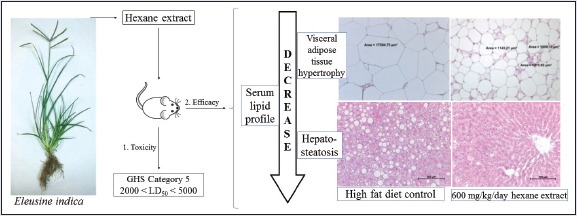
Abstract
Background:
To date, anti-obesity agents based on natural products are tested for their potential using lipase inhibition assay through the interference of hydrolysis of fat by lipase resulting in reduced fat absorption without altering the central mechanisms. Previous screening study had indicated strong anti-obesity potential in Eleusine indica (E. indica), but to date, no pharmacologic studies have been reported so far.
Objective:
This study was performed to investigate the lipid-lowering effects of E. indica using both in vitro and in vivo models.
Methods:
The crude methanolic extract of E. indica was fractionated using hexane (H-Ei), dichloromethane (DCM-Ei), ethyl acetate (EA-Ei), butanol (B-Ei), and water (W-Ei). All the extracts were tested for antilipase activity using porcine pancreatic lipase. Because H-Ei showed the highest inhibition, it was further subjected to chemical profiling using high-performance liquid chromatography. Subsequently, oral toxicity analysis of H-Ei was performed [Organization for Economic Cooperation and Development guidelines using fixed dose procedure (No. 420)]; efficacy analysis was performed using high-fat diet (HFD)-induced hyperlipidemic female Sprague–Dawley rats.
Results:
According to the toxicity and efficacy analyses, H-Ei did not demonstrate any noticeable biochemical toxicity or physiologic abnormalities and did not cause any tissue damage as per histologic analysis. Furthermore, H-Ei significantly reduced body weight and improved serum profile and did not show hepatotoxicity and nephrotoxicity based on the serum profile. Moreover, H-Ei alleviated HFD-induced hepatosteatosis and ameliorated induced adiposity in both visceral and subcutaneous adipose tissue.
Conclusion:
Our results demonstrate that H-Ei effectively improved hyperlipidemia. Further studies to explore its possibility as an alternative pharmacologic agent to treat obesity are warranted.
SUMMARY
Hexane extract of Eleusine indica (H-Ei) showed strong potential in the inhibition of porcine pancreatic lipase (27.01 ± 5.68%).
The acute oral toxicity of E. indica hexane extract on animal model falls into Globally Harmonized System Category 5 (low hazard), since mortality, clinical toxicity symptoms, gross pathologic, or histopathologic damage was not observed.
The hexane extract of E. indica had significantly reduced the body weight and improved serum lipid profile, with reduction in serum triglycerides, total cholesterol, low-density lipoprotein, and elevation in high-density lipoprotein when comparing against the high-fat diet control group.
Microscopic evaluation on histologic slides of liver and adipose tissues suggested that E. indica hexane extract had greatly improved liver steatosis and adipose tissue hypertrophy in high-fat diet control group.
Abbreviation used: ALT: Alanine transaminase; AST: Aspartate transaminase; B-Ei: Butanol extract of E. indica; DCM-Ei: Dichloromethane extract of E. indica; EA-Ei: Ethyl acetate extract of E. indica; GHS: Globally Harmonized System; HDL: High-density lipoprotein; H-Ei: Hexane extract of E. indica; HFD: High-fat diet; HPLC: High-performance liquid chromatography; LDL: Low-density lipoprotein; NFD: Normal fed diet; PPL: Porcine pancreatic lipase; SEM: Standard error of mean; SD: Standard deviation; TC: Total cholesterol; TG: Triglycerides; W-Ei: Water extract of E. indica.
Keywords: Anti-obesity, Eleusine indica, high-fat diet, hyperlipidemia, lipase inhibition, toxicity
INTRODUCTION
Obesity, the fifth leading cause of global deaths, is one of the leading non-communicable diseases leading to vascular diseases. It causes elevation in serum lipid profile and increase in blood pressure and sometimes leads to diabetes.[1,2] The prevalence of obesity has doubled since 1980 compared with that of 2014.[2] Current trend of combating obesity is focused more toward lifestyle changes, which may not be prompt and effective in treating obesity. Therefore, several therapeutic approaches, such as inhibition of pancreatic lipase, might provide a prospective future toward controlling obesity.
Lipase inhibitory activity is the most widely used methodology, with the aim of searching potential anti-obesity agents, without altering central mechanisms.[3] Furthermore, according to previous study, where 32 local medicinal plants were screened, Eleusine indica (E. indica) demonstrated highest pancreatic lipase inhibitory activity.[4] Therefore, in this study, we aimed to explore the toxicity and efficacy of E. indica extracts using in vivo model.
E. indica (Poaceae), also known as goosegrass, is native to the tropical and subtropical regions.[5,6] Traditionally, its root is known to possess depurative, febrifugal, diuretic, and laxative properties. It is commonly used in treating hypertension, influenza, oliguria, and urine retention.[5] The decoction of whole plant is commonly used as an anthelmintic and as a febrifuge.[7] The seeds of E. indica have been occasionally used as famine food, as well as in the treatment of liver complaints.[8] To date, only a handful of research has been conducted on E. indica: anti-inflammatory activity,[9] antioxidant activity,[5,8] antimicrobial activity,[5] hepatoprotective effect,[8] antiplasmodial and antidiabetic effect,[10] cytotoxic activity performed on various cancel cell lines, including MCF-7, HT-29, CEM-SS, A549, HeLa,[5,11] and anti-inflammatory activity.[9]
To the best of our knowledge, the data on oral toxicity of E. indica hexane extract (H-Ei) are still lacking. Recently, a study demonstrated the efficacy of 150 and 300 mg/kg/day aqueous extract of E. indica on hepatic damaged rats; however, their toxicity was not analyzed.[8] Therefore, it is crucial to investigate the acute toxicity effects of H-Ei via oral administration. In view of its potential use as a medicinal drug, in this study, we aimed to identify the oral toxicity and efficacy of E. indica hexane fraction on high-fat diet (HFD)-induced hyperlipidemic rats.
