 0.5-µm optical sections were taken by confocal microscopy to assess staining in ren:LifeAct-RFP-expressing cells. A: a juxtaglomerular ren-RFP cell cluster with regions of punctate intracellular LysoTracker staining (white arrows). B: preglomerular arteriolar renin cell cluster also with regions of punctate LysoTracker staining (white arrows). C: efferent arteriole showing very occasional LysoTracker-stained vesicles (arrows). All scale bars = 10 µm.
F-actin visualized in ren:LifeAct-RFP-expressing cells was most prominent at the luminal side of the cells, and to a lesser extent at renin cell-to-renin cell boundaries (Fig. 2, B–E). Mammalian renin cells with an intermediate SMA and renin phenotype contain visible myofilaments, which are difficult to detect in fully differentiated cells of the JGA (22, 73).
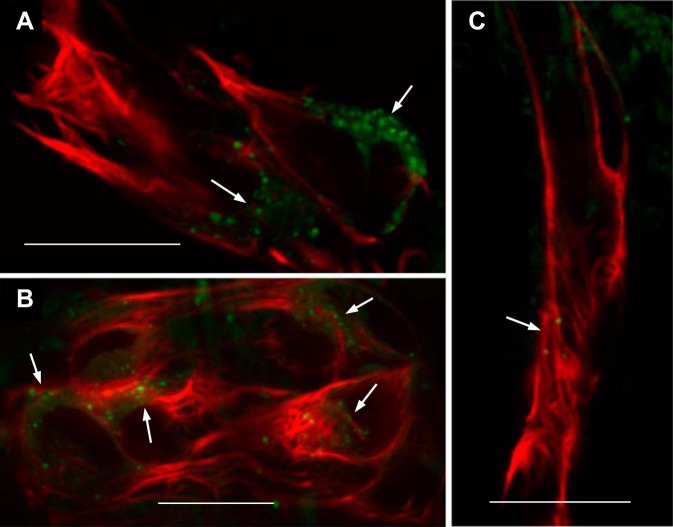
Immunogold-stained ren:LifeAct-RFP cells contained either a highly vacuolated structure with small 50–200-nm electron-dense granules of various sizes, or a cytoplasm packed with numerous uniformly sized electron-dense granules. As with mammalian renin cells, different intracellular structures suggest different stages of cell maturity. Renin cells (Fig. 4, A–D) with a more vacuolated structure are believed to be representative of an immature endocrine structure. The partially filled protogranules (Fig. 4B) and paracrystalline granules (Fig. 4C) observed in zebrafish renin cells are also reported in immature mammalian counterparts (74). Cells with highly packed granules (Fig. 4, E–H), ranging from 150 to 400 nm in size, are expected to represent a fully endocrine renin-secreting cell. Renin granules in zebrafish are similar to the mean 230-nm size of carp renin granules (32). No large mammalian-like granules (~500 nm in size) are present in the zebrafish kidney (71).
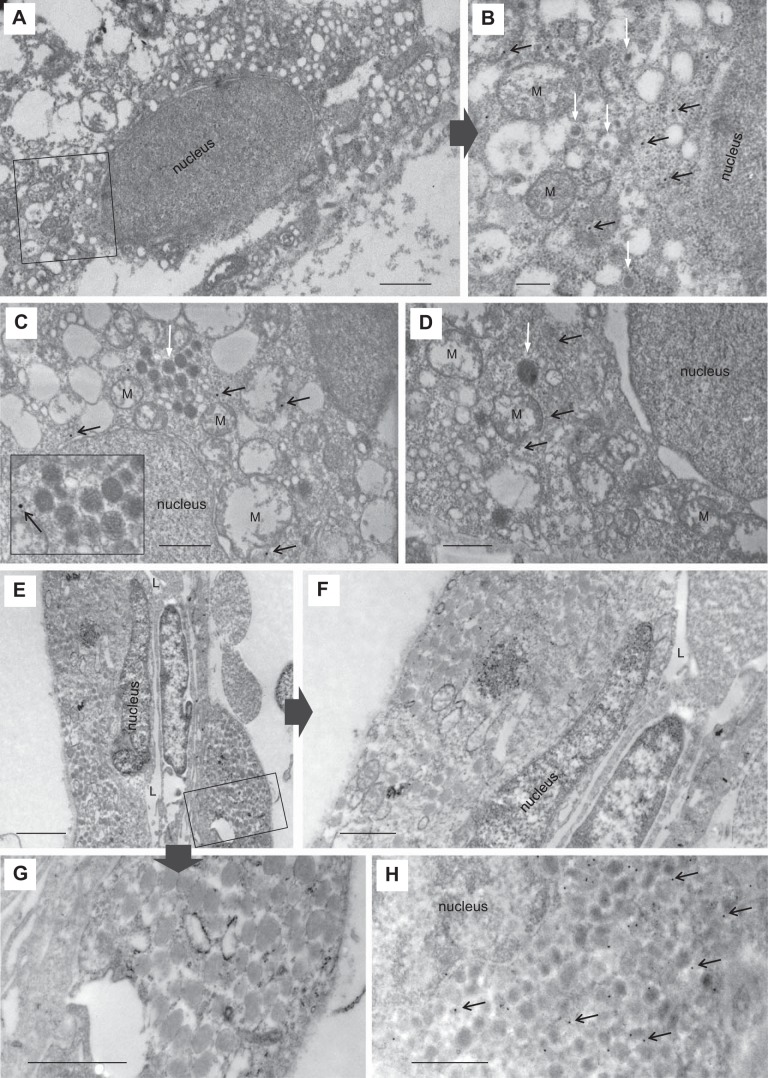
Fig. 4.
