Abstract
Fetal insulin secretion is inhibited by acute hypoxemia. The relationship between prolonged hypoxemia and insulin secretion, however, is less well defined. To test the hypothesis that prolonged fetal hypoxemia impairs insulin secretion, studies were performed in sheep fetuses that were bled to anemic conditions for 9 ± 0 days (anemic, n = 19) and compared with control fetuses (n = 15). Arterial hematocrit and oxygen content were 34% and 52% lower, respectively, in anemic vs. control fetuses (P < 0.0001). Plasma glucose concentrations were 21% higher in the anemic group (P < 0.05). Plasma norepinephrine and cortisol concentrations increased 70% in the anemic group (P < 0.05). Glucose-, arginine-, and leucine-stimulated insulin secretion all were lower (P < 0.05) in anemic fetuses. No differences in pancreatic islet size or β-cell mass were found. In vitro, isolated islets from anemic fetuses secreted insulin in response to glucose and leucine as well as control fetal islets. These findings indicate a functional islet defect in anemic fetuses, which likely involves direct effects of low oxygen and/or increased norepinephrine on insulin release. In pregnancies complicated by chronic fetal hypoxemia, increasing fetal oxygen concentrations may improve insulin secretion.
Keywords: β-cell, islet, oxygen, pancreas, fetus
in human pregnancies complicated by placental insufficiency and intrauterine growth restriction (IUGR), fetuses are characterized by chronic hypoxemia, decreased glucose-stimulated insulin secretion (GSIS), and impaired development of the pancreatic islet (32, 46). Severely intrauterine growth-restricted fetuses have smaller islets and lower β-cell mass (46). In both humans and animal models, the chronic fetal hypoxemia seen in pregnancies complicated by placental insufficiency is accompanied by other defects, such as impaired glucose transfer to the fetus and fetal hypoglycemia (13, 21–23, 26, 27, 41). Our laboratory has already demonstrated in sheep that chronic fetal hypoglycemia independent of chronic fetal hypoxemia and placental insufficiency impairs fetal insulin secretion and pancreatic islet development (19). Furthermore, acute fetal hypoxemia inhibits GSIS in normal fetal sheep (25, 51). Whether chronic hypoxemia that is independent of fetal hypoglycemia and placental insufficiency can impair insulin secretion has not been determined. The relative impact of both chronic hypoxemia and hypoglycemia on pancreatic islet development and fetal insulin secretion is important to determine, however, as both oxygen and glucose supplementation of growth-restricted fetuses has been considered as potential therapies of fetal growth restriction in pregnancies complicated by placental insufficiency (39, 43).
To test the effect of fetal hypoxemia independent of fetal hypoglycemia on fetal insulin secretion, we used an animal model of chronic fetal anemic hypoxemia in pregnant sheep (10, 17). We have previously shown that fetal glucose concentrations are not decreased in this model, but oxygen utilization is lower (10). We hypothesized that chronic experimental anemic hypoxemia attenuates fetal GSIS. We also measured fetal insulin secretion in response to the amino acids leucine and arginine, which stimulate insulin secretion by increasing β-cell metabolism and directly depolarizing the β-cell plasma membrane, respectively (7, 40). Additionally, we also measured other circulating fetal hormones, including norepinephrine, cortisol, and glucagon, which have their own independent effects on insulin secretion. Pancreatic islet structure and mRNA concentrations of hormones and regulatory genes for insulin secretion were quantified. We also measured insulin secretion from pancreatic islets isolated from chronically anemic and control fetuses to determine whether in vivo differences in insulin secretion persisted in vitro.
MATERIALS AND METHODS
Fetal sheep model of chronic anemic hypoxemia.
Studies were conducted on pregnant Columbia-Rambouillet sheep carrying a singleton fetus at the Perinatal Research Center in Aurora, Colorado, with the approval of the Institutional Animal Care and Use Committee of the University of Colorado School of Medicine. This center is accredited by The Association for Assessment and Accreditation of Laboratory Animal Care International. At 119 ± 0 days gestational age (term ~148 days), fetal catheters were surgically placed into the descending aorta and femoral vein via pedal incisions (10). Maternal catheters were placed into the femoral artery and vein via a groin incision (10). Surgery was performed with anesthesia and pain control as follows. A maternal jugular venous catheter was placed for administration of diazepam (10 mg) and ketamine (1 g), and ewes were then maintained on isoflurane inhalation anesthesia (2–4%) for the remainder of the surgical procedure. Postsurgical pain control was achieved with flunixin meglumine (2.2 mg/kg im) on the day of the operation and the following 1–3 days based on assessment of the animal’s comfort. Immediately before surgery, the ewe also received procaine penicillin G (600,000 U im), and ampicillin (500 mg) was instilled into the amniotic cavity before closure of the hysterotomy and then administered three times a week.
Beginning at least 5 days after surgery, fetuses were bled (anemic; n = 19) with isovolumetric replacement by 0.9% NaCl to anemic conditions for an average of 9 ± 0 days before insulin secretion studies were performed. Anemic fetuses were compared with control fetuses who were not bled but were otherwise treated the same as the anemic fetuses with daily monitoring of blood gases and hematocrit (control, n = 15). The target arterial blood oxygen content for the anemic fetuses was 2.0 mmol/l, which is the mean arterial blood oxygen content in IUGR sheep fetuses with placental insufficiency (6, 45). The amount of blood removed daily was determined using a previously established formula taking into account fetal hematocrit and the target arterial blood oxygen content (17).
Biochemical analysis.
Fetal and maternal arterial plasma glucose and lactate concentrations were measured using Yellow Springs Instrument 2700 (Yellow Springs Instruments, Yellow Springs, OH). Blood hematocrit, pH, partial pressure of oxygen (), partial pressure of carbon dioxide (), and hemoglobin-O2 saturation were measured with the blood gas analyzer ABL825 (Radiometer, Copenhagen, Denmark). Oxygen content of the blood was calculated by the ABL825 analyzer. Arterial plasma insulin, insulin-like growth factor-1 (IGF-1), and cortisol were measured by ELISA [Insulin: ALPCO, Windham, NH; intra-assay and interassay coefficients of variation (CVs) = 5.6% and 4.7%, respectively; sensitivity = 0.14 ng/ml; IGF-1: ALPCO; intra-assay and interassay CVs, 3.1% and 5.6%, respectively; sensitivity, 0.09 ng/ml; cortisol: ALPCO; intra-assay and interassay CVs = 4.6% and 5.8%, respectively; sensitivity = 1.0 ng/ml] and norepinephrine by HPLC (model no. 2475, Waters; intra-assay and interassay CVs = 9.2% and 9.0%, respectively; sensitivity = 170 pg/ml). Fetal arterial plasma glucagon was measured by radioimmunoassay [Millipore, Billerica, MA; intra-assay and interassay CVs: 4.8% and 11.7%; sensitivity = 18.5 pg/ml] (3). Fetal arterial plasma proteins were measured in triplicate by the Bradford protein assay (Bio-Rad Laboratories, Hercules, CA).
In vivo insulin secretion studies.