MATERIALS AND METHODS
Plant materials
The whole plant of E. indica (L.) Gaertn. was collected from a herb farm under the patronage of the Traditional Herb Association of Negeri Sembilan in Pantai, Negeri Sembilan, Malaysia (coordinates: 2 46’ 13"N, 101 59’40"E). The plant specimen was authenticated by Dr. Fadzureena Jamaludin, a botanist from the Forest Research Institute of Malaysia (FRIM). A voucher specimen (Code 003/15) was also deposited in Taylor's University (Lakeside Campus), Subang Jaya, Malaysia.
Extraction and preparation of active extract
The whole plant of E. indica was first cleaned to remove residual dirt by washing with tap water. The samples were then freeze-dried and pulverized into a fine powder. Methanol (analytical grade, Merck) was added (1:10, w/v) then left for an hour and sonicated intermittently. The extracts were repeatedly extracted (three times) until the filtrate turned light colored. The extract was filtered through Whatman (Maidstone, UK) Grade 1 filter paper (pore size: 11 μm) under reduced pressure. All filtrates were subsequently pooled together and the solvent was evaporated using rotary evaporator (Heidolph, Schwabach Germany). The dried, solvent-free extract was freeze dried and stored at -20°C until further use.
The crude methanolic extract was subjected to sequential extraction employing a solvent gradient with increasing polarity: hexane (H-Ei), dichloromethane (DCM-Ei), ethyl acetate (EA-Ei), butanol (B-Ei), and water (W-Ei). Each solvent extract obtained was subjected to porcine pancreatic lipase (PPL) inhibition assay as described previously.[4] Solvent extract with highest PPL inhibitory activity (H-Ei) was then subjected to in vivo study for its toxicity and efficacy.
High-performance liquid chromatography
The fingerprinting of the H-Ei extract was performed on Shimadzu Prominence Series coupled with photodiode array detector SPD-M20A using Phenomenex Luna Silica column (250 mm × 4.6 mm, 100 Å, 5 μm). The mobile phase consisted of solvent A, hexane, and solvent B, 2-propanol, with an injection volume of 20 µL of 1 mg/mL H-Ei. The gradient program was as follows: 100% A (0-5 min) and 100-0% A (5-25 min) at a constant flow rate of 1 mL/min.
Animals and housing conditions
Animal protocol was approved by Animal Ethics Committee of Taylor's University (Lakeside Campus) through its reference no. TUL 2013-005. Single-sex (female) Sprague–Dawley rats, aged 6–8 weeks were obtained from Monash University (Sunway Campus, Malaysia). The animals were acclimatized in polypropylene cages with free access to food and water for at least 1 week in temperature-controlled room (22 ± 2°C) under a photoperiod of 12 h. During the acclimatization period, the animals were supplied with standard pellet diet and water ad libitum.
Sample size calculation
Calculation of sample size was based on the following formula[12]:
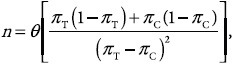
where n = sample size; α = significance level; (1- β) = power θ = (Zα + Z2β)2 = ordinates of normal distribution, when α = 0.05 and (1 - β) = 0.8, θ = 7.8;
π = proportion of anticipated response, where πc = proportion in the control group = 0, πT = proportion in the treated group = 0.5.
When values are put into the equation: 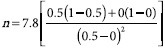
n = 7.8 Therefore, 10 rats were used in each group as a safety margin.
Administration of doses
Orlistat/H-Ei was orally administered using a 16-G (3 in.) gavage needle, with each administration not more than 1 mL in total volume. The emulsion of orlistat/H-Ei was prepared using Tween 80 (<5% v/v) and water as the vehicle. Control group was administered with vehicle without test compound.
Toxicity analysis of H-Ei-fixed-dose procedure
The toxicity analysis was conducted based on the fixed-dose procedure of the Organization for Economic Cooperation and Development guidelines test no. 420 (2002),[13] which comprises a sighting study and a main study. The starting dose used was 300 mg/kg. The classification of the test extract was determined based on Globally Harmonized System (GHS).
Efficacy study of H-Ei
Induction of hyperlipidemia
The animals were divided randomly into six groups. Five groups fed with HFD pellet (where 60% of the calories contained were contributed by fat) for 21 days. The rats were deemed as hyperlipidemic based on the significant elevation of the serum lipid profile [total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL)] when compared with the normal control group.
