Abstract
Pulmonary fibrosis contributes to morbidity and mortality in a range of diseases, and there are no approved therapies for reversing its progression. To understand the mechanisms underlying pulmonary fibrosis and assess potential therapies, mouse models are central to basic and translational research. Unfortunately, metrics commonly used to assess murine pulmonary fibrosis require animals to be grouped and euthanized, increasing experimental difficulty and cost. We examined the ability of magnetic resonance imaging (MRI) to noninvasively assess lung fibrosis progression and resolution in a doxycycline (Dox) regulatable, transgenic mouse model that overexpresses transforming growth factor-α (TGF-α) under control of a lung-epithelial-specific promoter. During 7 wk of Dox treatment, fibrotic lesions were readily observed as high-signal tissue. Mean weighted signal and percent signal volume were found to be the most robust MRI-derived measures of fibrosis, and these metrics correlated significantly with pleural thickness, histology scores, and hydroxyproline content (R = 0.75–0.89). When applied longitudinally, percent high signal volume increased by 1.5% wk−1 (P < 0.001) and mean weighted signal increased at a rate of 0.0065 wk−1 (P = 0.0062). Following Dox treatment, lesions partially resolved, with percent high signal volume decreasing by −3.2% wk−1 (P = 0.0034) and weighted mean signal decreasing at −0.015 wk−1 (P = 0.0028). Additionally, longitudinal MRI revealed dynamic remodeling in a subset of lesions, a previously unobserved behavior in this model. These results demonstrate MRI can noninvasively assess experimental lung fibrosis progression and resolution and provide unique insights into its pathobiology.
Keywords: magnetic resonance imaging, pulmonary fibrosis, interstitial lung disease, TGF-α, IPF
pulmonary fibrosis is defined as the replacement of normal lung interstitium with excessive extracellular matrix and the accumulation of apoptosis-resistant myofibroblasts. Fibrotic lung disease contributes significantly to morbidity and mortality in a diverse spectrum of chronic illnesses including cystic fibrosis, some systemic connective tissue disorders, and interstitial lung diseases such as idiopathic pulmonary fibrosis (IPF). It also occurs in response to chronic environmental exposures and acute injuries, including radiation and chemotherapeutic drugs (22, 33, 39). Understanding of the molecular processes that initiate lung fibrosis and that cause it to progress have been advanced through ongoing basic and translational studies, as highlighted by the development of pirfenidone and nintedanib for IPF, both of which slow disease advancement (31, 40). However, therapies to promote lesion resolution have yet to be identified, underscoring the need to better understand the events that underlie fibrotic lung disease. To this end, animal studies play a vital role in lung fibrosis research (20), with rodents, particularly mice, being the most tractable animal models (37).
Unfortunately, studies in mice are typically constrained by two key factors. First, the experimental tools used to understand disease mechanisms and to quantify fibrotic burden require mice be separated into experimental cohorts, so the lungs can be assessed for physiologic changes or excised for histological and biochemical analysis. This need for terminal, cross-sectional studies limits temporal information, prevents dynamic structural changes from being observed, and increases the number of animals needed to achieve statistical significance, thereby contributing substantially to the cost and complexity of experiments (30). To overcome this experimental constraint, noninvasive imaging has shown promise for longitudinally examining lung fibrosis in small animal models (17, 50).
Currently three-dimensional (3-D), X-ray computed tomography (CT) is the most developed preclinical imaging modality for studying lung diseases, and CT findings correlate significantly with conventional measures of experimental lung fibrosis, including histology and collagen content (3, 4, 42, 44, 47). Like CT, magnetic resonance imaging (MRI) provides 3-D visualization of the lungs, albeit at somewhat reduced spatial resolution (50). However, MRI is sensitive to a richer variety of contrast mechanisms, in particular tissue-level water diffusion and relaxation-based contrast (T1, T2, and T2*), providing this modality the ability to probe a range of biophysical tissues properties (8). As such, MRI has the potential to differentiate different types of high density lung tissues (9, 14). MRI can also readily be combined with contrast agents (e.g., gadolinium-based agents, magnetic nanoparticles, and hyperpolarized gases) to enable molecular and functional imaging in rodents (10, 13, 16, 41). However, the sensitivity of MRI to detect fibrosis progression and resolution in the absence of acute insults has not been studied in detail.
The second experimental constraint for studying pulmonary fibrosis with mice involves replicating the distribution and progression of lesions seen in human disease. In murine models, lung fibrosis is typically induced using acute insults, such as radiation exposure, mineral particle inhalation (e.g., asbestos or silica), and most commonly intratracheal bleomycin exposure (38). Because these insults are acute, they are typically characterized by an early inflammatory response, resulting from epithelial and vascular damage followed by a fibroproliferative phase. Often the spatial distribution of fibrotic lesions in these models do not replicate the pattern seen in progressive human disease, and lesions typically resolve spontaneously after initial injury (12). To overcome some of these obstacles, more progressive and durable models have been developed, such as repetitive exposure to injury-inducing agents like bleomycin. Alternatively fibrogenic growth factors or cytokines can be expressed specifically in the lungs, using direct gene transfer or transgenic techniques (37). Persistent and progressive models further allow the fibroproliferative mechanisms that promote and maintain fibrosis to be more fully investigated, making them essential for preclinical testing of future treatments.
Here we report the first MRI study of a transgenic mouse model of pulmonary fibrosis. In this model, mice overexpress transforming growth factor-α (TGF-α), a fibrogenic ligand of the epidermal growth factor receptor. When administered doxycycline (Dox), TGF-α is overexpressed in the lung epithelium, and mice develop fibrotic lesions with pathologic features, including mesenchymal transformation and proliferation, progressive migration of fibrotic lesions from the subpleural region into the interstitium, extracellular matrix deposition, severe restrictive lung function changes, secondary pulmonary hypertension, cachexia, and ultimately death (23, 24). Fibrotic lesions in the TGF-α model are distinctive in that they occur in the absence of detectable acute inflammation or inflammatory cytokine expression, allowing fibrotic changes to be assessed in the absence of these potential confounders. When Dox administration is ceased, fibrosis partially resolves over the course of weeks, thereby providing a unique in vivo model to assess the sensitivity of MRI to detect fibrosis resolution in addition to progression.
Using this TGF-α model, we demonstrate that three MRI-derived biomarkers of fibrotic burden: weighted mean lung signal, percent high signal volume, and signal coefficient of variation, correlate strongly with conventional experimental measures of fibrosis (i.e., collagen content and histology). These image-derived biomarkers also allowed fibrosis progression and resolution to be visualized and quantified without sacrificing animals. By imaging mice longitudinally, we identified previously unrecognized dynamic processes in this model. Specifically, transient, high-signal parenchymal structures formed and resolved during Dox treatment, suggesting that fibrosis progression can involve a spatially and temporally heterogeneous, dynamic interplay between the lungs’ healing processes and fibrotic remodeling.
MATERIALS AND METHODS
Transgenic mice.
Mice were bred and housed under pathogen-free conditions and studied using protocols approved by the Institutional Animal Use and Care Committee of the Cincinnati Children's Hospital Research Foundation. Double transgenic, TGF-α expressing mice were generated from two single transgenic strains that were originally derived from the FVB/NJ inbred strain. CCSP-rtTA activator mice expressed reverse tetracycline-responsive transactivator (rtTA) under control of the 2.3-kb rat Club Cell Secretory Protein (CCSP) gene promoter (45). Conditional, Dox-regulated transgenic mice contained human TGF-α cDNA under the control of seven copies of the tetracycline operon [(TetO)7-cmv TGFα] and a minimal CMV promoter (24). Single transgenic CCSP-rtTA+/− mice (hereafter abbreviated CCSP/−) and bitransgenic CCSP-rtTA+/−/(TetO)7-cmv TGF-α+/− mice (abbreviated CCSP/TGF-α) were produced in the same litter by mating homozygous CCSP/− mice to hemizygous (TetO)7-cmv TGF-α+/− mice. CCSP/− litter-matched controls were used as controls. To induce TGF-α expression in experimental cohorts (i.e., double transgenic mice), Dox (Sigma, St. Louis, MO) was administered via doxycycline-treated food (62.5 mg/kg), which was available ad libitum throughout the treatment period.
In vivo study design.