Fetal GSIS and glucose-potentiated arginine-stimulated (ASIS) were measured in one subset of fetuses (anemic, n = 11; control, n = 7), and leucine-stimulated insulin secretion (LeuSIS) was measured in a different subset of fetuses (anemic, n = 8; control, n = 8) on the final day of the experimental period. Fetal GSIS was measured using a primed, continuous, variable-rate hyperglycemic clamp beginning at time 0, as previously described (3). Baseline plasma insulin and glucose concentrations were obtained at −60, −45, −30, and −15 min. At time 0, a hyperglycemic clamp was initiated with a 33% dextrose (wt/vol in saline) bolus (825 mg glucose) into the fetus. This was followed by a constant infusion of 33% dextrose, titrated to keep plasma glucose concentration near 2.5–3.0 mmol/l, which elicits 90% maximal insulin concentrations in this breed of fetal sheep (14). The dextrose infusion rate was then held constant from minute 45 until the completion of the GSIS and through the ASIS study. Fetal arterial samples were collected for measurement of glucose and insulin concentrations at 5, 10, 15, 20, 30, 45, 60, 75, 90, and 105 min for the GSIS study. GSIS was quantified as the difference between mean insulin concentration during the hyperglycemic clamp (minutes 60–105) and the mean of the four baseline samples. Fetal arterial plasma glucagon was measured at minutes 90 and 105. To measure ASIS, beginning at time 110 min, 0.5 mmol/kg of arginine dissolved in 5 ml of 0.4 mol/l sodium acetate and 0.9% NaCl was infused over 4 min into the fetal venous circulation. Arginine-stimulated insulin and glucagon secretion were defined as the difference between hormone concentrations 5 min after the start of the arginine infusion and the average of the two baseline concentrations obtained before the arginine infusion (3).
LeuSIS was measured as previously described (36). Baseline fetal arterial insulin concentrations were measured 10 and 5 min before the leucine infusion. Leucine (0.5 mmol/kg), dissolved in 15 ml 0.9% NaCl, was infused into the fetal venous circulation over 4 min. LeuSIS was defined as the difference between insulin concentrations 30 min after the start of the infusion and the average of the two baseline concentrations. During all insulin secretion studies, fetal blood that was removed was replaced with a fetal transfusion of heparinized maternal whole blood diluted with 0.9% NaCl to maintain the hematocrit of the fetal blood at the start of the study.
Necropsy.
The day after insulin secretion studies, ewes were sedated with diazepam (10 mg) and ketamine (1 g) intravenously, and fetuses were delivered via maternal laparotomy and hysterotomy. The ewe and fetus then received intravenous Fatal-Plus Solution (12 ml and 2 ml, respectively; Vortech Pharmaceuticals, Dearborn, MI). Fetal organs were collected and weighed. The pancreases from fetuses in which GSIS was measured were dissected, weighed, and processed for morphometric analysis (splenic portion) and snap frozen in liquid nitrogen and transferred to −80°C for pancreatic RNA extraction (hepatic portion) (anemic, n = 10; control, n = 7; one anemic fetus did not survive to necropsy). The pancreases from fetuses in which LeuSIS was measured were left in situ for perfusion and islet isolation (anemic, n = 8; control, n = 8) (35).
Quantitative real-time PCR.
RNA was extracted from pulverized −80°C pancreas (100 mg) and reverse transcribed into complementary DNA (cDNA), as previously described (anemic, n = 10; control, n = 7) (12). Extraction was by immersion in TRIzol (Invitrogen, Carlsbad, CA) and homogenization. To separate nucleic acids and proteins, the homogenate was mixed with chloroform and centrifuged at 12,000 g. The aqueous phase was then removed, and an RNeasy minicolumn (Qiagen, Germantown, MD) was used to isolate total RNA. Purity and quantity of RNA were determined with a spectrophotometer (Nanodrop 1000). Two micrograms of total RNA were reverse transcribed using Superscript III (Invitrogen) and Oligo dT 18–20 (Invitrogen) at 50°C for 1 h.
Quantitative PCR assays were performed on a 1:10 dilution (with sterile water) of the reverse transcription cDNA for INS (insulin; for-TCA GCA AAC AGG TCC TCG CAA G, rev-GGG CCA GGT CTA GTT ACA GTA G), GCG (glucagon; for-TCA CTC TCT CTT CAC CTG CTC TGT, rev-GAC ACA CTT ACT TCC TGT CAG), PDX1 (pancreatic and duodenal homeobox-1; for-TTT CCC GTG GAT GAA GTC TAC, rev-CGG TGC GTG TCC GCT TGT TCT), GCK (glucokinase; for-TTT CCT GTG AGG CAC GAA GAC, rev-CGT GCT CAG GAT GTT GTA GA), SLC2A2 [glucose transporter-2 (GLUT-2); for-CTT TGC AGT TGG TGG AAT GAT, rev-GCT GAT GAA GAG CAC CGA TAG], IGF1 [insulin like growth factor-1 (IGF-1); for-GAG ACC CTC TGC GGG GCT GA, rev-CTG CTC GAG CCG TAC CCC GT], IGF2 (IGF-2; for-TGT GGG GAC CGC GGC TTC TA, rev-CAG GGC CAG GTC GCA GCT TC), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase; for-TGG AGG GAC TTA TGA CCA CTG, rev-TAG AAG CAG GGA TGA TGT TCT) were performed and validated, as previously described (9, 12). The total volume of each reaction was 10 µl: 4 µl cDNA, 0.5 µl for each primer (0.5 mol/l), and 5 µl Faststart Universal SYBR Green MM (Roche, Indianapolis, IN). The temperature cycles were 5 min at 95°C to activate the enzyme; then 40 cycles of 30 s at 60°C, 30 s at 72°C, and 10 s at 96°C. To verify the correct amplification of the reaction, we examined the melt-curve analysis to ensure a single peak. The cDNA samples were analyzed in triplicate, and the standard curve method of relative quantification was used (49). Genes of interest were normalized to the reference gene GAPDH, which was not different between treatment groups. Results are presented relative to controls.
Histology of the fetal pancreas.
The morphology of the fetal pancreas was analyzed by an individual blinded to treatment groups, as previously described (anemic, n = 10; control, n = 7) (3, 35). The splenic portion of the pancreas was cut into 5-µm thick sections in the ventral-dorsal plane. For β- and α-cell area and mass, the sections were blocked with 1.5% normal donkey serum in PBS (vol/vol) for 30 min. Sections were incubated overnight in blocking buffer with the following combination of four primary antibodies: guinea pig anti-porcine insulin (Dako, Glostrup, Denmark; 1:250), mouse monoclonal anti-human glucagon, (Sigma, St. Louis, MO; 1:500), rabbit anti-human somatostatin, (Dako; 1:500), and rabbit anti-human pancreatic polypeptide, (Dako; 1:500). Immunocomplexes were detected with affinity-purified secondary antiserum (Jackson ImmunoResearch Laboratories, Bar Harbor, ME): AMCA anti-guinea pig IgG (blue); Alexa Fluor 594 goat anti-mouse IgG (red); and CY2 goat anti-rabbit IgG (green). All secondary antibodies were diluted 1:500 in blocking buffer and placed on tissue sections for 1 h at room temperature. Determination of the fluorescent positive area was performed with ImagePro 6.3 software (Media Cybernetics, Silver Spring, MD). Four pancreatic sections, at least 100 µm apart, were used for each animal, and 25 randomly selected fields of view (FOV) for each section were analyzed. Results for all FOVs were averaged to produce a mean value for each section, and then each of the four sections were averaged to produce a mean value for each animal. β- and α-cell area and mass were quantified, as previously described, and β- and α-cell mass was calculated as the product of the average percent insulin+ and glucagon+, respectively, and the weight of the fetal pancreas (3).
For pancreatic islet vessel density, tissue sections were blocked with 0.5% NEN Block (Perkin-Elmer, Waltham, MA) for 1 h, followed by the addition of the anti-insulin, glucagon, somatostatin, and pancreatic polypeptide primary antibodies noted above diluted in PBS with 1% BSA. FITC-conjugated Griffonia simplicifolia (GS-I) agglutinin (green, 15 µg/ml; Vector Laboratories, Burlingame, CA) was added to the mixture of primary antibodies. Samples were incubated overnight at 4°C. Secondary antibodies were as described above except that Texas Red goat anti-rabbit (1:500; Jackson Immuno-Research Laboratories) replaced the CY2 goat anti-rabbit IgG antibody. Pancreatic islets were defined as clusters of endocrine cells with an area of >500 µm2. Two to four sections at least 100 µm apart were imaged per animal, and eight FOVs per section were evaluated for an average of 99 ± 6 islets/animal. Vessel density of all islets from one fetus was averaged to produce a mean value for use in summary and comparative statistics (35).
