Abstract
Severe primary graft dysfunction affects 15–20% of lung transplant recipients and carries a high mortality risk. In addition to known donor, recipient, and perioperative clinical risk factors, numerous biologic factors are thought to contribute to primary graft dysfunction. Our current understanding of the pathogenesis of lung injury and primary graft dysfunction emphasizes multiple pathways leading to lung endothelial and epithelial injury. Protein biomarkers specific to these pathways can be measured in the plasma, bronchoalveolar lavage fluid, and lung tissue. Clarification of the pathophysiology and timing of primary graft dysfunction could illuminate predictors of dysfunction, allowing for better risk stratification, earlier identification of susceptible recipients, and development of targeted therapies. Here, we review much of what has been learned about the association of protein biomarkers with primary graft dysfunction and evaluate this association at different measurement time points.
Keywords: biomarkers, lung transplantation, primary graft dysfunction
despite advances in care for recipients of lung transplantation and strict donor lung selection criteria, 15–20% of recipients develop primary graft dysfunction (PGD), which increases short-term mortality risk by fourfold (13, 24, 42). The current understanding of the pathogenesis of PGD emphasizes multiple pathways leading to endothelial and alveolar epithelial injury in the lung; pathways involving inflammation, innate immunity, platelet and coagulation dysfunction, fibrinolysis, and others suggest a complex framework for the development of PGD (20). Protein and other biomarkers specific to these pathways can be measured in plasma, bronchoalveolar lavage (BAL) fluid, and lung tissue, supporting the proposed mechanistic pathways of development of PGD after lung transplantation. Further clarification of the biomarkers associated with PGD could illuminate important predictors of PGD, allowing for better risk stratification, earlier identification of susceptible recipients, and development of targeted therapies.
Here we review studies of protein biomarkers specific to these pathways of injury in severe PGD and evaluate their potential prognostic value. Since it remains unclear whether individual biomarkers are specific to certain time points and which of these time points are ideal, we examine different time points of biomarker measurement starting with the donor and extending to the early postoperative period in the recipient. Finally, we discuss future directions for biomarker research in relation to PGD, including the need for a risk-prediction model that combines biologic and clinical risk factors.
Literature Search
Two databases were searched: PubMed and EMBASE, through July 21, 2016. Search terms were: (“biomarker” OR “biomarkers”) AND (“primary graft dysfunction” OR “PGD”) AND (“lung transplant” OR “lung transplantation”). A total of 116 articles were found. Articles were excluded for the following reasons: duplicates (n = 21), abstract only (n = 29), unrelated or inappropriate topic (n = 32), review article without original data (n = 17), and case report (n = 1). The 16 remaining studies were included. There were 15 additional studies added following review of the references of the included articles and as the manuscript was finalized.
Time Point 1: the Donor
The ability to identify donor lungs at risk for PGD even before recipient selection, the earliest possible time point for intervention and risk stratification, has the potential to significantly affect recipient outcomes. Several studies highlight the predictive potential of donor protein biomarker measurements.
Interleukin-8.
Interleukin (IL)-8 is a proinflammatory cytokine. During lung reperfusion, IL-8 activates neutrophils and recruits them to areas of lung injury (26). In 26 donor BAL and lung tissue specimens (26), BAL IL-8 levels were not associated with standard clinical donor characteristics such as traumatic cause of death, duration of mechanical ventilation, inotropic support, or smoking status. However, elevated BAL IL-8 levels were negatively correlated with the / ratio in the intensive care unit (ICU), positively correlated with development of PGD, and associated with 6-mo mortality in the recipient. IL-8 mRNA expression levels were higher in donor tissue from recipients who developed PGD than in those who did not. The association of donor IL-8 levels with PGD development highlights this biomarker as a unique indicator of risk, outside of traditional donor-lung selection criteria. This is consistent with prior studies using donor inflammatory cytokine measurements as risk factors for 30-day mortality beyond standard selection criteria (35).
Receptor for advanced glycation end products.
The receptor for advanced glycation end products (RAGE) is a marker of alveolar epithelial type I cell injury in acute respiratory distress syndrome (ARDS). In mouse and human lung models, elevated levels of RAGE in the plasma or BAL are associated with impaired alveolar fluid clearance and severity of lung injury (27, 33, 68). These findings have stimulated further investigation of RAGE as a biomarker predictive of PGD.
In 35 consecutive lung transplant recipients, donor BAL RAGE levels correlated with recipient PGD grade (48); donor BAL RAGE levels were higher in donor lungs that developed severe PGD than lungs that did not develop PGD. Postoperative recipient BAL RAGE levels also correlated with severity of PGD; recipients who developed severe PGD had higher BAL RAGE levels than those who did not. However, timing of recipient sample collection was unclear and was simply at some point before extubation. These findings indicate that donor and recipient levels of RAGE, suggestive of alveolar epithelial injury, are associated with the development of PGD.
Endothelin-1.
The endothelin family of vasoconstrictors may participate in vascular-mediated lung injury. Endothelin-1 (ET-1) is released by endothelial cells and smooth muscle cells and expressed predominantly in the lung (54). High levels of ET-1 are associated with increased vascular endothelial growth factor (VEGF) expression and increased vascular permeability, leading to pulmonary edema. An analysis of 105 lung transplant donors and recipients compared serum concentration and mRNA expression of ET-1 between recipients that developed grade 3 PGD and those that did not (54). They found that donor serum ET-1 concentration did not differ between groups but that donor ET-1 expression was higher in grafts that developed grade 3 PGD than grafts that did not develop any PGD (54). Recipients who developed grade 3 PGD had higher levels of ET-1 in their explanted lung tissue and also in preoperative serum than those who did not develop PGD. These results suggest a combined contribution from the donor and recipient since elevation of both donor ET-1 tissue mRNA and pretransplantation recipient serum levels of ET-1 were associated with the development of grade 3 PGD. This study highlights the synergism between donor and recipient characteristics and supports a role for ET-1 in PGD development.
Time Point 2: the Preoperative Recipient
Club cell secretory protein.
Club cell secretory protein (CC-16) is a plasma marker of respiratory epithelial cell injury. Preoperative lung epithelial injury may predispose the recipient to development of PGD, as was shown in a cohort study of 714 recipients in which biomarker levels were measured in preoperative recipient plasma (62). In recipients who did not have idiopathic pulmonary fibrosis (IPF), elevated preoperative levels of CC-16 were associated with progression to PGD, despite significant variance in preoperative collection time (from recipient listing time to within 24 h of transplant). This result suggests that preexisting epithelial injury in the recipient confers a higher risk of PGD posttransplantation. A similar relationship between CC-16 levels and PGD was not seen in recipients with IPF, perhaps because they had higher baseline levels of CC-16 regardless of PGD development, making differences difficult to measure. In this study, preoperative plasma biomarkers indicative of alveolar epithelial injury (RAGE), inflammation [IL-8, intercellular adhesion molecule-1 (ICAM-1)], and abnormalities in coagulation (protein C) were not associated with PGD. These findings underline the potential importance of preexisting recipient respiratory epithelial injury, as measured via plasma levels of CC-16, over other candidate pathways.
Vascular endothelial growth factor.
Alveolar epithelial type 2 cells release vascular endothelial growth factor (VEGF), and increased VEGF expression promotes vascular endothelial permeability. This effect of the lung epithelium on the vascular endothelium can be illustrated in patients with ARDS, in whom elevated plasma levels of VEGF have been associated with worsening respiratory failure (64). Moreover, serum VEGF levels measured preoperatively between hospital admission and transfer to the operating room for lung transplantation were higher in recipients who developed PGD than in recipients who did not (39). This result suggests that preexisting vascular endothelial injury is a risk factor for recipient development of PGD.
Collagen type V.
Collagen type V [col(V)] is another marker of lung epithelial injury and is implicated in the innate immune response during lung transplantation. A self-antigen present in the lung matrix and expressed on the apical surface of lung epithelial cells, col(V) is unmasked during ischemia-reperfusion and triggers the release of IL-17, a potent proinflammatory cytokine produced by memory T cells (7, 32). Posttransplant reactivity specific to col(V) has been associated with recipient development of bronchiolitis obliterans syndrome (8).