Treatment
The following six groups (n = 10 for each group) were formed: animals fed with normal diet (NFD), HFD group, orlistat (30 mg/kg/day) as the standard drug group, and the treatment group with H-Ei at 150, 300, and 600 mg/kg/day. The treatment was started on the 29th day after acclimatization and the induction of hyperlipidemia and then continued further for 14 days.[14] On 43rd day after an overnight fast, all animals were sacrificed after performing anesthesia using a cocktail of ketamine (100 mg/kg) and xylazine (10 mg/kg) intraperitoneally. Subsequently, cardiac puncture was performed followed by infusion of 10% (v/v) formaldehyde-saline solution prior to organ harvesting. Hepatic, visceral, and subcutaneous adipose tissues were collected and stored immediately in a 10% (v/v) formaldehyde solution for histopathologic analysis.
Determination of body weight and food intake
Body weight of each rat was measured once in a week and the food intake was assessed once every 2 days.
Determination of serum lipid, hepatic, and renal function profiles
Serum was collected on 29th day (pretreatment and confirmation of hyperlipidemia) via tail vein and on 43rd day (posttreatment) via cardiac puncture. The following parameters were tested: TC, TG, HDL, LDL, albumin, total and direct bilirubin, aspartate transaminase (AST), alanine transaminase (ALT), urea, and creatinine. All serum parameters were determined using the automated chemical analyzer, LWC100 (Landwind, Hungary).
Histopathologic evaluation
The fixed-tissue samples (in 10% v/v formaldehyde solution) were impregnated using sequential immersion in increasing concentrations of ethanol to remove any residual water. This was followed by a clearing step involving increasing concentrations of xylene until the tissue became transparent. Tissue infiltration was done right after the clearing step, where the tissue samples were submerged into paraffin:xylene solution (1:1), with gradually increasing temperature (40, 50, and 55°C) for 24 h each. Finally, the samples were immersed in fresh molten paraffin at 60°C for 3 days. The impregnation and infiltration were done by SLEE Mainz MTP Tissue Processor (SLEE Medical GmbH, Germany).
The 5 µm thick tissue samples were prepared at an angle of 10° using a SLEE Mainz Microtome CUT 5062 (SLEE Medical GmbH, Germany) followed by staining with hematoxylin (Merck KGaA, Germany) and eosin (Merck KGaA, Germany). The stained sections were then mounted on a glass slide with a drop of DPX-mounting medium (Merck KGaA, Germany). A glass cover slip was placed on top to protect the specimens.
Images of the stained slides were captured using Nikon Eclipse 55i connected to a camera head (Nikon DS-Fi2). The quantitative analysis of these images was done using NIS-Elements D (Documentation) (version 4.20.00).
Statistical analysis
All results are expressed as either mean ± standard deviation (SD) or mean ± standard error of the mean (SEM). The data set were explored to determine the model of statistic (either parametric or nonparametric) to be used. Independent sample t-test and paired t-test were used for parametric data set; Kruskal-Wallis and Dunnett's T3 test were used for nonparametric data set. A P value less than 0.05 was considered significant. IBM SPSS software (version 16.0) was used for data analysis.
RESULTS AND DISCUSSION
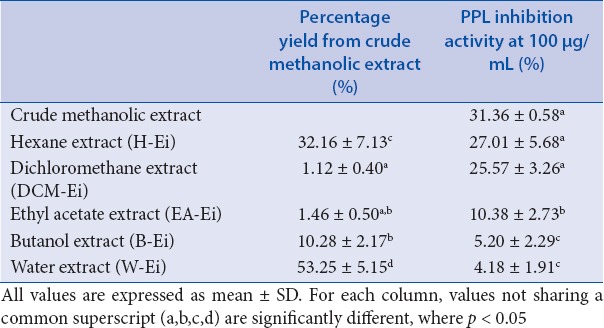
We previously reported that the crude methanolic extract of E. indica possessed the highest PPL inhibitory activity of 31.36 ± 0.58% (at 100 μg/mL) among the 32 plants that were evaluated.[4] In continuation with the aforementioned study, crude methanolic extract was fractionated into hexane (H-Ei), dichloromethane (DCM-Ei), ethyl acetate (EA-Ei), butanol (B-Ei), and water (W-Ei) extracts. All the fractionated extracts were tested for PPL inhibitory activity at the concentration 100 μg/mL. Among the tested extracts, H-Ei exhibited the strongest PPL inhibitory activity of 27.01 ± 5.68% at 100 mg/mL [Table 1]. In addition to the highest PPL inhibitory activity, H-Ei also recorded a high percentage of yield (0.73% yield of the dried plant materials). Therefore, H-Ei was selected for further in vivo toxicity evaluation and detection of lipid-lowering potential in HFD-fed animal models. To the best of our knowledge, there are no studies reporting on the anti-obesity property of E. indica. Therefore, in this study, we aimed to explore its potential as an effective and safer alternative medicine to treat obesity.
Table 1.
Percentage yield and PPL inhibitory activity of different solvent extracts of E. indica
High-performance liquid chromatography fingerprinting
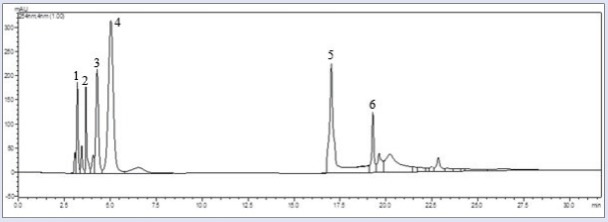
High-performance liquid chromatography (HPLC) fingerprints of H-Ei [Figure 1] showed the presence of six major peaks with their retention time of 3.2, 3.7, 4.2, 5.0, 17.0, and 19.3 min. The subsequent batches of fractionated H-Ei extracts were analyzed based on this HPLC fingerprint to ensure that a similar profile was obtained prior to subjecting them to in vivo studies.