Islet isolation and in vitro insulin secretion.
Islet isolation and in vitro insulin secretion studies were performed as previously described (anemic, n = 8; control, n = 8) (23). The fetal pancreatic ducts were perfused with a collagenase solution (Clostridium histolyticum, type V, 0.5 mg/ml; Sigma) containing 0.02% DNase I (Roche) in Krebs-Ringer buffer (KRB). The pancreas was removed and incubated at 37°C for 15–30 min with gentle mixing every 5–10 min and then washed three times. Islets were purified with a discontinuous gradient of Histopaque (density 1.119 mg/mg; Sigma-Aldrich) and KRB (2:1 vol:vol) and then washed three times in KRB-BSA media. Islets were recovered from the isolation procedure for 60–90 min in KRB-BSA at 37°C in 95% O2: 5% CO2.
Islet insulin secretion was assayed in static incubations of 10 islets. Each in vitro incubation condition was tested in four replicates for each fetal islet incubation. After recovery from isolation, the islets were washed twice in KRB with 0.5% BSA (wt/vol) and 10 μmol/l forskolin (Sigma-Aldrich). After this step, 10 islets were handpicked and placed into an incubation vial. One milliliter of pregassed (95% O2-5% CO2) and prewarmed (37°C) KRB-BSA with 10 μmol/l forskolin (Sigma-Aldrich) and supplemental nutrients (as indicated) was added to each incubation vial. Islets also were incubated with 11 mmol/l of glucose on ice to determine β-cell integrity. In addition, incubations were performed in media without nutrient supplementation. Islets were incubated at 37°C for 1 h and then pelleted at 2,300 g for 3 min. Media were then removed and insulin concentrations were measured by ELISA (ALPCO). Insulin concentrations of the islet pellet was measured by ELISA (ALPCO) after an overnight acid ethanol extraction. Insulin secretion was calculated as the percentage of total islet insulin that was secreted into the medium. Total islet insulin was defined as the sum of the amount of residual insulin in the islets after the 1-h incubation and the amount of insulin present in the incubation medium.
Statistical analysis.
Statistical analysis was performed using SAS version 9.4 (SAS Institute), GraphPad Prism version 6.0, and Microsoft Excel (2016). Results are expressed as means ± SE. A mixed-model ANOVA was performed and included terms (as appropriate) for treatment group (anemic or control), time (days of treatment), in vitro incubation conditions, and interactions. A term was included to account for repeated measures made in the same animal. If the overall ANOVA was significant (P < 0.05), then Fisher's protected least squares difference was used for post hoc test comparisons. Measurements made once were analyzed by Student’s t-test or Mann-Whitney U-test. P values <0.05 were accepted as significant. After determining that fetal sex did not impact outcomes in a preliminary mixed-model ANOVA, we pooled data from male and female fetuses.
RESULTS
Maternal and fetal biochemistry.
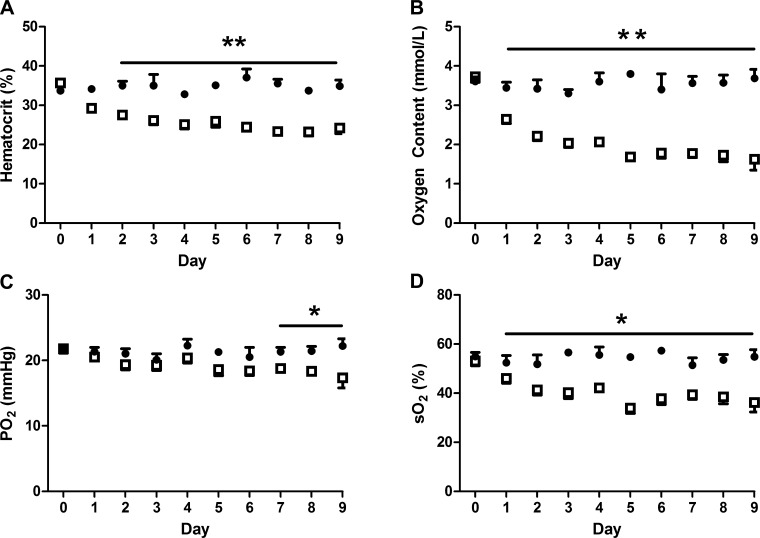
Maternal values for blood pH, , , hemoglobin-O2 saturation, O2 content, hematocrit, plasma glucose, and plasma lactate were similar between groups (Table 1). Fetal hematocrit, O2 content, , and hemoglobin-O2 saturation were 34% (P < 0.0001), 52% (P < 0.0001), 14% (P < 0.05), and 28% lower (P < 0.05), respectively, in the anemic group compared with controls (Fig. 1). Fetal blood was 4% higher in anemic sheep compared with controls (P < 0.05, Table 1), although pH and plasma lactate and protein concentrations were similar. Fetal glucose concentrations were 21% higher in the anemic group (P < 0.05, Table 1). Fetal IGF-1 concentrations were 26% lower in anemic sheep compared with controls (P < 0.005, Table 1). Fetal norepinephrine and cortisol concentrations were over 70% higher in anemic sheep compared with controls (P < 0.05), and norepinephrine concentrations were inversely correlated to fetal blood oxygen concentrations (Table 1 and Fig. 2).
Table 1.
Maternal and fetal arterial biochemistry at the end of the study
| Control | Anemic | |
|---|---|---|
| Blood pH | 7.44 ± 0.01 | 7.43 ± 0.01 |
| Blood , mmHg | 35.2 ± 1.1 | 37.7 ± 1.3 |
| Blood , mmHg | 92.9 ± 1.5 | 89.0 ± 1.4 |
| Blood So2, % | 95.7 ± 0.7 | 95.8 ± 0.6 |
| Blood O2 content, mmol/l | 5.75 ± 0.20 | 5.71 ± 0.17 |
| Blood hematocrit, % | 31.0 ± 1.1 | 30.8 ± 0.9 |
| Plasma glucose, mmol/l | 3.88 ± 0.19 | 4.00 ± 0.14 |
| Plasma lactate, mmol/l‡ | 0.85 ± 0.05 | 0.87 ± 0.08 |
| Blood pH | 7.35 ± 0.00 | 7.34 ± 0.00 |
| Blood , mmHg | 51.2 ± 0.6 | 53.2 ± 0.6* |
| Plasma glucose, mmol/l | 1.03 ± 0.05 | 1.25 ± 0.06† |
| Plasma lactate, mmol/l | 2.16 ± 0.13 | 2.37 ± 0.13 |
| Plasma insulin, ng/ml | 0.31 ± 0.03 | 0.33 ± 0.03 |
| Plasma protein, µg/ml | 44.0 ± 5.1 | 42.5 ± 3.7 |
| Plasma IGF-1, ng/ml | 119.6 ± 8.4 | 88.4 ± 5.6† |
| Plasma glucagon, pg/ml‡ | 39.3 ± 4.7 | 64.6 ± 7.9* |
| Plasma cortisol, ng/ml | 9.7 ± 1.5 | 16.6 ± 2.4* |
| Plasma norepinephrine, pg/ml | 554.9 ± 100.7 | 952.7 ± 95.3* |
Values are expressed as means ± SE; n = 15 for control and n = 19 for anemic. IGF, insulin-like growth factor.
P < 0.05, by Student's t-test or the Mann-Whitney U-test.
P < 0.001, by Student's t-test.
Mann-Whitney U-test was performed.
Fig. 1.
Arterial hematocrit and oxygen are decreased in anemic fetuses. Fetal arterial hematocrit (A), oxygen content (B), Po2 (C), and hemoglobin-oxygen saturation (D) were significantly lower in anemic fetuses (□, n = 19) compared with controls (●, n = 15), *P < 0.05, **P < 0.0001. Values are expressed as means ± SE; statistical analysis was performed by mixed-model ANOVA.