One study sought to understand if baseline reactivity to col(V), thus suggesting pretransplant exposure to col(V) with autoimmune activation, could predispose lung transplant recipients to development of PGD. This study measured preoperative baseline reactivity to col(V) in the peripheral blood of lung transplant recipients using trans-vivo delayed-type hypersensitivity testing (7) [this involves collecting human peripheral blood mononuclear cells from the recipient and injecting them along with col(V) antigen subcutaneously into a mouse, followed by later assessment of reactive swelling (34)]. Recipients were then categorized as reactive or non-reactive, and the groups were followed for development of PGD. Recipients in the col(V) reactive group had worse early allograft function and were more likely to develop PGD. When multiple potential risk factors were analyzed (recipient age, diagnosis, transplant type, human leukocyte antigen matching, panel reactive antibody, reactivity to col(V), ischemic time, 6-min walk test), only cold ischemic time and col(V) reactivity were associated with recipient development of PGD. Further investigation was conducted to delineate the pathogenicity of col(V) unmasking. In some of the above experiments, following recipient blood draw but before reactivity measurement, cytokines were blocked by the addition of anticytokine antibodies to the murine injection. Cytokine blockade of IL-17, IL-1β, and TNF-α blocked the response to col(V). In addition, cell depletion assays showed that CD4 T cells and CD14 monocytes were integral to the response to col(V). Different experimental studies done on animals demonstrated that rats exposed to col(V) preoperatively had impaired gas exchange and severe monocyte infiltration in the transplanted lung compared with either unexposed rats or their native un-transplanted lung. The association of reactivity to col(V) with PGD is further supported by earlier work, in which recipients who developed PGD had higher plasma levels of anti-col(V) antibodies at 24, 48, and 72 h posttransplantation than recipients who did not develop PGD (32). Given that humoral immunity and antibody production occur over weeks and not hours, these early levels of anti-col(V) Ab indicate preformed antibodies.
Collectively, these results support the activation of the innate immune system, specifically to col(V), in the pathophysiology of PGD. Memory T cells, when exposed to col(V), may act to promote rapid monocyte activation contributing to development of PGD. Thus preoperative evaluation of col(V) reactivity, followed by col(V)-specific T-cell depletion, may open therapeutic possibilities. In addition, the localization of col(V) expression to the lung epithelium highlights the importance of the epithelium in PGD (32).
Endothelin-1.
As discussed in Time Point 1: the Donor, a study that measured serum ET-1 levels in preoperative recipients found that recipients who developed PGD had higher levels than those who did not (54).
Time Point 3: Intraoperative
Interleukin-6, interleukin-8, interleukin-10, and monocyte chemoattractant protein-1.
Ischemia and reperfusion during lung transplantation activate multiple pathways in a cascade of injury. Numerous pro and anti-inflammatory cytokines, such as IL-6, -8, and -10, mediate these pathways. These cytokines are measurable biomarkers that moderate interactions between cells in this cascade (20). IL-6 is produced by several cells, including bronchial and lung epithelium, vascular endothelium, macrophages, and fibroblasts (6). IL-8, a chemokine released by multiple cell types during lung reperfusion, recruits and activates neutrophils at sites of injury in the lung (26). IL-10 is an anti-inflammatory cytokine released primarily by type 2 T-helper cells to inhibit inflammatory cytokine production (6). Elevated levels of these biomarkers signify a heightened inflammatory state and may have prognostic potential.
According to a study of 28 recipients of lung transplantation (2), a heightened inflammatory state of the recipient even before reperfusion increases the risk of PGD. In that study, serum levels of IL-6, IL-8, and IL-10, as well as monocyte chemoattractant protein (MCP)-1, and matrix metalloprotease (MMP)-9 levels, were measured before and after allograft reperfusion. Recipients who developed grade 2 or 3 PGD had higher prereperfusion levels of IL-6, IL-8, IL-10, and MCP-1 than did recipients without PGD. Serum levels at 24 h were only higher for IL-10 and MCP-1. These findings suggest the importance of the inflammatory cascade during the intraoperative time point in the development of PGD. The significance of this time point was further suggested by another study that found higher intraoperative levels of IL-10 immediately after reperfusion in recipients who developed PGD compared with those who did not (45).
One study used gene array to perform gene set enrichment analysis on 46 lung transplant recipients (11). Changes in gene expression were measured between two time points: BAL samples collected from each donor before procurement, and recipient samples collected 1-h postreperfusion. Eight gene sets had greater upregulation between these two time points in recipients who developed PGD compared with those who did not. All eight gene sets belong to a pathway involved in inflammasome and innate immune function. The highest ranked individual transcript was for IL-1β, an inflammasome-regulated effector cytokine. Significant upregulation of these genes in recipients who develop PGD suggests the presence of subclinical lung injury in the donor that is exacerbated during the transplantation process.
Receptor for advanced glycation end products.
One recent study measured plasma levels of RAGE, ICAM-1, and endocan in 47 lung transplant recipients preoperatively and at 0, 6, 12, 24, 48, and 72 h after pulmonary artery unclamping (49). Traditional clinical risk factors (including donor age, donor smoke exposure, recipient pulmonary artery hypertension, recipient diagnosis, and recipient obesity) were not associated with progression to PGD, but recipients that developed PGD had higher RAGE levels and lower / ratios immediately after pulmonary artery unclamping (time 0) than recipients who did not develop PGD. These results signify the contribution of early alveolar epithelial injury to PGD and suggest that relevant biomarkers could be sampled even before the recipient leaves the operating room.
Endothelin-1.
As discussed in Time Point 1: the Donor, a study that measured ET-1 levels in explanted recipient lung tissue found that recipients who developed PGD had higher levels of ET-1 in their explanted lung tissue than those who did not develop PGD (54).
Time Point 4: the Postoperative Recipient
Interleukin-6, interleukin-8, and interleukin-10.
Several studies that examined the association among IL-6, IL-8, IL-10, and PGD found that early postoperative inflammation in the recipient is associated with PGD development. A study of 31 lung transplant recipients examined IL-6 in blood and BAL samples that were collected preoperatively and 12, 24, and 48 h after reperfusion (47). In all recipients, IL-6 levels in blood and BAL were elevated compared with preoperative measurements. However, IL-6 levels in blood and BAL at 12 h were more elevated in recipients who developed PGD than those who did not. Another study measured baseline and postoperative serum levels of IL-6, IL-8, IL-10, and tumor necrosis factor-α (TNF-α) in 22 recipients of lung transplantation (45). Baseline biomarker measurements and all measurements of TNF-α were similar between recipients who developed PGD and recipients who did not. Recipients with PGD had higher IL-6 levels at 4 h after reperfusion, higher IL-8 levels at 4, 8, and 24 h, and higher IL-10 levels immediately after reperfusion and at 8 h. Yet another study measured multiple inflammatory markers in postoperative recipient plasma (30). The strongest association was between elevated levels of MCP-1 and IP-10 in the postoperative period and development of PGD. The association of elevated MCP-1 in postoperative recipient plasma with the development of PGD is consistent with other work (60).
Intercellular adhesion molecule-1.
ICAM-1 is a marker of endothelial cell dysfunction although it is also expressed by alveolar epithelium. In a study that measured pre and postoperative plasma levels of ICAM-1 and von Willebrand factor, recipients who developed PGD had higher levels of ICAM-1 at each postoperative time point (6, 24, 48, and 72 h) compared with those who did not develop PGD (17). Although preoperative ICAM-1 levels only tended to be higher in recipients who developed PGD, elevated preoperative levels were positively associated with preoperative pulmonary artery pressure. These results suggest the prognostic significance of ICAM-1 in PGD.
Plasminogen activator inhibitor 1 and protein C.
The normal lung maintains a balance between fibrin deposition and degradation, modulated partly by conversion of plasminogen to plasmin, a fibrinolytic enzyme. Plasminogen activator inhibitor 1 (PAI-1) inhibits this conversion. PAI-1, generated by multiple cells in the lung, is necessary for normal fibrinolysis in the lung, whereas other molecules, such as protein C, help regulate coagulation (56). Protein C is an endogenous anticoagulant activated by endothelial receptors in the coagulation and fibrinolysis pathway. Low circulating levels of protein C are associated with a procoagulant and antifibrinolytic state. Elevated plasma levels of PAI-1 and low levels of circulating protein C, resulting in an antifibrinolytic and procoagulant state, have been implicated in worse clinical outcomes and increased mortality in patients with ARDS (67). Thus PAI-1 and protein C may contribute to acute lung injury, including PGD, after lung transplantation.
A prospective cohort study of 128 lung transplant recipients found that postoperative plasma levels of PAI-1 were higher at 6, 24, and 48 h in recipients who developed PGD than in those who did not (15). Preoperative protein C levels did not differ between the two groups but were lower at each postoperative time point (6, 24, 48, and 72 h) in recipients with PGD. The odds of PGD increased as protein C levels decreased and as PAI-1 levels increased. Thus low protein C and elevated PAI-1 plasma levels in the early postoperative period were associated with PGD, suggesting impaired fibrinolysis and hypercoagulability may be important early in the time course of PGD.
Proadrenomedullin.
Adrenomedullin (ADM), an immune-modulating peptide with vasodilatory activity stimulated by hypoxia, has shown promise in reducing endothelial permeability, lipopolysaccharide-induced lung injury, and myocardial ischemia-reperfusion injury. Proadrenomedullin (proADM) is cosynthesized with ADM but has a longer half-life and is easier to measure. One study found that recipients with PGD at 72 h after lung transplantation had higher plasma levels of proADM at 24 and 72 h than those without PGD (51). Elevated plasma levels of proADM at 48 and 72 h were also associated with recipient mortality in the ICU. Furthermore, proADM levels at each time point were inversely correlated to the / ratio. Early elevated plasma levels of proADM may therefore act as a marker of ischemia-reperfusion injury during transplantation. Elevated proADM early in the postoperative period could help distinguish those at risk for PGD (51).