Mice (n = 18) were divided into four groups: three cross-sectional cohorts and one longitudinal cohort. Single transgene control mice (n = 5) underwent a single MRI session. One cohort of bitransgenic mice (n = 4) were treated with Dox for 3 wk before a single MRI session, and a second bitransgenic cohort (n = 5) was treated with Dox for 7 wk before MRI. The longitudinal cohort of double transgenic mice (n = 4) was also imaged immediately before Dox treatment and then weekly for 7 wk during Dox treatment. To assess the ability of MRI to capture fibrosis resolution, this longitudinal cohort was removed from Dox treatment (i.e., Dox-treated food was replaced with standard feed) and were imaged at five time points (0.5, 1, 2, 3, and 4 wk). After each imaging session for the cross-sectional cohorts and the final MRI session for the longitudinal cohort, animals were euthanized by overdose with sodium pentobarbital (65 mg/ml; Fort Dodge Animal Health, Fort Dodge, IA). Following nonterminal studies (longitudinal cohort only), mice were provided food and water and allowed to fully recover from anesthesia before being returned to animal housing.
MRI.
Experiments were performed using a Bruker 7 T Avance horizontal, 30-cm bore, small animal MRI scanner (Bruker, Billerica, MA) with a home-built, quadrature birdcage transmitter/receiver coil (diameter = 35 mm; length = 50 mm). Before experiments, automated Bruker routines were used to shim, set the scanner frequency, and determine the flip angle. Free-breathing mice were anesthetized with 1.5–2% isoflurane in air to maintain a respiration rate of ~100 breaths/min. A small-animal physiological monitoring system (SA Instruments, Stony Brook, NY) was used to monitor and maintain body temperature via a thermocouple placed near the mouse. Throughout imaging, the respiratory pattern was monitored via a respiratory bellows, and the heart rate was monitored via 2 electrocardiogram (ECG) leads.
Images were acquired using a partial Fourier, multislice, fast low angle shot (FLASH) sequence from free-breathing mice. Acquisitions were cardiac and respiratory gated and performed at end-expiration, with 30−60% of the breath duration used for imaging depending on breathing cycle regularity. MRI acquisition parameters included receiver bandwidth = 150 kHz, echo time (TE) = 0.85 ms, repetition time (TR) = 104 ms, flip angle = 45°, slice thickness = 0.75 mm, number of slices = 29, field of view (FOV) = 28.4 mm, and in-plane resolution = 160 μm × 160 μm. Total acquisition time was ~15 min. In total, eight individual images were acquired from each animal at each treatment time-point. After reconstruction these images were averaged, slice-by-slice to increase signal-to-noise ratio and reduce the influence of motion (5).
MRI analysis.
Averaged images were manually segmented in Amira (FEI, Hillsboro, OR) to create masks of the low signal intensity volume within the lungs, the high signal intensity volume, and total lung volume. Briefly, a “seed point” was selected within the low signal intensity region within the thoracic cavity (i.e., lung parenchyma). Region growing within a user-defined signal threshold was then used to partition the low intensity volume from the remainder of the image. If needed, additional manual intervention was performed to refine this lung parenchyma mask. The remainder of the lung volume was then manually segmented, and the high signal volume was defined as all regions lying within the lung volume mask but outside the low signal intensity mask. A mask of all soft tissues outside the lungs was also generated using manually directed region growing. The mean signal from soft-tissue within this mask was used to weight lung signal to compensate for breathing-rate-induced variations in T1 weighting and for animal-to-animal signal variations caused by differences in coil loading.
In one animal (longitudinal cohort, Dox week 5), motion artifacts were noted in two of the eight individually acquired images. These motion-corrupted images were omitted from signal averaging. Additionally, in one animal (longitudinal cohort, post-Dox week 2), the bellows used for respiratory gating was mispositioned, causing the thoracic cavity to be slightly compressed. This time point was omitted from analysis. Three imaging-based metrics were calculated from the final averaged images and the Amira-derived masks using routines written in MATLAB (The MathWorks, Natick, MA). These metrics included the following: 1) mean weighted signal intensity (i.e., mean signal intensity within the lung volume divided by the mean nonpulmonary, soft-tissue signal); 2) percentage of high signal lung volume (the percentage of lung volume within the lung mask but outside the low signal parenchyma mask); and 3) signal coefficient of variation (CV, ratio of the signal standard deviation divided by the mean within the lung mask).
Histology.
Following euthanasia, the lungs and heart were removed, and the left and right lungs were separated. The right lung was inflation-fixed overnight at 4°C using 4% paraformaldehyde at a pressure of 25 cm H2O, washed with phosphate-buffered saline (PBS), dehydrated through a graded series of ethanol washes, and processed for paraffin embedding. Paraffin-embedded sections (5 μm thick) were loaded onto polysine slides for hematoxylin and eosin (H&E) staining. To assess pleural thickness, nine randomly selected lung areas were photographed per mouse at high-magnification (×20 ) using a Leica DM2700 M bright-field microscope (Leica Microsystems, Buffalo Grove, IL). Histomorphometric measurements of subpleural thickness were obtained from lung sections using the measured distance function in MetaMorph (Molecular Devices, Sunnyvale, CA) as previously described (36). Ten subpleural measurements per photograph were taken for a total of 90 measurements per mouse, which were then averaged. Lung tissue sections from mice were also scored for the degree of lung fibrosis using a system developed specifically for these TGF-α, transgenic mice (35). To calculate the score, each tissue section was assigned an integer score between zero and five corresponding to the degree of lung fibrosis in the pleural surface, perivascular, and terminal peribronchial adventitial regions of the lung.
Hydroxyproline measurement.
Total lung collagen content was quantified from the left lung by measuring hydroxyproline levels and total soluble collagen (measured by Sircol Collagen Assay, Biocolor) as previously described (34, 36). Briefly, the left lung was homogenized in 5 ml of 0.5 M acetic acid containing pepsin (1 mg/10 mg tissue; Sigma-Aldrich) and incubated for 24 h (24°C, with 240 rpm shaking). Sircol dye was added (1 ml/100 μl, 30 min), and the sample was centrifuged (12,000 rpm for 12 min). The pellet was then suspended in 1 ml of 0.5 M NaOH. The optical density of the sample was measured spectrophotometrically (absorbance at 540 nm), with sample concentrations determined using a standard curve.
Statistical analysis.
To correlate traditional fibrosis metrics with MRI-derived metrics, linear regression was performed in MATLAB. Additionally, to examine differences between each of the euthanized cohorts (controls, Dox week 3, Dox week 7, and post-Dox week 4), a one-way ANOVA was performed to compare the means of both the traditional histology metrics and the MRI-derived metrics. Following ANOVA, a post hoc Tukey multiple comparisons test was performed using routines written in the R language to assess difference in means between each of the cohorts, with significance differences defined as P < 0.05.
Longitudinal analysis of MRI-derived metrics was performed in R. Data were separated into two sets: 1) progressive-phase data collected from Dox week 0 to Dox week 7; and 2) resolution-phase data collected from Dox week 7 to post-Dox week 4. Single transgene control data were treated as equivalent to Dox week 0 data. Progression and resolution data sets were fit separately to a random slope, linear mixed model to capture each subject’s unique intercept and slope. The model was compared with the null model (no correlation with time) using one-way ANOVA to determine correlation significance, with significant differences defined as P < 0.05.
RESULTS
Comparing histology and hydroxyproline content with MRI.
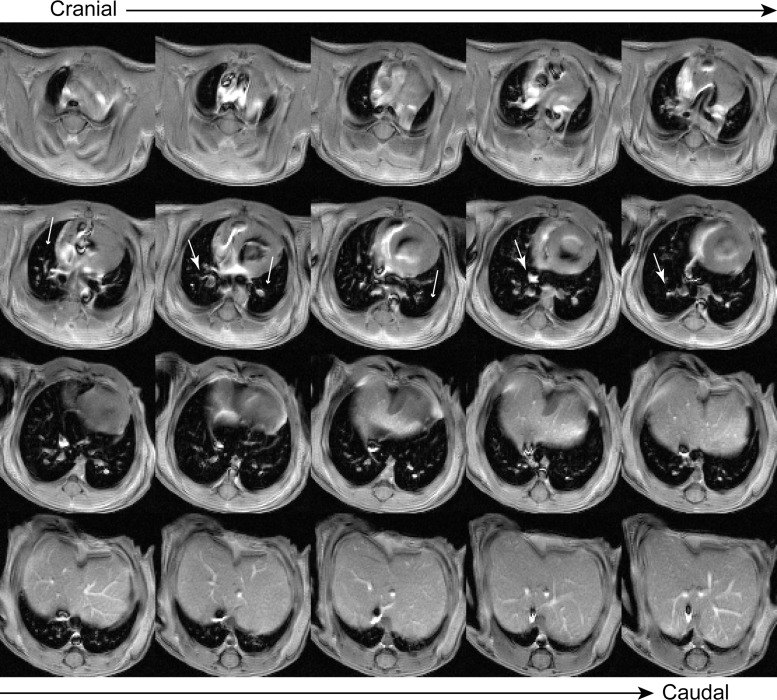
Averaged MRIs spanning the entire 3-D volume of the lungs were successfully obtained from all mice at all time points. Because all images were respiratory and cardiac-gated acquisitions, motional artifacts (e.g., image blurring or streaking) were minimal. In healthy mice (e.g., see Fig. 1), low and high tissue density regions were readily apparent as low and high MRI signal intensity regions, respectively.