Fig. 2.
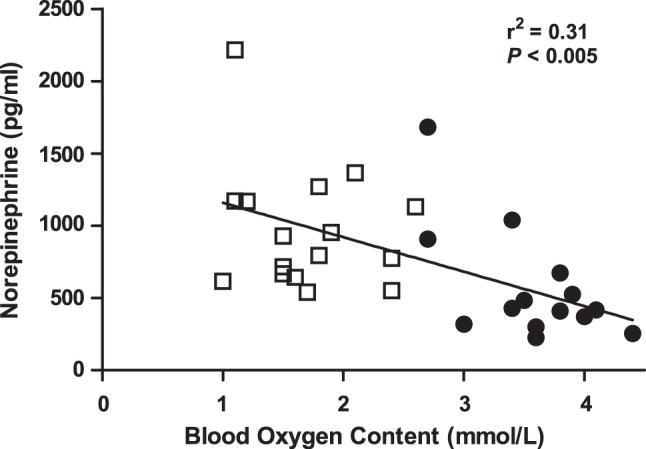
Norepinephrine is inversely associated with fetal oxygen concentrations. Fetal arterial plasma norepinephrine concentrations are inversely associated with fetal arterial blood oxygen content in anemic (□, n = 19) and control (●, n = 15) animals by linear regression analysis.
Fetal insulin and glucagon secretion.
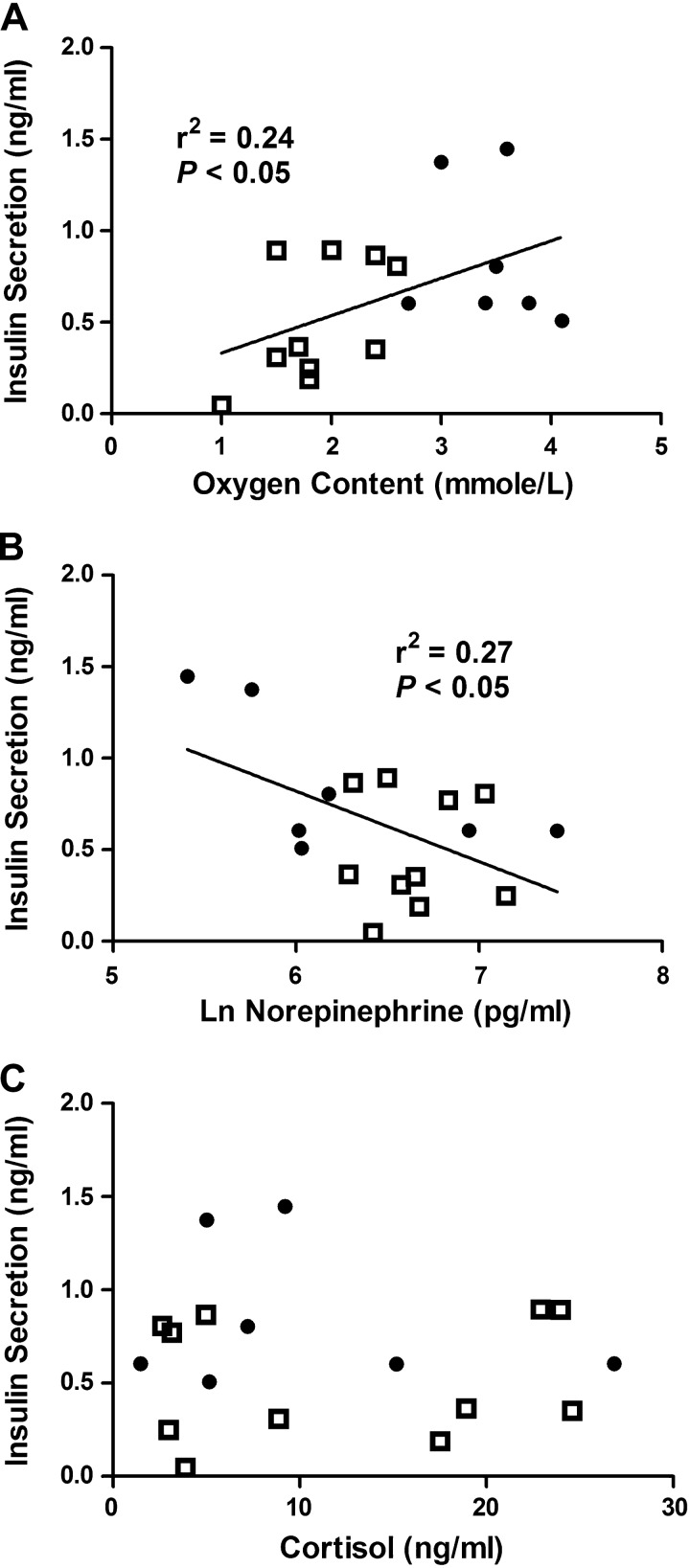
Before insulin secretion studies, insulin concentrations were similar between groups (Table 1). Fetal GSIS was attenuated in anemic animals (P < 0.05; Fig. 3A), despite slightly higher glucose concentrations achieved during the hyperglycemic clamp (P < 0.05; Fig. 3B). The glucose infusion rate required to maintain the hyperglycemic clamp was lower in anemic animals compared with controls (0.49 ± 0.03 vs. 0.68 ± 0.03 mmol·kg−1·min−1, P < 0.01). ASIS was lower in anemic fetuses compared with controls (1.35 ± 0.32 vs. 2.10 ± 0.20 ng/ml, P < 0.05). GSIS was directly correlated with fetal arterial oxygen concentrations and inversely correlated with log-transformed norepinephrine concentrations, but not with cortisol concentrations (Fig. 4). LeuSIS was lower in anemic fetuses compared with controls (0.07 ± 0.03 vs. 0.15 ± 0.04 ng/ml, P < 0.05). Before nutrient stimulation, fetal arterial plasma glucagon concentrations were 64% higher in anemic animals than controls (P < 0.05, Table 1). After the arginine infusion, glucagon concentrations increased to a greater degree in the anemic fetuses compared with controls (124.1 ± 35.6 vs. 38.2 ± 8.3 ng/ml, P < 0.05, data log transformed for analysis).
Fig. 3.
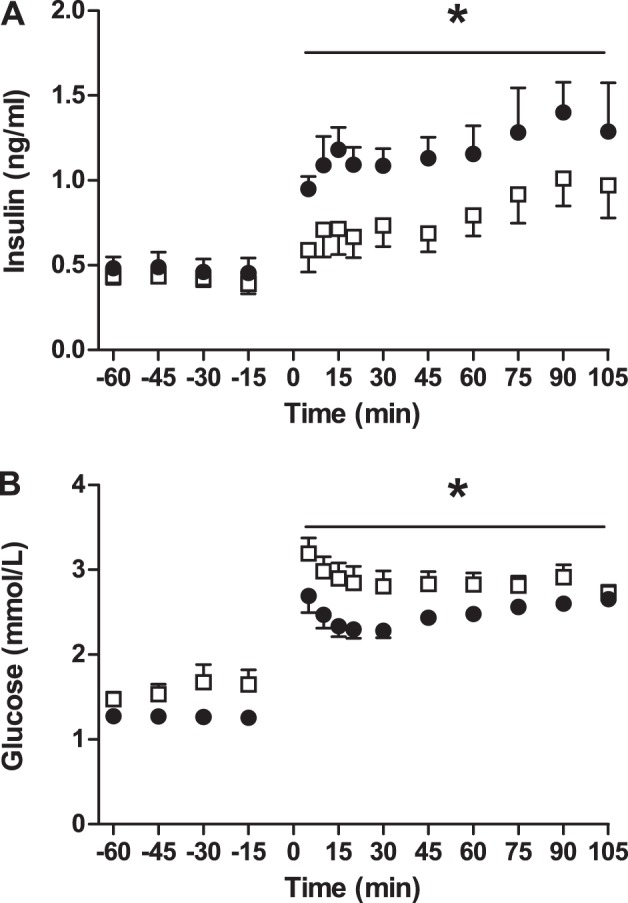
In vivo glucose stimulated insulin secretion is attenuated in anemic fetuses. Values are expressed as means ± SE. Fetal arterial plasma insulin (A) and glucose (B) concentrations are plotted relative to the start of a primed continuous variable-rate fetal hyperglycemia clamp (time = 0 min) in anemic (□, n = 11) and control (●, n = 7) animals. *Significantly lower insulin concentrations (A) and higher glucose concentrations (B) in anemic fetuses compared with controls by mixed models ANOVA, P < 0.05).