Intracellular ultrastructure of mesonephric renin cells. The intracellular structure of renin-expressing cells was determined by electron microscopy (EM). Renin-expressing cells in Tg(ren:LifeAct-RFP) were identified using 15-nm immunogold labeling against ren:LifeAct-RFP. Example immunogold particles are highlighted with black arrows, and mitochondria are highlighted with “M.” Immunogold staining is specific, only being present in the cytoplasm and not nuclei. A: gold-labeled ren:LifeAct-RFP cell with a highly vacuolated intracellular structure and several mitochondria. The rectangle outline is of B. Scale bar = 2 µm. B: a higher magnification of a portion of A shows detail of clear vesicles, some of which are partially filled with electron-dense material (white arrows). Scale bar = 400 nm. C and D: immunogold-labeled cells with a vacuolated intracellular structure and occasional 50–200-nm electron-dense granules with paracrystalline content (white arrows). Paracrystalline granules in C are highlighted at higher magnification in insert. Scale bars = 200 (C) and 500 nm (D). E: standard EM showing renin and endothelial cells, the latter recognizable by elongated nuclei. The vessel lumen is visible (L). The rectangular outline is of G. Scale bar = 2 µm. F and G: higher magnification of E showing numerous 150–400-nm electron-dense granules. Scale bars (F and G) = 1 µm. H: intracellular structure of immunogold-labeled cell showing electron-dense granules of similar size (150–350 nm). Scale bar = 1 µm.
Physiological challenge and ren transcription.
In mammals, low sodium (23, 85) or RAS inhibition by ACE inhibitors (18, 36, 66) both increase renin transcription, plasma renin activity, and renin cell distribution down the afferent arteriole (17, 41). RAS inhibition blocks the ANG II-mediated homeostatic negative feedback mechanism that suppresses renin secretion (22). Consistent with a functional RAS, our data show that ren is upregulated by both captopril and decreasing salinity (Fig. 5), as also reported in larval zebrafish (25, 58). This supports a RAS-mediated sodium homeostasis in adult zebrafish. In mammalian whole kidney, upregulation of renin transcription results from renin cell recruitment or upregulation within individual cells (66).
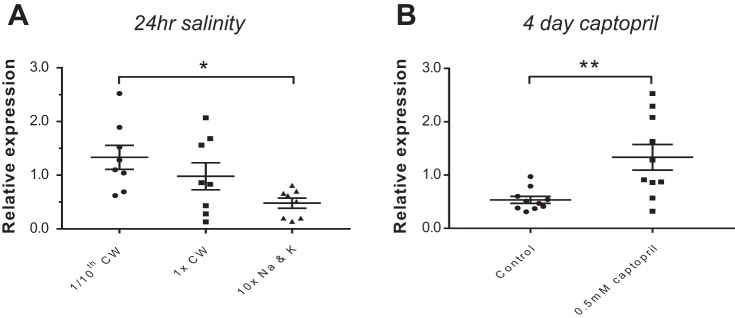
Fig. 5.
Transcriptional response of whole kidney ren to salinity and captopril. To determine whether variation in salinity initiated a homeostatic response, adult fish were exposed to varying ambient salinities. A: the 24-h exposure to varied salinity decreased ren expression with increasing salinity and vice versa. B: the upregulation of ren due to the lack of a negative feedback on transcription associated with RAS inhibition was determined in fish treated with 0.5 mM waterborne captopril for 4 days. This resulted in a significant upregulation of renin mRNA. P value summary: *P ≤ 0.033 and **P ≤ 0.002.
Transcriptional ren responses to kidney injury.
The response of ren transcription to kidney injury was assessed by use of a well-characterized acute kidney injury (AKI) model. The aminoglycoside antibiotic gentamicin is toxic to proximal tubular cells (28, 43, 86). Postinjury, adult zebrafish undergo nephron repair followed by de novo nephrogenesis to fully restore kidney function by 21 days. The response of whole kidney renin transcription was tested during the injury phase and during nephron repair and regeneration (43). Renal injury was confirmed 2 days postinjection by a marked upregulation of kidney injury molecule 1 (kim1) and a concurrent decrease of the proximal tubular marker slc20a1a, a sodium-dependent phosphate transporter (Fig. 6, A and B). This was associated with a significant upregulation of ren transcription (Fig. 6F) implying RAS activation in response to renal injury.
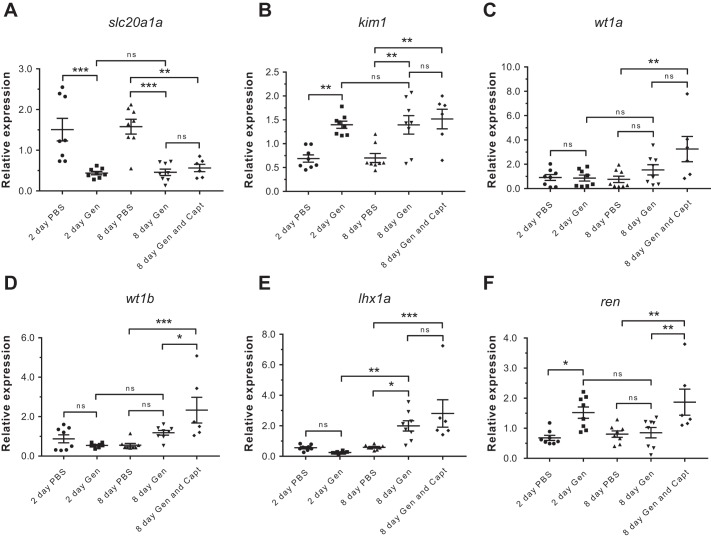
Fig. 6.