Fig. 1.
Multislice MR image of a complete three-dimensional (3-D) lung volume from a healthy, bitransgenic mouse before doxycycline (Dox) treatment arranged from cranial (top left) to caudal (bottom right). High signal intensity features within the thoracic cavity correspond to the pulmonary vasculature (e.g., second row: large white arrows). The remainder of the thoracic cavity is occupied by low signal intensity lung parenchyma (e.g., second row: small arrows).
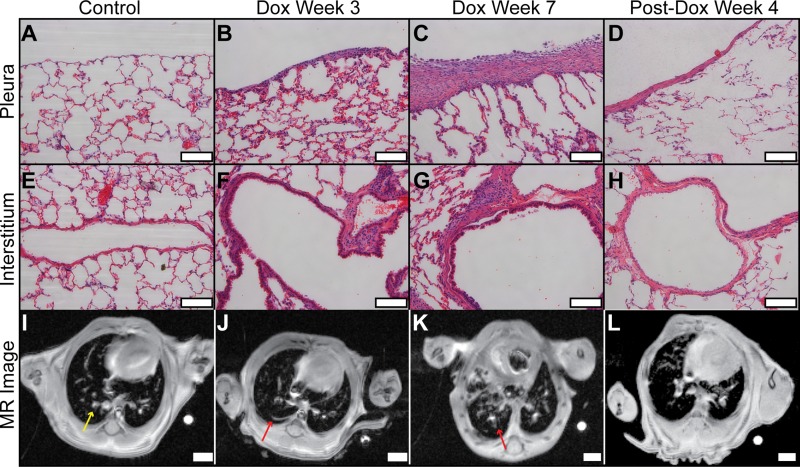
Consistent with previous studies of this model, histology readily distinguished between fibrotic and nonfibrotic tissue and detected differences in fibrotic burden between treatment cohorts (24). This can be seen qualitatively in representative micrographs provided in Fig. 2. Histology from a control animal shows the pleura is relatively thin and of comparable thickness to the alveolar walls. However, increasing pleural thickness was observed in animals treated with Dox for 3 and 7 wk. In mice evaluated 4 wk after Dox treatment ended, pleural thickening had partially resolved. Similar histological patterns are observed within the interstitium, with vascular-adventitial fibrosis being absent in the control, increasing in prevalence from Dox week 3 to Dox week 7, and then partially resolving 4 wk after Dox treatment ceased.
Fig. 2.
Comparison of histology and MRI. Histology scale bars (A−H) are 100 μm (×20 magnification); MR image scale bars (E−H) are 1 mm. The pleura of the control (A) was thin but was progressively thicker in animals treated with Dox for 3 and 7 wk (B and C, respectively). Four weeks after Dox treatment (D), pleural thickening partially resolved. Similar histological patterns are observed for vascular-adventitial fibrosis (I−L). In the MR images from the same animals, the control (I) displayed low signal from lung parenchyma and high signal from larger pulmonary vessels (e.g., yellow arrow). In contrast, animals undergoing Dox treatment (J and K) increasingly displayed high signal structures that extended from the subpleural region and adventitia into the interstitium (e.g., red arrows). High signal volume decreased 4 wk after Dox treatment ended (L), consistent with progression and resolution patterns observed with conventional histology.
The corresponding MRIs (Fig. 2, I–L), obtained from these animals on the same day as they were euthanized, are also provided in Fig. 2. These images display patterns that are in qualitative agreement with fibrosis progression and resolution observed using histology. Specifically, the control mouse displayed low MRI signal throughout the lung parenchyma, and the high signal intensity features corresponded to the larger pulmonary vessels. However, animals undergoing Dox treatment displayed additional high-signal structures that extended from the subpleural and adventitial regions into the interstitium. Importantly, the volume of these nonvascular, high signal structures increased from Dox week 3 to week 7, again consistent with the increased fibrotic burden observed using histology. After Dox treatment ceased, this pattern reversed, with the high-signal volume decreasing substantially by post-Dox week 4, again consistent with histological observations.
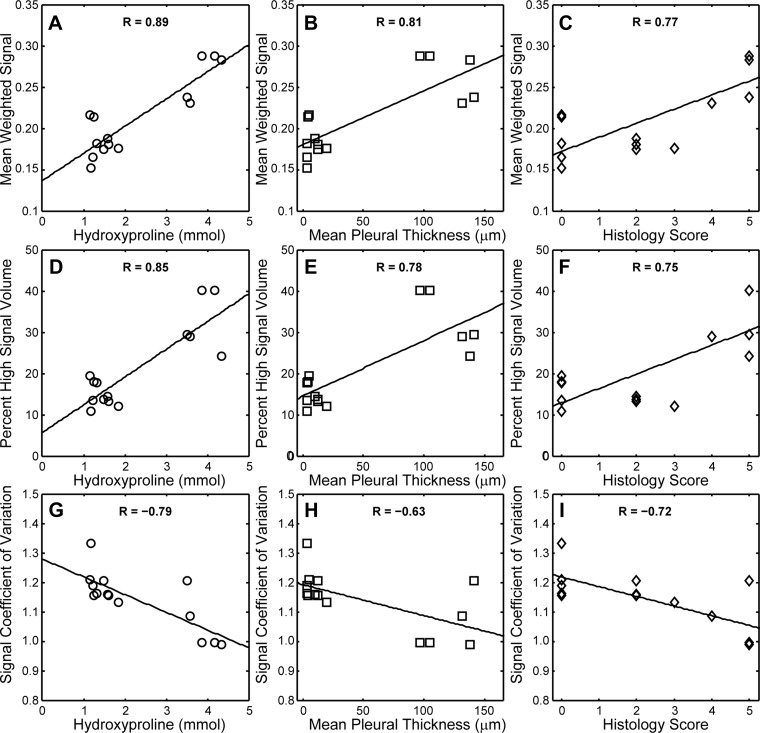
MRI metrics of fibrosis also correlated quantitatively with conventional histology and hydroxyproline content (see Fig. 3). The strongest correlation (R = 0.89) was observed between mean weighted signal and hydroxyproline content. Mean signal was also positively correlated with pleural thickness (R = 0.81) and histology score (R = 0.77). Similarly, percent high signal volume increased with increasing hydroxyproline content, pleural thickness, and histology score (R = 0.85, 0.78, and 0.75, respectively). The final MRI-derived metric assessed, CV, a signal intensity independent measure of heterogeneity, decreased with increasing hydroxyproline content (R = −0.79), pleural thickness (R = −0.63), and histology score (R = −0.72).
Fig. 3.
Correlations between MRI-derived and conventional metrics during fibrosis progression. Lines are least-squares fits; correlation coefficients (R values) are provided for each regression. Mean weighted signal increased with hydroxyproline content (P = 2 × 10−5), mean pleural thickness (P = 0.005) and histology score (P = 0.001) in A–C, respectively. Similarly, percent high signal intensity lung volume increased with hydroxyproline content (P < 0.001), mean pleural thickness (P < 0.001) and histology score (P = 0.002) in D, E, and F, respectively. The signal coefficient of variation (a measure of image heterogeneity) decreased with hydroxyproline content (P < 0.001), mean pleural thickness (P = 0.016) and histology score (P = 0.004) in G, H, and I, respectively.