Fig. 4.
Lower glucose-stimulated insulin secretion is associated with lower oxygen and higher norepinephrine concentrations in anemic and control fetal sheep. Lower fetal arterial oxygen (A) and higher fetal norepinephrine (B) concentrations are associated with impaired fetal glucose-stimulated insulin secretion. Cortisol (C) concentrations are not associated with fetal insulin secretion. Insulin secretion is defined as the difference between insulin concentrations during the hyperglycemic clamp and at baseline and is compared with oxygen, norepinephrine, and cortisol by linear regression in anemic (□, n = 11) and control (●, n = 7) fetuses. Norepinephrine concentrations are log transformed.
Characteristics of the fetal pancreas.
Pancreatic weight was the same in both groups. There were no differences between groups for islet size, islet vessel density, or the proportion of the pancreatic sections staining insulin+ or glucagon+ (Table 2). There also were no differences for β- or α-cell mass (Table 2). Insulin and PDX1 mRNA expression were 20% and 30% lower, respectively, in pancreases from anemic fetuses compared with controls (P < 0.05, Table 2). Pancreatic glucokinase, GLUT2, glucagon, IGF-1, and IGF-2 mRNA expression were not different between groups (Table 2).
Table 2.
Characteristics of the fetal pancreas
| Control | Anemic | |
|---|---|---|
| Pancreas weight, g | 2.96 ± 0.25 | 3.08 ± 0.20 |
| Islet size, µm2 | 2,444 ± 187 | 2,954 ± 311 |
| Insulin-positive area, %‡ | 2.90 ± 0.66 | 2.73 ± 0.26 |
| Glucagon-positive area, % | 0.76 ± 0.08 | 0.87 ± 0.07 |
| β-Cell mass, mg | 83.50 ± 16.70 | 82.85 ± 8.49 |
| α-Cell mass, mg | 21.83 ± 2.47 | 26.67 ± 2.34 |
| Islet vessel density, % | 16.54 ± 0.69 | 17.23 ± 0.46 |
| Pancreatic mRNA (ratio) | ||
| Insulin | 1.00 ± 0.08 | 0.79 ± 0.04* |
| Glucagon‡ | 1.00 ± 0.21 | 0.84 ± 0.09 |
| PDX1‡ | 1.00 ± 0.12 | 0.70 ± 0.04* |
| Glucokinase | 1.00 ± 0.12 | 0.85 ± 0.10 |
| GLUT2 | 1.00 ± 0.08 | 1.05 ± 0.09 |
| IGF-1 | 1.00 ± 0.13 | 0.88 ± 0.18 |
| IGF-2 | 1.00 ± 0.09 | 0.86 ± 0.11 |
Values are expressed as means ± SE; n = 7 for control and n = 10 for anemic.
P < 0.05, by Student's t-test or the Mann-Whitney U-test.
Mann-Whitney U-test was performed.
In vitro insulin secretion.
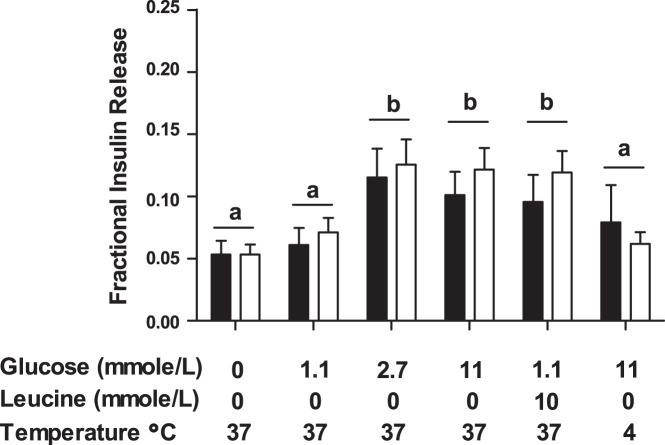
Pancreatic islet insulin content was similar between groups (7.9 ± 1.8 vs. 6.6 ± 1.2 ng/islet, anemic and control, respectively). Glucose (2.7 and 11 mmol/l) and leucine both stimulated insulin secretion compared with basal conditions (P < 0.0005), but the degree of insulin secretion was not different between groups (Fig. 5).
Fig. 5.
In vitro glucose- and leucine-stimulated insulin secretion is equivalent in isolated anemic and control fetal islets. Pancreatic islets were isolated from anemic (open bars; n = 8) and control (solid bars; n = 8) fetuses. In vitro insulin secretion was assayed in 1-h static incubations with Krebs-Ringer bicarbonate buffer and additional glucose and leucine as indicated. Although glucose and leucine stimulated insulin secretion, there were no differences between anemic and control islets. Data are presented as means ± SE, and statistics were obtained through mixed-model ANOVA. Incubations with different letters were significantly different from each other (P < 0.0005).
Fetal organ weights.
Fetal gestational age, placentome number, placental weight, fetal weight, and fetal crown-to-rump length ratio were similar between groups (Table 3). Relative to fetal weight, fetal liver (P < 0.01) and right ventricle of the heart (P < 0.001) were heavier in anemic fetuses, and the ratio of the right ventricle to left ventricle was higher in anemic fetuses (P < 0.05). All other fetal organ weights were similar between the two groups. Anemic fetuses had hindlimbs that were 6% shorter than controls (P < 0.001, Table 3).
Table 3.
Fetal necropsy data
| Control | Anemic | |
|---|---|---|
| Gestational age, days | 134.3 ± 0.3 | 134.4 ± 0.3 |
| Total number of placentomes | 75.6 ± 2.5 | 81.0 ± 3.4 |
| Total placental weight, g | 313.2 ± 18.3 | 337.6 ± 15.9 |
| Fetal weight, g | 3,053 ± 95 | 2,994 ± 75 |
| Percent male, % | 33.0 | 31.6 |
| Crown rump length, cm | 48.7 ± 1.0 | 48.1 ± 1.1 |
| Lower limb length, cm | 34.8 ± 0.5 | 32.5 ± 0.4† |
| Organ weights relative to fetal weight, % | ||
| Liver | 2.91 ± 0.10 | 3.42 ± 0.13† |
| Kidneys | 0.67 ± 0.02 | 0.64 ± 0.02 |
| Perirenal adipose tissue | 0.40 ± 0.03 | 0.39 ± 0.02 |
| Spleen | 0.23 ± 0.01 | 0.20 ± 0.01 |
| Adrenal glands | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Lungs | 3.71 ± 0.10 | 3.63 ± 0.10 |
| Heart | 0.70 ± 0.02 | 0.78 ± 0.02* |
| Left ventricle+septum | 0.27 ± 0.01 | 0.29 ± 0.01 |
| Right ventricle# | 0.13 ± 0.01 | 0.17 ± 0.01‡ |
| Right:left ventricle (ratio)# | 0.46 ± 0.04 | 0.62 ± 0.05* |
| Brain | 1.57 ± 0.04 | 1.56 ± 0.05 |
Values are expressed as means ± SE; n = 15 for control and n = 19 for anemic.
P < 0.05, by Student's t-test or the Mann-Whitney U-test.
P < 0.01, by Student's t-test.
P < 0.001,by Student's t-test or the Mann-Whitney U-test.