Effect of renal damage on renin expression and effect of RAS inhibition on kidney regeneration. To test responses of ren transcription to renal injury and regeneration, mRNA expression was tested in whole kidney. The role for RAS during regeneration was tested using captopril RAS inhibition from 24 h postinjection. Renal damage was induced by 65 mg/kg intraperitoneal (ip) gentamicin (Gen) injection and analyzed 2 days postinjection (dpi). Whole kidneys were analyzed both with and without 0.5 mM waterborne captopril (Capt) during kidney regeneration at 8 dpi. A: the decrease of the proximal tubular marker, solute carrier slc20a1a, confirms damage of the proximal tubule at both 2 and 8 dpi. B: kidney damage is also confirmed by the upregulation of kidney injury molecule (kim1) at 2 and 8 dpi. C and D: the slight increase of both Wilm's tumor homologs is not significant at 8 dpi. Wilm's tumor 1b is upregulated in regenerating kidneys subject to captopril treatment. E: the nephron progenitor marker LIM homeobox 1a (lhx1a) is upregulated 8 days postinjection confirming a regenerative response. Expression of lhx1a is not affected by captopril treatment. F: renin mRNA is upregulated with the renal injury at 2 dpi. Expression of ren subsequently decreases to control levels after the renal tissue progresses from an injury phase to regeneration at 8 dpi. As occurs under normal conditions, renin expression is increased by captopril in regenerating kidneys. P value summary: ns, P ≥ 0.123; *P ≤ 0.033; **P ≤ 0.002; and ***P ≤ 0.001.
Early markers of nephron progenitors were upregulated during nephron repair and neonephrogenesis. Upregulation of lhx1a (Fig. 6E) 8 days postinjury confirmed a reparative response involving activation of renal progenitor cells. There was a trend toward increased transcription of both Wilm's tumor (wt1) homologs, but these were not statistically significant (Fig. 6, C and D). De novo nephrogenesis is in its early stages 8 days postinjury and was demonstrated in our study by a maintained low and high transcription of slc20a1a and kim1, respectively. During this early phase of kidney repair, ren transcripts returned to normal levels suggesting that the RAS may have a limited function during early kidney repair and neonephrogenesis. In the zebrafish mesonephros, renin cells are not associated with individual new nephrons until the latter stages of neonephrogenesis (Fig. 2, L and M).
The requirement for RAS in renal repair and regeneration was tested using pharmacological RAS inhibition during injury recovery. Expression of ren was significantly upregulated as expected with RAS blockade by captopril. Captopril treatment had no marked effect on the resolution of AKI, as determined by the lack of any change in expression of slc20a1a and kim1 transcripts 8 days postinjury (Fig. 6F).
Spatial variation of ren expression.
Early nephron progenitor markers are ubiquitously expressed across the head, trunk, and tail regions of the kidney (13). Transcripts of ren and wt1 were determined in the head kidney and compared with the trunk and tail regions. No significant spatial differences were observed in either wt1 homolog, and both genes were similarly upregulated across the kidney during regeneration (Fig. 7, A and B). Conversely, ren:LifeAct-RFP expression (Fig. 1D) and ren mRNA transcripts (Fig. 7C) were significantly higher in the tail and trunk regions than the head kidney. As previously observed (Fig. 6F), ren was not significantly upregulated during regeneration.
DISCUSSION
This first study of renin-expressing cells in the zebrafish mesonephros reveals two distinct morphologies of renin cell. Only epithelioid-like renin cells contained a secretory intracellular structure consistent with active renin secretion. The ren:LifeAct-RFP transgene faithfully recapitulates renin expression in both adult and larval zebrafish (58). As characteristic of the mammalian mesonephros and developing metanephros (27), zebrafish ren is exclusively detected in perivascular cells (19). A low expression of renin mRNA is detectable in the mammalian metanephric proximal tubule (10, 64), but not in zebrafish. The localization of perivascular renin cells in zebrafish is similar to that observed in developing mammals (26, 62). In the human mesonephros (9), and initially the developing mammalian metanephros (9, 26, 62), renin expression is associated with preglomerular arteries and arterioles before a postnatal restriction to the JGA (20, 22, 26, 47, 62, 73). In the mammalian and piscine mesonephroi (12, 33), there is no association between renin cells and the distal tubules. Consequently, although present at the pole of the afferent arteriole, mesonephric JG renin cells are not part of a structured JGA.
The lack of granulation in efferent renin cells suggests that these postglomerular perivascular cells differ in their function from their secretory counterparts. In mice, 20–40% of efferent arterioles are renin positive, of which a portion are granulated (73). By virtue of their low cell volume compared with the cuboidal secretory renin cells, acidic granules are sparse in their efferent counterparts. Without secretory granules, efferent renin cells are expected to have a limited capacity for the regulated excretion of active renin but may constitutively secrete prorenin.
