Abstract
The bladder urothelium is essentially quiescent but regenerates readily upon injury. The process of urothelial regeneration harkens back to the process of urothelial development whereby urothelial stem/progenitor cells must proliferate and terminally differentiate to establish all three urothelial layers. How the urothelium regulates the level of proliferation and the timing of differentiation to ensure the precise degree of regeneration is of significant interest in the field. Without a carefully-orchestrated process, urothelial regeneration may be inadequate, thereby exposing the host to toxins or pathogens. Alternatively, regeneration may be excessive, thereby setting the stage for tumor development. This review describes our current understanding of urothelial regeneration. The current controversies surrounding the identity and location of urothelial progenitor cells that mediate urothelial regeneration are discussed and evidence for each model is provided. We emphasize the factors that have been shown to be crucial for urothelial regeneration, including local growth factors that stimulate repair, and epithelial-mesenchymal cross talk, which ensures feedback regulation. Also highlighted is the emerging concept of epigenetic regulation of urothelial regeneration, which additionally fine tunes the process through transcriptional regulation of cell cycle genes and growth and differentiation factors. Finally, we emphasize how several of these pathways and/or programs are often dysregulated during malignant transformation, further corroborating their importance in directing normal urothelial regeneration. Together, evidence in the field suggests that any attempt to exploit regenerative programs for the purposes of enhanced urothelial repair or replacement must take into account this delicate balance.
Keywords: urothelium, regeneration, progenitor cells, superficial cells, label retention, lineage tracing, epithelial-mesenchymal cross talk, epigenetics
the urothelium is a unique epithelial surface that lines most of the genitourinary tract, including the renal pelvis, ureters, bladder, and proximal urethra. Urothelium consists of multiple layers of epithelial cells that can change size and shape to accommodate fluctuating volumes of urine. This mucosal epithelial surface also serves as a barrier to prevent absorption of toxic substances like acid and urea from the urine and to defend against entry of pathogens from the external environment (30). Implicit in this latter function is the ability of urothelium to “sense” and respond to the presence of pathogens within the genitourinary tract (88). Coordinating other cues from the external environment, such as chemical, thermal, and mechanical stimuli, requires an additional layer of sophistication (38). Besides direct expression of neuronal sensory receptors and ion channels on urothelial cells and their ability to release chemicals and neurotransmitters, afferent nerves also innervate the detrusor muscle and extend into the urothelial layer to help the bladder respond to external stimuli (8, 9, 21, 64, 107).
Urothelium Comprises Three Major Cell Types
Despite this wide range of functions, the urothelium has a relatively simple structure comprising three main cell types that are distinguished by their location, size, and expression of molecular markers. Directly facing the luminal surface are large (50–120 μm in diameter) multinucleated hexagonal cells known as superficial or umbrella cells, which are principally responsible for the barrier function of the urothelium (103). Adjacent superficial cells are connected by tight junction proteins including claudin-8 and zona occludens 1 (ZO-1) that restrict exchange of ions and solutes between cells and between urine and blood (1, 32, 75). Superficial cells are covered by a crystalline lattice comprising four major uroplakin proteins that together form asymmetric unit membrane (AUM) plaques. These plaques further restrict permeability to water, solutes, and toxins (37, 90, 105, 106). Superficial cells also contribute to the plasticity in urothelial cell surface area through a regulated process of endocytosis or exocytosis of discoidal/fusiform-shaped vesicles (DFVs) containing uroplakins (47, 50, 109). Underlying the superficial cell layer is a layer of intermediate cells that are significantly smaller (20 μm in diameter) than superficial cells. Finally, along the basement membrane is a layer of basal cells. Despite being the smallest population in size (5–10 μm in diameter), basal cells constitute the most abundant population of cells in adult urothelium. Given the substantial size discrepancy between superficial and either basal or intermediate cells, it is no surprise that histological analysis of whole-mount adult mouse bladders has revealed that one superficial cell spans the area of ~40 underlying intermediate/basal cells (36). Depending on the species, there can be as few as three discrete layers of urothelial cells in the mouse bladder and up to seven layers in the human bladder, with the additional layers contributed by intermediate cells (11).
In addition to the size discrepancy among urothelial cells in the different layers, urothelial cells can also be distinguished by molecular differentiation markers, which begin to be expressed at different stages of embryogenesis (Fig. 1). Superficial cells represent terminally differentiated cells and are the only cell layer in the bladder to express the low-molecular-weight cytokeratin 20 (Krt20) (29, 68, 82). Superficial cells also express several uroplakins (Upk) but lack expression of the high-molecular-weight cytokeratin Krt5, the transcriptional factor p63, and signaling molecule Sonic Hedgehog (Shh) (25). Similar to superficial cells, most intermediate cells are Upk+ and Krt5−, but in contrast, intermediate cells also express p63. Diverging from superficial and intermediate cells, basal cells distinguish themselves by expression of high levels of Krt5 and p63 but are negative for Upk and Krt20. Notably, despite the previous assumption that each of the three urothelial layers comprises a homogenous population of cells based on these five markers, our recent findings suggest that there is, in fact, significant urothelial cell heterogeneity. For example, variable levels of histone H3 lysine 27 trimethylation (H3K27me3), an epigenetic modification often associated with gene silencing, are apparent among urothelial cells within the Krt5+ basal as well as Krt5− intermediate and superficial cells (26a). In addition, ~14% of Krt5+ basal cells also express Krt14. While the significance of urothelial cell heterogeneity remains to be determined, the Krt5+/Krt14+ subpopulation of basal cells may play an important role in urothelial regeneration and tumorigenesis (to be discussed in subsequent sections) (55, 77, 81). Emerging techniques like single-cell RNA sequencing (scRNA-seq) may further stratify cells within each discrete layer and potentially identify functional differences among cells in each layer.
Fig. 1.
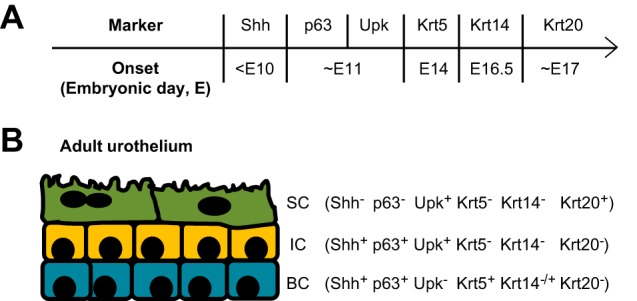
Urothelium is stratified into three major cell types. A: temporal expression of several key urothelial cell markers is depicted. Shh is the earliest of these markers to be expressed in the urothelium, while the cytokeratins are expressed much later in embryogenesis. B: adult urothelium comprises basal cells (BC), intermediate cells (IC), and superficial cells (SC), each of which have discrete patterns of cell marker expression.
Normally Quiescent Urothelium Rapidly Regenerates in Response to Injury
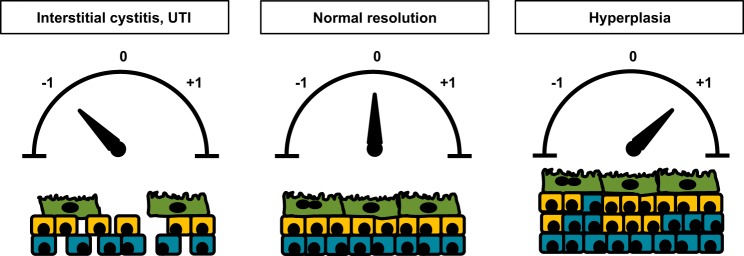
Unlike the epithelium of the skin and intestine, mature urothelium has a very low mitotic index and turnover rate. Pulse-labeling of unstimulated rat bladders with tritiated thymidine revealed a labeling index of ~0.2–0.5% (14, 30, 61, 65) while uninjured mouse bladders had an even lower labeling index of 0.11% (36, 58). Similarly, bladder biopsies from normal human patients that were cultured with tritiated thymidine in vitro had a labeling index of 0.12% (28). Based on these low labeling indexes, turnover rates of quiescent rodent urothelium have been estimated to be approximately once every 200 days (14, 30). The prevailing quiescence of the urothelium makes its ability to awaken rapidly in response to damage even more remarkable. Within hours of chemical injury with cyclophosphamide (CPP) or protamine sulfate (PS) or biological insult with uropathogenic Escherichia coli (UPEC), the urothelium begins to proliferate and initiate the process of regeneration (Fig. 2) (25, 71, 84). One can imagine that urothelial regeneration needs to be carefully controlled. Incomplete regeneration results in potential breaches in barrier function (Fig. 3) whereby toxic substances or pathogens in the urine can gain access to the bloodstream, stimulate local tissue inflammation, and/or depolarize afferent nerve fibers. In fact, this last process has been hypothesized as being a potential cause of bladder pain syndrome or interstitial cystitis (44, 83, 94). Conversely, unrestrained regeneration can lead to urothelial hyperplasia and possible malignant transformation (Fig. 3). An understanding of the molecular mechanisms responsible for maintaining the delicate balance between urothelial quiescence and regeneration is critical for devising new clinical strategies to prevent or treat diseases of the urothelium.
Fig. 2.
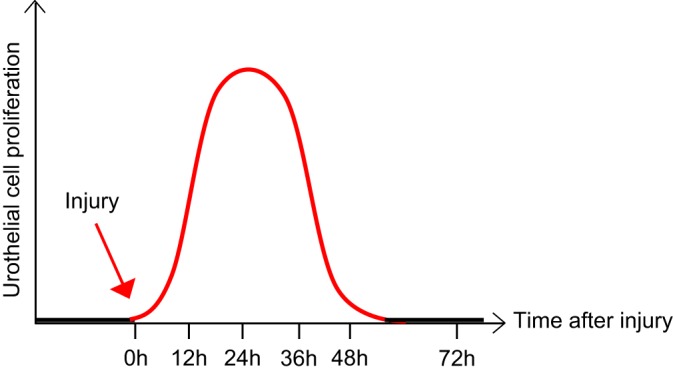
Adult urothelium is normally quiescent but rapidly responds and proliferates upon urothelial injury. At baseline, mature urothelium remains in a quiescent state, with extremely slow turnover. However, in response to injury, the urothelium rapidly awakens and undergoes proliferation and differentiation to restore the damaged epithelium. Maximal proliferation occurs within 12–36 h, depending on the stimulus, followed by differentiation and a return to the dormant state.
Fig. 3.