Mann-Whitney U-test was performed.
DISCUSSION
In this study, we determined that low blood oxygen concentration as a result of chronic fetal anemia (average of 9 days) impairs in vivo fetal insulin secretion in response to glucose, arginine, and leucine. These results show that experimentally replicating a specific complication of placental insufficiency, late-gestation fetal hypoxemia, is sufficient to replicate in vivo pancreatic endocrine dysfunction present in IUGR fetuses (23). Impaired fetal insulin secretion in anemic fetuses represents an in vivo constraint on the function of the pancreatic β-cells, as there were no islet structural abnormalities identified and the in vivo insulin secretion defect did not persist when isolated islets were stimulated in vitro with glucose or leucine. Impaired insulin secretion may be a direct response of the islet to decreased fetal oxygen concentrations or to increased fetal norepinephrine concentrations, as a significant direct correlation between both low fetal oxygen and high fetal norepinephrine with impaired insulin secretion was found. This is the first study to test the impact of chronic fetal anemic hypoxemia independent of placental insufficiency and fetal hypoglycemia on the structure and function, both in vivo and in vitro, of the fetal pancreatic islet, thereby establishing that experimental hypoxemia reduces in vivo fetal GSIS.
Factors that are potentially responsible for decreased insulin secretion include decreased oxygen and increased norepinephrine. However, these factors are dependent on each other in the fetus, making them difficult to study in isolation in vivo. Experimentally increasing fetal norepinephrine concentrations with a chronic norepinephrine infusion increases fetal oxygen concentrations (8). Furthermore, experimentally decreasing fetal oxygen concentrations stimulates catecholamine secretion from the fetal adrenal gland (25, 51). Experimentally increasing fetal norepinephrine concentrations by infusion inhibits fetal insulin secretion (8, 16). Similar impairments in fetal sheep islet insulin secretion are observed with in vitro catecholamine incubations (23). Although in vitro studies have clearly defined a direct role for reduced oxygen in inhibiting insulin secretion in adult islets (11), most in vivo experimental protocols have not differentiated the role of low oxygen independent of increased catecholamines. In acute experimental hypoxemia produced by decreasing the maternal fraction of inspired oxygen, the inhibitory effects on fetal insulin secretion could be prevented by prior removal of the catecholamine-secreting adrenal medulla (51).
Whether due to reduced blood oxygen content or increased plasma norepinephrine concentrations, it is clear that impaired insulin secretion does not result from a permanent alteration in the intrinsic ability of the fetal islet to respond to glucose. While the pancreatic content of insulin and PDX1 mRNA were 20–30% lower in anemic fetuses, isolated islets from anemic fetuses secrete insulin in response to glucose and leucine to a similar degree as control islets. Unlike fetal islets isolated from growth-restricted pregnancies following chronic and severe placental insufficiency (23), the anemic islets did not have a decrement in the amount of insulin present. This may be due to the longer exposure to fetal hypoxemia in the chronic placental insufficiency model of fetal growth restriction relative to the anemic fetuses in the current study (22, 24). Furthermore, unlike islets isolated from chronically hypoglycemic fetuses (36, 37), anemic islets were able to secrete normal amounts of insulin in vitro.
There also was no change in the structure of the pancreatic islets to explain decreased in vivo fetal insulin secretion. Fetal sheep exposed to hypoxemia from placental insufficiency for longer periods of time have decreased islet size, β-cell mass, and islet vascularity (21, 35); none of these were found in anemic fetuses. Longer periods of fetal anemic hypoxemia will be required to impair islet growth and reduce pancreatic insulin and PDX1 mRNA content to the same degree (50–70% reductions), as seen in severely IUGR fetal sheep (5, 9, 21). Although there are other sheep models of long-term hypoxemia that do not use placental insufficiency (2, 18), the impact of long-term hypoxemia on fetal pancreatic islet structure has not been quantified in these models, as we have done in this study.
It is notable that pancreatic expression of insulin and PDX1 mRNA are both decreased in anemic fetuses, despite normal β-cell mass. However, it is unlikely that the modest (20–30%) reduction in expression of these two genes is responsible for decreased in vivo insulin secretion, given that isolated islets from anemic fetuses have normal insulin concentrations. Furthermore, although the PDX1 target INS (insulin) mRNA was decreased in the anemic pancreases, two other important PDX1 target genes, GCK (glucokinase) and SLC2A2 (GLUT-2), that regulate GSIS were not decreased. Although direct inhibition of insulin gene transcription by catecholamine signaling has been identified in an insulin-secreting cell line (34), increasing fetal norepinephrine concentrations for 7 days with a direct fetal infusion did not impact islet insulin mRNA levels (8). The decrease in insulin gene expression in the current study, therefore, could be due to the direct effects of hypoxemia, as seen in insulin-secreting cell lines and cultured adult islets (47).
There were several other noteworthy findings in the current study. First, we found that experimental anemic hypoxemia increased circulating fetal glucagon concentrations and glucagon secretion, despite normal pancreatic α-cell mass and glucagon mRNA levels. Perhaps the role of glucagon as a “stress” hormone (48) explains increased circulating concentrations in the current study, consistent with increased cortisol and norepinephrine. In fact, in adult animals, a direct pancreatic norepinephrine infusion resulted in increased baseline and arginine-stimulated glucagon secretion that were independent of other systemic effects (1). However, the well-described stimulation of β-cell insulin secretion by glucagon was likely countered by the low oxygen and high norepinephrine concentrations in the anemic fetuses (15, 33, 38). Another noteworthy observation in the current study was that while fetal weights were unchanged, the lower limb lengths of the anemic fetuses were consistently slightly shorter than controls. This may be related to lower circulating concentrations of IGF-1 and oxygen, or increased concentrations of cortisol and norepinephrine, or other unmeasured responses to fetal anemia (28–30, 50). Studies are under way to determine the mechanisms for this unexpected finding, including experiments testing the impact of anemic hypoxemia on fetal amino acid and protein metabolism.
Experimental fetal anemia was used to produce fetal hypoxemia. This allowed us to isolate the effects of fetal hypoxemia from placental insufficiency without compensatory increases in fetal hemoglobin concentrations (2, 18). It also allowed us to better match the mean oxygen content of PI-IUGR fetuses (2.0 mmol/l) (6, 45). However, our protocol did not match the timing of fetal hypoxemia observed in sheep models of human placental insufficiency and IUGR (31). To address this, future studies will be required to induce experimental anemia earlier in gestation. However, our experimental design models the fetal anemia and hypoxemia observed in other pathological conditions of pregnancy, such as chronic fetomaternal hemorrhage, subacute placental abruption, twin-twin transfusion syndrome, and some cases of diabetic pregnancy and postdate pregnancies (4, 42, 44).