The mesenchymal precursor of the renin-expressing cell is postulated to share a lineage with pericytes. Mammalian pericyte markers (Rgs5, NG2, and CD146) are detected in both adult and embryonic renin cells (4, 7, 67, 70). Pericytes and renin cells both derive from mesenchymal FoxD1 cells (16, 39, 63, 65). Their relationship is evident in experimental renal injury where CoRL repopulate multiple glomerular cell niches, including the mesangium (2, 53–55, 68). The expression of individual pericyte markers in renin cells may be species specific, since neither adult nor embryonic murine renin cells express pdgfrb, nor do they rely on its expression for their differentiation (49). In larval fish, pdgfrb- and ren-expressing cells both require Notch signaling for their differentiation, and both arise from the lateral mesoderm to occupy the same cell niche at the ventral dorsal aorta (3, 58). As for mammalian renin cells (49), zebrafish pdgfrb-expressing cells do not rely on pdgfr signaling for their differentiation (3).
Mammalian renin cells have a reversible phenotype switching from contractile smooth muscle cells to an endocrine renin phenotype during development or physiological challenge (7, 17, 40, 62, 66). In some instances, renin cells of the JGA lose detectable SMA (29, 49, 51). Zebrafish renin cells do not lose SMA expression toward the glomerulus and maintain expression of early pericyte markers. Although the differentiation and phenotypic switch of renin cells may differ across vertebrates, their physiological function appears to be conserved.
Prior to their maturation into JG cells with a full endocrine phenotype (6, 15, 57), embryonic renin cells present at branch points may release paracrine tropic factors required for angiogenesis of nascent renal vessels (56, 58). Experimental ablation of renin cells or RAS during development results in renal vascular defects (21, 39, 77). In the renal vasculature of rats (56) and the anterior mesenteric artery of larval zebrafish (58), renin-expressing cells are associated with branch points. In mice, renin is not preferentially expressed at intrarenal vessel branch points (62). Despite the ubiquitous distribution of nephron progenitors expressing wt1 across the zebrafish kidney tissue (86), renin cells are lower in density in the head kidney. In our studies of the zebrafish mesonephros, which continually undergoes de novo nephrogenesis (86), no angiogenic sprout tips associated with renin cells were observed. In mammals, the RAS may be implicated in angiogenesis via the angiotensin II receptor type 2-mediated activation of Vegf, a potent stimulator of vasculogenesis and angiogenesis (30).
The endocrine phenotype of mesonephric renin cells in zebrafish was confirmed by the presence of either an immature or fully granulated ultrastructure, as characteristic of mammalian renin cells. Embryonic or intermediate mammalian renin cells contain a variable number of small electron-dense protogranules (74), as observed in adult zebrafish. Paracrystalline granules, which largely contain prorenin in mammals (72), were also present in zebrafish renin cells. The numerous uniformly sized electron-dense granules characteristic of mature renin cells are also reported in other fish species (32) and are approximately half the size of mammalian granules. The activation of mammalian renin by the cleavage of prorenin is proposed to occur because of the acidic milieu of secretory granules (84). The presence of acidic granules suggests that renin activation is likely to be conserved in the zebrafish.
The response of mesonephric renin cells to salinity variation, pharmacological RAS inhibition, and renal injury is consistent with their endocrine function within a functional RAS. With the exception of the Mas receptor, zebrafish contain all components of the RAS including ACE 1 and 2, and both angiotensin receptors (14). Our data in adult zebrafish showing the modulation of renin mRNA with varying salinity are consistent with a role for the RAS in ion homeostasis, which is also evident in larval zebrafish (25, 34). The robust response of ren transcription to tubular injury may be due to tubular obstruction and a reduction in glomerular filtration, or the impaired tubular reabsorption of solutes stimulating tubuloglomerular feedback and associated renin secretion (5, 46). Indeed, the zebrafish gentamicin injury model is known to result in tubular obstruction due to the formation of epithelial casts (24, 44). Impaired solute transport is also expected with a significant decrease in slc20a1a-expressing proximal tubular cells.
RAS activity is required for mammalian renal development (21, 58, 77) and may be activated during nephron repair and regeneration (78). Eight to nine days postinjury, both tubular repair and de novo nephrogenesis occur in the zebrafish (43). Aggregates of nephron progenitor cells expressing wt1 and lhx1a reach a peak by 9 days postinjury (13, 86). In our study, upregulation of lhx1a confirms the activation of nephron progenitors during repair and regeneration, but this was not associated with an upregulation of ren. These data show that although the RAS is activated during renal injury, RAS activity is similar to baseline levels during the initial phase of renal repair.
These data from zebrafish show that although forming two distinct morphological populations, mesonephric renin cells share numerous similarities to their embryonic mammalian counterparts. The characteristic granular and epithelioid renin cell phenotype is maintained in fish. Functionally, mesonephric renin cells respond to RAS-mediated challenges in a similar manner to mammals demonstrating the conservation of the physiological actions of the RAS across vertebrates. Our studies demonstrate the relevance of adult zebrafish as an excellent model species for evaluating the mechanisms associated with the clinical improvement of renal function under RAS inhibition.