Figure 1.
HPLC profile of H-Ei at 254 nm
Toxicity analysis of H-Ei in Sprague–Dawley model fixed-dose procedure
In this study, we used Sprague-Dawley rats to determine the toxicity and efficacy of H-Ei. Sprague-Dawley rats are commonly used in vivo model for studying obesity and its related metabolic disorders.[15] A total of six animals were used: two for the initial sighting study (300 and 2000 mg/kg, respectively) and another four for the subsequent main study (2000 mg/kg each). All six animals were reported healthy and showed no signs of clinical toxicity symptoms (11 clinical symptoms were evaluated that included salivation, lacrimation, exophthalmos, piloerection, aggressiveness, tremors, convulsions, gasping, straub tail, ptosis, circling, and jumping). All rats also recorded normal increment of body weight [Table 2]. The serum profile for both tested concentrations showed no significant difference with one another [Table 3], and the internal organs were free from any gross pathologic (data not shown) and histopathologic [Figure 2] changes or damage. Consequently, the acute toxicity of H-Ei falls into GHS Category 5, where the hazard of H-Ei is low (2000 < LD50 < 5000).[13]
Table 2.
Effects of H-Ei on the body weight of the Sprague--Dawley female rats during the 14-day toxicity study
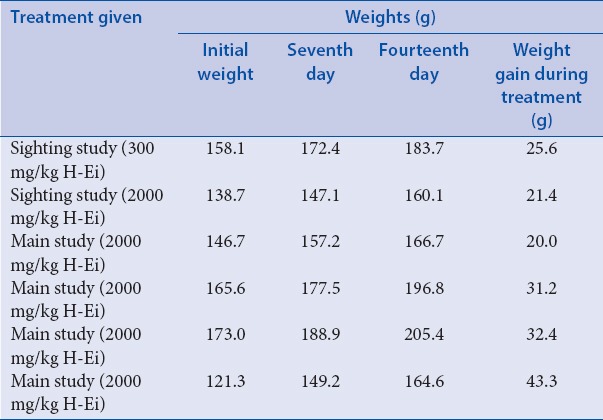
Table 3.
Effects of H-Ei on the serum profile of the Sprague--Dawley female rats during the toxicity study
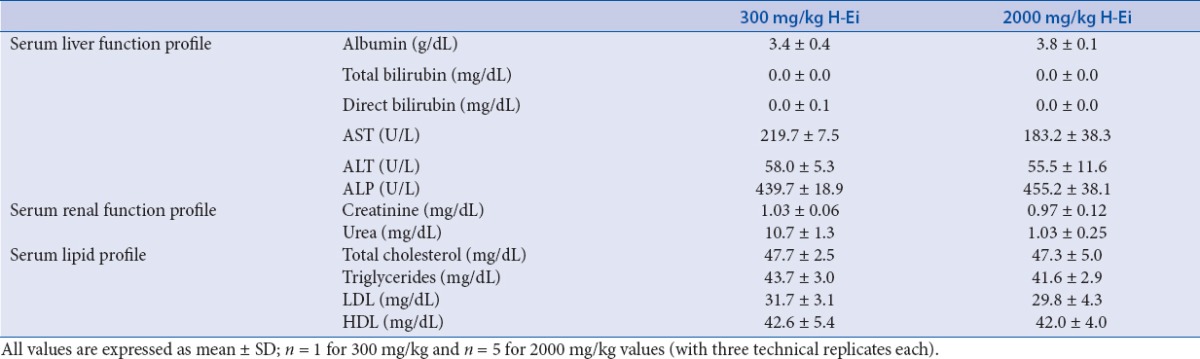
Figure 2.
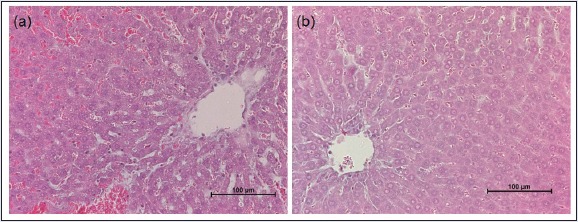
Histology of stained rat liver specimen tested with (a) 300 mg/kg and (b) 2000 mg/kg of H-Ei. Hematoxylin and eosin, ×200
Effect on food intake and anthropometric parameters in the rats
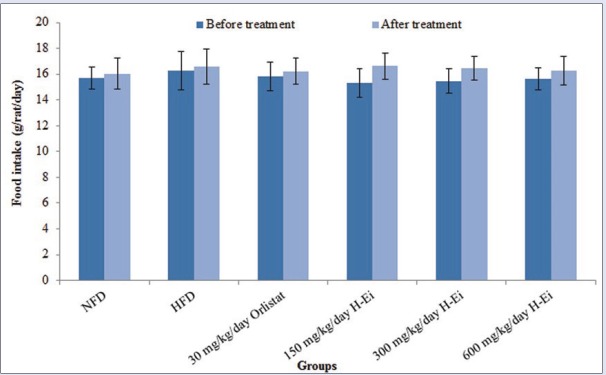
During the oral administration of H-Ei to their respective treatment group, the food intake by the rats was recorded and compared with NFD, HFD, and orlistat groups [Figure 3]. There were no significant differences in terms of the amount of food intake among all the treatment groups and NFD and HFD group when compared with their respective pretreatment group. Throughout the treatment period, the food intake was found to be in a consistent range of 15–17 g/rat/day. This result agrees with that of Karmase et al.,[16] who also reported a consistent food intake of 10–16 g/rat/day by male Sprague–Dawley rats. This signifies that H-Ei does not possess appetite-suppressing effects.