Comparing treatment cohorts:
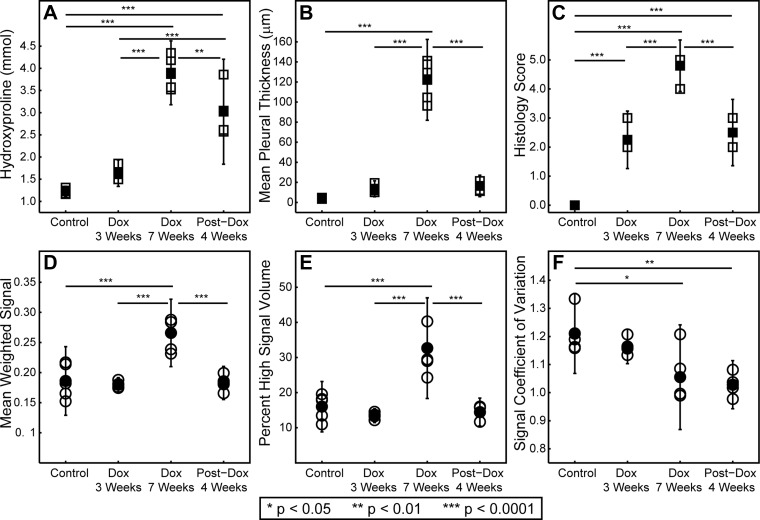
Group-wise comparisons between MRI-derived and conventional metrics are summarized in Fig. 4 and Table 1. Significant differences between cohort means were found for all conventional metrics (P < 0. 001 for hydroxyproline, pleural thickness, and histology score). Pleural thickness was able to discriminate between the Dox week 7 cohort and the control, Dox week 3, and post-Dox week 4 cohorts (P < 0.001 for all comparisons). Histology score displayed superior sensitivity, failing only to discriminate Dox week 3 from post-Dox week 4. Finally, hydroxyproline was best able to discriminate between cohorts, failing only to discriminate between the control and Dox week 3 cohorts.
Fig. 4.
Cross-sectional comparison of experimental cohorts. Traditional fibrosis metrics, hydroxyproline content (A), mean pleural thickness (B), and histology score (C), are represented by square symbols. MRI-derived metrics, mean weighted signal (D), percent high signal volume (E), and coefficient of variation (F), are represented by circles. Open symbols are values obtained from individual mice, and closed symbols are cohort means. Vertical bars are 95% confidence intervals around the means. Significant differences are denoted with horizontal lines, and the level of significance is denoted by asterisks (see legend).
Table 1.
Summary of fibrosis measures for treatment cohorts*
| Control | Dox Week 3 | Dox Week 7 | Post-Dox Week 4 | |
|---|---|---|---|---|
| Hydroxyproline, mmol | 1.22 [1.16, 1.31] | 1.64 [1.50, 1.85] | 3.90 [3.52, 4.35] | 3.02 [2.57, 3.86] |
| Pleural thickness, μm | 3.94 [3.51, 4.75] | 13.6 [10.2, 19.4] | 122.2 [96.4, 141.2] | 16.5 [11.3, 21.3] |
| Histology score | 0 [0, 0] | 2.25 [2, 3] | 4.80 [4, 5] | 2.50 [2, 3] |
| Mean signal | 0.186 [0.152, 0.217] | 0.180 [0.174, 0.188] | 0.266 [0.243, 0.403] | 0.183 [0.166, 0.199] |
| High signal volume, % | 16.0 [13.5, 19.6] | 13.4 [12.1, 14.5] | 32.7 [24.3, 43.1] | 14.5 [11.68, 16.04] |
| Signal CV | 1.21 [1.16, 1.33] | 1.16 [1.13, 1.21] | 1.06 [1.00, 1.21] | 1.03 [0.98, 1.08] |
CV, coefficient of variation.
Cohort means with the range of values within the cohort in brackets.
Significant differences between cohort means were also found for MRI-derived metrics (P < 0.001 for percent high signal volume, P < 0.001 for mean weighted signal, and P = 0.003 for CV). CV was able to discriminate between the controls and the Dox week 7 and post-Dox week 4 cohorts. Mean weighted signal displayed greater sensitivity and was able to discriminate between the Dox week 7 cohort and the control, Dox week 3, and post-Dox week 4 cohorts. Finally, percent high signal volume displayed the highest sensitivity and discriminated between the Dox week 7 cohort and the control, Dox week 3, and post-Dox week 4 cohorts.
Quantifying longitudinal changes with MRI.
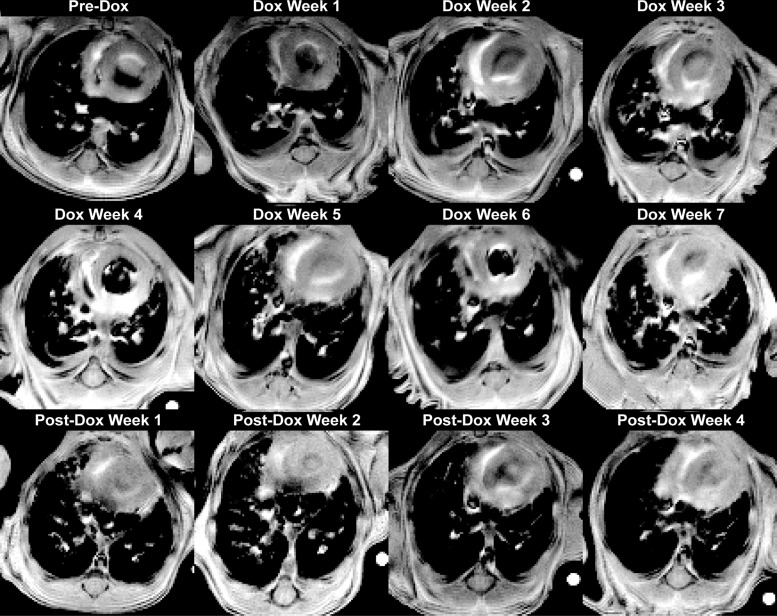
An example of a series of noninvasive, longitudinal experiments is shown in Fig. 5, which displays image slices from a single, bitransgenic mouse obtained weekly over 12 wk. Before Dox treatment, a similar signal intensity pattern is observed to that displayed by the control animal (Fig. 2I). Low signal is observed throughout the lung parenchyma, and the high signal structures correspond to pulmonary vasculature. After a single week of Dox treatment, high signal intensity lesions are observed that extended from the pleura and vasculature into the interstitium. These lesions grow in both number and volume throughout the 7 wk of Dox treatment. After Dox treatment ended, these pathological features began to resolve. By 4 wk post-Dox, the high signal intensity regions are again predominantly confined to the larger pulmonary vasculature.
Fig. 5.
Longitudinal imaging. Representative image slices from approximately the same anatomical location in a single mouse obtained weekly over 12 wk (pre-Dox, Dox week 7, and post-Dox week 4). Before treatment, low signal was seen from lung parenchyma and high signal only from larger pulmonary vasculature. During Dox treatment, high signal lesions formed that extend from the pleura and adventitia into the interstitium. After Dox treatment, these features partially resolved, with the volume of high-signal regions decreasing almost to their pre-Dox treatment levels.