GRANTS
This work was financially supported by a British Heart Foundation Centre of Research Excellence award and Kidney Research UK. The authors also acknowledge financial support from the Wellcome Trust for the zebrafish facility.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A.R., H.C.C., L.J.M., C.A.M., and J.J.M. conceived and designed research; S.A.R., H.C.C., L.J.M., and A.R.H. performed experiments; S.A.R. and H.C.C. analyzed data; S.A.R., H.C.C., L.J.M., C.A.M., and J.J.M. interpreted results of experiments; S.A.R. prepared figures; S.A.R. drafted manuscript; S.A.R., H.C.C., L.J.M., C.A.M., and J.J.M. edited and revised manuscript; S.A.R., H.C.C., L.J.M., C.A.M., and A.R.H. approved the final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Prof. K. Kawakami, Prof. D. Lyons, and Dr. T. Czopka for Tol2 kit plasmids; Prof. Didier Stainier for the acta2:GFP line; Prof. B. Peault, Dr. Charlotte Buckley, and Dr. C. Tucker for helpful discussions; and facility staff for fish husbandry.
REFERENCES
- 1.Abel MH, Charlton HM, Huhtaniemi I, Pakarinen P, Kumar TR, Christian HC. An investigation into pituitary gonadotrophic hormone synthesis, secretion, subunit gene expression and cell structure in normal and mutant male mice. J Neuroendocrinol 25: 863–875, 2013. doi: 10.1111/jne.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altintas MM, Reiser J. Bridges to cross, burn, and mend: cells of renin lineage as podocyte progenitors. Am J Physiol Renal Physiol 309: F499– F500, 2015. doi: 10.1152/ajprenal.00301.2015. [DOI] [PubMed] [Google Scholar]
- 3.Ando K, Fukuhara S, Izumi N, Nakajima H, Fukui H, Kelsh RN, Mochizuki N. Clarification of mural cell coverage of vascular endothelial cells by live imaging of zebrafish. Development 143: 1328– 1339, 2016. doi: 10.1242/dev.132654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21: 193–215, 2011. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2: 1303–1353, 2012. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncol 7: 452–464, 2005. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunskill EW, Sequeira-Lopez MLS, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA. Genes that confer the identity of the renin cell. J Am Soc Nephrol 22: 2213–2225, 2011. doi: 10.1681/ASN.2011040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capréol SV, Sutherland LE. Comparative morphology of juxtaglomerular cells. I. Juxtaglomerular cells in fish. Can J Zool 46: 249–256, 1968. doi: 10.1139/z68-038. [DOI] [Google Scholar]
- 9.Celio MR, Groscurth P, Inagami T. Ontogeny of renin immunoreactive cells in the human kidney. Anat Embryol (Berl) 173: 149–155, 1985. doi: 10.1007/BF00316297. [DOI] [PubMed] [Google Scholar]
- 10.Chen M, Harris MP, Rose D, Smart A, He XR, Kretzler M, Briggs JP, Schnermann J. Renin and renin mRNA in proximal tubules of the rat kidney. J Clin Invest 94: 237–243, 1994. doi: 10.1172/JCI117312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi J, Dong L, Ahn J, Dao D, Hammerschmidt M, Chen J-N. FoxH1 negatively modulates flk1 gene expression and vascular formation in zebrafish. Dev Biol 304: 735–744, 2007. doi: 10.1016/j.ydbio.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Martino C, Zamboni L. A morphologic study of the mesonephros of the human embryo. J Ultrastruct Res 16: 399–427, 1966. doi: 10.1016/S0022-5320(66)80072-2. [DOI] [PubMed] [Google Scholar]
- 13.Diep CQ, Ma D, Deo RC, Holm TM, Naylor RW, Arora N, Wingert RA, Bollig F, Djordjevic G, Lichman B, Zhu H, Ikenaga T, Ono F, Englert C, Cowan CA, Hukriede NA, Handin RI, Davidson AJ. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature 470: 95–100, 2011. doi: 10.1038/nature09669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fournier D, Luft FC, Bader M, Ganten D, Andrade-Navarro MA. Emergence and evolution of the renin-angiotensin-aldosterone system. J Mol Med (Berl) 90: 495–508, 2012. doi: 10.1007/s00109-012-0894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaengel K, Genové G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 29: 630–638, 2009. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 16.Gomez IG, Duffield JS. The FOXD1 lineage of kidney perivascular cells and myofibroblasts: functions and responses to injury. Kidney Int Suppl (2011) 4: 26–33, 2014. doi: 10.1038/kisup.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez RA, Belyea B, Medrano S, Pentz ES, Sequeira-Lopez MLS. Fate and plasticity of renin precursors in development and disease. Pediatr Nephrol 29: 721–726, 2014. doi: 10.1007/s00467-013-2688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez RA, Lynch KR, Chevalier RL, Everett AD, Johns DW, Wilfong N, Peach MJ, Carey RM. Renin and angiotensinogen gene expression and intrarenal renin distribution during ACE inhibition. Am J Physiol Renal Physiol 254: F900–F906, 1988. [DOI] [PubMed] [Google Scholar]