A fine balance is necessary to ensure normal urothelial regeneration after injury. Following injury, several outcomes are possible. Most commonly, regeneration results in restoration of the urothelium to its original state (designated as “0”). However, failure to fully regenerate the urothelium (designated as “−1”) results in potential breaches in barrier function that may increase susceptibility to infection or increase sensory fiber stimulation and set the stage for interstitial cystitis. Alternatively, unrestrained regeneration (designated as “+1”) can lead to urothelial hyperplasia that may ultimately lead to bladder tumor formation.
Given the priority of maintaining a protective barrier, it is not surprising that one of the first steps in urothelial regeneration is re-establishment of tight junctions between the remaining and regenerated superficial cells (54, 56). Ultrastructural analysis reveals that the de novo superficial cells undergo successive stages of differentiation, first involving expression of microvilli, then formation of cells with rounded microridges that begin to express uroplakins, and finally terminal differentiation in which superficial cells enlarge, adopt a rigid-appearing plasma membrane, and robustly express Upk and Krt20. In contrast to superficial urothelial injuries, full-thickness wounds such as partial cystectomy or bladder augmentation presumably require a more elaborate process to repair all layers of the urothelium and/or smooth muscle. Using primary explant cultures of mouse bladder to examine full-thickness wounds, Kreft et al. demonstrated that basal cells at the wound edge first proliferate (54). Through concentration of their actin skeleton in lamellipodia and at the tip of filopodia, basal cells at the leading edge of the wound migrate and cover the basolateral exposed surface. At the same time, overlying superficial cells flatten and stretch out to cover these new urothelial cells before reestablishing tight junctions. Within 48 h of injury, cells at the leading edge begin to express the junctional proteins ZO-1, claudin-4, occludin, and E-cadherin as well as the superficial differentiation marker Krt20.
Identifying the Urothelial Progenitor Cells Responsible for Regeneration Has Been Challenging
In recent years, much emphasis has been placed on identifying a putative population of resident urothelial stem and/or progenitor cells as these cells can potentially be harnessed to repair chronically damaged or transformed urothelium, augment bladders, or repair ureteral or urethral strictures. While the existence of this population is generally accepted, a major debate in the field centers on the identity and location of these progenitor cells within urothelium. Also disputed is whether different populations of progenitor cells are responsible for urothelial development and differentiation in embryogenesis vs. regeneration and repair of injured adult urothelium.
Label retention studies.
Two major methods have been used to characterize these cells, namely DNA label retention studies and lineage tracing analysis (Table 1). During development and early post-natal life, proliferating cells can incorporate nucleoside analogs such as bromodeoxyuridine (BrdU), 5-ethynyl-2=-deoxyuridine (EdU), or tritiated thymidine. DNA label retention studies rely on the concept that stem cells are the slow-dividing cells and thereby retain the nucleoside analogs for extended periods of time. These label retention studies do not rely on prior knowledge of specific stem/progenitor cell markers which is advantageous, given the paucity of known markers of urothelial progenitor cells. Initial DNA label retention studies by Kurzrock et al. demonstrated that, after BrdU labeling of 6-wk-old rats, ~9% of urothelial cells retained label after 1 yr and these cells resided exclusively in the basal cell layer (55). Subsequent studies uncovered that label retention cells (LRCs) could actually be found in all three layers of the urothelium provided that the label was administered between embryonic day (e)15 and postnatal day (P)1 (13, 91, 110). The later one administers the label, the more restricted the LRCs become, ultimately becoming restricted to the basal cell layer. Consistent with the quiescent nature of urothelium, the baseline proliferation of urothelial cells dramatically decreases and becomes limited to a small (~1%) population of basal cells as the animal ages. While basal cells appear to be important in maintaining urothelial homeostasis, Colopy et al. observed that upon injury with UPEC, proliferation occurs within both basal and intermediate cell layers (13). In fact, the percentage of proliferating intermediate cells was reportedly twice that of basal cells. These findings raised the possibility that adult regenerating urothelium may recapitulate embryogenesis, whereby progenitor cells can be activated in both basal and intermediate cell layers. However, contrary to the assumption that LRCs represent the definitive urothelial progenitors, LRCs in both layers comprised only a small proportion of proliferating cells after injury.
Table 1.
Comparison of label retention and lineage tracing methods
| Advantages | Limitations | |
|---|---|---|
| Label retention | Does not require prior knowledge of stem cells | Assumes that label retention is an intrinsic property of stem cells (which is not always true) |
| Pulse label replicating DNA with BrdU, EdU, or 3H-TdR, then chase and observe washout | Technically simple to perform | Does not allow one to track the fate of labeled cells if label gets diluted over time |
| Genetic lineage tracing | Allows one to track the fate of labeled cells and their progeny over long periods of time | Requires preexisting knowledge of a putative stem cell marker |
| Uses genetic tools (e.g., Cre/LoxP) to indelibly label specific cells and then track their progeny over time | Labeled cells can be extensively analyzed using advanced genomic technologies | Requires sophisticated genetic tools to perform |
BrdU, bromodeoxyuridine; EdU, 5-ethynyl-2=-deoxyuridine; 3H-TdR, tritiated thymidine.
Although label retention studies were previously considered the gold standard for identifying epithelial stem cells, the universal reliability of this method has been called into question. One significant criticism of using this technique is that while LRCs in adult urothelium may include the slowly dividing population of progenitor cells, they may also simply represent labeled, terminally differentiated cells. From the existing literature it appears that LRC analysis may be more helpful in predicting the “birth date” of cells within urothelium than in distinguishing progenitor cells.
Lineage tracing studies.
Perhaps a more powerful and reliable method for identifying urothelial stem/progenitor cells is lineage tracing. Unlike label retention studies, lineage tracing does rely on existing knowledge of putative stem cell markers. By placing Cre recombinase expression under control of a cell/tissue-specific promoter, one can indelibly label a cell and all of its progeny by using a gene reporter and determine whether a particular cell is truly pluripotent. However, like LRC studies, lineage tracing does carry a few caveats (Table 1). One caveat is that lineage tracing, which relies on the use of a Cre transgene, can suffer from positional effects depending on the site of Cre insertion within the genome. An additional caveat is that lineage tracing using a constitutive promoter does not allow one to distinguish specifically whether labeled cells represent the progeny of a single multipotent progenitor cell or the progeny of multiple unipotent progenitor cells. Nevertheless, through this method, Pignon et al. were able to demonstrate that urothelial stem cells express the transcription factor p63 (78). P63 encodes for two distinct isoforms, transactivating (TA) p63 and NH2-terminal truncated (ΔN) p63, which are generated by alternative promoters (108). Urothelial cells expressing the ΔNp63 isoform in embryogenesis were shown to give rise to all urothelial cell lineages. However, over time, terminally differentiated superficial cells lose ΔNp63 expression. Cheng et al. additionally highlighted a specific antiapoptotic role for p63 in development of the ventral bladder urothelium (12). Deletion of p63 leads to absence of the ventral abdominal and bladder walls in association with markedly enhanced apoptosis. Furthermore, urothelial cells along the ventral bladder remain in a state of limbo whereby they remain undifferentiated and uncommitted. In contrast, the dorsal urothelium exhibits reduced thickness but superficial cells still develop, implying that p63 exerts a predominant role in ventral epithelium during bladder development. Nevertheless, while p63 may not be essential for differentiation of the dorsal bladder urothelium, it still appears to play a role in promoting stem cell survival and proliferation along this axis.
Lineage tracing, combined with urothelial organoid cultures, have likewise revealed that Shh is a marker for urothelial stem cells in both embryogenesis and adulthood. Using ShhCreER;R26mTmG mice injured with multiple rounds of UPEC, Shin et al. demonstrated that Shh+ cells have a long-standing capacity for self-renewal in adult regenerating urothelium and can give rise to all three urothelial cell lineages (84). Based on apparent exclusive localization of mG-labeled reporter cells in the basal layer prior to injury, followed by regeneration of all urothelial layers with these mG-labeled cells after injury, this group argued that urothelial stem/progenitor cells derive from a subpopulation of basal cells that are specifically Shh+. These data seem to corroborate earlier studies by Mysorekar et al., who demonstrated that UPEC infection of wild-type mice results in BrdU labeling of basally located cells only (71). These results also seem consistent with the prevailing notion that, like epidermis, urothelial differentiation and regeneration occur in a linear fashion, with basal stem cells progressively differentiating into the suprabasal cell layers (Fig. 4A) (23, 31). A more recent report by Papafatiou et al. expands upon this notion and further suggests that a minor population of Krt5+/Krt14+ basal cells serves as the urothelial progenitors responsible for superficial cell regeneration both during normal homeostasis and after injury (77).
Fig. 4.

Proposed models of urothelial formation and regeneration. A: the linear model of urothelial formation and regeneration suggests that the urothelial stem/progenitor cells resides within the basal cell population, likely the Krt5+/Krt14+ basal cells. These cells are capable of self renewal but also give rise to intermediate cells, which subsequently give rise to superficial cells. B: in the nonlinear model, the transient population of P cells, distinguished by Foxa2 expression, gives rise to intermediate and superficial cells during embryogenesis. In contrast to the linear model, the nonlinear model argues that a separate progenitor cell (unidentified at present) initially gives rise to basal cells during development. In adults, stem cells are thought to reside within both the intermediate and the basal cell populations and are capable of self-renewal and/or differentiation into superficial cells. It is unclear, however, whether stem cells in the basal layer need to go through an intermediate step, such as transient amplifying cells, before they differentiate into superficial cells.
Notably, a separate report by Gandhi et al. challenges this linear model of regeneration (Fig. 4B) (25). Arguing that Krt5+ cells are detectable only after intermediate and superficial cells are first formed (Fig. 1A), the authors hypothesized that an alternative population of cells is likely to be the progenitor for superficial cells during bladder development. Using a combination of strategies, including in situ hybridization analysis of Shh mRNA, lineage tracing with ShhCreERT2;R26mTmG embryos, and marker analysis of ShhGFP/Cre bladders, this group was able to demonstrate that Shh+ cells can actually be found in both basal and intermediate cell layers, as well as in P cells, a transient population of Foxa2+ cells that are abundant only during discrete stages in bladder development before basal cell formation. To test directly whether Krt5+ basal cells are the definitive progenitor cell for all embryonic urothelial layers, Gandhi et al. performed lineage tracing in Krt5CreER;R26mTmG mice during embryogenesis. They observed that Krt5+ cells exclusively give rise to basal cells and not to intermediate or superficial cells. Instead, they characterized a discrete population of P cells, which they define as progenitors for intermediate and superficial cells during embryogenesis. Notably, the authors do highlight that P cells are only a transient population of cells, and, while important during bladder development, they are no longer detected in mature bladders. The authors also performed lineage tracing in adult Krt5CreER;R26mTmG mice after chemical injury with CPP. Consistent with their findings in embryogenesis that Krt5+ cells are not progenitors for terminally differentiated superficial cells, mG-labeled Krt5+ genetic lineages never gave rise to either intermediate or superficial cells in the adult urothelium. Rather, lineage tracing with Upk3aGCE;mCherry adult mice injured with CPP reinforced the concept that in mature urothelium intermediate cells become the source of progenitors that give rise to superficial cells.