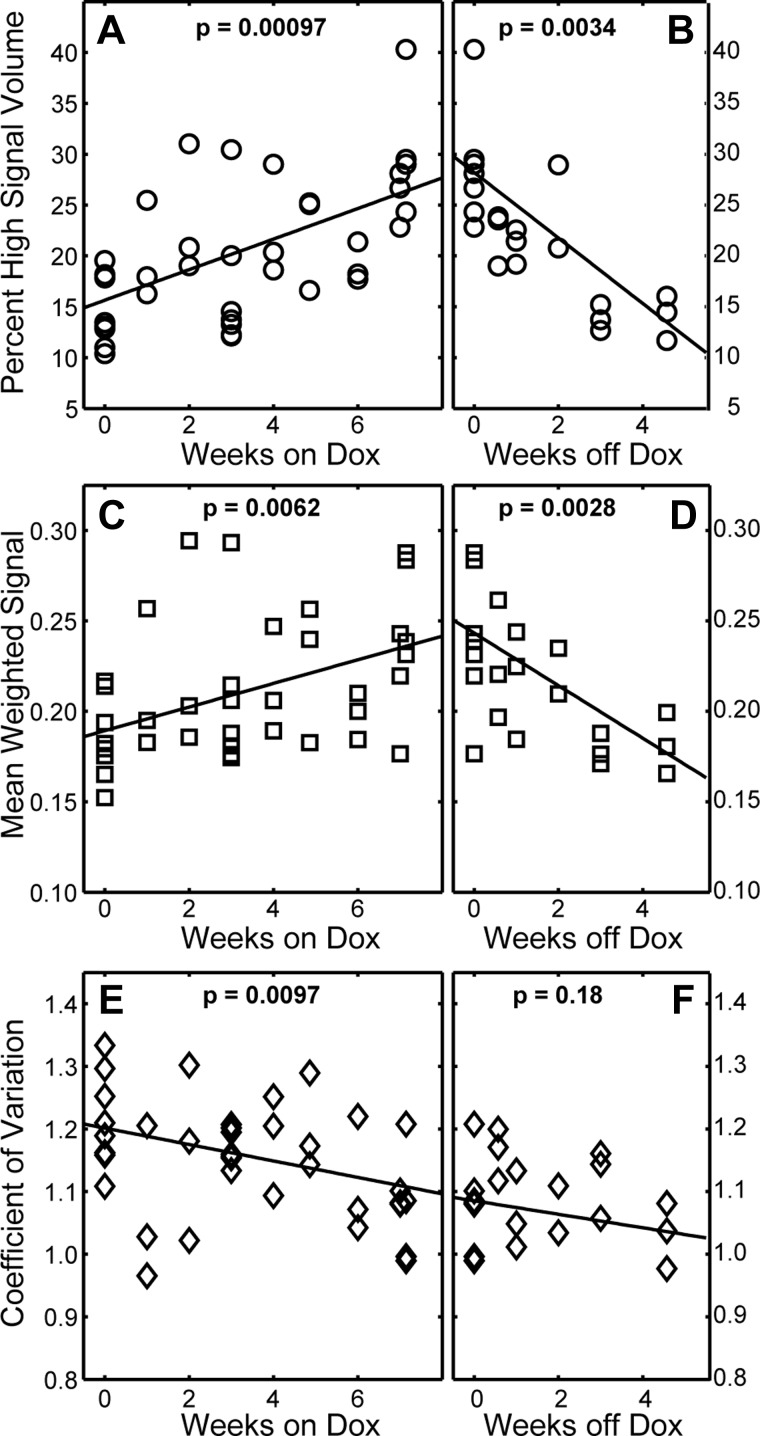
Figure 6 and Table 2 summarize all MRI-derived metrics (longitudinal and cross-sectional cohorts) as a function of time. During Dox treatment, the most robust measure of fibrosis progression was percent high signal intensity volume, which increases at a rate of 1.5% wk−1 (P < 0.001). A mean weighted signal also significantly increased with time (slope = 0.0065 wk−1; P = 0.0062), whereas signal CV significantly decreased with time (slope = −0.013 wk−1; P = 0.0097). While CV did not change significantly in the 4 wk following Dox treatment (P = 0.18), the percent high signal volume decreased significantly at a rate of −3.2% wk−1 (P = 0.0034), and weighted mean signal decreased at a rate of −0.015 wk−1 (P = 0.0028).
Fig. 6.
Longitudinal changes in fibrosis burden observed with MRI. During fibrosis progression, percent high signal intensity volume increased significantly at a rate of 1.5% per week (A) and mean weighted signal (C) increased significantly at 0.0065 wk−1, whereas the signal coefficient of variation (E) decreases significantly at −0.13 wk−1. During fibrosis resolution, CV (F) did not change significantly, whereas percent high signal intensity volume (B) decreased significantly at −3.2% wk−1 and mean weighted signal (D) decreased significantly at −0.011 wk−1.
Table 2.
Fibrosis progression and resolution assessed by MRI‡
| Progression |
Resolution |
|||||
|---|---|---|---|---|---|---|
| Slope | Intercept | P | Slope | Intercept | P | |
| High signal volume, % | 1.50 ± 0.30 | 15.6 ± 1.4 | 0.00097* | −3.22 ± 0.56 | 28.2 ± 2.4 | 0.0034* |
| Mean signal | 0.0065 ± 0.002 | 0.19 ± 0.01 | 0.0062* | −0.0150.003 | 0.24 ± 0.01 | 0.0028* |
| CV | −0.013 ± 0.005 | 1.20 ± 0.02 | 0.0097* | −0.011 ± 0.006 | 1.09 ± 0.02 | 0.18 |
Slopes and intercepts ± SE from linear least-squares fits.
Statistically significant.
Dynamic remodeling in TGF-α-induced lung fibrosis.
The majority of fibrotic lesions displayed the expected pattern of progressive and persistent fibrotic remodeling during Dox treatment. For example, Fig. 7 shows image slices taken in a single mouse (longitudinal cohort) at approximately the same anatomical location but at four different time points. A lesion (white arrows) is seen forming between Dox week 5 and Dox week 6 and then persisting into Dox week 7. This lesion persisted even into the post-Dox treatment period, during which fibrosis partially resolved (not shown). Interestingly, more dynamic behavior can also be seen in Fig. 7. That is, between Dox week 4 and Dox week 5, a high-signal-to-noise ratio lesion formed (black arrow) that protruded from the subpleural region into the lung parenchyma. By Dox week 6, however, this structure had completely resolved. Similar examples of lesion regression during Dox treatment were observed in three of the four animals in the longitudinal cohort.
Fig. 7.
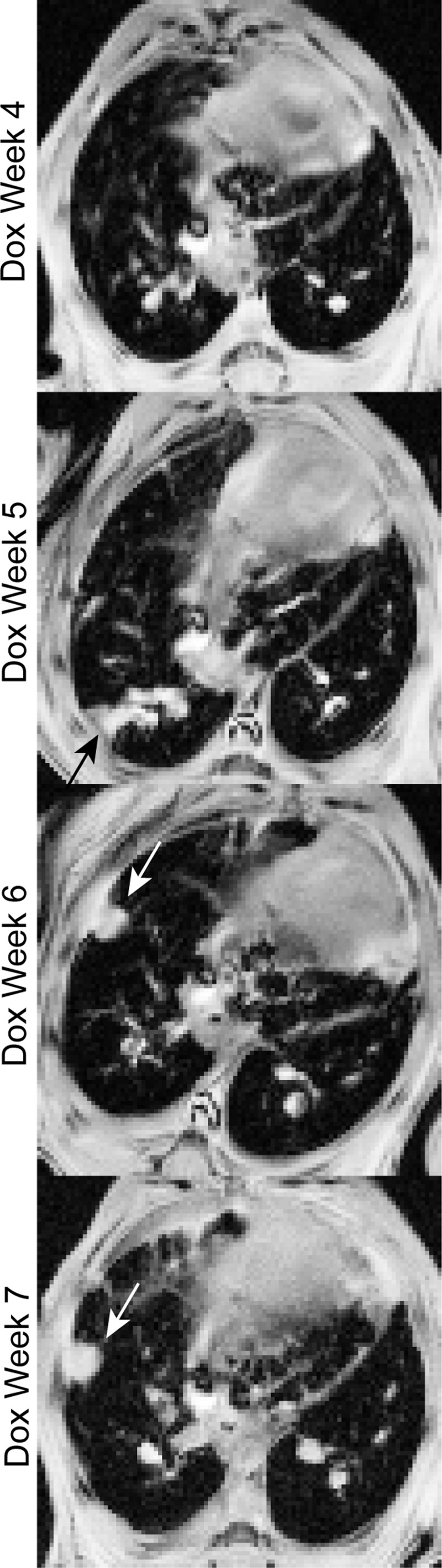
Dynamic remodeling during fibrosis progression. A high-signal-to-noise ratio, fibrotic structure is observed protruding from the subpleural region at Dox week 6, and this structure persisted into Dox week 7 (white arrows). In contrast, a second structure (black arrow) formed by Dox week 5 and resolved by Dox week 6. Similar patterns were observed in 3 of the 4 animals studied longitudinally, indicating that fibrosis progression in this model involves a previously unrecognized, dynamic component.
DISCUSSION
MRI-derived metrics of fibrosis.