P-selectin.
P-selectin, a measure of platelet activation, has also been associated with PGD. In a study that measured preoperative and 6- and 24-h postoperative levels of plasma soluble P-selectin, median levels at baseline did not differ significantly between recipients who developed PGD and those who did not (36). However, elevated levels at 6 and 24 h after lung transplantation were associated with recipient development of PGD. These results signify the potential contribution of increased platelet activation in recipient development of PGD.
Angiopoietin 2.
Elevated plasma levels of angiopoietin 2 (Ang-2) are a marker of the severity of endothelial injury in patients with lung injury and have been associated with increased mortality in noninfection-related ARDS as well as pediatric ARDS (10, 69). Ang-2 is rapidly released from the vascular endothelium upon endothelial cell activation and may mediate the endothelial inflammatory response. Elevated plasma levels of Ang-2 were also associated with the development of ARDS in critically ill patients in the emergency department (1).
Only one study has analyzed plasma level of Ang-2 before and after lung transplantation and its association with PGD (25). Forty recipients with grade 3 PGD were frequency matched by diagnosis of IPF or chronic obstructive pulmonary disease (COPD) to 79 control subjects. In all subjects, Ang-2 levels increased over time from pre- to posttransplantation, but the slope of the increase was greater for the PGD group. In recipients with IPF, cases had higher plasma Ang-2 levels at 6 h posttransplant than controls, as well as a greater increase between pre- and posttransplantation levels. This was not true for recipients with COPD. Pretransplantation Ang-2 levels were also higher in recipients with IPF than in recipients with COPD, although pretransplantation levels did not differ between cases and controls with either diagnosis. Therefore, elevated Ang-2 levels posttransplantation may be associated with PGD development. Replication of these results would further support the significance of the endothelial pathway of injury.
Receptor for advanced glycation end products.
In a study of 317 recipients of lung transplantation, elevated postoperative plasma levels of RAGE were associated with PGD (16). RAGE levels were higher in recipients with PGD than in recipients without PGD at 6 and 24 h postoperatively and, at both time points, were associated with duration of mechanical ventilation. As discussed in Time Point 1: the Donor, postoperative recipient BAL RAGE levels have also been shown to correlate with development and severity of PGD (48). These results provide evidence for the association of RAGE with short-term outcomes following lung transplantation.
Early postoperative plasma levels of RAGE may also be associated with later complications. Plasma levels of RAGE measured in 106 transplant recipients at 6 and 24 h postreperfusion were associated with bronchiolitis obliterans syndrome, the leading contributor to death after the first postoperative year (57). The highest quartile of RAGE at 24 h had the greatest risk. The RAGE level at 24 h was also higher in recipients who developed PGD than in those who did not. The association of RAGE with both early and late outcomes may signify the potential for RAGE to propagate and sustain an acute inflammatory reaction that leads to chronic alveolar epithelial inflammation and injury.
Cell death markers (cytokeratin 18).
Epithelial cell death can occur via two pathways generally understood as apoptosis (caspase dependent) or necrosis (caspase independent). Previous work has suggested that reperfusion after transplantation activates the apoptosis pathway and that inhibition of this pathway improves posttransplantation graft function (28). Apoptosis, however, is not always a negative event. Leukocyte apoptosis is one mechanism by which inflammation resolves. Inhibition of caspases in leukocyte apoptosis is associated with worse outcomes. Therefore, selective inhibition of apoptosis in certain cell types such as graft parenchymal cells would be better than broad inhibition of apoptosis.
Cytokeratin 18 (CK18) is an epithelial-cell death marker, released intact during necrotic cell death and as a caspase-cleaved fragment during apoptosis. In one study, recipient plasma levels of both caspase-cleaved and total CK18 at 24 and 48 h posttransplantation were higher in those that developed PGD than in those who did not (28). Higher CK18 levels at 48 h posttransplantation, both caspase cleaved and total, were also associated with worse 1-yr survival. Following adjustment for PGD, total CK18 remained elevated in recipients who died within 1 yr, whereas caspase-cleaved CK18 did not. This indicates that epithelial cell death by apoptosis, and more significantly necrosis, are associated with progression to PGD and longer term recipient outcomes. Measurement of epithelial cell necrosis could thus provide both short and long-term prognosis posttransplant, and therapies to target epithelial cell necrosis could improve both short and long-term recipient mortality.
Club cell secretory protein (CC-16).
The respiratory epithelium also may play a role in recipient development of PGD. In 104 lung transplant recipients, plasma levels of CC-16 were measured preoperatively and at 6 and 24 h postoperatively (22). Recipients who developed PGD had elevated levels of CC-16 at 6 h postoperatively compared with recipients who did not develop PGD. In contrast to findings from a larger study (62), preoperative levels did not differ between groups, although the association with PGD was similarly attenuated by the diagnosis of IPF. However, in recipients without IPF, the association between elevated 6-h levels of CC-16 and development of PGD was independent of multiple donor, recipient, and surgical characteristics, suggesting the value of this biomarker apart from more traditional risk factors.
Long pentraxin-3.
Innate immune activation may also be involved in the pathogenesis of PGD. Long pentraxin-3 (PTX3) is an acute-phase reactant involved in inflammation and innate immunity. In 119 lung transplant recipients with either IPF or COPD, plasma levels of PTX3 were higher at baseline in recipients with IPF than in those with COPD (23). In addition, at both 6 and 24 h postoperatively, PTX3 levels in recipients with IPF were higher in those who developed PGD than in those who did not. No such association was seen in recipients with COPD. These results suggest the importance of the innate immune reaction in the early postoperative period and the development of PGD and further suggest that the strength of this association is diagnosis dependent.
Complement (C3a, C4a, and C5a).
The role of complement activation further implicates innate immunity in development of PGD. In 190 lung transplant recipients, plasma levels of C3a, C4a, and C5a were measured preoperatively and at 6 and 24 h postoperatively (61). While there was no difference in complement levels at a single time point between those who did and did not develop PGD, there were significant differences in the change of levels between two time points and development of PGD. Furthermore, increased preoperative levels of C5a and at 24 h postoperatively were associated with an increased risk of death, as were elevated C3a levels at 6 h after transplantation. These results provide further support for the pathogenicity of the innate immune system in lung transplantation.
Neutrophil extracellular traps.
Neutrophils form complexes of DNA, histones, and neutrophil granular proteins called neutrophil extracellular traps (NETs). Released from neutrophils upon activation, these complexes act as a defense against bacteria, fungi, and potentially viruses. NETs form in the lung during transfusion-related acute lung injury. According to one study, the level of NETs in BAL fluid obtained within 24 h after transplantation was higher in recipients who developed PGD than in recipients who did not (55). Plasma samples before and after transplantation were also studied, but there were no differences in NET levels. A mouse model that was part of the same study showed NET formation is platelet-dependent as well as pathogenic in PGD. Treatment with a platelet aggregation inhibitor (aspirin) or a NET degrading substance (DNaseI) reduced NET formation and lung injury. These results give insight into the potential pathogenic contribution and thus therapeutic target of both platelets and neutrophils in PGD (55).
Antibodies to collagen type V.
As discussed in Time Point 2: the Preoperative Recipient, a study that measured levels of antibodies to col(V) in recipient plasma at 24, 48, and 72 h postoperatively found that recipients who developed PGD had higher levels of anti-col(V) antibodies than recipients who did not develop PGD (32).
Biomarker combinations.
The studies discussed thus far provide evidence of the association of individual protein biomarkers with PGD. However, a combination of these markers may provide a more accurate predictive approach, as was shown in a cohort study of 128 recipients that measured a panel of plasma biomarkers known to be associated with ARDS (58). Levels of ICAM-1, RAGE, surfactant protein D (SP-D), PAI-1, and protein C were measured at 6 and 24 h after allograft reperfusion and two primary end points were examined: development of PGD within 72 h posttransplantation and 90-day mortality. Biomarker levels at 24 h were more strongly associated with PGD than levels at 6 h. Levels of all but protein C at 24 h were higher in recipients who developed PGD than in those who did not. Individually, levels of RAGE and PAI-1 discriminated the best. Combinations of two biomarkers, PAI-1 with ICAM-1, or PAI-1 with SP-D, were more predictive of PGD than each biomarker alone or combinations of three, four, or five biomarkers. Individually, ICAM-1 and PAI-1 had the best discrimination for 90-day mortality, improved somewhat by a combination of ICAM-1 and RAGE. Notably, including the presence or absence of PGD as a variable in combination with any individual biomarker in predicting 90-day mortality was stronger than using PGD alone. Thus this study demonstrates the value of single biomarkers and combinations in predicting PGD. It also illustrates the potential for using biomarkers as adjuncts with clinical factors in predicting early death following lung transplantation.