Perspectives and Significance
Chronic fetal anemic hypoxemia leads to lower in vivo insulin secretion. These differences do not persist in isolated in vitro pancreatic islets removed from low oxygen and high norepinephrine conditions. Additionally, β-cell mass, islet size, and islet vascularity were unchanged between anemic and control fetuses. Collectively, these data point to a functional islet defect that is likely due to either direct effects of low oxygen or increased concentrations of catecholamines, or a combination of the two. These results are consistent with recent observations in models of chronic placental insufficiency, showing that acutely restoring oxygen concentrations and inhibiting adrenergic signaling result in an improvement in GSIS (20, 25). We have shown that experimental fetal hypoxemia, independent of maternal hypoxemia or other conditions that impair placental function, is sufficient to inhibit fetal insulin secretion. These are important findings, as chronic fetal hypoxemia may be one mechanism by which placental insufficiency leads to impaired fetal islet function and insulin secretion that reduce fetal growth independent of reduced nutrient supplies.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01 DK-088139 [P. J. Rozance, principle investigator (PI)]. L. D. Brown was supported by NIH Grants K12 HD-057022 Building Interdisciplinary Careers in Women's Health Scholar Award, The University of Colorado Center for Women’s Health Research, and R01 HD-079404-01A1. S. R. Wesolowski was supported by K01 DK-090199 and R03 DK-102972. W. W. Hay Jr. was supported by NIH Grants T32007186 [PI and program director (PD)], K12HD068372 (PD), and NIH-NCATS UL1TR001082, TL-1TR001081, and KL2TR-001080 (codirector), and a Grand Challenges Exploration Grant from the Bill and Melinda Gates Foundation (OPP1061082). S. W. Limesand was supported by NIH Grant R01 DK-084842. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or National Institute of Child Health and Human Development.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.S.B., C.B.C., and P.J.R. conceived and designed research; J.S.B., C.B.C., L.D.B., S.R.W., M.A.D., S.W.L., R.B.W., W.W.H.J., and P.J.R. performed experiments; J.S.B., C.B.C., L.D.B., S.R.W., S.S.J., M.A.D., S.W.L., and P.J.R. analyzed data; J.S.B., C.B.C., L.D.B., S.R.W., S.S.J., M.A.D., S.W.L., R.B.W., W.W.H.J., and P.J.R. interpreted results of experiments; J.S.B. and P.J.R. prepared figures; J.S.B. and P.J.R. drafted manuscript; J.S.B., C.B.C., L.D.B., S.R.W., S.S.J., M.A.D., S.W.L., R.B.W., W.W.H.J., and P.J.R. edited and revised manuscript; J.S.B., C.B.C., L.D.B., S.R.W., S.S.J., M.A.D., S.W.L., R.B.W., W.W.H.J., and P.J.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank David Caprio, Nicole Isenberg, Larry Toft, Dan LoTurco, Karen Trembler, Gates Roe, David Goldstrohm, and Alex Cheung for technical assistance.
REFERENCES
- 1.Ahrén B, Taborsky GJ Jr. Effects of pancreatic noradrenaline infusion on basal and stimulated islet hormone secretion in the dog. Acta Physiol Scand 132: 143–150, 1988. doi: 10.1111/j.1748-1716.1988.tb08311.x. [DOI] [PubMed] [Google Scholar]
- 2.Allison BJ, Brain KL, Niu Y, Kane AD, Herrera EA, Thakor AS, Botting KJ, Cross CM, Itani N, Skeffington KL, Beck C, Giussani DA. Fetal in vivo continuous cardiovascular function during chronic hypoxia. J Physiol 594: 1247–1264, 2016. doi: 10.1113/JP271091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews SE, Brown LD, Thorn SR, Limesand SW, Davis M, Hay WW Jr, Rozance PJ. Increased adrenergic signaling is responsible for decreased glucose-stimulated insulin secretion in the chronically hyperinsulinemic ovine fetus. Endocrinology 156: 367–376, 2015. doi: 10.1210/en.2014-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry SM, Puder KS, Bottoms SF, Uckele JE, Romero R, Cotton DB. Comparison of intrauterine hematologic and biochemical values between twin pairs with and without stuck twin syndrome. Am J Obstet Gynecol 172: 1403–1410, 1995. doi: 10.1016/0002-9378(95)90469-7. [DOI] [PubMed] [Google Scholar]
- 5.Brown LD, Davis M, Wai S, Wesolowski SR, Hay WW Jr, Limesand SW, Rozance PJ. Chronically increased amino acids improve insulin secretion, pancreatic vascularity, and islet size in growth-restricted fetal sheep. Endocrinology 157: 3788–3799, 2016. doi: 10.1210/en.2016-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown LD, Rozance PJ, Thorn SR, Friedman JE, Hay WW Jr. Acute supplementation of amino acids increases net protein accretion in IUGR fetal sheep. Am J Physiol Endocrinol Metab 303: E352–E364, 2012. doi: 10.1152/ajpendo.00059.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charles S, Tamagawa T, Henquin JC. A single mechanism for the stimulation of insulin release and 86Rb+ efflux from rat islets by cationic amino acids. Biochem J 208: 301–308, 1982. doi: 10.1042/bj2080301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Green AS, Macko AR, Yates DT, Kelly AC, Limesand SW. Enhanced insulin secretion responsiveness and islet adrenergic desensitization after chronic norepinephrine suppression is discontinued in fetal sheep. Am J Physiol Endocrinol Metab 306: E58–E64, 2014. doi: 10.1152/ajpendo.00517.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Rozance PJ, Hay WW Jr, Limesand SW. Insulin-like growth factor and fibroblast growth factor expression profiles in growth-restricted fetal sheep pancreas. Exp Biol Med (Maywood) 237: 524–529, 2012. doi: 10.1258/ebm.2012.011375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culpepper C, Wesolowski SR, Benjamin J, Bruce JL, Brown LD, Jonker SS, Wilkening RB, Hay WW Jr, Rozance PJ. Chronic anemic hypoxemia increases plasma glucagon and hepatic PCK1 mRNA in late-gestation fetal sheep. Am J Physiol Regul Integr Comp Physiol 311: R200–R208, 2016. doi: 10.1152/ajpregu.00037.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dionne KE, Colton CK, Lyarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes 42: 12–21, 1993. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- 12.Gadhia MM, Maliszewski AM, O’Meara MC, Thorn SR, Lavezzi JR, Limesand SW, Hay WW Jr, Brown LD, Rozance PJ. Increased amino acid supply potentiates glucose-stimulated insulin secretion but does not increase β-cell mass in fetal sheep. Am J Physiol Endocrinol Metab 304: E352–E362, 2013. doi: 10.1152/ajpendo.00377.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatford KL, Mohammad SN, Harland ML, De Blasio MJ, Fowden AL, Robinson JS, Owens JA. Impaired β-cell function and inadequate compensatory increases in β-cell mass after intrauterine growth restriction in sheep. Endocrinology 149: 5118–5127, 2008. doi: 10.1210/en.2008-0233. [DOI] [PubMed] [Google Scholar]
- 14.Green AS, Macko AR, Rozance PJ, Yates DT, Chen X, Hay WW Jr, Limesand SW. Characterization of glucose-insulin responsiveness and impact of fetal number and sex difference on insulin response in the sheep fetus. Am J Physiol Endocrinol Metab 300: E817–E823, 2011. doi: 10.1152/ajpendo.00572.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huypens P, Ling Z, Pipeleers D, Schuit F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia 43: 1012–1019, 2000. doi: 10.1007/s001250051484. [DOI] [PubMed] [Google Scholar]
- 16.Jackson BT, Piasecki GJ, Cohn HE, Cohen WR. Control of fetal insulin secretion. Am J Physiol Regul Integr Comp Physiol 279: R2179–R2188, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Jonker SS, Scholz TD, Segar JL. Transfusion effects on cardiomyocyte growth and proliferation in fetal sheep after chronic anemia. Pediatr Res 69: 485–490, 2011. doi: 10.1203/PDR.0b013e3182181e01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitanaka T, Alonso JG, Gilbert RD, Siu BL, Clemons GK, Longo LD. Fetal responses to long-term hypoxemia in sheep. Am J Physiol Regul Integr Comp Physiol 256: R1348–R1354, 1989. [DOI] [PubMed] [Google Scholar]