We assessed the suitability of three MRI-derived biomarkers: 1) weighted mean signal, 2) percent high signal lung volume, and 3) signal coefficient of variation, to assess disease progression and resolution in a double transgenic, TGF-α-induced mouse model of lung fibrosis. Of these markers, mean signal and high signal volume had been used previously to assess lung fibrosis in bleomycin-treated mice and rats (1, 2, 18, 47). Signal CV was used previously to quantify regionally impaired lung function, specifically gas exchange, in rats with bleomycin induced lung fibrosis (13). These metrics were selected because they were expected to be robust to common, nonbiological sources of signal variation. That is, CV is a scale invariant measure of heterogeneity and thus expected to be relatively insensitive to factors such as global shimming imperfections and differences in coil loading. Mean weighted signal, while possibly somewhat affected by shimming, is expected to be relatively insensitive to complications such as loading and breathing-rate induced differences in T1 weighting. Finally, percent high signal volume is determined by the level of contrast between the lung parenchyma and other tissues rather than total signal.
For simplicity, all high signal tissues were treated equivalently in quantitative analyses, meaning that no attempt was made to segment vasculature from fibrotic tissues. This approach was adopted, because a substantial fraction of the lesion volume in this model arises from the adventitia. Thus attempts to segment adventitial fibrosis from pulmonary vasculature would have been burdensome and subject to user bias. Additionally, vascular volume within the lungs is expected to be only moderately impacted by fibrosis progression. That is, in MRI longitudinal studies, the primary metrics of interest are temporal changes in MR signal (i.e., the slope of a regression model). As such, contributions from the pulmonary vascular data simply appear as a positive, y-intercept, when the data are plotted as a function of time (see Fig. 6) and therefore do not interfere with data interpretation.
Correlating MRI-derived and traditional metrics.
All MRI metrics correlated significantly with the conventional metrics of fibrotic progression. Mean weighted signal followed by percent high signal volume correlated most strongly with conventional metrics. Correlations were positive, because mean MRI signal and high-signal volume, like the conventional metric, are measures of the extent of fibrotic remodeling. In contrast, signal CV correlated negatively with conventional metrics, because CV is a global metric of tissue density heterogeneity, and as the lungs become more homogeneously filled with high density tissues, CV decreased. Of the conventional metrics, the strongest correlations were consistently observed between hydroxyproline content and the MRI-derived metrics. This superior agreement is reasonable, given that histologically derived metrics assess only a small fraction of the tissue volume. In contrast, hydroxyproline content, like MRI, is a whole-lung metric of fibrotic burden.
In general, the correlation strengths observed between MRI-derived and conventional metrics in this study are similar to those reported previously. For instance, when comparing weighted mean signal and hydroxyproline in C57BL/6 mice, Vande Velde et al. (47) observed correlation coefficients of R = 0.91, 0.87, and 0.87, using gradient recalled echo (GRE) MRI, ultra-short echo-time (UTE), and micro-CT, respectively. Egger et al. (18) examined bleomycin-induced, pseudo-progressive fibrosis with UTE and found significant correlation of R = 0.48 (P = 0.005) between hydroxyproline content and high signal lung volume. Taken as a whole, our results combined with previously published MRI studies (primarily using bleomycin models), indicate the MRI-derived metric, particularly those based on high signal volume and mean signal, provide comparable information to that provided by conventional metrics.
Quantifying time-dependent changes.
Significant differences between cross-sectional cohort means were found for all conventional and all MRI-derived measures of fibrosis. Additionally, all metrics (conventional and MRI-derived) were able to differentiate the control cohort from the cohort treated with Dox for 7 wk, and all metrics except CV were able to differentiate the 7 wk on Dox cohort from the 4 wk postdoxycycline-treatment cohort. These results confirm the ability to of MRI to assess lung fibrosis in time-dependent, cross-sectional studies.
When analyzed as a function of time, all three MRI-derived metrics readily quantified the rate of fibrosis progression. Percent signal volume and mean weighted signal were the most robust measures, with percent high signal displaying the greatest sensitivity to progression. While CV did not display a significant trend over the post-Dox week 4 period, both percent high signal volume and mean weighted signal were able to quantify fibrosis resolution. These results argue that percent high signal volume and mean weighted signal are suitable measures of fibrosis progression and fibrosis resolution in longitudinal studies. Moreover, if these disease trajectories (i.e., slope of progression or resolution as a function of time) were used as end points, preclinical investigations of drug efficacy would benefit from the higher statistical power afforded by repeated observations of individual animals.
In addition to quantifying disease trajectories, noninvasive imaging affords the opportunity to detect dynamic changes. For instance, longitudinal imaging in this study led to the unexpected observation of transient dense structures in the lung parenchyma that developed and then resolved during Dox treatment (Fig. 7). Because of the noninflammatory nature of this model, these structures unlikely to be explained by inflammation-induced edema. Furthermore, these transitory structures were only seen during fibrosis progression and were never observed in control animals or during fibrosis resolution. As such, they are unlikely to be explained by simple anesthesia-induced atelectasis, which would have been apparent in all cohorts.
We hypothesize that these transient image features represent a dynamic interchange between profibrotic and resolution processes during fibrogenesis. This hypothesis is supported by the imaging, biochemical, and histological data presented, demonstrating that much of the fibrotic tissue generated during Dox treatment is ultimately reversible following cessation of TGF-α overexpression. Furthermore, previous microarray analysis of lung homogenates from TGF-α mice, taken at intervals during fibrosis progression, demonstrated activation of protease genes believed to be important in extracellular matrix degradation (23). Together, these findings support the concept of a yin-yang interplay of pro- and antifibrotic processes during the progression of pulmonary fibrosis. Moreover, if these fibrotic lesions can spontaneously regress in the face of a continuous profibrotic stimulus, these findings support future work to identify and ultimately augment these antifibrotic pathways as a means of reversing fibrogenesis.
Selecting imaging methods.
The protocols used in this study were chosen for their expected robustness and the ease with which they could be adopted. While both micro-CT and MRI have been used to assess fibrotic burden, MRI was chosen, because it benefits from a much richer range of contrast mechanisms, both intrinsic and generated by select contrast agent, suggesting MRI will ultimately provide greater sensitivity to the functional, chemical, and biophysical changes involved in fibrotic remodeling (10, 13, 16, 41). Moreover, unlike CT, MRI delivers no ionizing radiation. Although the radiation doses typically used for longitudinal micro-CT are not known to produce lung toxicity in common mouse strains (15, 48), the impact of X-ray irradiation on the mouse strain used in this study (primarily FVB/NJ background), particularly during fibrosis progression, has never been assessed. Because sensitivity to radiation-induced lung injury is highly strain dependent (26, 27), and because TGF-α is a mediator in radiation induced lung injury (11), the use of micro-CT could have introduced an added complication for experiments.
However, multiple MRI sequences are used in small animal studies (6, 7, 49), GRE sequences, such as the FLASH used in this work, and UTE and best suited to assess experimental lung fibrosis (5, 18). UTE sequences are robust to motion and suffer less from T2*-induced signal losses than GRE sequences, making them superior for quantifying lung density (19, 21, 28, 32, 43, 46). However, the contrast between high-signal, fibrotic tissue and low-signal, healthy parenchyma is reduced using UTE, unless contrast-enhancing dual-echo approaches are employed (28). Additionally, UTE sequences generate non-Cartesian data that are more sensitive to MRI gradient imperfections, and these data typically require custom, offline reconstruction software. In contrast, data from GRE sequences are simpler (Cartesian), allowing the use of the online reconstruction typically packaged with commercially available preclinical scanners.
Another important experimental choice for preclinical imaging is whether to synchronize the acquisition (i.e., gate) with the cardiac and respiratory cycle to mitigate motional artifacts. Several previous studies of murine fibrosis employed nongated, free-breathing acquisitions and averaged the resulting images to partially compensate for motion (1, 5, 18, 29). In contrast, both respiratory and cardiac gating were employed in our study, because unlike bleomycin-based models that have been characterized with imaging, the spatial and temporal dynamics of this transgenic model have not been examined. Without this higher spatial fidelity made possible by gating, it is unlikely that the dynamic changes or in this study would have been observed.
As a final point, image acquisition time was ~15 min, with ~10 additional minutes needed to anesthetize animals, position them in the MRI scanner, setup physiological monitoring, and determine necessary imaging parameters (flip angle, shimming, etc.). However, acquisition time could be reduced by employing higher signal intensity-UTE sequences that are robust to cardiac motion and do not require signal averaging. Setup time could also be reduced three- to fourfold by omitting prospective gating and using retrospective, self-gated UTE sequences (25). Thus, with relatively minor changes in imaging protocols, the total experimental time would be reduced to ~10 min per animal.