Recipient adiposity (leptin).
Obesity, a risk factor that has been linked to acute lung injury, has also been associated with recipient development of PGD (40). Leptin, a proinflammatory cytokine produced by adipose tissue (an “adipokine”), has been linked to lung fibrosis after injury. To investigate the association of obesity, as well as adipokines, with PGD, one study measured recipient obesity as well as plasma levels of leptin preoperatively and at 6 and 24 h after transplantation (40).
Obese recipients had a twofold increase in their risk of developing PGD compared with nonobese recipients. Furthermore, higher plasma leptin levels at 24 h after transplantation were also associated with progression to PGD. Plasma leptin levels were also positively correlated with recipient body mass index (BMI). Recipients who were overweight and obese also had different baseline and operative characteristics than normal or underweight recipients. Those who were overweight or obese tended to have longer ischemic times and higher systolic pulmonary artery pressure.
The association between adiposity and PGD may be explained by multiple cytokines associated with hypoxic adipose tissue, such as IL-6, TNFα, IL-1β, and leptin (38). This proinflammatory environment may be exacerbated by other more mechanical factors related to obesity, such as the longer ischemic times and higher systolic pulmonary arterial pressure found in this study.
Ex vivo human lung perfusion.
As we have seen, the timing of sample collection for protein biomarkers of PGD is a complicated issue. The same holds for donor lung evaluation. Ex vivo lung perfusion (EVLP) has extended our ability to evaluate donor lungs and has gained support both in research and in clinical practice. A study of 51 analytes in perfusate obtained from 50 donor lungs placed on EVLP found that at 1 h, IL-8 and macrophage colony stimulating factor (M-CSF) were elevated in the perfusate of transplanted lungs that developed PGD compared with lungs that did not (43). After 4 h, IL-8 remained elevated, and growth-regulated oncogene-Α, granulocyte colony-stimulating factor, macrophage inhibitory protein-1α, and macrophage inhibitory protein-1β were also higher in lungs that developed PGD than those that did not. When a cutoff value was retrospectively applied to IL-8, discrimination for PGD prediction was excellent (area under the curve = 0.93). There were no significant physiologic differences between transplanted lungs that developed PGD and lungs that did not. These findings indicate that elevated levels of IL-8 in the perfusate could potentially predict the risk of PGD before transplant.
EVLP was also used to investigate the contribution of the donor to PGD development in the recipient, as mediated by ET-1 (44). Perfusate samples were collected at 1 and 4 h of perfusion and levels of ET-1, big ET-1, endothelin-converting enzyme, and nitric oxide metabolites (NOx) were measured. The most interesting results emerged from studying the lungs that progressed to develop PGD, specifically those from donors after cardiac death (DCD), whereby lungs destined to develop PGD had higher levels of ET-1 and big ET-1 at 4 h than lungs that were transplanted but did not develop PGD. At this 4-h time point, the ET-1 level differentiated those lungs that would develop PGD and those that would not. In DCD donors and brain-dead donors, lungs that developed PGD also had higher NOx levels at 1 h than lungs that did not develop PGD. The involvement of NOx is interesting because of prior research showing alterations in nitric oxide synthase linked to ischemia-reperfusion injury in lung transplantation (12, 41). Importantly, physiologic measurements during the time on EVLP were not associated with progression to PGD, highlighting the potential impact for biomarker measurements in predicting transplantation success. The difference between the donor types raises the question of different pathways of injury depending on the mechanism of death.
Discussion
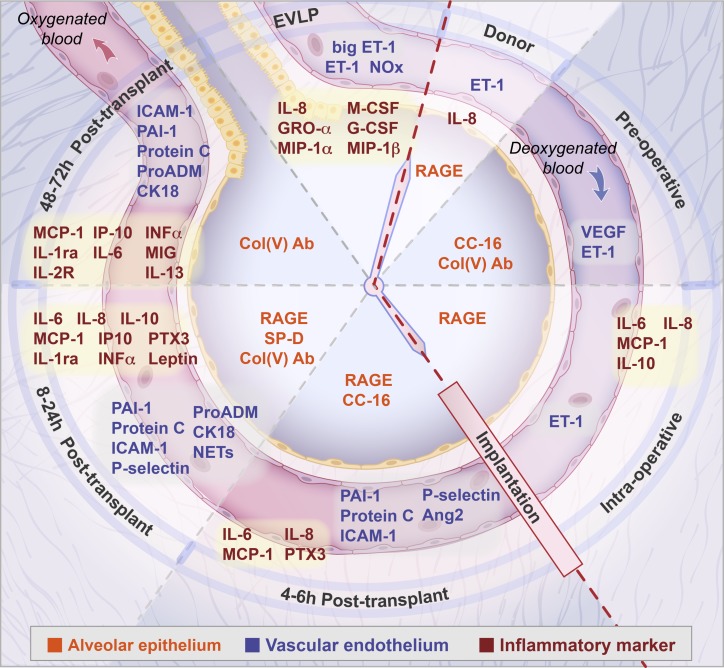
The studies reviewed here have identified protein biomarkers in donors and recipients associated with increased risk for severe PGD (Table 1). Multiple protein biomarkers consistent with various pathways have been identified, in addition to important time points for sampling (Fig. 1). However, it is not feasible at this point in time to further clarify which biomarkers may be pathogenic, which may be markers of cellular injury or dysfunction, or which may be diagnostic, predictive, or prognostic. Indeed, there is no consensus regarding the pathways that lead to PGD. Another problem is that the most frequently evaluated sampling period is in the first 24 h postoperatively; by then, PGD has often already developed, leaving little time for use of targeted preventative therapies. IL-6, IL-8, RAGE, and PAI-1 have shown promise at multiple time points in multiple samples (plasma, tissue, and BAL) for identifying those at risk for PGD. However, we must acknowledge the possibility that additional pathways of injury exist that are yet to be discovered, using biomarkers yet to be studied. Further research for biomarker discovery using metabolomics, proteomics, microarray, and gene expression technology, as has already been done by some (3, 21, 31, 37, 50, 52), may detect novel biomarkers associated with PGD and yield a more comprehensive understanding of the pathways of injury.
Table 1.
Summary of biomarkers associated with primary graft dysfunction
| Biomarker | Source | Vehicle | Authors and Year (Reference) |
|---|---|---|---|
| Donor | |||
| IL-8 | Inflammatory marker | BAL, tissue | Fisher et al. 2001 (26) |
| RAGE | Alveolar epithelium | BAL | Pelaez et al. 2010 (48) |
| ET-1 | Vascular endothelium | Tissue | Salama et al. 2010 (54) |
| Recipient preoperative | |||
| CC-16 | Respiratory epithelium | Blood | Shah et al. 2014 (62) |
| Antibodies to collage type V | Respiratory epithelium | Blood | Bobadilla et al. 2012 (7) |
| ET-1 | Vascular endothelium | Blood | Salama et al. 2010 (54) |
| VEGF | Vascular endothelium | Blood | Krenn et al. 2007 (39) |
| Intraoperative | |||
| IL-6, IL-8, IL-10, MCP-1 | Inflammatory marker | Blood | Allen et al. 2012 (2) |
| IL-10 | Inflammatory marker | Blood | Mathur et al. 2006 (45) |
| RAGE | Alveolar epithelium | Blood | Pottecher et al. 2016 (49) |
| ET-1 | Vascular endothelium | Tissue | Salama et al. 2010 (54) |
| 4–6 h Posttransplant | |||
| IL-6, IL-8 | Inflammatory marker | Blood | Mathur et al. 2006 (45) |
| MCP-1 | Inflammatory marker | Blood | Hoffman et al. 2009 (30) |
| PTX3 | Inflammatory marker | Blood | Diamond et al. 2011 (23) |
| PAI-1, protein C | Coagulation | Blood | Christie et al. 2007 (15) |
| P-selectin | Platelet activation | Blood | Kawut et al. 2009 (36) |
| Ang-2 | Vascular endothelium | Blood | Diamond et al. 2012 (25) |
| ICAM-1 | Vascular endothelium | Blood | Covarrubias et al. 2007 (17) |
| RAGE | Alveolar epithelium | Blood | Christie et al. 2009 (16) |
| CC-16 | Respiratory epithelium | Blood | Diamond et al. 2011 (22) |
| 8–24 h Posttransplant | |||
| IL-10, MCP-1 | Inflammatory marker | Blood | Allen et al. 2012 (2) |
| IL-8, IL-10 | Inflammatory marker | Blood | Mathur et al. 2006 (45) |
| MCP-1, IP-10, INFα, IL-1ra | Inflammatory marker | Blood | Hoffman et al. 2009 (30) |
| PTX3 | Inflammatory marker | Blood | Diamond et al. 2011 (23) |
| IL-6 | Inflammatory marker | Blood, BAL | Moreno et al. 2007 (47) |
| MCP-1 | Inflammatory marker | Blood | Shah et al. 2012 (60) |
| PAI-1, protein C | Coagulation | Blood | Christie et al. 2007 (15) |
| PAI-1, ICAM-1, SP-D, RAGE | Epithelium, endothelium, coagulation | Blood | Shah et al. 2012 (58) |
| ICAM-1 | Vascular endothelium | Blood | Covarrubias et al. 2007 (17) |
| RAGE | Alveolar epithelium | BAL | Pelaez et al. 2010 (48) |
| RAGE | Alveolar epithelium | Blood | Christie et al. 2009 (16) |
| RAGE | Alveolar epithelium | Blood | Shah et al. 2013 (57) |
| Antibodies to collagen type V | Respiratory epithelium | Blood | Iwata et al. 2008 (32) |