While these latter results provide compelling evidence against a linear model of urothelial regeneration that begins with basal progenitor cells giving rise to intermediate cells that then differentiate into superficial cells (Fig. 4A), one needs to consider the potential reasons for this discrepancy among the various groups. One possible explanation is the different injury models used (i.e., UPEC vs. CPP). This notion that distinct injuries may result in activation of different pools of urothelial progenitor cells was described by Mysorekar et al., who noted that UPEC-induced injury was associated with basal cell proliferation whereas protamine sulfate-based injury awakened the intermediate cell compartment (71). One obvious question that is raised from these studies is whether the degree of inflammation induced by the urothelial injury influences which subset of urothelial progenitor cells is stimulated. Regardless, it is possible to ascribe Gandhi’s results to the use of chemical injury with CPP. To further clarify this point, it would be informative to repeat lineage tracing results in Krt5CreER;R26mTmG or Upk3aGCE;mCherry mice infected with UPEC. Additionally, it would be instructive to examine which cells serve as progenitors for regeneration in a surgical model of injury such as bladder resection and/or augmentation. Given the increased appreciation of the heterogeneity of urothelial cells (26a), it is possible that genetic lineage tracing strategies have caught only a glimpse of the complete repertoire of urothelial progenitors, thus accounting for the discrepancy among these studies. For example, Krt14+ progenitor cells only make up less than 1% of the total urothelial cells in the adult bladder (77). Of course, this argument is less plausible during development, since we have observed that ~14% of murine urothelial cells are Krt14+ at e18.5 (26a). Moreover, the observation by Papafatiou et al. that Krt14+ cells are first observed at e16.5 (77) casts some doubt on these cells being progenitors for superficial and intermediate cells during embryogenesis, as these latter cells emerge several days earlier. Could a nonlinear model account for urothelial formation in embryogenesis (Fig. 4B) while a linear model explains urothelial regeneration in adulthood (Fig. 4A)? The chapter on this fascinating topic has yet to be closed.
A Presumptive Anatomical Urothelial Stem Cell Niche Has Yet To Be Identified
One challenging point to reconcile in these models of urothelial regeneration is the absence of a clear anatomical stem/progenitor cell “niche” within the urothelium. For instance, intestinal stem cells reside at the base of intestinal crypts and are supported by soluble factors and cell-associated ligands from the surrounding Paneth and stromal cells (92). Likewise, within hair follicles, the bulge area serves as a niche for stem cells (31). In contrast, there is no obvious structural niche within the urothelium. Attempts have been made to define the proliferation unit within the bladder urothelium by using either X chromosome inactivation analysis (98) or mitochondrial mutation-based analysis (24) of human bladder specimens. These studies revealed variably sized patches of urothelial cells carrying the same genetic signature and continuously extending from the basal layer toward the luminal surface. Similarly, in the setting of UPEC-induced injury, Shin et al. similarly demonstrated that regeneration occurs in clonal units that are vertically oriented and include all three layers of urothelium (84). Collectively, these findings reinforce one existing theory that the urothelial stem cell niche may simply represent cells in contact with the basement membrane. Notably, this finding does not necessarily discount the argument of Gandhi et al. (25) that intermediate cells, rather than basal cells, are progenitors for superficial cells, as there is evidence that intermediate cells do send downward projections that contact the basement membrane (30, 62). Whether there is a specialized population of supporting stromal cells that are anatomically restricted to specific regions within the lamina propria remains to be determined.
Epithelial-Mesenchymal Cross Talk Is Critical for Urothelial Regeneration
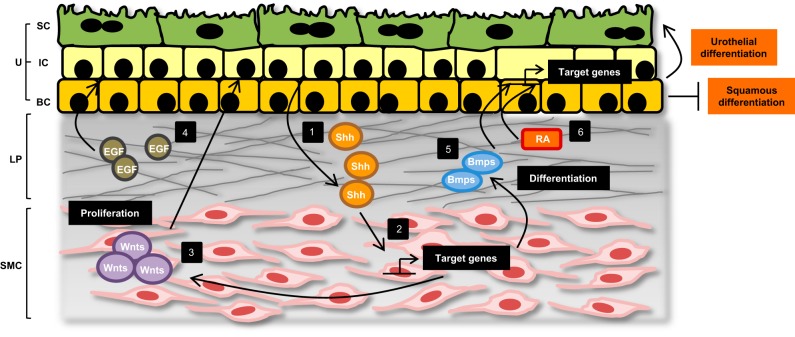
Regardless of the debate surrounding the identity of urothelial progenitors, several studies have underscored the importance of epithelial-mesenchymal interactions in directing processes of both urothelial formation and regeneration. One prime example is the cross talk between urothelium and the underlying stroma through the retinoid signaling pathway (Fig. 5). In situ hybridization analysis of both embryonic and adult bladders identified the suburothelial stroma as the source of retinaldehyde dehydrogenase 2 (Raldh2), while P cells, intermediate cells, and superficial cells were identified as RA-responsive cells (25). Using ShhCre;RaraDN−/− mice, which are deficient in retinoic acid signaling within Shh+ progenitor cells, Ghandi et al. further illustrated that differentiation of intermediate and superficial cells in embryogenesis, as well as regeneration of the superficial cell layer after injury, depend on an intact RA signaling pathway. In a separate experiment, Shin et al. demonstrated that mesenchymal cells also undergo Gli1-mediated upregulation of Wnt2 and Wnt4 expression in response to increased soluble Shh ligand that is released from injured urothelium (84). A paracrine feedback loop is established in which Wnt signals back to urothelial progenitor cells to promote their proliferation and differentiation with restoration of urothelial integrity (Fig. 5). Without Gli-mediated proliferation after injury, the urothelial barrier remains compromised and mice are more susceptible to ascending infections of their kidneys. Whether this increased risk of upper tract infection is simply due to reduced urothelial regeneration or whether additional Gli-mediated signaling pathways are responsible for limiting the spread of infection remains uncertain. Similarly, Mysorekar et al. illustrated the importance of epithelial-mesenchymal cross talk through the BMP signaling pathway (Fig. 5) in ensuring proper urothelial regeneration (71). Deletion of Bmpr1a on urothelial cells abrogates BMP4 signaling and leads to reduced urothelial cell proliferation in response to UPEC infection. More remarkably, disruption of this signaling pathway results in failure of regenerating superficial cells to undergo terminal differentiation and to enter a state of quiescence.
Fig. 5.
Urothelial regeneration is achieved through multiple layers of regulation. The urothelium comprises three main cell types: superficial cells (SC), intermediate cells (IC), and basal cells (BC). Below the urothelium lies the lamina propria (LP) and smooth muscle cell (SMC) layers. Significant epithelial-mesenchymal cross talk facilitates urothelial regeneration after injury (1). In response to damage, urothelial cells upregulate and secrete the soluble molecule Shh. Shh acts on the underlying mesenchymal cells and promotes SMC proliferation and differentiation (2). Shh signaling also leads to activation of target genes, including the Gli family of transcription factors, and (3) increased stromal expression of Wnt molecules. Wnt signals subsequently promote urothelial proliferation and differentiation (4). Injured urothelium also secretes local growth factors like EGF, which function in an autocrine manner to stimulate urothelial cell proliferation (5). Likewise, BMP signaling through BMP receptors on the urothelium is important in ensuring terminal differentiation of regenerating superficial cells (6). Finally, RA produced by stromal cells acts on RA-responsive urothelial cells to promote superficial cell regeneration and inhibit squamous differentiation, in part through activation of transcription factors like FOXA1.
Local Growth Factors Promote Urothelial Regeneration and Repair
In addition to receiving signals from mesenchymal cells, urothelial cells also respond to local growth factors that promote regeneration and repair. Glycosaminoglycans, including heparan sulfate, in the extracellular matrix of the urothelium help to restrain diffusion of growth factors and provide a concentrated pool of factors that can be temporally released (33). One conspicuous growth factor axis involved in this process is the epidermal growth factor receptor (EGFR) and its ligands (Fig. 5). EGF produced by injured urothelial cells functions in an autocrine fashion to promote urothelial cell proliferation and regeneration (22, 100). EGF achieves this function, in part, through induction of the transcription factor Sox9. Despite high levels of Sox9 expression in the developing urothelium, Sox9 becomes undetectable in quiescent adult urothelium (60). However, with different types of urothelial injury, Sox9 is transiently upregulated in all three urothelial layers, coinciding with the timing of urothelial repair. Other growth factors noted to enhance urothelial regeneration are the EGFR ligands transforming growth factor-α (TGFα), keratinocyte growth factor (KGF), heparan-binding EGF (HB-EGF), and amphiregulin, as well as fibroblast growth factor 2 (FGF2) (3, 7, 15). Several groups have begun to take advantage of this knowledge to design better bladder scaffolds impregnated with individual or combination growth factors to facilitate bladder regeneration and ingrowth of urothelial cells in bladder augmentation procedures (reviewed in Ref. 63). Kanematsu et al. illustrated that sustained release of FGF-2 from a bladder acellular matrix could promote VEGF production and improve angiogenesis within the graft (39). By combining nerve growth factor (NGF) with VEGF in a rat model of spinal cord injury, Kikuno et al. were able to enhance smooth muscle and nerve fiber content in the regenerating bladder while significantly improving bladder capacity and compliance (48). It is possible that additional incorporation of immunomodulatory factors into scaffolds may further enhance bladder repair.