Figure 3.
Effects of H-Ei on the food intake of rats of pretreatment (21st day) and posttreatment (35th day). All values are expressed as mean ± SEM; n = 10 values. Statistically significant effects were compared using one-way ANOVA and paired samples t-tests (P < 0.05)
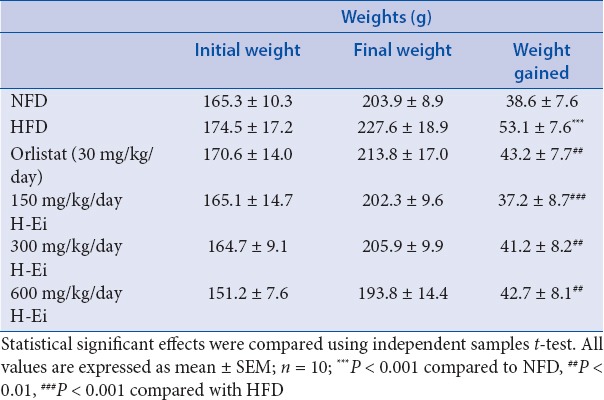
After 21 days of diet initiation and 14 days of treatment, significant (P < 0.001) weight gain was observed in the HFD group when compared with the NFD group [Table 4]. The weight gain in orlistat and all the treatment groups (treated with H-Ei) were significantly (P < 0.01) lower than the group fed with HFD. In the group treated with 600 mg/kg/day of H-Ei, an increase in the body weight was recorded with only 37.2 ± 8.7 g (P < 0.001), which was the lowest among the six studied groups.
Table 4.
Effects of H-Ei on the body weight in Sprague--Dawley female rats before and after 14 days of treatment
Effect on serum lipid profile
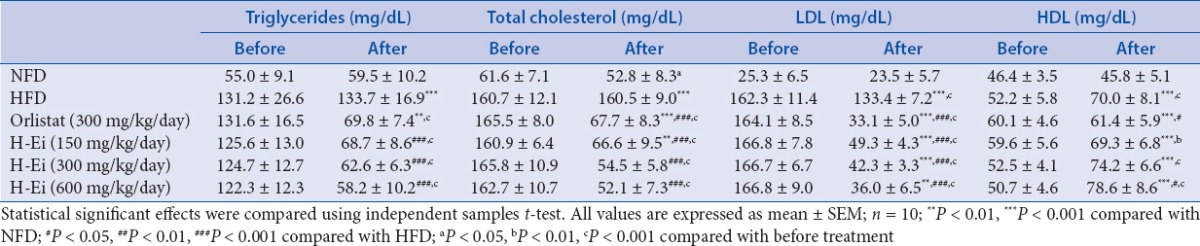
[Table 5] presents the results of the serum lipid profile. It is observed that HFD group showed significant elevation (P < 0.001) in serum TG, TC, and LDL levels by 2.25-, 3.04-, and 5.68- fold, respectively, as compared with those of the NFD control group. Another comparison among the pre- and posttreatment data showed significant (P < 0.05) reductions in all the tested parameters (except HDL in orlistat). H-Ei administration demonstrated a significant decrease (P < 0.05) in dietary TG absorption, where inhibition of pancreatic lipase plays a major role in the absorption of dietary fat.[17] Apart from improving the lipid profile, H-Ei was also found to reduce the development of hypertension in HFD-fed obese rats. When compared with the NFD group, the H-Ei group showed significant elevation in HDL concentrations. Obesity is commonly associated with dyslipidemia, which is identified by the elevation of TG and reduced HDL concentrations.[18] Several factors such as age, family history, and lifestyle play a role in causing heart disease, but TC and TG are reported to be strongly associated withcoronary heart disease.[19,20]
Table 5.
Effects of H-Ei on the serum lipid profile in Sprague--Dawley female rats before and after 14 days of treatments
Effect of H-Ei on hepatic function
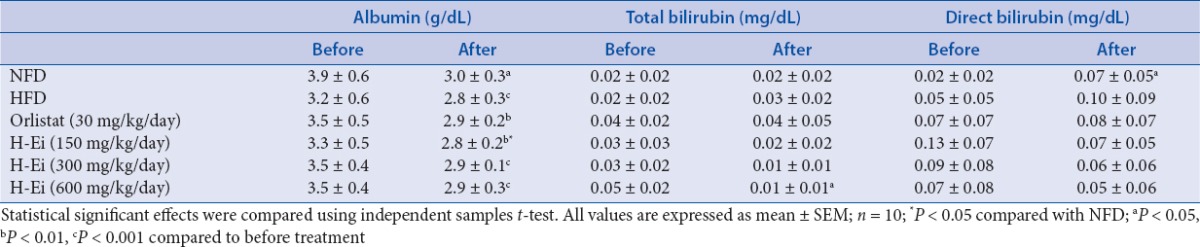
Both AST and ALT were reported to be sensitive indicators of hepatic injury.[21] In this study, the level of AST and ALT [Table 6] were elevated significantly (P < 0.01) after the induction of hyperlipidemia for 3 weeks followed by 2 weeks of blank vehicle treatment. However, the H-Ei-treated group in higher concentration had significantly lowered the level of AST, ALT, and albumin [Table 7], which was comparable to that of the NFD control group, suggesting the hepatoprotective effect of E. indica. In addition, this finding is consistent with the report by Iqbal and Gnanaraj,[8] wherein aqueous extract of E. indica had demonstrated hepatoprotective effects in carbon tetrachloride (CCl4)-induced hepatic injury in rats. Other than that, total bilirubin and direct bilirubin levels were not significantly altered in all groups [Table 7].