Conclusions.
We have reported the first use of noninvasive imaging to assess disease severity in a transgenic model of pulmonary fibrosis. MRI-derived metrics correlated well with histology and biochemical measures and were able to differentiate cross-sectional groups that underwent different treatments (i.e., time on and off Dox). These metrics were also used to track fibrosis progression and resolution over time in individual animals, paving the way for studies in which disease trajectories rather than group-wide summary statistics are used as experimental end points. Finally, we observed dynamic progression and resolution within individual lesion, a pathological pattern previously unknown in this model and that would have been difficult to detect with conventional metrics. Together, these results demonstrate MRI can quantify fibrosis progression and resolution longitudinally, which will be particularly advantageous when using transgenic models that are expensive to generate, breed, and house.
GRANTS
This study was supported in part by the National Center for Advancing Translational Sciences Grant 1UL1TR001425-01; National Heart, Lung, and Blood Institute Grants R00-HL-111217-03, P50-HL107-159-01, and T35-HL-113229-04; and the Cincinnati Children’s Hospital Medical Center, Trustee Award & Procter Scholar (TAPS) Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Z.I.C. and W.D.H. conceived and designed research; Z.I.C., R.S.D., and C.R.D. performed experiments; Z.I.C., Y.M.Z., T.G.A., C.R.D., J.G., and W.D.H. analyzed data; Z.I.C., Y.M.Z., T.G.A., R.S.D., C.R.D., J.C.W., and W.D.H. interpreted results of experiments; Z.I.C., Y.M.Z., T.G.A., and C.R.D. prepared figures; Z.I.C., Y.M.Z., T.G.A., and W.D.H. drafted manuscript; Z.I.C., Y.M.Z., T.G.A., J.C.W., and W.D.H. edited and revised manuscript; Z.I.C., Y.M.Z., T.G.A., R.S.D., C.R.D., J.G., J.C.W., and W.D.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Diana Lindquist and Satish Madala for helpful suggestions and for assisting with some of the experiments, Drs. David Roach for helpful discussions involving image segmentation, and Dr. Matthew Freeman for carefully proofreading the manuscript.
REFERENCES
- 1.Babin AL, Cannet C, Gérard C, Saint-Mezard P, Page CP, Sparrer H, Matsuguchi T, Beckmann N. Bleomycin-induced lung injury in mice investigated by MRI: model assessment for target analysis. Magn Reson Med 67: 499–509, 2012. doi: 10.1002/mrm.23009. [DOI] [PubMed] [Google Scholar]
- 2.Babin AL, Cannet C, Gérard C, Wyss D, Page CP, Beckmann N. Noninvasive assessment of bleomycin-induced lung injury and the effects of short-term glucocorticosteroid treatment in rats using MRI. J Magn Reson Imaging 33: 603–614, 2011. doi: 10.1002/jmri.22476. [DOI] [PubMed] [Google Scholar]
- 3.Badea C, Hedlund LW, Johnson GA. Micro-CT with respiratory and cardiac gating. Med Phys 31: 3324–3329, 2004. doi: 10.1118/1.1812604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badea CT, Johnston SM, Subashi E, Qi Y, Hedlund LW, Johnson GA. Lung perfusion imaging in small animals using 4D micro-CT at heartbeat temporal resolution. Med Phys 37: 54–62, 2010. doi: 10.1118/1.3264619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckmann N, Tigani B, Mazzoni L, Fozard JR. MRI of lung parenchyma in rats and mice using a gradient-echo sequence. NMR Biomed 14: 297–306, 2001. doi: 10.1002/nbm.706. [DOI] [PubMed] [Google Scholar]
- 6.Biederer J, Beer M, Hirsch W, Wild J, Fabel M, Puderbach M, Van Beek EJ. MRI of the lung (2/3). Why . . . when . . . how? Insights Imaging 3: 355–371, 2012. doi: 10.1007/s13244-011-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biederer J, Mirsadraee S, Beer M, Molinari F, Hintze C, Bauman G, Both M, Van Beek EJ, Wild J, Puderbach M. MRI of the lung (3/3)—current applications and future perspectives. Insights Imaging 3: 373–386, 2012. doi: 10.1007/s13244-011-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown RW, Cheng YC, Haake EM, Thompson MR, Venkatesan R. Magnetic Resonance Imaging: Physical Principles and Sequence Design. Hoboken, NJ: Wiley, 2014. doi: 10.1002/9781118633953. [DOI] [Google Scholar]
- 9.Buzan MTA, Eichinger M, Kreuter M, Kauczor HU, Herth FJ, Warth A, Pop CM, Heussel CP, Dinkel J. T2 mapping of CT remodelling patterns in interstitial lung disease. Eur Radiol 25: 3167–3174, 2015. doi: 10.1007/s00330-015-3751-y. [DOI] [PubMed] [Google Scholar]
- 10.Caravan P, Yang Y, Zachariah R, Schmitt A, Mino-Kenudson M, Chen HH, Sosnovik DE, Dai G, Fuchs BC, Lanuti M. Molecular magnetic resonance imaging of pulmonary fibrosis in mice. Am J Respir Cell Mol Biol 49: 1120–1126, 2013. doi: 10.1165/rcmb.2013-0039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung EJ, Hudak K, Horton JA, White A, Scroggins BT, Vaswani S, Citrin D. Transforming growth factor alpha is a critical mediator of radiation lung injury. Radiat Res 182: 350–362, 2014. doi: 10.1667/RR13625.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung MP, Monick MM, Hamzeh NY, Butler NS, Powers LS, Hunninghake GW. Role of repeated lung injury and genetic background in bleomycin-induced fibrosis. Am J Respir Cell Mol Biol 29: 375–380, 2003. doi: 10.1165/rcmb.2003-0029OC. [DOI] [PubMed] [Google Scholar]
- 13.Cleveland ZI, Virgincar RS, Qi Y, Robertson SH, Degan S, Driehuys B. 3D MRI of impaired hyperpolarized 129Xe uptake in a rat model of pulmonary fibrosis. NMR Biomed 27: 1502–1514, 2014. doi: 10.1002/nbm.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutillo AG, Chan PH, Ailion DC, Watanabe S, Rao NV, Hansen CB, Albertine KH, Laicher G, Durney CH. Characterization of bleomycin lung injury by nuclear magnetic resonance: correlation between NMR relaxation times and lung water and collagen content. Magn Reson Med 47: 246–256, 2002. doi: 10.1002/mrm.10082. [DOI] [PubMed] [Google Scholar]
- 15.Detombe SA, Dunmore-Buyze J, Petrov IE, Drangova M. X-ray dose delivered during a longitudinal micro-CT study has no adverse effect on cardiac and pulmonary tissue in C57BL/6 mice. Acta Radiol 54: 435–441, 2013. doi: 10.1177/0284185113475608. [DOI] [PubMed] [Google Scholar]
- 16.Driehuys B, Cofer GP, Pollaro J, Mackel JB, Hedlund LW, Johnson GA. Imaging alveolar-capillary gas transfer using hyperpolarized 129Xe MRI. Proc Natl Acad Sci USA 103: 18278–18283, 2006. doi: 10.1073/pnas.0608458103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Driehuys B, Hedlund LW. Imaging techniques for small animal models of pulmonary disease: MR microscopy. Toxicol Pathol 35: 49–58, 2007. doi: 10.1080/01926230601132048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger C, Cannet C, Gérard C, Jarman E, Jarai G, Feige A, Suply T, Micard A, Dunbar A, Tigani B, Beckmann N. Administration of bleomycin via the oropharyngeal aspiration route leads to sustained lung fibrosis in mice and rats as quantified by UTE-MRI and histology. PLoS One 8: e63432, 2013. doi: 10.1371/journal.pone.0063432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gewalt SL, Glover GH, Hedlund LW, Cofer GP, MacFall JR, Johnson GA. MR microscopy of the rat lung using projection reconstruction. Magn Reson Med 29: 99–106, 1993. doi: 10.1002/mrm.1910290117. [DOI] [PubMed] [Google Scholar]
- 20.Gharaee-Kermani M, Ullenbruch M, Phan SH. Animal models of pulmonary fibrosis. In: Fibrosis Research: Methods and Protocols, edited by Varga J, Brenner DA, Phan SH. Totowa, NJ: Humana, 2005, p. 251–259. doi: 10.1385/1-59259-940-0:251. [DOI] [PubMed] [Google Scholar]