Urothelial Homeostasis and Regeneration Is Also Modulated at the Epigenetic Level
Recent work from our laboratory and others' also suggests that progenitor cell behavior during urothelial development and injury-induced regeneration is additionally regulated by epigenetic factors. Epigenetics refers to the study of changes in gene expression that are not the result of changes in actual DNA sequence. These changes in gene expression are, instead, the result of chemical modifications to DNA or histone proteins which result in local chromatin remodeling that alters accessibility of the transcriptional machinery (52). While many epigenetic programs exist, one program that has been significantly implicated in stem cell biology is the polycomb repressive complex (PRC)2 (18). The PRC2-dependent H3K27me3 epigenetic modification is tightly associated with local chromatin compaction and gene repression. Our laboratory made the initial observation that core components of the PRC2 complex, including Ezh2 and Eed, are expressed within murine bladders, and H3K27me3 is highly enriched within the urothelium (26a). These data suggest that PRC2 may play a role in the normal function of urothelium. By deleting Eed, the obligatory structural component of PRC2, or Ezh2, the major enzymatic component, in Shh+ progenitor cells, we discovered that the PRC2-mediated epigenetic program represses both the cell cycle inhibitors p15 and p16 and Shh signaling molecules, and it is critical for maintaining urothelial progenitor cell proliferation during development. The study also reveals that PRC2 not only ensures the proper timing of differentiation of Krt5+ basal cells and Upk3a+ superficial cells, but it also prevents aberrant induction of squamous-like differentiation programs within the urothelium and prevents ectopic expression of Krt14 and Krt17. In adult urothelium, we have observed that PRC2 plays a similar role. In the quiescent state, PRC2 activity is important in preventing precocious differentiation of Shh+ progenitor cells to superficial cells, thereby regulating normal homeostasis. In the setting of repeated urothelial injury in vivo with CPP, PRC2 is needed to ensure maximal proliferative and regenerative potential of the progenitor cells. These results have been corroborated in an in vitro model of UPEC-infection, where Ting et al. demonstrated that UPEC induces expression of Ezh2 in human bladder cancer cells (93). Pharmacological inhibition of Ezh2 resulted in reduced urothelial cell proliferation in response to UPEC, confirming the role of PRC2 in maintaining the early proliferative response to infection. Last but not least, we have observed that PRC2 not only plays a role in promoting urothelial proliferation but may also regulate the host inflammatory response to UPEC infection (our unpublished data). Together, these emerging data point to the importance of epigenetic regulation throughout the lifespan of urothelial cells, i.e., in establishing the identity of urothelial cells, in maintaining their quiescence, and in quickly responding to injury to promote proliferation and differentiation. Whether other epigenetic programs play a role in the urothelial regenerative process remains an active area of investigation.
Dysregulated Urothelial Regeneration Can Lead to Carcinogenesis
From this discussion, it is clear that maintaining urothelial homeostasis for extended periods of time while facilitating rapid regeneration upon injury is a complicated process governed by epithelial-mesenchymal signaling, secreted growth factors, and epigenetic mechanisms. A disruption in any one of these processes could lead to abnormal urothelial regeneration and malignant transformation. Not surprisingly, then, additional knowledge has been gleaned about the molecular mechanisms of urothelial regeneration by studying the factors that drive urothelial carcinogenesis.
Altered epithelial-mesenchymal signaling.
One popular rodent model of bladder cancer involves administration of the pro-carcinogen N-butyl-N-4-hydroxybutyl nitrosamine (BBN) in the drinking water. BBN exposure induces urothelial hyperplasia and carcinoma in situ (CIS) within 3–4 mo (reviewed in Ref. 102). Unlike the normal hyperplastic response seen in urothelium after acute injury with CPP or UPEC, which recedes quickly after the exposure is removed, BBN-induced hyperplasia develops progressively over time and fails to regress. Shin et al. made the important observation that Shh+ cells, which are urothelial progenitors and are responsible for the normal regenerative response after injury, are also responsible for bladder carcinogenesis, because ablation of Shh+ cells attenuates BBN-induced bladder cancer development (85). Remarkably, however, during progression of CIS to invasive carcinoma, Shh expression is frequently lost. Supporting the hypothesis that Shh secretion normally helps curb development of advanced bladder cancer, genetic ablation of the Shh signaling pathway led to accelerated progression to invasive disease (86). This observation further highlights the role of epithelial-mesenchymal signaling in keeping the regenerative process in check. While Shh/Wnt signaling promotes urothelial progenitor cell proliferation after injury (84), Shh signaling in the mesenchyme through Smo and Gli is also critical to feedback on the urothelium and induces upregulation of genes such as BMP4 and BMP5, which tip the balance toward urothelial terminal differentiation (86). The finding that enhanced BMP signaling can delay progression of BBN-treated animals to invasive cancer is somewhat analogous to the observation of Mysorekar et al. that the BMP4/Bmpr1a axis is important in ensuring terminal differentiation of superficial cells after UPEC injury (71). Together, these examples highlight how specific signaling pathways can be manipulated to maximize regeneration, but they must be carefully titrated to avoid progression to cancer.
Abnormal growth factor signaling.
In addition to the growing evidence that dysregulation of normal epithelial-mesenchymal interactions may promote carcinogenesis, an imbalance in growth factor signaling, which normally functions to promote urothelial repair, has also been associated with increased bladder cancer risk. Overexpression of EGFR has been reported in 40–60% of bladder tumors and has been correlated with invasive bladder cancer (5, 66, 73, 87). In dysplastic and malignant urothelium, EGFR begins to be expressed throughout all urothelial cell layers, rather than remaining confined to the basal cells (67). The increased expression of EGFR on tumor cells leads to enhanced signaling through urinary EGF and other ligands, thereby promoting tumor cell growth and proliferation (4, 67, 74). Additional studies have shown a correlation between bladder cancer prognosis and the specific cellular localization of HB-EGF, a potent growth factor ligand for EGFR (2, 49, 76). Cleavage of proHB-EGF leads to increased secretion of the soluble amino terminal fragment sHB-EGF, which can function as an autocrine or paracrine activator of EGFR and several family members. Concomitant with this process, increased nuclear translocation of the carboxy terminal fragment HB-EGF-C is associated with increased cyclin A expression and cell cycle progression (72). Not surprisingly, patients with an increase in both the secreted and nuclear forms of HB-EGF, as opposed to the cytosolic pro form, exhibit worse prognosis due to increased tumor cell survival and proliferation (2, 53).
While the EGFR axis is one of the most prominent growth factor pathways altered in bladder cancer, other growth factors have also been shown to be dysregulated in their expression. Both VEGF and VEGFR1 can be overexpressed in bladder tumors, but they are more significantly upregulated in superficial as opposed to invasive tumors (51). FGF signaling pathways are also altered in a significant proportion of bladder cancer patients (reviewed in Ref. 19). Patients with either superficial or muscle-invasive disease may demonstrate overexpression of FGFR1, whereas aggressive disease has been correlated with reductions in FGFR2 (20, 96, 97). Furthermore, mutations in FGFR3 have been reported in ~80% of low-grade superficial tumors, and FGFR3 overexpression has been detected in ~20–50% of muscle-invasive tumors (6, 27, 95). It is no surprise, then, that several new therapies for urothelial cancer have centered on blockade of growth factor signaling as a method of inhibiting tumor cell proliferation (27, 35).
Mutations in epigenetic programs.
Consistent with our observation that PRC2-mediated repression controls urothelial progenitor cell proliferation and regenerative potential, several groups have observed that dysregulation of this epigenetic program is associated with urothelial carcinoma in human bladder tissue samples (57, 79, 104). Specifically, human EZH2 is upregulated in urothelial carcinoma samples compared with adjacent benign urothelium, although debate remains whether degree of EZH2 elevation is predictive of oncologic outcome. Conversely, whole exome sequencing of human bladder cancer as part of the Cancer Genome Atlas project found that 24% of bladder tumors studied had inactivating mutations in KDM6A, the histone demethylase that works in opposition to PRC2 (10). Moreover, we found that urothelium-specific knockout of murine Kdm6a significantly increases the risk of BBN-induced bladder cancer (Kaneko S and Li X, unpublished observations). Collectively, these data suggest that tipping the overall balance in favor of increased H3K27me3 modification in urothelial cells may predispose to bladder cancer. Other chromatin remodeling genes that are frequently mutated in bladder cancer include the histone acetyltransferase (HAT) genes CREBBP and EP300, the SWI/SNF-related chromatin remodeling gene ARID1A, and the H3K4 methyltransferase MLL (10, 26). However, because epigenetic programs regulate a broad range of genes, it can be challenging to know whether specific epigenetically-targeted genes responsible for normal urothelial regeneration are the same genes disrupted in urothelial cancer.
Urothelial metaplasia.
While the examples above focus on how abnormal regeneration pathways can lead to dysregulated urothelial cell growth and urothelial cell carcinoma, it is important to keep in mind that aberrant urothelial regeneration can alternatively result in squamous metaplasia that has the potential to progress to squamous cell carcinoma (SCC). It has long been appreciated that Vitamin A deficiency in rats and mice can lead to keratinizing squamous metaplasia that occurs heterogeneously throughout the bladder (59, 69). This phenomenon suggested the importance of retinoic acid signaling in maintaining the urothelial differentiation phenotype in mature bladders and potentially deterring neoplastic transformation. And, in fact, in vitro cultures of normal human urothelial (NHU) cells deprived of exogenous retinoids adopt a squamous phenotype that can revert to a basal phenotype following introduction of RA derivatives (89). Intersecting with RA signaling is the peroxisome proliferator-activated receptor-γ (PPARγ) pathway, a nuclear hormone receptor that, when bound by ligand, heterodimerizes with RXRa to form a transcription factor complex that activates several genes including the forkhead box A1 (FOXA1) (16, 34, 101). FOXA1 plays a role in urothelial differentiation by binding to the promoters of Upk1a, Upk2, and Upk3a and enhancing expression of these terminal differentiation markers (101). Not only is FOXA1 expression significantly reduced in human samples of aggressive bladder cancer, there is also an increased frequency of FOXA1 loss in cases of SCC compared with transitional cell carcinoma (TCC) (17, 80). Likewise, female mice deleted in FOXA1 within the urothelium developed keratinizing squamous metaplasia with increased Krt14 expression, further confirming that this axis helps maintain the terminal differentiation state of urothelium (81). Remarkably, using lineage-tracing analysis, Van Batavia et al. were able to show that, although intermediate cells could give rise to noninvasive papillary tumors, they were not responsible for SCC; rather, Krt5+ basal cells were identified as the cell of origin for SCC (99). These results suggest that despite common themes the molecular mechanisms governing the regenerative process in Krt5+ cells may truly be distinct from that in intermediate cells. Regardless, these data collectively highlight that successful regeneration of urothelium does not simply involve carefully regulated cell proliferation but also progression along the correct differentiation pathway.