Table 6.
Effects H-Ei on the serum liver function profile of the Sprague--Dawley female rats before and after 14 days of treatments
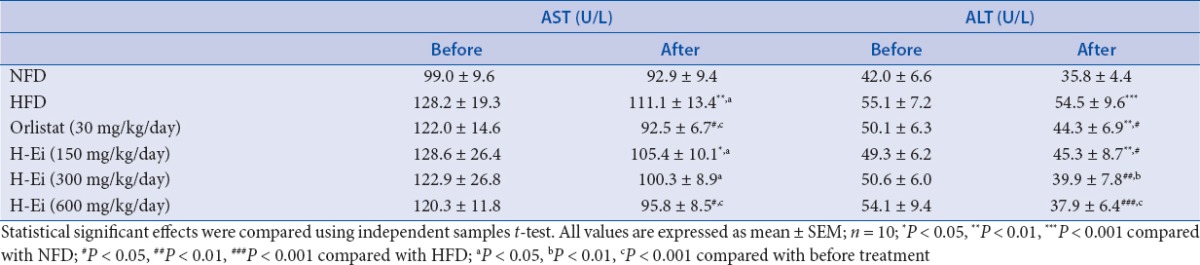
Table 7.
Effects H-Ei on the serum liver function profile of the Sprague--Dawley female rats before and after 14 days of treatments
Effect of H-Ei on histopathology hepatic, visceral, and subcutaneous adipocytes
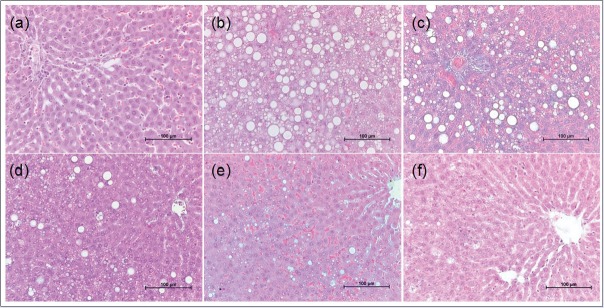
Histologic sections of hepatic tissue from HFD group presented an intense microvacuolization indicating steatosis and degenerative changes [Figure 4]. These changes were noticeably reduced in H-Ei-treated group, and to a smaller extent in the orlistat-treated group. As shown in Figure 5, both the lipid droplet count and its diameter in the hepatic tissue were significantly (P < 0.05) reduced compared with that of the hepatic lipid globules found in the HFD group. It is noteworthy that TGs are often involved in the ectopic accumulation of lipid storage in the liver and are associated with a number of diseases, including metabolic syndrome.[18] Our results showed improvements in the histologic features of hepatic tissue induced by HFD in rats, which correspond well to the serum lipid profiles.
Figure 4.
Histology of liver tissue of six different treatment groups including control: (a) NFD; (b) HFD; (c) HFD + 30 mg/kg/day orlistat; (d) HFD + 150 mg/kg/day H-Ei; (e) HFD + 300 mg/kg/day H-Ei; (f) HFD + 600 mg/kg/day H-Ei. Hematoxylin and eosin, ×200
Figure 5.
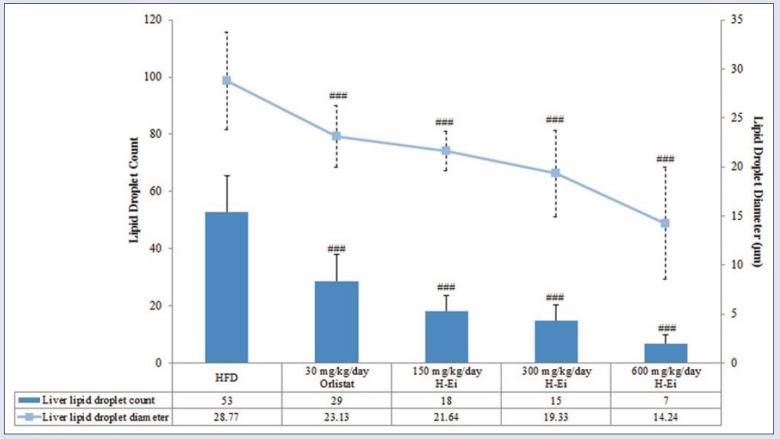
Liver lipid droplet count and diameter in each treatment group after 14 days of treatment prior to 21 days of induced diet condition. Statistical analysis was conducted using nonparametric test using Kruskal–Wallis and Dunnett's T3 as posthoc test. All values were expressed as mean ± SEM, where n = 10; ###P < 0.001 compared with HFD
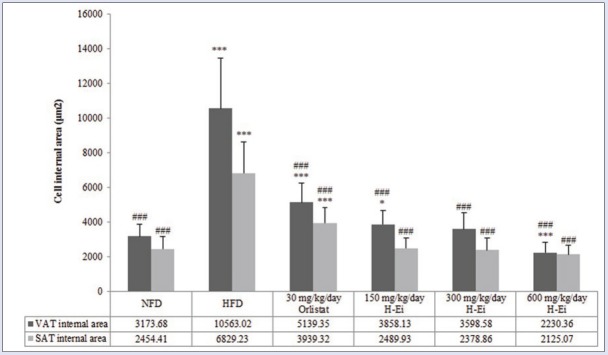
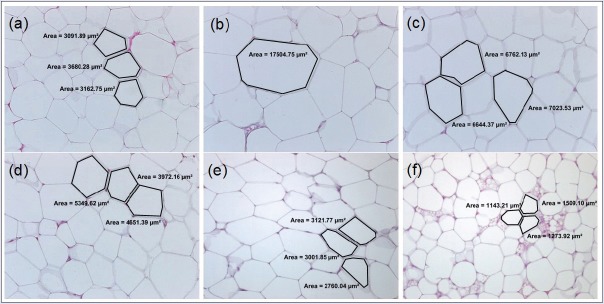
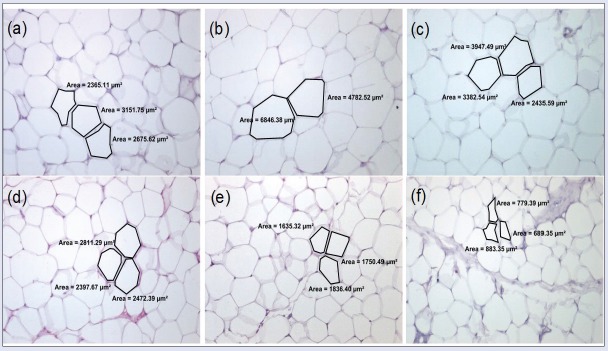
In general, as shown in [Figure 6], the adipose cell size (internal surface area) in H-Ei-treated group did not differ from that of NFD control group, especially in higher dosage. In 600 mg/kg/day H-Ei-treated group, a significantly lower (P < 0.001) internal surface area in adipose cell was seen (2230.36 µm2). In addition, [Figures 7 and 8] show the histologic sections of both visceral and subcutaneous adipose tissues. HFD group presented enhanced adipose cell size when compared with that of NFD group. It can be understood that H-Ei supplementation in Sprague–Dawley rats reversed the changes in the histo-architecture of the adipocytes, thus further justifying the role of H-Ei in improving HFD-induced adiposity. Moreover, it was previously reported that the weight of adipose tissue, adipose cell size, and cell number were increased in obese animals that were induced with HFD.