| P-selectin | Platelet activation | Blood | Kawut et al. 2009 (36) |
| ProADM | Hypoxic injury | Blood | Riera et al. 2016 (51) |
| CK18 | Cell death marker | Blood | Hashimoto et al. 2016 (28) |
| NETs | Neutrophil activation | BAL | Sayah et al. 2015 (55) |
| Leptin | Adipokine | Blood | Lederer et al. 2011 (40) |
| 48–72 h Posttransplant | |||
| MCP-1, IP-10, INFα, IL-1ra, IL-6, MIG, IL-2R, IL-13 | Inflammatory marker | Blood | Hoffman et al. 2009 (30) |
| PAI-1, protein C | Coagulation | Blood | Christie et al. 2007 (15) |
| ICAM-1 | Vascular endothelium | Blood | Covarrubias et al. 2007 (17) |
| Antibodies to collagen type V | Respiratory epithelium | Blood | Iwata et al. 2008 (32) |
| ProADM | Hypoxic injury | Blood | Riera et al. 2016 (51) |
| CK18 | Cell death marker | Blood | Hashimoto et al. 2016 (28) |
| EVLP | |||
| IL-8, M-CSF, GRO-α, G-CSF, MIP-1α, MIP-1β | Inflammatory marker | EVLP | Machuca et al. 2015 (43) |
| ET-1, big ET-1, NOx | Vascular endothelium | EVLP | Machuca et al. 2015 (44) |
IL, interleukin; BAL, bronchoalveolar lavage; RAGE, receptor for advanced glycation end products; ET, endothelin; CC-16, club cell secretory protein; VEGF, vascular endothelial growth factor; MCP, monocyte chemoattractant protein; PAI, plasminogen activator inhibitor; Ang-2, angiopoietin 2; PTX3, long pentraxin-3; IP, interferon-γ inducible protein; INF, interferon; ICAM, intracellular adhesion molecule; SP-D, surfactant protein D; proADM, proadrenomedullin;CK18, cytokeratin 18; NETs, neutrophil extracellular traps; MIG, monokine induced by interferon-γ; EVLP, ex vivo lung perfusion; M-CSF, macrophage colony-stimulating factor; GRO, growth-regulated oncogene; G-CSF, granulocyte-colony stimulating factor; MIP, macrophage inflammatory protein; NOx, nitric oxide metabolites.
Fig. 1.
Representation of the time course, including ex vivo lung perfusion (EVLP), from the donor through to the postoperative period (clockwise direction), of biomarkers associated with primary graft dysfunction in the recipient. Biomarkers specific to the epithelium are shown centrally in the alveolus (orange print). Biomarkers specific to the vascular endothelium as well as coagulation, fibrinolysis, platelet activation, hypoxic injury, cell death markers and neutrophil activation are shown in the capillary lumen (blue print). Inflammatory cytokines are in red print. Epithelial markers: RAGE, receptor for advanced glycation end products; CC-16, club cell secretory protein; col(V), antibodies to collage type V; SP-D, surfactant protein D. Endothelial markers: ET-1, endothelin; big ET-1; NOx, nitric oxide metabolites; VEGF, vascular endothelial growth factor; Ang-2, angiopoietin 2; ICAM-1, intracellular adhesion molecule-1. Marker of hypoxic injury: proADM, proadrenomedullin. Cell death marker: CK18, cytokeratin 18. Marker of neutrophil activation: NETs, neutrophil extracellular traps. Markers of coagulation and fibrinolysis: PAI-1, plasminogen activator inhibitor 1; protein C. Marker of platelet activation: P-selectin. Markers of inflammation: IL, interleukin-6, 8, 10, 13, 1ra, and 2R; M-CSF, macrophage colony stimulating factor; G-CSF, granulocyte-colony stimulating factor; GRO-A, growth-regulated oncogene; MIP-1α and -1β, macrophage inflammatory protein-1α and -1β; MCP-1, monocyte chemoattractant protein; PTX3, long pentraxin-3; INF-α, interferon-α; IP, interferon-γ; MIG, monokine induced by interferon-γ.
This review leads us to propose pulmonary epithelial injury as a critical factor in the development of alveolar edema in the setting of PGD. Historically, damage to the vascular endothelium during preservation and reperfusion was highlighted as the initial insult for subsequent pulmonary edema and lung injury (29). However, alveolar epithelial injury has been also implicated numerous times in ARDS, both in mouse and human lung models (27, 33, 68). The role of the epithelium in alveolar fluid clearance and recovery from injury has been well established (9, 65). At each time point, including in the donor (RAGE) and preoperative recipient [CC-16 and reactivity to col(V)], markers of alveolar or respiratory epithelial injury are apparent. Subtle epithelial injury in the donor and preoperative recipient, not yet detectable by physiologic measurements before organ recovery or during EVLP, may set the stage for later additional epithelial dysfunction. Further epithelial injury during the intraoperative time point may occur, most likely during reperfusion. In this review, the most consistent and repeated finding was an elevation of RAGE in the recipient blood compartment during the early postoperative period. We propose that during the early postoperative period, RAGE acts as a marker of alveolar epithelial injury and epithelial barrier breakdown with subsequent pulmonary edema as in PGD. What may be most important in the early posttransplant period is the ability of the recipient to recover from the insult to pulmonary function up to this point, including injurious contributions from the donor, the preoperative recipient, and the operation itself. The elevated levels of RAGE in the postoperative period as discussed in this review may identify those transplant recipients with persistent alveolar epithelial injury, and thus explain the association with PGD.
Our review also indicates that PGD has been associated with an elevation of inflammatory biomarkers in the donor and recipient at all postoperative time points, as well as with recipient abnormalities of coagulation, fibrinolysis, platelet activation, neutrophil activation, adipokines, and circulating cell death markers, some of which may contribute directly or indirectly to alveolar epithelial injury. The complexity of injury in the postoperative period is evidenced by the elevation of several protein biomarkers, indicating the confluence of different pathways.
An important finding in our review is the association of protein biomarkers of epithelial [CC-16 and col(V) reactivity] and endothelial injury (VEGF and ET-1), measured in recipient blood before transplantation, with postoperative progression to PGD. The role of these biomarkers as pathogenic components rather than simply markers of dysfunction deserves further consideration. Comparing measurements of these biomarkers in recipients that do and do not develop PGD does not prove their pathogenicity. However, work in rats has shown that the postoperative venous injection of purified antibodies to col(V) will induce a pathology and physiology in the transplanted lung similar to PGD (32). In another study, the addition of VEGF to a cell monolayer derived from human pulmonary artery endothelial cells caused an increase in endothelial cell permeability (64). The breakdown of this endothelial barrier was reduced when VEGF was neutralized. Collectively, the results of these studies suggest that exposure of the transplanted allograft to biologically active proteins in the preoperative recipient may prime the lung for the development of PGD. Further confirmation of specific pathogenic proteins could identify targeted prophylactic and therapeutic treatments for PGD.
Biomarkers can be used to develop targeted therapies and a prediction model for PGD risk. Targeted treatments, such as viral vectors that drive expression of IL-10, have shown promise in lung injury repair and are entering early-phase clinical trials in ex vivo lung perfusion (18). Other treatments aimed at ischemia-reperfusion injury (63) also show promise, including human mesenchymal stem cells (46). EVLP can be used to determine effects of targeted treatments and assess changes in lung function before implantation. Development of a risk prediction model requires knowledge of which donor and recipient clinical characteristics contribute most to the risk of PGD, rapid biomarker analysis, and an understanding of what sampling time points are the most valuable. How the interaction of donor and recipient biomarkers may contribute to PGD risk remains unclear.