Inadequate Urothelial Regeneration May Weaken the Defense Barrier and Contribute to Interstitial Cystitis and/or Increased Susceptibility to Infection
While an overabundant regenerative process has the danger of progressing to malignancy, there can also potentially be deleterious effects from insufficient regeneration of the urothelium. Consequences may include increased exposure to urinary toxins, inadequate clearance of microorganisms, chronic low-level bladder inflammation, and increased sensory nerve activation leading to chronic pain. In patients diagnosed with interstitial cystitis/painful bladder syndrome (IC/PBS), bladder biopsies frequently reveal erosion and/or thinning of the urothelium concerning for insufficient urothelial regeneration (94). The discovery of antiproliferative factor (APF) in the urine of IC/PBS patients, along with reduced levels of HB-EGF, has led to the hypothesis that an initial insult to the bladder epithelium can lead to upregulation of APF in a select group of patients (40–43, 46). Not only does APF impair urothelial cell proliferation, but it may also inhibit the production of autocrine or paracrine growth factors like HB-EGF (41, 45, 46). Together, this process results in blunted reepithelialization of the urothelium characterized by fewer urothelial cell layers, altered tight junction formation, and increased paracellular permeability.
One can similarly imagine that exposure of incompletely regenerated urothelium to UPEC may result in enhanced susceptibility to and/or reduced clearance of infection. Using a mouse model in which bladders were pretreated with PS to induce chemical damage of the urothelium before UPEC infection, Mysorekar and Hultgren observed that UPEC was now able to gain access and establish infection within the underlying intermediate and/or basal cell layers cells (70). Furthermore, compared with vehicle-treated controls, PS-treated bladders contained increased numbers of quiescent intracellular reservoirs (QIR) within the deeper urothelial cell layers where they are presumably protected from exfoliation and/or host defense mechanisms. Notably, with a new stimulus, bacteria present within these QIR could be triggered to reemerge from these underlying compartments and seed a new acute infection. These studies underscore the possibility that conditions leading to an incompletely regenerated urothelial layer may set the stage for increased susceptibility to recurrent UTI.
Conclusions
Urothelium is a dynamic epithelial surface that relies upon its ability to maintain a state of quiescence while remaining poised for rapid regeneration in the face of injury. Identifying the key molecular players in this process is important to improve our understanding of what host factors may predispose to IC/PBS, recurrent UTI, and malignant transformation. Furthermore, capitalizing on this knowledge may improve our efforts to safely and effectively regenerate or replace the bladder, ureters, or urethra. Work in the past few years has focused on characterization of the urothelial progenitor cell population responsible for regeneration. Debate still exists regarding the precise identity and location of these progenitor cells. While this may be related to variability in the genetic models tested, a more intriguing possibility that merits further investigation is that different types or severities of injury (chemical vs. biological vs. surgical) may stimulate different progenitor cell pools within the urothelium. Whether the degree of injury and/or the degree of inflammation are key determinants of this process remains to be determined.
Regardless, judicious progenitor cell proliferation and differentiation are necessary to ensure proper regeneration. This is achieved through multiple layers of regulation including growth factor stimulation and epithelial-mesenchymal cross talk (Fig. 5). More recently, there has been a growing appreciation for the role of epigenetic programs, more specifically the PRC2 epigenetic program, in modulating urothelial homeostasis and regeneration. Additional work is warranted to examine the role of other epigenetic programs in urothelial regeneration. When this fine balance of regulatory networks is disrupted, regeneration can go awry and lead to extremes of insufficient or overabundant regeneration. Identifying methods to restore the equilibrium between urothelial regeneration and quiescence should be the focus of translational research in this area.
GRANTS
This work was supported by American Urological Association scholar awards (Z. R. Balsara) and National Institute of Diabetes and Digestive and Kidney Diseases (1R01 DK-091645 and 1R01 DK-110477, X. Li) and National Cancer Institute (1R21 CA-198544, X. Li).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Z.R.B. prepared figures; Z.R.B. drafted manuscript; Z.R.B. and X.L. edited and revised manuscript; X.L. interpreted results of experiments; X.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all members of the Li laboratory, particularly Dr. Chunming Guo, for helpful discussions.
REFERENCES
- 1.Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, Birder L, Apodaca G. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol 287: F305–F318, 2004. doi: 10.1152/ajprenal.00341.2003. [DOI] [PubMed] [Google Scholar]
- 2.Adam RM, Danciu T, McLellan DL, Borer JG, Lin J, Zurakowski D, Weinstein MH, Rajjayabun PH, Mellon JK, Freeman MR. A nuclear form of the heparin-binding epidermal growth factor-like growth factor precursor is a feature of aggressive transitional cell carcinoma. Cancer Res 63: 484–490, 2003. [PubMed] [Google Scholar]
- 3.Baskin LS, Sutherland RS, Thomson AA, Nguyen HT, Morgan DM, Hayward SW, Hom YK, DiSandro M, Cunha GR. Growth factors in bladder wound healing. J Urol 157: 2388–2395, 1997. doi: 10.1016/S0022-5347(01)64786-4. [DOI] [PubMed] [Google Scholar]
- 4.Bellmunt J, Hussain M, Dinney CP. Novel approaches with targeted therapies in bladder cancer. Crit Rev Oncol Hematol 46, Suppl: 85–104, 2003. doi: 10.1016/S1040-8428(03)00067-2. [DOI] [PubMed] [Google Scholar]
- 5.Berger MS, Greenfield C, Gullick WJ, Haley J, Downward J, Neal DE, Harris AL, Waterfield MD. Evaluation of epidermal growth factor receptors in bladder tumours. Br J Cancer 56: 533–537, 1987. doi: 10.1038/bjc.1987.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billerey C, Chopin D, Aubriot-Lorton MH, Ricol D, Gil Diez de Medina S, Van Rhijn B, Bralet MP, Lefrere-Belda MA, Lahaye JB, Abbou CC, Bonaventure J, Zafrani ES, van der Kwast T, Thiery JP, Radvanyi F. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol 158: 1955–1959, 2001. doi: 10.1016/S0002-9440(10)64665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bindels EM, van der Kwast TH, Izadifar V, Chopin DK, de Boer WI. Functions of epidermal growth factor-like growth factors during human urothelial reepithelialization in vitro and the role of erbB2. Urol Res 30: 240–247, 2002. doi: 10.1007/s00240-002-0260-7. [DOI] [PubMed] [Google Scholar]
- 8.Birder L, Andersson KE. Urothelial signaling. Physiol Rev 93: 653–680, 2013. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birder LA, Apodaca G, De Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol Renal Physiol 275: F226–F229, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Network; Weinstein JN, Akbani R, Broom BM, Wang W, Verhaak RGW, McConkey D, Lerner S, Morgan M, Creighton CJ, Smith C, Kwiatkowski DJ, Cherniack AD, Kim J, Sekhar Pedamallu C, Noble MS, Al-Ahmadie HA, Reuter VE, Rosenberg JE, Bajorin DF, Bochner BH, Solit DB, Koppie T, Robinson B, Gordenin DA, Fargo D, Klimczak LJ, Roberts SA, Au J, Laird PW, Hinoue T, Schultz N, Ramirez R, Hansel D, Hoadley KA, Kim WY, Damrauer JS, Baylin SB, Mungall AJ, Gordon Robertson A, Chu A, Kwiatkowski DJ, Sougnez C, Cibulskis K, Lichtenstein L, Sivachenko A, Stewart C, Lawrence MS, Getz G, Lander E, Gabriel SB, Creighton CJ, Donehower L, Cherniack AD, Kim J, Carter SL, Saksena G, Schumacher SE, Sougnez C, Freeman SS, Jung J, Sekhar Pedamallu C, Bhatt AS, Pugh T, Getz G, Beroukhim R, Gabriel SB, Meyerson M, Mungall AJ, Gordon Robertson A, Chu A, Ally A, Balasundaram M, Butterfield YSN, Dhalla N, Hirst C, Holt RA, Jones SJM, Lee D, Li HI, Marra MA, Mayo M, Moore RA, Schein JE, Sipahimalani P, Tam A, Thiessen N, Wong T, Wye N, Bowlby R, Chuah E, Guin R, Jones SJM, Marra MA, Hinoue T, Shen H, Bootwalla MS, Triche T Jr, Lai PH, Van Den Berg DJ, Weisenberger DJ, Laird PW, Hansel D, Hoadley KA, Balu S, Bodenheimer T, Damrauer Alan P. Hoyle JS, Jefferys SR, Meng S, Mose LE, Simons JV, Soloway MG, Wu J, Kim WY, Parker JS, Neil Hayes D, Roach J, Buda E, Jones CD, Mieczkowski PA, Tan D, Veluvolu U, Waring S, Todd Auman J, Perou CM, Wilkerson MD, Santoso N, Parfenov M, Ren X, Pantazi A, Hadjipanayis A, Seidman J, Kucherlapati R, Lee S, Yang L, Park PJ, Baylin SB, Wei Xu A, Protopopov A, Zhang J, Bristow C, Mahadeshwar HS, Seth S, Song X, Tang J, Zeng D, Chin L, Guo C, Weinstein JN, Akbani R, Broom BM, McConkey D, Casasent TD, Liu W, Ju Z, Motter T, Peng B, Ryan M, Wang W, Verhaak RGW, Su X, Yang J-Y, Lorenzi PL, Yao H, Zhang N, Zhang J, Mills GB, Kim J, Noble MS, Cho J, DiCara D, Frazer S, Gehlenborg N, Heiman DI, Lin P, Liu Y, Stojanov P, Voet D, Zhang H, Zou L, Chin L, Getz G, Bernard B, Kreisberg D, Reynolds S, Rovira H, Shmulevich I, Ramirez R, Schultz N, Gao J, Jacobsen A, Arman Aksoy B, Antipin Y, Ciriello G, Dresdner