- 19.Lavezzi JR, Thorn SR, O’Meara MC, LoTurco D, Brown LD, Hay WW Jr, Rozance PJ. Increased fetal insulin concentrations for one week fail to improve insulin secretion or β-cell mass in fetal sheep with chronically reduced glucose supply. Am J Physiol Regul Integr Comp Physiol 304: R50–R58, 2013. doi: 10.1152/ajpregu.00413.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leos RA, Anderson MJ, Chen X, Pugmire J, Anderson KA, Limesand SW. Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 298: E770–E778, 2010. doi: 10.1152/ajpendo.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Limesand SW, Jensen J, Hutton JC, Hay WW Jr. Diminished β-cell replication contributes to reduced β-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 288: R1297–R1305, 2005. doi: 10.1152/ajpregu.00494.2004. [DOI] [PubMed] [Google Scholar]
- 22.Limesand SW, Rozance PJ, Macko AR, Anderson MJ, Kelly AC, Hay WW Jr. Reductions in insulin concentrations and β-cell mass precede growth restriction in sheep fetuses with placental insufficiency. Am J Physiol Endocrinol Metab 304: E516–E523, 2013. doi: 10.1152/ajpendo.00435.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW Jr. Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 147: 1488–1497, 2006. doi: 10.1210/en.2005-0900. [DOI] [PubMed] [Google Scholar]
- 24.Macko AR, Yates DT, Chen X, Green AS, Kelly AC, Brown LD, Limesand SW. Elevated plasma norepinephrine inhibits insulin secretion, but adrenergic blockade reveals enhanced β-cell responsiveness in an ovine model of placental insufficiency at 0.7 of gestation. J Dev Orig Health Dis 4: 402–410, 2013. doi: 10.1017/S2040174413000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macko AR, Yates DT, Chen X, Shelton LA, Kelly AC, Davis MA, Camacho LE, Anderson MJ, Limesand SW. Adrenal demedullation and oxygen supplementation independently increase glucose-stimulated insulin concentrations in fetal sheep with intrauterine growth restriction. Endocrinology 157: 2104–2115, 2016. doi: 10.1210/en.2015-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marconi AM, Paolini CL, Zerbe G, Battaglia FC. Lactacidemia in intrauterine growth restricted (IUGR) pregnancies: relationship to clinical severity, oxygenation and placental weight. Pediatr Res 59: 570–574, 2006. doi: 10.1203/01.pdr.0000205477.70391.3e. [DOI] [PubMed] [Google Scholar]
- 27.Marconi AM, Paolini C, Buscaglia M, Zerbe G, Battaglia FC, Pardi G. The impact of gestational age and fetal growth on the maternal-fetal glucose concentration difference. Obstet Gynecol 87: 937–942, 1996. doi: 10.1016/0029-7844(96)00048-8. [DOI] [PubMed] [Google Scholar]
- 28.Milley JR. Effects of increased cortisol concentration on ovine fetal leucine kinetics and protein metabolism. Am J Physiol Endocrinol Metab 268: E1114–E1122, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Milley JR. Ovine fetal metabolism during norepinephrine infusion. Am J Physiol Endocrinol Metab 273: E336–E347, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Milley JR. Ovine fetal leucine kinetics and protein metabolism during decreased oxygen availability. Am J Physiol Endocrinol Metab 274: E618–E626, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol 35: 730–743, 2008. doi: 10.1111/j.1440-1681.2008.04975.x. [DOI] [PubMed] [Google Scholar]
- 32.Nicolini U, Hubinont C, Santolaya J, Fisk NM, Rodeck CH. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm Metab Res 22: 426–430, 1990. doi: 10.1055/s-2007-1004939. [DOI] [PubMed] [Google Scholar]
- 33.Pipeleers DG, Schuit FC, In’t Veld PA, Maes E, Hooghe-Peters EL, Van de Winkel M, Gepts W. Interplay of nutrients and hormones in the regulation of insulin release. Endocrinology 117: 824–833, 1985. doi: 10.1210/endo-117-3-824. [DOI] [PubMed] [Google Scholar]
- 34.Redmon JB, Towle HC, Robertson RP. Regulation of human insulin gene transcription by glucose, epinephrine, and somatostatin. Diabetes 43: 546–551, 1994. doi: 10.2337/diab.43.4.546. [DOI] [PubMed] [Google Scholar]
- 35.Rozance PJ, Anderson M, Martinez M, Fahy A, Macko AR, Kailey J, Seedorf GJ, Abman SH, Hay WW Jr, Limesand SW. Placental insufficiency decreases pancreatic vascularity and disrupts hepatocyte growth factor signaling in the pancreatic islet endothelial cell in fetal sheep. Diabetes 64: 555–564, 2015. doi: 10.2337/db14-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozance PJ, Limesand SW, Hay WW Jr. Decreased nutrient-stimulated insulin secretion in chronically hypoglycemic late-gestation fetal sheep is due to an intrinsic islet defect. Am J Physiol Endocrinol Metab 291: E404–E411, 2006. doi: 10.1152/ajpendo.00643.2005. [DOI] [PubMed] [Google Scholar]
- 37.Rozance PJ, Limesand SW, Zerbe GO, Hay WW Jr. Chronic fetal hypoglycemia inhibits the later steps of stimulus-secretion coupling in pancreatic β-cells. Am J Physiol Endocrinol Metab 292: E1256–E1264, 2007. doi: 10.1152/ajpendo.00265.2006. [DOI] [PubMed] [Google Scholar]
- 38.Samols E, Marri G, Marks V. Promotion of insulin secretion by glucagon. Lancet 286: 415–416, 1965. doi: 10.1016/S0140-6736(65)90761-0. [DOI] [PubMed] [Google Scholar]
- 39.Say L, Gülmezoglu AM, Hofmeyr GJ. Maternal oxygen administration for suspected impaired fetal growth. Cochrane Database Syst Rev 1: CD000137, 2003. doi: 10.1002/14651858.CD000137. [DOI] [PubMed] [Google Scholar]
- 40.Sener A, Malaisse WJ. l-Leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase. Nature 288: 187–189, 1980. doi: 10.1038/288187a0. [DOI] [PubMed] [Google Scholar]
- 41.Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 50: 2279–2286, 2001. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- 42.Stanek J. Comparison of placental pathology in preterm, late-preterm, near-term, and term births. Am J Obstet Gynecol 210: 234.e1–234.e6, 2014. doi: 10.1016/j.ajog.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Tchirikov M, Kharkevich O, Steetskamp J, Beluga M, Strohner M. Treatment of growth-restricted human fetuses with amino acids and glucose supplementation through a chronic fetal intravascular perinatal port system. Eur Surg Res 45: 45–49, 2010. doi: 10.1159/000318859. [DOI] [PubMed] [Google Scholar]
- 44.Teramo KA. Obstetric problems in diabetic pregnancy–The role of fetal hypoxia. Best Pract Res Clin Endocrinol Metab 24: 663–671, 2010. doi: 10.1016/j.beem.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Thorn SR, Brown LD, Rozance PJ, Hay WW Jr, Friedman JE. Increased hepatic glucose production in fetal sheep with intrauterine growth restriction is not suppressed by insulin. Diabetes 62: 65–73, 2013. doi: 10.2337/db11-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol 84: 751–753, 1977. doi: 10.1111/j.1471-0528.1977.tb12486.x. [DOI] [PubMed] [Google Scholar]
- 47.Vasir B, Aiello LP, Yoon KH, Quickel RR, Bonner-Weir S, Weir GC. Hypoxia induces vascular endothelial growth factor gene and protein expression in cultured rat islet cells. Diabetes 47: 1894–1903, 1998. doi: 10.2337/diabetes.47.12.1894. [DOI] [PubMed] [Google Scholar]
- 48.Weise K, Zaritsky A. Endocrine manifestations of critical illness in the child. Pediatr Clin North Am 34: 119–130, 1987. doi: 10.1016/S0031-3955(16)36185-5. [DOI] [PubMed] [Google Scholar]
- 49.Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques 39: 75–85, 2005. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- 50.Woods KA, Camacho-Hübner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med 335: 1363–1367, 1996. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- 51.Yates DT, Macko AR, Chen X, Green AS, Kelly AC, Anderson MJ, Fowden AL, Limesand SW. Hypoxaemia-induced catecholamine secretion from adrenal chromaffin cells inhibits glucose-stimulated hyperinsulinaemia in fetal sheep. J Physiol 590: 5439–5447, 2012. doi: 10.1113/jphysiol.2012.237347. [DOI] [PMC free article] [PubMed] [Google Scholar]