- 21.Glover GH, Pauly JM. Projection reconstruction techniques for reduction of motion effects in MRI. Magn Reson Med 28: 275–289, 1992. doi: 10.1002/mrm.1910280209. [DOI] [PubMed] [Google Scholar]
- 22.Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol 175: 3–16, 2009. doi: 10.2353/ajpath.2009.081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardie WD, Korfhagen TR, Sartor MA, Prestridge A, Medvedovic M, Le Cras TD, Ikegami M, Wesselkamper SC, Davidson C, Dietsch M, Nichols W, Whitsett JA, Leikauf GD. Genomic profile of matrix and vasculature remodeling in TGF-alpha induced pulmonary fibrosis. Am J Respir Cell Mol Biol 37: 309–321, 2007. doi: 10.1165/rcmb.2006-0455OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardie WD, Le Cras TD, Jiang K, Tichelaar JW, Azhar M, Korfhagen TR. Conditional expression of transforming growth factor-alpha in adult mouse lung causes pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 286: L741–L749, 2004. doi: 10.1152/ajplung.00208.2003. [DOI] [PubMed] [Google Scholar]
- 25.Higano NS, Hahn AD, Tkach JA, Cao X, Walkup LL, Thomen RP, Merhar SL, Kingma PS, Fain SB, Woods JC. Retrospective respiratory self-gating and removal of bulk motion in pulmonary UTE MRI of neonates and adults. Magn Reson Med 77: 1284–1295, 2016. doi: 10.1002/mrm.26212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson IL, Vujaskovic Z, Down JD. A further comparison of pathologies after thoracic irradiation among different mouse strains: finding the best preclinical model for evaluating therapies directed against radiation-induced lung damage. Radiat Res 175: 510–518, 2011. doi: 10.1667/RR2421.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson IL, Vujaskovic Z, Down JD. Revisiting strain-related differences in radiation sensitivity of the mouse lung: recognizing and avoiding the confounding effects of pleural effusions. Radiat Res 173: 10–20, 2010. doi: 10.1667/RR1911.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med 70: 1241–1250, 2013. doi: 10.1002/mrm.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karmouty-Quintana H, Cannet C, Zurbruegg S, Blé FX, Fozard JR, Page CP, Beckmann N. Bleomycin-induced lung injury assessed noninvasively and in spontaneously breathing rats by proton MRI. J Magn Reson Imaging 26: 941–949, 2007. doi: 10.1002/jmri.21100. [DOI] [PubMed] [Google Scholar]
- 30.Kiessling F, Pichler BJ, Hauff P, editors. Small Animal Imaging—Basics and Practical Guide. Berlin, Germany: Spinger-Verlag, 2011. doi: 10.1007/978-3-642-12945-2. [DOI] [Google Scholar]
- 31.King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW, Group AS; ASCEND Study Group . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083–2092, 2014. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 32.Kuethe DO, Filipczak PT, Hix JM, Gigliotti AP, Estépar RS, Washko GR, Baron RM, Fredenburgh LE. Magnetic resonance imaging provides sensitive in vivo assessment of experimental ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 311: L208–L218, 2016. doi: 10.1152/ajplung.00459.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuwano K. Epithelial cell apoptosis and lung remodeling. Cell Mol Immunol 4: 419–429, 2007. [PubMed] [Google Scholar]
- 34.Madala SK, Edukulla R, Davis KR, Schmidt S, Davidson C, Kitzmiller JA, Hardie WD, Korfhagen TR. Resistin-like molecule α1 (Fizz1) recruits lung dendritic cells without causing pulmonary fibrosis. Respir Res 13: 51, 2012. doi: 10.1186/1465-9921-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madala SK, Korfhagen TR, Schmidt S, Davidson C, Edukulla R, Ikegami M, Violette SM, Weinreb PH, Sheppard D, Hardie WD. Inhibition of the αvβ6 integrin leads to limited alteration of TGF-α-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 306: L726–L735, 2014. doi: 10.1152/ajplung.00357.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madala SK, Schmidt S, Davidson C, Ikegami M, Wert S, Hardie WD. MEK-ERK pathway modulation ameliorates pulmonary fibrosis associated with epidermal growth factor receptor activation. Am J Respir Cell Mol Biol 46: 380–388, 2012. doi: 10.1165/rcmb.2011-0237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore B, Lawson WE, Oury TD, Sisson TH, Raghavendran K, Hogaboam CM. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol 49: 167–179, 2013. doi: 10.1165/rcmb.2013-0094TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mouratis MA, Aidinis V. Modeling pulmonary fibrosis with bleomycin. Curr Opin Pulm Med 17: 355–361, 2011. doi: 10.1097/MCP.0b013e328349ac2b. [DOI] [PubMed] [Google Scholar]
- 39.Pinzani M. PDGF and signal transduction in hepatic stellate cells. Front Biosci 7: d1720–d1726, 2002. doi: 10.2741/A875. [DOI] [PubMed] [Google Scholar]
- 40.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR; INPULSIS Trial Investigators . Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370: 2071–2082, 2014. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 41.Santyr G, Fox M, Thind K, Hegarty E, Ouriadov A, Jensen M, Scholl TJ, Van Dyk J, Wong E. Anatomical, functional and metabolic imaging of radiation-induced lung injury using hyperpolarized MRI. NMR Biomed 27: 1515–1524, 2014. doi: 10.1002/nbm.3180. [DOI] [PubMed] [Google Scholar]
- 42.Scotton CJ, Hayes B, Alexander R, Datta A, Forty EJ, Mercer PF, Blanchard A, Chambers RC. Ex vivo micro-computed tomography analysis of bleomycin-induced lung fibrosis for preclinical drug evaluation. Eur Respir J 42: 1633–1645, 2013. doi: 10.1183/09031936.00182412. [DOI] [PubMed] [Google Scholar]
- 43.Shattuck MD, Gewalt SL, Glover GH, Hedlund LW, Johnson GA. MR microimaging of the lung using volume projection encoding. Magn Reson Med 38: 938–942, 1997. doi: 10.1002/mrm.1910380613. [DOI] [PubMed] [Google Scholar]
- 44.Shofer S, Badea C, Qi Y, Potts E, Foster WM, Johnson GA. A micro-CT analysis of murine lung recruitment in bleomycin-induced lung injury. J Appl Physiol (1985) 105: 669–677, 2008. doi: 10.1152/japplphysiol.00980.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem 275: 11858–11864, 2000. doi: 10.1074/jbc.275.16.11858. [DOI] [PubMed] [Google Scholar]
- 46.Togao O, Tsuji R, Ohno Y, Dimitrov I, Takahashi M. Ultrashort echo time (UTE) MRI of the lung: assessment of tissue density in the lung parenchyma. Magn Reson Med 64: 1491–1498, 2010. doi: 10.1002/mrm.22521. [DOI] [PubMed] [Google Scholar]
- 47.Vande Velde G, De Langhe E, Poelmans J, Dresselaers T, Lories RJ, Himmelreich U. Magnetic resonance imaging for noninvasive assessment of lung fibrosis onset and progression: cross-validation and comparison of different magnetic resonance imaging protocols with micro-computed tomography and histology in the bleomycin-induced mouse model. Invest Radiol 49: 691–698, 2014. doi: 10.1097/RLI.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 48.Vande Velde G, De Langhe E, Poelmans J, Bruyndonckx P, d’Agostino E, Verbeken E, Bogaerts R, Lories R, Himmelreich U. Longitudinal in vivo microcomputed tomography of mouse lungs: No evidence for radiotoxicity. Am J Physiol Lung Cell Mol Physiol 309: L271–L279, 2015. doi: 10.1152/ajplung.00098.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wild JM, Marshall H, Bock M, Schad LR, Jakob PM, Puderbach M, Molinari F, Van Beek EJ, Biederer J. MRI of the lung (1/3): methods. Insights Imaging 3: 345–353, 2012. doi: 10.1007/s13244-012-0176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y, Chen H, Ambalavanan N, Liu G, Antony VB, Ding Q, Nath H, Eary JF, Thannickal VJ. Noninvasive imaging of experimental lung fibrosis. Am J Respir Cell Mol Biol 53: 8–13, 2015. doi: 10.1165/rcmb.2015-0032TR. [DOI] [PMC free article] [PubMed] [Google Scholar]