G, Gross B, Lee W, Reva B, Shen R, Sinha R, Onur Sumer S, Weinhold N, Ladanyi M, Sander C, Benz C, Carlin D, Haussler D, Ng S, Paull EO, Stuart J, Zhu J, Liu Y, Zhang W, Taylor BS, Lichtenberg TM, Zmuda E, Barr T, Black AD, George M, Hanf B, Helsel C, McAllister C, Ramirez NC, Tabler TR, Weaver S, Wise L, Bowen J, Gastier-Foster JM, Weinstein JN, Lerner S, Jian W, Tello S, Ittman M, Castro P, McClenden WD, Morgan M, Gibbs R, Liu Y, Saller C, Tarvin K, DiPiero JM, Owens J, Bollag R, Li Q, Weinberger P, Czerwinski C, Huelsenbeck-Dill L, Iacocca M, Petrelli N, Rabeno B, Swanson P, Shelton T, Curley E, Gardner J, Mallery D, Penny R, Van Bang N, Thi Hanh P, Kohl B, Van Le X, Duc Phu B, Thorp R, Viet Tien N, Quang Vinh L, Sandusky G, Burks E, Christ K, Gee J, Holway A, Moinzadeh A, Sorcini A, Sullivan T, Al-Ahmadie HA, Bajorin DF, Bochner BH, Garcia-Grossman IR, Regazzi AM, Solit DB, Rosenberg JE, Reuter VE, Koppie T, Boice L, Kimryn Rathmell W, Thorne L, Bastacky S, Davies B, Dhir R, Gingrich J, Hrebinko R, Maranchie J, Nelson J, Parwani A, Bshara W, Gaudioso C, Morrison C, Alexopoulou V, Bartlett J, Engel J, Kodeeswaran S, Antic T, O’Donnell PH, Smith ND, Steinberg GD, Egea S, Gomez-Fernandez C, Herbert L, Jorda M, Soloway M, Beaver A, Carter S, Kapur P, Lewis C, Lotan Y, Robinson B, Hansel D, Guo C, Bondaruk J, Czerniak B, Akbani R, Broom BM, Liu Y, Zhang W, Weinstein JN, Lerner S, Morgan M, Kim J, Cherniack AD, Freeman SS, Sekhar Pedamallu C, Noble MS, Kwiatkowski DJ, Al-Ahmadie HA, Bajorin DF, Bochner BH, Solit DB, Rosenberg JE, Reuter VE, Koppie T, Robinson B, Skinner E, Ramirez R, Schultz N, Hansel D, Kim WY, Guo C, Bondaruk J, Aldape K, Czerniak B, Jensen MA, Kahn AB, Pihl TD, Pot DA, Srinivasan D, Wan Y, Ferguson ML, Claude Zenklusen J, Davidsen T, Demchok JA, Mills Shaw KR, Sheth M, Tarnuzzer R, Wang Z, Yang L, Hutter C, Ozenberger BA, Sofia HJ, Eley G. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507: 315–322, 2014. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castillo-Martin M, Domingo-Domenech J, Karni-Schmidt O, Matos T, Cordon-Cardo C. Molecular pathways of urothelial development and bladder tumorigenesis. Urol Oncol 28: 401–408, 2010. doi: 10.1016/j.urolonc.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Cheng W, Jacobs WB, Zhang JJ, Moro A, Park JH, Kushida M, Qiu W, Mills AA, Kim PC. DeltaNp63 plays an anti-apoptotic role in ventral bladder development. Development 133: 4783–4792, 2006. doi: 10.1242/dev.02621. [DOI] [PubMed] [Google Scholar]
- 13.Colopy SA, Bjorling DE, Mulligan WA, Bushman W. A population of progenitor cells in the basal and intermediate layers of the murine bladder urothelium contributes to urothelial development and regeneration. Dev Dyn 243: 988–998, 2014. doi: 10.1002/dvdy.24143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper EH, Cowen DM, Knowles JC. The recovery of mouse bladder epithelium after injury by 4-ethylsulphonylnaphthalene-1-sulphonamide. J Pathol 108: 151–156, 1972. doi: 10.1002/path.1711080209. [DOI] [PubMed] [Google Scholar]
- 15.de Boer WI, Schuller AG, Vermey M, van der Kwast TH. Expression of growth factors and receptors during specific phases in regenerating urothelium after acute injury in vivo. Am J Pathol 145: 1199–1207, 1994. [PMC free article] [PubMed] [Google Scholar]
- 16.DeGraff DJ, Cates JM, Mauney JR, Clark PE, Matusik RJ, Adam RM. When urothelial differentiation pathways go wrong: implications for bladder cancer development and progression. Urol Oncol 31: 802–811, 2013. doi: 10.1016/j.urolonc.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeGraff DJ, Clark PE, Cates JM, Yamashita H, Robinson VL, Yu X, Smolkin ME, Chang SS, Cookson MS, Herrick MK, Shariat SF, Steinberg GD, Frierson HF, Wu XR, Theodorescu D, Matusik RJ. Loss of the urothelial differentiation marker FOXA1 is associated with high grade, late stage bladder cancer and increased tumor proliferation. PLoS One 7: e36669, 2012. doi: 10.1371/journal.pone.0036669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Croce L, Helin K. Transcriptional regulation by Polycomb group proteins. Nat Struct Mol Biol 20: 1147–1155, 2013. doi: 10.1038/nsmb.2669. [DOI] [PubMed] [Google Scholar]
- 19.di Martino E, Tomlinson DC, Knowles MA. A decade of FGF receptor research in bladder cancer: past, present, and future challenges. Adv Urol: 429213: 2012. doi: 10.1155/2012/429213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diez de Medina SG, Chopin D, Marjou A, Delouvée A, LaRochelle WJ, Hoznek A, Abbou C, Aaronson SA, Thiery JP, Radvanyi F. Decreased expression of keratinocyte growth factor receptor in a subset of human transitional cell bladder carcinomas. Oncogene 14: 323–330, 1997. doi: 10.1038/sj.onc.1200830. [DOI] [PubMed] [Google Scholar]
- 21.Everaerts W, Vriens J, Owsianik G, Appendino G, Voets T, De Ridder D, Nilius B. Functional characterization of transient receptor potential channels in mouse urothelial cells. Am J Physiol Renal Physiol 298: F692–F701, 2010. doi: 10.1152/ajprenal.00599.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman MR, Yoo JJ, Raab G, Soker S, Adam RM, Schneck FX, Renshaw AA, Klagsbrun M, Atala A. Heparin-binding EGF-like growth factor is an autocrine growth factor for human urothelial cells and is synthesized by epithelial and smooth muscle cells in the human bladder. J Clin Invest 99: 1028–1036, 1997. doi: 10.1172/JCI119230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuchs E, Horsley V. More than one way to skin. Genes Dev 22: 976–985, 2008. doi: 10.1101/gad.1645908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaisa NT, Graham TA, McDonald SA, Cañadillas-Lopez S, Poulsom R, Heidenreich A, Jakse G, Tadrous PJ, Knuechel R, Wright NA. The human urothelium consists of multiple clonal units, each maintained by a stem cell. J Pathol 225: 163–171, 2011. doi: 10.1002/path.2945. [DOI] [PubMed] [Google Scholar]
- 25.Gandhi D, Molotkov A, Batourina E, Schneider K, Dan H, Reiley M, Laufer E, Metzger D, Liang F, Liao Y, Sun TT, Aronow B, Rosen R, Mauney J, Adam R, Rosselot C, Van Batavia J, McMahon A, McMahon J, Guo JJ, Mendelsohn C. Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Dev Cell 26: 469–482, 2013. doi: 10.1016/j.devcel.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gui Y, Guo G, Huang Y, Hu X, Tang A, Gao S, Wu R, Chen C, Li X, Zhou L, He M, Li Z, Sun X, Jia W, Chen J, Yang S, Zhou F, Zhao X, Wan S, Ye R, Liang C, Liu Z, Huang P, Liu C, Jiang H, Wang Y, Zheng H, Sun L, Liu X, Jiang Z, Feng D, Chen J, Wu S, Zou J, Zhang Z, Yang R, Zhao J, Xu C, Yin W, Guan Z, Ye J, Zhang H, Li J, Kristiansen K, Nickerson ML, Theodorescu D, Li Y, Zhang X, Li S, Wang J, Yang H, Wang J, Cai Z. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat Genet 43: 875–878, 2011. doi: 10.1038/ng.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Guo G, Balsara ZR, Hill WG, Li X. Stage- and subunit-specific functions of polycomb repressive complex 2 in bladder urothelial formation and regeneration. Development 144: 400–408, 2017. doi: 10.1242/dev.143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gust KM, McConkey DJ, Awrey S, Hegarty PK, Qing J, Bondaruk J, Ashkenazi A, Czerniak B, Dinney CP, Black PC. Fibroblast growth factor receptor 3 is a rational therapeutic target in bladder cancer. Mol Cancer Ther 12: 1245–1254, 2013. doi: 10.1158/1535-7163.MCT-12-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hainau B, Dombernowsky P. Histology and cell proliferation in human bladder tumors. An autoradiographic study. Cancer 33: 115–126, 1974. doi:. [DOI] [PubMed] [Google Scholar]
- 29.Harnden P, Eardley I, Joyce AD, Southgate J. Cytokeratin 20 as an objective marker of urothelial dysplasia. Br J Urol 78: 870–875, 1996. doi: 10.1046/j.1464-410X.1996.23511.x. [DOI] [PubMed] [Google Scholar]
- 30.Hicks RM. The mammalian urinary bladder: an accommodating organ. Biol Rev Camb Philos Soc 50: 215–246, 1975. doi: 10.1111/j.1469-185X.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 31.Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med 20: 847–856, 2014. doi: 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu P, Meyers S, Liang FX, Deng FM, Kachar B, Zeidel ML, Sun TT. Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol 283: F1200–F1207, 2002. doi: 10.1152/ajprenal.00043.2002. [DOI] [PubMed] [Google Scholar]
- 33.Hurst RE. Structure, function, and pathology of proteoglycans and glycosaminoglycans in the urinary tract. World J Urol 12: 3–10, 1994. doi: 10.1007/BF00182044. [DOI] [PubMed] [Google Scholar]
- 34.Jacob A, Budhiraja S, Reichel RR. The HNF-3alpha transcription factor is a primary target for retinoic acid action. Exp Cell Res 250: 1–9, 1999. doi: 10.1006/excr.1999.4512. [DOI] [PubMed] [Google Scholar]
- 35.Jordan EJ, Iyer G. Targeted therapy in advanced bladder cancer: what have we learned? Urol Clin North Am 42: 253–262, 2015. doi: 10.1016/j.ucl.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jost SP. Cell cycle of normal bladder urothelium in developing and adult mice. Virchows Arch B Cell Pathol Incl Mol Pathol 57: 27–36, 1989. doi: 10.1007/BF02899062. [DOI] [PubMed] [Google Scholar]
- 37.Kachar B, Liang F, Lins U, Ding M, Wu XR, Stoffler D, Aebi U, Sun TT. Three-dimensional analysis of the 16 nm urothelial plaque particle: luminal surface exposure, preferential head-to-head interaction, and hinge formation. J Mol Biol 285: 595–608, 1999. doi: 10.1006/jmbi.1998.2304. [DOI] [PubMed] [Google Scholar]