- 19.Gomez RA, Lynch KR, Sturgill BC, Elwood JP, Chevalier RL, Carey RM, Peach MJ. Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol Renal Physiol 257: F850–F858, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Gomez RA, Pupilli C, Everett AD. Molecular and cellular aspects of renin during kidney ontogeny. Pediatr Nephrol 5: 80–87, 1991. doi: 10.1007/BF00852854. [DOI] [PubMed] [Google Scholar]
- 21.Guron G, Friberg P. An intact renin-angiotensin system is a prerequisite for normal renal development. J Hypertens 18: 123–137, 2000. doi: 10.1097/00004872-200018020-00001. [DOI] [PubMed] [Google Scholar]
- 22.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev 70: 1067–1116, 1990. [DOI] [PubMed] [Google Scholar]
- 23.Harding P, Sigmon DH, Alfie ME, Huang PL, Fishman MC, Beierwaltes WH, Carretero OA. Cyclooxygenase-2 mediates increased renal renin content induced by low-sodium diet. Hypertension 29: 297–302, 1997. doi: 10.1161/01.HYP.29.1.297. [DOI] [PubMed] [Google Scholar]
- 24.Hentschel DM, Park KM, Cilenti L, Zervos AS, Drummond I, Bonventre JV. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol 288: F923–F929, 2005. doi: 10.1152/ajprenal.00386.2004. [DOI] [PubMed] [Google Scholar]
- 25.Hoshijima K, Hirose S. Expression of endocrine genes in zebrafish larvae in response to environmental salinity. J Endocrinol 193: 481–491, 2007. doi: 10.1677/JOE-07-0003. [DOI] [PubMed] [Google Scholar]
- 26.Jones CA, Hurley MI, Black TA, Kane CM, Pan L, Pruitt SC, Gross KW. Expression of a renin/GFP transgene in mouse embryonic, extra-embryonic, and adult tissues. Physiol Genomics 4: 75–81, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Jones CA, Sigmund CD, McGowan RA, Kane-Haas CM, Gross KW. Expression of murine renin genes during fetal development. Mol Endocrinol 4: 375–383, 1990. doi: 10.1210/mend-4-3-375. [DOI] [PubMed] [Google Scholar]
- 28.Kamei CN, Liu Y, Drummond IA. Kidney regeneration in adult zebrafish by gentamicin induced injury. J Vis Exp 102: e51912, 2015. doi: 10.3791/51912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karger C, Kurtz F, Steppan D, Schwarzensteiner I, Machura K, Angel P, Banas B, Risteli J, Kurtz A. Procollagen I-expressing renin cell precursors. Am J Physiol Renal Physiol 305: F355–F361, 2013. doi: 10.1152/ajprenal.00079.2013. [DOI] [PubMed] [Google Scholar]
- 30.Khakoo AY, Sidman RL, Pasqualini R, Arap W. Does the renin-angiotensin system participate in regulation of human vasculogenesis and angiogenesis? Cancer Res 68: 9112–9115, 2008. doi: 10.1158/0008-5472.CAN-08-0851. [DOI] [PubMed] [Google Scholar]
- 31.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 32.Kon Y, Hashimoto Y, Kitagawa H, Kudo N. Morphological and immunohistochemical studies of juxtaglomerular cells in the carp, Cyprinus carpio. Nippon Juigaku Zasshi 49: 323–331, 1987. doi: 10.1292/jvms1939.49.323. [DOI] [PubMed] [Google Scholar]
- 33.Krishnamurthy VG, Bern HA. Correlative histologic study of the corpuscles of Stannius and the juxtaglomerular cells of teleost fishes. Gen Comp Endocrinol 13: 313–335, 1969. doi: 10.1016/0016-6480(69)90255-X. [DOI] [PubMed] [Google Scholar]
- 34.Kumai Y, Bernier NJ, Perry SF. Angiotensin-II promotes Na+ uptake in larval zebrafish, Danio rerio, in acidic and ion-poor water. J Endocrinol 220: 195–205, 2014. doi: 10.1530/JOE-13-0374. [DOI] [PubMed] [Google Scholar]
- 35.Kurt B, Kurtz A. Plasticity of renal endocrine function. Am J Physiol Regul Integr Comp Physiol 308: R455–R466, 2015. doi: 10.1152/ajpregu.00568.2013. [DOI] [PubMed] [Google Scholar]
- 36.Kurtz A. Renin release: sites, mechanisms, and control. Annu Rev Physiol 73: 377–399, 2011. doi: 10.1146/annurev-physiol-012110-142238. [DOI] [PubMed] [Google Scholar]
- 37.Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien C-B. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn 236: 3088–3099, 2007. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- 38.Liang P, Jones CA, Bisgrove BW, Song L, Glenn ST, Yost HJ, Gross KW. Genomic characterization and expression analysis of the first nonmammalian renin genes from zebrafish and pufferfish. Physiol Genomics 16: 314–322, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Lin EE, Sequeira-Lopez MLS, Gomez RA. RBP-J in FOXD1+ renal stromal progenitors is crucial for the proper development and assembly of the kidney vasculature and glomerular mesangial cells. Am J Physiol Renal Physiol 306: F249–F258, 2014. doi: 10.1152/ajprenal.00313.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol 281: F345–F356, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Lopez ML, Gomez RA. The renin phenotype: roles and regulation in the kidney. Curr Opin Nephrol Hypertens 19: 366–371, 2010. doi: 10.1097/MNH.0b013e32833aff32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lumbers ER. Functions of the renin-angiotensin system during development. Clin Exp Pharmacol Physiol 22: 499–505, 1995. doi: 10.1111/j.1440-1681.1995.tb02057.x. [DOI] [PubMed] [Google Scholar]