Biologic markers alone are not sufficient to identify those recipients at risk of developing PGD. Numerous clinical risk factors have been identified in both donors and recipients. Donor risk factors include donor age (<21 yr, >45 yr), female gender, African American race, smoking history, and increased ischemic time (4, 14, 19, 24, 66). Recipient risk factors that have been identified include age, female gender, African American race, elevated BMI, diagnosis of pulmonary hypertension, sarcoidosis, idiopathic pulmonary fibrosis, hepatic impairment, and use of cardiopulmonary bypass (4, 5, 24, 42, 66). The interplay of recipient anthropomorphic characteristics with the activity of protein biomarkers and this effect on the risk of PGD deserves more study. For example, recent work has shown the impact of ischemia-reperfusion injury on gene expression profiles specifically in adipose tissue (21). This study further supports the association of several protein biomarkers with levels of adiposity, in addition to cytokines specifically associated with hypoxic adipose tissue as discussed above. These findings illustrate the complex interactions between biologic and clinical characteristics that are likely occurring in the pathogenic pathways leading to PGD. As with the heterogeneity of acute lung injury in patients with ARDS, classifiable subgroups of donors exist that likely interact with certain subgroups of recipients. A study of multiple risk prediction models (59) risk-stratified 1,255 recipients into three different models based on their BMI, diagnosis, and pulmonary artery pressure. The impact of donor smoking on PGD by the resulting risk classification was also evaluated. However, only clinical characteristics were considered. To develop a parsimonious risk-prediction model for PGD, the permutations of donor and recipient biologic and clinical predictors require further study.
Additionally, a feasible model would require rapid point-of-care biomarker analysis to facilitate matching of donor and recipient lungs based on biologic and clinical characteristics. Recent work has shown the potential for novel methods for rapid biomarker analysis (53). These new technologies could allow sample-to-results times of 20–30 min. Rapid point-of-care testing for two or three biomarkers could generate an enhanced risk profile for donors at three potential time points: before organ recovery, immediately before implantation, or after recovery during EVLP. A donor risk profile would allow further risk stratification during recipient selection. How to incorporate recipient biologic or clinical characteristics into this risk stratification profile will depend on the development of a feasible risk-prediction model.
GRANTS
M. A. Matthay and L. B. Ware were supported in part by National Heart, Lung, and Blood Institute Grant H-126176. L. B. Ware was also supported by National Heart, Lung, and Blood Institute Grant HL-103836.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.C.H. prepared figures; B.C.H. drafted manuscript; B.C.H., J.K., L.B.W., and M.A.M. edited and revised manuscript; B.C.H., J.K., L.B.W., and M.A.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Pamela Derish in the University of California San Francisco Department of Surgery for assistance in the preparation of this manuscript and Diana Lim for artwork in the figure.
REFERENCES
- 1.Agrawal A, Matthay MA, Kangelaris KN, Stein J, Chu JC, Imp BM, Cortez A, Abbott J, Liu KD, Calfee CS. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med 187: 736–742, 2013. doi: 10.1164/rccm.201208-1460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JG, Lee MT, Weiss ES, Arnaoutakis GJ, Shah AS, Detrick B. Preoperative recipient cytokine levels are associated with early lung allograft dysfunction. Ann Thorac Surg 93: 1843–1849, 2012. doi: 10.1016/j.athoracsur.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 3.Anraku M, Cameron MJ, Waddell TK, Liu M, Arenovich T, Sato M, Cypel M, Pierre AF, de Perrot M, Kelvin DJ, Keshavjee S. Impact of human donor lung gene expression profiles on survival after lung transplantation: a case-control study. Am J Transplant 8: 2140–2148, 2008. doi: 10.1111/j.1600-6143.2008.02354.x. [DOI] [PubMed] [Google Scholar]
- 4.Arcasoy SM, Fisher A, Hachem RR, Scavuzzo M, Ware LB; ISHLT Working Group on Primary Lung Graft Dysfunction . Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part V: predictors and outcomes. J Heart Lung Transplant 24: 1483–1488, 2005. doi: 10.1016/j.healun.2004.11.314. [DOI] [PubMed] [Google Scholar]
- 5.Barr ML, Kawut SM, Whelan TP, Girgis R, Böttcher H, Sonett J, Vigneswaran W, Follette DM, Corris PA; ISHLT Working Group on Primary Lung Graft Dysfunction . Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part IV: recipient-related risk factors and markers. J Heart Lung Transplant 24: 1468–1482, 2005. doi: 10.1016/j.healun.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Binnie A, Tsang JLY, dos Santos CC. Biomarkers in acute respiratory distress syndrome. Curr Opin Crit Care 20: 47–55, 2014. doi: 10.1097/MCC.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 7.Bobadilla JL, Love RB, Jankowska-Gan E, Xu Q, Haynes LD, Braun RK, Hayney MS, Munoz del Rio A, Meyer K, Greenspan DS, Torrealba J, Heidler KM, Cummings OW, Iwata T, Brand D, Presson R, Burlingham WJ, Wilkes DS. Th-17, monokines, collagen type V, and primary graft dysfunction in lung transplantation. Am J Respir Crit Care Med 177: 660–668, 2008. doi: 10.1164/rccm.200612-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, Gopalakrishnan B, Cai J, Brand DD, Yoshida S, Cummings OW, Wilkes DS. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest 117: 3498–3506, 2007. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calfee CS, Budev MM, Matthay MA, Church G, Brady S, Uchida T, Ishizaka A, Lara A, Ranes JL, deCamp MM, Arroliga AC. Plasma receptor for advanced glycation end-products predicts duration of ICU stay and mechanical ventilation in patients after lung transplantation. J Heart Lung Transplant 26: 675–680, 2007. doi: 10.1016/j.healun.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calfee CS, Gallagher D, Abbott J, Thompson BT, Matthay MA; NHLBI ARDS Network . Plasma angiopoietin-2 in clinical acute lung injury: prognostic and pathogenetic significance. Crit Care Med 40: 1731–1737, 2012. doi: 10.1097/CCM.0b013e3182451c87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantu E, Lederer DJ, Meyer K, Milewski K, Suzuki Y, Shah RJ, Diamond JM, Meyer NJ, Tobias JW, Baldwin DA, Van Deerlin VM, Olthoff KM, Shaked A, Christie JD; CTOT Investigators . Gene set enrichment analysis identifies key innate immune pathways in primary graft dysfunction after lung transplantation. Am J Transplant 13: 1898–1904, 2013. doi: 10.1111/ajt.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee S, Nieman GF, Christie JD, Fisher AB. Shear stress-related mechanosignaling with lung ischemia: lessons from basic research can inform lung transplantation. Am J Physiol Lung Cell Mol Physiol 307: L668–L680, 2014. doi: 10.1152/ajplung.00198.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christie JD, Kotloff RM, Ahya VN, Tino G, Pochettino A, Gaughan C, DeMissie E, Kimmel SE. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med 171: 1312–1316, 2005. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, Kimmel SE. Clinical risk factors for primary graft failure following lung transplantation. Chest 124: 1232–1241, 2003. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 15.Christie JD, Robinson N, Ware LB, Plotnick M, De Andrade J, Lama V, Milstone A, Orens J, Weinacker A, Demissie E, Bellamy S, Kawut SM. Association of protein C and type 1 plasminogen activator inhibitor with primary graft dysfunction. Am J Respir Crit Care Med 175: 69–74, 2007. doi: 10.1164/rccm.200606-827OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christie JD, Shah CV, Kawut SM, Mangalmurti N, Lederer DJ, Sonett JR, Ahya VN, Palmer SM, Wille K, Lama V, Shah PD, Shah A, Weinacker A, Deutschman CS, Kohl BA, Demissie E, Bellamy S, Ware LB; Lung Transplant Outcomes Group . Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am J Respir Crit Care Med 180: 1010–1015, 2009. doi: 10.1164/rccm.200901-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Covarrubias M, Ware LB, Kawut SM, De Andrade J, Milstone A, Weinacker A, Orens J, Lama V, Wille K, Bellamy S, Shah C, Demissie E, Christie JD; Lung Transplant Outcomes Group . Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. Am J Transplant 7: 2573–2578, 2007. doi: 10.1111/j.1600-6143.2007.01981.x. [DOI] [PubMed] [Google Scholar]