- 38.Kanai A, Andersson KE. Bladder afferent signaling: recent findings. J Urol 183: 1288–1295, 2010. doi: 10.1016/j.juro.2009.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanematsu A, Yamamoto S, Noguchi T, Ozeki M, Tabata Y, Ogawa O. Bladder regeneration by bladder acellular matrix combined with sustained release of exogenous growth factor. J Urol 170: 1633–1638, 2003. doi: 10.1097/01.ju.0000084021.51099.8a. [DOI] [PubMed] [Google Scholar]
- 40.Keay S. Cell signaling in interstitial cystitis/painful bladder syndrome. Cell Signal 20: 2174–2179, 2008. doi: 10.1016/j.cellsig.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Keay S, Kleinberg M, Zhang CO, Hise MK, Warren JW. Bladder epithelial cells from patients with interstitial cystitis produce an inhibitor of heparin-binding epidermal growth factor-like growth factor production. J Urol 164: 2112–2118, 2000. doi: 10.1016/S0022-5347(05)66980-7. [DOI] [PubMed] [Google Scholar]
- 42.Keay S, Warren JW. A hypothesis for the etiology of interstitial cystitis based upon inhibition of bladder epithelial repair. Med Hypotheses 51: 79–83, 1998. doi: 10.1016/S0306-9877(98)90260-2. [DOI] [PubMed] [Google Scholar]
- 43.Keay S, Zhang CO, Chai T, Warren J, Koch K, Grkovic D, Colville H, Alexander R. Antiproliferative factor, heparin-binding epidermal growth factor-like growth factor, and epidermal growth factor in men with interstitial cystitis versus chronic pelvic pain syndrome. Urology 63: 22–26, 2004. doi: 10.1016/j.urology.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 44.Keay S, Zhang CO, Kagen DI, Hise MK, Jacobs SC, Hebel JR, Gordon D, Whitmore K, Bodison S, Warren JW. Concentrations of specific epithelial growth factors in the urine of interstitial cystitis patients and controls. J Urol 158: 1983–1988, 1997. doi: 10.1016/S0022-5347(01)64198-3. [DOI] [PubMed] [Google Scholar]
- 45.Keay S, Zhang CO, Shoenfelt JL, Chai TC. Decreased in vitro proliferation of bladder epithelial cells from patients with interstitial cystitis. Urology 61: 1278–1284, 2003. doi: 10.1016/S0090-4295(03)00005-0. [DOI] [PubMed] [Google Scholar]
- 46.Keay SK, Szekely Z, Conrads TP, Veenstra TD, Barchi JJ Jr, Zhang CO, Koch KR, Michejda CJ. An antiproliferative factor from interstitial cystitis patients is a frizzled 8 protein-related sialoglycopeptide. Proc Natl Acad Sci USA 101: 11803–11808, 2004. doi: 10.1073/pnas.0404509101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khandelwal P, Ruiz WG, Balestreire-Hawryluk E, Weisz OA, Goldenring JR, Apodaca G. Rab11a-dependent exocytosis of discoidal/fusiform vesicles in bladder umbrella cells. Proc Natl Acad Sci USA 105: 15773–15778, 2008. doi: 10.1073/pnas.0805636105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kikuno N, Kawamoto K, Hirata H, Vejdani K, Kawakami K, Fandel T, Nunes L, Urakami S, Shiina H, Igawa M, Tanagho E, Dahiya R. Nerve growth factor combined with vascular endothelial growth factor enhances regeneration of bladder acellular matrix graft in spinal cord injury-induced neurogenic rat bladder. BJU Int 103: 1424–1428, 2009. doi: 10.1111/j.1464-410X.2008.08129.x. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Adam RM, Freeman MR. Trafficking of nuclear heparin-binding epidermal growth factor-like growth factor into an epidermal growth factor receptor-dependent autocrine loop in response to oxidative stress. Cancer Res 65: 8242–8249, 2005. doi: 10.1158/0008-5472.CAN-05-0942. [DOI] [PubMed] [Google Scholar]
- 50.Kong XT, Deng FM, Hu P, Liang FX, Zhou G, Auerbach AB, Genieser N, Nelson PK, Robbins ES, Shapiro E, Kachar B, Sun TT. Roles of uroplakins in plaque formation, umbrella cell enlargement, and urinary tract diseases. J Cell Biol 167: 1195–1204, 2004. doi: 10.1083/jcb.200406025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kopparapu PK, Boorjian SA, Robinson BD, Downes M, Gudas LJ, Mongan NP, Persson JL. Expression of VEGF and its receptors VEGFR1/VEGFR2 is associated with invasiveness of bladder cancer. Anticancer Res 33: 2381–2390, 2013. [PubMed] [Google Scholar]
- 52.Kouzarides T. Chromatin modifications and their function. Cell 128: 693–705, 2007. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Kramer C, Klasmeyer K, Bojar H, Schulz WA, Ackermann R, Grimm MO. Heparin-binding epidermal growth factor-like growth factor isoforms and epidermal growth factor receptor/ErbB1 expression in bladder cancer and their relation to clinical outcome. Cancer 109: 2016–2024, 2007. doi: 10.1002/cncr.22627. [DOI] [PubMed] [Google Scholar]
- 54.Kreft ME, Sterle M, Veranic P, Jezernik K. Urothelial injuries and the early wound healing response: tight junctions and urothelial cytodifferentiation. Histochem Cell Biol 123: 529–539, 2005. doi: 10.1007/s00418-005-0770-9. [DOI] [PubMed] [Google Scholar]
- 55.Kurzrock EA, Lieu DK, Degraffenried LA, Chan CW, Isseroff RR. Label-retaining cells of the bladder: candidate urothelial stem cells. Am J Physiol Renal Physiol 294: F1415–F1421, 2008. doi: 10.1152/ajprenal.00533.2007. [DOI] [PubMed] [Google Scholar]
- 56.Lavelle J, Meyers S, Ramage R, Bastacky S, Doty D, Apodaca G, Zeidel ML. Bladder permeability barrier: recovery from selective injury of surface epithelial cells. Am J Physiol Renal Physiol 283: F242–F253, 2002. doi: 10.1152/ajprenal.00307.2001. [DOI] [PubMed] [Google Scholar]
- 57.Lee SR, Roh YG, Kim SK, Lee JS, Seol SY, Lee HH, Kim WT, Kim WJ, Heo J, Cha HJ, Kang TH, Chung JW, Chu IS, Leem SH. Activation of EZH2 and SUZ12 Regulated by E2F1 Predicts the Disease Progression and Aggressive Characteristics of Bladder Cancer. Clin Cancer Res 21: 5391–5403, 2015. doi: 10.1158/1078-0432.CCR-14-2680. [DOI] [PubMed] [Google Scholar]
- 58.Levi PE, Cooper EH, Anderson CK, Path MC, Williams RE. Analyses of DNA content, nuclear size and cell proliferation of transitional cell carcinoma in man. Cancer 23: 1074–1085, 1969. doi:. [DOI] [PubMed] [Google Scholar]
- 59.Liang FX, Bosland MC, Huang H, Romih R, Baptiste S, Deng FM, Wu XR, Shapiro E, Sun TT. Cellular basis of urothelial squamous metaplasia: roles of lineage heterogeneity and cell replacement. J Cell Biol 171: 835–844, 2005. doi: 10.1083/jcb.200505035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ling S, Chang X, Schultz L, Lee TK, Chaux A, Marchionni L, Netto GJ, Sidransky D, Berman DM. An EGFR-ERK-SOX9 signaling cascade links urothelial development and regeneration to cancer. Cancer Res 71: 3812–3821, 2011. doi: 10.1158/0008-5472.CAN-10-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Locher GW, Cooper EH. Repair of rat urinary bladder epithelium following injury by cyclophosphamide. Invest Urol 8: 116–123, 1970. [PubMed] [Google Scholar]
- 62.Martin BF. Cell replacement and differentiation in transitional epithelium: a histological and autoradiographic study of the guinea-pig bladder and ureter. J Anat 112: 433–455, 1972. [PMC free article] [PubMed] [Google Scholar]
- 63.Mauney JR, Adam RM. Dynamic reciprocity in cell-scaffold interactions. Adv Drug Deliv Rev 82–83: 77–85, 2015. doi: 10.1016/j.addr.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Merrill L, Gonzalez EJ, Girard BM, Vizzard MA. Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nat Rev Urol 13: 193–204, 2016. doi: 10.1038/nrurol.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Messier B, Leblond CP. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am J Anat 106: 247–285, 1960. doi: 10.1002/aja.1001060305. [DOI] [PubMed] [Google Scholar]
- 66.Messing EM. Clinical implications of the expression of epidermal growth factor receptors in human transitional cell carcinoma. Cancer Res 50: 2530–2537, 1990. [PubMed] [Google Scholar]
- 67.Messing EM. Growth factors and bladder cancer: clinical implications of the interactions between growth factors and their urothelial receptors. Semin Surg Oncol 8: 285–292, 1992. doi: 10.1002/ssu.2980080507. [DOI] [PubMed] [Google Scholar]
- 68.Moll R, Schiller DL, Franke WW. Identification of protein IT of the intestinal cytoskeleton as a novel type I cytokeratin with unusual properties and expression patterns. J Cell Biol 111: 567–580, 1990. doi: 10.1083/jcb.111.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Molloy CJ, Laskin JD. Effect of retinoid deficiency on keratin expression in mouse bladder. Exp Mol Pathol 49: 128–140, 1988. doi: 10.1016/0014-4800(88)90027-5. [DOI] [PubMed] [Google Scholar]
- 70.Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci USA 103: 14170–14175, 2006. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mysorekar IU, Isaacson-Schmid M, Walker JN, Mills JC, Hultgren SJ. Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe 5: 463–475, 2009. doi: 10.1016/j.chom.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nanba D, Mammoto A, Hashimoto K, Higashiyama S. Proteolytic release of the carboxy-terminal fragment of proHB-EGF causes nuclear export of PLZF. J Cell Biol 163: 489–502, 2003. doi: 10.1083/jcb.200303017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neal DE, Bennett MK, Hall RR, Marsh C, Abel PD, Sainsbury JRC, Harris AL. Epidermal-growth-factor receptors in human bladder cancer: comparison of invasive and superficial tumours. Lancet 325: 366–368, 1985. doi: 10.1016/S0140-6736(85)91386-8. [DOI] [PubMed] [Google Scholar]
- 74.Neal DE, Mellon K. Epidermal growth factor receptor and bladder cancer: a review. Urol Int 48: 365–371, 1992. doi: 10.1159/000282357. [DOI] [PubMed] [Google Scholar]