- 43.McCampbell KK, Springer KN, Wingert RA. Atlas of cellular dynamics during zebrafish adult kidney regeneration. Stem Cells Int 2015: 547636, 2015. doi: 10.1155/2015/547636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKee RA, Wingert RA. Zebrafish renal pathology: emerging models of acute kidney injury. Curr Pathobiol Rep 3: 171–181, 2015. doi: 10.1007/s40139-015-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Methot D, Reudelhuber TL. Knockout of renin-angiotensin system genes: effects on vascular development. Curr Hypertens Rep 3: 68–73, 2001. doi: 10.1007/s11906-001-0083-x. [DOI] [PubMed] [Google Scholar]
- 46.Metzger R, Bohle RM, Pauls K, Eichner G, Alhenc-Gelas F, Danilov SM, Franke FE. Angiotensin-converting enzyme in non-neoplastic kidney diseases. Kidney Int 56: 1442–1454, 1999. doi: 10.1046/j.1523-1755.1999.00660.x. [DOI] [PubMed] [Google Scholar]
- 47.Minuth M, Hackenthal E, Poulsen K, Rix E, Taugner R. Renin immunocytochemistry of the differentiating juxtaglomerular apparatus. Anat Embryol (Berl) 162: 173–181, 1981. doi: 10.1007/BF00306489. [DOI] [PubMed] [Google Scholar]
- 48.Neubauer B, Machura K, Chen M, Weinstein LS, Oppermann M, Sequeira-Lopez ML, Gomez RA, Schnermann J, Castrop H, Kurtz A, Wagner C. Development of vascular renin expression in the kidney critically depends on the cyclic AMP pathway. Am J Physiol Renal Physiol 296: F1006–F1012, 2009. doi: 10.1152/ajprenal.90448.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neubauer B, Machura K, Rupp V, Tallquist MD, Betsholtz C, Sequeira-Lopez ML, Ariel Gomez R, Wagner C. Development of renal renin-expressing cells does not involve PDGF-B-PDGFR-β signaling. Physiol Rep 1: e00132, 2013. doi: 10.1002/phy2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishimura H, Ogawa M. The renin-angiotensin system in fishes. Am Zool 13: 823–838, 1973. doi: 10.1093/icb/13.3.823. 4349179 [DOI] [Google Scholar]
- 51.Park S, Harrison-Bernard LM. Augmented renal vascular nNOS and renin protein expression in angiotensin type 1 receptor null mice. J Histochem Cytochem 56: 401–414, 2008. doi: 10.1369/jhc.2007.950220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perner B, Englert C, Bollig F. The Wilms tumor genes wt1a and wt1b control different steps during formation of the zebrafish pronephros. Dev Biol 309: 87–96, 2007. doi: 10.1016/j.ydbio.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 53.Pippin JW, Glenn ST, Krofft RD, Rusiniak ME, Alpers CE, Hudkins K, Duffield JS, Gross KW, Shankland SJ. Cells of renin lineage take on a podocyte phenotype in aging nephropathy. Am J Physiol Renal Physiol 306: F1198–F1209, 2014. doi: 10.1152/ajprenal.00699.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pippin JW, Kaverina NV, Eng DG, Krofft RD, Glenn ST, Duffield JS, Gross KW, Shankland SJ. Cells of renin lineage are adult pluripotent progenitors in experimental glomerular disease. Am J Physiol Renal Physiol 309: F341–F358, 2015. doi: 10.1152/ajprenal.00438.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pippin JW, Sparks MA, Glenn ST, Buitrago S, Coffman TM, Duffield JS, Gross KW, Shankland SJ. Cells of renin lineage are progenitors of podocytes and parietal epithelial cells in experimental glomerular disease. Am J Pathol 183: 542–557, 2013. doi: 10.1016/j.ajpath.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reddi V, Zaglul A, Pentz ES, Gomez RA. Renin-expressing cells are associated with branching of the developing kidney vasculature. J Am Soc Nephrol 9: 63–71, 1998. [DOI] [PubMed] [Google Scholar]
- 57.Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol 55: 261–268, 2011. doi: 10.1387/ijdb.103167dr. [DOI] [PubMed] [Google Scholar]
- 58.Rider SA, Mullins LJ, Verdon RF, MacRae CA, Mullins JJ. Renin expression in developing zebrafish is associated with angiogenesis and requires the Notch pathway and endothelium. Am J Physiol Renal Physiol 309: F531–F539, 2015. doi: 10.1152/ajprenal.00247.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rider SA, Tucker CS, del-Pozo J, Rose KN, MacRae CA, Bailey MA, Mullins JJ. Techniques for the in vivo assessment of cardio-renal function in zebrafish (Danio rerio) larvae. J Physiol 590: 1803–1809, 2012. doi: 10.1113/jphysiol.2011.224352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, Sixt M, Wedlich-Soldner R. Lifeact: a versatile marker to visualize F-actin. Nat Methods 5: 605–607, 2008. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romagnani P, Lasagni L, Remuzzi G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat Rev Nephrol 9: 137–146, 2013. doi: 10.1038/nrneph.2012.290. [DOI] [PubMed] [Google Scholar]
- 62.Sauter A, Machura K, Neubauer B, Kurtz A, Wagner C. Development of renin expression in the mouse kidney. Kidney Int 73: 43–51, 2008. doi: 10.1038/sj.ki.5002571. [DOI] [PubMed] [Google Scholar]