- 18.Cypel M, Liu M, Rubacha M, Yeung JC, Hirayama S, Anraku M, Sato M, Medin J, Davidson BL, de Perrot M, Waddell TK, Slutsky AS, Keshavjee S. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med 1: 4ra9–4ra9, 2009. doi: 10.1126/scitranslmed.3000266. [DOI] [PubMed] [Google Scholar]
- 19.De Perrot M, Bonser RS, Dark J, Kelly RF; ISHLT Working Group on Primary Lung Graft Dysfunction . Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part III: donor-related risk factors and markers. J Heart Lung Transplant 24: 1460–1467, 2005. doi: 10.1016/j.healun.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 20.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med 167: 490–511, 2003. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 21.Diamond JM, Arcasoy S, McDonnough JA, Sonett JR, Bacchetta M, D’Ovidio F, Cantu E III, Bermudez CA, McBurnie A, Rushefski M, Kalman LH, Oyster M, D’Errico C, Suzuki Y, Giles JT, Ferrante A, Lippel M, Singh G, Lederer DJ, Christie JD; Lung Transplant Body Composition Study . Adipose gene expression profile changes with lung allograft reperfusion. Am J Transplant 17: 239–245, 2017. doi: 10.1111/ajt.13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diamond JM, Kawut SM, Lederer DJ, Ahya VN, Kohl B, Sonett J, Palmer SM, Crespo M, Wille K, Lama VN, Shah PD, Orens J, Bhorade S, Weinacker A, Demissie E, Bellamy S, Christie JD, Ware LB; Lung Transplant Outcomes Group . Elevated plasma clara cell secretory protein concentration is associated with high-grade primary graft dysfunction. Am J Transplant 11: 561–567, 2011. doi: 10.1111/j.1600-6143.2010.03431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamond JM, Lederer DJ, Kawut SM, Lee J, Ahya VN, Bellamy S, Palmer SM, Lama VN, Bhorade S, Crespo M, Demissie E, Sonett J, Wille K, Orens J, Shah PD, Weinacker A, Weill D, Kohl BA, Deutschman CC, Arcasoy S, Shah AS, Belperio JA, Wilkes D, Reynolds JM, Ware LB, Christie JD; Lung Transplant Outcomes Group . Elevated plasma long pentraxin-3 levels and primary graft dysfunction after lung transplantation for idiopathic pulmonary fibrosis. Am J Transplant 11: 2517–2522, 2011. doi: 10.1111/j.1600-6143.2011.03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL, Lederer DJ, Cantu E, Kohl BA, Lama VN, Bhorade SM, Crespo M, Demissie E, Sonett J, Wille K, Orens J, Shah AS, Weinacker A, Arcasoy S, Shah PD, Wilkes DS, Ware LB, Palmer SM, Christie JD; Lung Transplant Outcomes Group . Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 187: 527–534, 2013. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamond JM, Porteous MK, Cantu E, Meyer NJ, Shah RJ, Lederer DJ, Kawut SM, Lee J, Bellamy SL, Palmer SM, Lama VN, Bhorade SM, Crespo M, Demissie E, Wille K, Orens J, Shah PD, Weinacker A, Weill D, Arcasoy S, Wilkes DS, Ware LB, Christie JD; Lung Transplant Outcomes Group . Elevated plasma angiopoietin-2 levels and primary graft dysfunction after lung transplantation. PLoS One 7: e51932–e51938, 2012. doi: 10.1371/journal.pone.0051932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher AJ, Donnelly SC, Hirani N, Haslett C, Strieter RM, Dark JH, Corris PA. Elevated levels of interleukin-8 in donor lungs is associated with early graft failure after lung transplantation. Am J Respir Crit Care Med 163: 259–265, 2001. doi: 10.1164/ajrccm.163.1.2005093. [DOI] [PubMed] [Google Scholar]
- 27.Frank JA, Briot R, Lee JW, Ishizaka A, Uchida T, Matthay MA. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. Am J Physiol Lung Cell Mol Physiol 293: L52–L59, 2007. doi: 10.1152/ajplung.00256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto K, Besla R, Zamel R, Juvet S, Kim H, Azad S, Waddell TK, Cypel M, Liu M, Keshavjee S. Circulating cell death biomarkers may predict survival in human lung transplantation. Am J Respir Crit Care Med 194: 97–105, 2016. doi: 10.1164/rccm.201510-2115OC. [DOI] [PubMed] [Google Scholar]
- 29.Hidalgo MA, Shah KA, Fuller BJ, Green CJ. Cold ischemia-induced damage to vascular endothelium results in permeability alterations in transplanted lungs. J Thorac Cardiovasc Surg 112: 1027–1035, 1996. doi: 10.1016/S0022-5223(96)70104-6. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman SA, Wang L, Shah CV, Ahya VN, Pochettino A, Olthoff K, Shaked A, Wille K, Lama VN, Milstone A, Ware LB, Orens J, Weinacker A, Demissie E, Bellamy S, Kawut SM, Hancock WW, Christie JD; Lung Transplant Outcomes Group . Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. Am J Transplant 9: 389–396, 2009. doi: 10.1111/j.1600-6143.2008.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsin MK, Zamel R, Cypel M, Wishart D, Han B, Keshavjee S, Liu M. Metabolic profile of ex vivo lung perfusate yields biomarkers for lung transplant outcomes. Ann Surg 2016. doi: 10.1097/SLA.0000000000002016. [DOI] [PubMed] [Google Scholar]
- 32.Iwata T, Philipovskiy A, Fisher AJ, Presson RG Jr, Chiyo M, Lee J, Mickler E, Smith GN, Petrache I, Brand DB, Burlingham WJ, Gopalakrishnan B, Greenspan DS, Christie JD, Wilkes DS. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol 181: 5738–5747, 2008. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jabaudon M, Blondonnet R, Roszyk L, Bouvier D, Audard J, Clairefond G, Fournier M, Marceau G, Déchelotte P, Pereira B, Sapin V, Constantin JM. Soluble receptor for advanced glycation end-products predicts impaired alveolar fluid clearance in acute respiratory distress syndrome. Am J Respir Crit Care Med 192: 191–199, 2015. doi: 10.1164/rccm.201501-0020OC. [DOI] [PubMed] [Google Scholar]
- 34.Jankowska-Gan E, Hegde S, Burlingham WJ. Trans-vivo delayed type hypersensitivity assay for antigen specific regulation. J Vis Exp 75: e4454–e4454, 2013. doi: 10.3791/4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaneda H, Waddell TK, de Perrot M, Bai XH, Gutierrez C, Arenovich T, Chaparro C, Liu M, Keshavjee S. Pre-implantation multiple cytokine mRNA expression analysis of donor lung grafts predicts survival after lung transplantation in humans. Am J Transplant 6: 544–551, 2006. doi: 10.1111/j.1600-6143.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 36.Kawut SM, Okun J, Shimbo D, Lederer DJ, De Andrade J, Lama V, Shah A, Milstone A, Ware LB, Weinacker A, Demissie E, Christie JD; Lung Transplant Outcomes Group . Soluble p-selectin and the risk of primary graft dysfunction after lung transplantation. Chest 136: 237–244, 2009. doi: 10.1378/chest.08-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosanam H, Sato M, Batruch I, Smith C, Keshavjee S, Liu M, Diamandis EP. Differential proteomic analysis of bronchoalveolar lavage fluid from lung transplant patients with and without chronic graft dysfunction. Clin Biochem 45: 223–230, 2012. doi: 10.1016/j.clinbiochem.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Kotloff RM, Heffner JE. Obesity and primary graft dysfunction: weighing the evidence. Am J Respir Crit Care Med 184: 994–996, 2011. doi: 10.1164/rccm.201108-1431ED. [DOI] [PubMed] [Google Scholar]
- 39.Krenn K, Klepetko W, Taghavi S, Lang G, Schneider B, Aharinejad S. Recipient vascular endothelial growth factor serum levels predict primary lung graft dysfunction. Am J Transplant 7: 700–706, 2007. doi: 10.1111/j.1600-6143.2006.01673.x. [DOI] [PubMed] [Google Scholar]
- 40.Lederer DJ, Kawut SM, Wickersham N, Winterbottom C, Bhorade S, Palmer SM, Lee J, Diamond JM, Wille KM, Weinacker A, Lama VN, Crespo M, Orens JB, Sonett JR, Arcasoy SM, Ware LB, Christie JD; Lung Transplant Outcomes Group . Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. Am J Respir Crit Care Med 184: 1055–1061, 2011. doi: 10.1164/rccm.201104-0728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu M, Tremblay L, Cassivi SD, Bai XH, Mourgeon E, Pierre AF, Slutsky AS, Post M, Keshavjee S. Alterations of nitric oxide synthase expression and activity during rat lung transplantation. Am J Physiol Lung Cell Mol Physiol 278: L1071–L1081, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Liu Y, Su L, Jiang SJ. Recipient-related clinical risk factors for primary graft dysfunction after lung transplantation: a systematic review and meta-analysis. PLoS One 9: e92773, 2014. doi: 10.1371/journal.pone.0092773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machuca TN, Cypel M, Yeung JC, Bonato R, Zamel R, Chen M, Azad S, Hsin MK, Saito T, Guan Z, Waddell TK, Liu M, Keshavjee S. Protein expression profiling predicts graft performance in clinical ex vivo lung perfusion. Ann Surg 261: 591–597, 2015. doi: 10.1097/SLA.0000000000000974. [DOI] [PubMed] [Google Scholar]