[22] Similarly, HFD-fed obese mice fed with aqueous extract of leaf of Clerodendron glandulosum (CG) recorded significant increment in size and mass of abdominal, renal, and epididymal fat pads, whereas this increment was prevented in CG-supplemented obese mice.[23]
Figure 6.
Mean surface area of visceral adipose tissue and subcutaneous adipose tissue (SAT) in each treatment group after 14 days of treatment prior to 21 days of induced diet condition. Statistical analysis was conducted using nonparametric test using Kruskal–Wallis and Dunnett's T3 as posthoc test. All values were expressed as mean ± SEM, where n = 10; *P < 0.05, ***P < 0.001 compared with NFD; ###P < 0.001 compared with HFD
Figure 7.
Histology of visceral adipose tissue of six different treatment groups including control: (a) NFD; (b) HFD; (c) HFD + 30 mg/kg/day orlistat; (d) HFD + 150 mg/kg/day H-Ei; (e) HFD + 300 mg/kg/day H-Ei; (f) HFD + 600 mg/kg/day H-Ei. Hematoxylin and eosin, × 200
Figure 8.
Histology of subcutaneous adipose tissue (SAT) of six different treatment groups including control: (a) NFD; (b) HFD; (c) HFD + 30 mg/kg/day orlistat; (d) HFD + 150 mg/kg/day H-Ei; (e) HFD + 300 mg/kg/day H-Ei; (f) HFD + 600 mg/kg/day H-Ei. Hematoxylin and eosin, × 200
In conclusion, H-Ei demonstrated marked evidence in inhibiting the development of obesity and hyperlipidemia in HFD-induced hyperlipidemic Sprague-Dawley rats. This inhibition was not found to be dependent on decreased food or energy intake, as there was no significant difference between the HFD control group and H-Ei-treated groups. Furthermore, this is the first study reporting on the in vivo toxicity and efficacy data on E. indica. In this study, we demonstrated the anti-obesity properties of E. indica suggesting its potential role to be an anti-obesity agent from natural sources. Its effect was seen via pancreatic lipase inhibition activity. Further studies using animal models are necessary prior to human clinical investigations, as these tests form part of the nonclinical laboratory tests of pharmaceuticals.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest
Acknowledgements
This work was supported by grants from Taylor's Research Grant Scheme TRGS/2/2011SOBS/012 and Postgraduate Research Scholarship Programme. The authors are grateful to Dr. Nilesh Kumar Mitra from School of Medicine, Taylor's University (Lakeside Campus) for his expert guidance during the histopathologic analysis.