- 75.Negrete HO, Lavelle JP, Berg J, Lewis SA, Zeidel ML. Permeability properties of the intact mammalian bladder epithelium. Am J Physiol Renal Physiol 271: F886–F894, 1996. [DOI] [PubMed] [Google Scholar]
- 76.Ongusaha PP, Kwak JC, Zwible AJ, Macip S, Higashiyama S, Taniguchi N, Fang L, Lee SW. HB-EGF is a potent inducer of tumor growth and angiogenesis. Cancer Res 64: 5283–5290, 2004. doi: 10.1158/0008-5472.CAN-04-0925. [DOI] [PubMed] [Google Scholar]
- 77.Papafotiou G, Paraskevopoulou V, Vasilaki E, Kanaki Z, Paschalidis N, Klinakis A. KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat Commun 7: 11914, 2016. doi: 10.1038/ncomms11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pignon JC, Grisanzio C, Geng Y, Song J, Shivdasani RA, Signoretti S. p63-expressing cells are the stem cells of developing prostate, bladder, and colorectal epithelia. Proc Natl Acad Sci USA 110: 8105–8110, 2013. doi: 10.1073/pnas.1221216110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raman JD, Mongan NP, Tickoo SK, Boorjian SA, Scherr DS, Gudas LJ. Increased expression of the polycomb group gene, EZH2, in transitional cell carcinoma of the bladder. Clin Cancer Res 11: 8570–8576, 2005. doi: 10.1158/1078-0432.CCR-05-1047. [DOI] [PubMed] [Google Scholar]
- 80.Raman JD, Warrick JI, Caruso C, Yang Z, Shuman L, Bruggeman RD, Shariat S, Karam JA, Wood C, Weizer AZ, Remzi M, Haitel A, Bensalah K, Rioux-Leclerq N, Bolenz C, Roscigno M, Krabbe LM, Kapur P, Lotan Y, Margulis V, DeGraff DJ. Altered expression of the transcription factor forkhead box a1 (foxa1) is associated with poor prognosis in urothelial carcinoma of the upper urinary tract. Urology 94: 314.e1–314.e7, 2016. doi: 10.1016/j.urology.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 81.Reddy OL, Cates JM, Gellert LL, Crist HS, Yang Z, Yamashita H, Taylor JA III, Smith JA Jr, Chang SS, Cookson MS, You C, Barocas DA, Grabowska MM, Ye F, Wu XR, Yi Y, Matusik RJ, Kaestner KH, Clark PE, DeGraff DJ. Loss of FOXA1 Drives Sexually Dimorphic Changes in Urothelial Differentiation and Is an Independent Predictor of Poor Prognosis in Bladder Cancer. Am J Pathol 185: 1385–1395, 2015. doi: 10.1016/j.ajpath.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Romih R, Jezernik K, Masera A. Uroplakins and cytokeratins in the regenerating rat urothelium after sodium saccharin treatment. Histochem Cell Biol 109: 263–269, 1998. doi: 10.1007/s004180050226. [DOI] [PubMed] [Google Scholar]
- 83.Ruggieri MR, Chelsky MJ, Rosen SI, Shickley TJ, Hanno PM. Current findings and future research avenues in the study of interstitial cystitis. Urol Clin North Am 21: 163–176, 1994. [PubMed] [Google Scholar]
- 84.Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472: 110–114, 2011. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shin K, Lim A, Odegaard JI, Honeycutt JD, Kawano S, Hsieh MH, Beachy PA. Cellular origin of bladder neoplasia and tissue dynamics of its progression to invasive carcinoma. Nat Cell Biol 16: 469–478, 2014. doi: 10.1038/ncb2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shin K, Lim A, Zhao C, Sahoo D, Pan Y, Spiekerkoetter E, Liao JC, Beachy PA. Hedgehog signaling restrains bladder cancer progression by eliciting stromal production of urothelial differentiation factors. Cancer Cell 26: 521–533, 2014. doi: 10.1016/j.ccell.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith K, Fennelly JA, Neal DE, Hall RR, Harris AL. Characterization and quantitation of the epidermal growth factor receptor in invasive and superficial bladder tumors. Cancer Res 49: 5810–5815, 1989. [PubMed] [Google Scholar]
- 88.Song J, Abraham SN. TLR-mediated immune responses in the urinary tract. Curr Opin Microbiol 11: 66–73, 2008. doi: 10.1016/j.mib.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Southgate J, Hutton KA, Thomas DF, Trejdosiewicz LK. Normal human urothelial cells in vitro: proliferation and induction of stratification. Lab Invest 71: 583–594, 1994. [PubMed] [Google Scholar]
- 90.Sun TT, Zhao H, Provet J, Aebi U, Wu XR. Formation of asymmetric unit membrane during urothelial differentiation. Mol Biol Rep 23: 3–11, 1996. doi: 10.1007/BF00357068. [DOI] [PubMed] [Google Scholar]
- 91.Sun W, Wilhelmina Aalders T, Oosterwijk E. Identification of potential bladder progenitor cells in the trigone. Dev Biol 393: 84–92, 2014. doi: 10.1016/j.ydbio.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 92.Tan DW, Barker N. Intestinal stem cells and their defining niche. Curr Top Dev Biol 107: 77–107, 2014. doi: 10.1016/B978-0-12-416022-4.00003-2. [DOI] [PubMed] [Google Scholar]
- 93.Ting K, Aitken KJ, Penna F, Samiei AN, Sidler M, Jiang JX, Ibrahim F, Tolg C, Delgado-Olguin P, Rosenblum N, Bägli DJ. Uropathogenic E. coli (UPEC) Infection Induces Proliferation through Enhancer of Zeste Homologue 2 (EZH2). PLoS One 11: e0149118, 2016. doi: 10.1371/journal.pone.0149118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tomaszewski JE, Landis JR, Russack V, Williams TM, Wang LP, Hardy C, Brensinger C, Matthews YL, Abele ST, Kusek JW, Nyberg LM; Interstitial Cystitis Database Study Group . Biopsy features are associated with primary symptoms in interstitial cystitis: results from the interstitial cystitis database study. Urology 57, Suppl 1: 67–81, 2001. doi: 10.1016/S0090-4295(01)01166-9. [DOI] [PubMed] [Google Scholar]
- 95.Tomlinson DC, Baldo O, Harnden P, Knowles MA. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J Pathol 213: 91–98, 2007. doi: 10.1002/path.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tomlinson DC, Knowles MA. Altered splicing of FGFR1 is associated with high tumor grade and stage and leads to increased sensitivity to FGF1 in bladder cancer. Am J Pathol 177: 2379–2386, 2010. doi: 10.2353/ajpath.2010.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tomlinson DC, Lamont FR, Shnyder SD, Knowles MA. Fibroblast growth factor receptor 1 promotes proliferation and survival via activation of the mitogen-activated protein kinase pathway in bladder cancer. Cancer Res 69: 4613–4620, 2009. doi: 10.1158/0008-5472.CAN-08-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsai YC, Simoneau AR, Spruck CH III, Nichols PW, Steven K, Buckley JD, Jones PA. Mosaicism in human epithelium: macroscopic monoclonal patches cover the urothelium. J Urol 153: 1697–1700, 1995. doi: 10.1016/S0022-5347(01)67507-4. [DOI] [PubMed] [Google Scholar]
- 99.Van Batavia J, Yamany T, Molotkov A, Dan H, Mansukhani M, Batourina E, Schneider K, Oyon D, Dunlop M, Wu XR, Cordon-Cardo C, Mendelsohn C. Bladder cancers arise from distinct urothelial sub-populations. Nat Cell Biol 16: 982–991, 2014. doi: 10.1038/ncb3038. [DOI] [PubMed] [Google Scholar]
- 100.Varley C, Hill G, Pellegrin S, Shaw NJ, Selby PJ, Trejdosiewicz LK, Southgate J. Autocrine regulation of human urothelial cell proliferation and migration during regenerative responses in vitro. Exp Cell Res 306: 216–229, 2005. doi: 10.1016/j.yexcr.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 101.Varley CL, Bacon EJ, Holder JC, Southgate J. FOXA1 and IRF-1 intermediary transcriptional regulators of PPARgamma-induced urothelial cytodifferentiation. Cell Death Differ 16: 103–114, 2009. doi: 10.1038/cdd.2008.116. [DOI] [PubMed] [Google Scholar]
- 102.Vasconcelos-Nóbrega C, Colaço A, Lopes C, Oliveira PA. Review: BBN as an urothelial carcinogen. In Vivo 26: 727–739, 2012. [PubMed] [Google Scholar]
- 103.Walker BE. Polyploidy and differentiation in the transitional epithelium of mouse urinary bladder. Chromosoma 9: 105–118, 1957. doi: 10.1007/BF02568069. [DOI] [PubMed] [Google Scholar]
- 104.Wang H, Albadine R, Magheli A, Guzzo TJ, Ball MW, Hinz S, Schoenberg MP, Netto GJ, Gonzalgo ML. Increased EZH2 protein expression is associated with invasive urothelial carcinoma of the bladder. Urol Oncol 30: 428–433, 2012. doi: 10.1016/j.urolonc.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 105.Wu XR, Manabe M, Yu J, Sun TT. Large scale purification and immunolocalization of bovine uroplakins I, II, and III. Molecular markers of urothelial differentiation. J Biol Chem 265: 19170–19179, 1990. [PubMed] [Google Scholar]
- 106.Wu XR, Medina JJ, Sun TT. Selective interactions of UPIa and UPIb, two members of the transmembrane 4 superfamily, with distinct single transmembrane-domained proteins in differentiated urothelial cells. J Biol Chem 270: 29752–29759, 1995. doi: 10.1074/jbc.270.50.29752. [DOI] [PubMed] [Google Scholar]
- 107.Yamada T, Ugawa S, Ueda T, Ishida Y, Kajita K, Shimada S. Differential localizations of the transient receptor potential channels TRPV4 and TRPV1 in the mouse urinary bladder. J Histochem Cytochem 57: 277–287, 2009. doi: 10.1369/jhc.2008.951962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dötsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 2: 305–316, 1998. doi: 10.1016/S1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 109.Yu W, Khandelwal P, Apodaca G. Distinct apical and basolateral membrane requirements for stretch-induced membrane traffic at the apical surface of bladder umbrella cells. Mol Biol Cell 20: 282–295, 2009. doi: 10.1091/mbc.E08-04-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang H, Lin G, Qiu X, Ning H, Banie L, Lue TF, Lin CS. Label retaining and stem cell marker expression in the developing rat urinary bladder. Urology 79: 746.e1–746.e6, 2012. doi: 10.1016/j.urology.2011.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]