- 44.Machuca TN, Cypel M, Zhao Y, Grasemann H, Tavasoli F, Yeung JC, Bonato R, Chen M, Zamel R, Chun YM, Guan Z, de Perrot M, Waddell TK, Liu M, Keshavjee S. The role of the endothelin-1 pathway as a biomarker for donor lung assessment in clinical ex vivo lung perfusion. J Heart Lung Transplant 34: 849–857, 2015. doi: 10.1016/j.healun.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Mathur A, Baz M, Staples ED, Bonnell M, Speckman JM, Hess PJ, Klodell CT, Knauf DG, Moldawer LL, Beaver TM. Cytokine profile after lung transplantation: correlation with allograft injury. Ann Thorac Surg 81: 1844–1849, 2006. doi: 10.1016/j.athoracsur.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 46.McAuley DF, Curley GF, Hamid UI, Laffey JG, Abbott J, McKenna DH, Fang X, Matthay MA, Lee JW. Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Physiol Lung Cell Mol Physiol 306: L809–L815, 2014. doi: 10.1152/ajplung.00358.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreno I, Vicente R, Ramos F, Vicente JL, Barberá M. Determination of interleukin-6 in lung transplantation: association with primary graft dysfunction. Transplant Proc 39: 2425–2426, 2007. doi: 10.1016/j.transproceed.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 48.Pelaez A, Force SD, Gal AA, Neujahr DC, Ramirez AM, Naik PM, Quintero DA, Pileggi AV, Easley KA, Echeverry R, Lawrence EC, Guidot DM, Mitchell PO. Receptor for advanced glycation end products in donor lungs is associated with primary graft dysfunction after lung transplantation. Am J Transplant 10: 900–907, 2010. doi: 10.1111/j.1600-6143.2009.02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pottecher J, Roche AC, Dégot T, Helms O, Hentz JG, Schmitt JP, Falcoz PE, Santelmo N, Levy F, Collange O, Uring-Lambert B, Bahram S, Schaeffer M, Meyer N, Geny B, Lassalle P, Diemunsch P, Massard G, Kessler R, Steib A; Groupe de Transplantation Pulmonaire des Hôpitaux Universitaires de Strasbourg . Increased extravascular lung water and plasma biomarkers of acute lung injury precede oxygenation impairment in primary graft dysfunction after lung transplantation. Transplantation 101: 112–121, 2017. doi: 10.1097/TP.0000000000001434. [DOI] [PubMed] [Google Scholar]
- 50.Ray M, Dharmarajan S, Freudenberg J, Zhang W, Patterson GA. Expression profiling of human donor lungs to understand primary graft dysfunction after lung transplantation. Am J Transplant 7: 2396–2405, 2007. doi: 10.1111/j.1600-6143.2007.01918.x. [DOI] [PubMed] [Google Scholar]
- 51.Riera J, Senna A, Cubero M, Roman A, Rello J; Vall d’Hebron Lung Transplant Study Group Investigators . Primary graft dysfunction and mortality following lung transplantation: a role for proadrenomedullin plasma levels. Am J Transplant 16: 634–639, 2016. doi: 10.1111/ajt.13478. [DOI] [PubMed] [Google Scholar]
- 52.Rogers AJ, Matthay MA. Applying metabolomics to uncover novel biology in ARDS. Am J Physiol Lung Cell Mol Physiol 306: L957–L961, 2014. doi: 10.1152/ajplung.00376.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sage AT, Besant JD, Mahmoudian L, Poudineh M, Bai X, Zamel R, Hsin M, Sargent EH, Cypel M, Liu M, Keshavjee S, Kelley SO. Fractal circuit sensors enable rapid quantification of biomarkers for donor lung assessment for transplantation. Sci Adv 1: e1500417–e1500417, 2015. doi: 10.1126/sciadv.1500417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salama M, Andrukhova O, Hoda MA, Taghavi S, Jaksch P, Heinze G, Klepetko W, Aharinejad S. Concomitant endothelin-1 overexpression in lung transplant donors and recipients predicts primary graft dysfunction. Am J Transplant 10: 628–636, 2010. doi: 10.1111/j.1600-6143.2009.02957.x. [DOI] [PubMed] [Google Scholar]
- 55.Sayah DM, Mallavia B, Liu F, Ortiz-Muñoz G, Caudrillier A, DerHovanessian A, Ross DJ, Lynch JP III, Saggar R, Ardehali A, Ware LB, Christie JD, Belperio JA, Looney MR; Lung Transplant Outcomes Group Investigators . Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 191: 455–463, 2015. doi: 10.1164/rccm.201406-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sebag SC, Bastarache JA, Ware LB. Therapeutic modulation of coagulation and fibrinolysis in acute lung injury and the acute respiratory distress syndrome. Curr Pharm Biotechnol 12: 1481–1496, 2011. doi: 10.2174/138920111798281171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shah RJ, Bellamy SL, Lee JC, Cantu E, Diamond JM, Mangalmurti N, Kawut SM, Ware LB, Christie JD. Early plasma soluble receptor for advanced glycation end-product levels are associated with bronchiolitis obliterans syndrome. Am J Transplant 13: 754–759, 2013. doi: 10.1111/ajt.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shah RJ, Bellamy SL, Localio AR, Wickersham N, Diamond JM, Weinacker A, Lama VN, Bhorade S, Belperio JA, Crespo M, Demissie E, Kawut SM, Wille KM, Lederer DJ, Lee JC, Palmer SM, Orens J, Reynolds J, Shah A, Wilkes DS, Ware LB, Christie JD. A panel of lung injury biomarkers enhances the definition of primary graft dysfunction (PGD) after lung transplantation. J Heart Lung Transplant 31: 942–949, 2012. doi: 10.1016/j.healun.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shah RJ, Diamond JM, Cantu E, Flesch J, Lee JC, Lederer DJ, Lama VN, Orens J, Weinacker A, Wilkes DS, Roe D, Bhorade S, Wille KM, Ware LB, Palmer SM, Crespo M, Demissie E, Sonnet J, Shah A, Kawut SM, Bellamy SL, Localio AR, Christie JD. Objective estimates improve risk stratification for primary graft dysfunction after lung transplantation. Am J Transplant 15: 2188–2196, 2015. doi: 10.1111/ajt.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shah RJ, Diamond JM, Lederer DJ, Arcasoy SM, Cantu EM, Demissie EJ, Kawut SM, Kohl B, Lee JC, Sonett J, Christie JD, Ware LB. Plasma monocyte chemotactic protein-1 levels at 24 hours are a biomarker of primary graft dysfunction after lung transplantation. Transl Res 160: 435–442, 2012. doi: 10.1016/j.trsl.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shah RJ, Emtiazjoo AM, Diamond JM, Smith PA, Roe DW, Wille KM, Orens JB, Ware LB, Weinacker A, Lama VN, Bhorade SM, Palmer SM, Crespo M, Lederer DJ, Cantu E, Eckert GJ, Christie JD, Wilkes DS. Plasma complement levels are associated with primary graft dysfunction and mortality after lung transplantation. Am J Respir Crit Care Med 189: 1564–1567, 2014. doi: 10.1164/rccm.201312-2121LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shah RJ, Wickersham N, Lederer DJ, Palmer SM, Cantu E, Diamond JM, Kawut SM, Lama VN, Bhorade S, Crespo M, Demissie E, Sonett J, Wille K, Orens J, Weinacker A, Shah P, Arcasoy S, Wilkes DS, Christie JD, Ware LB; Lung Transplant Outcomes Group . Preoperative plasma club (clara) cell secretory protein levels are associated with primary graft dysfunction after lung transplantation. Am J Transplant 14: 446–452, 2014. doi: 10.1111/ajt.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stone ML, Sharma AK, Zhao Y, Charles EJ, Huerter ME, Johnston WF, Kron IL, Lynch KR, Laubach VE. Sphingosine-1-phosphate receptor 1 agonism attenuates lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol 308: L1245–L1252, 2015. doi: 10.1152/ajplung.00302.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thickett DR, Armstrong L, Christie SJ, Millar AB. Vascular endothelial growth factor may contribute to increased vascular permeability in acute respiratory distress syndrome. Am J Respir Crit Care Med 164: 1601–1605, 2001. doi: 10.1164/ajrccm.164.9.2011071. [DOI] [PubMed] [Google Scholar]
- 65.Ware LB, Golden JA, Finkbeiner WE, Matthay MA. Alveolar epithelial fluid transport capacity in reperfusion lung injury after lung transplantation. Am J Respir Crit Care Med 159: 980–988, 1999. doi: 10.1164/ajrccm.159.3.9802105. [DOI] [PubMed] [Google Scholar]
- 66.Ware LB, Lee JW, Wickersham N, Nguyen J, Matthay MA, Calfee CS; California Transplant Donor Network . Donor smoking is associated with pulmonary edema, inflammation and epithelial dysfunction in ex vivo human donor lungs. Am J Transplant 14: 2295–2302, 2014. doi: 10.1111/ajt.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ware LB, Matthay MA, Parsons PE, Thompson BT, Januzzi JL, Eisner MD; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network . Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med 35: 1821–1828, 2007. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamakawa N, Uchida T, Matthay MA, Makita K. Proteolytic release of the receptor for advanced glycation end products from in vitro and in situ alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L516–L525, 2011. doi: 10.1152/ajplung.00118.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zinter MS, Spicer A, Orwoll BO, Alkhouli M, Dvorak CC, Calfee CS, Matthay MA, Sapru A. Plasma angiopoietin-2 outperforms other markers of endothelial injury in prognosticating pediatric ARDS mortality. Am J Physiol Lung Cell Mol Physiol 310: L224–L231, 2016. doi: 10.1152/ajplung.00336.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]