REFERENCES
- 1.Bustanji Y, Al-Masri IM, Mohammad M, Hudaib M, Tawaha K, Tarazi H, et al. Pancreatic lipase inhibition activity of trilactone terpenes of Ginkgo biloba.J. Enzyme Inhib Med Chem. 2011;26:453–9. doi: 10.3109/14756366.2010.525509. [DOI] [PubMed] [Google Scholar]
- 2.WHO. int [Obesity and overweight] Geneva: World Health Organisation; [updated 2015 Jan; cited 2015 Nov 24]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/ [Google Scholar]
- 3.Yun JW. Possible anti-obesity therapeutics from nature: a review. Phytochemistry. 2010;71:1625–41. doi: 10.1016/j.phytochem.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Ong SL, Paneerchelvan S, Lai HY, Rao NK. In vitro lipase inhibitory effect of thirty two selected plants in Malaysia. Asian J Pharm Clin Res. 2014;7:19–24. [Google Scholar]
- 5.Al-Zubairi AS, Abdul AB, Abdelwahab SI, Peng CY, Mohan S, Elhassan MM. Eleusine indica possesses antioxidant, antibacterial and cytotoxic properties. Evid Based Complement Alternat Med. 2011:96537. doi: 10.1093/ecam/nep091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haber RM, Semaan MT. Two new records fron Lebanon: Chamaesyce nutans (Lag.) small (Euphorbiaceae) and Eleusine indica (L.) Gaertner (Poaceae) Turk J Botany. 2007;31:341–3. [Google Scholar]
- 7.Kulip J. A preliminary survey of traditional medicinal plants in the west coast and interior of Sabah. J Trop for Sci. 1997;10:271–4. [Google Scholar]
- 8.Iqbal M, Gnanaraj C. Eleusine indica L possesses antioxidant activity and precludes carbon tetrachloride (CCl4)-mediated oxidative hepatic damage in rats. Environ Health Prev Med. 2012;17:307–15. doi: 10.1007/s12199-011-0255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melo G, De Muzitano M. C-Glycosylflavones from the aerial parts of Eleusine indica inhibit LPS-induced mouse lung inflammation. Planta Med. 2005;71:362–3. doi: 10.1055/s-2005-864104. [DOI] [PubMed] [Google Scholar]
- 10.Okokon J, Effiong I. Antiplasmodial and antidiabetic activities of Eleusine indica. Int J Drug Dev Res. 2010;2:493–500. [Google Scholar]
- 11.Hansakul P, Ngamkitidechakul C, Ingkaninan K, Sireeratawong S, Panunto W. Apoptotic induction activity of Dactyloctenium aegyptium (L.) P.B and Eleusine indica (L) Gaerth Extracts on human lung and cervical cancer cell lines. Songklanakarin J Sci Technol. 2009;31:273–9. [Google Scholar]
- 12.Machin D, Campbell MJ, Walters SJ. Comparison of proportions. Medical statistics West Sussex, UK: Wiley; 2007. p. 273. [Google Scholar]
- 13.OECD OECD Guidelines for the Testing of Chemicals, Section 4, Test No 420: Acute Oral Toxicity – Fixed Dose Procedure. 2002 doi: 10.1787/9789264070943-en. [Google Scholar]
- 14.Bustanji Y, Mohammad M, Hudaib M, Tawaha K, Al-Masri IM, AlKhatib HS, et al. Screening of some medicinal plants for their pancreatic lipase inhibitory potential. Jordan J Pharm Sci. 2011;4:81–8. [Google Scholar]
- 15.Birari R, Javia V, Bhutani K. Antiobesity and lipid lowering effects of Murraya koenigii (L.) Spreng leaves extracts and mahanimbine on high fat diet induced obese rats. Fitoterapia. 2010;81:1129–33. doi: 10.1016/j.fitote.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Karmase A, Birari R, Bhutani KK. Evaluation of anti-obesity effect of Aegle marmelos leaves. Phytomedicine. 2013;20:805–12. doi: 10.1016/j.phymed.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Lei F, Zhang XN, Wang W, Xing DM, Xie WD, Su H, et al. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int J Obes. 2007;31:1023–9. doi: 10.1038/sj.ijo.0803502. [DOI] [PubMed] [Google Scholar]
- 18.Bhandari U, Chaudhari HS, Bisnoi AN, Kumar V, Khanna G, Javed K. Anti-obesity effect of standardized ethanol extract of Embelia ribes in murine model of high fat diet-induced obesity. Pharmanutrition. 2013;1:50–7. [Google Scholar]
- 19.Chang CJ, Tzeng TF, Liou SS, Chang YS, Liu IM. Kaempferol regulates the lipid-profile in high-fat diet-fed rats through an increase in hepatic PPARα levels. Planta Med. 2011;77:1876–82. doi: 10.1055/s-0031-1279992. [DOI] [PubMed] [Google Scholar]
- 20.Palmer A, Nova E, Anil E, Jackson K. Differential uptake of subfractions of triglyceride-rich lipoproteins by THP-1 macrophages. Atherosclerosis. 2005;180:233–44. doi: 10.1016/j.atherosclerosis.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 21.Krishna K, Mruthunjaya K, Patel J. Antioxidant and hepatoprotective activity of leaf extract of Justicia gendarussa Burm. Int J Biol Chem. 2009;3:99–110. [Google Scholar]
- 22.Han LK, Kimura Y, Kawashima M, Takaku T, Taniyama T, Hayashi T, et al. Anti-obesity effects in rodents of dietary teasaponin, a lipase inhibitor. Int J Obes Relat Metab Disord. 2001;25:1459–64. doi: 10.1038/sj.ijo.0801747. [DOI] [PubMed] [Google Scholar]
- 23.Jadeja RN, Thounaojam MC, Ramani UV, Devkar RV, Ramachandran V. Anti-obesity potential of Clerodendron glandulosum Coleb leaf aqueous extract. J Ethnopharmacol. 2011;135:338–43. doi: 10.1016/j.jep.2011.03.020. [DOI] [PubMed] [Google Scholar]