- 63.Sequeira-Lopez ML, Lin EE, Li M, Hu Y, Sigmund CD, Gomez RA. The earliest metanephric arteriolar progenitors and their role in kidney vascular development. Am J Physiol Regul Integr Comp Physiol 308: R138–R149, 2015. doi: 10.1152/ajpregu.00428.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sequeira-Lopez ML, Nagalakshmi VK, Li M, Sigmund CD, Gomez RA. Vascular versus tubular renin: role in kidney development. Am J Physiol Regul Integr Comp Physiol 309: R650–R657, 2015. doi: 10.1152/ajpregu.00313.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sequeira Lopez ML, Gomez RA. Development of the renal arterioles. J Am Soc Nephrol 22: 2156–2165, 2011. doi: 10.1681/ASN.2011080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sequeira López MLS, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004. doi: 10.1016/S1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 67.Smith SW, Chand S, Savage CO. Biology of the renal pericyte. Nephrol Dial Transplant 27: 2149–2155. doi: 10.1093/ndt/gfs134. [DOI] [PubMed] [Google Scholar]
- 68.Starke C, Betz H, Hickmann L, Lachmann P, Neubauer B, Kopp JB, Sequeira-Lopez ML, Gomez RA, Hohenstein B, Todorov VT, Hugo CP. Renin lineage cells repopulate the glomerular mesangium after injury. J Am Soc Nephrol 26: 48–54, 2015. doi: 10.1681/ASN.2014030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stefanska A, Kenyon C, Christian HC, Buckley C, Shaw I, Mullins JJ, Péault B. Human kidney pericytes produce renin. Kidney Int 90: 1251–1261, 2016. doi: 10.1016/j.kint.2016.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stefańska A, Péault B, Mullins JJ. Renal pericytes: multifunctional cells of the kidneys. Pflugers Arch 465: 767–773, 2013. [Erratum in Pflugers Arch 465: 919,, 2013 ]. doi: 10.1007/s00424-013-1294-0. [DOI] [PubMed] [Google Scholar]
- 71.Steppan D, Zügner A, Rachel R, Kurtz A. Structural analysis suggests that renin is released by compound exocytosis. Kidney Int 83: 233–241, 2013. doi: 10.1038/ki.2012.392. [DOI] [PubMed] [Google Scholar]
- 72.Taugner R, Murakami K, Kim SJ. Renin activation in juvenile secretory granules? Immunocytochemical experiments with an antiserum directed against the prosegment of human renin. Histochemistry 85: 107–109, 1986. [DOI] [PubMed] [Google Scholar]
- 73.Taugner R, Hackenthal E. Morphology of the juxtaglomerular apparatus. In: The Juxtaglomerular Apparatus: Structure and Function, edited by Taugner R and Hackenthal E. Berlin: Springer, 1989, p. 5–43. doi: 10.1007/978-3-642-88426-9_2. [DOI] [Google Scholar]
- 74.Taugner R, Hackenthal E. Synthesis and traffic of renin in epithelioid cells. In: The Juxtaglomerular Apparatus: Structure and Function, edited by Taugner R and Hackenthal E. Berlin: Springer, 1989, p. 103–126. doi: 10.1007/978-3-642-88426-9_5 [DOI] [Google Scholar]
- 75.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3: 59–69, 2008. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- 76.Tikellis C, Bernardi S, Burns WC. Angiotensin-converting enzyme 2 is a key modulator of the renin-angiotensin system in cardiovascular and renal disease. Curr Opin Nephrol Hypertens 20: 62–68, 2011. doi: 10.1097/MNH.0b013e328341164a. [DOI] [PubMed] [Google Scholar]
- 77.Tufro-McReddie A, Romano LM, Harris JM, Ferder L, Gomez RA. Angiotensin II regulates nephrogenesis and renal vascular development. Am J Physiol Renal Physiol 269: F110–F115, 1995. [DOI] [PubMed] [Google Scholar]
- 78.van der Meer IM, Cravedi P, Remuzzi G. The role of renin angiotensin system inhibition in kidney repair. Fibrogenesis Tissue Repair 3: 7, 2010. doi: 10.1186/1755-1536-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y, Pan L, Moens CB, Appel B. Notch3 establishes brain vascular integrity by regulating pericyte number. Development 141: 307–317, 2014. doi: 10.1242/dev.096107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio). Eugene, OR: University of Oregon Press, 1995. [Google Scholar]
- 81.White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, Bourque C, Dovey M, Goessling W, Burns CE, Zon LI. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2: 183–189, 2008. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Whitesell TR, Kennedy RM, Carter AD, Rollins E-L, Georgijevic S, Santoro MM, Childs SJ. An α-smooth muscle actin (acta2/αSMA) zebrafish transgenic line marking vascular mural cells and visceral smooth muscle cells. PLoS One 9: e90590, 2014. doi: 10.1371/journal.pone.0090590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wiens KM, Lee HL, Shimada H, Metcalf AE, Chao MY, Lien CL. Platelet-derived growth factor receptor beta is critical for zebrafish intersegmental vessel formation. PLoS One 5: e11324, 2010. doi: 10.1371/journal.pone.0011324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xa LK, Lacombe MJ, Mercure C, Lazure C, Reudelhuber TL. General lysosomal hydrolysis can process prorenin accurately. Am J Physiol Regul Integr Comp Physiol 307: R505–R513, 2014. doi: 10.1152/ajpregu.00467.2013. [DOI] [PubMed] [Google Scholar]
- 85.Yang T, Endo Y, Huang YG, Smart A, Briggs JP, Schnermann J. Renin expression in COX-2-knockout mice on normal or low-salt diets. Am J Physiol Renal Physiol 279: F819–F825, 2000. [DOI] [PubMed] [Google Scholar]
- 86.Zhou W, Boucher RC, Bollig F, Englert C, Hildebrandt F. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am J Physiol Renal Physiol 299: F1040–F1047, 2010. doi: 10.1152/ajprenal.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]